OSHA Salt Lake Technical Center (SLTC) Project
Utilization of Direct Reading Monitors on
Hazardous Waste Sites to Improve Worker Protection
While Reducing Costs of Unnecessary
PPE (Personal Protective Equipment) and Sample Analyses
PURPOSE: As documented during the incinerator project inspections, many hazardous waste sites are not conducting effective worker exposure monitoring. A major result of this deficiency is that workers are using unnecessarily high levels of PPE with its associated costs (Millions of dollars in PPE, maintenance, work-rest regiments, etc.) and risks (e.g., heat stress, impaired vision). This project would implement "model" worker exposure monitoring programs at representative hazardous waste sites to demonstrate the utility of direct-reading and/or on-site analyses sampling methods to both improve exposure characterization and allow for altering required PPE and other controls with changing exposure conditions (e.g., a worker would only wear a respirator when it is needed). Example techniques include direct-reading warning devices for transient high levels of toxic compounds and field tests, such as immunoassay or PCR, to classify appropriate PPE levels for normal operating conditions.
Field analysis of air samples to determine the source and extent of releases would be performed at reduced cost compared to laboratory analyses and within a much shorter time frame. The short sample analysis turnaround for field analyses will allow a much faster response to releases, return from evacuations, and selection of appropriate PPE levels.
This project will utilize existing technologies and exposure monitors (self reading and alarmed) that have already been developed and field evaluate them on hazardous waste site workers. Example contaminants which are found at hazardous waste sites and which have potential for this application include PCB's, chlorinated hydrocarbons, heavy metals, total VOC's, pesticides, PNA's, etc.
This project will involve the analytical chemistry section of the SLTC to evaluate the monitors and field analyses under laboratory conditions to determine their accuracy and reliability and the HRT section of the SLTC or contractors to perform the field evaluations.
The following report describes the results of the laboratory and limited field tests of a personal-sized direct-reading/alarmed, photoionization detector, monitoring instrument as utilized for this project.
After evaluating both the laboratory and field tests, the following points stand out:
- The MicroRAE Turbo PID can be used in the field when proper factors of safety are built into the alarm settings. The unit will require a trained person to determine the alarm settings and later interpret the output. This is especially true considering the problems identified in the laboratory tests
- The MicroRAE Turbo PID can be used as a personal monitor to indicate when PPE should be upgraded.
-
- The MicroRAE Turbo PID should be used as a means to alert the industrial hygienist of a potential problem. It should not be used to determine compliance or where accuracy is important.
Use of the MicroRAE Turbo Photoionization Detector (PID) For Aiding the Selection of Appropriate Personal Protective Equipment (PPE) in Hazardous Waste Operations
Table of Contents
1.0
Introduction
2.0
Methods and Materials
3.0
Discussion
4.0
Conclusions
Appendix 1
Field Testing Report of the Model PGM-22 MicroRAE Turbo Photoionization Detector
Appendix 2
MicroRAE Turbo Field Evaluation Protocol
Appendix 3
MicroRAE Turbo Laboratory Evaluation Protocol
Appendix 4
Laboratory Evaluation of the MicroRAE Model PGM-22 Portable Photoionization Detector
1.0 Introduction
The purpose of this final report is to summarize the findings of the field and laboratory testing of the MicroRAE Turbo Photoionization detector made by RAE Systems Inc. The MicroRAE PID was evaluated to determine if it could be used as a personal monitor on a hazardous waste site to warn the user, through an alarm, if they were exceeding some predetermined organic chemical concentration. The alarm would also alert the on-site industrial hygienist and help him decide if an upgrade in personal protective equipment (PPE) would be needed for the task being undertaken.
2.0 Methods and Materials
This study consisted of three phases. The first phase was a literature review of products available on the market that could be used by an industrial hygienist on a hazardous waste site to quickly evaluate changing working conditions and make appropriate modifications to PPE. The equipment needed to be light weight and easily worn. It also needed to be responsive to a broad range of organic compounds and with few interfering agents.
The second phase was to take the selected equipment and test it in a laboratory environment to identify inherent problems. A laboratory protocol (Appendix 4) was developed which tested the equipment and documented equipment responses to chemical concentrations under various physical conditions. A laboratory test report was prepared which summarized the equipment responses (Appendix 2).
The final phase was to take the equipment to a hazardous waste site for field testing (Appendix 1) per a protocol (Appendix 3) during actual hazardous waste site activities. In this particular case, Operative Unit 2 at Hill AFB base was selected as the test site. This particular site offered a wide variety of chemicals in the waste stream and an opportunity to visually observe the facility operator while he performed all of his field activities without having to have the persons conducting the test don protective equipment. The site was especially useful because the normal working environment did not present an airborne hazard. However, during the course of each day, the facility operator undertook specific activities that did have significant acute organic chemical exposures. This variety of exposures provided an unique opportunity to test the unit as a personal monitor under a wide range of organic chemical concentrations.
3.0 Discussion
The MicroRAE Turbo photoionization detector (PID) made by RAE Systems Inc. was the unit chosen to be tested on a hazardous waste site where the airborne hazard was from organic compounds that could be detected by photoionization techniques. During the preliminary evaluation of the MicroRAE unit, it was discovered that the 11.7 eV lamp had a short shelf-life and that the 10.2 eV lamp was the highest eV lamp that could be practically used. This limitation reduced the unit's ability to distinguish between some organic compounds but it did not eliminate its usefulness.
During the laboratory testing of the MicroRAE PID, the unit was found to be sensitive to humidity, temperature, and electromagnetic interference. The results of the laboratory tests raised doubts about whether the unit could be used in the field as a personal monitor. In the report that interpreted the laboratory test results, the author noted that "[i]n the event that the meters are used in the field, it is suggested that these physical conditions be carefully accounted for in calibration and use procedures so that meaningful and accurate data may be obtained". This point is well founded based on a scientific evaluation of the data. However, from a practical prospective and with the full knowledge of the unit's limitations, an industrial hygienist can successfully use the unit with a proper factor of safety built into the alarm settings. These inherent problems with the MicroRAE limits the usefulness of the unit for a company that does not have an industrial hygienist or other individual with a comparable practical and theoretical background. The person using the unit must be able to initially establish an appropriate alarm protocol and later properly interpret the hazard of the work environment where the alarm went off.
During the field testing of the unit, the lamp, temperature, humidity, and electromagnetic limitations did not adversely affect the units perceived performance. It should be noted that the chemical compound chosen as the surrogate for the chemicals on the hazardous waste site was trichloroethylene which does have a good response with the 10.2 eV lamp. Also, the test environment was not extreme relative to either the temperature or humidity. The humidity did exceed fifty percent for a brief period of time on two mornings but dropped to less than forty percent as each day progressed. Electromagnetic interference was suspected of causing the alarm to sound on the personal monitor for a brief period of time during the second day of testing. However, for all cases, with the exception of the suspected electromagnetic interference instance referenced above, the unit only alarmed during work activities that could reasonably produce airborne levels sufficient to exceed the alarm settings. Several times while the alarm was going off, the environment was checked with the contractor's PID and confirmed to be approximately the same airborne concentration as detected by the MicroRAE unit.
4.0 Conclusions
After evaluating both the laboratory and field tests, the following points stand out:
- The MicroRAE Turbo PID can be used in the field when proper factors of safety are built into the alarm settings. The unit will require a trained person to determine the alarm settings and later interpret the output. This is especially true considering the problems identified in the laboratory tests.
