- Publication Date:
- Publication Type:Final Rule
- Fed Register #:68:1494-1619
- Standard Number:
- Title:Occupational Exposure to Methylene Chloride
DEPARTMENT OF LABOR
Occupational Safety and Health Administration
29 CFR Parts 1910, 1915 and 1926
RIN 1218-AA98
Occupational Exposure to Methylene Chloride
AGENCY: Occupational Safety and Health Administration (OSHA),Department of Labor.
ACTION: Final rule.
SUMMARY: The Occupational Safety and Health Administration (OSHA) hereby amends its existing regulations for employee exposure to methylene chloride (MC), (also known as methylene dichloride, dichloromethane or DCM). OSHA has determined, based on animal and human data, that the current permissible exposure limits (PELs) allow employee exposure to a significant risk of material impairment of health. OSHA is reducing the existing 8-hour time-weighted average (TWA) exposure from 500 parts MC per million parts (ppm) of air to 25 ppm. Also, OSHA is deleting the existing ceiling limit concentration of 1,000 ppm and is reducing the existing short-term exposure limit from 2,000 ppm (measured over five minutes in any 2 hour period) to 125 ppm, measured as a 15-minute TWA. In addition, the Agency is setting an "action level" of 12.5 ppm, measured as an 8-hour TWA. The final rule also contains provisions for exposure control, personal protective equipment, employee exposure monitoring, training, medical surveillance, hazard communication, regulated areas, and recordkeeping. Together, these provisions will substantially reduce significant risk to the extent feasible. This standard applies to all employment in general industry, shipyards and construction. Small employers, for purposes of the Regulatory Flexibility Act, 5 U.S.C. 601, are defined as firms with fewer than twenty employees. The final standard will prevent an estimated 31 cancer deaths per year and an estimated three deaths per year from acute central nervous system and carboxyhemoglobinemic effects, and will also reduce cardiovascular disease and material impairment of the central nervous system. The estimated cost, on an annualized basis, is $101 million per year.
DATES: This final rule becomes effective April 10, 1997.
Compliance: Start-up dates for specific provisions are set in 1910.1052(n) of the regulatory text. However, affected parties do not have to comply with the information collection requirements in 1910.1052(d) exposure monitoring, 1910.1052(e) regulated areas, 1910.1052(j) medical surveillance, 1910.1052(l) employee information and training; and 1910.1052(m) recordkeeping, until the Department of Labor publishes in the Federal Registerthe control numbers assigned by the Office of Management and Budget (OMB). Publication of the control numbers notifies the public that OMB has approved these information collection requirements under the Paperwork Reduction Act of 1995.
Comments: Interested parties may submit comments on the information collection requirements for this standard until March 11, 1997.
ADDRESSES: In compliance with 28 U.S.C. 2112(a), the Agency designates the Associate Solicitor for Occupational Safety and Health, Office of the Solicitor, Room S-4004, U.S. Department of Labor, 200 Constitution Avenue, NW., Washington, D.C. 20210, as the recipient of petitions for review of the standard.
Comments on the paperwork requirements of this final rule are to be submitted to the Docket Office, Docket No. ICR96-15, U.S. Department of Labor, Room N-2625, 200 Constitution Ave., NW., Washington D.C. 20210, telephone (202) 219-7894. Written comments limited to 10 pages or less in length may also be transmitted by facsimile to (202) 219-5046.
Copies of the referenced information collection request are available for inspection and copying in the Docket Office and will be mailed immediately to persons who request copies by telephoning Vivian Allen at (202) 219-8076. For electronic copies of the Methylene Chloride Final Standard and the Information Collection Request, contact OSHA's WebPage on Internet at http://www.osha.gov/.
FOR FURTHER INFORMATION CONTACT: Bonnie Friedman, Director, OSHA Office of Public Affairs, Room N-3647, U.S. Department of Labor, 200 Constitution Avenue, NW, Washington, D.C. 20210; Telephone (202) 219-8148.
SUPPLEMENTARY INFORMATION:
Collections of Information:Comment Request
The Department of Labor, as part of its continuing effort to reduce paperwork and respondent burden, conducts a preclearance consultation program to provide the general public and Federal agencies with an opportunity to comment on proposed and/or continuing collections of information in accordance with the Paperwork Reduction Act of 1995 (PRA95) (44 U.S.C. 3506(c)(2)(A)). This program helps to ensure that requested data can be provided in the desired format, reporting burden (time and financial resources) is minimized, collection instruments are clearly understood, and the impact of collection requirements on respondents can be properly assessed. Currently, OSHA is soliciting comments concerning the proposed approval for the paperwork requirements of the Methylene Chloride Final Standard. Written comments should:
-
Evaluate whether the proposed collection of information is necessary for the proper performance of the functions of the agency, including whether the information will have practical utility;
-
Evaluate the accuracy of the agency's estimate of the burden of the proposed collection of information, including the validity of the methodology and assumptions used;
-
Enhance the quality, utility, and clarity of the information to be collected; and
-
Minimize the burden of the collection of information on those who are to respond, including through the use of appropriate automated, electronic, mechanical, or other technological collection techniques or other forms of information technology, e.g., permitting electronic submissions of responses.
Background:The Methylene Chloride Standard and its information collection requirements are designed to provide protection for employees from adverse health effects associated with occupational exposure to MC. The standard requires employers to monitor employee exposure to MC and inform employees of monitoring results. If monitoring results are above the 8-hour TWA PEL or the STEL, then employers must also inform employees of the corrective action that will be taken to reduce employee exposure to or below the 8-hour PEL or STEL. Employers may also be required to provide medical surveillance to employees who are or may be exposed to MC. Employers are also required to provide information and training to employees on the following: health effects of MC, specifics regarding use of MC in the workplace, the contents of the standard, and means the employee can take to protect themselves from overexposure to MC.
Current Actions:This notice requests public comment on the paperwork requirements in the Methylene Chloride Final Standard. The Agency previously sought clearance on three Methylene Chloride Notice of Proposed Rulemaking Information Collection Requests: Shipyards, 1218-0177; Construction, 1218-0178; and General Industry, 1218-0179. Since the information requirements are identical for each industry, the Agency has combined these three packages into one entitled Methylene Chloride 1910.1052, OMB number 1218-0179.
Type of Review:Revision of a currently approved collection.
Agency:Occupational Safety and Health Administration.
Title:Methylene Chloride 1910.1052.
OMB Number:1218-0179.
Agency Number:Methylene Chloride Docket Number H-71.
Recordkeeping:Employers must maintain employee medical records for at least the duration of employment plus thirty years. Employee exposure monitoring records must be maintained for at least 30 years. Objective data, data showing that any materials in the workplace containing MC will not release MC at levels which exceed the action level or the STEL under foreseeable condition of exposures, must be maintained as long as the employer is relying on the data in support of the initial monitoring exemption.
Affected Public:Business or other for-profit, Federal government, State and Local governments.
Total Respondents:92,000.
Frequency:On Occasion.
Total Responses:Initial 719,948; Recurring 299,620.
Average Time per Response:0.26 hour.
Estimated Total Burden Hours:Initial 188,728; Recurring 74,299.
Estimated Total Burden Cost:Initial $32,496,380; Recurring $12,282,420.
Comments submitted in response to this notice will be summarized and/or included in the request for the Office of Management and Budget approval of the information collection request; they will also become a matter of public record.
Federalism
This standard has been reviewed in accordance with Executive Order 12612, 52 FR 41685 (October 30, 1987), regarding Federalism. This Order requires that agencies, to the extent possible, refrain from limiting State policy options, consult with States prior to taking any actions that would restrict State policy options, and take such actions only when there is a clear constitutional authority and the presence of a problem of national scope. The Order provides for preemption of State law only if there is a clear Congressional intent for the Agency to do so. Any such preemption is to be limited to the extent possible.
Section 18 of the Occupational Safety and Health Act (OSH Act), expresses Congress' clear intent to preempt State laws with respect to which Federal OSHA has promulgated occupational safety or health standards. Under the OSH Act, a State can avoid preemption only if it submits, and obtains Federal approval of, a plan for the development of such standards and their enforcement. Occupational safety and health standards developed by such State Plan-States must, among other things, be at least as effective in providing safe and healthful employment and places of employment as the Federal standards. Where such standards are applicable to products distributed or used in interstate commerce, they may not unduly burden commerce and must be justified by compelling local conditions (See section 18(c)(2)).
The final MC standard is drafted so that employees in every State will be protected by general, performance-oriented standards. States with occupational safety and health plans approved under section 18 of the OSH Act will be able to develop their own State standards to deal with any special problems which might be encountered in a particular state. Moreover, the performance nature of this standard, of and by itself, allows for flexibility by States and employers to provide as much leeway as possible using alternative means of compliance.
This final MC rule addresses a health problem related to occupational exposure to MC which is national in scope.
Those States which have elected to participate under section 18 of the OSH Act would not be preempted by this regulation and will be able to deal with special, local conditions within the framework provided by this performance-oriented standard while ensuring that their standards are at least as effective as the Federal Standard.
State Plans
The 23 States and two territories with their own OSHA-approved occupational safety and health plans must adopt a comparable standard within six months of the publication of this final standard for occupational exposure to methylene chloride or amend their existing standards if it is not "at least as effective" as the final Federal standard. The states and territories with occupational safety and health state plans are: Alaska, Arizona, California, Connecticut (for State and local government employees only), Hawaii, Indiana, Iowa, Kentucky, Maryland, Michigan, Nevada, New Mexico, New York (for State and local government employees only), North Carolina, Oregon, Puerto Rico, South Carolina, Tennessee, Utah, Vermont, Virginia, the Virgin Islands, Washington, and Wyoming. Until such time as a State standard is promulgated, Federal OSHA will provide interim enforcement assistance, as appropriate, in these states and territories.
Unfunded Mandates
The MC final rule has been reviewed in accordance with the Unfunded Mandates Reform Act of 1995 (UMRA) (2 U.S.C. 1501 et seq.) and Executive Order 12875. As discussed below in the Summary of the Final Economic Analysis (FEA) (Section VIII of this document), OSHA estimates that compliance with the revised MC standard will require the expenditure of slightly more than $100 million each year by employers in the private sector. Therefore, the MC final rule establishes a federal private sector mandate and is a significant regulatory action, within the meaning of Section 202 of UMRA (2 U.S.C. 1532). OSHA has included this statement to address the anticipated effects of the MC final rule pursuant to Section 202.
OSHA standards do not apply to state and local governments, except in states that have voluntarily elected to adopt an OSHA State Plan. Consequently, the MC standard does not meet the definition of a "Federal intergovernmental mandate" (Section 421(5) of UMRA (2 U.S.C. 658(5)). In addition, the Agency has concluded, based on review of the rulemaking record, that few, if any, of the affected employers are state, local and tribal governments. Further, OSHA has found that any impact on such entities would be insignificant. In sum, the MC standard does not impose unfunded mandates on state, local and tribal governments.
The anticipated benefits and costs of this final standard are addressed in the Summary of the FEA (Section VIII of this document), below, and in the FEA [Ex. 129]. In addition, pursuant to Section 205 of the UMRA (2 U.S.C. 1535), having considered a reasonable number of alternatives as outlined in this Preamble and in the FEA [Ex. 129], the Agency has concluded that the final rule is the most cost-effective alternative for implementation of OSHA's statutory objective of reducing significant risk to the extent feasible. This is discussed at length in the FEA [Ex. 129] and in the Summary and Explanation (Section X of this document) for the various provisions of the MC standard.
I. General
The preamble to the final rule on occupational exposure to Methylene Chloride (MC) discusses the events leading to the final rule, the physical and chemical properties of MC, the health effects of exposure, the degree and significance of the risk presented by MC exposure, the Final Economic Analysis and Regulatory Flexibility Analysis, and the rationale behind the specific provisions set forth in the final standard. The discussion follows this outline:
- I. General
II. Pertinent Legal Authority
III. Events Leading to the Final Standard
IV. Chemical Identification
V. Health Effects
VI. Quantitative Risk Assessment
VII. Significance of Risk
VIII. Summary of the Final Economic Analysis
IX. Environmental Impact
X. Summary and Explanation of the Final Standard
- A. Scope and Application
B. Definitions
C. Permissible Exposure Limits
D. Exposure Monitoring
E. Regulated Areas
F. Methods of Compliance
G. Respiratory Protection
H. Protective Clothing and Equipment
I. Hygiene Facilities
J. Medical Surveillance
K. Hazard Communication
L. Employee Information and Training
M. Recordkeeping
N. Dates
O. Appendices
XII. Final Rule and Appendices
Appendix A: Substance Safety Data Sheet and Technical Guidelines for Methylene Chloride
Appendix B: Medical Surveillance for Methylene Chloride
Appendix C: Questions and Answers -- Methylene Chloride Control in Furniture Stripping - A. Scope and Application
II. Pertinent Legal Authority
The purpose of the Occupational Safety and Health Act, 29 U.S.C. 651 et seq. ("the Act") is to "assure so far as possible every working man and woman in the nation safe and healthful working conditions and to preserve our human resources." 29 U.S.C. section 651(b). To achieve this goal, Congress authorized the Secretary of Labor to promulgate and enforce occupational safety and health standards. U.S.C. Secs. 655(a) (authorizing summary adoption of existing consensus and federal standards within two years of the Act's enactment), 655(b) (authorizing promulgation of standards pursuant to notice and comment), 654(b) (requiring employers to comply with OSHA standards.) A safety or health standard is a standard "which requires conditions, or the adoption or use of one or more practices, means, methods, operations, or processes, reasonably necessary or appropriate to provide safe or healthful employment or places of employment." 29 U.S.C. section652(8).
A standard is reasonably necessary or appropriate within the meaning of section 652(8) if it substantially reduces or eliminates significant risk, and is economically feasible, technologically feasible, cost effective, consistent with prior Agency action or supported by a reasoned justification for departing from prior Agency actions, supported by substantial evidence, and is better able to effectuate the Act's purposes than any national consensus standard it supersedes. See 58 FR 16612-16616 (March 30, 1993).
The Supreme Court has noted that a reasonable person would consider a fatality risk of 1/1000 to be a significant risk, and would consider a risk of one in one billion to be insignificant. Industrial Union Department v. American Petroleum Institute, 448 U.S. 607, 646 (1980) (the "Benzene decision"). So a risk of 1/1000 (10(-3)) represents the uppermost end of a million-fold range suggested by the Supreme Court, somewhere below which the boundary of acceptable versus unacceptable risk must fall. The Court further stated that "while the Agency must support its findings that a certain level of risk exists with substantial evidence, we recognize that its determination that a particular level of risk is significant will be based largely on policy considerations." See, e.g., International Union, UAW v. Pendergrass, 878 F.2d 389 (D.C. Cir. 1989) (formaldehyde standard); Building and Constr. Trades Department, AFL-CIO v. Brock, 838 F.2d 1258, 1265 (D.C. Cir. 1988) (asbestos standard).
A standard is technologically feasible if the protective measures it requires already exist, can be brought into existence with available technology, or can be created with technology that can reasonably be expected to be developed. American Textile Mfrs. Institute v. OSHA 452 U.S. 490, 513 (1981) ("ATMI "), American Iron and Steel Institute v. OSHA, 939 F.2d 975, 980 (D.C. Cir 1991) ("AISI ").
A standard is economically feasible if industry can absorb or pass on the cost of compliance without threatening its long term profitability or competitive structure. See ATMI, 452 U.S. at 530 n. 55; AISI, 939 F. 2d at 980.
A standard is cost effective if the protective measures it requires are the least costly of the available alternatives that achieve the same level of protection. ATMI, 453 U.S. at 514 n. 32; International Union, UAW v. OSHA, 37 F. 3d 665, 668 (D.C. Cir. 1994) ("LOTO III ").
All standards must be highly protective. See 58 FR 16614-16615; LOTO III, 37 F. 3d at 668. However, health standards must also meet the "feasibility mandate" of section 6(b)(5) of the Act, 29 U.S.C. 655(b)(5). Section 6(b)(5) requires OSHA to select "the most protective standard consistent with feasibility" that is needed to reduce significant risk when regulating health hazards. ATMI, 452 U.S. at 509.
Section 6(b)(5) also directs OSHA to base health standards on "the best available evidence," including research, demonstrations, and experiments. 29 U.S.C. section655(b)(5). OSHA shall consider "in addition to the attainment of the highest degree of health and safety protection * * * the latest scientific data * * * feasibility and experience gained under this and other health and safety laws." Id.
Section 6(b)(7) of the Act authorizes OSHA to include among a standard's requirements labeling, monitoring, medical testing and other information gathering and transmittal provisions. 29 U.S.C. section655(b)(7).
III. Events Leading to the Final Standard
The present OSHA standard for MC requires employers to ensure that employee exposure does not exceed 500 ppm as an 8-hour TWA, 1000 ppm as a ceiling concentration, and 2000 ppm as a maximum peak for a period not to exceed five minutes in any two hours (29 CFR 1910.1000, Table Z-2). This standard was adopted by OSHA in 1971 pursuant to section 6(a) of the OSH Act, 29 U.S.C. 655, from an existing Walsh-Healey Federal Standard. The source of this Walsh-Healey Standard [Ex. 7-1] was the American National Standards Institute (ANSI) standard for acceptable concentrations of MC (ANSI-Z37.23-1969), which was intended to protect workers from injury to the neurological system including loss of awareness and functional deficits linked to anesthetic and irritating properties of MC which had been observed from excessive, acute or large chronic exposures to MC in humans and experimental animals.
In 1946, the American Conference of Governmental Industrial Hygienists (ACGIH) recommended a Threshold Limit Value (TLV) of 500 ppm for MC [Ex. 2]. In 1975, the ACGIH lowered the recommended TLV to 100 ppm [Ex. 7-11].
In March 1976, the National Institute for Occupational Safety and Health (NIOSH) published "Criteria for a recommended standard for Methylene Chloride" [Ex. 2], which recommended a reduction of occupational exposures to MC to 75 ppm as an 8-hour TWA, and a lower peak exposure not to exceed 500 ppm. Further exposure reduction based on the ambient level of carbon monoxide was also recommended.
In February 1985, the National Toxicology Program (NTP) reported the final results of animal studies indicating that MC is a potential cancer causing agent [Ex. 7-8]. Subsequently, the U.S. Environmental Protection Agency (EPA), upon receipt of the NTP studies, initiated a risk assessment evaluation to determine whether or not MC presents an unreasonable risk to human health or the environment and to determine if regulatory actions are needed to eliminate or reduce exposures.
On May 14, 1985, EPA announced its determination that MC was a probable human carcinogen. EPA classified MC as Group B2, in accordance with its interim guidelines for cancer risk (49 FR 46294), and hence announced the initiation of a 180-day priority review (50 FR 20126) under section 4(f) of the Toxic Substances Control Act (TSCA). In meeting its mandate under section 4(f) of TSCA to initiate a regulatory action, on October 17, 1985, EPA published an Advance Notice of Proposed Rulemaking (ANPR) (50 FR 42037) for the purpose of collecting the necessary information required for initiating a rulemaking. In this notice, EPA established December 16, 1985, as its deadline for receiving comments.
On April 11, 1985, the U.S. Consumer Product Safety Commission (CPSC) released its risk assessment findings for MC and began to consider a regulatory action to ban MC containing products and to develop a voluntary hazard communication program for consumers.
On December 18, 1985, the U.S. Food and Drug Administration (FDA) published a proposal to ban the use of MC as an ingredient in aerosol cosmetic products (50 FR 51551). This proposal was based on a risk assessment that used the NTP animal data.
On July 19, 1985, Owen Bieber, President of International Union, United Automobile, Aerospace and Agricultural Implement Workers of America (UAW), petitioned OSHA to act expeditiously on reducing workers' exposure to MC. Specifically, Mr. Bieber requested that OSHA: (1) Publish a hazard alert; (2) issue an emergency temporary standard (ETS); and (3) begin work on a new permanent standard for controlling MC exposure. Subsequently, the following unions joined UAW in petitioning OSHA to act on revising the current standard:
A. International Union, Allied Industrial Workers of America; B. Glass, Pottery, Plastics and Allied Workers International Union; C. United Furniture Workers of America; D. The Newspaper Guild; E. Communication Workers of America; and F. United Steelworkers of America.
In March 1986, as a preliminary response to this petition, OSHA issued "Guidelines for Controlling Exposure to Methylene Chloride." That document, which was canceled by OSHA Notice ADM 8 (July 12, 1994), provided information to employers and workers on risks of MC exposure and methods for controlling such exposure [Ex. 8-11].
In April 1986, NIOSH published a Current Intelligence Bulletin #46 (CIB) on MC reflecting the findings of the NTP study [Ex. 8-26]. The CIB concluded that MC should be regarded as a potential occupational carcinogen and that exposure should be controlled to the lowest feasible level.
On August 20, 1986, the CPSC issued a proposed rule [51 FR 29778] "that would declare household products containing other than contaminant levels of MC to be hazardous substances." The CPSC noted the proposal was prompted by evidence that inhalation of MC vapor increased the incidence of various malignant and benign tumors in rats and mice. Accordingly, the Commission proposed to require that household products which can expose consumers to MC vapor be treated as hazardous substances and be labeled as provided by section 2(p)(1) of the Federal Hazardous Substances Act (FHSA) (15 U.S.C. 1261(p)(1)). The FHSA requires the use of labels which (1) indicate that exposure to a product may present a cancer risk; (2) explain the factors (such as level and duration of exposure) that control the degree of risk; and (3) explain the precautions to be taken.
On November 17, 1986, OSHA denied the petition for an Emergency Temporary Standard, but agreed that work on a permanent standard should commence [Ex. 3A]. On November 24, 1986, OSHA announced, in an Advance Notice of Proposed Rulemaking (ANPR) [51 FR 42257], that it was considering revision of the occupational health standard for MC. The Agency based this action on animal studies which indicated that the PEL of 500 ppm did not provide adequate protection against potential cancer risks and other adverse health effects. The ANPR summarized OSHA's information regarding the production and use of MC, occupational exposure to MC, and the potential adverse health effects associated with MC exposure. In addition, the notice invited interested parties to submit comments, recommendations, data, and information on a variety of issues related to the regulation of MC. OSHA received 43 comments in response to the ANPR. Those comments are discussed, as appropriate, below.
On December 5, 1986, the FDA reopened the comment period for 30 days on the above-cited proposal to ban the use of MC in cosmetic products [51 FR 43935]. The reopening enabled interested parties to submit comments on studies received after the close of the initial comment period regarding MC comparative pharmacokinetics, metabolism, and genotoxicity.
On September 14, 1987, the CPSC issued a statement of interpretation and enforcement policy, in lieu of continuing with rulemaking, which expressed the Commission's determination that consumer products containing MC and capable of exposing consumers to significant amounts of MC may pose cancer risk to humans and, therefore, are subject to the above-described hazardous substance labeling requirements. The CPSC explicitly retained the option of resuming the rulemaking if voluntary compliance with and enforcement of the Commission's interpretation did not adequately induce firms to label their products appropriately.
In 1988, based on the response to the ANPR, OSHA began contacting small businesses and conducting a number of site visits, to develop a clear understanding of how revisions to OSHA's MC standard would affect small entities. For example, on April 27, 1989, OSHA participated in a NIOSH conference on MC controls for the furniture stripping industry (54 FR 11811, March 22, 1989) to learn how that industry, which is dominated by small businesses, was dealing with MC exposure. That conference focused on the progress of a NIOSH pilot program aimed at developing affordable engineering controls for the furniture stripping industry. OSHA continued to seek input from small businesses throughout the MC rulemaking, as discussed below in the Preamble and in the Final Economic Analysis [Ex. 129].
Also, in 1988, ACGIH officially lowered the TLV for MC to 50 ppm as an 8-hour TWA. OSHA considered whether the TLV recommended by the ACGIH would be an appropriate OSHA standard. The ACGIH is a professional society devoted to administrative and technical aspects of occupational and environmental health. Voting members of ACGIH are scientists who work for government agencies or educational institutions. Every year the ACGIH adopts new or revised TLVs for several substances by a majority vote, not by consensus. OSHA has not adopted the MC TLV (50 ppm) as the 8-hour TWA PEL because the Agency's criteria for setting standards differ from those used by the ACGIH. OSHA standards must eliminate significant risks to the extent feasible, whereas the ACGIH sets limits under which it is believed that nearly all workers may be repeatedly exposed day after day without adverse health effects. Also, as evidenced by their "Documentation of the TLVs," the ACGIH does not perform quantitative risk assessments. This difference between OSHA and ACGIH practice is critical because the Supreme Court has required OSHA to perform quantitative risk assessments when data permit, and to use these assessments to set exposure limits.
On June 29, 1989, the FDA issued a final rule that banned the use of MC in cosmetic products [54 FR 27328]. The Agency based its final rule on scientific studies that showed inhalation of MC caused cancer in laboratory animals. The FDA concluded, accordingly, "that continued use of MC in cosmetic products may pose a significant risk to human health * * * " The Agency considered comments and information regarding the application of a physiologically-based pharmacokinetic model to the prediction of human cancer risk. The FDA determined that the risk assessment developed using animal studies should not be changed to reflect the "pharmacokinetic and metabolic data and hypothesized GST metabolic mechanism of carcinogenicity."
On August 8, 1990, the Consumer Product Safety Commission (CPSC) issued a General Order (55 FR 32282) that required manufacturers, importers, packagers and private labelers of consumer products containing 1% or more of MC to report to the CPSC information on the labeling and marketing of those products. The CPSC indicated that the information obtained would aid the Commission in evaluating the CPSC's policy concerning the labeling of MC-containing products as hazardous substances, pursuant to the Federal Hazardous Substances Act.
On November 11, 1990, then-President Bush signed the Clean Air Act Amendments (CAAA) of 1990. Title VI of the CAAA requires the phaseout of ozone-depleting chemicals by the year 2000 (section 604) and requires the EPA to determine which alternatives to ozone-depleting chemicals are safe for use (section 612). MC was among the potential substitutes studied by the EPA. In addition, section 112 of the CAAA requires the EPA to address the residual risks of MC and other specified Hazardous Air Pollutants (HAPs) by establishing Maximum Achievable Control Technology (MACT) standards. In particular, section 112(d) requires EPA to promulgate National Emission Standards for Hazardous Air Pollutants (NESHAP) (40 CFR part 63) over a 10-year period. In addition, EPA regulates MC as a priority pollutant under the Clean Water Act as amended (33 U.S.C. 1251, et seq.) On February 12-13, 1991, EPA convened an international conference on "Reducing Risk in Paint Stripping" that was well attended by representatives of small businesses which use MC or its substitutes in a wide range of operations. OSHA actively participated in the workgroup and panel discussions to elicit information regarding the anticipated impacts of a revised MC standard on paint stripping operations.
OSHA determined, based on animal and human data, that the existing PELs for MC did not adequately protect employee health. Accordingly, on November 7, 1991, OSHA issued a notice of proposed rulemaking (NPRM) (56 FR 57036) to address the significant risks of MC-induced health effects. The proposed rule required employers to reduce occupational exposure to MC and to institute ancillary measures, such as employee training and medical surveillance, for further protection of MC-exposed workers. The provisions of the proposed rule are discussed in detail in the Summary and Explanation, Section X, below. The Agency published a correction notice on January 6, 1992 (57 FR 387). The NPRM solicited comments on the proposed rule and raised 48 specific issues to elicit information about MC health effects, use, and exposure controls, as well as input regarding the appropriateness and impacts of particular provisions. The written comment period, which ended on April 6, 1992, produced 58 comments, including several hearing requests.
On February 11, 1992, then-President Bush announced an accelerated phaseout schedule for ozone depleting substances and ordered the EPA to accelerate its review of substitutes (such as MC) whose use would reduce damage to the ozone layer.
On May 19, 1992, OSHA presented the MC proposal to the newly reconstituted Advisory Committee on Construction Safety and Health (ACCSH) for consultation. The Advisory Committee established a MC work group to generate information and recommendations regarding MC use and exposure in the construction industry.
In response to the hearing requests and to concerns raised by commenters, the Agency issued a notice of informal public hearing (57 FR 24438, June 9, 1992), which scheduled hearings to start in Washington, D.C. on September 16, 1992 and in San Francisco, California on October 14, 1992. That notice also reopened the written comment period until August 24, 1992. The hearing notice raised 16 issues, based on the NPRM comments, which solicited input regarding the human health risks of MC exposure and the impact of the proposed rule on MC users. San Francisco was selected as a hearing site to facilitate participation by small businesses, particularly foam blowers and furniture refinishers, for whom attendance at the Washington, D.C. hearing would have been economically burdensome.
On July 28, 1992, the MC work group's report was presented to the ACCSH and was adopted as the Advisory Committee's recommendation to OSHA. Based on the input from the ACCSH, OSHA issued a supplemental hearing notice (57 FR 36964, August 17, 1992) which raised MC use, exposure and control issues specific to the construction industry. The supplemental notice extended the deadline for submission of comments regarding the construction issues until September 22, 1992.
OSHA convened public hearings in Washington, D.C. on September 16-24, 1992 and in San Francisco on October 14-16, 1992, with Administrative Law Judge James Guill presiding. At the conclusion of the hearings, Judge Guill set a post hearing period for the submission of additional data, which ended on January 14, 1993, and for the submission of additional briefs, arguments and summations, which ended on March 15, 1993. The posthearing comment period elicited 35 comments.
On March 31, 1993, pursuant to section 112 of the CAAA, the EPA issued a notice (58 FR 16808) requesting information on the anticipated impacts of a National Emission Standard for Hazardous Air Pollutants (NESHAP) for the halogenated solvent cleaning-vapor degreasing source category. This notice characterized MC as the third most commonly used halogenated solvent, based on 1991 data. On November 29, 1993, the EPA issued a notice of proposed rulemaking (58 FR 62566) describing MACT rules for the use of MC and other HAPs in halogenated solvent cleaning-vapor degreasing operations.
On March 11, 1994, OSHA reopened the rulemaking record for 45 days (59 FR 11567) to receive public comment on reports related to engineering controls for MC exposure in the furniture refinishing industry, MC carcinogenicity, and the availability of water-based substitutes for MC-based adhesives in the manufacture of flexible foam products. In particular, OSHA solicited input regarding the extent to which it was feasible for small businesses with furniture stripping operations to comply with the proposed PELs using engineering controls addressed in an OSHA contractor's report [Ex. 114]. The limited reopening, which ended on April 25, 1994, elicited 29 comments.
OSHA has evaluated the impact of the final rule on the identified application groups (except for farm equipment [Ex. 115-23], insofar as this rulemaking does not address agricultural employment). The Agency's analysis and conclusions are presented in the Final Economic Assessment for this rulemaking [Ex.129], summarized in Section VIII, below.
On March 18, 1994, the EPA issued a final rule (59 FR 13044) which addressed the use of MC as a substitute for ozone-depleting chemicals being phased out under section 612 of the CAAA of 1990. The EPA has found the use of MC to be acceptable in the production of flexible polyurethane foam; polyurethane integral skin foams; metal cleaning; electronics cleaning; precision cleaning; and adhesives, coatings and inks. That Agency expressed concern regarding MC toxicity, stating "methylene chloride use will be subject to future controls for hazardous air pollutants under Title III section 112 of the CAA. In addition, use of the compound must conform to all relevant workplace safety standards * * * Use is also subject to waste disposal requirements under RCRA (59 FR at 13088)." The EPA also noted that it is encouraging companies to decrease emissions of MC through the "30/50" pollution prevention program, under which companies voluntarily commit to reduce emissions 33 percent by the end of 1992 and 50 percent by the end of 1995 (59 FR at 13093).
On April 21, 1994, the Department of Housing and Urban Development (HUD) issued a notice (59 FR 19084) announcing that funds were available for the removal of lead-based paint. That notice explicitly provided that paint removal activities funded by HUD could not use products containing MC.
On May 31, 1994, Judge Guill closed and certified the hearing record for OSHA's MC rulemaking.
Pursuant to section 112(d) of the CAAA, the EPA has already finalized NESHAP rulemakings that cover halogenated solvent cleaning (59 FR 61801, December 4, 1994, 40 CFR part 63, subpart T), aerospace manufacture and rework facilities (September 1, 1995, 40 CFR part 63, subpart ) and wood furniture manufacturing (60 FR 62930, December 7, 1995, 40 CFR part 63, subpart JJ). MC-related NESHAP proceedings for several industries (e.g., pharmaceuticals, flexible polyurethane foam, polycarbonates and nylon 6 are currently underway.
Pursuant to its CAAA, CWA, RCRA and PPA mandates, EPA has proposed effluent limitation guidelines for the pharmaceutical industry (60 FR 21592, May 2, 1995) which characterize MC as one of the most significant priority pollutants to be addressed under the CWA. In particular, EPA has addressed the use of stream stripping and distillation technology to recover MC from wastewater for reuse or sale for use in other industries. That Agency has also proposed requirements for compliance monitoring of MC that, due to dilution with wastewater, would be found at levels below current analytical limits of detection.
OSHA has attempted to consider the foreseeable impact of EPA action on the use of MC because EPA-driven changes in such use would affect the data on which OSHA relies to estimate the impact of this final rule. In brief, while EPA action to reduce HAP exposure may encourage employers to reduce or eliminate MC use, simultaneous EPA efforts to reduce the emission of ozone-depleting chemicals may encourage employers to maintain or increase MC use. Given the time frame for EPA action and that Agency's need to coordinate proceedings that arise from several statutory mandates, it is inappropriate to draw conclusions regarding the impact of EPA regulatory action on the need for OSHA action.
OSHA has also consulted with EPA to determine whether any potential overlapping or conflicting requirements exist in OSHA's MC standard and various EPA NESHAPs, and has committed to continue working with EPA on future NESHAP compliance issues. OSHA discussed the MC regulation with project officers for all recent, current and planned NESHAPs projects and has determined that there are no overlapping or conflicting requirements in the NESHAPs and OSHA's MC standard. Indeed, employers can choose among a variety of means to comply which would not entail any conflict in OSHA and EPA regulations.
In particular, OSHA conducted a thorough analysis of the EPA Solvent Degreasing NESHAP. OSHA determined, and EPA agreed, that there are no conflicting requirements in the two regulations. OSHA does not require or recommend specific compliance strategies. One common method of reducing worker exposure is local exhaust ventilation. In addition, some of the alternative compliance strategies suggested in the EPA solvent degreasing NESHAP include reducing room draft. OSHA has determined that even if an employer chooses reducing room draft as its compliance strategy for the EPA NESHAP, employers may use some local exhaust ventilation to reduce worker MC exposures and still be in compliance with both the OSHA MC standard and the EPA NESHAP. There are also other combinations of compliance strategies that can be utilized to comply with both regulations. OSHA plans further discussion of this issue in its compliance assistance documents. The purpose of these documents is to assist employers in selecting among the many appropriate control strategies which satisfy requirements under both OSHA and EPA regulations.
On October 25, 1995, OSHA reopened the rulemaking record (60 FR 54462) to obtain input regarding studies submitted by the Halogenated Solvents Industry Alliance (HSIA) [Ex. 118-125] which address the use of animal data to estimate human cancer risk from MC exposure. The comments received on those studies [Exs. 126-1 through 126-37] are discussed in relation to the Quantitative Risk Assessment (Section VI), below.