- The MicroRAE Turbo PID can be used as a personal monitor to indicate when PPE should be upgraded.
- The MicroRAE Turbo PID should be used as a means to alert the industrial hygienist of a potential problem. It should not be used to determine compliance or where accuracy is important.
Appendix 1
Field Testing Report of the MicroRAE Turbo Photoionization Detector
Table of Contents
1.0
Introduction
2.0
Methods and Materials
3.0
Discussion
4.0
Conclusions
Table 1
Daily MicroRAE Turbo PID STEL Value in PPM
Table 2
MicroRAE Turbo PID Peak and TWA Daily Values
Table 3
Personal Air Sampling Analytical Results
Table 4
Daily Temperature and Humidity Readings
1.0 Introduction
On July 26-31, 1995, Mr. Dick Jordan, a Certified Industrial Hygienist (CIH), and Mr. Todd Jordan, an industrial hygiene graduate student, conducted a field level evaluation of the MicroRAE Turbo photoionization detector (PID) made by RAE Systems Inc. of Sunnyvale, California. The evaluation consisted of using the PID in accordance with a field evaluation protocol developed earlier for the unit. The purpose of the field test was to evaluate the performance of the PID under actual use on a hazardous waste site. This field test was performed at the Operable Unit #2 site located at Hill AFB, Utah. This site is being managed by Radian Corporation.
2.0 Methods and Materials
2.1 MicroRAE Turbo PID Direct Reading Instrument
Two MicroRAE Turbo PIDs were used to monitor acute chemical exposures on the hazardous waste site. One was used as an area sampler inside the chemical process building and the other used as a personal monitor and worn by the facilities operator. Each PID had its STEL and Peak alarms set for 91 and 182 ppm respectively. These settings were based upon trichloroethylene because this specific chemical constituted the majority of the waste as identified in the Radian Corporation report titled Site Health and Safety Plan Source Removal System Operable Unit 2, and the trichloroethylene concentration in the groundwater was an order of magnitude greater than any of the other organic chemicals. The STEL alarm setting of 91 ppm is approximately one-half of the ACGIH STEL for trichloroethylene (100 ppm) and adjusted by a MicroRAE correction factor of 0.52 because the unit was calibrated with isobutylene instead of trichloroethylene. The Peak alarm setting of 182 ppm was established in the same manner as the STEL except the OSHA Ceiling (200 ppm) was used as the reference point.
Each PID was calibrated before and after each day's use. Calibration followed the manufacturer's recommended protocol and consisted of checking each unit against a standard isobutylene mixture. Temperature and humidity readings were monitored each day because the MicroRAE units are sensitive to both. Also, the contractor's PID was routinely used to verify readings from the two MicroRAE units.
STELs were recorded every fifteen minutes following activation of the PID. The Peak reading was the highest reading recorded during the monitoring period. Each unit retains only the highest Peak. All concentrations recorded by the MicroRAE units are in parts per million (ppm) and are based upon the isobutylene standard.
2.2 Personal Air Monitoring
Personal air sampling was conducted on the contractor's facility operator at the same time he was wearing the MicroRAE unit. The sampling pump was calibrated both before and after each day's use to ensure the air flow rate was accurately known and remained relatively constant. The pump was checked periodically throughout each day to ensure it was functioning properly. All samples were delivered to the OSHA Salt Lake City laboratory for analysis.
The personal air sampling train consisted of a MSA Flow Lite low flow pump in series with a Gilian manifold. One port of the manifold was connected to two charcoal tubes in series and adjusted for a flow rate of approximately 0.4 liters per minute for about eight hours with the exception of the third day when the operator had to leave the site. For this day the unit ran for about four hours. Each of the other two ports on the manifold were closed.
The personal samples were analyzed for: (1) acetone; (2) 1,2-dichloroethylene; (3) methyl chloroform; (4) methylene chloride; (5) trichloroethylene; (6) tetrachloroethylene; (7) toluene; (8) ethyl benzene; (9) xylene; (10) chlorobenzene. Each of these compounds were selected because they represented the major chemical compounds in the ground water as identified in Radian Corporation report titled Site Health and Safety Plan Source Removal System Operable Unit 2.
3.0 Results
Table 1
Daily MicroRAE Turbo PID STEL Values in PPM
These tables are best viewed on tablets, notebooks, or desktop computer screens.
| STEL Sample Interval | Personal Sampler | Area Sampler | ||||||
|---|---|---|---|---|---|---|---|---|
| July 26, 1995 | July 27, 1995 | July 28, 1995 | July 31, 1995 | July 26, 1995 | July 27, 1995 | July 28, 1995 | July 31, 1995 | |
| 0730-0745 | 0.2 | 0.0 | 1.2 | 0.0 | ||||
| 0745-0800 | 0.1 | 0.0 | 1.6 | 0.0 | ||||
| 0800-0815 | 0.0 | 0.2 | 0.0 | 0.4 | 1.9 | 0.0 | ||
| 0815-0830 | 0.0 | 0.1 | 1.1 | 0.0 | 0.3 | 2.2 | 0.4 | 0.0 |
| 0830-0845 | 0.0 | 0.1 | 0.1 | 0.0 | 0.1 | 2.6 | 0.2 | 0.0 |
| 0845-0900 | 0.0 | 0.0 | 0.5 | 0.0 | 0.2 | 1.8 | 0.0 | 0.0 |
| 0900-0915 | 0.01 | 0.0 | 0.3 | 0.1 | 0.4 | 1.2 | 0.0 | 0.0 |
| 0915-0930 | 7.5 | 0.1 | 0.1 | 0.3 | 0.4 | 0.9 | 0.0 | 0.0 |
| 0930-0945 | 0.0 | 0.4 | 0.0 | 0.0 | 0.4 | 0.6 | 0.0 | 0.0 |
| 0945-1000 | 0.0 | 0.1 | 0.0 | 0.2 | 0.5 | 0.4 | 0.0 | 0.1 |
| 1000-1015 | 0.0 | 0.1 | 0.0 | 0.5 | 0.5 | 0.4 | 0.0 | 0.0 |
| 1015-1030 | 0.3 | 0.1 | 0.1 | 0.1 | 0.5 | 0.4 | 0.0 | 0.0 |
| 1030-1045 | 0.0 | 6.3 | 4.2 | 0.0 | 0.6 | 0.4 | 0.0 | 0.0 |
| 1045-1100 | 0.0 | 0.2 | 0.0 | 0.7 | 0.4 | 0.0 | ||
| 1100-1115 | 1.1 | 0.2 | 0.0 | 0.8 | 0.4 | 0.0 | ||
| 1115-1130 | 0.1 | 0.1 | 0.0 | 0.8 | 0.4 | 0.0 | ||
| 1130-1145 | 0.0 | 0.0 | 0.0 | 0.7 | 0.5 | 0.0 | ||
| 1145-1200 | 0.0 | 0.0 | 0.0 | 0.8 | 0.8 | 0.0 | ||
| 1200-1215 | 0.0 | 0.0 | 0.0 | 0.7 | 0.8 | 0.0 | ||
| 1215-1230 | 0.0 | 0.1 | 0.3 | 0.7 | 0.8 | 0.0 | ||
| 1230-1245 | 0.0 | 0.1 | 0.0 | 0.8 | 0.8 | 0.0 | ||
| 1245-1300 | 0.0 | 0.2 | 0.0 | 0.6 | 0.8 | 0.0 | ||
| 1300-1315 | 0.0 | 0.2 | 0.0 | 0.6 | 0.9 | 0.0 | ||
| 1315-1330 | 0.0 | 0.2 | 0.0 | 0.6 | 0.9 | 0.0 | ||
| 1330-1345 | 0.0 | 0.2 | 0.0 | 0.6 | 0.9 | 0.0 | ||
| 1345-1400 | 0.0 | 0.2 | 7.2 | 0.6 | 0.9 | 0.0 | ||
| 1400-1415 | 0.0 | 0.6 | 0.0 | 0.6 | 0.9 | 0.0 | ||
| 1415-1430 | 0.1 | 0.2 | 0.0 | 0.6 | 0.9 | 0.0 | ||
| 1430-1445 | 4.8 | 0.2 | 0.0 | 0.6 | 0.9 | 0.0 | ||
| 1445-1500 | 0.1 | 0.2 | 122.7 | 0.6 | 0.9 | 4.1 | ||
| 1500-1515 | 5.3 | 0.2 | 55.3 | 0.8 | 1.1 | 15.7 | ||
| 1515-1530 | 32.1 | 0.2 | 1.0 | 1.2 | ||||
| 1530-1545 | 1.2 | 0.2 | 0.6 | 1.2 | ||||
| 1545-1600 | 0.0 | 0.2 | 0.1 | 0.6 | 1.4 | 0.5 | ||
| 1600-1615 | 0.0 | 0.2 | 0.6 | 1.2 | ||||
| 1615-1630 | 0.3 | 0.1 | 0.5 | 1.0 | ||||
| 1630-1645 | 0.1 | 1.0 | ||||||
| 1645-1700 | 16.1 | 0.9 | ||||||
| 1700-1715 | 0.8 | |||||||
Table 2
MicroRAE Turbo PID Peak and TWA Daily Values
These tables are best viewed on tablets, notebooks, or desktop computer screens.