The rulemaking record contains 129 exhibits, and 2717 pages of hearing transcript. A wide range of employees, employers, union representatives, trade associations, government agencies and other interested parties contributed to the development of the rulemaking record. The Agency appreciates these efforts to help OSHA develop a record that provides a sound basis for the promulgation of this final rule.
Throughout the ten years since OSHA initiated MC proceedings, the Agency has sought and evaluated input regarding the anticipated impact of a MC health standard on small entities. For example, Issue K of OSHA's Advance Notice of Proposed Rulemaking for MC (ANPRM) (51 FR 42257, November 24, 1986) solicited comments, recommendations, data and information regarding the anticipated impacts of a MC standard on small entities. Responses from manufacturers of flexible polyurethane foam [Exs. 10-4 and 10-17] and industrial paint removers [Ex. 10-7] indicated that rulemaking regarding MC would affect small entities. Based on the response to the ANPRM, OSHA initiated contacts with small businesses and conducted a number of site visits, to develop a clear understanding of how revisions to OSHA's MC standard would affect small entities.
Based on OSHA's contacts with small business and the response to the ANPRM, the Preliminary Regulatory Impact Analysis (PRIA) for the MC NPRM (56 FR 57036, November 7, 1991) considered small firms to be those with fewer than 20 total employees. In addition, the PRIA estimated that 45 percent of establishments using MC were "small businesses."
Issue 25 of the NPRM for MC stated that OSHA had analyzed the impacts of the proposed rule on small businesses and had adapted the standard to take into account the circumstances of small businesses, where appropriate. The performance-oriented language covering the demarcation of regulated areas (proposed paragraph (e)(4)) and the 30/10 days of exposure thresholds for medical surveillance (proposed paragraph (i)(1)(i)) reflected the Agency's determination to avoid imposing unnecessary burdens on small entities. In addition, Issue 25 solicited information regarding anticipated small business impacts so that OSHA could update the initial regulatory flexibility analysis performed pursuant to 5 U.S.C. 604 of the Regulatory Flexibility Act.
Small businesses, particularly in the furniture refinishing [Exs. 19-1, 19-4, 19-6, 19-8, 19-10 and 19-11] and polyurethane foam blowing industries [Ex. 19-3], expressed concern that the proposed rule would impose excessive compliance burdens on their operations. Based in part on these concerns, the Agency convened informal public hearings (57 FR 24438, June 9, 1992) in Washington, D.C. and San Francisco, CA. San Francisco was selected as a hearing site to facilitate participation by small businesses, particularly foam blowers and furniture refinishers, for whom attendance at the Washington, D.C. hearing would have been economically burdensome.
Hearing Notice Issue 8 solicited comments and testimony, with supporting documentation, regarding the impact of the proposed rule on small businesses, particularly in the furniture refinishing sector. A significant number of small businesses participated in the Washington, D.C. and San Francisco hearings, providing OSHA with useful testimony and posthearing submissions. For example, Harold Markey of the Markey Restoration Company proposed [Tr. 2660, 2672, 10/16/92] that "furniture refinishing businesses be exempt from [25 ppm PEL] due to the financial hardship that enforcement would cause." In addition, Mr. Markey expressed appreciation for OSHA's efforts to facilitate his participation in the hearing. As discussed above, OSHA subsequently solicited (59 FR 11567, March 11, 1994) additional input regarding the extent to which it was feasible for small businesses with furniture stripping operations to comply with the proposed PELs using the engineering controls addressed in an OSHA contractor's report [Ex. 114].
OSHA has had numerous contacts with furniture refinishers, particularly with members of the National Association of Furniture Refinishers and Refurbishers (NAFRR), the trade association for the industry. In 1994, OSHA was represented at the NAFRR's annual conference in Williamsburg, VA. The Agency has continued to provide assistance to NAFRR members and other furniture refinishers regarding appropriate industrial hygiene measures for workplaces where MC is used. For example, OSHA has disseminated information about the engineering controls developed by NIOSH for the furniture stripping industry. OSHA will continue to strive for a cooperative relationship with the small businesses affected by the MC final rule through careful compliance with the Small Business Regulatory Enforcement Fairness Act (SBREFA) (5 U.S.C. Chapter 8) and the Regulatory Flexibility Act (5 U.S.C. 601, et seq.), as amended. In addition, the Agency's "Outreach Program" for the MC final rule will involve a commitment of significant consultation and other resources by OSHA and other concerned parties, building on the relationships established during the rulemaking.
OSHA has developed a multifaceted outreach plan to provide information and compliance assistance to the regulated community. In particular, OSHA:
- -- Has developed a booklet which summarizes the provisions of the MC standard;
-- Has developed a compliance directive for the MC standard which answers compliance-related questions about the MC standard;
-- Is developing compliance guides directed at assisting small businesses in complying with the MC standard, consistent with section 212 of the Small Business Regulatory Enforcement Fairness Act of 1996;
-- Has recruited interested trade associations to assist in the distribution of MC standard-related information, and the convening of workshops to help small businesses understand available compliance strategies;
-- Has spoken to trade association meetings and distributed MC standard-related materials;
-- Has contacted manufacturers of MC to develop a strategy for inclusion of OSHA MC-standard information in existing product stewardship programs; and
-- Is working with individuals interested in conducting workshops for impacted industries, such as polyurethane foam manufacturers and furniture refinishers, to train small businesses on compliance with OSHA and EPA regulations.
all 50 states and the territories covered by the OSH Act provide free consultation services for small businesses to assist them in achieving compliance with OSHA standards. Those services are funded by federal OSHA but supplied by the states in state plan states and by private contractors in other areas. Those consultation services will provide free assistance for small business so it will be easier to come into compliance with the MC standard.
OSHA will also set up Cooperative Assessment Programs (CAP's) for individual employers to assist them in achieving compliance in a reasonable manner. In a CAP, an OSHA industrial hygienist works with the employer and employee representatives, to determine a reasonable number of cost-effective engineering controls and work practices to bring the employer into compliance. A reasonable schedule is determined for the implementation of those controls. Good faith efforts to implement a CAP are generally considered to be in compliance with the provisions of the standard. OSHA has had success in implementing CAP's for the arsenic, lead and other standards. Employers have found that working with OSHA or CAP's has led to cost effective compliance with OSHA standards.
IV. Chemical Identification
Methylene chloride (MC), also called dichloromethane (DCM) [Chemical Abstracts Service Registry Number 75-09-2] is a halogenated aliphatic hydrocarbon with a chemical formula of CH(2Cl)(2), a molecular weight of 84.9, a boiling point of 39.8 deg. C (104 deg. F) at 760 mm Hg, a specific gravity of 1.3, a vapor density of 2.9 and a vapor pressure of 350 mm Hg at 20 deg. C (68 deg. F). Concentration of MC in saturated air at 25 deg. C reaches 550,000 ppm. MC has low water solubility (1.3 gm per 100 gm of water at 20 deg. C), an extensive oil and fat solubility, and a low flammability potential. It is used as a flame suppressant in solvent mixtures (lower explosive limit of 12% and upper explosive limit of 19%). It is a colorless volatile liquid with a chloroform-like odor and its odor threshold varies between 100 and 300 ppm. Contact with strong oxidizers, caustics and active metal powder may cause explosions and fires. Decomposition products during combustion or fire include phosgene, hydrogen chloride and carbon monoxide.
V. Health Effects
A. Introduction
The toxicology of MC is summarized below. A more detailed review of MC toxicology can be found in the NPRM [56 FR 57036].
B. Absorption and Disposition of Methylene Chloride
Inhalation is the most significant route of entry for MC in occupational settings. The quantity of MC taken into the body depends on the concentration of MC in inspired air, the breathing rate, the duration of exposure to MC, and the solubility of MC in blood and tissues. Because MC is volatile, inhalation exposures to MC can be quite high, especially in poorly ventilated spaces.
Dermal absorption of MC is a slow process relative to inhalation. In the NPRM, OSHA described the rate of skin absorption of pure MC as insignificant relative to inhalation. In contrast, Mr. Harvey Clewell, in comments prepared for the U.S. Navy [Ex. 19-59], stated that substantial occupational exposure could occur through the dermal route when the employee is exposed to high concentrations of MC vapor and protective clothing is not worn [Ex. 19-59]. Mr. Clewell provided a physiologically-based pharmacokinetic (PBPK) model to describe the potential absorption through skin exposed to high vapor concentrations of MC. Where the employee is protected from inhalation exposure by use of an air-supplied respirator and the skin (exposed surface area = two hands) is unprotected in high MC-vapor concentrations, the primary route of exposure in this case will be dermal exposure. Mr. Clewell has determined that sufficient MC may be absorbed by the dermal route over an 8-hour shift to give an internal concentration which would exceed that experienced by workers exposed to MC through inhalation of 25 ppm for 8 hours.
In the NPRM, OSHA also indicated that the burning sensation associated with dermal exposure to liquid MC would likely lead employers and employees to limit skin absorption. However, exposure to high concentrations of vapor may not be associated with a burning sensation, and there is evidence in the record [Tr. 2468-70, 10/15/92] to suggest that employees are exposed to liquid MC without protective clothing. OSHA believes that dermal exposure to liquid and high vapor concentrations of MC should be limited to the extent feasible to protect the employee from overexposure. For this reason, in this standard OSHA has required that employers provide personal protective clothing and equipment appropriate to the hazard. For example, if an employee will be at risk of hand contact with liquid MC, impermeable gloves must be provided.
C. Metabolism of MC
Once MC is absorbed into the body, it is widely distributed in the body fluids and in various tissues. The uptake and elimination of MC has been well described in human and animal studies [Exs. 7-156, 7-157, 7-174].
The carcinogenic mechanism of action for MC has not been clearly established. Although it has not been proven whether MC is carcinogenic through a genotoxic or non-genotoxic mechanism, current evidence supports the hypothesis that MC is a genotoxic carcinogen. Genotoxic carcinogens typically are reactive compounds or metabolized to reactive compounds. MC is unreactive in the body until it is metabolized. Therefore, many investigators believe that one or more of the metabolites of MC, and not MC itself, is the ultimate carcinogen.
It has been established by Kubic and Anders [Ex. 7-167] and Ahmed and Anders [Ex. 7-25] that MC is metabolized by rat liver enzymes in vitro by two distinct pathways. The first pathway is the mixed function oxidase system (MFO pathway) associated with the microsomal cell fraction and the second is the glutathione dependent pathway localized primarily in the cytoplasm and mediated by glutathione-S-transferase (GST pathway). The metabolism of MC is illustrated in Figure 1.

Figure V-1. Proposed metabolic pathways for methylene chloride metabolism. (Adapted from Andersen et al. (1987) [Ex. 7-125]
The MFO pathway metabolizes MC via a cytochrome-P450 dependent oxidative dehalogenation [Ex. 7-167] which produces formyl chloride. The formyl chloride decomposes to give chloride ion and carbon monoxide. It has been postulated that if the MFO pathway contributes to the carcinogenicity of MC, it is through the production of the reactive compound, formyl chloride. The end product of the MFO pathway, carbon monoxide, can be detected in the blood and breath of humans and animals exposed to MC, and has been used as a surrogate measure of MC exposure in humans.
The GST pathway metabolizes MC to formaldehyde and chloride ions via a postulated S-chloromethylglutathione conjugate [Ex. 7-25]. Formaldehyde is further metabolized to carbon dioxide in mammalian systems. Potential reactive metabolites in this pathway are the S-chloromethylglutathione conjugate and formaldehyde (known to react with protein, RNA and DNA).
Animal data indicate that the MFO pathway is saturated at ambient concentrations less than 500 ppm, while the GST pathway remains linear throughout the exposure levels examined [Exs. 7-161, 7-171]. Saturation of the MFO pathway in humans has been estimated to occur at a level which is within the range of the animal data (estimates range from 200 to 1000 ppm MC) [Exs. 7-114, 7-115, 8-32]. The GST pathway is not thought to be saturated for any of the species investigated at doses up to 4000 ppm.
D. Carcinogenicity
The evidence for the carcinogenicity of MC has been derived from mutagenicity studies, animal bioassays and human epidemiological studies. OSHA analyzed data from each of these sources in determining that MC is carcinogenic to test animals and a potential occupational carcinogen. The evidence that OSHA evaluated in making this determination is summarized below. Additional evidence pertaining to the hazard identification of MC is discussed in the Quantitative Risk Assessment, Section VI, below.
1. Mutagenicity Studies
Mutagenicity and genotoxicity studies are useful in describing the possible carcinogenic mechanism of action of MC. Evidence for the interaction of MC or MC metabolites with DNA (producing mutations or toxicity) is consistent with a genotoxic mechanism for the carcinogenic action of MC, rather than a non-genotoxic action (i.e., by acting as a promoter, increasing cell turnover). The EPA reviewed the literature on the mutagenic potential of MC in their "Health Assessment Document for Dichloromethane (Methylene Chloride)" (HAD) [Ex. 4-5] and studies conducted by ECETOC in the "Technical Analysis of New Methods and Data Regarding Dichloromethane Hazard Assessments" [Ex. 7-129].
As described in the MC Notice of Proposed Rulemaking (56 FR 57036), the documentation of positive responses in the production of mutations in bacteria, yeast and Drosophila, chromosomal aberrations in CHO cells and sister chromatid exchanges (SCE) in CHO and V79 cells and equivocal responses in other systems indicated the potential genotoxicity of MC.
A paper submitted to the record by Dr. Trevor Green [Ex. L-107], for the Halogenated Solvents Industry Alliance (HSIA), investigated the role of metabolites of the GST pathway in the bacterial mutagenicity of MC. The authors of this study found that in glutathione-deficient strains of Salmonella typhimurium there was approximately a two-fold decrease in mutations. Mutation rates returned to normal when bacteria were supplemented with exogenous glutathione. They also investigated whether individual metabolites in the GST pathway were likely to be responsible for mutagenesis. Experiments in S. typhimurium strains were consistent with the S-chloromethylglutathione conjugate as the mutagenic moiety. Experiments in Escherichia coli strains implicated formaldehyde as the active mutagen. Overall, these results support the hypothesis that MC may act as a genotoxic carcinogen, but the ultimate reactive species still remains to be identified.
Dillon et al. [Ex. 21-89] also conducted experiments on the mechanism of MC mutagenicity in bacterial cells, using wild type and glutathione-deficient Salmonella typhimurium TA100. Dose-related increases in mutagenicity were observed with and without metabolic (cytosolic or microsomal) activation. The authors characterized the mutagenicity as marginally highest in the presence of cytosol at the highest MC concentrations. The glutathione-deficient strain was slightly less responsive to MC-induced mutation than the wild type. In contrast to the study by Green, Dillon et al. found that MC mutagenicity was not appreciably enhance by the addition of microsomal or cytosolic liver fractions or exogenous glutathione. They concluded that it was not clear to what extent, if any, glutathione was involved in MC mutagenicity, and noted that "* * * the residual glutathione present in the glutathione-deficient strain may have been sufficient to facilitate the mutagenic responses observed."
The differing results in these studies suggest that the exact mechanism of MC mutagenicity, even in bacterial cells, has not been determined with certainty. However, OSHA has concluded that the evidence that MC is genotoxic is compelling. Additional studies supporting classification of MC as a genotoxin were submitted to the Agency in late 1995 and are discussed in the Quantitative Risk Assessment, Section VI, below.
2. Animal Studies
The evidence for the carcinogenicity of MC has been derived primarily from data obtained in chronic toxicity studies in rodents. Table V-1 contains a summary of the major bioassays. These bioassays have been conducted in three rodent species (rat, mouse and hamster) using two routes of administration (oral and inhalation) and a wide range of doses (from 5 mg/kg/d, oral to 4000 ppm inhaled for 6 hr/d, 5 d/wk).
The National Toxicology Program conducted two 2-year inhalation bioassays [Ex. 7-8] using B6C3F1 mice and Fischer 344 rats. In the NTP mouse study [Ex. 7-8], groups of 50 male and 50 female B6C3F1 mice were exposed to 0, 2000 or 4000 ppm MC, 6 hr/day, 5 d/wk for 102 weeks. All animals were necropsied and examined histopathologically.
Treated male and female mice had increased incidences of alveolar or bronchiolar adenomas and carcinomas as compared with control animals. In addition, there was an increased number of lung tumors per tumor-bearing animal (multiplicity of tumors) with increasing dose of MC.
In the liver, the toxic effects of MC were expressed as cytologic degeneration in male and female mice which was not present in the controls. An increased incidence of hepatocellular adenomas and carcinomas (combined) was observed in male mice. The incidence of hepatocellular carcinomas in male mice was statistically significantly increased at 4000 ppm. Female mice also experienced dose-related increases in the incidences of hepatocellular adenomas and carcinomas. An increased multiplicity of liver tumors was also found in both male and female mice.
| Reference | Species/strain | Route and dosing schedule | Dosage (No. of animals) | comments |
|---|---|---|---|---|
| NTP (1985) | B6C3F(1) mouse | Inhalation 6 hr/day, 5 days/week | 0,2000,4000 ppm (50 mice/sex/ dose) | Lung and liver tumors both sexes, both doses |
| Serota (NCA) (1986) | B6C3F(1) mouse | Daily in water | 0 (125M, 100F), 60 (200M, 100F), 125 (100M, 50F), 185 (100M, 50F), and 250 (125M, 50F) mg/kg/d | No tumors observed |
| NTP (1985) | Fischer 344 rat | Inhalation 6 hr/day, 5 days/week | 0, 1000, 2000 and 4000 ppm (50 rats/sex/dose) | Mammary and integumentary and fibromas and fibrosarcomas in both sexes |
| Burek (DOW) (1980) | Sprague-Dawley rat | Inhalation 6 hr/day, 5 days/week | 0, 500, 1500 and 3500 ppm (95 rats/sex/dose) | Malignant salivary gland tumors at 3500 ppm, dose-related increase in mammary tumors |
| Nitschke (DOW) (1982) | Sprague-Dawley rat | Inhalation 6 hr/day, 5 days/week | 0, 50, 200 and 500 ppm (70 rats/sex/dose) | No tumors observed |
| Serota (NCA) (1986) | Fischer 344 rat | Daily in water | 0, 5, 50, 125 and 250 mg/kg/d (135/sex at 0, 85/sex/dose) | No tumors observed |
| Burek (DOW) (1980) | Syrian Golden hamster | Inhalation 6 hr/day, 5 days/week | 0, 500, 1500, 3500 ppm (90 hamsters/sex/ dose) | No tumors observed |
The dose-related increase in the incidence of lung and liver tumors in mice, and the increased multiplicity of these tumors, present the strongest evidence for the carcinogenicity of MC. NTP concluded that, based on the evidence from these lung and liver tumors, there was clear evidence of the carcinogenicity of MC in both male and female mice.
In a second two-year bioassay, the NTP examined the effects of inhalation of MC at 0, 1000, 2000 and 4000 ppm in F344 rats [Ex. 7-8]. Body weights of all exposure groups were comparable. The highest dose female rats experienced reduced survival after 100 weeks of exposure.
The incidence of mammary tumors in the high dose group in both sexes was statistically significantly higher than in control animals (concurrent and historical). The incidence of mammary fibroadenomas alone and the combined incidence of fibroadenomas and adenomas in male and female rats occurred with statistically significant positive trends. When subcutaneous fibromas or sarcomas in the male rat, which were believed to have originated in the mammary chain, were included in comparisons, differences between control and exposed animals were even greater.
MC-exposed male and female rats also showed increased incidence of liver effects, characterized by hemosiderosis, hepatocytomegaly, cytoplasmic vacuolization and necrosis. Neoplastic nodules alone and combined incidence of neoplastic nodules and hepatocellular carcinomas in female rats occurred with significant positive trends by the life table test. Pair-wise comparisons did not indicate statistically significant effects at any one dose. Although this is suggestive of a carcinogenic response in the female rat liver, NTP did not use this response in their determination of the carcinogenicity of MC.
NTP based its determination of the carcinogenicity of MC in the rat on the mammary tumor incidence data. NTP has concluded that the increased incidences of mammary gland tumors in the female rats provided clear evidence of carcinogenicity and, in the male rats, some evidence of carcinogenicity.
The Dow Chemical Company [Ex. 7-151] conducted experiments in which Sprague-Dawley rats and Syrian Golden hamsters were exposed to 0, 50, 1500 or 3500 ppm MC, 6 hr/d, 5 d/wk for 2 years. A dose-related statistically-significant increase in the number of mammary tumors per tumor-bearing female rat was observed. These results support the NTP findings of increased mammary tumors in F344 rats. The background mammary tumor response in the Sprague-Dawley rat is higher than in F344 rats, so a quantitative analysis of risk is easier to perform on the data from the NTP study.
A statistically significant increase in male rat salivary tumors was also observed in this study, although the authors believed that this response should be discounted because of the presence of sialodacryoadenitis virus in the rats. OSHA believes that the presence of this virus in the rats would complicate the interpretation of the data, and so has relied on the NTP studies for its quantitative risk assessments.
No statistically significant excess incidence of tumors was observed in either sex of hamsters at any exposure level. This suggests that hamsters are less sensitive to the carcinogenic effects of MC than either mice or rats. Metabolism data gathered in hamsters indicate that hamsters have less capability to metabolize MC by the GST pathway than rats or hamsters (or humans). This correlation between lack of GST metabolism capacity and lack of tumor response supports the hypothesis that GST metabolism is important in MC carcinogenesis and also indicates that it would not be protective to use the hamster response to MC as the basis for a carcinogenic risk assessment.
A second inhalation study in Sprague-Dawley rats conducted by investigators at Dow Chemical [Ex. 7-173], with exposures up to 500 ppm, showed an increase in the number of mammary tumors per tumor- bearing animal in female rats at the highest dose level only. This study extended the finding of excess mammary tumors in rats to the 500 ppm level. However, because of the high background rates of mammary tumors in Sprague-Dawley rats, the NTP study showed a clearer dose-response relationship between MC exposure and incidence of mammary tumors.
In a study conducted for the National Coffee Association [Ex. 7-180], no statistically significant increased incidence of tumors was observed in B6C3F1 mice or F344 rats exposed to up to 250 mg/kg/d MC in drinking water. These studies used the drinking water route of exposure instead of inhalation and exposed animals to lower doses (on an mg/kg/d basis) than the NTP and high-dose Dow studies. These factors most likely accounted for the lack of a positive tumor response. The NCA studies were used by Reitz et al. in the development of the physiologically-based pharmacokinetic models for MC. Specifically, these studies helped to determine that the lack of tumor development was consistent with model predictions of the amount of GST metabolites in lung and liver of mice and that the MFO pathway was most likely not primarily responsible for the mouse tumor response.
The Agency believes that the NTP studies show the clearest evidence of a carcinogenic effect of MC and has used these studies as the basis of its risk assessment for the following reasons: (1) The studies were well conducted and underwent extensive peer review. (2) The inhalation route of exposure was used, which is the most appropriate route for extrapolation to occupational exposures. (3) Dose-related, statistically significant increases in tumor incidence were observed in both sexes in mice and in female rats. OSHA believes that because of the clear tumor response, and quality of the studies, the NTP studies provide the best data for quantitative cancer risk assessment. OSHA concludes from these studies that MC causes cancer in two species of test animals by the inhalation route, and that a clear dose-response has been demonstrated.
3. Epidemiological Studies
Epidemiological studies of occupational exposure to MC have been conducted in the manufacturing of triacetate fibers, photographic film production, and the manufacturing of paint and varnish. Those studies were reviewed by OSHA in the preamble to the proposed rule [56 FR 57075] and are summarized and updated in this document. In addition, an epidemiological study of MC exposure and astrocytic brain cancer is reviewed in this text.
a. Studies of triacetate fiber production workers. Ott et al. [Ex. 7-76] performed a retrospective cohort study using a cellulose diacetate and triacetate plant in Rock Hill, South Carolina to examine the effects of MC on a working population. In particular, Ott et al. evaluated the effects that were possibly mediated through the metabolism of MC to carboxyhemoglobin. Employees at this plant had MC exposures close to OSHA's time weighted average (TWA) permissible exposure limit (PEL) of 500 ppm. Ott et al. used workers in a plant in Narrows, Virginia as a comparison population because it had operations similar to those at the Rock Hill plant, but did not use MC. In this study, Ott et al. compared the number of deaths within the exposed cohort with the United States population and the Narrows, Virginia referent group. Ott et al. observed that the overall mortality of the cohort was comparable to that of the age, sex, and race-matched U.S. population. Comparing exposed and referent cohorts, statistical differences in risk were observed in white men for "all causes" (risk ratio=2.2, p< 0.01), "diseases of the circulatory system" (risk ratio=2.2, p< 0.5), and "ischemic heart disease" (risk ratio=3.1, p< 0.05).
In interpreting the results of this study, Ott noted that there may have been differences in hiring practices in the two plants which could have contributed to the observed differences in mortality. In their conclusion, Ott et al. stated that a healthy worker effect (HWE) and the low power of their study did not permit them to dismiss the possibility of increased health risks within the working population exposed to MC.
Dr. Mirer of UAW testified [Tr. 1896-6, 9/24/92] that there is some evidence that there is excess work-related heart disease mortality in epidemiological studies that have observed SMRs greater than 80% for ischemic heart disease or any other cardiovascular disease. Furthermore, when the MC epidemiological studies are looked at together, there is evidence, although limited, that MC exposure has an effect on cardiovascular mortality.
On the other hand, Kodak [Ex. 91D] questioned the appropriateness of the referent population in the Rock Hill study, alleging that the SMR for ischemic heart disease in the referent population was unusually low, and that this fact, rather than an effect of MC exposure, caused the observed differences in ischemic heart disease rates.
In contrast, NIOSH considered the Rock Hill study to be suggestive of an effect of MC on risk of cardiac disease. According to NIOSH [Tr.879, 9/21/92] the Ott study did not use appropriate analytic techniques that would allow the acute effects of MC on cardiac disease risk to be examined. Furthermore, NIOSH suggested [Tr. 969, 9/21/92] that future epidemiological studies should examine risks from MC exposure during the period when employees are actively working.
In an update to the Rock Hill study, Lanes et al. followed the Ott et al. cohort through September 1986 [Ex. 7-260] and December 1990 [Ex. 106]. Lanes et al. used the population of York County, South Carolina as the comparison group. Statistically significant excess mortality was observed for cancer of the liver and biliary passages (SMR=5.75, CI:1.82-13.78) in the study group. Excess mortality was also observed for buccal cavity and pharynx cancer (SMR=2.31, 95% CI:0.39-7.60) and melanoma (SMR=2.28, CI:0.38-7.51), although mortality from these causes did not reach statistical significance. No excess mortality was observed for ischemic heart disease (SMR=0.90, CI:0.62-1.27).
Examination of the liver and biliary cancers indicated that the workers had ten or more years of employment and at least 20 years since first employment (4 observed v. 0.35 expected). Three of the four employees who died from liver/biliary cancer had tumor sites in the intrahepatic and common bile duct, common bile duct, and ampulla of Vater. Approximate durations of employment for these three cases were 28 years, 20 years, and less than one year. No medical record for the third case could be obtained. However, an autopsy report indicated adenocarcinoma of the liver for this case. To estimate the expected number of biliary cancer deaths, Lanes et al. used Surveillance, Epidemiology, and End Results (SEER) mortality rates of the continental United States. The computed risk estimate, based on 0.15 cases expected, was SMR=20 (95% CI:5.2-56.0).
The authors hypothesized that the biliary duct cancer cases may have been due to factors such as oral contraceptive use, gallstones, or ulcerative colitis. However, it appeared that medical records showed no indication of gallstones or ulcerative colitis in workers who died of biliary cancer. Moreover, although these factors were not specifically controlled for, there is no reason to believe the rates of these factors would be different in the exposed cohort compared to the general U.S. population.
Lanes et al. updated their study through December 31, 1990 [Ex. 106] using the National Death Index and focused on mortality from pancreatic cancer, biliary and liver cancer, and ischemic heart disease. Lanes et al. ascertained fifty more death certificates from the end of the last follow-up period on September 1, 1986. As before, York County, South Carolina was used as the comparison population.
The overall SMR from all causes of death was 0.90, and for malignant neoplasms, the SMR was 0.82. In this follow-up, the SMR for liver and biliary cancer dropped from 5.75 to 2.98 (95% CI:0.81-7.63). No additional deaths from biliary or liver cancer were observed. In the original and updated studies combined, four deaths from biliary/liver cancer were observed and 0.64 were expected. Using a Poisson distribution, Lanes et al. calculated the probability of failing to observe any liver/biliary cancer deaths in this update if the "true" value of the SMR for liver/biliary cancer was 5.75 (from the previous study) and then expecting 3.68 deaths in this follow-up (0.64 x 5.75). They estimated the probability that this update would have no observed biliary/liver cancer deaths if the true SMR were 5.75, as e(-3) 68=0.025. On the other hand, if MC had no effect on liver and biliary cancer mortality, Lanes et al. estimated that the probability of observing zero deaths would have been 0.527 (e(-0.64)). Lanes et al. used the likelihood ratio (0.527/0.025=21.08) to compare these two hypotheses. The authors concluded that the null hypothesis that the SMR=1.0 was 21 times more probable than the hypothesis that the SMR=5.75.
Because of the small number of cases involved and the instability of the numbers generated in this type of statistical analysis, OSHA believes that this study, overall, is suggestive (but not definitive) of an association between occupational exposure to MC and elevation of human cancer risk. Furthermore, the Agency has determined that the study results are not inconsistent with the results of the NTP cancer bioassay.
Hoechst-Celanese [Ex. 19-65, pp. 6-8; Ex. 19-19] was concerned that OSHA considered the incidence of biliary cancer as evidence of a positive effect. They argued that the reported excess in biliary tract cancer did not support the conclusion that MC exposure is associated with an increased risk of cancer. Specifically, they noted that,
(1) Biliary cancers have not been reported in any of the animal cancer studies of MC; (2) no statistically significant increase in biliary cancers was seen in the Cumberland study (described below); (3) no statistically significant excess in biliary cancers was reported in the Kodak studies (described below); (4) It was unlikely that MC could have been responsible for the biliary tract cancer observed in one employee who had been exposed to MC for less than one year; and (5) the Rock Hill study did not control for other chemical exposures.
Comments by the Halogenated Solvents Industry Alliance (HSIA) [Ex. 19-45, p. 47] were in accord with those of Hoechst-Celanese.
Dr. Shy, on behalf of Kodak, asserted [Tr. 1303, 9/22/92; Ex. 91F] that MC exposure failed to meet Bradford Hill's criteria for causality (e.g., biological plausibility, dose-response, and consistency) for producing biliary tract cancer. Dr. Shy acknowledged that animal bioassays have demonstrated liver tumors from MC exposure, but he noted that there is no evidence in humans that liver and biliary tract cancers have the same etiology. Furthermore, Dr. Shy argued that, (1) the results from the Lanes study is not supported by in vitro or pharmacokinetic studies.
(2) a dose-response relationship could not be determined from the Lanes study because there were no direct measurements of worker exposure to MC.
(3) the observed association between MC exposure and liver/biliary cancer was an isolated finding and the existence of a causal relationship could not be concluded.
(4) the excess biliary tract cancer in the Lanes study was not consistent with the other three epidemiological studies (Hearne, 1987, 1990, 1992; Hearne, 1992; Gibbs, 1992).
Dr. Shy did recognize that there was a strong association between MC exposure and biliary tract cancer in the Lanes study (SMR=20). Moreover, the 20 year time interval between first exposure and death from biliary tract cancer provided evidence that "exposure preceded cancer with an appropriate interval for induction of the tumor [Ex. 91F]."
OSHA disagrees with the conclusions reached by Dr. Shy. The Agency believes that the risks of biliary cancer observed in these studies is consistent with risks derived from its pharmacokinetic analysis (see the Quantitative Risk Assessment, Section VI). Since the occupational exposures in these studies are likely to have been among the highest in any of the epidemiologic cohorts, there is no evidence that the increased biliary/liver cancer result is inconsistent with other reported epidemiological findings. Regarding the biological plausibility, the Agency notes that human biliary cells appear to contain high concentrations of the mRNA for GST (the enzyme many investigators believe to be responsible for MC-induced carcinogenesis) [Exs. 124 and 124A]. Although this requires more investigation to determine if there is a direct relationship, OSHA believes there is a plausible mechanistic argument for MC causality in human biliary tract cancers. The Agency agrees with Dr. Shy, however, that the lack of dose-response data and the small number of cases in this cohort limit the strength of conclusions that can be drawn from this study. After weighing these considerations, the Agency has determined that there is suggestive evidence of a causal role for MC in these cases of biliary cancer.
Gibbs et al. conducted a study of another cellulose acetate and triacetate fibers plant in Cumberland, Maryland [Ex. 54] to evaluate the possible relationship between MC exposure and biliary/liver cancer. This plant, which ceased to operate in 1982, had operations similar to the plant in Rock Hill, and it was assumed to have had similar MC exposure levels as well. However, exposure measurements were not submitted for the Cumberland plant and it is unknown whether the Cumberland employees experienced the same exposures as their Rock Hill counterparts.
The Gibbs study investigated the mortality of 3,211 workers who were employed at this plant on or after January 1970. There were 2,187 men and 1,024 women in the cohort. Most of the workers in the cohort were hired prior to 1979 (2,566 total). The study population was divided into three subcohorts based on their estimated exposure to MC: 1) 834 men and 146 women in the "high exposure" group (estimated to be 350-700 ppm), 2) 1095 men and 832 women in the "low but never high exposure" group (estimated to be 50-100 ppm), and 3) 256 men and 46 women in the "no exposure" group. This cohort was followed through December 1989. The observed mortality was compared to expected death rates for Allegany County, Maryland (where the plant was located and where most of the cohort deaths occurred), the State of Maryland, and the United States.
The author of this study believed that the county rates were the most appropriate to use because the city of Cumberland is located in a rural area of Maryland and the state rates may have been influenced by rates in large urban areas such as Baltimore. In addition, local rates tend to adjust for social, economic, ethnic, and cultural factors which may be related to disease risk, access to medical care, etc. However, if the fiber plant was the major employer in this rural area, then county rates may reflect the cohort's mortality rather than the background risk, in which case, state rates or U.S. population rates would be more appropriate. The overall mortality rate for the high MC-exposed group was below the expected rates for Allegany County, Maryland, and the U.S. population.
As in the Rock Hill study, mortality from biliary tract cancer was observed in the Cumberland study, although no statistically significant elevated incidence of biliary cancer was found (two cases of biliary tract cancer were observed). In the high exposure group, there was one death (1.24 expected with Allegany rates (SMR=80.5) and 1.42 expected with Maryland rates (SMR=70.4)). In the low MC-exposed group, there was also one death from biliary/liver cancer. For the high MC-exposed subcohort, Gibbs et al. estimated SMRs of 80.4, 70.3, and 75.1 when comparisons were made with Allegany County, Maryland, and U.S. rates, respectively. In the low MC-exposed subcohort, the SMRs using Allegany and Maryland rates were 75.4 and 76.4, respectively. This cohort should be followed for a longer period of time to help clarify the suggested association between MC exposure and biliary cancer observed in the Rock Hill cohort.