| Date (m/d/y) |
Sample Type |
Peak (ppm) |
TWA (ppm) |
Time On (hrs) |
Description of Peak |
|---|---|---|---|---|---|
| 7/26/95 | Personal | 1002 | 0.3 | 8.5 | Peak occurred between 1515-1530 hrs. during filter replacement |
| 7/26/95 | Area | 199.4 | 0.6 | 8.7 | Not observed |
| 7/27/95 | Personal | 213 | 0.4 | 8.4 | Peak occurred between 1030-1045 hrs. settling tank sampling |
| 7/27/95 | Area | 2.7 | 1.1 | 8.7 | Not observed |
| 7/28/95 | Personal | 593.8 | .7 | 4.0 | Peak occurred between 1645-1700 hrs. during settling tank sampling |
| 7/28/95 | Area | 1.8 | 0.2 | 4.2 | Not observed |
| 7/31/95 | Personal | 689.2 | 5.9 | 7.9 | Peak occurred between 1445-1500 hrs. during filter maintenance |
| 7/31/95 | Area | 270.9 | 0.6 | 7.9 | Not observed |
Table 3
Personal Air Sampling Analytical Results
These tables are best viewed on tablets, notebooks, or desktop computer screens.
| Sample Number | Analyte | Avg. Flow (lpm) |
Sample Duration (min) |
Total Volume (m3) |
Sample Results (ppm) |
OSHA PEL Standard (ppm) |
|---|---|---|---|---|---|---|
| OS950726-01 & OS950726-02 (two tubes in series) |
Acetone 1,2-Dichloroethylene Methyl Chloroform Methylene Chloride Trichloroethylene Tetrachloroethylene Toluene Ethyl Benzene Xylene Chlorobenzene |
0.394 | 423 | 0.167 | <0.010 <0.006 0.18 0.23 1.10 <0.004 <0.006 <0.006 <0.006 <0.005 |
1000 200 350 500 100 100 200 100 100 75 |
| OS950727-03 & OS950727-04 (two tubes in series) |
Acetone 1,2-Dichloroethylene Methyl Chloroform Methylene Chloride Trichloroethylene Tetrachloroethylene Toluene Ethyl Benzene Xylene Chlorobenzene |
0.409 | 525 | 0.215 | <0.008 <0.005 0.021 0.078 0.16 <0.003 <0.005 <0.004 <0.004 <0.004 |
1000 200 350 500 100 100 200 100 100 75 |
| OS950728-05 &OS950728-06 (two tubes in series) |
Acetone 1,2-Dichloroethylene Methyl Chloroform Methylene Chloride Trichloroethylene Tetrachloroethylene Toluene Ethyl Benzene Xylene Chlorobenzene |
0.386 | 252 | 0.097 | <0.017 <0.010 0.17 0.11 1.3 0.045 <0.011 <0.009 <0.009 <0.009 |
1000 200 350 500 100 100 200 100 100 75 |
| OS950731-07 &OS950731-08 (two tubes in series) |
Acetone 1,2-Dichloroethylene Methyl Chloroform Methylene Chloride Trichloroethylene Tetrachloroethylene Toluene Ethyl Benzene Xylene Chlorobenzene |
0.447 | 465 | 0.208 | <0.008 <0.005 0.98 0.33 5.5 0.055 0.0075 <0.004 <0.004 <0.004 |
1000 200 350 500 100 100 200 100 100 75 |
Table 4
Daily Temperature and Humidity Readings
These tables are best viewed on tablets, notebooks, or desktop computer screens.
| Date (m/d/y) | Time | Dry Bulb (°F) |
Wet Bulb (°F) |
Location | Relative Humidity1 (%) |
|---|---|---|---|---|---|
| 7/26/95 | 0835 | 79.0 | 57.8 | outside bldg. | 39 |
| 7/26/95 | 1000 | 81.2 | 57.3 | inside bldg | 36 |
| 7/26/95 | 1100 | 83.0 | 56.3 | inside bldg | 33 |
| 7/26/95 | 1100 | 94.0 | 58.1 | outside bldg | 25 |
| 7/26/95 | 1315 | 85.4 | 56.8 | inside bldg | 31 |
| 7/26/95 | 1320 | 95.5 | 59.7 | outside bldg | 25 |
| 7/26/95 | 1440 | 87.7 | 56.3 | inside bldg | 28 |
| 7/26/95 | 1445 | 90.7 | 57.5 | outside bldg | 27 |
| 7/26/95 | 1645 | 96.0 | 59.7 | outside bldg | 24 |
| 7/27/95 | 0845 | 64.8 | 48.9 | outside bldg | 51 |
| 7/27/95 | 1000 | 75.5 | 56.2 | outside bldg | 41 |
| 7/27/95 | 1120 | 82.5 | 59.0 | outside bldg | 35 |
| 7/27/95 | 1205 | 89.2 | 62.2 | outside bldg | 31 |
| 7/27/95 | 1210 | 83.6 | 59.0 | inside bldg | 33 |
| 7/27/95 | 1345 | 85.3 | 57.2 | inside bldg | 32 |
| 7/27/95 | 1445 | 87.3 | 57.4 | inside bldg | 29 |
| 7/27/95 | 1600 | 87.0 | 58.1 | inside bldg | 30 |
| 7/28/95 | 0750 | 67.8 | 51.4 | outside bldg | 50 |
| 7/28/95 | 0910 | 71.5 | 53.6 | outside bldg | 45 |
| 7/28/95 | 0950 | 76.9 | 56.5 | outside bldg | 41 |
| 7/28/95 | 1420 | 100.3 | 64.5 | outside bldg | 22 |
| 7/28/95 | 1610 | 100.0 | 63.9 | outside bldg | 22 |
| 7/31/95 | 0745 | 55.4 | 45.2 | outside bldg | 67 |
| 7/31/95 | 0910 | 61.5 | 48.6 | outside bldg | 60 |
| 7/31/95 | 1000 | 70.1 | 52.2 | outside bldg | 47 |
| 7/31/95 | 1115 | 74.5 | 55.2 | outside bldg | 43 |
| 7/31/95 | 1200 | 77.0 | 55.2 | outside bldg | 40 |
| 7/31/95 | 1310 | 77.9 | 54.8 | outside bldg | 38 |
| 7/31/95 | 1410 | 83.9 | 57.3 | outside bldg | 33 |
| 7/31/95 | 1505 | 83.8 | 57.2 | outside bldg | 33 |
Notes:
1. Extracted from a Psychrometric Chart where the properties of mixtures of air and water vapor are for a total pressure of 29.92 in. Hg.
4.0 Discussion
The personal PID alarmed during times when the waste stream was being directly handled. The alarms occurred during work activities that were suspected to have the greatest potential for airborne exposures. A review of Table 1, which is a listing of the STELs as they occurred throughout each day, shows large periods of time when the personal PID had very low readings. There were occasions when the readings were elevated but these corresponded to times when the facility operator interfaced directly with the waste stream. The area PID measurements fluctuated less throughout each day but also showed elevated levels of a much lower concentration. These elevated levels for the area PID corresponded to those times when the facility operator was performing routine sampling or maintenance inside the process building and can be matched to elevated levels on the personal PID.
The personal air sampling supported the initial assumption that trichloroethylene was the chemical of greatest concentration and could be used as a surrogate to measure the airborne chemical hazard on the site. A review of the analysis indicates that time-weighted average (TWA) exposures are minimal. A review also indicates that the trichloroethylene airborne concentrations were an order of magnitude greater than the other analytes which matches the environmental sampling data from previous site characterization. These air sampling results are as expected, considering the closed system facilities and processes on the site. The facility operator manages a chemical process system that is not open to the atmosphere. Chemical transfers are automated and can be controlled from control panels in the control room. The facility operator is required to minimally interface with the waste stream. This interface occurs primarily during well sampling, periodic maintenance, and change out of the charcoal filters.
5.0 Conclusions
The following points can be concluded from the data collected on the site:
- A surrogate chemical can be used for monitoring. This chemical can be chosen based upon the site characterization studies.
- The MicroRAE Turbo PID will alarm at predetermined levels and can provide worker protection from acute exposures.
- Humidity and temperature did not visibly interfere with either of the MicroRAE Turbo's performance during this field test.
- The MicroRAE Turbo PID alarmed during activities when alarming would be expected and was consistent over the four days of testing.
- The MicroRAE Turbo PID did not interfere with the worker to any more extent than would an air sampling pump's sampling train or another type of personal monitor.
- The MicroRAE Turbo PID can be used in conjunction with a field level protocol of using minimal PPE until the PID alarmed. Once the PID alarmed an industrial hygienist would evaluate the circumstances surrounding the alarming and decide if the PPE needed upgrading.
Appendix 2
MicroRAE Turbo Field Evaluation Protocol
PURPOSE
This protocol describes the tests and procedures required to perform the field analysis of the MicroRAE Turbo photoionization detector (PID).
SCOPE
This protocol consists of an outline of steps and tests designed to both assist in the use of the MicroRAE Turbo in evaluating workers' chemical exposures, and obtain information on the performance on the MicroRAE Turbo during actual field use. In order to account for variability between individual units and working conditions, a minimum of two units, if possible, should be used in each work zone.
Procedure
Evaluate the Site-Assessment Data
Use the environmental site data and any other background data available to identify and quantify the chemical agents that are present on the site.
Use Site Characterization to Determine Principal Chemical Agents of Concern
Based upon the site characterization data, preliminary concentration estimates should be made of the airborne and skin contact hazardous chemicals. The ratios of these contaminants to each other in the work area should also be made. Raoult's Law, Henry's Law, partition coefficients, published physical/chemical environmental fate data, and other techniques can be employed by the Health and Safety professional to assist in transforming the environmental data into data that can be used to provide a "best estimate" of a worker's exposure to the chemical contaminants.
Principal chemical agents of concern on the site should be identified. This identification should be based upon:
- Toxicity of the chemical.
- Concentration in the work area.
- Potential of exposure at or near levels determined to be hazardous.
- Routes of entry.
- Synergistic, additive, or other effects that may occur with other chemicals present in the work zone.
- Any other factors the Health and Safety professional deems important.
Divide the Site Into Independent Zones
After determining the approximate concentrations of contaminants on the site, divide the site into independent monitoring zones. Factors to consider when creating the zones include:
- Homogeneity of waste and representiveness of proposed analyte in the waste.
- Variety of types of air contaminants (organic, inorganic, biohazard, radioactive).
- Physical state of air contaminants (gas, liquid, solid).
- Level of air contaminant emission.
- Feasibility of air monitoring for the analyte.
- Potential interferences affecting sample collection and/or analysis.
Select Analytes to Monitor
Where there is more than one contaminant present it is useful to select a surrogate analyte or parameter as being representative of a group of analytes, rather than sampling all analytes. Characteristics to consider in the selection of the surrogate include:
- Present in the air emissions.
- Non-reactive or stable species.
- Present at levels above analytical detection limits.
- Unique to the hazardous waste site (not in background levels).
- Representative of the "worst-case" toxicity for the compound or compounds in each zone.
- Applicable for existing measurement and monitoring technologies.
- Has known toxicity and exposure criteria.
- Present in a known ratio to the "worst case" compound or compounds of concern in the zone.
- The sensitivity of the surrogate to the meter as compared with the sensitivity to other chemical contaminants present and/or the sensitivity of the contaminants of most concern.
Once the above factors have been considered, select the surrogate of interest for each zone on the site.
Select Appropriate Instrumentation
For this protocol, the instrumentation needed will include the MicroRAE Turbo, personal sampling pumps, and collection media. Chemical specific detector tubes, patches, and other instrumentation may be used if desired. The specific tubes, patches, etc. used will be based upon the surrogate as well as the other chemical species present.
Select Alarm Protocol
Once the surrogate has been chosen, an alarm setting that includes a factor of safety must be chosen for the meter. This alarm setting will be the level at which a predetermined course of action will be followed. This action would include a stop in work and an evacuation of the area.
Select Workers to Sample
Review the work plan and select the workers with the highest potential of chemical exposure in each zone. These individuals will wear the MicroRAE Turbo and be monitored for the surrogates in their zones. The alarm level should be based upon the ratios of the surrogate to the chemical compounds in the zone and include a factor of safety.
Perform Baseline Chemical Analysis
Conduct a side-by-side baseline analysis for each zone utilizing approved OSHA and/or NIOSH collection and analysis techniques. The samples should be broad indicators of all potential chemical exposures in each zone including the surrogates. Three 8-hour TWA samples should be taken for each zone utilizing personal sampling pumps and appropriate collection media. If STEL and/or Ceiling levels for the chemical agents are of concern, then appropriate samples should be taken in order to determine these exposure levels.