Statistically significant excess mortality was also observed from prostate, uterine, and cervical cancers, although these also represented small numbers of cases: 13, 2, and 1, respectively.
The excess of prostate cancer in the Gibbs et al. study suggested an exposure-response relationship (3 deaths in no MC-exposure group, 9 in low MC-exposure group, and 13 in high MC-exposure group). According to Gibbs et al. and Shy [Tr. 1303, 9/22/92; Exs. 19-64, 91F], this response may have been related to other chemical exposures (occupational or non-occupational). In support of this hypothesis, no other epidemiological or animal studies of MC exposure have suggested a relationship between prostate cancer and MC. Hoechst-Celanese [Ex. 19-65, pp. 10-12; Ex. 91D, p. 12] cautioned OSHA not to overinterpret the excess of prostate cancer in the Cumberland study for the following reasons:
(1) of all the epidemiological studies, only the Cumberland study has shown an excess of prostate cancer; (2) of the thirteen high subcohort men who died of prostate cancer, twelve worked in the extrusion area of the Cumberland plant before methylene chloride was used as a solvent in cellulose triacetate fiber production. Thus, these men may have had longer exposure to other chemicals; (3) the study did not control for other personal risk factors; (4) Gibbs reported an increased incidence of prostate cancer elsewhere in the textile industry; and (5) the large number of statistical tests may have increased the probability of finding the death rate of a specific cause to be elevated or depressed.
OSHA believes that the increased risk of prostate cancer should be noted as a possible positive effect of MC exposure on cancer risk, particularly considering the exposure-response relationship. However, because of potential confounding factors and lack of corroborating findings in other studies, OSHA believes this is suggestive rather than conclusive evidence of a human carcinogenic effect.
b. Studies of film production workers. In their original study of film production workers, Friedlander et al. [Ex. 4-27] conducted both a proportionate mortality study and a retrospective mortality cohort study to determine if workers exposed to MC experienced an increased risk for specific causes of mortality. The cohort in these studies consisted of workers who worked in any department in film production that used MC as its primary solvent for approximately thirty years. The cohort was followed through 1976.
Proportionate mortality analysis for those workers ever employed in the study area versus a comparison group of workers in other Kodak Park departments produced a proportionate mortality ratio (PMR) of 143.88 for liver (intrahepatic ducts-primary) cancer. For ischemic heart disease, Friedlander et al. calculated a PMR of 94.74. No statistically significant differences were observed at p less than or equal to 0.05.
For the cohort mortality study, Friedlander et al. used rates from the 1964-70 hourly males age group exposed to MC in the film department and the other Kodak Park departments for internal comparison. Mortality rates for New York State, excluding New York City, males age group were used for external comparisons.
Forty-five deaths from circulatory diseases were observed in the MC-exposed cohort versus 38.5 expected in the Kodak Park referent group. Also, 6 deaths from respiratory diseases were reported in the MC-exposed group versus 3.2 expected for the Kodak Park comparison group. No liver deaths were observed in this cohort. Thirty-three deaths from ischemic heart disease were observed in this cohort compared with 28.7 expected in the Kodak Park population. None of these observed differences in mortality reached statistical significance.
Hearne et al. conducted several updates to the cohort study involving MC exposure and mortality among workers in film production areas at the Kodak plant in Rochester, New York [Exs. 7-122, 7-163, 49 A-1]. In the first update, the study cohort was followed through 1983. Two referent groups were utilized in this study: the general population of upstate New York men, excluding New York City, and Kodak Park employees.
No statistically significant findings were observed for any cause of death. However, Hearne et al. did find a relatively large number (8 observed) of pancreatic cancer deaths compared with the New York State (3.2 expected) and Kodak (3.1 expected) populations. This observation did not achieve statistical significance and a dose-response relationship was not observed when Hearne et al. considered latency and dose.
Hearne et al. then updated this study through 1988 [Ex. 7-163] and 1990 [Ex. 49 A-2]. In the 1988 update, nonsignificant deficits in observed-expected ratios for lung and liver cancer were found. Also, overall mortality from 1964 to 1988 was significantly less than in both referent groups. Since 1986, the number of pancreatic cancer deaths remained the same. As before, dose-response analysis showed no statistically significant pattern when latency or dose were considered.
The 1990 update showed that deaths due to liver cancer, lung cancer, and ischemic heart disease were below the expected numbers in both referent groups. Also, no additional pancreatic cancer deaths were observed in this second update. Since the start of the follow-up, Hearne et al. observed 8 deaths from pancreatic cancer compared with 4.5 expected (SMR = 1.78, p = 0.17).
Hearne et al. [Ex. 49 A-1] conducted a second Kodak cohort study involving workers in cellulose triacetate preparation and film base manufacturing between 1946 and 1970. Hearne et al. addressed the potential selection bias in the 1964-70 Kodak cohort by including only workers exposed primarily to MC after it was introduced in these areas and making the study more complete by adding workers in the Dope Department, which prepares the viscous cellulose triacetate mixture used in the film base coating, and the Distilling Department, which redistills and reblends solvents recovered from the coating operations.
The 1,311 men in the cohort were followed through 1990. An occupational control group could not be formed because death rates for Kodak employees before 1964 were unavailable. Instead, male residents of upstate New York living outside of the five New York City counties were used.
Hearne et al. combined exposures by job and time period with occupational history information to produce a career exposure estimate for each individual in the study for dose-response analyses. The mean career individual exposure was approximately 40 ppm for 17 years and the average interval between first exposure and end of follow-up was about 32 years.
Total mortality for this cohort was 22% below the expected mortality (statistically significant). Circulatory diseases and ischemic heart disease mortality were also statistically significantly below expectation. For lung cancer there were 22 deaths (28.7 expected) and for liver/biliary cancer there was one death (1.5 expected). Hearne et al. found that the number of pancreatic cancer deaths observed (4) was similar to the expected number (4.4). In this cohort, the number of observed deaths was greater than expected for diseases of the colon/rectum (13 observed v. 10.8 expected), brain (5 v. 2.3), and for leukemia (7 v. 3.4), but were not statistically significant.
Hearne et al. concluded that the findings in the 1964-70 cohort were consistent with the 1946-70 cohort: mortality from all causes, cancer (including lung and liver malignancies), and ischemic heart disease was lower than expected. Also, since the number of observed pancreatic cancer deaths in this cohort was similar to the expected number, Hearne et al. believed that this provided further evidence that the earlier finding of an excess of pancreatic cancer in the 1964-70 cohort was due to chance or to factors other than MC exposure.
Kodak [Tr. 1287-88, 9/22/92] also investigated the risk of adverse health effects during active occupational exposure to MC, as suggested by NIOSH [Tr. 970, 9/21/92]. Using person-years of active employment only in their analysis, Hearne observed 27 deaths (36 were expected in the internal Kodak reference group) from ischemic heart disease in the 1964-70 Kodak cohort; in the 1946-70 cohort, Kodak recorded 33 deaths compared with 43 expected in the New York State comparison population.
NIOSH testified [Tr. 877-83, 9/21/92] that the healthy worker effect (HWE) could have obscured any excess mortality from ischemic heart disease caused by MC exposure. NIOSH has stated that the HWE may be particularly strong for cardiovascular diseases.
The HWE is likely to be less of a factor when occupational comparison groups are used. Kodak's use of the Kodak Park employees as a comparison group should reduce the HWE in its studies. However, there are two potential problems with using occupational comparison groups in this instance:
(1) Cancer rates are more stable in larger populations, so comparison with state and national rates may be more appropriate.
(2) Due to the volume of MC used in the Kodak plant, the occupational comparison group may be exposed to air-or water-borne environmental concentrations of MC which could obscure the impact of occupational exposure to MC on cancer incidence.
c. Study of workers in paint and varnish manufacturing. The NPCA submitted to the record an epidemiological study of employees who worked for at least one year in the manufacture of paint or varnish [Ex. 10-29B]. OSHA's review of this study was published in the proposed rule [56 FR 57077]. Although no statistically significant excess of mortality was reported, OSHA noted that there were 4 pancreatic cancers (1.93 expected) and 15 cancers of digestive organs and peritoneum (10.66 expected) among MC-exposed workers.
d. Astrocytic brain cancer among workers in electronic equipment production and repair. In its March 11, 1994 Notice of Limited Reopening of the Rulemaking Record, OSHA solicited comments on a case-control study submitted to the Agency by the National Cancer Institute (NCI) [Exs. 112 and 113].
Heineman et al. conducted a case-control study to examine the potential association between brain cancer and exposure to organic solvents as a group and six chlorinated aliphatic hydrocarbons (CAHs) including MC. Cases were defined as white males who died from brain or other central nervous system tumors in southern Louisiana, northern New Jersey, and Philadelphia, Pennsylvania. Controls were randomly selected from death certificates and included white males who died of causes other than brain tumors, cerebrovascular diseases, epilepsy, suicide, and homicide. Controls were frequency-matched to cases by age, year of death, and geographic area.
Four-digit Standard Industrial Classification (SIC) and 4-digit Standard Occupational Classification (SOC) codes were employed to code occupational histories of study subjects. These codes linked work histories to job-exposure matrices which "characterized likely exposure to the six CAHs and to organic solvents" [Ex. 112]. Gomez et al. [Ex. 112] used an algorithm to assign estimates of probability and intensity of exposure to each industry/occupation combination in subjects' work histories. As noted by Gomez et al., these estimates were based on "occupation alone, industry alone, or both occupation and industry, depending on the specificity of the exposure environment that could be inferred from the occupational (SOC) code."
The following surrogate measures of dose, for each substance, were used to summarize "likely" exposure histories for each study subject: duration of employment in occupation/industry combinations considered exposed, a cumulative exposure score, and "average" intensity of exposure. Odds ratios were calculated for exposure intensity categories to refrain from using weights. These categories did not include duration in jobs with lower intensity for subjects with high or medium intensity jobs. In their statistical analyses, Heineman et al. controlled for age, geographic area, and employment in electronics-related occupations/industries.
Astrocytic brain cancer was not found to be associated with "ever"
being exposed to organic solvents as a group or to any of the six CAHs examined in this study. However, as probability of exposure to organic solvents as a group, and MC in particular, increased, the risk of brain cancer increased (chi-squared statistics for trend for organic solvents and MC were 1.93 and 2.29 (< 0.05), respectively). For MC there was a 2.4-fold increase in risk for subjects with a high probability of exposure (confidence interval=1.0-5.9).
Risk of brain cancer significantly increased with duration of exposure for subjects with high probabilities of MC exposure (OR=6.1; CI=1.1-43.8). Heineman et al. found that, in the high probability of MC exposure category, risk significantly increased with duration (chi for trend=2.58, < 0.01). Similar results were seen for organic solvents and methyl chloroform for all probabilities combined (chi-squared statistics for trend were 2.35 (p< 0.01) and 1.87 (p< 0.05), respectively).
Lagging exposure by 10 years produced findings analogous to those noted above. Higher risks and a sharper increase with duration was observed for organic solvents when exposure was lagged by 20 years (all probabilities: 2-20 years, OR=1.3 (95% CI=0.9-2.0); 21+ years, OR=2.8 (1.1-3.7); p for trend=0.006; high probability: 2-20 years, OR=1.2 (95% CI=0.7-1.9); 21+ years, OR=3.1 (1.3-7.4), p=0.009).
Subjects with a high probability of MC exposure experienced a statistically significant increased risk as the cumulative exposure score increased (chi-squared statistics for trend=2.18, p< 0.05). However, risk did not increase monotonically with cumulative exposure.
Lagging exposure 20 years supported the odds ratios and the trends for organic solvents, particularly in men with a high probability of exposure (low cumulative score: OR=1.1 (95% CI=0.5-2.3); medium: OR=1.4 (0.8-2.5); high: OR=2.2 (1.0-4.5); p for trend=0.02). Few individuals had high cumulative scores when exposure was lagged 20 years for the individual CAHs.
Compared with jobs with medium or low intensity exposures to organic solvents and all six CAHs, risk of brain cancer was higher for subjects who worked in jobs with high intensity exposures. Brain cancer was associated most strongly, and increased with probability of exposure, among subjects who worked 20 or more years with high intensity exposure to MC (all probabilities: OR=6.7, CI=1.3-47.4; high probability: OR=8.8, CI=1.0-200.0).
Since many subjects were determined to have been exposed to more than one of the CAHs, sometimes even in the same job, Heineman et al. used logistic regression to examine, simultaneously, the effects of MC, carbon tetrachloride, tetrachloroethylene, and trichloroethylene, controlling for age, geographic area, and employment in electronics-related occupations/industries. MC was the only substance to show a statistically significant increase in risk as the probability of exposure increased (low: OR=0.9, CI=0.5-1.6); medium: OR=1.4, CI=0.6-3.1; high: OR=2.4, CI=0.9-6.4; chi-squared statistics for trend=2.08, p< 0.05). Risks associated with MC increased when adjustments for exposure to the other agents were made. In addition, subjects employed for 20 years or more in jobs with high average intensity MC exposure showed an eight-fold excess of brain cancer (OR=8.5, CI=1.3-55.5), taking all probabilities into consideration.
Among the six CAHs examined in this study Heineman et al. found the strongest association between brain cancer and MC-exposure, for which relative risks rose with probability, duration, and average intensity of exposure, though not with the cumulative exposure index.
According to Heineman et al., the major weakness of this study was not having direct information on exposure to solvents. Next-of-kin data, poor specificity of some work histories for specific solvents, and the interchangeability of solvents may have resulted in misclassification of individuals with respect to any of the exposure measurements used in this study. However, Heineman et al. pointed out that the potential sources of error probably did not significantly bias risk estimates away from the null or generate the observed trends.
Another limitation of this study, pointed out by Heineman et al., was that over one-third of the next-of-kin of eligible cases and controls were not interviewed. According to Heineman et al., this could have artificially created the associations seen in this study "only by underrepresenting cases who were unexposed, and/or controls who were exposed, to solvents in general, and MC in particular" [Ex. 113]. Heineman further remarked that differential misclassification was probably not a problem in this study because occupational histories came from next-of-kin of both cases and controls.
In light of the limitations of this study, however, Heineman et al. commented that the consistency of exposure-response trends for MC was surprising and suggestive. Moreover, Heineman et al. believed that the trends and consistency of the associations between brain cancer and MC could not be explained by chance alone.
Several commenters [Exs. 115-1, 115-31, 115-32, 115-36] indicated that Heineman et al. relied too heavily on next-of-kin information. Information provided by next-of-kin concerning jobs held, job descriptions, dates of employment, and hours worked per week may be flawed with recall bias. Next-of-kin may not be able to accurately recall job-related information, especially for jobs held early in life. If next-of-kin for cases or controls had better recall than the other group, differential misclassification could occur. HSIA [Ex. 115-36] stated that even small differences in error rates between cases and controls could produce false associations. Both HSIA and NIOSH [Ex.115-31] agreed that this indirect source of exposure information was likely to produce some degree of misclassification. However, NIOSH noted that misclassification "is a typical problem in population based case-control studies of this type [Ex. 115-31]" and that this misclassification could also explain the fact that no associations were found between brain cancer and the cumulative exposure score.
Organization Resources Counselors (ORC) [Ex. 115-2] and Abbott Laboratories [Ex. 115-30] were concerned that the lack of exposure verification made this NCI study unreliable for setting MC exposure limits. ORC stated that exposure values were assigned to all SIC and SOC codes, and not developed based on job history information, which would have given the study more validity. Kodak also expressed some concern regarding this study due to lack of accurate records of past exposures, reliance on expert judgement to a large degree, use of next-of-kin to determine potential exposure, and undocumented qualifications of those making judgements concerning the different occupations and industries involved. In addition, Kodak felt that the exposure data were "at best, unsubstantiated semi-qualitative judgements of likelihood and intensity of exposure [Ex. 115-1]." Organization Resources Counselors [Ex. 115-2] and Abbott Laboratories [Ex. 115-30] asserted that it was impossible to tell if those who died of cancer had been exposed to MC because there was no exposure verification. Vulcan Chemicals [Ex. 115-32] criticized the investigators for not going to work sites and determining the actual magnitude of exposure to the CAHs. HSIA [Ex. 115-36] argued that "concordance of proxy reports with actual work histories may range from 0-50% for decedents' first jobs and from 50-70% for last jobs." OSHA believes that exposure verification would have increased the validity of the findings of this study. However, lack of exposure verification does not nullify the results of the study. The Agency believes that the associations observed are suggestive of a human carcinogenic effect of MC.
Another issue that Kodak [Ex. 115-1] and Vulcan [Ex. 115-32] emphasized was the possible exposure to other chemicals or sources of potential human carcinogens, such as ionizing radiation, electromagnetic fields, smoking history, and place of residence. Vulcan [Ex. 115-32] noted that there may have been selection bias in this study because of the large ratio of astrocytic brain cancer tumors to the total number of brain tumors. Although they offered no explanation of how this selection bias would operate, Vulcan did suggest that this issue should be investigated further.
Vulcan was also concerned that the matching of controls and cases with respect to occupations and socioeconomic status may be inadequate. In particular, Vulcan criticized the Heineman study for not presenting the occupations of the control group and for not matching the socioeconomic status of the two groups. Similarly, Kodak [Ex. 115-1] stated that some adjustment should have been made in order to match across educational levels.
Kodak [Ex. 115-1] also believed that the estimates of trends observed in this study could have been affected, if workers in the longest duration or the higher probability of exposure categories had longer dates of employment, worked in more stable industries, and had better health benefits, better access to medical care, and more sophisticated diagnostic procedures. OSHA believes that there is no evidence that this is the case in this study.
HSIA [Ex. 115-36] criticized the methodology for assessing the number of industries with exposures to CAHs. HSIA argued that Gomez et al. did not fully explain how they determined that workplaces in the specific SICs would have CAH exposures. According to HSIA, Gomez et al. reported inaccurate information regarding industry use of MC. HSIA cited EPA's "Toxic Air Pollutant/Source Crosswalk, A Screening Tool for Locating Possible Sources Emitting Toxic Air Pollutants (EPA-450/4-87-023A, Dec. 1987)" which revealed a higher number of SIC codes using MC. In conclusion, HSIA asserted that Gomez et al.'s "exposure scenario" was incorrect.
Several commenters [Exs. 115-1, 115-31, 115-36] argued that the Heineman et al. study should only be considered a hypothesis-generating study and should not be used to adjust the PEL.
OSHA agrees with NIOSH that the Heineman et al. study was well-conducted because there was a systematic attempt to estimate] exposure by work experience. Furthermore, there was a remarkably high correlation between exposure to MC and brain tumors. OSHA concludes that the results from this study strongly suggest a possible association between MC and brain cancer. However, in the absence of quantified exposure data for these workers, it remains relatively speculative to attempt to estimate a quantitative dose-response relationship. Therefore, OSHA concludes that the risk estimate based on the animal data is the best available and accordingly it retains that estimate for its significant risk analysis.
e. Summary of epidemiological studies. Considered as a whole, the available epidemiologic evidence did not demonstrate a strong, statistically significant cancer risk associated with occupational exposures to MC. However, the positive trend for biliary tract/liver cancer deaths, the association between occupational MC exposure and astrocytic brain cancer and the statistically significant excess prostate cancer results are suggestive of an association between MC exposure and cancer risk. In addition, the non-positive epidemiological studies summarized here are not of sufficient power to rule out the positive results from the animal studies. This issue is addressed further in the Quantitative Risk Assessment section of this document.
In summary, the epidemiological results are suggestive of an association between occupational exposure to MC and elevated cancer risk which offers supporting evidence to the positive animal bioassay results.
4. Conclusion
OSHA concludes from the mutagenicity, animal bioassay and human epidemiology data that MC causes cancer in test animals and that it is a potential occupational carcinogen. The Agency has determined that, because of the quality of the studies, the clear dose-response relationship and the appropriateness of the route of administration, the NTP rodent bioassay data are the best available for quantitative cancer risk assessment.
OSHA also concludes that the epidemiology data, in some cases, suggest a positive association between human MC exposure and cancer incidence, but the dose-response relationships are not clear. The Agency has determined that the remaining epidemiology data (the non-positive studies) are not of sufficient power to rule out the results obtained in the animal bioassay data and that the animal data provide the best available data for quantitative risk assessment.
E. Other Toxic Responses
1. Central Nervous System Toxicity
MC acts on the central nervous system (CNS) as a CNS depressant. CNS depression has been described in humans exposed to MC concentrations as low as 175 ppm (8-hour TWA). This depression in CNS activity was manifested as increased tiredness, decreased alertness and decreased vigilance. These effects could compromise worker safety by leading to an increased likelihood of accidents following MC exposure.
a. Animal studies. In the NPRM, OSHA reviewed two animal studies of MC CNS toxicity (briefly summarized below) and concluded that the CNS was potentially susceptible to reversible and irreversible effects due to MC exposure.
Savolainen et al. [Ex. 7-178] studied biochemical changes in the brains of rats exposed to MC. Rats were exposed to 500 ppm MC for 6 hr/ d. On the fifth day, after 3 and 4 hours of exposure to MC, levels of acid proteinase in rat brains were significantly increased, but no change in brain RNA levels was reported. The authors suggested that the increase in acid proteinase may have been the result of increased levels of CO from metabolism of MC. OSHA believes that this study shows that MC can cause specific changes in the neurological system at a biochemical level. The Agency intends to monitor the scientific literature for additional developments on these effects, but has not used this information in setting the MC exposure limits because it is presently unclear how changes in acid proteinase are related to the observed CNS depressive effects of MC in humans.
Rosengren et al. [Ex. 7-56] looked at the effects of MC on glial cell marker proteins and DNA concentrations in gerbil brains after continuous exposure to 210, 350 or 700 ppm MC. Because of high mortality in the 2 higher doses, no data were collected at 700 ppm and exposure was terminated after 10 weeks at 350 ppm. Exposure to 210 ppm was continued for three months. Exposure to MC was followed by four months of no exposure before animals were examined for irreversible CNS effects. The authors found increased levels of glial cell marker proteins in the frontal cerebral cortex and sensory motor cortex after exposure to 350 ppm MC. These findings are consistent with glial cell hypertrophy or glial cell proliferation. Levels of DNA were decreased in the hippocampus of gerbils exposed to both 210 and 350 ppm and in the cerebellar hemispheres after 350 ppm MC. Decreased DNA concentrations indicate decreased cell density resulting from cell death or inhibition of DNA synthesis.
The neurotoxic mechanism of action of MC in gerbil brains is not understood. However, since the metabolism of MC to CO was determined to be saturated at both 210 and 350 ppm (COHb levels were equivalent at both exposure concentrations), the changes in glial cell proteins and DNA concentrations was attributed to either a direct effect of MC or an effect of a metabolite of the GST pathway. Although this study describes biochemical changes in the CNS subsequent to MC exposure, the high mortality of the experimental animals and the lack of MC toxicity data in the gerbil make it difficult to determine the significance of this study for extrapolation to other species. It is also unclear how these effects would relate to CNS depression observed in humans after MC exposure. In addition, continuous exposure to MC has been shown in other experimental situations [Exs. 7-14 and 7-130] to elicit more severe health effects than exposure to similar or higher concentrations when the animals are allowed a recovery period (for example, 6 hours' exposure per day). Exposure on a 6 or 8-hour per day schedule is also more like occupational exposure scenarios and therefore those experiments are generally easier to interpret when assessing risk to workers.
In summary, OSHA believes that the rat and gerbil data described above shows that MC can cause specific changes in the neurological system at a biochemical level. The Agency intends to monitor the scientific literature for additional developments on these effects to determine if these types of effects have implications for human CNS risks.
b. Human studies. The CNS depressant effects of MC have been well described in the literature [Exs. 7-4, 7-153, 7-154, 7-160, 7-175, 7-182, 7-183, 7-184]. MC causes CNS depression which is characterized by tiredness, difficulty in maintaining concentration, decreased task vigilance, dizziness, headaches, and, at high concentrations, loss of consciousness and death. Accidental human overexposures to MC [Exs. 7-18, 7-19] (for example, at concentrations greater than 10,000 ppm) have resulted in narcosis and death. CNS depression has been described after humans were exposed to experimental MC concentrations as low as 200 ppm [Ex. 7-175] and occupational concentrations as low as 175 ppm [Ex. 7-153].
i. Experimental studies. CNS depression was detected in human subjects exposed to MC at concentrations as low as 200 ppm for 4 hours or 300 ppm for 1.5 hours [Exs. 7-4, 7-160, 7-175, 7-182 and 7-184]. In these experiments, which measured subtle CNS depression (such as dual task performance and visual evoked response), it was not possible to determine a no observed effect level (NOEL), because the lowest experimental concentration used (200 ppm) elicited CNS effects. Since a NOEL was not determined for the CNS effects of MC, those effects may occur at lower exposures or after exposure for shorter durations.
The HSIA questioned whether bias was introduced into the results of these studies by inadequate procedures to establish a "double blind." This criticism raises a legitimate concern about the validity of the study. However, since Putz et al. did not describe the blinding procedures used in their experiments, the Agency concludes that there is not enough evidence publicly available to make the conclusion that the study is biased. OSHA believes that these studies were well conducted and is relying on the quality of the studies overall as evidence of the validity of the results. Absent evidence demonstrating the inadequacy of the blinding procedures, OSHA has determined that these studies show that MC can cause mild CNS depression in humans exposed at concentrations as low as 200 ppm.
NIOSH expressed concern regarding the potential for neurobehavioral impairment (expressed as CNS depression) at lower exposures and shorter durations, particularly in relation to the setting of a STEL for MC [Exs. 23-18 and 94]. In order to assess the potential impact of the CNS effects of MC, NIOSH looked at data gathered from several studies and compared breath concentrations of MC (as a surrogate for brain tissue MC concentrations) at different ambient exposure levels with the CNS depression described by Putz et al. [Ex. 7-175]. NIOSH concluded that:
At the proposed STEL of 125 ppm, increased uptake of MC in active workers may place them in the breath concentration range associated with mild neurobehavioral impairment. Although there are insufficient data to draw firm conclusions, extrapolation from existing studies suggests that the proposed STEL of 125 ppm may not fully protect physically active workers from CNS impairment. Therefore, a lower STEL should be considered, if feasible.
In response to concerns raised by NIOSH, the HSIA [Ex. 105] noted that NIOSH's analysis of breath MC concentration versus neurobehavioral impairment "seemed highly speculative." HSIA emphasized that the exposures which produced the reported neurobehavioral effects were observed only after 2 to 4 hours of exposure and that the effects were observed only when difficult tasks were measured.
To support their position, the HSIA asked Mr. Richard Reitz to use a PBPK model to estimate the concentration of MC in brain tissue. This analysis [Ex. 105] indicated that at exposures of 200 ppm for 15 minutes with persons exercising at 50 watts, the brain concentration of MC would be predicted to be similar to that observed in the Putz et al. study for subjects engaged in "light activity" for 2 hours at 200 ppm MC, which did not produce measurable CNS depression. (Putz et al. did not detect CNS depression in subjects exposed to 200 ppm for 2 hours). The model also predicted that 15-minute exposures to 125 ppm while the subject was exercising at 50 watts would produce brain MC concentrations substantially less than that predicted for the 4 hour exposure to 200 ppm MC.
OSHA considered the PBPK analysis presented by the HSIA, but was concerned that there has been no experimental validation of the predicted brain MC concentrations or any evidence as to what MC concentration would produce detectable CNS depression. OSHA believes the primary value of both the NIOSH and HSIA analyses is in demonstrating the relative effect that exercise and duration of exposure is likely to have on brain (or breath) concentrations of MC. The PBPK analysis clearly demonstrates that increasing exercise level increases brain concentration of MC, which is consistent with the detected CNS depression. Workers engaged in strenuous activity while exposed to MC should take special precautions, such as frequent breaks in fresh air, especially if dizziness or lightheadedness occurs.
Although OSHA found the PBPK model to be useful for demonstrating the interaction between exercise and brain concentration of MC, the Agency did not use the model quantitatively (for example, in determining the STEL). OSHA believes that the data suggest that there may be CNS effects at levels below those tested. There are no studies which directly address whether there are CNS effects after exposure to STEL concentrations of MC. To the extent that these effects occur, the STEL would not be protective. Mild and reversible CNS depression was detected at 200 ppm for 4 hours and 300 ppm for 1.5 hours. The Agency shares NIOSH's concern, based on extrapolation of breath MC concentrations, that the proposed STEL may not be adequately protective for physically-active workers.
OSHA concludes that there are clearly sufficient data to determine that a 125 ppm 15-minute STEL is needed to prevent a significant risk of material impairment to the CNS. Impairment of the CNS would also increase the risk from accidents. Measured data show risks at 200 ppm for four hours of exposure. A lower level at shorter duration is needed to avoid that risk. NIOSH's calculations show that for active workers a level lower than 125 ppm may be needed. However, because of feasibility concerns, which would be greater at lower levels and the suggestion that short duration of exposure (i.e., 15-minutes) may mitigate the effects, OSHA is retaining the proposed level, but will carefully monitor and follow up data to determine if this level eliminates significant risk.
ii. Occupational exposure studies. In the NPRM, OSHA summarized studies which it believed described a neuropathy associated with chronic occupational exposure to solvents. Weiss [Ex. 7-196] described the case of a 39-year old chemist who worked for 5 years with airborne concentrations of MC as high as 660 ppm to 3600 ppm in a room with poor ventilation. After 3 years of exposure, the worker developed neurological symptoms, characterized by restlessness, palpitations, forgetfulness, poor concentration, sleep disorders, and finally, acoustical delusions and optical hallucinations. No hepatic damage or cardiac toxicity was found. At the first appearance of symptoms, cessation of exposure produced an immediate cessation of symptoms. Later, longer and longer periods were required after termination of exposure in order to alleviate the symptoms. The increasing persistence of symptoms is consistent with a diagnosis of toxic encephalosis.
Hanke et al. [Ex. 7-195] examined 32 floor tile setters who were exposed primarily to MC at concentrations from 400 to 5300 ppm for an average tenure of 7.7 years. Clinical examination of 14 of the workers who had neurological symptoms (headache, vertigo, sleep disturbance, digestive complaints and lapses in concentration and memory) revealed changes in the EEG patterns of the exposed workers which persisted over a weekend pause in exposure. These EEG changes were characteristic of a toxic encephalosis produced by chronic intoxication with a halogenated solvent (MC). The persistence of the EEG changes over the weekend break indicated a prolonged effect of MC exposure on EEG patterns. (Additional changes in the EEG found during exposure could be attributed to an acute effect of MC). Although these studies represent a small number of cases with very high chronic exposures, the evidence is suggestive of a relationship between chronic MC exposure and toxic encephalosis.
In a case study report, Barrowcliff et al. [Ex. 7-123] attributed cerebral damage in a case study to CO poisoning caused by exposure to MC. Axelson [Ex. 7-150] has described an increased number of neuropsychiatric disorders among occupations with high solvent exposures.
In the NPRM, OSHA expressed the opinion that these studies, taken together, "provide suggestive evidence of a permanent toxicity [different from the observed reversible CNS depression] which may be the result of chronic exposure to MC." NIOSH stated that this assessment was too speculative and stated,
in the Hanke study, MC was apparently only one component of a solvent mixture and may not have been the only neurotoxic agent* * * In addition, the observation interval of 2.5 days was not long enough to provide convincing evidence of irreversible effect, regardless of the active agent.
Upon reexamination of these studies, OSHA agrees with NIOSH [Ex. 19-46] that although a prolonged effect (over a weekend break in exposure) of MC on EEG patterns has been demonstrated, these studies do not support a determination that MC exposure is associated with irreversible brain damage in humans.
OSHA reviewed several other studies of occupational exposure to MC for evidence of CNS effects of MC. The first study was provided as an English translation of a Czechoslovakian paper by Kuzelova et al. [Ex. 7-26]. These investigators examined workers in a film production plant who were exposed to MC concentrations from 29 to 4899 ppm. Several workers suffered frank MC intoxication and many workers showed signs of MC-induced CNS depression. Toxicity associated with chronic MC exposure was observed in workers exposed to MC for up to two years, but the authors recommended continuing studies of the long-term health effects.
OSHA believes that this study shows CNS depression in workers exposed to MC. The Agency agrees with the authors that this study was not sufficient to adequately characterize the long-term CNS health effects that may be induced by MC exposure.
Cherry et al. [Ex. 7-154] studied the effects of occupational exposure to MC at 28 to 175 ppm in two exposed populations. In a 1981 study, the authors found a marginal increase in self-reported neurological symptoms among exposed workers. This increase disappeared when an appropriate reference group was used for comparison. However, in a 1983 investigation, Cherry [Ex. 7-153] showed statistically significant increases in tiredness and deficits in reaction time and digit symbol substitution which correlated with MC in blood. Ambient MC exposures for this population ranged from 28 to 175 ppm for the full shift. This study demonstrated CNS effects due to occupational MC exposures below 200 ppm (the lowest dose which was administered in the experimental studies).
The HSIA [Ex. 105, p. 34] commented as follows:
Decades of experience with worker populations exposed even at levels up to the current 500 ppm TWA have provided no evidence that such workers have higher rates of accidents or other signs of significant neurobehavioral impairment.
To the contrary, OSHA believes that the occupational studies discussed above demonstrate that MC has an effect on the CNS at occupational exposure levels as low as 175 ppm.
The Agency believes that the 1983 study by Cherry shows that occupational exposure to MC concentrations below the former 8-hour TWA PEL of 500 ppm can produce detectable CNS effects. Although the 1981 study, which relied on self-report of neurological symptoms, did not demonstrate a CNS effect, the 1983 study examined more objective measures of CNS depression and correlated the observed effects with a direct measure of MC exposure. OSHA believes that this study demonstrates that, although the CNS depression may be mild, it is demonstrable in occupational settings and at concentrations in the range of the STEL (although the exposures in this study were over an 8-hour work day). As described above, OSHA is sufficiently concerned about the potential for health effects at concentrations below the STEL of 125 ppm that it will continue to gather information and revisit this issue, if warranted.
2. Cardiac Toxicity
As described in the section on the metabolism of MC, MC is metabolized in vivo (in animals and humans) to CO and CO(2). Cardiovascular stress has been observed after exposure to CO, so it is reasonable to suspect that similar health effects would be observed after exposure to MC (and metabolism to CO) [Ex. 7-73, 4-33]. Carbon monoxide successfully competes with oxygen and blocks the oxygen binding site on hemoglobin, producing carboxyhemoglobin (COHb) and reducing delivery of oxygen to the tissues. This reduces the oxygen supply to the heart itself, which can result in myocardial infarction (heart attack) [Ex. 4-33].
Generally, humans have a baseline level of COHb of less than 1% COHb due to the endogenous production of CO from normal metabolic processes. The measured level of COHb in the general non-smoking population is from 1% to 3% because of direct exposure to CO from combustion sources such as automobiles, etc. In smokers, COHb generally ranges from 2% to 10% because of the additional CO exposure during smoking. CO generated from exposure to MC would be additive to the COHb burden already experienced by an individual from direct exposure to CO. The cardiac health effects anticipated from exposure to MC itself or CO as the result of metabolism of MC are described below.
a. Animal studies. There is no evidence from animal studies in the MC rulemaking record that MC has a direct toxic effect on cardiac tissue. After lethal doses of MC, death has been primarily attributed to CNS and respiratory depression [Exs. 7-27, 7-28]. Also, chronic studies (in which COHb levels have been maintained at 10% and higher) [Exs. 7-3, 7-8, 7-14, 7-130, 7-151] have not shown direct cardiotoxicity.