Review Baseline Data and Make Adjustments
Review the side-by-side baseline chemical sampling data to verify that the proper surrogate was chosen, the chemical agents are in concentrations and ratios predicted by the environmental data, and that the MicroRAE Turbo's alarm setting is at an appropriate level to protect the workers. Based on the baseline data, make any necessary changes in the instrumentation, alarm setting, and/or surrogate. If changes are made in the choice of the surrogate and/or instrumentation, then the alarm protocol and side-by-side sampling should be repeated and reevaluated.
Establish Final Alarm Setting
Establish the final alarm setting for the meter based upon the baseline data and any changes that were made.
Follow-up Monitoring
One 8-hour TWA personal sample (or other appropriate sample) for each zone should be taken every two weeks while workers continue to work in the zone to assure that conditions have not changed. Adjustments should be made if the follow-up monitoring indicates significant changes in contaminant exposures.
If at any time during the operation there are significant changes in work conditions that would impact the ability and/or reliability of the MicroRAE Turbo to accurately protect the workers (e.g. significant changes in the weather, work practices, changes in contaminant concentrations, etc.) then the entire field evaluation protocol procedure should be repeated.
General Considerations
During the field analysis the following items should be documented:
- Temperature, humidity, and general weather conditions.
- Map of the work area.
- Locations and work practices of the workers
- PPE levels of workers
- Relevant site assessment data.
Appendix 3
MicroRAE Turbo Laboratory Evaluation Protocol
Purpose
This protocol describes the tests and procedures required to perform the laboratory analysis of the MicroRAE Turbo photoionization detectors (PIDs).
Scope
The test plan employed in this study consists of a set of functional tests (see below bullets) designed to obtain information on the performance of the MicroRAE Turbo units. If a unit being evaluated does not perform adequately during any single functional test in this protocol then a second unit will be tested against the specific test in question in order to determine if the poor performance is characteristic of the product or the specific unit being tested. To minimize laboratory time, the functional tests can be divided among the four units with different functional tests being performed simultaneously on different units. However, a particular functional test can not be split between several units. If during any of the functional tests a unit fails to properly function in any of the extreme temperature and humidity conditions, the laboratory personnel will be allowed to modify the extreme conditions and determine the parameters under which the unit will properly function.
All four MicroRAE units will be evaluated against functional tests two through five at room temperature to ensure each will be able to work properly on-site.
- Determine best calibration method
- Alarm response
- Battery life
- Battery recharge time
- Zero drift
- Accuracy
- Calibration drift
- Linearity of response
- Response time
- Recovery time
- Temperature and humidity effects
- Electromagnetic interferences
Determine Best Calibration Method
Developing the best calibration method will be the first test performed and will be the calibration method used for the remaining tests. The laboratory will determine which of the following equipment combinations is best or develop a better alternate procedure.
- Gas cylinder of known concentration connected directly to the unit.
- A large bag of a known calibration gas connected directly to the unit.
- A tee connection consisting of a gas cylinder of known concentration on one end of the tee, a rotometer connected to the other end to monitor a pre-defined flow rate, and the unit connected to the last end of the tee.
Alarm Response
The alarm response is a check of the unit's alarm to sound at prescribed settings. The test procedure is as follows:
- Turn the test unit on and allow sufficient time for warm-up.
- Calibrate the unit using the zero gas filter and 100 ppm isobutylene in the manner finalized by the laboratory.
- Set the unit's alarm to 25 ppm. At room temperature expose the unit to this concentration of isobutylene. If the unit does not alarm at this setting, reduce the alarm setting in 5 ppm increments until the alarm does activate. Record the alarm setting and gas concentration.
- Repeat this procedure for a 100 ppm alarm setting and 100 ppm isobutylene.
Battery Life
The battery life is the time over which the battery will provide sufficient power for the uninterrupted operation of the instrument. The test procedure is as follows:
- Fully charge the unit in the charging station provided by the manufacturer.
- Remove the unit from the charging station. Turn on the unit and allow it to run continuously at room temperature. Record the amount of time it takes for the unit to automatically shut off.
Battery Recharge Time
The battery recharge time is the time necessary to charge the battery to full capacity. The test procedure is as follows:
- Discharge the unit's battery by allowing the unit to run continuously in an non-alarm condition until it automatically shuts off.
- Place the meter in the charging station provided by the manufacturer and record the time it takes for the battery to fully charge at room temperature (indicator light turns green).
Zero Drift
Zero drift is the change in the unit's output over a stated period of unadjusted, continuous operation. The test procedure is as follows:
- Turn the test unit on and allow sufficient time for warm-up.
- Calibrate the unit using the zero gas filter and 100 ppm isobutylene in the manner finalized by the laboratory.
- Allow the unit to operate at room temperature for a full shift in a non-contaminated environment.
- Check the unit's zero calibration point every thirty minutes to evaluate the stability of the zero setting. Graph the readings vs. time in thirty minute increments. The slope of this graph represents the long term zero drift in ppm/half-hour.
Accuracy
This is the degree of agreement between a measured value and the true value. The test procedure is as follows:
- Turn the test unit on and allow sufficient time for warm-up.
- Calibrate the meter using the zero gas filter and 100 ppm isobutylene gas in the manner finalized by the laboratory.
- Expose the meter to vapor concentrations of 0.25, 0.5, and 1 times the OSHA PEL for toluene at room temperature. Record the unit's response.
Calibration Drift
Calibration drift is the change in a unit's output over a stated time period of unadjusted, continuous operation. The test procedure is as follows:
- Turn the test unit on and allow sufficient time for warm-up.
- Calibrate the unit using the zero gas filter and 100 ppm isobutylene in the manner finalized by the laboratory.
- Allow the instrument to run at room temperature for a full 8-hr work shift in a non-contaminated environment. Every thirty minutes, connect the unit to the 100 ppm isobutylene calibration gas per the manner finalized by the laboratory and record the unit's reading. Graph the unit's readings vs. time in thirty minute increments. The resulting slope of this graph represents the long term calibration drift in ppm/half-hour.
Linearity of Response
Linearity of response is the deviation between the unit's actual readings and the readings predicted by a specified straight line. For this report, the linearity will be expressed as the line of best fit as determined by a linear regression analysis. Since this unit is calibrated to a zero and a span concentration, these will be two of the points used for specifying a straight line for evaluating linearity. The test procedure is as follows:
- Turn the test unit on and allow sufficient time for warm-up.
- Calibrate the unit using the zero gas filter and 100 ppm isobutylene in the manner finalized by the laboratory.
- Expose the unit to three additional concentrations of isobutylene gas (25 ppm, 250 ppm, 1000 ppm) at room temperature. Graph the unit's readings vs. the actual concentration of gas. Determine the line of best fit as determined by a linear regression analysis.
Response Time
Response times include the positive and negative response times, rise time, fall time, and lag time. For this report, the 90% response time will be defined as the time interval from a step change in the input concentration and the time the unit's readout settles at ±10% of its final value. If the response time is due to a step increase in the concentration it is a positive (+90%) response time and is therefore the sum of the lag time and the rise time. If the response time is due to a step decrease in the concentration it is a negative response time (-90%) and is therefore the sum of the lag time and the fall time. The test procedure is as follows:
- Turn the test unit on and allow sufficient time for warm-up.
- Calibrate the unit using the zero gas filter and 100 ppm isobutylene in the manner finalized by the laboratory.
- Connect the unit to a cylinder of 250 ppm isobutylene gas at room temperature. Minimize the length of tubing and time needed to connect the cylinder to the unit to achieve the most accurate response time.