Chlorinated solvents have been shown to sensitize the cardiac tissue to epinephrine-induced fatal cardiac arrhythmias [Ex. 7-226]. However, MC is less effective in sensitizing cardiac tissue than other chlorinated analogues. MC caused sensitization of cardiac tissues only at doses well above doses which produce a narcotic effect. This finding indicates that compliance with an 8-hour TWA of 25 ppm MC would likely be sufficient to protect against such sensitization.
b. Human studies. The metabolism of MC to CO and measurement of COHb in human subjects exposed to MC were described in detail in the NPRM. In summary, it was found that exercising increased MC uptake and, subsequently, increased blood COHb levels compared to that of sedentary individuals [Ex. 7-222]. In addition, COHb levels due to smoking were found to be additive to the COHb produced by MC metabolism. Taken together, these results suggested that smokers or individuals engaged in physical exertion (as in a workplace) may be at increased risk from CO-induced toxicity from MC exposure. This risk may be especially elevated in individuals with silent or symptomatic cardiac disease who may be susceptible to very small increases in COHb because of an already impaired blood supply to the heart. Many American workers have silent or symptomatic heart disease. This increased OSHA's concern for the potential cardiac effects of MC and its metabolites.
Elevated COHb has been measured in humans experimentally and occupationally exposed to MC [Exs. 7-4, 7-5-R0327, 7-102, 7-115, 7-157, 7-159, 7-169, 7-174, 7-176]. The effects of elevated COHb are primarily increased risk of myocardial infarction, especially in susceptible individuals. Atkins and Baker [Ex. 7-198] described two cases of myocardial infarction in workers subsequent to CO exposure. COHb was measured at 30% and 24% in these individuals, which is much higher than normal general population levels of COHb. Humans exposed to MC would not be expected to experience COHb at those levels unless the exposure to MC was extremely high (greater than 500 ppm).
In a laboratory study of humans with coronary artery disease, subjects were exposed to CO and observed for cardiac health effects during exercise. In subjects with 3 to 10% COHb, decreased exercise tolerance and increased anginal pain were observed [Ex. 7-198]. In an epidemiological study submitted to OSHA by NIOSH during the MC public hearings, the investigators observed a statistically significant excess of ischemic heart disease mortality among tunnel workers when compared with rates for the New York City population [Ex. 23-18]. This increase in mortality is supported by clinical findings. Allred et al. [Ex. 23-18] observed that elevation of COHb from 0.6% to as low as 2% decreased time to myocardial ischemia and anginal pain during laboratory tests. OSHA believes that these studies, taken together, suggest that small increases in COHb can adversely affect persons with compromised cardiac health. The results observed in the tunnel workers are particularly relevant because they show an increased risk in a working population. NIOSH used these studies to support its recommendation that the COHb effects of MC be carefully considered in the MC rulemaking [Tr. 881-2, 9/21/92]. OSHA agreed with NIOSH that the effects observed at low levels of COHb are cause for concern about the risks of MC metabolism to CO.
In the NPRM, OSHA also reviewed case reports in which individuals exposed to MC experienced myocardial infarctions [Exs. 7-102, 7-73]. These case reports suggested that exposure to MC increased cardiac stress, although it was not determined whether this was a direct effect of MC or as the result of metabolism of MC to CO. OSHA believes that these case studies support the hypothesis that CO generated through metabolism of MC would have the same adverse health effects as direct CO exposure.
Two epidemiological studies (in film coating and fiber production workers) [Exs. 7-75, 7-76, 7-122, 7-163] examined cardiac mortality due to occupational exposure to MC. Ott [Ex. 7-76] compared mortality from a plant in South Carolina that used MC to a reference plant in Virginia. An increased risk ratio for ischemic heart disease (risk ratio = 3.1) was observed in the MC-exposed workers compared to the reference population.
This approach controls for the healthy worker effect by comparing two working populations, and excess risk was demonstrated. The authors believed that the apparent excess risk was due to geographical variability in the incidence of ischemic heart disease. The population from the reference plant was found to have an unusually low death rate due to ischemic heart disease in comparison to the general population rate.
In an update of the study [Ex. 7-75], the ischemic heart disease rate in the exposed population was compared to that in the surrounding York County, S.C. population instead of a reference plant. No difference in ischemic heart disease rates was detected between exposed workers and controls, although this approach would not control for the healthy worker effect. The SMR was 0.94 (32 observed, 34.2 expected).
NIOSH disagreed with the conclusion of the authors of this study, and indicated that the studies summarized above would be cause for concern regarding the cardiac effects of MC. NIOSH suggested that the raw data from the epidemiological studies of cellulose acetate film production workers and the studies of workers in cellulose acetate fiber manufacture be reviewed for cardiac mortality occurring during the period of occupational exposure for the workers. OSHA is concerned about the potential CO effects from metabolism of MC and will continue to monitor the scientific literature on this topic. However, the Agency is setting the exposure limits based on cancer and CNS effects and has not reached final conclusions on this issue.
3. Hepatic Toxicity
Chlorinated hydrocarbons as a class, such as carbon tetrachloride and chloroform, are toxic to the liver. In general, chlorinated hydrocarbons cause cytotoxicity (cell death) in rodent livers. Therefore, there was suspicion that the liver would also be a target organ for MC (a chlorinated hydrocarbon) toxicity. OSHA evaluated the available literature on the hepatic effects of MC in animal and human studies.
a. Animal studies. Studies of the effects of MC exposure on the rodent liver have not demonstrated significant acute liver toxicity, even at lethal or near-lethal doses. As summarized in the NPRM, Kutob et al. [Ex. 7-27] and Klaassen et al. [Ex. 7-28] conducted experiments on halogenated methanes and hepatotoxicity. MC was determined to be the least hepatotoxic of the halogenated methanes examined. The only injury described was a mild inflammatory response associated with lethal MC concentrations. These studies demonstrated that liver was not the primary target organ for the acute toxicity of MC.
Weinstein et al. [Ex. 7-181] examined the hepatic effects of MC on female mice who were continuously exposed for up to 7 days to MC concentrations of up to 5000 ppm. Mild, nonlethal injury to the livers was noted, characterized by balloon degeneration of the rough endoplasmic reticulum (RER), transient severe triglyceride accumulation (fatty liver), partial inhibition of protein synthesis and breakdown of polysomes into individual ribosomes. The injury is similar to a mild form of carbon tetrachloride toxicity (a structural analog of MC) and suggests that although the toxicity due to MC is not as severe as that produced by carbon tetrachloride, the mechanism of toxicity may be similar.
In subchronic experiments more severe effects were observed in the liver after continuous exposure. MacEwen et al. [Ex. 7-14] studied the effects of continuous exposure of mice, rats, dogs and rhesus monkeys to 1000 and 5000 ppm MC for up to 14 weeks. Fatty liver, icterus, elevated SGPT and ICDH were reported in dogs at both concentrations. These effects appeared at 6-7 weeks of exposure to 1000 ppm MC and at 3 weeks of exposure to 5000 ppm. Monkeys were less sensitive to hepatic injury, and showed no changes in liver enzymes and only mild to moderate liver changes at 5000 ppm MC. No liver alterations were detectable in monkeys exposed to 1000 ppm MC. Mice and rats developed liver toxicity at both exposure levels, characterized by increased hemosiderin pigment, cytoplasmic vacuolization, nuclear degeneration and changes in cellular organization.
Hepatic effects associated with chronic MC exposure were observed in lifetime cancer bioassays in three rodent species: rats, mice and hamsters. In studies conducted by the NTP and Dow Chemical Co., rats were exposed to inhalation concentrations of MC from 50 ppm to 4000 ppm 6 hours per day, 5 days per week [Exs. 7-8, 7-151, 7-173]. Hepatic effects were observed after exposure to MC concentrations as low as 500 ppm. These effects were characterized by increased fatty liver, cytoplasmic vacuolization and an increased number of multinucleated hepatocytes. At higher doses (greater than 1500 ppm), increased numbers of altered foci and hepatocellular necrosis became apparent.
Serota et al. [Ex. 7-180] administered 5 to 250 mg MC/kg body weight to rats in drinking water. Hepatic toxicity similar to that observed in the inhalation studies was reported at doses from 50 to 250 mg/kg.
In mice, the chronic hepatic effects of MC were investigated in two bioassays: NTP [Ex. 7-8] and Serota et al. [Ex. 7-179]. In the NTP study, mice were exposed by inhalation to 2000 or 4000 ppm MC. Cytologic degeneration was observed in both male and female mice and increased incidences of hepatocellular adenomas and carcinomas were found at both concentrations. The carcinogenic effects of MC are described in greater detail above, in the discussion of MC carcinogenicity.
In a drinking water study, Serota et al. found that mice exposed to 50 to 250 mg/kg/d MC had dose-related increases in the fat content of the liver (a sign of liver toxicity). Although some proliferative hepatocellular lesions were identified in this study, they were distributed across all exposure groups. Hepatocellular tumor incidences were not elevated above historical control incidences.
In the hamster, Burek et al. [Ex. 7-151] found minimal treatment-related changes in the livers of the MC-exposed animals after exposure to 500, 1500 or 3500 ppm MC. A dose-related increase in hemosiderin was found in male hamsters at 6 months and at 3500 ppm at 12 months. No other changes in liver physiology were reported.
OSHA believes that these studies demonstrate that the rodent liver is not sensitive to acute affects of MC, but that chronic exposure to MC caused toxic effects in rat and mouse liver and cancer in mouse liver. These studies appear to have been well conducted and the differences in toxicity observed across studies were likely due to differences in dose or route of exposure. The hamsters appeared to be insensitive to liver toxicity. OSHA believes that this is most likely due to inherent species differences in response to toxicants.
b. Human studies. OSHA evaluated epidemiological studies and case reports to determine the extent of hepatic effects detected after exposure of humans to MC. Liver toxicity was measured as alterations in the blood levels of any of several normal liver enzymes in these studies.
i. Epidemiological studies. In a cross-sectional analysis of the health of workers in an acetate fiber production plant in which workers were exposed to 140 to 475 ppm MC, Ott et al. [Ex. 4-33c] reported statistically significant increases in serum bilirubin and alanine aminotransferase (ALT) (also known as serum glutamic pyruvic transaminase (SGPT)) when compared with a reference group of industrial workers. The elevation in bilirubin levels showed a dose-response relationship, but the ALT levels were not associated with MC exposure. The authors felt that the increase in ALT in MC-exposed workers could not be attributed to MC because a dose-response relationship was not demonstrated and, therefore, the increase in ALT between the exposed and reference populations could be disregarded as a sign of liver toxicity. The authors concluded that although bilirubin elevation may be interpreted as a sign of liver toxicity, this interpretation was not supported by alterations in other liver parameters. OSHA feels that ALT cannot be disregarded as unrelated to MC exposure based on the lack of dose response within the exposure group. The high variability of this parameter and the low numbers of individuals within certain exposure subgroups (e.g., 10 men exposed at 280 ppm), make a dose-response relationship more difficult to demonstrate. Any mistake made in the characterization in an exposure group would result in obscuring the dose-response relationship. Although the evidence is not unequivocal, OSHA believes that the elevated bilirubin coupled with the elevated ALT values indicate suggestive evidence of a hepatotoxic response to MC exposure in this worker population.
In an update to the study described above, Cohen et al. [Ex. 7-75] found 4 cases of liver/biliary duct cancer in workers with more than 10 years of exposure to MC and after 20 years from first hire. Further description of this study can be found in the discussion of MC carcinogenicity, above.
In an English translation of a 1968 Czechoslovakian study, Kuzelova et al. [Ex. 7-26] found no liver enzyme abnormalities in workers exposed to MC concentrations from 29 ppm to 4899 ppm for up to two years. In contrast, in an English translation of a German study which focussed on neurological changes due to MC exposure, Hanke et al. [Ex. 7-195] observed pathological liver function tests and hepatomegaly (enlarged liver) in 4 of 14 floor tile setters examined. These workers were chronically exposed to MC at concentrations as high as 400 to 5300 ppm. The average tenure of employment of these workers was 7.7 years. The authors of the Hanke study noted that although MC with its impurities could be responsible for the liver damage, the evidence was not conclusive. OSHA has determined that there is insufficient evidence from the Kuzelova and Hanke studies to conclude that MC causes chronic human hepatotoxic effects.
ii. Case reports. In addition to the cross-sectional analyses of worker morbidity described above [Exs. 4-33c and 7-26], the relationship of MC exposure and hepatotoxicity has been studied by analysis of case reports. Welch [Ex. 7-73] collected 144 case reports of clinical disease reported subsequent to occupational MC exposure. Quantitative exposure estimates for individuals were unreliable, but the presence of MC in the work environment was ascertained for each employee. The most prevalent findings in these case reports were CNS symptoms, upper respiratory syndrome and alterations in liver enzymes. The patterns of alteration in liver enzymes were not consistent among individuals, but may be suggestive of a MC-associated hepatotoxic effect. One case of hepatitis of unknown etiology was identified. The case physician believed that the hepatitis was secondary to solvent exposure. The solvents to which this employee was exposed included xylene and methylethyl ketone as well as MC. OSHA believes that the confounding solvent exposures in the hepatitis case and the unknown exposure histories of the individuals with altered liver enzymes limit the interpretation of these studies. OSHA has determined that these case reports provide insufficient evidence to conclude that MC was the causative agent in these cases.
Analysis of cases of fatal and near-fatal human exposures [Exs. 7-18, 7-19] indicated no apparent acute alterations of liver function. Acute concentrations of MC which caused narcosis and even death were not associated with changes in liver enzymes.
OSHA concludes that limited evidence supports the hypothesis that MC causes human hepatotoxicity, based on the data in the Ott study. The remaining studies and case reports do not provide clear evidence of a causative role of MC in hepatotoxicity. The Agency has set the exposure limits based on cancer and CNS effects and has not reached final conclusions on this issue.
4. Reproductive Toxicity
There are only limited data available regarding the potential adverse teratogenic or reproductive effects due to MC exposure. Teratogenicity studies have been conducted in rats and mice and limited epidemiology and case reports have been described for humans.
a. Animal studies. A study [Ex. 4-5] using chicken embryos indicated that MC disrupts embryogenesis in a dose-related manner. Since the application of MC to the air space of chicken embryos is not comparable to MC administration to animals with a placenta, the exposure effect seen in the chick embryos can only be considered as suggestive evidence that an effect may also occur in mammalian systems.
The teratogenicity of inhaled MC has also been studied in rats and mice [Exs. 7-20, 7-21, 7-22]. In 1975, Schwetz et al. [Ex. 7-21] conducted a study on Swiss Webster mice. Mice were exposed to 1250 ppm MC for 7 hours/day, on days 6-15 of gestation. On day 18 of gestation, Caesarian sectioning of dams was performed. A statistically significant increase in mean maternal body weight (11-15%) was observed in dams exposed to 1250 ppm MC; however, food consumption was not measured. The only effect on fetal development associated with MC exposure was a statistically significant increase in the number of fetuses which contained a single extra center of ossification in the sternum. The incidence of gross anomalies observed in the MC-exposed fetuses was not significantly different from that in the control litters. Maternal COHb level during exposure reached 12.6%; however, 24 hours after the last exposure, COHb had returned to control levels.
In the same study by Schwetz et al. [Ex. 7-21], Sprague-Dawley rats were exposed to 1250 ppm MC via inhalation for 7 hours daily on days 6-15 of gestation. No MC-associated effects were observed in food consumption or maternal body weight. Among litters from MC-exposed dams, the incidence of lumbar ribs or spurs was significantly decreased when compared to controls, while the incidence of delayed ossification of sternebrae was significantly increased compared to controls. No increased incidence of gross anomalies were observed in the fetuses from exposed rats compared to fetuses from control litters. No MC-associated effects were observed on the average number of implantation sites per litter, litter size, the incidence of fetal resorptions, fetal sex ratios or fetal body measurements, in the 19 litters that were evaluated. As observed in the MC-exposed mice, there was significant elevation of the COHb level in the dams, but the level returned to control values within 24 hours of cessation of exposure.
In 1980, Hardin and Manson [Ex. 7-22] evaluated the effect of MC exposure in Long-Evans rats after inhalation of 4500 ppm for 6 hours/day, 7 days/week prior to and during gestation. Four exposure groups were described. The first group was exposed to MC for 12 to 14 days prior to gestation and during the first 17 days of pregnancy. The second group was exposed to MC only during the 12 to 14 days prior to gestation. The third group was exposed to MC only during the first 17 days of pregnancy. The fourth group (control group) was exposed only to filtered air. The purpose of this study was to test whether MC exposure prior to and/or during gestation was more detrimental to reproductive outcome in female rats than exposure during gestation alone.
In rats exposed to MC during gestation, there were signs of maternal toxicity, characterized by a statistically significant increase in maternal liver weights. The only fetal MC effects observed were statistically significant decreases in mean fetal body weights. No significantly increased incidence of skeletal or soft tissue anomalies was observed in the offspring.
In 1980, Bornschein et al. [Ex. 7-224] tested some of the offspring of the Long-Evans rats from Hardin and Manson's study described above. All four treatment groups were used to assess the postnatal toxicity of MC exposure at 4500 ppm. The general activity measurements of groups of 5-day old pups showed no exposure-related effects. At 10-days of age, however, significant MC-associated effects were observed in both sexes in the general activity test. These effects were still apparent in male rats at 150-days of age. This study showed that maternal exposure to MC prior to and/or during pregnancy altered the manner in which the offspring react and adapt to novel test environments at up to 150-days of age. These effects suggest that MC exposure prior to, or during pregnancy may influence the processes of orientation, reactivity, and/or behavioral habituation. No changes in growth rate, long-term food and water consumption, wheel running activity or avoidance learning were reported.
OSHA concluded from the animal studies that maternal exposure to high concentrations of MC during pregnancy may have some adverse effects on the offspring, in particular with regard to behavioral effects. The Agency has set the exposure limits based on cancer and CNS effects and has not reached final conclusions on this issue.
b. Human studies. Limited data have been collected on the reproductive effects of MC in male workers. In a study reported in the Occupational Safety and Health Reporter [Ex. 7-43], a greater risk of male sterility was found in male workers exposed to MC. In 1988, Kelly [Ex. 7-165] reported 4 cases of oligospermia in MC-exposed workers. This study was described in detail in the NPRM. Although the study provided some evidence of an effect of MC on male fertility, the observations were based on a small number of cases and OSHA believes that more research is necessary before causative conclusions can be drawn about the human male reproductive toxicity of MC.
The reproductive and developmental effects due to MC exposure in female workers have also been studied. According to information reported in an English translation of an abstract of a Russian article by Vozovaya et al. [Ex. 7-16], detectable levels of MC were found in the blood, milk, embryonal, fetal and placental tissues of nursing women exposed to MC in a rubber product plant. No other information was provided in the abstract. In a study by Taskinen et al. [Ex. 7-199], increased rates of spontaneous abortions were observed in female pharmaceutical workers exposed to MC. Exposure data were not reported in this study and it is unclear what confounding factors or other chemical exposures were present. OSHA believes that more research is necessary in order to evaluate the potential effect of MC on pregnancy outcomes, and so has not reached a conclusion on this issue.
Carbon monoxide has well known adverse reproductive effects in humans. Since MC is metabolized to CO, OSHA was concerned about the adverse reproductive effects of CO as a metabolite of MC. The EPA has reviewed the literature on the effects of maternal CO exposure on the development of the fetus in the Air Quality Criteria for Carbon Monoxide [Ex. 7-201]. Very high maternal CO exposures have resulted in fetal or infant death or severe neurological impairment of the offspring. CO reduces the amount of oxygen available to the tissues. The developing fetus is very sensitive to these effects. According to Fechter et al. [Ex. 7-200], low levels of CO exposure in animals have been shown to adversely affect the fetus, producing CNS damage or reduced fetal growth. These effects suggest that the fetus may be especially sensitive to the toxic effects of MC through its metabolism to CO.
As described above, OSHA is sufficiently concerned about the potential for reproductive health effects of carbon monoxide as a result of MC metabolism that it has decided to continue to gather information and revisit this issue, if warranted.
F. Conclusion
OSHA's determination that MC is a potential occupational carcinogen was based primarily on the positive findings of chronic inhalation bioassays in rodents. MC is carcinogenic to mice of both sexes, producing lung and liver neoplasms. In rats, MC produced dose-related increases in mammary tumors and increases in the number of tumors per tumor-bearing rat. The evidence in rodents is supported by epidemiologic findings from cellulose triacetate fiber production workers and a case-control study of individuals with astrocytic brain cancer. The study of fiber production workers suggests an association between liver and biliary cancer and long term (greater than 10 years) exposure to MC. The case-control study indicates an association between risk of astrocytic brain cancer and occupational exposure to MC. This evidence is further supported by the findings of genotoxic activity of MC in bacterial and mammalian cell systems. OSHA has set the 8-hour TWA PEL of 25 ppm primarily to protect employees from the risk of cancer due to MC exposure in the workplace.
CNS depression has been demonstrated in humans and animals at relatively low inhalation concentrations of MC. The CNS depression observed in those studies was relatively mild, although the effects occurred at concentrations in the range of the STEL of 125 ppm. OSHA believes that the STEL will be protective against CNS depression for most employees exposed to MC most of the time, but the Agency is sufficiently concerned about the potential for CNS health effects at concentrations below the STEL and have decided to continue to gather information and revisit this issue, if warranted.
VI. Quantitative Risk Assessment
Summary
After examining all the available data, both animal and human, and both quantitative and qualitative, OSHA has concluded that MC is a multi-species, multi-site carcinogen in various rodent species, and is likely to be so in humans, and that it most probably acts via one or more genotoxic metabolite(s). The evidence for this conclusion is quite strong: there exist several positive bioassays with low background incidence and dose-related increases; there is an unusually large amount of mechanistic information; and there are several positive epidemiological studies and no negative epidemiological studies of sufficient power to rule out the animal-based potency estimates.
Furthermore, OSHA has conducted a quantitative risk assessment based on the highest-quality animal tumor data, constructing a state-of-the-art physiologically-based pharmacokinetic (PBPK) model incorporating rodent and human metabolic information. That analysis shows a final estimate of risk of 3.62 deaths per 1000 workers occupationally exposed to 25 ppm MC for a working lifetime. [An alternative analysis, which incorporated all of the data used in the main analysis plus the assumption that human enzymes are even less active to MC (as compared to mice) than that predicted by the main analysis, gave a risk estimate of 1.23 deaths per 1000]. Both estimates are clearly well above any plausible upper boundary of the "significant risk" range defined by the Supreme Court, used by OSHA in its prior rulemakings, and reported in the scientific/economic literature on risk. The estimated risk at the current PEL of 500 ppm is 126 excess cancers per 1000 workers; clearly, the 25 ppm standard will effect a substantial reduction in a very high risk. The Final Economic Analysis shows that the average risk at current exposure levels is approximately 7.6 deaths per 1000 and ranges up to 126 per 1000; at post-regulatory exposure levels (which account for the fact that the action level will encourage some employers, where feasible, to lower exposures below 25 ppm), average risk is estimated to be 1.7 deaths per 1000 (and nowhere higher than 3.62 per 1000 risk at the new PEL of 25 ppm) -- also a substantial reduction of a highly significant risk.
Prior to the October 1995 record reopening, there was strong evidence to support the determination that MC is a human carcinogen, using well-established risk assessment models based on substantial biologically-based evidence and theories: there were two multi-site positive bioassays with dose-response trends and low background, and suggestive epidemiology with no clearly conflicting epidemiology. The only question was whether to use an administered-dose scaling or a PBPK model.
Data submitted in the reopening of the record in late 1995 shed light both on the hazard identification and the quantitative risk assessment. Studies of isoenzyme activity and intracellular distribution across species were interpreted by the Halogenated Solvents Industry Alliance (HSIA) to suggest that MC is not a human carcinogen. OSHA has concluded that the HSIA interpretation of the studies is not supported by the evidence. There are numerous methodological problems with the studies: for example, in the experiment in which Graves et al. examined MC-induced mutations [Ex. 123], OSHA agrees with Dr. Douglas Bell [Ex. 126-26] that insufficient numbers of doses and mutants were examined to reach any conclusions whatsoever regarding differences in mutation spectra between chemicals.
More importantly, OSHA and most commenters agreed that the data showed a quantitative -- and quantifiable -- difference between mice and humans, not an infinite, qualitative one. In other words, there is substantial evidence that humans and mice metabolize MC similarly, only at different rates. HSIA's qualitative argument rests on two questionable assumptions, both of which are contradicted by other data: first, that the DNA single strand break assay is infinitely sensitive -- but the investigators do not even know if it is sensitive enough to show the 7-fold difference in enzyme activity between mice and humans that OSHA's main PBPK analysis uses; and second, that the human isoenzyme most active against MC, although clearly present in human cells, is located in a different part of the cell. This interpretation: 1) contradicts some basic beliefs of comparative physiology (Why would the cell structures of humans and mice be so fundamentally different?); 2) would require OSHA to do a "subcellular PBPK analysis" to predict risk -- no one has ever developed, let alone parameterized and validated, such a model; and 3) contradicts other data on activation by mouse cytosolic preparations -- MC has been shown to have enhanced mutagenicity in bacterial and mammalian cell preparations when mouse cytosolic preparations were used to metabolize the MC. This requires metabolism by cytoplasmic (not nuclear) GST and for the metabolites to be stable enough to cross membranes and interact with DNA.
Therefore, the new studies do not cast doubt on the MC hazard identification -- in fact, they should probably increase the level of concern because it is now more clear that MC is likely to act by a genotoxic mechanism [animal tests are most relevant to humans when clear genotoxic agents are involved] and that that pathway exists in humans, and may be concentrated in cells of concern in human cancers, such as the bile duct epithelium. OSHA notes that an epidemiologic study of cellulose triacetate fiber workers has shown a statistically significant increase in biliary duct tumors [Ex. 7-260].
On the other hand, the new data did reinforce OSHA's decision to proceed with a PBPK-based risk assessment and helped OSHA to incorporate the best available scientific data into a PBPK model. Here OSHA presents two PBPK-based risk analyses, both of which represent substantial refinements over the applied-dose risk assessment and over previous PBPK analyses. OSHA's final risk assessment incorporates all reliable data -- OSHA's alternative analysis, in addition to the data in the final risk assessment, also incorporates some suggestive/sparse data found in new studies. As stated above, both analyses estimate risks at 25 ppm well in excess of any possible boundary line between significant and insignificant risk.
Both of OSHA's PBPK analyses made two major advances: 1) the use of non-independent Monte Carlo simulation -- Monte Carlo simulation is a well-developed computational technique that allows the modeler to take estimates of uncertainty in each of the many variables in a complex model and generate a quantitative estimate of the total uncertainty in the result. Others have used Monte Carlo simulation in PBPK modeling, but OSHA added information on the covariance structure of all the parameters, so that the uncertainty estimate would not be biased (exaggerated, probably) by incorrectly assuming that all the variables could simultaneously be at their lowest or highest values; and 2) the use of Bayesian analysis -- this allows uncertainty distributions to be better estimated (narrowed) by cross-checking them against other independently-collected data from laboratory experiments, rather than simply guessing how big the uncertainties are and not refining the estimates as the model runs.
Both these advances enabled OSHA to strike a balance between two unsatisfactory extremes -- a) the extreme overconfidence of using estimates for each variable that did not allow for any uncertainty -- or b) the extreme "underconfidence" of assuming that all uncertainties are independent of each other and of other laboratory data. The result is an analysis that tells what science knows and does not know about the relationship between ambient concentrations and the putative relevant dose measure (concentration of GST metabolites in the target organ) in mice and humans.
Again, OSHA's final risk assessment regards the very limited human data base on GST-0 activity [a total of 39 liver samples and 5 lung samples] as useful, but insufficient to discard the traditional "allometric" assumption (the well-validated assumption that, as a general rule, metabolic parameters scale proportional to a function of the animal's body weight). OSHA's alternative analysis accepts the limited human data at face value to extrapolate without using allometry. OSHA has concluded that the main analysis is better supported by available evidence than is the alternative analysis, but both yield significant risks. An important caveat is that both models are strictly applicable to humans who are physiologically similar to the six subjects analyzed by Dow (see the discussion later in this document for a fuller explanation). Since the population of 200,000 workers will be much more heterogeneous than those six subjects, we regard these estimates as "overconfident" -- some workers exposed at 25 ppm will have higher risks than 3.6 per 1000 (although some may have lower risks as well).
Introduction
OSHA performs quantitative risk assessment, when information permits, to help determine the Permissible Exposure Limit (PEL) for toxic substances (contingent on the feasibility determination). The first step of assessing risks to human health is hazard identification. This step results in the determination that an exposure to a toxic substance causes, is likely to cause, or is unlikely or unable to cause, one or more specific adverse health effect(s) in workers. This identification also shows which studies have data that would allow a quantitative estimation of risk.
If studies are available that contain information regarding the amount of exposure and disease, mathematical modeling allows extrapolation of the information in the study to conditions of concern in the workplace. OSHA uses these risk estimates to determine whether exposure results in significant risk, and whether the standards considered by OSHA substantially reduce the risk.
This section describes the record evidence received during the public rulemaking concerning OSHA's quantitative risk assessment and the reasons OSHA has maintained or modified its opinion from the proposal. In the following sections, the evidence supporting and casting doubt on the hypothesis that MC is a probable carcinogen (the "Hazard Identification" issues) is discussed first. Then the results of OSHA's quantitative risk assessments, conducted to estimate the carcinogenic potency of MC, are discussed.
A. Methylene Chloride Hazard Identification
Animal and human evidence, summarized in the health effects section, indicates that MC can cause cancer, cardiac effects, central nervous system damage and other health effects. As described in the NPRM, OSHA's preliminary quantitative risk assessment was based on cancer and relied on rodent bioassay data for quantitation of risks. In 1986, the National Toxicology Program (NTP) concluded that the mouse bioassay data provided "clear evidence" of carcinogenesis in male and female mice, based on the liver and lung tumors. The NTP also determined that the rat mammary tumors observed in the bioassay provided clear evidence of carcinogenesis in female rats and some evidence of carcinogenesis in male rats. This evidence of cancer in multiple species and in both sexes underlies the concern for MC as a potential human carcinogen. On the basis of these studies, IARC has classified MC as a 2B carcinogen, the EPA has classified MC as a B2 carcinogen and NIOSH has classified MC as a potential occupational carcinogen. OSHA concurred with these assessments.
Animal bioassays are a critical tool in determining the potential hazard of a substance for humans. Virtually all of the toxic substances that have been demonstrated to be carcinogenic in humans are also carcinogenic in laboratory animals. Although it is possible that a substance may be carcinogenic in a laboratory species, but not in humans, it is reasonable to suspect that substances that cause cancer in multiple animal species and at multiple target organ sites would be carcinogenic in humans. Therefore, in the absence of sufficiently powerful negative epidemiological studies or mechanistic studies demonstrating that the purported carcinogenic mechanism of action of the substance is irrelevant to humans, OSHA and other federal agencies rely on well-conducted, high-quality bioassays as the primary basis for their hazard identification and risk assessment. This is the case with MC.
During this rulemaking, some commenters have supported and others have questioned the hazard identification of MC as a potential human carcinogen. Most recently, some commenters contested the relevance of the mouse bioassay data for extrapolating to human cancer risks. Although these issues were raised by some rulemaking participants earlier in the rulemaking process, they were most thoroughly explored in connection with the information received by the Agency in late 1995. On October 24, 1995, OSHA reopened the MC record to receive comments on several studies submitted to the Agency by the Halogenated Solvents Industry Alliance (HSIA) pertaining to the mechanism of action of MC carcinogenesis in mice, and the implications of these studies for estimating human risks. The record closed on November 29, 1995, but was reopened in order to give the public additional opportunity to comment on the submitted studies. The record then closed on December 29, 1995. Thirty-seven comments were received on this topic and reviewed as part of this rulemaking.
The papers submitted by the HSIA consisted of a cover letter [Ex. 117], an overview of the sponsored research [Ex. 118] and seven research papers on the mechanism of MC carcinogenesis [Ex. 119-124A]. The hypothesis under investigation in these seven studies was that the pathways of MC metabolism and the mechanism of carcinogenesis in the mouse represented a unique situation that would not take place in humans, making the mouse unsuitable as the basis for extrapolating risks of cancer to humans. The specific studies are described briefly here and the comments received during the reopening of the rulemaking record are discussed in detail below.
1. Summary of Studies Submitted by HSIA
Exhibit 119 "Methylene Chloride: an inhalation study to investigate toxicity in the mouse lung using morphological, biochemical and Clara cell culture techniques," J.R. Foster, T. Green, L.L. Smith, S. Tittensor, and I. Wyatt, Toxicology 91 (1994) 221-234.
This study investigated the potential role of MC as a mouse lung carcinogen via non-genotoxic mechanisms and the Clara cell as the cell of origin in mouse lung cancer. The hypothesis was that MC acts specifically to produce toxicity (vacuolation) in Clara cells which leads to cell proliferation and production of mouse lung tumors. The authors investigated the toxicity of MC in bronchiolar Clara cells by measuring the production of vacuoles after exposure to MC. The investigators also measured DNA synthesis in Clara cells isolated from mice exposed to MC as a measure of cell proliferation.
The authors observed a transient vacuolation of bronchiolar Clara cells in mice exposed to 2000 and 4000 ppm MC, but not in mice exposed to 0, 125, 250, 500 or 1000 ppm MC. When the mixed function oxidase (MFO) pathway was inhibited, the bronchiolar cell vacuolation observed after exposure to 2000 and 4000 ppm MC was reduced. Inhibition of the glutathione S-transferase pathway (GST) had no effect on Clara cell vacuolation. The researchers also found that exposure of mice to 1000 ppm MC or greater for 6 hours induced an increase in DNA synthesis in Clara cells cultured in vitro from exposed animals.
Clara cells are present in mice, rats and humans, but appear to be more abundant in mice than other species. Clara cells contain enzymes for both the MFO and glutathione S-transferase (GST) pathways of MC metabolism. According to the authors, the results of this study suggest that metabolism of MC via the MFO pathway induces a transient toxicity in Clara cells and a transient increase in DNA synthesis.
Exhibit 120 "Methylene chloride-induced DNA damage: an interspecies comparison," R.J. Graves, C. Coutts and T. Green, Carcinogenesis, vol. 16 no. 8 pp. 1919-1926, 1995.
This study investigated the role of MC as a mouse carcinogen via a genotoxic mechanism of action. The hypothesis under investigation was that MC is metabolized to a genotoxic carcinogen via the GST pathway to different extents in different species and that expression of this genotoxicity correlates with risk of developing cancer across species. The authors used production of single strand (ss) DNA breaks as a measure of genotoxicity. The researchers measured DNA ss breaks in lung and liver cells from mouse, rat, hamster and humans. They observed increased DNA ss breaks in mouse liver cells, after in vivo exposure to 4000-8000 ppm MC for 6 hr and in mouse lung cells after exposure to 2000-6000 ppm MC. Depletion of glutathione in the liver (after administration of buthionine sulfoximine) reduced the amount of ss breaks observed. No increase in ss breaks was observed in Clara cells isolated from mice exposed to MC in vivo. However, in experiments on isolated mouse Clara cells, the authors observed increased DNA ss breaks in cells exposed to concentrations of MC of 5 mM and above.
No increases in ss breaks above control levels were detected in rat livers after exposure to 4000 ppm for 6 hr or in rat lungs after exposure to 4000 ppm for 3 hr. Increases in ss breaks were also not detected in hamster and human liver cells after exposure to MC in vitro at concentrations up to 90 and 120 mM.
In Chinese hamster ovary (CHO) cells, MC plus mouse liver cytosol (which contains the GST enzymes) also induced ss breaks, while incubation of CHO cells with MC in the presence of mouse liver microsomes (which contain the MFO enzymes) did not increase ss breaks.
The results suggest that mouse liver and lung cells are more susceptible to MC-induced ss breaks than cells from rats, hamsters or humans. Assuming that ss breaks are a relevant surrogate for carcinogenicity, the authors infer from this study that humans, rats and hamsters are insensitive to MC-induced liver cancer, because those species lack the high level of GST metabolic activity to MC found in the mouse liver cell and lung Clara cell.
Exhibit 121 "Isolation of two mouse theta glutathione S-transferases active with methylene chloride," G.W. Mainwaring, J. Nash and T. Green, Zeneca Central Toxicology Laboratory, 1995.
This study was conducted in order to characterize the mouse GST isozyme(s) responsible for MC metabolism. The results of this work could be used to explore the hypothesis that a particular GST isozyme was responsible for metabolizing MC to the carcinogenic metabolite and that there may be different concentrations of this enzyme across species.
The researchers used a variety of chromatography methods to isolate two mouse glutathione S-transferases (MT-1 and MT-2, also known as T1-1(*) and T2-2(*), respectively) which metabolize MC, comparing the observed enzyme activity with that described in rats. Rats were found previously to have two GST isomers in the theta class (GST 5-5 and GST 12-12) which metabolized MC. The mouse MT-1 and MT-2 enzymes were found to be closely related to rat GST 5-5 and 12-12, respectively, and the specific activity of mouse MT-1 was found to be similar to rat GST 5-5. GST 12-12 and MT-2 were found to be extremely labile during purification, and so the specific activities of those isozymes have not been measured.
The results of this study suggest that the mouse and rat contain GST theta enzymes similar in amino acid sequence and in specific activity (GST 5-5 and MT-1). The authors postulate that the greater conjugating activity seen in mice in other studies is "probably due to a difference in expression of the enzyme or to a significant contribution from MT-2" [Ex. 121].
Exhibit 122 "Mouse Liver glutathione S-Transferase Mediated Metabolism of Methylene Chloride to a Mutagen in the CHO/HPRT Assay," R.J. Graves and T. Green, Zeneca Central Toxicology Laboratory, 1995.
This study investigated the mutagenicity of MC as a potential carcinogenic mechanism of action. The purposes of this study were to clarify the ability of MC to act as a mutagen, because studies in mammalian systems have yielded mixed results regarding the mutagenicity of MC, and to more fully characterize the metabolite purportedly responsible for MC mutagenicity by comparing the results to formaldehyde (one metabolite of MC by the GST pathway). Mutagenicity was measured by assaying CHO cells in vitro for mutations at the HPRT locus of DNA. Ss DNA breaks were also monitored. Cells were exposed in culture to MC mouse liver cytosol metabolites (which include metabolic enzymes for the GST but not the MFO pathway), formaldehyde (one of the MC GST metabolites) or 1,2-dibromoethane (1,2-DBE) (a reference genotoxin).
Using standard techniques, MC GST metabolites were shown to be weakly mutagenic using the CHO/HPRT assay. Formaldehyde was also determined to be weakly mutagenic in this assay, but the effect was not as great as with MC GST metabolites. 1,2-DBE, as expected, showed a potent mutagenic response. The mutagenicity of MC GST metabolites and formaldehyde was increased when cell density was increased, cells were exposed in suspension rather than as attached cultures and cytosol concentration was optimized.
MC mouse liver cytosol metabolites were observed to increase ss DNA breaks in CHO cells exposed in suspension, but caused only marginal increases in DNA-protein cross-links. In contrast, the researchers found that formaldehyde induced both DNA ss breaks and DNA-protein cross-links. Slight increases in ss DNA breaks were also seen with exposure to either MC alone or the cytosol fraction alone.
Based on a comparison of the mutagenic effects of the three compounds, particularly on the lack of MC-induced DNA-protein cross-linking in this experimental system, the authors concluded that formaldehyde does not play a major role in MC mutagenicity. Accordingly, the researchers viewed the results of this study as supporting the hypothesis that the DNA ss breaks induced by MC, and the resultant DNA mutations, are caused by interaction of S-chloromethyl-glutathione (formed by the GST pathway) with DNA.
Exhibit 123 "DNA Sequence Analysis of Methylene Chloride-Induced HPRT Mutations in CHO Cells: Comparison with the Mutation Spectrum Obtained for 1,2-Dibromethane and Formaldehyde," R.J. Graves, P. Trueman, S. Jones and T. Green, Zeneca Central Toxicology Laboratory, 1995.
The purpose of this study was to describe the types of mutations induced by MC in order to further characterize the GST metabolite likely to cause MC mutations and therefore perhaps be responsible for the carcinogenicity of MC in the mouse. The spectrum of mutations in the HPRT locus of CHO DNA induced by MC plus mouse liver cytosol was compared to mutations induced by formaldehyde (a GST metabolite of MC) or 1,2-dibromoethane (1,2-DBE, a reference genotoxin).
The results were expressed as a sequence analysis of 11 MC-induced mutations, 6 formaldehyde-induced mutations and 13 1,2-DBE-induced mutations. In comparing the distribution of types of mutations, the results suggested to the researchers that formaldehyde-induced DNA damage can contribute to MC mutagenicity, but that the majority of the mutations were derived from other types of DNA damage, probably via an interaction of S-chloromethylglutathione with DNA. The researchers noted that a glutathione conjugate also plays a role in the mutagenicity of 1,2-DBE. The increases above background mutation frequency detected through this study were 24.7-fold for 1,2-DBE, 4.7-fold for formaldehyde, and 8-fold for MC.
Exhibit 124 "The distribution of glutathione S-transferase 5-5 in the lungs and livers of mice, rats and humans" [Preliminary communication, T. Green, 1995].
Exhibit 124A "The distribution of theta class glutathione S-transferases in the liver and lung of mouse, rat and human." G.W. Mainwaring, S.M. Williams, J.R. Foster and T. Green,1995.
The preliminary communication [Ex. 124] and the unpublished report which followed [Ex. 124A] summarized the results of a study comparing the inter- and intra-cellular distribution of the messenger RNA (mRNA) for a glutathione S-transferase (GST) isoenzyme which metabolizes MC in the lungs and livers of mice, rats and humans. The purpose of the experiments summarized in these reports was to describe the distribution of the mRNA for the GST theta isozyme believed to be responsible for metabolism of MC to a carcinogenic metabolite in different species. The researchers believed that differences in distribution of the mRNA for this isozyme would correlate with differences in distribution (and activity) of the isozyme itself, and might explain differences in sensitivities of the species to the carcinogenicity of MC.
The distribution of GST theta mRNA was visualized using DNA oligonucleotide anti-sense probes complementary to the nucleotide sequences for the GST theta isozymes. This technique is used to visualize the mRNA coding for a specific protein (such as the GST theta isozymes) within cells in tissues. The mRNA is a nucleotide sequence transcribed from the DNA containing the gene for the specific protein. After transcription, mRNA is transported to the cytoplasm, where it is translated into the amino acid sequence which becomes the specific protein (in this case, the GST theta isozyme). The finished protein then migrates to its final site of activity within the cell. Localization of the mRNA does not necessarily correspond to localization of the specific protein.
The results of the study showed that the GST-specific mRNA could be found in lungs and livers of all three species. Mouse liver cells (particularly the nuclei) and mouse lung cells appeared (from the photomicrographs shown in the article) to stain more heavily for the GST mRNA than the lung or liver cells from rats or humans. Although the amount of GST-specific mRNA was not quantified in this study, the authors interpreted the photographs to suggest that, "* * * mouse tissues are stained much more heavily than sections from either rat or human." Based on the intracellular and intercellular distribution of the GST mRNA, the authors stated,
The most significant findings are the presence of very high concentrations of GST 5-5 mRNA in specific cells and nuclei of mouse liver and lung. Metabolism of methylene chloride at high rates and within nuclei to a reactive but highly unstable glutathione conjugate is believed to facilitate alkylation of DNA by this metabolite. The lack of high or nuclear GST 5-5 concentrations in rat and human tissue, provides an explanation for the lack of genotoxicity in these species. [Ex. 124]
In the letter submitting the studies summarized above to OSHA, HSIA characterized the studies as follows:
This research, which is now complete, shows that B6C3F1 mice * * * are uniquely sensitive at high exposure levels to methylene chloride-induced lung and liver cancer, and that other species, including humans, are not at similar risk. [Ex. 117]
They went on to conclude:
As a result of this research program, it appears that there are no foreseeable conditions of human exposure in which the carcinogenic effects seen in mice would be expected to occur in man. * * * The risk assessment that is the basis for the methylene chloride standard, which is in turn based on the increased liver and lung tumor incidence observed in the mouse bioassay, must be discarded in favor of scientific data that are relevant to human risk.
In response to the request by HSIA, OSHA has reviewed the cancer hazard identification of MC based on all of the evidence in the MC record, with particular emphasis on the validity of the conclusion stated immediately above. This review is presented below.
2. Carcinogenesis of Methylene Chloride
a. Animal evidence. Several long-term MC bioassays have been conducted and are summarized in the Health Effects section. These included studies in which the route of exposure was inhalation [Burek et al., Ex. 4-25, Nitschke et al., Ex. 7-29, and NTP, Ex. 4-35] and two studies in which the route of exposure was drinking water [National Coffee Association, Exs. 7-30, 7-31]. In order to ensure full consideration of the data, OSHA analyzed in its preliminary assessment all data sets which showed an elevated incidence of tumors in a MC-exposed group, compared to controls, whether or not the elevation of tumor response was statistically significant. This analysis and the individual datasets used were described in detail in the NPRM.
In the NTP bioassay [Ex. 4-35], groups of 50 nine-week old B6C3F 1 mice of each sex were exposed by inhalation to 0, 2000 or 4000 ppm MC. Groups of 50 eight-week old F344/N rats of each sex were exposed to MC at concentrations of 0, 1000, 2000, or 4000 ppm. The inhalation exposures were administered 6 hours a day, 5 days a week for 102 weeks. Food was provided to the animals ad libitum except during the exposure periods, while water was available at all times via an automatic watering system. All animals were observed twice a day for mortality and moribund animals were sacrificed. Clinical examinations were performed once a week for 3.5 months, then twice a month for 4.5 months, and once a month thereafter. Each animal was also weighed weekly for 12 weeks, then monthly until the conclusion of the study at 102 weeks. All animals were necropsied and histologically examined. Three different neoplastic lesions were observed to have significantly increased incidence over the controls: adenomas and carcinomas of the lung in male and female mice, adenomas and carcinomas of the liver in male and female mice, and mammary gland fibroadenomas and fibromas in male and female rats.
HSIA and others argued that benign tumors, especially the mammary tumors in the rats, should not be counted as a carcinogenic response. The NTP has addressed that issue in its Technical Report [Ex. 4-35] and has concluded that the benign mammary tumors observed in the F344 female rats are "clear evidence" of carcinogenicity and noted that such tumors may proceed to malignancy. OSHA agrees with this determination and has considered the rat mammary tumors as part of its cancer hazard identification for MC. However, OSHA's quantitative risk assessment does not consider rat mammary tumor responses.
OSHA believes that the NTP studies provide the strongest evidence of carcinogenicity of MC in animals. Many commenters and hearing participants [Exs. 19-46, 7-128, 7-126, 25-E, 126-11,126-12, 126-16 and others] supported the use of the NTP mouse study as the basis for quantitative risk assessment. There are several reasons for this described in the proposal and earlier in this document. In brief, the NTP study used well established standard operating procedures that are generally considered a predictor of a potential carcinogenic response in humans. This study was also replicated by a second partial bioassay, conducted by NTP, in which groups of female mice were exposed to 2000 ppm MC for 2 years. Statistically significant increases in alveolar/bronchiolar and hepatocellular tumors were observed [Ex. 27].
Before the 1995 record reopening, some commenters had raised specific arguments why a mouse study might not predict human carcinogenic response to MC. Mr. Krenson of Besway Systems [Tr. 397, 9/17/92] objected to OSHA using the NTP mouse study as the basis for setting the PELs for MC. He believed that the mouse was irrelevant to human risk because the doses used were "extremely high" and that he believed that tests conducted on rats, hamsters and human epidemiological investigations showed "no conclusive proof of cancer in human beings." OSHA disagrees with Mr. Krenson's conclusion. In general, high doses in rodent bioassay studies are appropriate to elicit a response due to the practical limitations on the number of animals that can be used in a study. In MC, there was no observed acute toxicity at the levels used in the study, which is an indication that the doses were not too high. Use of high doses in bioassay studies is common and its practical necessity has been affirmed by numerous expert bodies, including several committees of the National Academy of Sciences. In addition, for every known human carcinogen, positive results were obtained at high rodent doses. Also, quantitative comparisons, as conducted by Allen and Crump in 1988, demonstrate that, in general, observations of cancer potency from epidemiology studies agree with estimates of potency derived from rodent bioassay data. In the case of MC, statistically significant excess tumors were observed in mice after exposure to only 2000 ppm, or only four times the former PEL of 500 ppm (8-hour TWA), and excess tumors were seen in rats at 4000 ppm. This level is within the range of human exposures experienced in occupational settings. Certainly the lower exposure showing substantial effect was not "extremely high" in relation to the exposure limit, as Mr. Krenson claimed.
The HSIA and several others [Exs. 117, 126-1, 126-3, 126-5, 126-6,126-8,126-10, 126-13,126-20, 126-21, 126-29] also objected to using the mouse data as the basis of human risk assessment, based on the mechanism of action studies submitted to the Agency by HSIA on December 6, 1995. OSHA's analysis of the individual studies follows, but overall, the Agency has determined that the mouse cancer data are appropriate for assessment of the cancer risks to humans (although, as discussed later in this section, OSHA has made extensive use of the submitted data to modify the quantitative e stimates of risk derived from the mouse model).
b. Evidence pertaining to the mechanism of action of methylene chloride. Several lines of evidence relate to the mechanism of carcinogenesis of MC. The issues discussed in the papers submitted by the HSIA and subsequent comments can be divided into those pertaining to genotoxicity, those discussing potential non-genotoxic modes of action, and those related to the enzymatic metabolism of MC. Although some comments overlap these divisions, this organization is used in this discussion to simplify consideration of the issues.
(1) Genotoxicity. It has not been conclusively demonstrated that MC or its metabolites act by a genotoxic mechanism in mice and rats. Substance-specific DNA adducts, which are among the strongest evidence of direct genotoxicity, have not been identified from MC exposure. However, evidence has been accumulating that MC is likely to be carcinogenic through a genotoxic mechanism of action. For example, DNA-protein cross-links have been demonstrated in mouse liver [Ex. 21-16], increases in unscheduled DNA synthesis have been demonstrated in mouse lung [Ex. 126-25] and other evidence of MC metabolite interaction with mammalian DNA (such as increases in ss DNA breaks) has been observed. It is not necessary for a substance to bind covalently with DNA in order to act via a genotoxic mechanism, although evidence of covalent binding is a strong indication of genotoxicity. In the case of MC, although the reactive metabolites are presumed to exert a genotoxic effect by binding to DNA, no MC metabolite-DNA adducts have yet been identified. However, RNA adducts have been identified after MC exposure, which supports the hypothesis that MC acts by a genotoxic mechanism. Substance-specific DNA adducts have also not been identified for some other carcinogens which are presumed to act via a genotoxic mechanism.
In addition, as discussed in the Health Effects section, MC has been found to be mutagenic in bacterial, yeast, Drosophila and mammalian systems; associated with chromosomal aberrations in CHO cells; and associated with sister chromatid exchanges in mammalian cell culture systems, such as CHO and V79 cells.
Investigations of the role of metabolites of the GST pathway in the bacterial mutagenicity of MC found that in glutathione-deficient strains of Salmonella typhimurium MC-induced mutations were reduced [Ex. L107]. Mutation rates returned to normal when bacteria were supplemented with exogenous glutathione. This study supports the hypothesis that MC may act as a genotoxic carcinogen via its GST metabolites, although a study of similar design by Dillon et al. [Ex. 21-89] did not replicate these results.
(i) MC induced mutuations. Studies on the MC mechanism of carcinogenesis included two studies on the mutations induced by MC in the CHO/hypoxanthine phosphoribosyl transferase (HPRT) assay. In the 1995 study by Graves et al. [Ex. 122], the investigators compared mutations induced by MC with those induced by formaldehyde and 1,2-dibromoethane. The authors characterized the results of the studies as follows:
Using the CHO/HPRT assay we have shown that MC is metabolized to a mutagen by mouse liver cytosol in a reaction which is dependent upon GST and GSH. Mutagenicity was enhanced by exposing the cells at high density in suspension rather than as attached cultures, which is consistent with the critical metabolites being extremely short-lived.
The authors also observed that the MC-induced mutations were associated with an increase in DNA ss breaks. They remarked, "The results suggest that MC-induced DNA ss breaks seen in other cell types are associated with DNA damage which can lead to mutation."
In a follow-on to the CHO/HPRT study, Graves et al. [Ex. 123] conducted a sequence analysis of HPRT mutations in CHO cells, comparing the spectrum of MC-induced mutations with those induced by 1,2-dibromoethane or formaldehyde. The investigators analyzed 28 HPRT mutations: 13 from 1,2-dibromoethane experiments, 6 from formaldehyde experiments, and 11 from MC experiments. The authors characterized their results as follows,
All three compounds induced primarily point mutations, with a small number of insertions and deletions. * * * The mutation sequence results for MC suggest that formaldehyde may also play a role in MC mutagenesis, although the majority of mutations arise from other types of DNA damage, probably DNA adducts formed by reaction of S-chloromethyl glutathione with DNA.
Dr. Douglas A. Bell of NIEHS [Ex. 126-26] had specific comments regarding the study on the mutation spectra [Ex. 123]. He stated,
This experiment is extremely weak scientifically and not of publication quality. It is unlikely that such a naive experiment could detect differences in spectra between the different chemicals tested. To test the hypothesis that there are chemical specific mutation spectra requires analysis of hundreds of mutants at several different doses. This exhibit contains no useful information for risk assessment.
OSHA agrees with Dr. Bell that there are serious methodological problems with the paper. The Agency also agrees with Dr. Bell that the important information in these two studies is that MC increases the mutation frequency, showing a clear genotoxic effect.
(ii) Single strand DNA breaks. In a 1995 study, Graves et al. [Ex. 120] investigated the role of MC exposure in development of single strand (ss) DNA breaks in the lung and liver of mice and rats and in hamsters and human cell cultures. The authors observed a transient, dose-related increase in DNA ss breaks in mouse hepatocytes after inhalation exposure to MC. No increased amount of ss breaks was observed in rat liver cells exposed to MC as compared to control cells. The authors also reported a decrease in the amount of ss DNA breaks in liver and lung when a glutathione depletor was administered to mice immediately before MC exposure.
In mouse and rat hepatocytes incubated with MC, the authors found increases in ss breaks, but no increases in ss breaks in hamster or human hepatocytes exposed in vitro were observed. No increase in DNA damage was observed in CHO cells exposed to MC plus mouse liver microsomes, while MC plus mouse liver cytosol induced detectable ss DNA breaks.
The authors characterized their findings in the lung as follows:
Here we show that Clara cells are also sensitive to MC-induced DNA ss breaks and that the DNA-damaging metabolites are derived from the GST pathway. * * * Overall, these findings support the proposal that Clara cells are the cell of origin of MC-induced mouse lung tumors.
For liver cancer, the investigators concluded:
These studies suggest that humans (and rats and hamsters) are insensitive to MC-induced liver cancer.
Commenters raised issues about the relevance and utility of ss DNA breaks in assessing the genotoxicity of MC. Dr. Karl T. Kelsey [Ex. 126-34] and Dr. Miriam Poirier [Ex. 126-37] raised concerns about the sensitivity of the DNA ss break assay for detecting genotoxic effects.
Specifically, Dr. Kelsey stated,
Reviewing the literature, considerable weight seems to fall upon the measure of DNA single strand breaks. I have serious concerns about this assay. It is well known that the assay is extraordinarily difficult to standardize and is sensitive only to very high doses of genotoxic compounds. This data, therefore, is certainly not compelling; persuading any competent independent scientist of its relevance to humans would be difficult.
Dr. Poirier was concerned with the sensitivity of the DNA single strand break assay and the relevance of DNA ss breaks to carcinogenesis. She remarked that ss DNA breaks and mutagenicity are secondary indicators of DNA damage. She indicated that a better measure of genotoxicity would be formation of DNA adducts. Dr. Errol Zeiger [Ex. 126-28] of NIEHS agreed, stating,
If the mechanism of carcinogenicity is through an alkylating S-chloromethyl GSH complex, there should be evidence of DNA adducts in vitro and in vivo.
OSHA agrees that DNA adducts are strong evidence of genotoxicity and that ss DNA breaks and mutagenicity are not as specific or relevant as indications of a genotoxic mechanism of action. However, the Agency has determined that, even in the absence of identified MC-specific DNA adducts, the accumulated evidence suggests that MC interacts with DNA via a genotoxic mechanism of action and that the GST pathway is a plausible carcinogenic pathway.
Dr. Melnick [Ex. 126-33] stated, "* * * it has not been demonstrated that the carcinogenicity of MC in mice is dependent solely on the induction of DNA single strand breaks." Dr. Andrew G. Salmon concurred with this analysis and also raised a serious concern about the ability of the assay even to detect increases in ss breaks, regardless of their relevance:
Green's account states that "mouse hepatocytes were * * * 20-fold * * * more sensitive to the effects of methylene chloride [i.e., DNA strand breaks] than rat hepatocytes * * * " and no breaks were detected in hamster or human liver cells. This is translated in the discussion to an assertion that not only humans and hamsters but also rats are completely immune to the carcinogenic effect of methylene chloride. However, the data simply do not support the assertion of a categorical difference as proposed by the HSIA. This particular work also raises a number of other issues, such as whether the liver is an appropriate model tissue, and whether single-strand breaks are an appropriate indicator of the type of genetic damage produced by the putative genotoxic metabolites of methylene chloride.
OSHA agrees that the ss DNA break assay is not as sensitive as other methodologies for assessing the genotoxic potential of MC in different systems and therefore data from the ss DNA break study must be interpreted in a quantitative, not qualitative context, with allowance for uncertainty in assay sensitivity. It is also unclear whether ss DNA breaks are the appropriate surrogate measure for carcinogenic potential. In light of the issues raised by commenters, the Agency believes that the ss DNA break data should be interpreted with caution.
(iii) DNA-protein cross-linking. Casanova and Heck [Ex. 21-16] observed DNA-protein crosslinks in mouse liver, but not mouse lung, after exposure to 500, 1500 and 4000 ppm. This study indicated that metabolites of MC have the ability to interact with DNA. However, the quantity of DNA-protein crosslinks did not show a strong correlation with tumor incidence, and so the DNA-protein crosslinks were not used as a dose-surrogate for MC exposure in OSHA's risk assessment.
The Chemical Industry Institute of Toxicology (CIIT) [Ex. 126-25] submitted further evidence that MC exposure causes DNA-protein cross-links in mouse liver but not mouse lung, hamster liver or hamster lung. These investigators also observed RNA adducts in mouse, rat and human cells after incubation with MC, but DNA-protein cross links were only observed in the mice. In addition, they submitted a pharmacokinetic model which modeled the DNA-protein cross-links as the dose surrogate for MC exposure. Finally, they made extensive comparisons of their model with the PBPK model submitted by Clewell [Ex. 96] and EPA's risk assessment for MC. Dr. Roger McClellan summarized the conclusions they reached as follows,
The pharmacokinetic results suggest that at very low concentrations of DCM [methylene chloride], the yield of DPX [DNA-protein cross-links] is almost linearly proportional to DCM concentration * * * DPX cannot be used directly as a surrogate for the internal dose in humans, however, because human hepatocytes, unlike mouse hepatocytes, do not appear to form DPX in measurable amounts in vitro. * * * These results suggest that the mouse may not be an appropriate animal model for human risk assessment due to its unusual susceptibility to DPX formation and to the fact that cell proliferation is a uniquely high-dose phenomenon that may occur only in this species.
OSHA believes that this work provides more evidence for the formation of genotoxic metabolites in mouse liver after MC exposure. However, OSHA is not convinced that the DNA-protein cross-linking is the appropriate dose-surrogate for pharmacokinetic modeling. One of the strengths of Reitz's and subsequent PBPK models was that the dose surrogate used in the modeling was linearly related to tumor incidence. That is one reason that many investigators have focused on the GST pathway, instead of the MFO pathway of metabolism as the carcinogenic pathway. As explained by Dr. Lorenz Rhomberg [Ex. 126-16],
* * * if this proportionality in the case of GST is broken by a deeper analysis, the rationale for focusing only on GST must be reevaluated.
Dr. Rhomberg was referring to results presented by HSIA on the distribution of GST theta isozymes within and among cells, but the same sentiment applies here; if OSHA were to abandon PBPK modeling using GST metabolites, all of the HSIA and other studies would have to be re-evaluated and considerable more research might need to be done. Finally, in the CIIT study, RNA adducts, a more direct measure of genotoxicity than DNA ss breaks, were observed in human hepatocytes after incubation with MC. The amount of RNA adducts in human cells was only about 3-fold lower than the amount in mouse hepatocytes. It is therefore clear that human hepatocytes in this system are forming genotoxic metabolites after exposure to MC.
OSHA notes that, in mouse lung, the DNA-protein cross-links were not observed, even though a clear dose-response relationship for tumors has been established at this site. OSHA is not convinced that the explanation for carcinogenesis in mice is DNA-protein cross-links in liver. Overall, it is unclear whether the interspecies difference in DNA-protein cross-linking is related in any way to the carcinogenic mechanism of action.
OSHA concludes that there continue to be strong reasons for using the mouse data as the basis for its quantitative risk assessment because there is a clear dose-response relationship in the mouse liver and lung tumor incidence data; the mouse metabolizes MC by the same pathways as humans; PBPK models have been developed which account for inter-species differences in MC metabolism; statistical techniques have been developed to quantify the uncertainty and variability in the parameters used in the PBPK models; and there are no data that demonstrate that the mouse is an inappropriate model for assessing human cancer risks. In fact, OSHA finds further evidence in the studies described above which suggest that MC acts via a genotoxic mechanism in human cells as well as in mice and rats, which further supports OSHA's use of the mouse tumor incidence as the basis for quantitative risk.
(iv) Interpreting the genotoxicity studies. Several other issues were raised regarding interpretation of the results of these studies on the genotoxic mechanism of action of MC. NIOSH and others [Exs. 126-30, 126-11, 126-32] commented that, in general, the data presented by HSIA supported the hypothesis that the carcinogenic metabolite(s) of MC were derived from the GST pathway. They agreed with HSIA's interpretation of the data that the studies presented here helped to confirm that the mechanism of MC carcinogenesis is through one or more genotoxic metabolites of the GST pathway.
Interpretation of short-term effects in explaining chronic mechanisms of action.
Concerns were raised about the generalizability of the results of short-term genotoxicity assays to tumor incidence, especially when the observed effect is transient, as in the vacuolation of Clara cells, the appearance of ss DNA breaks in mouse liver and lung cells, etc. Dr. Mirer of the UAW [Ex. 126-31] commented,
1. The evidence cited concerns acute effects which appear after a few hours of high level exposure of the animal to methylene chloride vapor, or the glassware (in vitro) mixing of homogenized animal or human tissue with the solvent. In a number of studies the effect in the whole animal is transient.
2. There is no evidence to connect the acute toxic effect, or single strand breaks of DNA after acute exposure, to the chronic effect of lung or liver injury, or cancer. * * *
Dr. Maronpot [Ex. 126-22] was concerned that the vacuolation observed in Clara cells was not reproduced in the NIEHS mechanistic studies. HSIA responded to this concern by remarking that the vacuolation could only be found after single exposures to MC, and that the vacuolation of Clara cells was also associated with increased DNA synthesis in these cells. The fact that this response was only observed after single exposures to MC again raises the issue of the transience of this response and its relevance to MC carcinogenesis.
Increased cell turnover. In these studies on genotoxicity, the authors remarked that increased cell turnover was observed in the lung (transient increase in DNA synthesis after single exposures to MC). Dr. Daniel Byrd [Ex. 126-32] also commented on the DNA synthesis issue. Citing an HSIA study, he contended that there appeared to be a common mechanism of action between the lung and the liver since increased DNA synthesis was observed in both tissues. Dr. Maronpot of the NIEHS [Ex. 126-22] disagreed, stating,
The purported "liver growth" in methylene chloride-exposed mice is actually an increase in liver weight attributable to accumulation of glycogen within hepatocytes. There is no evidence of replicative DNA synthesis (cell proliferation) in the liver of methylene chloride-treated mice, and, hence, actual increases in the numbers of hepatocytes did not occur. * * * It is noteworthy that recovery to normal liver weight occurs within two weeks after cessation of exposure to methylene chloride.
OSHA agrees with Dr. Maronpot that no data in the rulemaking record show increases in liver cell proliferation as the result of MC exposure, although increased DNA synthesis was actively searched for in the NIEHS mechanistic and other studies. The increased DNA synthesis observed in mouse Clara cells is a transient phenomenon that has not been clearly linked to carcinogenesis in the mouse. In any event, cell proliferation is not necessarily related in any way to carcinogenesis and is often uncorrelated with the doses used in bioassays and the tumor rates themselves. Many substances that cause prolonged cell proliferation do not cause tumor formation and vice versa [Ex. 126-22], and many experts believe that transient increases in cell proliferation, such as seen with MC, cannot account for the carcinogenic effect. Further discussion of cell turnover as a mechanism of carcinogenicity is discussed below under "Non-genotoxic mechanisms."
Clara cell as the mouse lung tumor cell of origin.
Another issue raised by commenters concerned the cell of origin of the mouse lung tumors. The mouse lung has a higher proportion of Clara cells than the human lung. The investigators hypothesized that if the Clara cell were the mouse lung tumor cell of origin, the risk estimated from the mouse lung tumor data may overstate human risk because humans have fewer Clara cells, and therefore fewer potential target cells.
Green et al. have focused much of their research efforts into determining the mechanism of action of MC in mouse lung and liver. In lung tissue, as described above, they concentrated on experiments addressing the hypothesis that the mouse Clara cell is the cell of origin of the mouse lung tumors observed in the NTP bioassay. Dr. Daniel Byrd [Ex. 126-32] indicated that he believed that the data presented supported this conclusion. He stated, "Mouse lung tumors most likely arise from damaged Clara cells, although a few pathologists continue to speculate that mouse lung tumors arise from other lung cells, such as Type II pneumocytes."
In contrast, Dr. Maronpot of the NIEHS [Ex. 126-22] disagreed with that statement, indicating that "* * * current belief among researchers is that mouse lung tumors arise from Type II pneumocytes rather than Clara cells." Dr. Melnick [Ex. 126-33] suggested that the HSIA data are not consistent with the hypothesis that the Clara cell is the tumor cell of origin. He stated,
DNA damage was detected in lungs of mice exposed to 2000 ppm methylene chloride; however, no significant increase in DNA single strand breaks was observed in Clara cells isolated from mice exposed to 4000 ppm methylene chloride. This observation does not support the conclusion that Clara cells were the cells of origin of methylene chloride-induced mouse lung tumors.
In their paper, Graves et al. [Ex. 120] explain their results as follows,
Attempts to measure DNA damage in Clara cells isolated from mice which had been exposed to MC in vivo were unsuccessful. * * * [I]t is possible that cells extensively damaged by MC do not survive the isolation procedure. The observation that the in vivo vacuolation of Clara cells observed after MC treatment is not seen in vitro when the cells are isolated from the damaged lungs supports this proposal.
This means that the authors could induce ss breaks in the DNA of Clara cells in vitro, but in mice exposed to MC in vivo, it is not clear that the DNA ss breaks observed in lung tissue were concentrated in the Clara cells. In fact, the authors state,
Since Clara cells represent only 5% of the total lung cell population, the DNA ss breaks observed in vivo may not exclusively result from damage to this cell population.
OSHA believes that these issues raise serious doubts as to whether current evidence supports the determination that the Clara cell is the cell of origin of the mouse lung tumors. Although the absence of increased ss breaks is not necessarily an indication of lack of genotoxicity, the presence of ss breaks in lung tissue (and apparently not concentrated in Clara cells) reveals an inconsistency in HSIA's argument: either the ss breaks are irrelevant or Clara cells are not the cells of origin, or both. Further discussion of the issues surrounding identification of the Clara cell as cell of origin for mouse lung tumors is contained below under "Non-genotoxic mechanisms of carcinogenesis."
Ability of MC reactive metabolites to cross membranes. Although no data were presented by the HSIA to address this issue directly, several of the HSIA papers and the accompanying letters postulate that the reactive metabolites of the GST pathway are too short-lived to cross membranes. This argument is used in combination with the claim of high concentrations of the mRNA for the GST T1-1(*) in the nuclei of mouse cells (but not those of rats and humans) to support the contention that humans are not at risk of developing cancer after exposure to MC. The reasoning is as follows: (1) Mice are the only species to have high concentrations of GST T1-1(*) in the nucleus of lung and liver cells. (2) The reactive metabolites of the GST pathway are too short-lived to cross the nuclear membrane. (3) In order to produce a carcinogenic effect, reactive metabolites must be produced inside the nucleus in proximity to the DNA. (4) Because the mouse has high concentrations of these enzymes in the nucleus (and rats and humans do not), the mouse is uniquely susceptible to lung and liver cancer after exposure to MC. (5) Therefore, there is no risk of humans developing cancer after exposure to MC.