- Record the time required between introducing the gas and the instrument's first response (lag time). Also record the time required to settle within ±10% of the final meter reading (positive response time = lag time + rise time).
- After the unit has settled to constant value, immediately switch it to a cylinder of 100 ppm isobutylene gas at room temperature. Minimize the length of tubing and time needed to connect the cylinder to the unit to achieve the most accurate response time.
- Record the time required between introducing the gas and the instrument's first response (lag time). Also record the time required to settle within ±10% of the final meter reading (negative response time = lag time + fall time).
Recovery Time
The recovery time is the time required for a PID to recover from exposure to high concentrations of organic vapors.
- Turn the test unit on and allow sufficient time for warm-up.
- Calibrate the unit using the zero gas filter and 100 ppm isobutylene in the manner finalized by the laboratory.
- Expose the unit to 1000 ppm of isobutylene and allow it to stabilize for 30 seconds at room temperature. Remove the unit from the 1000 ppm environment and immediately place the unit in a 100 ppm isobutylene environment. Record the time necessary for the unit to register 100 ppm within ±10%.
Temperature and Humidity Effects
The effects of temperature and humidity on the unit are measured by stabilizing it at a specific temperature and humidity and determining the unit's response to known physical and chemical parameters. The test procedure is as follows:
- Repeat tests three through eight at 2 0C and 20% relative humidity, and 40 0C and 80% relative humidity.
Electromagnetic Interferences
The unit's response to nearby electromagnetic fields will be examined in this test. The test procedure is as follows:
- Turn the test unit on and allow sufficient time for warm-up.
- Calibrate the meter using the zero gas filter and 100 ppm isobutylene in the manner finalized by the laboratory.
- At a concentration of 100 ppm isobutylene and room temperature, activate a hand-held walkie-talkie at distances of 0.5, 1, 3, and 10 feet from the meter.
- Record the unit's response.
Appendix 4
- A Laboratory Evaluation of the MicroRAE Model PGM-22
- Portable Photoionization Detector
- Report date: July 14, 1995
Tests conducted by: Duane Lee, Chemist, OSHA Analytical Laboratory
Test protocol by: Dick Jordan, CIH, Jordan Associates
Data analysis and report by: Jeff Throckmorton, CIH, Health and Safety Services
Project Officer: Earl Cook, CIH, OSHA Health Response Team
Table of Contents
- Overview
- Executive summary
- Equipment tested
- Accuracy
- Battery: Life
- Battery: Recharge Time
- Best Calibration Method
- Calibration: Drift
- Drift: Zero
- Electromagnetic Interference
- Response: Alarm
- Response: Linearity
- Response: Time
- Recovery time
- Temperature and Humidity Effects
Overview
A laboratory evaluation has been made of RAE Systems' MicroRAE Model PGM-22 Turbo portable personal PID's (photoionization detectors). This report presents that evaluation.
The test protocol was developed by Dick Jordan, CIH, of Jordan Associates. The evaluation was overseen by, and the summary report prepared by Jeff Throckmorton, CIH, of Health and Safety Services. Laboratory tests were conducted in late June and early July 1995 in the OSHA Salt Lake Technical Center by Duane Lee, OSHA Chemist. The project officer was Earl Cook, OSHA Health Response Team. A copy of the evaluation protocol is included in Appendix A. In some cases the original protocol could not be followed due to practical or physical limitations of test equipment. Specific test protocols followed for each of the tests are reported within the individual sections of the report. The evaluation parameters included:
- Accuracy
- Battery: life
- Battery: recharge time
- Calibration: "best" method
- Calibration: drift
- Drift: zero
- Electromagnetic Interference
- Response: alarm
- Response: linearity
- Response: time
- Recovery time
- Temperature and humidity effects
Executive Summary
Although the meters met expectations in a number of areas, performance was disappointing in tests related to temperature, humidity, and electromagnetic interference. Details on instrument performance as applied to each test protocol can be found in the respective test sections of the report.
The following table presents summary comments, as well as a simple "pass-fail" check off. The "pass-fail" check was arbitrarily given, based upon the opinions of individuals with field experience, as to their comfort with the ability of the MicroRAE's to perform satisfactorily in the field. Areas where instrument performance was not satisfactory have been underlined and are in a larger size font for quick reference.
These tables are best viewed on tablets, notebooks, or desktop computer screens.
| Pass | Fail | |
|---|---|---|
| X | Accuracy: High humidity adversely affects instrument accuracy. | |
| X | Battery life: Acceptable, although cold will shorten run time below 8 hours. | |
| X | Battery recharge time: Good. Never more than 13 hours. | |
| X | Calibration, "best" method: In line, per manufacturer's directions. Potential leakage problems with the present calibration cup make the "T" method unacceptable. The bag method seems to allow a larger large margin of error than the direct method. | |
| X | Calibration, drift: Up to 19.5% drift observed. Variations between meters were noted. NOTE: High humidity or temperature extremes, which were not considered in this test, might produce different results. | |
| X | Drift, Zero: No drift observed. NOTE: High humidity or temperature extremes, which were not considered in this test might produce different results. | |
| X | Electromagnetic interference: All meters failed the test. They cannot be used in the proximity of transceivers. | |
| X | Response, alarm: Good meter performance. | |
| X | Response, linearity: Good meter performance. | |
| X | Response, time: Good meter performance, for both + and - changes. | |
| X | Recovery time: Good meter performance. | |
| X | Temperature and humidity effects: All meters failed some part of the test. The degree of inaccuracy with both temperature and humidity effects was so great as to question the usefulness of this model in the field. |
As highlighted in the above table, the meters tested were subject to substantial interference and variance due to temperature changes, humidity, and electromagnetic pulses. In the event that the meters are used in the field, it is suggested that these physical conditions be carefully accounted for in calibration and use procedures so that meaningful and accurate data may be obtained. In the OSHA tests, meters were calibrated with a vendor supplied span gas of low humidity. The test protocol did not include calibrating the meters with a gas of the same humidity as that encountered during the actual tests. In the event that such precautions were taken, or in the case of low humidity environments (15% or less), it is anticipated that the meters would produce relatively accurate results. Individual report sections follow.
Equipment Tested
4 MicroRAE Turbo model PGM-22 personal photoionization detectors
- Lab Designation
- Serial Number
- C
002007
- B
002008
- A
002009
- D
002010
It should be noted that during much of the test period only three of the four instruments were available for testing due to one meter (Unit D) being used in by OSHA field personnel.
Additional:
Although not the instrument being evaluated, a MicroTip model IS-3000 was also used during most of the testing. In some of the tables presented in this report, comparative readings indicated by this instrument will also be presented.
ACCURACY
Summary Comments: Due to the manner in which this test was performed, it is difficult to fully separate the issue of accuracy from humidity effects. Using only a single concentration range, and low humidity, accuracy was within 6% - 12%. At higher humidities variances of between 52% and 151% were observed. More detail on humidity and temperature effects can be found in the temperature and humidity effects section.
Test Protocol Followed: Meters were calibrated with 100 ppm isobutylene (bag method), and then subjected to known concentrations of toluene in an environmental chamber. These tests were coupled with humidity tests, reported in a later section. Only the first section of tests, where toluene concentrations were verified by charcoal tube sampling will be reported in this section.
It should be mentioned as to why charcoal tube sampling was used only during part of the testing. Charcoal tube testing is well documented, although it is more costly and time consuming than simply calculating concentrations in an environmental chamber. Once charcoal tube testing, at both low and high humidities, demonstrated good correlation with the calculated chamber concentrations, it was dropped in favor of the more cost effective and faster latter method. In all of the following tables, comparisons are given against the calculated chamber concentrations.