Some commenters [Exs. 126-12, 126-30, 126-33] maintained that HSIA's submitted studies do not support this argument. As discussed subsequently, the probe used in these experiments measured GST T1-1(*) mRNA, not the isozyme itself. There is not necessarily a correlation between the intracellular concentration of mRNA and the concentration of enzyme at a specific locus. In addition, one would expect there to be higher mRNA outside the nucleus (since that is where the enzyme is transcribed from the mRNA), even if the enzyme were subsequently concentrated inside the nucleus. Additionally, as discussed previously, some of the evidence presented by HSIA suggests that the metabolites can be generated outside the cell (not simply outside the nuclear membrane) and interact with the DNA. Specifically, Dr. Dale Hattis [Ex. 126-12] has remarked that,
* * as long as these reaction and detoxification processes are not infinitely fast (and in principle they cannot be infinitely fast), a finite fraction of the activated metabolite molecules must reach the DNA and react. Even though this chain of events is required by our basic understanding of the relevant kinetic processes, in this case we also have direct empirical evidence that active metabolites need not be generated in a cell's nucleus in order to reach DNA and do damage. The DNA sequence mutations of Graves and Green [Ex. 122] and Graves et al. [Ex. 123], and the DNA single strand breaks reported by Graves et al. [Ex. 120] for CHO cells were all produced by exposing mammalian cells to a tissue culture medium that had been supplemented with mouse metabolizing enzymes and methylene chloride. The active metabolites in those cases were necessarily generated from outside of the cells, not just in the cytoplasm of the cells that manifested the DNA damage. Therefore, the claim that the active glutathione transferase metabolite(s) must be generated in the nucleus and would be ineffective if generated in the cytoplasm is flatly contradicted by HSIA's own evidence.
HSIA [Ex. 126-29] strongly disagreed that their results should be interpreted in this way and countered as follows:
The investigators had to use a suspension assay to maximize the concentration ratio of methylene chloride to cells to about 10(14), and to optimize the GST activity from mouse liver preparation. Only under these extreme nonphysiological conditions with a transformed cell line could any increase in mutation frequency be observed. There is absolutely no justification for assuming similar conditions in humans, where GST activity is absent or at very low levels in the cytoplasm and absent in the nucleus.
OSHA disagrees with HSIA, however, and finds Dr. Hattis' and the other commenters' reasoning more sound. The results of these experiments indicate that the metabolites of MC are stable enough to cross the cellular and the nuclear membrane to interact with DNA. The Agency recognizes that these are not physiological conditions, but the conditions of the experiment do support the common-sense assumption that enzymatic metabolism takes place in the cytoplasm of mouse cells and show that some fraction of the GST metabolite(s) is stable enough to cross membranes in the cell. Thus, the Agency believes that the observed tumorigenesis in the mouse is not the exclusive result of nuclear MC metabolism.
Other issues pertaining to genotoxicity. The remaining comments on these studies focused on more general issues such as the genotoxicity of MC and other factors related to the GST metabolic pathway and MC-induced carcinogenesis. Dr. Melnick [Ex. 126-33] remarked:
Some fundamental questions related to this mechanism and its uniqueness to mouse liver and mouse lung carcinogenesis are also not addressed by the present research. For example, why do tumors not develop in other organs in mice that also have high levels of GST theta (e.g., kidney)?
OSHA believes this is an important question that reduces the strength of HSIA's contention that the mouse responds in a unique way to MC. The investigators have attempted to explain differences in potency of MC with respect to liver and lung carcinogenesis by invoking differences in DNA repair rates and GST metabolism within the nuclei of critical cells. However, there are other tissues which, based on the HSIA hypothesis, ought to be prime candidates for carcinogenesis. The kidney, besides having high levels of GST theta, also has a slower rate of DNA repair than the liver. It would appear to be a logical site of carcinogenesis if HSIA's hypothesis is correct. OSHA believes that the lack of tumor response in this organ (and perhaps other logical sites) indicates that the hypothesis proposed by HSIA fails to account for all relevant observations.
(2) Non-genotoxic mechanisms of carcinogenesis. Non-genotoxic mechanisms of action have also been hypothesized for MC. Increased cell turnover, due to cell death caused by MC toxicity, could theoretically increase the available number of sites for mutation and subsequent tumor formation. However, there is only limited evidence of increased cell turnover after MC exposure. Casanova and Heck [Ex. 21-16] observed increased DNA synthesis in lung tissue of mice exposed to MC. Green et al. [Ex. 105] observed Clara cell vacuolation, and both studies measured increased DNA synthesis on the first day of exposure to MC, but not on subsequent days of exposure. Clara cells may be targets of MC-induced toxicity because they contain higher levels of MC-metabolizing enzymes and are therefore more likely to generate toxic MC metabolites (for example, carbon monoxide is known to poison MFO enzymes). Green et al. suggested that the Clara cell was the cell of origin of the lung tumors observed in the NTP mouse study, because of the metabolic properties of these cells and the increased cell turnover observed within a day of MC exposure (in addition to the DNA damage described above under the section entitled, "Genotoxic mechanisms of carcinogenesis").
Green et al. further suggested that if the cell of origin of the mouse lung tumors was the Clara cell, humans would be at substantially less risk of lung cancer, because humans have proportionally fewer Clara cells than mice do. However, OSHA believes that there is no clear evidence confirming that Clara cells were the cell of origin of the mouse lung tumors (see discussion above). Other cell types in the lung, such as the Type II lung cell, also have relatively high metabolic activity and could be the site of origin of lung tumors. These cells have not been studied separately. Further studies are needed to clarify the role of the Clara cell and other lung cell types and cells in other tissues in MC carcinogenesis.
(i) Increased cell division. In 1994, Foster et al. [Ex. 119] investigated increased cell division as the mechanism of action of MC in mouse lung cells. Specifically, they examined the mechanism of MC action on the transient vacuolation of bronchiolar cells observed following single exposures to MC. In mice exposed to 2000 and 4000 ppm MC, they observed increased numbers of vacuolated cells in the bronchiolar epithelium. Pretreatment of mice with a cytochrome P450 inhibitor decreased the number of vacuolated cells, while pretreatment with a glutathione depletor did not. In a replication of the observation made by Green et al. and described above, the authors found increased cell division (measured as incorporation of [3H]-thymidine) in Clara cells isolated from mice exposed to 4000 ppm MC. They concluded:
We believe that these results strongly support the supposition that the vacuolation of the Clara cells is due to a toxic metabolite produced by the CYP [cytochrome P-450] pathway of metabolism. Furthermore the most likely candidate for inducing the change is thought to be formyl chloride.
OSHA agrees that these observations indicate that increased cell turnover occurs in Clara cells of mice. This may possibly be a partial explanation of the mechanism, but only a partial one. In cases where cytotoxicity has been considered to be an explanation for risk occurring only at "high" doses, this argument is confined to chemicals believed to act non-genotoxically. MC is likely to be a genotoxic carcinogen, so even if cell proliferation is a factor, the genotoxic mechanism would be the primary mechanism of concern. Genotoxic carcinogens are not generally believed to have a threshold and the dose-response function is believed to be approximately linear at low doses. In addition, the study focused on one type of cell, which may not be the cell of origin for lung tumors. Carcinogenicity in humans (as well as in mice and rats) seems to originate from various cell types in various tissues.
(3) Metabolism of MC. As described above, the mechanism of carcinogenesis for MC is not known. Numerous studies over many years have explored numerous possible mechanisms and have provided substantial information regarding the metabolism and the probable metabolite responsible for the carcinogenic effect. As discussed in the Health Effects section, MC is metabolized by two pathways: the mixed function oxidase pathway (MFO) and the glutathione S-transferase (GST) pathway. Both pathways produce reactive intermediates which potentially could contribute to a genotoxic mechanism of carcinogenicity. During development of the PBPK model for MC, Reitz et al. found that tumor incidence correlated with the estimated amount of GST metabolite, as well as with the amount of parent compound administered, but not with the amount of MFO metabolite [Ex. 7-225]. The parent MC is not likely to act as a genotoxic carcinogen because it is a fairly non-reactive compound. In addition, MC blood levels in mice were lower than in rats, so if MC was the carcinogenic moiety, one would expect the risk of cancer in rats to be higher than mice, whereas the opposite was observed. Consideration of these factors has led many investigators to conclude that the GST pathway is responsible for carcinogenesis and that it is likely to produce a genotoxic carcinogenic moiety. OSHA has reviewed the data available on mechanism of action and has concluded that the most plausible assumption is that the GST pathway is responsible for the carcinogenic action of MC and that this should be taken into account in the quantitative risk assessment. This represents a case-specific departure from the default assumption that the administered dose of the parent compound is the relevant metric for exposure.
(i) Specific GST isozyme(s) responsible for MC metabolism to the carcinogenic metabolite. Recent work sponsored by the HSIA was directed at further characterization of the metabolism of MC by the GST pathway [Exs. 121, 124, 124A]. Specifically, the HSIA work on MC metabolism has focused on the isolation and description of isozymes in the GST theta class of enzymes, which HSIA believes are responsible for the metabolism of MC to the carcinogenic metabolite in mice. Mainwaring et al. have shown that the GST isomer with the greatest specific activity for MC is a member of the theta class of GST. [Ex. 121] In rats, three members of the theta class have been identified, GST 5-5, GST 12-12 and GST 13-13. In humans, two theta class enzymes have been identified, GST T1-1 and GST T2-2 and in mice, two theta enzymes have been described, GST T1-1(*) and GST T2-2(*) (also known as GST MT-1 and GST MT-2). According to Mainwaring et al. [Ex. 121], rat GST 5-5 and mouse GST T1-1(*) have similar specific activity toward MC and sequencing studies have shown "* * * that rat 5-5, mouse T1-1(*) and human T1-1 are orthologous proteins as are rat 12-12 and mouse T2-2(*) and human T2-2" [Ex. 124A].
The hypothesis under investigation in this work was that the enzyme similar to rat GST 5-5 (mouse T1-1(*) and human T1-1) was the critical enzyme responsible for metabolism of MC to the carcinogenic metabolite, and that differences in the interspecies intra- and inter-cellular distributions of this isozyme and differences in genotoxicity would be important for characterizing the risk of carcinogenesis after exposure to MC.
In order to examine the distribution of the GST isozymes of interest, the investigators used DNA oligonucleotide anti-sense probes complementary to three regions of the protein nucleotide sequences of rat GST 5-5, mouse GST T1-1(*) and human GST T1-1 to localize specific mRNA sequences in mouse, rat and human liver and lung tissue. They also used an antibody raised against rat GST 12-12 to localize the protein itself [Exs. 124, 124A]. In the full paper describing these experiments [Ex. 124A], Mainwaring characterized the results of this study, as follows:
The mouse enzymes [T1-1(*) and T2-2(*)] were present in significantly higher concentrations in both liver and lung than the equivalent enzymes in rat and human tissues. In mouse liver, both enzymes were localized in limiting plate hepatocytes surrounding the central vein, in bile duct epithelial cells and in the nuclei of hepatocytes. In rat liver the distribution of GST 12-12 was comparable to that seen for T2-2(*) in the mouse. GST 5-5 was not localized in limiting plate hepatocytes or in nuclei of rat liver. The levels of human transferase T1-1 in the liver were very low, with an even distribution throughout the lobule. The GST 12-12 antibody did reveal high concentrations of this enzyme in human bile ducts. The relative amounts of the theta enzymes in the lungs of the three species followed the pattern seen in the liver, with very high concentrations in Clara cells and ciliated cells of the mouse lung and much lower levels in the Clara cells only of rat lung. Low levels of human transferase T1-1 were detected in Clara cells and ciliated cells found at the alveolar/bronchiolar junction of one human lung sample. The enzyme was entirely absent from the large bronchioles.
Mainwaring et al. concluded that:
This study has demonstrated a highly specific distribution of the theta class GSTs 5-5 and 12-12 in liver and lung tissue from mice, rats and humans. * * * it was apparent from these studies that both the distribution and concentration of theses enzymes differed markedly between the three species. Whilst neither mRNA levels nor protein concentrations necessarily correspond to active enzyme, the distribution shown by the mRNA for GST 12-12 was quantitatively reflected by the antibody to the protein of this enzyme, suggesting that these techniques do, in this case, reflect the distribution of active enzyme. Although an antibody to GST 5-5 is not available, it is reasonable to assume that mRNA levels for this enzyme are similarly representative of the distribution of active enzyme.
An understanding of the cellular and sub-cellular distribution of GST 5-5 has provided an explanation for the species specificity of the mouse lung and liver carcinogen methylene chloride, and has provided reassurance that humans are not at risk from exposure to this chemical.
(ii) Issues raised pertaining to metabolic studies. Many commenters commended the HSIA for providing new information on the mechanism of action of MC and for confirming previous quantitative studies of the interspecies differences in MC metabolism. However, commenters also raised several specific issues regarding the conduct and interpretation of these experiments.
Correlation of mRNA concentrations with enzyme concentrations. Mainwaring et al. [Ex. 124A] correlated the inter- and intra-cellular distribution of the mRNA for GST 12-12 in the rat with the distribution of the antibody for GST 12-12. They stated that it is reasonable to assume that since the protein and mRNA for the 12-12 isomer have similar distributions, the protein for the 5-5 isomer would distribute in the same manner as the mRNA for the 5-5 isomer. In support of their assumption, they noted that there is 80% homology between the 5-5 and 12-12 isomer. Some commenters believed that this was not a reasonable assumption and that there was no reason to believe that the distribution of the GST 5-5 isomer protein would correlate with the distribution of the GST 5-5 mRNA simply because there seemed to be a correlation in the 12-12 isomer protein and mRNA distributions [Exs. 126-7, 126-16]. OSHA concurs with these commenters, and until there is actual measurement of the GST 5-5 protein, OSHA does not believe that the question of the actual distribution of GST 5-5 isozyme will have been settled.
More importantly, several commenters stressed that it was mRNA that was actually observed in these studies, and mRNA levels do not necessarily correspond to either protein levels or protein activity within a cell [Exs. 126-7, 126-16, 126-28, 126-30, 126-32]. Although Mainwaring et al. acknowledge this fact [Ex. 124A], the conclusions reached by the authors still suggest that measurement of mRNA is equivalent to measurement of enzyme activity. Referring to the conclusions drawn by Mainwaring et al., Dr. Lorenz Rhomberg [Ex. 126-16] commented:
This interpretation of mRNA distribution is profoundly in error and contradicts some of the most well established and fundamental principles of molecular biology.* * * Finding mRNA in the nucleus is unsurprising and uninformative about the eventual location of the protein products. Detecting mRNA only reveals that the cell may be presumed to be manufacturing the corresponding protein.
Dr. Rhomberg was also concerned that the concentration of GST T1-1* in the nucleus of mice may be an artifact of the experimental conditions, resulting, perhaps, from a burst of mRNA synthesis. The concern that the apparent nuclear concentration of GST may be an artifact was echoed by Dr. Douglas A. Bell of the National Institute for Environmental Health Sciences [Ex. 126-26]. He stated:
Why the [intracellular] distribution should be different among species is unclear and unusual. Differences in processing of the nuclear RNA transcript from full length pre-mRNA may be the underlying cause of this phenomenon (or perhaps there is a transcribed pseudogene that is complicating the process).
Because of the specific cellular mechanisms that would be required to concentrate a protein in the nucleus, Dr. Rhomberg [Ex. 126-16] indicated that translocation of the GST 5-5 protein to the nucleus only in mice seemed unlikely. He stated:
It seems implausible * * * that for a series of orthologous proteins, such localization would be found in a particular species and not in other species.
OSHA agrees with the comments made by Dr. Rhomberg and Dr. Bell on this issue, and concludes that the concentration of mRNA at a particular cellular site does not necessarily correlate with concentration of the enzyme itself. OSHA believes that caution should be used when interpreting the results of these experiments.
Attribution of GST metabolizing activity to a single GST isozyme. Concern was also raised about the validity of attributing all of the glutathione S-transferase metabolism of MC to one isomer of the theta class [Exs. 126-7, 126-12]. In particular, Dr. Dale Hattis noted that there was less enzyme activity eluting coincident with the peak identified as the 5-5 form than that eluting at pH 8, which was not believed to correspond to the 5-5 form. Dr. Ronald Brown described results from a paper by Blocki (1994) [Ex. 127-22] which showed that "expression of the [5-5] isozyme contributes 50% of the total GST activity toward this substrate." This leaves the question open as to whether isozymes which may have lower specific activity for MC but which may be expressed in much greater abundance (particularly u 4-4), could contribute as much as the remaining 50% of the total GST metabolism (see Table VI-1, reproduced below from Dr. Brown's comment [Ex. 126-7], original source Blocki et al. (1994) [Ex. 127-22]).
| - | - | - | - | Glutathione S-transferases | ||
|---|---|---|---|---|---|---|
| alpha Class | u Class | theta Class | ||||
| comparative parameter (units) | 1-1+1-2+2-2 | 3-3 | 3-4 | 4-4 | (b)5-5 | (b)13k |
| Specific activity (nmol/min/mg of protein) | < 0.1 | 7 | 11 | 23 | 11,000 | 9 |
| % Cytosolic pro- tein (% of total in liver) | 6.4 | 0.7 | 0.3 | 0.6 | 0.002 | 0.005 |
| Total activity (nmol/min/g of liver protein) | < 10 | 49 | 33 | 138 | 22 | 0.45 |
| % Total act- ivity(c) | < 1.5 | 11 | 7 | 32 | 50 | 0.1 |
| Footnote(a) Data from Meyers et al., 1991 | ||||||
| Footnote(b) Data for 13,000 molecular weight glutathione transferase from Blocki et al., 1992 | ||||||
| Footnote(c) Assuming Vmax conditions for each | ||||||
In addition, Mainwaring et al. [Ex. 124A] noted that the "substrate specificity of GST 12-12 is currently poorly characterized," although the purified enzyme has no activity toward MC. As described above, these enzymes appear to be very labile upon purification. Therefore, it is unclear how much the 12-12 isomer itself may contribute to MC metabolism. As Dr. Kenneth T. Bogen stated, "* * * while the substrate specificity of GST 12-12 may currently be poorly characterized, current data do not appear to rule out GST 12-12 specificity toward MC."
Limited human samples and human polymorphism in the GST theta genes. Several commenters expressed concern for the limited number of human samples (one pooled lung sample and less than 40 human liver samples have been assayed) and the potential effect of a known human polymorphism for the glutathione S-transferase theta class genes on risk estimations [Exs. 126-7, 126-16, 126-26, 126-35]. Specifically, commenters raised concerns that there may be a large subpopulation of GST conjugators who may be at increased risk from MC exposure that has not been adequately characterized in the limited number of human samples (especially lung samples) that have been tested. HSIA objected to these comments, stating,
The human tissue data base for the metabolism of methylene chloride by the GST pathway is one of the largest, if not the largest, available for this type of risk assessment. To discount it based on arguments concerning hypothetical polymorphisms, as these commenters urge OSHA to do, would be contrary to the message consistently put forward by the National Academy of Sciences and regulatory authorities for the past decade. * * *"
In fact, the National Academy of Sciences report cited by HSIA, "Science and Judgement in Risk Assessment" does encourage agencies to make use of biologically-based models, but cautions that using them without adequately considering human variability would be a step backwards:
EPA has not sufficiently accounted for interindividual variability in biologic characteristics when it has used various physiologic or biologically based risk-assessment models. The validity of many of these models and assumptions depends crucially on the accuracy and precision of the human biological characteristics that drive them. In a wide variety of cases, interindividual variation can swamp the simple measurement uncertainty or the uncertainty in modeling that is inherent in deriving estimates for the "average" person.
The Academy goes on to recommend specifically that making "reasonable inferences" about interindividual variation is required, rather than assuming that no such variation exists:
Even when the alternative to the default model hinges on a qualitative, rather than a quantitative, distinction, such as the possible irrelevance to humans of the alpha-2u-globulin mechanism involved in the initiation of some male rat kidney tumors, the new model must be checked against the possibility that some humans are qualitatively different from the norm. Any alternative assumption might be flawed, if it turns out to be biologically inappropriate for some fraction of the human population.
When EPA proposes to adopt an alternative risk-assessment assumption * * * it should consider human interindividual variability in estimating the model parameters or verifying the assumption of "irrelevance." If the data are not available that would enable EPA to take account of human variability, EPA should be free to make any reasonable inferences about its extent and impact (rather than having to collect or await such data), but should encourage interested parties to collect and provide the necessary data.
OSHA believes HSIA has misinterpreted the NAS recommendations, and further disagrees with HSIA that the polymorphism is "hypothetical." Investigators have demonstrated this polymorphism in human GST and have shown how the polymorphism varies across races [Exs. 127-7, 127-9, 127-17, 127-21, 127-23, 127-24, 127-25]. OSHA agrees with the commenters that a human polymorphism in the GST theta genes may increase concern for individuals that may be at higher risk from exposure to MC due to their genetic make-up. The Agency has considered sensitive subpopulations in the development of health standards, including this rulemaking. For example, the subpopulation of workers with silent or symptomatic heart disease was considered in assessing the cardiac risks of MC (due to its metabolism to carbon monoxide). The variation in enzyme activity raises additional uncertainty in the use of human data to support the hypothesis that mice are uniquely sensitive to MC carcinogenicity. However, for purposes of quantitative analysis, the Agency has not attempted to systematically adjust the risk estimates based on a "high GST metabolizing" individual because the frequency and impact of such polymorphisms have not been clearly worked out.
Target site of MC carcinogenesis in mice versus humans.
Drs. Brown and Melnick [Exs. 126-7, 126-33] also raised the possibility that the target site for MC carcinogenesis may be different in humans than in mice or rats. Specifically, research on the occurrence of theta isomers of GST in human blood was described. The characterization of GST metabolism in human erythrocytes [Exs. 127-11, 127-12] suggests the possibility of the bone marrow as a potential target of MC carcinogenesis and also the potential for metabolism in the blood and translocation of the metabolites to a variety of potential targets. The HSIA discounted human blood metabolism of MC, stating,
The very high capacity to conjugate methylene chloride' mentioned by Brown is in fact very low, approximately 40-fold lower than the highest activity detected in human liver.
OSHA believes that although the specific activity in the blood may be lower than the human liver activity, the total activity of the GST enzymes in blood and marrow may be significant when one also considers the volume of these compartments. OSHA also notes that interspecies tumor site concordance is not necessarily expected, and it is prudent to consider any human tissues which have the potential to metabolize MC to the putative carcinogen.
Concentration of protein complementary to rat GST 12-12 in human bile ducts.
Dr. Bogen [Ex. 126-15] commented specifically on the human liver protein complementary to the antibody to rat GST 12-12 protein. In particular he was concerned that high concentrations of this enzyme were reported in bile ducts of the human liver. He noted,
With regard to potential human carcinogenicity of MC relative to its known carcinogenic potential in mice, it seems to me that these particular data ought not to reduce regulatory concern, but rather ought to increase regulatory concern, in view of the fact that bile duct epithelium cells are the most likely stem cells for hepatocytes.
* * * Thus hepatocellular bile-duct epithelial cells are likely to play an important role in liver carcinogenesis in both mice and humans.
OSHA agrees with Dr. Bogen's concerns and also notes that in the cohort study of textile workers conducted by Hoescht-Celanese [Ex. 7-260], an excess of biliary cancers was observed in those workers exposed to the highest concentrations of MC and those with the longest latency period between exposure and disease. If the HSIA theory is correct (i.e., a single isozyme is the culprit), then finding high levels of this isozyme in human bile duct is strong evidence implicating MC in human carcinogenesis.
Interpretation of data as qualitative versus quantitative differences.
Perhaps most importantly for the purposes of MC risk assessment, several commenters remarked that OSHA should use caution when interpreting the data from the HSIA submissions, because any interspecies differences are rightly considered first as quantitative rather than qualitative ones. In part, the commenters cautioned that one should pay special attention to the threshold of detection in all assays. As Dr. Andrew Salmon stated,
Green and co-workers have consistently confused their inability to measure a result or parameter value due to its magnitude or frequency of occurrence being below their threshold for practical detection, with a true zero value for the parameter or zero risk of an occurrence [Ex. 126-36].
OSHA agrees that caution should be used when attempting to characterize a difference between species as an absolute qualitative difference. A much higher burden of proof is required to support a claim of zero risk than of diminished risk. (This higher burden is due to the need to consider assay sensitivity and other factors; the fact that the consequences of incorrectly concluding that humans are at zero risk are particularly dire only adds to the already high threshold of scientific evidence needed to successfully make such a claim). In the case of MC, humans clearly have the ability to metabolize MC via the GST pathway [Exs. 21-53, 127-16]. Even if the enzyme concentration of GST T1-1* itself actually occurs only in the nuclei of mouse lung or liver (as opposed to the concentration of mRNA, which may or may not be localized differently within mouse cells), it is still unclear what impact (if any) this fact would have on the characterization of human cancer risks for MC. OSHA believes that the statement that there are absolute species differences in the activity and intracellular distribution of GST 5-5 is highly speculative and is not supported by the data presented to date, because the data presented refers to the distribution of mRNA for GST 5-5, not the enzyme concentrations or activity levels of the enzyme; there is no quantification of the intracellular levels of the mRNA or enzyme levels, only photographic representations; and there is no evidence that any potential difference in enzyme activity (when those experiments are completed) would be greater than the difference already predicted from allometric scaling considerations.
Conclusions reached by the HSIA.
HSIA concluded from these studies that because of a qualitative inter-species difference in the distribution of the GST theta enzyme responsible for MC carcinogenesis, humans would not be at risk of developing cancer under "foreseeable conditions of exposure." Although some commenters agreed with the conclusions reached by the HSIA [e.g., Exs. 126-10, 126-13, 126-20], many commenters strongly disagreed with this interpretation of these data pertaining to the risk assessment for MC. These commenters [e.g., Exs. 126-7, 126-11, 126-12, 126-15, 126-16, 126-22, 126-26, 126-30, 126-36] were concerned that the question was in reality an issue of quantitation of enzyme, not a qualitative difference in metabolism. Dr. Lorenz Rhomberg commented:
The question is, is there any basis for believing that the species difference in activity suggested by the mRNA data is greater than has been previously supposed? It should be emphasized that some degree of species difference in metabolic activity is expected even under the default cross-species extrapolation methods. That is, in keeping with the general pattern of scaling of physiological processes across species, general metabolic rates are presumed to be lower on a per unit of tissue basis in larger animals. As a default, this pattern can be presumed to apply to individual metabolic pathways as well, although data on species-species activities can be used in place of such defaults if available.
If species-species activities are discovered by experiment to be less in humans than in mice to the degree already anticipated by allometry, then the experiments are simply confirming the default and no change in the human risk estimates is warranted. If humans have a metabolic activity different than the allometric prediction, the incorporation of such estimates into PBPK models can show different human risks from those predicted under the default. The allometric prediction is that, on a per unit of tissue basis, humans should have about 7-fold lower activity than mice and about 4-fold lower activity than rats.
Given the limit of detection of the assay methods, human metabolic activity (or mRNA levels) only a bit less than the allometric expectation of 7-fold less than mice are often difficult to distinguish from zero. That is, claims that humans have no activity (or no mRNA production) in certain tissues must be judged in the light of the fact that only a small change from the already acknowledged allometric difference can often make the human activity undetectable. A 20-fold mouse-human difference, for example, really only represents a 3-fold exaggeration of the 7-fold allometric pattern, yet many assays may fail to reliably characterize a 20-fold difference as a quantitative difference rather than a qualitative difference.
For the above reasons, claims that human metabolic activity in activating methylene chloride are so low as to be essentially qualitatively different than mice should be interpreted with great caution. In fact, existing assays have great difficulty in detecting species differences in metabolic activity great enough to markedly challenge existing risk assessments.
Another commenter discussed the fact that cellular levels of the GST 5-5 isoenzyme would be expected to be distributed unevenly across cells, putting some cells at greater or lesser risk. This would tend to average out over a tissue and would be best described by tissue metabolism data. Other commenters remarked that there was no need to adjust the risk estimates based on these studies because current pharmacokinetic models already account for interspecies differences in metabolism. Although OSHA has incorporated data from these studies, especially in its "alternative analysis," OSHA agrees with Dr. Rhomberg and the other commenters who have taken exception to the HSIA conclusions.
The Agency does not accept the HSIA characterization of the results of the summarized studies. OSHA has determined that no evidence has yet been presented that demonstrates that humans are not at risk of developing cancer after exposure to MC. At most, the presented studies suggest a quantitative inter-species difference in MC metabolism, which was established in previous scientific reports and is already accounted for by PBPK modeling. As discussed extensively in this document, OSHA has concluded that HSIA has undervalued certain strong evidence and has overemphasized some more speculative hypotheses. However, as is clear from this discussion OSHA has carefully considered all of the evidence. Substantial evidence in the record clearly supports OSHA's conclusions. Consequently, OSHA's approach of relying on the NTP mouse tumor data as the basis of its quantitative risk assessment continues to be the best approach to risk estimation.
c. Conclusions regarding the carcinogenesis of MC. The HSIA submitted these documents to OSHA with a request that the Agency consider the mouse tumor data in light of these additional studies and reject use of the mouse tumor response data as the basis of the Agency's quantitative risk assessment. OSHA believes it has given proper weight to all the evidence, giving greater weight to that which is of the highest scientific quality. However, in light of HSIA's request, the Agency reopened the rulemaking record and reviewed all the new data. After submitting these documents for review, the HSIA [Ex. 126-29] remarked on comments submitted to the docket by other scientists,
In general, the comments submitted by R. Maronpot, R. Brown, L. Rhomberg, K. Bogen and D. Hattis exhibit a reluctance to use the large body of mechanistic data now available in assessing the potential carcinogenic risk posed by methylene chloride, even though most other commenters agree that the pathway responsible for its observed carcinogenicity in mouse liver and lung, as well as species variations in activity of this critical pathway, have now been identified. Much of the comment addressed here appears to be motivated by a desire to maintain the "status quo" for assessing carcinogenic risk based on default principles that were developed twenty years ago.
The HSIA goes on to say,
Many of the conclusions reached by the commenters * * * are based, often erroneously, on single aspects of one or the other of these publications, rather than on the entire data base, as a "weight of evidence" approach would demand and as is necessary to understand the results.
OSHA finds it difficult to understand why HSIA believes that the scientists they listed are primarily interested in preserving the "status quo." Dr. Maronpot conducted the mechanistic studies on MC at NIEHS, which have generated mechanistic information useful to the risk assessment process. Dr. Rhomberg was instrumental in developing the pharmacokinetic approach used by the Environmental Protection Agency in its risk assessment of MC (an approach never used by the Agency previously). Dr. Hattis, Dr. Bogen and Dr. Brown are all experts in the application of pharmacokinetic modeling to risk assessment and have repeatedly called for incorporating more mechanistic and physiological data into pharmacokinetic models. These highly respected scientists, among others, reviewed the HSIA submissions critically and independently and reached conclusions different from those of the HSIA, conclusions which themselves depart significantly from the "status quo." This does not suggest to OSHA that they are trying to preserve some status quo in risk assessment, and OSHA finds nothing in the comments of these experts to suggest that this is the case.
In order to respond to HSIA's desire to have OSHA further review all of the data, the Agency has reviewed each submitted study carefully and critically on its own merits to determine how each piece of data fits into the overall picture of the mechanism of action for MC. OSHA believes that in this process the critical issues raised by the HSIA have received a full airing and the hazard identification and the risk assessment for MC have been improved because of it. OSHA believes, however, that looking only at the new studies submitted by HSIA, and examining them uncritically, would contradict every principle of scientific analysis.
In summary, in order to accept the HSIA's supposition that MC is not carcinogenic in humans, one must believe the following:
1. GST 5-5 is the only isozyme which can metabolize MC to the carcinogenic metabolite.
2. DNA single strand breaks are relevant and a sufficient measure of the tumorigenicity of a compound.
3. The absence of detectable increases in DNA ss breaks in a single experiment means that there are in fact no additional ss breaks.
4. The limited number of human samples (one sample of pooled lung tissues being the absolute extreme of "limited" data) used to determine metabolic parameters are truly representative of the range of human variability.
5. An apparent correlation in the distribution of the GST 12-12 protein and GST 12-12 mRNA means that the distribution of GST 5-5 protein will correlate similarly with the distribution of GST 5-5 mRNA.
6. Visual interpretation of photomicrographs staining for GST mRNA gives a true and accurate measure of GST activity in the cell.
And one must also ignore the following contradictory observations and conclusions about the mechanism of action (in addition to ignoring the suggestive epidemiologic evidence):
1. Metabolites of GST can cross cell and nuclear membranes and interact with DNA to induce DNA ss breaks and mutations.
2. GST mRNA and protein stain heavily in human bile duct cells (believed to be precursors of hepatocytes).
3. Human lung tissue has been shown to stain for GST mRNA. 4. Only 50% of the GST metabolism of MC can be accounted for by the GST 5-5 isozyme.
5. The metabolic capacity of GST 12-12 for MC has not been characterized.
OSHA concludes that these studies, even putting aside all technical objections to the methodology and interpretation of individual studies, do not change the conclusion that substantial evidence supports the carcinogenicity of MC. The bioassay results in mice are still qualitatively and quantitatively relevant to humans. Once the HSIA studies have been replicated and key components quantified (like the intracellular enzyme activity (instead of mRNA levels) of GST towards MC), the HSIA data may be useful in characterizing quantitative interspecies differences in MC GST metabolism. In particular, it would be useful to determine whether all of the evidence that HSIA submitted is consistent with an allometric difference (a difference expected based on the size of the animal) in sensitivity to MC or with a greater interspecies difference in sensitivity. (The specific activity of GST toward MC in mice is estimated to be about 7-fold that of humans, based on allometric considerations.) OSHA believes that its final risk assessment, which relies on an analysis of all available PBPK data, addresses both possible interpretations.
B. Selection of Database for Quantitative Risk Assessment
1. Animal Bioassays
The first step in performing a quantitative assessment of carcinogenic risk based on animal data is to choose a data set or sets from which to define the dose-response relationship. In its NPRM, OSHA had chosen the NTP female mouse lung and liver tumors to determine its estimates of risk. OSHA chose these responses because they provided clear dose-response relationships, had low background tumor rates and were more sensitive measures of dose-response than corresponding male mouse tumor sites.
The EPA, the CPSC and the FDA chose to use the combined incidence of adenomas and carcinomas of the lung and liver as the basis for their risk assessments. Specifically, the EPA [Exs. 25-D, 28] placed emphasis on the experimental species and sex group showing the highest risk: the number of female mice showing either adenoma or carcinoma in either lung or liver (or both). The CPSC [Ex. 25-I] pooled benign and malignant tumors of either the mammary gland, lung or liver and averaged male and female estimates to derive an overall risk estimate. The FDA [Ex. 6-1] used benign and malignant responses of female mice. The Crump report [Ex. 12] noted that it may be reasonable to combine lung and liver responses to give an indication of the potency of MC, due to the fact that metabolism of MC occurs by the same pathway in both lung and liver and thus results in the same ultimate metabolites. However, the report added that since both tissues have different background responses, combining responses may tend to complicate the interpretation of risk estimates.
In OSHA's final rule, the NTP study (rat and mouse, inhalation) was chosen for quantitative risk assessment because it provided the best toxicological and statistical information on the carcinogenicity of MC [Exs. 12, 7-127] and because the study was of the highest data quality. In the NTP study, MC induced significant increases both in the incidence and multiplicity of alveolar/bronchiolar and hepatocellular neoplasms in male and female mice. In rats, dose-related, statistically significant increases in mammary tumors were also observed. OSHA chose the female mouse tumor response as the basis of its quantitative risk assessment, because of the high quality of data, the clear dose response of liver and lung tumors and the low background tumor incidence. Although the female rat mammary tumor response was also dose-related, the data of high quality and amenable to quantitative risk assessment, the mouse data set had a clearer dose-response in both liver and lung tumors than the rat mammary tumor response and the mouse background tumor incidence was lower than in the rat. Therefore the mouse data set was chosen for quantitative analysis.
OSHA included the lung adenomas in the quantitative analysis. The evidence suggests that the presence of benign tumors with the potential to progress to malignancies should be interpreted as representing a potentially carcinogenic response. This belief is supported by the OSTP's views on chemical carcinogenesis (50 FR 10371). OSTP stated that at certain tissue sites, such as the lung, most tumors diagnosed as benign really represent a stage in the progression to malignancy. Additionally, NIOSH, the EPA, the CPSC and the FDA have also included benign responses in their assessments. Therefore, it is appropriate and sometimes necessary to combine certain benign tumors with malignant ones occurring in the same tissue and the same organ site. In particular, OSTP also stated that "the judgement of the pathologist as to whether the lesion is an adenoma or an adenocarcinoma is so subjective that it is essential they be combined for statistical purposes." (50 FR 10371).
OSHA chose female mouse lung tumors as the specific tumor site for its final quantitative risk assessment. There is no a priori reason to prefer the mouse lung tumor response over the liver tumor response, because both data sets were of high quality, showed a clear dose-response relationship and had low background tumor incidence. In fact, in the NPRM, the Agency reported estimates of risk generated using both sites. However, to reduce the complexity of the final PBPK analysis, which required highly intensive computations, OSHA chose one site (the female mouse lung tumor response) for its final risk estimates. The risks calculated using the female mouse liver response would likely be slightly lower than those calculated using the lung tumor response. On the other hand, pooling the total number of tumor-bearing animals having either a lung or liver tumor (or both) (which is the procedure EPA advocates [see its 1986 Guidelines for Cancer Risk Assessment]) would have yielded risk estimates higher than OSHA's final values.
The NTP study has been described in the Health Effects section and, above, in the discussion regarding hazard identification.
2. Epidemiologic Data
The epidemiology data are not as useful for quantitative risk assessment as the animal data because the animal data provide a clear dose-response, with fairly precise indices of exposure, which cannot be derived from the epidemiology data. All other things being equal, risk assessors would prefer to use epidemiologic data to assess cancer risk in humans over data from animal studies whenever good data on human risk exist. However, the uncertainty inherent in epidemiologic studies must be accounted for; in particular, "positive" studies often have lower confidence limits that do not rule out the no-effect hypothesis, while ostensibly "negative" studies often have UCLs that would support a substantial positive effect. OSHA believes (see discussion below) that the latter circumstance applies to some of the MC studies. Other factors, such as duration and intensity of a chemical exposure (which can rarely be controlled and accurately measured in an epidemiological study), difficulty in accurately defining the exposed population, and other confounding factors diffuse a study's predictive power of true risks.
Frequently, animal studies indicate a positive response to a particular chemical when epidemiologic studies of exposures to the same chemical fail to exhibit a statistically significant increase in risk. When animal studies show a substance to be a carcinogen but epidemiologic studies are non-positive, the minimum risk which could be detected by the human study should be estimated to assess the strength of the epidemiologic study and justify its importance in the risk assessment process. Similarly, the animal-based potency estimate can be used to predict the number of human deaths investigators would likely have seen in an epidemiologic study if the animal-based estimate was correct; if the observed number of human deaths is markedly inconsistent with this predicted number, the relevance of the animal-based estimate might well be called into question. If the human data are equivocal, or the epidemiologic study is not sufficiently sensitive to identify an increased risk predicted by a well-conducted animal bioassay, it is necessary to consider the animal data to protect workers from significant risk. OSHA concludes that the MC epidemiology studies do not have adequate information upon which to base a quantitative risk assessment. OSHA has, however, used the analyzed epidemiological data to determine whether the results are consistent with those estimated using the rodent models. This is discussed later in the document.
3. Conclusions
After reviewing the animal data and the quantifiable epidemiology data, OSHA has determined that the NTP female mouse lung tumor response is the appropriate data set on which to base its quantitative risk assessment, and has determined that the most scientifically-appropriate way to use these data involves constructing a PBPK model to extrapolate from animals to humans. OSHA believes that the non-positive epidemiology data, in particular those from Kodak, are of in sufficient power to rule out the risk estimates derived from the animal data.
C. Choice of Dose-Response Model
Several approaches have been used to estimate cancer risk from exposure to toxic agents. A standard approach uses mathematical models to describe the relationship between dose (airborne concentration or target tissue dose surrogate) and response (cancer). Generally, mathematical functions are fit to the data points observed at different exposure levels and these functions are used to estimate the risk that would occur at exposure levels below those observed. The shapes of these curves vary, ranging from linear extrapolations from the observed points through the origin (zero exposure and zero risk) to curves which may deviate far from linearity at the very highest or lowest doses. The use of a particular model or curve can be justified in part by statistical measures of "goodness-of-fit" to observed data points. That is, there are various statistical tests which measure how closely a predicted dose-response curve fits the observed data.
The most commonly used model for low-dose extrapolation is the multistage model of carcinogenesis. This model, derived from a theory proposed by Armitage and Doll in 1961, is based on the biological assumption that cancer is induced by carcinogens through a series of independent stages. The Agency believes that this model conforms most closely to what we know about the etiology of cancer. There is no evidence that the multistage model is biologically inappropriate, especially for genotoxic carcinogens, which MC most likely is. The most recent data submitted by the HSIA [Exs. 117-124A] clearly add substantial support to the previous body of evidence indicating that one or more metabolites of MC is a genotoxic carcinogen. The low-dose linearity feature of this model is scientifically required for any exposure that confers additional risk upon a pre-existing background level of risk produced by a similar or equivalent mechanism. Given the underlying connection between DNA mutations and cancer and the obvious background incidence of cancer in the human population, the overwhelming scientific consensus is that genotoxins follow low-dose linear functions.
The multistage model is generally considered to be a conservative model because it is approximately linear at low doses and because it assumes no threshold for carcinogenesis, although there are other plausible models of carcinogenesis which are more conservative at low doses. "No threshold" means that any incremental amount of exposure to a carcinogen is associated with some amount of increased risk. "Approximately linear at low doses" means that one unit of change in dose will result in one unit of change in risk at low doses.
The most common approach for setting the parameters in the multistage model is to assume that the dose-response curve is described by a polynomial of k-1 degrees, where k is the number of dose groups tested. The multistage model thus takes the form
P(Cancer) = 1-exp(-f(dose)),
with f(dose) given by:
f(dose) = a + b(1)(dose) + b(2)(dose)(2) + ...+ b(k-1)(dose)(k-1).
The number of stages is specified by k-1, and the parameters a (the background risk) and b(i) are estimated from the observed data.
Alternatives to the multistage model include the tolerance distribution models such as the probit model, the logit model and the Weibull model. The tolerance distribution models generally predict dose-response relationships which are sigmoid in shape. Thus, these models will approach zero more rapidly than a linear multistage model. This means that at low doses, these models will predict lower risks than will a linear multistage model.
In the MC rulemaking, most of the risk assessments submitted to the Agency used the linearized multistage model to predict risk. The differences in risk estimates were not generally due to the dose-response model used, but to whether the risk assessor used pharmacokinetic modeling to estimate target tissue doses, and what assumptions were used in the pharmacokinetic modeling.
D. Selection of Dose Measure
1. Estimation of Occupational Dose
The purpose of low dose extrapolation is to estimate risk of cancer at a variety of occupational exposures. This requires that the doses be converted into units comparable to those in which the experimental dose is measured.
In its NPRM, OSHA first converted the experimental dose, measured in ppm, to an inhaled dose, measured in mg/kg/day. The female mouse body weight used in these calculations was 0.0308 kg. The breathing rate for mice was 0.05 m(3)/day). The Agency then assumed that equivalent doses in mg/kg/day would lead to equivalent risk. Once the experimental dose (in mice) had been converted to mg/kg/day, it was then converted to ppm using the human breathing rate of 9.6 m(3)/workday and human body weight of 70 kg in order to estimate risks at various potential exposure levels. To determine the dose to humans corresponding to the risk estimated from the mouse data, OSHA used the following equations:
| Dose(M)(mg/m(3)) | = | Dose(M)(ppm)(84.9g/mol)(1000 mg)(1000 L) ----------------------------------------- 24.45 L/mol (g)(m(3)) |
| Dose(M)(mg/kg/d) | = | Dose(M)(mg/m(3))(0.05m(3)/d)(6hr/24hr)(5d/7d) ----------------------------------------- (0.0308 kg BW) |
OSHA assumed that risk estimates derived for mice at a given mg/kg/d would be equivalent to risks experienced by humans at that mg/kg/d. Doses in mg/kg/d in humans were converted to ppm to determine risk at various potential workplace exposures using the following equations:
Dose(H)(mg/m(3)) = Dose(H)(mg/kg/d)(70kg)
-----------------------------------
(9.6m(3)/workday)(5d/7d)(45yr/70yr) Dose(H)(ppm) = Dose(H)(mg/m(3))(24.45L/mol)/(84.9g/mol)
This process was used by K.S. Crump et al. in their risk assessment submitted to OSHA [Ex. 12]. Use of mg/kg/d as a measure of dose has been criticized by Mr. Harvey Clewell, representing the U.S. Navy [Ex. 19-59]. He stated,
Strictly speaking, the concept of a mg/kg/day dose applies only to exposures for which the term "administered dose" is well defined, which does not include inhalation exposure to a volatile, lipophilic chemical such as MC....If a non-pharmacokinetic dose surrogate is desired, the choice should be time-weighted average concentration (ppm) as used by the FDA.
Mr. Clewell preferred use of dose surrogates calculated in the PBPK models to estimate human risk. OSHA has given careful consideration to the issues raised by Mr. Clewell and, in the risk assessment presented here, considered dose surrogates estimated in PBPK models and time-weighted average concentration in addition to the mg/kg/d dose presented in the NPRM.
For all dose measures used to estimate human risk, the assumptions used by OSHA for body weights and exposure times and rates were those described above. In OSHA's final risk assessment, a Bayesian analysis was used and the prior distribution for breathing rate was centered on OSHA's preferred value of 9.6 m(3)/d).
2. mg/kg/d Versus Other Measures of Exposure
Quantitative risk assessments based on animal data are conducted under the assumption that animals and humans have equal risks from lifetime exposures to a chemical when exposure is measured in the same unit for both species. Opinions vary, however, on what is the correct measure of exposure. For site-of-contact tumors, a ppm-to-ppm conversion is a generally accepted measure of dose. For systemic tumors, commonly used dose conversions include mg/kg/day (as used by OSHA in its MC NPRM), mg/surface area/day (with surface area approximated by BW(2/3)), mg/BW(3/4)/day, and mg/kg/lifetime. When adequate and appropriate pharmacokinetic or metabolic data are available, these data are sometimes used to estimate internal dose. In the case of MC, metabolic data have been gathered and pharmacokinetic models have been used by various investigators to estimate target tissue doses for MC.
Some commenters [Exs. 19-28, 19-57] had expressed concern that OSHA used a surface area correction factor in its risk assessment in the NPRM. In fact, in the NPRM, OSHA extrapolated from mice to humans based on body weight rather than surface area. However, the Agency requested comment on which species conversion factor would be appropriate to use in OSHA's final risk assessment and whether incorporation of pharmacokinetic information should influence the choice of the conversion factor. Two commenters [Exs. 19-83, 23-21] referred to the interagency document on interspecies scaling which ultimately recommends BW(3/4) as the appropriate extrapolation factor in the absence of appropriate pharmacokinetic information, although the document also indicates that extrapolation factors based on BW or BW(2/3) would also be consistent with the available data (EPA Draft Report: "A cross-species scaling factor for carcinogen risk assessment based on equivalence of mg/kg(3/4)/day." 57 FR 24152, June 5, 1992).
There was also considerable discussion as to whether it was appropriate to apply an extrapolation factor such as BW(3/4) or BW(2/3) in addition to PBPK modeling of dose, to account for pharmacodynamic differences between species (such as differences in DNA repair rates and other non-metabolic differences in interspecies susceptibility to an agent). The EPA applied the BW(2/3) extrapolation factor after incorporation of the PBPK data for MC in their 1987 draft update of the MC risk assessment. In their previous risk assessment, which did not incorporate PBPK data, EPA also used BW(2/3) as the extrapolation factor. Since OSHA has preferred the BW extrapolation in other chemical-specific risk assessments and has used BW as the extrapolation factor in its best estimate of risk in the NPRM for MC, OSHA agrees with Dr. Lorenz Rhomberg's assessment [Ex. 28] that OSHA should continue to use body weight as its extrapolation factor in its final MC risk assessment. Thus, OSHA's risk estimate does not make any allowance for possible pharmacodynamic differences between rodents and humans, or within the diverse human population.
3. Pharmacokinetic Modeling of Dose
OSHA discussed issues relating to the use of pharmacokinetic data in its NPRM. These issues were further explored during the hearings and in pre-hearing and post-hearing comments. In response to the ANPR [51 FR 42257], Dow Chemical submitted documentation of a physiologically-based pharmacokinetic model (PBPK) [Exs. 8-14d and 10-6a], developed for MC by Reitz and Anderson, which described the rates of metabolism of the MFO and GST pathways and the levels of MC and its metabolites in various tissues of rats, mice, hamsters and humans. This model was presented as a basis for converting an applied (external) dose of MC to an internal dose of active metabolite in the lung and liver in various species under various MC exposure scenarios. Since publication of the NPRM, several parties have submitted pharmacokinetic models or comments on modeling to the rulemaking record. These are discussed in detail below.
a. General issues in PBPK modeling. Physiologically-based pharmacokinetic modeling can be a useful tool for describing the distribution, metabolism and elimination of a compound of interest under conditions of actual exposure and, if data are adequate, can allow extrapolation across dose levels, across routes of exposure and across species. One limitation of using PBPK modeling is a widespread lack of adequate and appropriate physiological and metabolic data to define the model. In particular, difficulties arise in attempting to define a model for which the mechanism of carcinogenesis has not been established, when it is unclear whether there would be tumor site concordance across species, and when the metabolic pathway(s) responsible for carcinogenesis has not been determined.
The concentration of a chemical in air or the total inhaled dose (mg/kg/d) may not be the most biologically relevant dose to use in comparing toxicity across doses or across species. The dose measure that would be most useful in risk assessment is the dose to the target tissue of the chemical or metabolite that is known to directly cause the toxic effect. Generally, this quantity is unknown in almost every case because the proximate carcinogenic moiety is usually highly reactive, and therefore very difficult to measure in biological systems. Since the proximate toxic agent is unlikely to be a quantity readily measured in the laboratory, it is sometimes desirable to use dose surrogate concentrations, calculated by methods such as PBPK modeling, to obtain a more direct estimate of a dose-response relationship. Examples of dose surrogates that may be relevant to the toxic mechanism of action of a chemical are peak concentrations of a particular metabolite at a target tissue site, area under the concentration-time curve of a metabolite at a target site, and blood concentration of the parent chemical or a relevant metabolite.
If the dose surrogate chosen is directly relevant to the mechanism of action of a chemical, there is greater confidence in the risk estimates generated using the dose surrogate than those generated using total inhaled concentration. If the mechanism of action of a chemical is uncertain, and therefore the relevance of the dose surrogate to carcinogenicity is in question, there is proportionally less confidence in the predicted risks estimated using that dose surrogate. Risk estimates from PBPK modeling can also be limited by the quality and quantity of available metabolic data. Since risk estimates are directly dependent upon the dose or dose surrogate chosen, reliable measures of all relevant physiological parameters and all relevant metabolic pathways in all target tissues from all species under investigation are critical. In addition, measures of the uncertainty and inter-individual variability of these parameters must be generated.
In its NPRM, OSHA solicited information on the appropriateness of physiologically-based pharmacokinetic modeling for the MC risk assessment. Specifically, OSHA asked the following questions:
(a) How can pharmacokinetics be best applied to the risk assessment of MC and what are the current limitations of this approach in the quantitation of health risks? What weight should OSHA give to pharmacokinetic data in its risk assessments and why? (b) Given that five separate risk assessments have utilized the pharmacokinetic models for MC in five different ways (resulting in from 0 to 170 fold reduction in the final risk when compared with assessments not utilizing pharmacokinetic data), how can OSHA best utilize the existing pharmacokinetic data and still be certain of protecting worker health? (c) Which parameters in the pharmacokinetic models are most sensitive to errors in measurement or estimation? Can an increased database reduce the uncertainties in these parameters? (d) How much confidence can be placed in the human in vitro MC metabolism data, especially that for lung tissue? How will human variability in these parameters affect the extrapolation of risk from rodent species? (e) Are there any studies in progress which attempt to verify the predictive ability of the model in vivo, (e.g., by giving doses in a lifetime bioassay which will produce cancer in a species other than the B6C3F1 mouse and the F344 and Sprague-Dawley rats)? (f) OSHA recognizes the large areas of uncertainty which exist in applied dose risk assessment procedures. If pharmacokinetic modeling reduces these uncertainties, can the reduction in uncertainty be quantified? Are additional uncertainties introduced into the risk assessment process by the use of pharmacokinetic models? (g) By using the pharmacokinetic models in the risk assessment process, one is making an assumption about the carcinogenic mechanism of action of methylene chloride. Are there any new studies on the carcinogenic mechanism of action of MC which would support or refute this assumption? (h) If the carcinogenic process is, in fact, not the result of the metabolite(s) from the GST pathway alone, but is due to a combination of metabolites or a combination of the parent compound plus the metabolites, how would the pharmacokinetic model and the subsequent risk assessments be affected? Can these effects be quantified? (i) One of the assumptions made in the pharmacokinetic model is that the target tissues for MC are liver and lung. Can this model predict cancer incidences at other sites? If not, is there a way to factor in consideration of possible MC-induced human cancers at other sites than liver and lung? (j) OSHA solicits information supporting or refuting interspecies allometric scaling based on body weight or body surface area.
OSHA reviewed comments and testimony on these issues from an expert witness [Ex. 25-E]; representatives of other U.S. government agencies, including NIOSH [Exs. 19-46, 41], EPA [Exs. 25-D, 28], CPSC [Ex. 25-I] and U.S. Navy [Exs. 19-59, 96]; the State of California [Ex. 19-17]; the Halogenated Solvents Industry Alliance (HSIA) [Exs. 19-45, 19-83, 105]; and the UAW [Exs. 19-22, 23-13, 61]. Comments and testimony from the expert witness, the other government agencies and the Halogenated Solvents Industry Alliance generally reflected the opinion that the pharmacokinetic information was sufficiently developed in the case of MC to justify its use in estimating human cancer risks. The predominant view among these commenters and hearing participants was that the data collected for MC and the pharmacokinetic model developed by Reitz and Andersen adequately represented the metabolism of MC in mice. Many commenters also believed that it was reasonable to conclude that the lung and liver tumor incidence in the B6C3F1 mice was the result of the GST metabolite. As described in further detail below, OSHA generally agrees that the PBPK approach is reasonable to assess cancer risks of MC. In fact, the Agency has evaluated the submitted PBPK models, determined that there were several deficiences in each of those models, and improved upon those in its final quantification of risks.
One rulemaking participant was strongly opposed to using pharmacokinetic data in the MC risk assessment. Dr. Franklin Mirer [Ex. 61], representing the UAW, stated:
The pharmacokinetic model advanced for methylene chloride carcinogenesis is incorrect and should not be used for quantitative risk assessment.
Dr. Mirer was particularly concerned that the PBPK model ignored the rat cancer bioassay data and that the model was based on a "mechanistic hypothesis."
Dr. Mirer reiterated his concerns in response to the October 24, 1995 reopening of the rulemaking record [Ex. 126-31], stating,
The simple message is that OSHA should give no additional weight to the pharmacokinetic argument. For OSHA to give the argument any additional weight would mean that OSHA was ignoring a substantial body of evidence regarding carcinogenicity of methylene chloride in additional animal species.
Dr. Mirer continued,
The pharmacokinetic hypothesis is unconvincing even as an explanation of the differences in lung and liver tumors in mice and rats.
OSHA shares Dr. Mirer's concerns that the mechanism of carcinogenicity for MC has not been clearly established and that using pharmacokinetic modeling may lead to risk estimates which ignore the rat tumor data. The Agency notes that it has used the NTP rat data in its hazard identification for MC. OSHA has also determined, however, that the mouse data represent the strongest data set on which to base a quantitative risk assessment, and notes that risk estimates based on the rat data (without PBPK-based adjustment of dose) are similar to OSHA's final risk estimates using mouse data and a PBPK analysis.
The determination that the mouse data set was the strongest on which to base a quantitative risk assessment was made without regard to the availability of information on pharmacokinetics. Incorporating pharmacokinetic modeling into the risk assessment for MC is a logical extension of OSHA's risk assessment decisionmaking process and reflects the Agency's review of the totality of data on tumor incidence, metabolism and mechanism of action. The extensive data base on MC metabolism and mechanism of action, although by no means complete, was the determining factor in the decision to incorporate pharmacokinetics into its final risk assessment. The Agency is aware of very few chemicals of regulatory interest for which the available data could match this body of information. The specific criteria utilized by the Agency in making this determination are enumerated below.
Comments on the specific issues enumerated above are discussed under the appropriate topics in the sections that follow.
b. Criteria for using PBPK in quantitative risk assessment. OSHA evaluated several criteria before deciding to use PBPK analysis in its final quantitative risk assessment for MC. In future rulemakings in which the use of pharmacokinetic information in risk assessment is at issue, it will be necessary to evaluate at least the criteria described below before reaching conclusions, in order to avoid adopting an alternative hypothesis that is less (rather than more) reflective of the true situation than the more generic applied-dose assumption. Further, it may be appropriate to evaluate additional criteria in some cases, depending on the metabolism and mechanism of action of the chemical. The criteria which OSHA considered before incorporation of PBPK in the final risk estimate for MC were:
(1) The predominant and all relevant minor metabolic pathways must be well described in several species, including humans. (Two metabolic pathways are responsible for the metabolism of MC in humans, mice, rats and hamsters).
(2) The metabolism must be adequately modeled (Only two pathways are responsible for the metabolism of MC as compared to several potential routes of metabolism for other compounds, such as benzene and the dioxins. This simplified the resulting PBPK models).
(3) There must be strong empirical support for the putative mechanism of carcinogenesis (e.g., genotoxicity) and the proposed mechanism must be plausible.
(4) The kinetics for the putative carcinogenic metabolic pathway must have been measured in test animals in vivo and in vitro and in corresponding human tissues (lung and liver) at least in vitro, although in vivo human data would be the most definitive.
(5) The putative carcinogenic metabolic pathway must contain metabolites which are plausible proximate carcinogens (for example, reactive compounds such as formaldehyde or S-chloromethylglutathione).
(6) The contribution to carcinogenesis via other pathways must be adequately modeled or ruled out as a factor. For example, there must be a reasonable analysis of why reactive metabolites formed in a second pathway would not contribute to carcinogenesis (e.g., formyl chloride produced via the MFO pathway is likely to be too short-lived to be important in MC carcinogenesis).
(7) The dose surrogate in target tissues (lung and liver in the case of MC) used in PBPK modeling must correlate with tumor responses experienced by test animals (mice, rats and hamsters).
(8) All biochemical parameters specific to the compound, such as blood:air partition coefficients, must have been experimentally and reproducibly measured. This must be true especially for those parameters to which the PBPK model is most sensitive.
(9) The model must adequately describe experimentally measured physiological and biochemical phenomena.
(10) The PBPK models must have been validated with data (including human data) which were not used to construct the models.
(11) There must be sufficient data, especially data from a broadly representative sample of humans, to assess uncertainty and variability in the PBPK modeling.
In the case of MC, to a large extent these criteria were met. This made evaluation of existing PBPK models and further development of the modeling strategy a viable option. Therefore, the Agency evaluated existing PBPK models and then contracted with Drs. Andrew Smith, Frederic Bois, and Dale Hattis to help OSHA improve on the MC PBPK model in the record, which would extend the application of modeling techniques beyond those models which had been submitted to the Agency and incorporate all of the data available and appropriate for quantitative analysis in the record. OSHA's evaluation of existing PBPK models, the development of a modified MC PBPK analysis, and OSHA's final risk assessment are described later in this document.
c. Choice of GST metabolic pathway as dose surrogate. The choice of "dose surrogate" for the MC PBPK model is a critical factor in estimating PBPK-based risks. The dose or "dose surrogate" used in a risk assessment should be a biologically-important quantity, should have a plausible mechanism of action at the target tissue and should correlate with the response of interest. The simplest choice of dose is the applied dose or ambient concentration of the contaminant measured as ppm or as the inhaled quantity in mg/kg/day (as used in the Preliminary Quantitative Risk Assessment in the NPRM). Such quantities have the advantage of being easily and directly measurable during the bioassay. Other meaningful dose surrogates could include the concentration of parent compound in the target organ, the concentration of specific metabolites in the target organ, the area under the time-concentration curve (integrated dose) of each metabolite and the parent compound, or peak blood or target organ levels of each metabolite and parent compound. These quantities are not as easily measured. Often only indirect measurements or computer modeling of these dose surrogates are available.
In the PBPK model developed by Reitz et al. [Ex. 7-225], the dose surrogates that correlated with the tumor response were the parent compound (MC) concentration and the amount of GST metabolites formed in the lung and liver. Reitz et al. discounted the parent compound as the dose surrogate because MC is not a chemically reactive compound and direct-acting carcinogens (and metabolites of carcinogenic compounds) are generally hypothesized to be reactive (usually, electrophilic). They also discounted the parent compound as a relevant dose surrogate because parent MC concentration was higher in the rat blood than in the mouse for any dose of MC, while the cancer response of the mouse was greater than the rat. If parent MC were the critical compound for MC carcinogenesis, one would expect the cancer response across species to correlate with blood levels of the compound.
(1) Metabolism via GST versus MFO pathway. Human metabolism of MC has been well studied. One clear finding from the human metabolic studies is that humans metabolize MC by both the MFO and GST pathways, as do mice, rats, and hamsters. Although human metabolism via the MFO pathway has been measured in vivo as well as in vitro, human MC metabolism via the GST pathway has been measured only in vitro. Metabolic data on the human GST pathway have been collected from several liver samples and one pooled lung sample (combined samples from four human subjects). However, it has not been possible to measure human GST metabolism of MC in vivo.
Reitz et al. measured the metabolic constants (K(m) and V(max) in vitro for the GST and the MFO metabolic pathways. Enzyme activities were determined by measuring the conversion of (36)Cl-labeled MC to water-soluble products. Metabolic constants were then compared across species (mouse, rat, hamster and human). In the liver, the MFO activity was highest in the hamster, followed by the mouse, human and rat. Human values were much more variable than those of the rodent species. Human V(max) for the liver MFO pathway ranged approximately an order of magnitude and human K(m) varied approximately three-fold. GST activity in the liver was determined for mouse and human tissues only. Mouse liver had approximately 18-fold greater activity (V(max)) than human liver, but the human tissue had about a three-fold greater affinity constant (K(m)) for MC than the mouse.
In the lung, the activity of the MFO and GST enzymes was determined for a single substrate concentration. For the MFO pathway, mouse tissue had the highest activity, followed by hamster and rat. No MFO activity specific for MC was detected in the human lung tissue, although other MFO isozymes were demonstrated to be active in the tissue. For the GST pathway in lung, mouse tissue was the most active, followed by rat and human. No GST activity was detected in the hamster lung.
In humans, the MFO pathway has been measured in vivo as well as in vitro [Ex. 7-225]. Human in vivo experimentation was conducted by several investigators. Metabolism via the MFO pathway is relatively easy to measure because the end product is carbon monoxide [Ex. 7-24]. The metabolic rates measured in vitro were not similar to those measured in vivo after exposure to known concentrations of MC, which means that in vitro measurements in human tissue (in particular for the GST pathway for which there are no human in vivo data) could not be used directly as a measure of metabolism. Human in vivo and in vitro MFO metabolism data were important in developing the pharmacokinetic models because they provided human data for MC-specific metabolism which could be used to help validate the models. Unfortunately, the modeling of the putative critical pathway for carcinogenesis (the GST pathway) could not be validated for humans. This is a weakness in the PBPK modeling for MC shared by all of the models, including OSHA's final PBPK analysis.
In the PBPK models submitted to OSHA, the human rate of metabolism of MC, particularly via the GST pathway, was based on data gathered from four liver samples and one pooled lung sample. Although the liver metabolic data were of the same magnitude as those collected by Green et al., Green's data were not considered in Reitz's model and the variability of those data was not assessed. Therefore, the estimates of the dose surrogates in Reitz's model were based on the average of four liver samples. Four liver samples are not nearly enough data to confidently estimate and account for human variability. Considerations of the variability and uncertainty of these data are discussed in more detail later in this document.
The human lung data were even more limited. Four human lung samples were pooled to provide a single data point. This lack of lung tissue data is particularly critical in PBPK modeling when calculating the ratios of A1 and A2 (the distribution of metabolism between liver and lung tissue in humans). Errors in calculating these ratios will significantly affect the final risk estimates, as discussed by Mr. Harvey Clewell for the U.S. Navy [Ex. 96].
HSIA submitted additional data on the human metabolism of MC in the form of a study of GST metabolism in human liver samples conducted by Bogaards et al. [Ex. 127-16]. The human GST liver metabolism data collected in this study were not directly comparable to the data collected by Reitz or Green, becausethe Bogaards data were measured using a colorimetric method which was not as sensitive as the (36Cl) method. Under contract to OSHA, Dr. Andrew Smith and Dr. Frederic Bois compared the data from different laboratories and collected under different methodologies and developed a correction factor across methodologies so that they could use all of the human metabolic data available in OSHA's final PBPK model [Ex. 128]. There are now over 30 data points for human liver in vitro metabolism by the GST pathway and 5 human lung data points (the additional lung data points were reported in Green et al., Ex. 124A). OSHA determined that it was important to use as much of the available human data in its PBPK model for MC as scientifically justifiable. These data were used to estimate the variability and uncertainty surrounding the measures of human GST metabolism. Although the methodologies differed across studies, OSHA has adjusted and incorporated all of the available human data in its PBPK model.
(2) Parallelogram approach. When the metabolic rates for the MFO pathway measured in vivo and in vitro within each species were compared, it was determined that those rates were not equivalent. This meant that, unlike the case for some other chemical compounds, the in vitro GST data could not substitute directly for an in vivo measurement of metabolism. Reitz and Andersen [Ex. 7-225] suggested a "parallelogram" approach to the problem of non-comparability of in vitro and in vivo rates. This approach makes the assumption that the ratio of in vivo to in vitro measurements is roughly comparable across species (including humans). They measured metabolic rates of both pathways in vitro and in vivo in rodents and then used the average ratio of the in vitro to in vivo metabolic rate in three rodent species to extrapolate from in vitro rates in humans [Ex. 7-225] to an estimated in vivo value.
Figure VI-1: Schematic diagram of the parallelogram approach.
| assumption: | rodent (in vitro) ----------------- rodent (in vivo) |
= | human (in vitro) ----------------- human (in vivo) |
| or, human (in vivo) | = | rodent (in vivo) x human (in vitro) --------------------------------- rodent (in vitro) |
Ron Brown [Ex. 25-E], an expert witness for OSHA, was concerned that "...the methodology used to extrapolate the in vitro data to the in vivo state is problematic and the accuracy of the human in vitro measurement of GST activity toward MC is uncertain." This may be due to the small sample size, variability in the laboratory analysis or inadequacy of the in vitro model. OSHA believes that this is a critical point of uncertainty in using the PBPK model for risk assessment. The Agency also notes that in the risk assessments using PBPK models submitted during the MC rulemaking, none used the parallelogram approach as the basis of determining human in vivo metabolic rates. Instead, allometric scaling was used to estimate human values. OSHA has conducted risk assessments using both the allometric approach (OSHA's final risk estimates) and the parallelogram approach (OSHA's alternative analysis). The Agency did this in order to determine what the risk estimates would be if all possible quantitative data were used to the fullest extent, regardless of the uncertainties in the data.
OSHA agrees that evidence presented in the record generally supports the GST pathway as a plausible carcinogenic mechanism of action of MC. The Agency remains concerned, however, that sole reliance on the GST pathway may show insufficient consideration for potential contributions of the parent compound and/or metabolites of the MFO pathway to the carcinogenesis of MC. It is clear that ambient MC concentration is dose-related to tumor response. It has not been shown with any certainty that MC GST metabolites are related to tumor response across species. Thus, there is greater confidence that the lifetime bioassays predict MC carcinogenicity in humans than there is that cancer occurred through a specific mechanism, and even less confidence that the metabolic rates measured in vitro accurately measure differences in species that correlate to tumor development. This is particularly true for lung metabolism where only one pooled and five individual human samples were analyzed. Notwithstanding the uncertainties described above, the Agency believes that the hypothesis that GST is the carcinogenic pathway presents a plausible mechanism of action for MC and is sufficiently well-developed to warrant the use of PBPK modeling of the GST pathway as the dose surrogate of choice in the quantitative risk assessment for MC.
d. Structure of the MC PBPK model. The PBPK models described below are based on the model originally submitted by Dr. Reitz on behalf of HSIA in 1992 [Ex. 7-225]. Over the years since the first submission of a MC PBPK model to OSHA, significant improvements have been made in model structure and in the data collected for PBPK modeling, especially in how the uncertainty and variability in the data are treated. The general structure of the models submitted to OSHA are described below, followed by a description of the parameters used in the various models. Next follows a description of how the variability, uncertainty, and sensitivity of the models to uncertainty have been assessed, noting the improvements that have been made in developing methods to handle these issues. This is followed by a comparison of the risk estimates generated by these models. Finally, OSHA's final risk assessment is described. This risk assessment incorporates lessons learned from previous models and uses all of the available, appropriate, quantifiable data in a Bayesian approach to modeling the dose metric for MC.
In the PBPK model submitted by Dr. Reitz of HSIA [Ex. 7-225], a series of differential equations was used to model the mass balance of MC and its metabolites in five physiologically-defined compartments, including the lung, liver, richly perfused tissue, slowly perfused tissue, and fat. Metabolism via the MFO pathway was described by saturable Michaelis-Menten kinetic equations and GST metabolism was modeled using first-order nonsaturable kinetics. With the exception of the PBPK model sumitted by ICI [Ex. 14A], all of the PBPK models submitted to the Agency followed these assumptions regarding the metabolism of MC. The rate constants for the metabolic equations were estimated based on measurements of the partition coefficients, allometric approximations of the physiological constants (e.g., lung weight), and estimations (i.e., allometric scaling of rodent data, estimations made using the parallelogram approach, etc.) of the biochemical constants (e.g., Michaelis-Menten constants).
NIOSH presented a PBPK model in 1993 [Ex. 94], also structurally based on the Reitz-Andersen model, but with modifications to the human breathing rate and cardiac output to account for uptake of MC in physically active workers, rather than at-rest humans or humans involved in light activity, and including an analysis of the variability of the human metabolic parameters. Specifically, NIOSH compared estimates derived from the arithmetic average of the human GST metabolism data with the individual human liver data points to estimate the uncertainty in an individual's risk of cancer from occupational MC exposure. This approach began to incorporate some necessary features, such as a special focus on physically active workers and the variability of human metabolic parameters, but did not attempt to quantify the uncertainty and variability of the individual parameters and their contribution to the uncertainty associated with the PBPK model.