For 15.7% Relative Humidity:
Calculated chamber toluene concentration 61.8 ppm.
Charcoal tube 1 reading: 43.5 ppm (Note--this tube is suspect of being low. No explanation has been developed.)
Charcoal tube 2 reading: 57.3 ppm
Meter calibration readings against 100 ppm isobutylene standard:
A: 99.8; B: 99.5; C: 99.5; MicroTip: 102.
A toluene PID response factor of 0.5 was used.
These tables are best viewed on tablets, notebooks, or desktop computer screens.
| Meter | Adjusted Reading-ppm (Facing) | % off calculated concentration | Adjusted Reading-ppm (Away) | % off calculated concentration |
|---|---|---|---|---|
| A | 67.8 | 9.7 % | 67.4 | 9.1 % |
| B | 69.2 | 12.0 % | 69.2 | 12.0 % |
| C | 65.5 | 6.0 % | 67.1 | 8.6 % |
| MicroTip | 74.5 | 20.5 % | 71.5(T) | 15.7 % |
NOTE: The term "facing" means that the instrument fan inlet was facing directly into the chamber airstream, while "away" means that it was facing downstream. The "(T)" notation means that the reading was taken using a T probe in the airstream.
For 80% Relative Humidity
Calculated chamber toluene concentration 59.8 ppm.
Charcoal tube 1 reading: 54.1 ppm
Charcoal tube 2 reading: 55.2 ppm
Meter calibration readings against 100 ppm isobutylene standard:
A: 99.8; B: 99.5; C: 99.5; MicroTip: 102. A toluene PID response factor of 0.5 was used.
These tables are best viewed on tablets, notebooks, or desktop computer screens.
| Meter | Adjusted Reading-ppm (Facing) | % off calculated concentration | Adjusted Reading-ppm (Away) | % off calculated concentration |
|---|---|---|---|---|
| A | 28.7 | 52.0 % | 28.6 | 52.2 % |
| B | 15.5 | 74.0 % | 17.6 | 70.6 % |
| C | 150.0 | 150.8 % | 130.0 | 117.0 % |
| MicroTip | 64.0 | 7.0 % | 59.5(T) | 0.5 % |
BATTERY: LIFE
Summary comments: Battery life under normal (room temperature) operating conditions, is not less than 8 hours. Cold adversely affects battery life. A meter which had previously demonstrated an operating time of over 8 hours shut down after being at a temperature of 32o F for 7 hours. It should be noted that new batteries were installed in all four meters before the test protocols began. None of the existing batteries in the four instruments, which were believed to be a year and a half old, had recharge capability to run any meter for 8 hours.
Test Protocol Followed: Determine battery life by timing how long it takes fully charged units to shut down due to low power. In this case, the individual doing the testing simply noted the approximate time when the meters shut down, thereby ending whatever testing was taking place.
These tables are best viewed on tablets, notebooks, or desktop computer screens.
| Meter | Day 1 Hours to Shutdown |
Day 2 Hours to Shutdown |
|---|---|---|
| A | 8.6 | 8.8 |
| B | >9.5 | >9.6 |
| C | 8.8 | 8.8 |
BATTERY: RECHARGE TIME
Summary comments: Recharge time is within 8-13 hours for a fully discharged instrument.
Test Protocol Followed: The time was noted during the charge cycle for the instrument charge light to change from red (charging) to green (fully charged). In the case of one instrument, this was done by checking the charger approximately every half hour.
Data: All 3 meters have successfully recharged within 13 hours. One instrument, closely monitored, recharged in 7.5 hours.
An additional advantage of the devices is noted in the optional side charging port. An instrument can recharge at the same time as a spare battery pack, although this increases the charge time for both.
"BEST" CALIBRATION METHOD
Discussion: In some ways, the term "best" is misleading, in that there are a variety of calibration methods possible, with different advantages and disadvantages. In this case "best" simply means the method which seems to be most accurate and practical for a field use instrument. The manufacturer, in Section 4.7.3 of the Operations and Maintenance Manual, recommends calibration by connecting the instrument via the calibration cap directly to the span gas cylinder regulator / flow controller. The calibration cup is a metal cup, which fits over the meter fan housing. Of the calibration methods evaluated, this method proved to be the most reliable. The rationale behind this statement, as well as a discussion of the other methods follows.
Three calibration methods were evaluated:
- Connecting the meter via the calibration cap directly to the span gas cylinder regulator / flow controller.
- Filling a calibration bag with span gas, and connecting it to the meter via the calibration cap.
- Connecting the meter via the calibration cap to a "T" fitting on the discharge line from the span gas cylinder regulator / flow controller.
In this test, instruments were calibrated with each of the above methods, using 100 ppm isobutylene span gas, and that calibration then compared with readings developed using the remaining two calibration methods.
The data is presented in the three following tables. There are a number of facts which can be seen from examining the data. Observations and comments follow:
Observation:
- Meters A and C tend to have close correlation, with meter B generally being an outlier. Looking more closely at the B readings, the B numbers are generally lowest (one exception) in the case when a method other than the T method was used as the primary method. When the T method was used as the primary method, the B reading are very high. This would be consistent with the originally calibration having been artificially low.
Comments:
- During the testing it was noted that the calibration cup seemed (subjectively) to fit more loosely in the case of meter B. This could result in a calibration error, especially in the case of the "T" method, where the fan could pull air in through a loose seal, diluting the calibration gas. The direct method would be relatively insensitive to cup leakage, as the gas running into the cup would likely result in an outward leakage preventing the dilution of the calibration gas as it entered the meter. In the case of the bag method, the bag was typically well filled, again likely allowing an outward, rather than inward leakage from the cup seal.
=================
Observation:
- Looking only at meters A and C, the direct and T methods have very close correlation. This is true even in the case of the bag method, where the two methods (direct and T) agree with each other more closely than with the bag calibration.
Comments:
- The consistency between the direct and T calibration methods (looking only at meters A and C), would tend to support that they are of similar merit as calibration procedures, assuming that there is no calibration cup seal leakage (as in the case of meter B).
=================
Observation:
- Looking only at meters A and C, and in the case when either the direct or T methods are the "primary" method, the bag method gives lower calibration readings. When the bag method is the "primary" method, the direct and T methods are higher. (This behavior is consistent with the bag method having given a lower initial calibration, which would make further reading artificially high.)
-
- Also, in general, the bag method, seems to produce greater differences in the correlation between meter A and C readings than in the case of the other two methods.
Comments:
- In general, bag meter readings seem to result in lower readings. This is demonstrated in the case of the direct and T "primary" calibrations, as well as in the case of the "primary" bag calibration. When the bag method was the "primary" calibration method, the other two comparison calibration methods give higher readings, probably due to the bag calibration having been low. This could be accounted for if there is some leakage in the case of the bag calibration method. In any case, it seems that the bag method results both in readings which are lower, as well as less consistent, than in the case of the other calibration methods.
=================
Conclusion:
The direct and T methods offer close correlation in cases where there is no potential leakage. This must be tempered by the fact that the calibration cup seemed to exhibit meaningful leakage in the case of meter B, however. In a field situation substantial dirt and particles which could only further interfere with a tight seal are likely to be encountered. The present seal is a relatively hard rubber, which does not necessarily allow for an air tight fit to the meter fan shrouds. For these reasons the T method is not recommended unless steps are taken to prevent any possible leakage between the fan housing and calibration cup.
The bag method seemed to result in data which was both less accurate and precise than in the case of the other two methods (barring leakage). For this reason, it is not recommended as the calibration method of choice. In brief, use the direct calibration method.
Calibration procedures used in this study:
For the record, it should be noted that the bag method was used extensively as a calibration procedure during this study. This was done before the data was fully analyzed. In the case of the widely varying effects noted with temperature, humidity, and electromagnetic effects, however, any error introduced by having used the bag calibration method was overcome by the far larger variations uncovered by the test protocols.
Finally, to avoid stating it repeatedly in this report, it should be noted that in the case of all calibrations performed for this report, the instruments were turned on and allowed to warm up and stabilize before tests were started.
Method 1--Direct Calibration
Meter readings in ppm after calibration: A: 98.1; B: 99.2; C: 98.7
These tables are best viewed on tablets, notebooks, or desktop computer screens.
| "T" method: | Bag method: | ||||
|---|---|---|---|---|---|
| Meter | ppm | Reading % Change | Meter | (ppm) | Reading % Change |
| A | 97 | 1.1 % | A | 89.7 | 9.5 % |
| B | 65.4 | 34.1 % | B | 85.0 | 14.3 % |
| C | 97.8 | 0.9 % | C | 93.0 | 5.8 % |
Method 2--"T" Calibration
Meter readings in ppm after calibration: A: 99.0; B: 100.0; C: 99.8
These tables are best viewed on tablets, notebooks, or desktop computer screens.
| Direct method: | Bag method: | ||||
|---|---|---|---|---|---|
| Meter | (ppm) | Reading % Change | Meter | (ppm) | Reading % Change |
| A | 99.2 | 0.2 % | A | 87.2 | 11.8 % |
| B | 164.9 | 64.9 % | B | 152.5 | 52.5 % |
| C | 100.2 | 0.4 % | C | 88.0 | 11.8 % |
Method 3--Bag Calibration
Meter readings in ppm after calibration: A: 99.8; B: 99.5; C: 99.5
These tables are best viewed on tablets, notebooks, or desktop computer screens.
| Direct method: | "T" method: | ||||
|---|---|---|---|---|---|
| Meter | (ppm) | Reading % Change | Meter | (ppm) | Reading % Change |
| A | 112.0 | 12.2 % | A | 110.7 | 10.9 % |
| B | 117.1 | 17.7 % | B | 64.2 | 34.5 % |
| C | 104.9 | 5.4 % | C | 105.0 | 5.5 % |
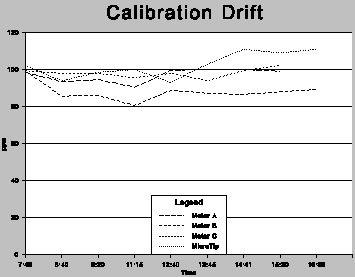
Calibration: Drift
Summary comments: The maximum drift observed during this test was 19.5%. The drift did not seem to follow a clear trend. Some error could be accounted for due to the bag method having been used in this test.
Test Protocol Followed: Instruments were calibrated to 100 ppm using the bag method with isobutylene, and checked every hour against the same calibration standard, also using the bag method.
Data: The data developed is presented in both tabular and chart form. Readings are in ppm.
These tables are best viewed on tablets, notebooks, or desktop computer screens.
| Time | A | B | C | MicroT |
|---|---|---|---|---|
| 7:40 | 98.4 | 99.2 | 99.4 | 102.0 |
| 8:40 | 93.5 | 85.7 | 98.0 | 94.0 |
| 9:20 | 94.5 | 86.0 | 97.9 | 98.6 |
| 11:15 | 90.5 | 80.5 | 95.6 | 100.0 |
| 12:40 | 99.4 | 88.8 | 98.0 | 93.1 |
| 13:45 | 100.3 | 87.2 | 94.0 | 103.0 |
| 14:41 | 100.0 | 86.6 | 99.6 | 111.0 |
| 15:30 | 99.1 | 88.0 | 102.2 | 109.0 |
| 16:30 | stopped | 89.2 | stopped | |
| 111.0 |
Drift: Zero
Summary comments: In the case of the three instruments tested, the zero remained stable and unchanged during an 8 hour and 50 minute run time. No drift was observed.
Test Protocol Followed: Instruments were calibrated at the beginning of the day to 100 ppm isobutylene standard using the bag method, and checked every hour to determine if the zero had changed. The same times were used as shown in the table above, under the "Calibration: Drift" section.
Electromagnetic Interference
Summary comments: Three instruments were tested against an electromagnetic pulse from a hand held transceiver. All three units were impacted at close range, experiencing unpredictable and significant effects. The units are considered to have failed this test, and are not considered to be usable in the close proximity (within 10 feet) of any source capable of generating an electromagnetic pulse similar to a walkie-talkie.
Test Protocol Followed: A 12 channel walkie-talkie was pulsed next to the units at respective distances of 1, 3, and 10 feet.
Tested meters A, B, and C. Various channels were used on the walkie-talkie. It should be noted that only certain channels had an effect on the units, indicating the interference is frequency related.
Results: The effects are summarized below.
- 10 feet
- No effects
- 3 feet
- Varying effects:
- * 10 ppm or less arbitrarily Added to scale during pulse
- One foot or less
- Varying effects:
- * Immediate meter shutoff with no warning of a problem.
- * Temporary addition of several hundred ppm to readout.
- * Subsequent brief triggering of meter alarm.
It should be highlighted that in the case of the meter shutdown, the unit turned itself off immediately with no indication of a problem. Although the unit has a faint operating "beep" (several minute interval), the unintentional shutdown is potentially a "fatal flaw", as a worker might not realize that the unit was inoperative and be exposed without warning.
Response: Alarm
Summary comments: There are three alarm set points on the instruments: Peak, STEL, and TWA. The first two alarm types were tested. The TWA alarm was not evaluated. It is believed that the alarms are keyed to the digital reading displayed, and respond when the target number set is encountered. Test results were satisfactory, with the meters responding at the designated set points.
Test Protocol Followed: After proper calibration, the instrument alarms were triggered at the following set points: Peak--200 ppm; STEL--100 ppm for 15 minutes. The tests were performed by exposing the meter to an isobutylene gas concentration of 250 ppm.
Response: Linearity
Summary comments: The instruments demonstrated a degree linearity, but at the high range the MicroRAE's readings were 9%-10% low and the MicroTip 41% high. It must be remembered that low humidity calibration gases were used, and that results would likely vary at higher humidities, or with temperature extremes.
Test Protocol Followed: The instruments were calibrated using the direct calibration method and 100 ppm isobutylene. Isobutylene gasses at concentrations of 50, 100, 250 and 1500 ppm were then respectively read.
These tables are best viewed on tablets, notebooks, or desktop computer screens.
| Meter A | Meter B | Meter C | MicroTip | |
|---|---|---|---|---|
| Concentration | (ppm) | (ppm) | (ppm) | (ppm) |
| 50 ppm | 47.9 | 50.6 | 49.3 | 45.7 |
| 100 ppm | 97.4 | 98.9 | 98.8 | 96.5 |
| 250 ppm | 233.1 | 229.0 | 230.2 | 280.0 |
| 1500 ppm | 1368.0 | 1358.0 | 1369.0 | 2112.0 |