Mr. Harvey Clewell, representing the U.S. Navy, also submitted several PBPK models to OSHA. In his initial submission (1992), Mr. Clewell modified an existing PBPK model [Ex. 7-125] to include more recent data on the mouse blood/air partition coefficient [Ex. 19-59]. In a second PBPK model, he "started from scratch" to construct a model based on data derived from sources independent of the previous work of Reitz and Andersen [Ex. 23-14], which was described in Mr. Clewell's testimony [Tr. 2361,10/15/92]. This model was structurally similar to the model presented by HSIA with the following exceptions: it featured three lumped compartments (slowly perfused, moderately perfused and rapidly perfused) based on tissue kinetic constants rather than the earlier two lumped compartment models based on tissue blood volumes; and the mouse blood/air partition coefficient was corrected to 19.4 instead of the earlier 8.29 on the basis of more recent data. A third model submitted by Mr. Clewell was identical in structure to the Reitz/Andersen model, but incorporated the more recent experimental data on the partition coefficients and the more recent mouse metabolism data [Ex. 96]. OSHA used Mr. Clewell's third model in its comparison of PBPK-derived risk estimates because of its similarity in structure to the original Reitz model and its incorporation of the most recent experimental data.
In his third model, Clewell either derived probability distributions for each parameter from the literature or estimated distributions for those parameters for which data were not available, and conducted Monte Carlo simulations to derive output distributions for the dose surrogates. These distributions of dose surrogates were then used to derive four risk estimates: the doses input into the multistage dose-response analysis of the tumor bioassay were derived either from the mean or from the 95th percentile of the output distribution of PBPK parameters, and these in turn were coupled with the either the MLE or the UCL of the distribution of possible values of the multistage model parameters. This analysis was an advance over that of previous models because it took into account some of the uncertainty and variability known to be associated with the data used in the PBPK model.
After evaluating these submitted models, OSHA determined that Clewell's model provided the best prototype on which to base its final PBPK modeling approach for MC. Therefore, the Agency worked with Drs. Smith and Bois to review Clewell's model and with the assistance of Dr. Hattis, to develop a refined PBPK modeling approach with a more sophisticated analysis of variability and uncertainty (and other refinements as described below). In this way the Agency developed an approach which would incorporate what was learned in the development of earlier PBPK models and make use of as much of the available physiological and metabolic data in the record as possible. Clewell's model was chosen for comparison, because this was the only model to provide a systematic analysis of the uncertainty, variability and sensitivity of the model using Monte Carlo techniques. OSHA's final risk assessment approach is described in greater detail below.
e. Choice of parameters for PBPK modeling. The definitions of the parameters used in the models described above are contained in Table VI-2. Note that not all parameters were used in each model and slightly different variable names were used by different investigators. For example, OSHA's final analysis contains a bone marrow compartment, while Clewell's model did not. OSHA refers to the blood flow for poorly (or slowly) perfused tissues as "QppC," while Clewell used "QSC."
| Parameter (units) | Definition |
|---|---|
| BW (kg) | Body weight in kg. Human body weights were assumed to be 70-kg (Reference Man). Mouse body weights were the average weight of mice in the NTP bioassay |
| QPC unscaled (1/hr, 1 kg BW) | Breathing rate. QPC = QP(1/hr)/BW(.75) where QP = alveolar ventilation rate. Human QP was based on rate of 9.6 m(3)/8-hr (converted 1/hr and adjusted to alveolar ventilation (= 0.70 total ventilation) except in NIOSH and OSHA-modified models. Mouse QP = (24.3 1/hr)(0.70 alveolar/total) |
| QCC unscaled (1/hr, 1 kg BW) | cardiac output. QCC = QC(1/hr)/BW(.75) where QC = cardiac output in 1/hr. Reitz set QC = QP. Clewell and NIOSH based human QC on Astrand et al. [Ex. 7-120] data on cardiac output and breathing rate vs. workload |
| VPR (ratio, unitless) | alveolar ventilation/perfusion ratio |
| Blood flows to tissues | |
| QGC or QgiC (fraction of cardiac output) | Blood flow to gastrointestinal tract as a fraction of cardiac output. QGC = QG/QC |
| QLC or QliC (fraction of cardiac output) | Blood flow to liver as a fraction of cardiac output. QLC = QL/QC |
| QFC or QfatC (fraction of cardiac output) | Blood flow to fat as a fraction of cardiac output. QFC = QF/QC |
| QSC or QppC (fraction of cardiac output) | Blood flow to slowly (or poorly) perfused tissues as a fraction of cardiac output. QSC = QS/QC |
| QRC or QwpC (fraction of cardiac output) | Blood flow to rapidly (or well) perfused tissues as a fraction of cardiac output. QRC = QR/QC |
| QmarC (fraction of cardiac output) | Blood flow to bone marrow as a fraction of cardiac output |
| Tissue volumes | |
| VGC or VgiC (fraction of body weight) | Volume of GI tract as a fraction of body weight. VGC = VG/BW |
| VLC or VliC (fraction of body weight) | Volume of liver as a fraction of body weight. VLC = VL/BW |
| VFC or VfatC (fraction of body weight) | Volume of fat as a fraction of body weight. VFC = VF/BW |
| VSC or VppC (fraction of body weight) | Volume of slowly (or poorly) perfused tissues as a fraction of body weight. VSC = VS/BW |
| VRC or VwpP (fraction of body weight) | Volume of rapidly (or well) perfused tissues as a fraction of body weight. VRC = VR/BW |
| VluC (fraction of body weight) | Volume of lung as a fraction of body weight |
| VmarC (fraction of body weight) | Volume of bone marrow as a fraction of body weight |
| Partition coefficients | |
| PB or Pblo | Blood/air partition coefficient |
| PG or Pgi | GI tract/blood partition coefficient (GI tract/air divided by PB) |
| PL or Pli | Liver/blood partition coefficient (Liver/air divided by PB) |
| PF or Pfat | Fat/blood partition coefficient (Fat/air divided by PB) |
| PS or Ppp | Slowly (or poorly) perfused tissue/blood partition coefficient (Slowly perfused tissue/air divided by PB) |
| PR or Pwp | Rapidly (or well) perfused tissue/blood partition coefficient (Rapidly perfused tissue/air divided by PB) |
| PLU or Plu | Lung/blood partition coefficient (Lung/air divided by PB) |
| Pmar | Bone marrow:air partition coefficient |
| Metabolic parameters | |
| VMAXC unscaled (mg/hr, 1 kg animal) | MFO pathway Michaelis-Menten maximum velocity for MC metabolism. VMAXC = VMAX (mg/hr)/BW(.75) |
| KM (mg/l) | MFO pathway Michaelis-Menten affinity constant for MC metabolism |
| KFC, unscaled, (/hr, 1 kg animal) | GST pathway 1st order kinetic rate constant for MC metabolism. KFC = KF (/hr)(BW(.25)) |
| a1 (ratio) | Ratio of distribution of MFO pathway MC metabolism between lung and liver. A1 = VMAXC(lung)/VMAXC(liver) |
| a2 (ratio) | Ratio of distribution of GST pathway MC metabolism between lung and liver. A2 = KFC(lung)/KFC(liver) |
| B1 (ratio) | Ratio of lung and liver tissue content of microsomal protein |
| B2 (ratio) | Ratio of lung and liver tissue content of cytosolic protein |
| Sp -- Kf | allometric scaling power for body weight scaling of KFC from mice to humans |
The MC physiologically-based pharmacokinetic (PBPK) models discussed here contain the following types of parameters as defined above: body weight, breathing rate, cardiac output, blood flows to tissue compartments (as a fraction of the cardiac output), volumes of tissue compartments (as a fraction of body weight), partition coefficients, the metabolic parameters (the Michaelis-Menten parameters, Vmax and Km, for the MFO pathway and the 1st-order rate constant, Kf, for the GST pathway) and the ratio of the pathway-specific metabolic capacity between the major metabolic sites (lung and liver). Differences in model structure (such as choice of lumped tissue compartments) and differences in sources of data for individual parameters lead to differences in the parameter values used in different models.
The parameter values (point estimates) used in the PBPK models reviewed by OSHA are presented in Table VI-3. The parameter distributions used by OSHA in its analysis are presented later.
As far as OSHA could determine, the parameters chosen by HSIA were those presented in Reitz's 1989 paper [Ex. 21-53] except that OSHA's preferred values for breathing rates (based on 9.6 m(3/workday)) and 8-hour human exposures were used. The model submitted by NIOSH used the parameters and computer code from the Reitz model, except for the human breathing rate, human cardiac output and human metabolic parameters. The parameters used by Clewell were summarized in his post-hearing submission [Ex. 96], which included more recent experimental data for the partition coefficients and mouse metabolic parameters and a different scaling for human cardiac output.
| Model | Clewell [Ex.96] | NIOSH [Ex. 23-18] | |
|---|---|---|---|
| Parameter | Mouse | Human | Mouse |
| BW (kg) | 0.0345 | 70 | 0.0345 |
| QPC, unscaled alveolar ventilation (1/hr, 1 kg animal) | 29.0 | 35 | 29.0 |
| QCC, unscaled cardiac output (1/hr, 1 kg animal) | 16.5 | 18 | 29.0 |
| QGC(a), flow to GI tract (fraction of cardiac output) | 0.165 | 0.195 | 0.0 |
| QLC(a), flow to liver (fraction of cardiac output) | 0.035 | 0.07 | 0.24 |
| QFC(a), flow to fat (fraction of cardiac output) | 0.03 | 0.05 | 0.05 |
| QSC(a), flow to slowly perfused tissues (fraction of cardiac output) | 0.25 | 0.24 | 0.19 |
| QRC(a), flow to rapidly perfused tissues (fraction of cardiac output) | 0.52 | 0.445 | 0.52 |
| VGC, GI volume (fraction of BW) | 0.031 | 0.045 | 0.0 |
| VLC, liver volume (fraction of BW) | 0.046 | 0.023 | 0.04 |
| VFC, fat volume (fraction of BW) | 0.100 | 0.16 | 0.07 |
| VSC, slowly perfused tissue volume (fraction of BW) | 0.513 | 0.48 | 0.75 |
| VRC, rapidly perfused tissue volume (fraction of BW) | 0.041 | 0.033 | 0.05 |
| VLUC, lung volume (fraction of BW) | 0.008 | 0.006 | 0.012 |
| PB, blood/air part. coeff | 23.0 | 12.9 | 8.29 |
| PG, GI tract/air part. coeff | 0.52 | 0.93 | NA |
| PL, liver/blood part. coeff | 1.6 | 2.9 | 1.71 |
| PF, fat/blood part. coeff | 5.1 | 9.1 | 14.5 |
| PS, slowly perf./blood part. coeff | 0.44 | 0.78 | 0.96 |
| PR, rapidly perf./blood part. coeff | 0.52 | 0.93 | 1.71 |
| PLU, lung/blood part. coeff | 0.46 | 0.82 | 1.71 |
| VMAXC mg/hr, 1 kg animal (unscaled) | 13.4 | 5.0 | 13.2 |
| KM (mg/L) | 1.35 | 0.4 | 0.396 |
| KFC /hr, 1 kg animal (unscaled) | 1.5 | 1.5 | 1.73 |
| a1 (Vmaxc(lung)/ Vmaxc(liver)) | 0.41 | 0.015 | 0.416 |
| a2 (KFC(lung)/ KFC(liver)) | 0.28 | 0.18 | 0.137 |
| Model | NIOSH [Ex. 23-18] (Cont'd | HSIA [Ex. 19-45] | |
| Parameter | Human | Mouse | Human |
| BW (kg) | 70 | 0.0345 | 70 |
| QPC, unscaled alveolar ventilation (1/hr, 1 kg animal) | 43.1 | 29.0 | 35.0 |
| QCC, unscaled cardiac output (1/hr, 1 kg animal) | 20.9 | 29.0 | 35.0 |
| QGC(a), flow to GI tract (fraction of cardiac output) | 0.0 | 0.0 | 0.0 |
| QLC(a), flow to liver (fraction of cardiac output) | 0.2093 | 0.24 | 0.24 |
| QFC(a), flow to fat (fraction of cardiac output) | 0.040 | 0.05 | 0.05 |
| QSC(a), flow to slowly perfused tissues (fraction of cardiac output) | 0.4319 | 0.19 | 0.19 |
| QRC(a), flow to rapidly perfused tissues (fraction of cardiac output) | 0.3188 | 0.52 | 0.52 |
| VGC, GI volume (fraction of BW) | 0.0 | 0.0 | 0.0 |
| VLC, liver volume (fraction of BW) | 0.0314 | 0.04 | 0.0314 |
| VFC, fat volume (fraction of BW) | 0.231 | 0.07 | 0.231 |
| VSC, slowly perfused tissue volume (fraction of BW) | 0.621 | 0.75 | 0.621 |
| VRC, rapidly perfused tissue volume (fraction of BW) | 0.0371 | 0.05 | 0.0371 |
| VLUC, lung volume (fraction of BW) | 0.011 | 0.012 | 0.011 |
| PB, blood/air part. coeff | 9.7 | 8.29 | 9.7 |
| PG, GI tract/air part. coeff | NA | NA | NA |
| PL, liver/blood part. coeff | 1.46 | 1.71 | 1.46 |
| PF, fat/blood part. coeff | 12.4 | 14.5 | 12.4 |
| PS, slowly perf./blood part. coeff | 0.82 | 0.96 | 0.82 |
| PR, rapidly perf./blood part. coeff | 1.46 | 1.71 | 1.46 |
| PLU, lung/blood part. coeff | 1.46 | 1.71 | 1.46 |
| VMAXC mg/hr, 1 kg animal (unscaled) | 3.98 | 13.2 | 4.9 |
| 1.15 | |||
| 9.81 | |||
| 4.71 | |||
| KM (mg/L) | 0.72 | 0.396 | 0.580 |
| 0.55 | |||
| 0.26 | |||
| 0.79 | |||
| KFC /hr, 1 kg animal (unscaled) | 1.56 | 1.73 | 1.24 |
| 0.00 | |||
| 1.62 | |||
| 1.79 | |||
| a1 (Vmaxc(lung)/ Vmaxc(liver)) | 0.00143 | 0.416 | 0.00143 |
| a2 (KFC(lung)/ KFC(liver)) | 0.18 | 0.137 | 0.18 |
| Footnote(a) QGC + QLC + QFC + QSC + QRC MUST = 1.00 | |||
f. Assessment of the sensitivity and uncertainty of the PBPK model. In the NPRM, OSHA expressed concern that, if PBPK models were used to adjust risk assessments, the uncertainty in PBPK modeling should be adequately addressed. Specifically, OSHA was concerned that the uncertainty in the mechanism of action and the lack of human lung metabolism data were the greatest obstacles to incorporation of pharmacokinetic data into the MC final risk assessment. Many of the uncertainties in model parameters have been quantified by various hearing participants and are summarized below. The quantification of these uncertainties, however, did not address OSHA's primary concerns regarding the mechanism of action and the distribution of metabolism between lung and liver. OSHA's analyses of the uncertainty and variability of parameters in the PBPK model are presented with its risk assessment later in this document.
The concepts of uncertainty, variability and sensitivity in PBPK modeling were defined in comments submitted by the U.S. Navy [Ex. 19-59]:
As it relates to the issue of using PBPK modeling in risk assessment, uncertainty can be defined as the possible error in estimating the "true" value of a parameter for a representative ("average") animal. Variability, on the other hand, should only be considered to represent true interindividual differences.
The normalized sensitivity coefficient gives the percentage change in a model output due to a percentage change in the parameter value and represents the relative importance of the parameter to the model output under the conditions of the simulation.
Each of these quantities is of concern for risk assessment and PBPK modeling. For example, we know that there is variability or inter-individual heterogeneity in the body weights of humans (and mice), yet we estimate risks for an average member of the population (70 kg in humans, average bioassay weight in mice). For many parameters, the interindividual variability may not be known and must be estimated.
Uncertainty in estimation of the value of a parameter representing an average member of a population is primarily due to laboratory measurement and related errors. Measurement errors, in many cases, can be quantified or estimated so that the potential impact of this uncertainty on the outcome of the PBPK modeling can be assessed.
The sensitivity of the model to particular parameters is useful for determining which experiments should be conducted to confirm parameters and to determine the amount of confidence that PBPK model outputs merit. For example, when a sensitivity analysis is conducted and it is determined that the model outcomes are not very sensitive to changes in the definitions of the lumped tissue volumes, it suggests that there is little need to conduct experiments to describe those relationships more precisely. Similarly, even though the lumped tissue volume does not represent a "true" biological quantity, there is confidence that its precise definition is not critically important in PBPK model outcomes. Therefore, if the only large (quantifiable) uncertainty resides in this measurement, one would have greater confidence that the model predictions were reasonably accurate. Therefore, it is instructive to understand which parameters influence the model outcomes to the greatest degree. Conversely, if the PBPK model outputs are sensitive to a parameter which has not been precisely described (such as the distribution of GST metabolism between lung and liver), the confidence in model outputs is correspondingly reduced.
Various investigators have attempted to determine the sensitivity of the PBPK models to parameter values and to characterize the uncertainty and variability within parameters in the models. The first attempt to describe the sensitivity of the Reitz's original PBPK model was performed by the Consumer Product Safety Commission (CPSC).
The CPSC conducted a sensitivity analysis of the metabolic parameters, Km, Vmax and Kf, in the "Updated Risk Assessment for Methylene Chloride" [Ex. 7-126]. They analyzed the sensitivity of the model by selecting alternative point estimates for the metabolic parameters and determining what the resulting ratio of GST metabolite at 4000 ppm vs. 1 ppm would be. This analysis shows how this ratio would vary if the metabolic parameters used in the model were higher or lower than the measured values as selected by CPSC. The results showed that the ratio of the GST metabolite in the liver at 4000 ppm to the GST metabolite at 1 ppm (or the ratio of the GST metabolite in the lung at 4000 ppm to the GST metabolite at 1 ppm) was relatively insensitive to the value of Kf (when CPSC varied Kf from 0.01 to 5.3, while Km and Vmax were held constant at Reitz-Andersen values).
HSIA presented a sensitivity analysis of the PBPK parameters from the Reitz (HSIA) model in the testimony of Dr. Reitz [Ex. 23-21A]. Results were presented for mice at 4000 ppm, mice at 1 ppm, humans at 1000 ppm and humans at 1 ppm. In the first analysis (mice at 4000 ppm), the most sensitive parameters were determined to be PB (blood:air partition coefficient) and Kf (metabolic parameter for the GST pathway). The authors observed that at high MC exposure levels the model output was at least an order of magnitude less sensitive to changes in the other sixteen parameters investigated.
When mice were exposed to lower concentrations of MC (1 ppm) Vmax and Km for the MFO pathway were the most sensitive parameters (sensitivity coefficient was over 120% for each of these parameters). In addition, several other parameters were found to exert a significant influence on model outputs: QP, QL, PB, VLu, and KF.
In humans, at high concentrations (> 1000 ppm) the results were similar to those observed in mice: the model was most sensitive to PB and KF, with sensitivity coefficients of 87% and 97%, respectively. In addition, the human model was also sensitive to the value chosen for the QP (sensitivity coefficient = 43%).
In humans, at 1 ppm MC, Km and Vmax for the MFO pathway were the most sensitive parameters out of the six parameters which had a significant effect upon model outputs: QP, QL, PB, Vmax, Km, and KF.
This type of sensitivity analysis improves on that conducted by the CPSC, because it looks at more of the parameters. It is still deficient, however, because it examines the effect of each parameter individually, and because it does not examine the effect of uncertainty in two key parameters, A1 and A2 (the ratios of distribution of the MFO and GST pathways between lung and liver), on the outcomes of the modeling.
Mr. Clewell [Ex. 19-59] also conducted a sensitivity analysis to determine the impact of uncertainty in PBPK parameters on the model outcomes. In contrast to the HSIA analysis, he examined the sensitivity of the outcomes to the ratios A1 and A2, and he chose a more realistic occupational exposure level (100 ppm). He found that for mice at 4000 ppm, the most sensitive parameters for estimation of lung tumors were KF, A2, and PB. In the liver, the most sensitive parameters were KF and PB, which agrees with the results of the HSIA analysis. For humans at 100 ppm, the most sensitive parameters for estimating lung tumors were KF and A2. Other parameters with significant effects on model outcomes were PB, QPC, BW, KM, QCC, and QLC. The most sensitive parameters for estimating liver tumors were VMAX, KF, QPC and BW, while PB, KM, QCC and QLC also produced significant effects on model outcomes.
In all of these analyses, the PBPK models were clearly sensitive to the values chosen for the metabolic parameters, especially the GST metabolic parameter (KF). Other parameters with consistently significant impact on the outcomes of the model included breathing rate (QP) and distribution of GST metabolism between lung and liver (A2). These analyses suggest that additional studies to quantify the metabolic parameters (KF, KM and VMAX), breathing rates (QP) and distribution of GST metabolism between lung and liver (A2) would increase confidence in the model outcomes. Characterization of the distribution of metabolism between lung and liver is particularly critical because estimates for human lung metabolism were initially based on one pooled sample of lung tissue, and the variability and uncertainty of the value of this parameter has not been quantified.
Some analysts [Ex. 21-52] have suggested that the uncertainty is increased in risk assessments based on PBPK as compared to applied-dose risk assessments, because some methods of quantifying the uncertainty result in rather broad distributions of uncertainties. OSHA, in contrast, agrees with most commenters that quantifying uncertainty in a PBPK model or risk assessment does not increase the uncertainty. The Agency stresses that the appearance of increasing uncertainty with the identification of sources of uncertainty almost certainly means that the original uncertainty was underestimated. (In fact, since many assessors have not attempted even to quantify the uncertainty in applied-dose risk assessments, the uncertainty has often been infinitely underestimated.) When conducting a risk assessment using PBPK that appears to increase the uncertainty over delivered-dose methodologies, the investigator should go back and recalibrate what the uncertainty in the original analysis likely was, in light of the sources of uncertainty identified using PBPK. This would tend to broaden the confidence limits of the traditional risk assessments, almost certainly beyond the limits generated in a thoughtful PBPK-based assessment. For example, many analyses using delivered dose assume that in the interspecies scaling factor, BW(x), x is known with perfect certainty (e.g., it is known to equal 2/3 or 1.0). An analysis that uses an empirically-derived probability distribution for x, which might reasonably extend from approximately 0.6 to approximately 1.0, would yield a rather broad distribution of uncertainty in the resulting estimate of risk.
The Agency also agrees that the primary uncertainties lie in the choice of the dose surrogate and assumptions regarding cross-species scaling. Clewell [Ex. 23-14] investigated the uncertainty of the PBPK parameters using Monte Carlo analyses of the assumed distributions of uncertainty of each parameter. The resulting estimates of dose surrogate values were characterized by a mean of the distribution and an upper 95th percentile estimate. Mr. Clewell stated [Ex. 19-59]:
[T]he use of the 95th percentile of the distribution of estimates accounts for additional uncertainty concerning the true values of the PBPK parameters for the bioassay animals and humans.
Mr. Clewell recommended that OSHA use the upper 95th percentile of the Monte Carlo distribution of GST metabolites (from PBPK modeling) as an input to the multistage model to generate risk estimates, and then use of the MLE from the multistage model in those risk estimates, in accordance with previous OSHA risk assessments. He remarked that use of the upper 95th percentile of the PBPK output would be a reasonable mechanism to account for the uncertainty quantified in these analyses. Using the upper 95th percentile of the distribution of GST metabolites, Mr. Clewell's risk estimate for lifetime occupational exposure to 25 ppm MC was 0.9 deaths per 1000 using the MLE of the multistage model, and 1.1 per 1000 using the 95th percentile upper confidence limit (UCL) from the multistage model. Using the mean of the distribtution of GST metabolites, his MLE risk estimate was 0.28 deaths per 1000 at the same exposure level, with an UCL of 0.35/1000.
The HSIA disagreed with using the upper 95th percentile for estimating risks, and stated [Ex. 105]:
[T]he analyses conducted by Clewell et al. indicate that consideration of model parameter variability does not contribute orders of magnitude to the uncertainty associated with PB-PK risk assessments. Further, the uncertainty associated with PB-PK risk assessments is significantly less than that associated with risk assessments that fail to consider pharmacokinetics. The uncertainty in PB-PK based procedures is simply more readily available for calculation.
OSHA disagrees with the HSIA that the uncertainty and variability associated with PBPK risk assessments is significantly less than that associated with risk assessments that fail to consider pharmacokinetics. Quantification of uncertainty does not equate with reducing uncertainty in an analysis. In fact, at a different level, the assumptions made regarding mechanism of action of MC and extrapolation of lung metabolic rates from one human in vitro sample may serve to underestimate the uncertainty inherent in the PBPK-based risk assessment if the underlying assumptions are wrong. Also, as stated above, identification of uncertainty may lead us to recalibrate the uncertainty associated with traditional risk assessment methods. In any event, the possibility that using PBPK significantly reduces uncertainty does not affect the need to account for whatever uncertainty remains.
In addition, OSHA agrees with Clewell that using the upper 95th percentile of the Monte Carlo distribution of GST metabolites as input to the multistage model is a reasonable way to incorporate the quantifiable uncertainty and variability into a risk assessment. In its final risk estimates, OSHA has used the upper 95th percentile on the distribution of GST metabolites from the Bayesian analysis as the input to the multistage model, as described later in this document.
E. Other Risk Estimates Based on PBPK Models Prior to OSHA's Final Analysis.
A PBPK model can produce estimates of target tissue doses (or dose surrogates) for different hypotheses of action of a chemical. The appropriate choice of target tissue dose can greatly influence risk estimates based on that dose. For MC, the dose surrogate that has been used most frequently to estimate cancer risks is the amount of GST metabolite produced. The amount of GST metabolite can then be used to extrapolate from a high bioassay dose of MC to a low occupational (or environmental) dose of MC and from mouse MC metabolic rates to human metabolic rates.
In the NPRM, OSHA reviewed available risk assessments for MC that used PBPK modeling in a variety of ways. The Food and Drug Administration risk assessment [Ex. 6-1] was not adjusted to account for pharmacokinetic information. The Consumer Product Safety Commission, in its "Updated risk assessment for methylene chloride" [Ex. 7-126], used pharmacokinetic data to adjust for differences in metabolism in extrapolating from high dose (4000 ppm mouse bioassay) to low dose (1 ppm) exposures, but did not adjust for interspecies differences in the metabolism of MC. The resulting risk estimate was approximately 2-fold lower than a risk estimate using applied dose.
The U.S. EPA analyzed the MC pharmacokinetic data in its documents, "Technical analysis of new methods and data regarding dichloromethane hazard assessment" [Ex. 7-129] and "Update to the Health Assessment Document and Addendum for dichloromethane (methylene chloride): pharmacokinetics, mechanism of action, and epidemiology" [Ex. 7-128]. The EPA used the PBPK data to adjust its risk estimates in its Integrated Risk Information System (IRIS) database. Adjustments were made for high-to-low dose and cross-species extrapolation. EPA's risk estimates for low human exposures to MC were decreased by approximately a factor of 9 from its risk estimates made without consideration of PBPK data.
The HSIA [Ex. 105] and ECETOC [Ex. 14] also submitted risk assessments based on PBPK data. The primary difference between the HSIA and the EPA risk estimates was that the HSIA did not use a surface area correction to account for interspecies differences other than pharmacokinetics (e.g., pharmacodynamic differences) while the EPA did. Also, HSIA's risk estimates used OSHA's preferred breathing rates and an occupational exposure scenario. ECETOC based its risk estimates on different measures of human MC metabolism. In a pre-hearing submission, "Using PB-PK Models for Risk Assessment with Methylene Chloride (Comparison of U.S. and U.K. procedures)" [Ex. 19-83A], scientists from the U.S. and the U.K. compared methodologies for using PBPK data in the MC risk assessment and presented a consensus opinion that OSHA should use the methodology developed by Dr. Richard Reitz [Ex. 7-225] for the U.S. For this reason, OSHA evaluated Dr. Reitz's analysis, as presented by the HSIA, and did not separately consider the ECETOC risk assessment.
As described previously, Clewell [Ex. 96] and NIOSH [Ex. 94] have submitted analyses of the PBPK data and risk assessments based on those analyses. Both of these analyses used PBPK modeling of the amount of GST metabolites produced in their estimates of carcinogenic risks.
OSHA has evaluated the data in the rulemaking record and has concluded that, if PBPK modeling is used to adjust estimates of risk, the weight of evidence supports using the amount of GST metabolites as the preferred surrogate for target tissue dose. The amount of GST metabolites predicted by the PBPK model varies depending upon the values or distributions chosen for the parameters in the model.
Of the risk assessments described above, OSHA has chosen to compare risks estimated using PBPK models submitted by Reitz et al., Clewell et al. and NIOSH with applied dose methodology using either of two scaling assumptions: the inhaled dose in mg/kg/day (the estimates of risk presented in the NPRM) and ppm-to-ppm extrapolation. OSHA evaluated the methodologies used in developing these risk estimates before developing its final risk estimates, which are presented in the next section.
The risk estimates derived from using PBPK with the multistage dose-response model submitted to the Agency by Reitz et al., Clewell et al., and NIOSH, and the risk estimates derived from applied dose methodologies, are shown in Table VI-4.
s
| Model | MLE (UCL)(**) | ||
|---|---|---|---|
| 25 ppm | 50 ppm | 500 ppm | |
| OSHA NPRM Risk Assessment (mg/kg/d,BW extrapolation) without PBPK Adjustment | 2.32 (2.97) | 4.64 (5.92) | 45.5 (57.7) |
| PPM to PPM extrapolation without PBPK Adjustment | 11.3 (14.4) | 22.4 (28.5) | 203 (251) |
| PBPK Reitz female mouse lung -- Reitz human (HSIA assumptions) | 0.43 (0.53) | 0.93 (1.17) | 14.3 (17.9) |
| PBPK Reitz female mouse lung -- Dankovic average human (NIOSH assumptions) | 0.81 (1.02) | 1.69 (2.12) | 15.0 (18.7) |
| PBPK Clewell female mouse lung -- Clewell human (Navy assumptions)(*) | 0.91 (1.14) | 1.88 (2.36) | 27.5 (34.2) |
| OSHA Final Risk Assessment (female mouse lung with PBPK) | 3.62 | 7.47 | 125.8 |
| Footnote(*) Upper 95th percentile of the GST metabolites distribution was used as input in the multistage model | |||
| Footnote(**) Maximum likelihood estimates and 95th percentile upper confidence limit (in parentheses) of the multistage dose-response function | |||
Of those risk estimates considered by OSHA prior to its final risk assessment, the risk estimates for lifetime occupational exposure to the 8-hour TWA PEL of 25 ppm ranged from 0.43 per 1000 to 11.3 per 1000. The risk assessment presented in the NPRM was based on a body weight extrapolation from mice to humans of a mg/kg/day dose of MC. Mr. Harvey Clewell [Ex. 19-59] stated that this dose was not a useful dose for estimating risks from volatile solvents such as MC. He suggested that, if PBPK modeling was not used to estimate target tissue dose (his preferred method of estimating risk), then a ppm-to-ppm extrapolation would be more appropriate. The ppm-to-ppm extrapolation resulted in an estimated risk of 11.3 deaths per 1000 after lifetime occupational exposure to 25 ppm. However, the ppm-to-ppm extrapolation is generally preferred for site-of-contact tumors. Although it is possible that the MC lung tumors were the result of a site-of-contact mechanism of action, the data are more supportive of a systemic, genotoxic mechanism mediated through metabolites of MC. In addition, the liver tumors are clearly not the result of a site-of-contact carcinogen because the liver is not a site of contact during inhalation bioassays.
Several commenters [Exs. 19-26, 19-28, 19-29, 19-45, 19-48, 19-57, 19-59, 25-E, 25-I] suggested using PBPK modeling to estimate target tissue dose and to account for differences in metabolism at high and low doses and differences in metabolism of MC across species. OSHA compared three sets of parameters in the PBPK models submitted by interested parties to adjust the dose across species and across doses. The risk estimates for those models (using the MLE of the multistage model parameters) ranged from 0.43 to 0.91 deaths per 1000 after lifetime occupational exposure to 25 ppm. Mr. Clewell's risk estimate (0.91/1000 MLE), unlike the other PBPK analyses, represent the upper 95th percentile of the Monte Carlo distribution of GST metabolites as input into the multistage model. The Monte Carlo simulation takes into account the assumed distribution of values for each parameter, including the parameters used to estimate human metabolism of MC. The other PBPK models used point estimates instead of distributions for the PBPK parameters, and therefore it is not known whether these are central estimates or upper bounds. OSHA agrees that the distributional approach used by Clewell is a reasonable way to account for the uncertainty and variability inherent in PBPK modeling, and that uncertainty and variability must be considered in any useful risk assessment. The Agency has used the upper 95th percentile on the distribution of GST metabolites from the Bayesian modeling, coupled with the MLEs of the multistage model parameters, for its final estimates of MC risk.
OSHA has concluded that all the risk estimates presented above support an 8-hour TWA PEL of 25 ppm or lower. The risks estimated from the PBPK models were less than an order of magnitude different from estimates of risk based on applied dose methodology. Either with or without PBPK modeling , the estimates of risk at 25 ppm clearly indicate a significant risk.
The risks estimated from these PBPK models and ppm-to-ppm extrapolation offer a range of risks which might be expected after lifetime occupational exposure to MC. OSHA has assessed these models and has decided to modify and expand on the submitted PBPK and uncertainty analyses in its final estimates of cancer risk, in order to give full consideration to all of the available data. This analysis is presented in the next section.
F. OSHA's PBPK Analysis and Final Risk Estimates
In developing an approach to PBPK modeling for MC, OSHA wished to use all of the available, appropriate and quantifiable biochemical and physiological data in its PBPK modeling and in assessing the uncertainty and variability in model parameters. The Agency determined that this approach would provide the best characterization of the variability and uncertainty in the data and the model. In addition, incorporation of as much of the available data as possible should give the most realistic PBPK model, and in turn, the most realistic risk estimate. Before development of OSHA's PBPK model, Clewell's approach (described above) was the most comprehensive pharmacokinetic approach submitted to the Agency. It addressed many of the issues of concern to the Agency, and OSHA believes that Clewell's approach was a reasonable template for using PBPK in risk assessment. However, since Clewell's work was done, PBPK modeling has continued to advance. Therefore OSHA modified Clewell's model to accommodate these advances and to allow incorporation of additional biochemical and physiological data that had been added to the rulemaking record. The following is a summary of OSHA's final (revised) PBPK analysis. A more detailed discussion can be found in the reports submitted to the Agency, reflecting OSHA's analysis in which the Agency was assisted by contractors [Ex. 128].
1. Review of Clewell's PBPK Analysis
a. Clewell's analytical approach. Clewell et al. [Ex. 96] employed Monte Carlo techniques to investigate imprecision in estimates of human health risk from occupational exposure to MC, as a function of imprecision in parameter values of the PBPK and dose-response models. (As described below, OSHA and its contractors believe that Clewell et al. did not correctly parse out uncertainty and variability, so their analysis is described as accounting for "imprecision" rather than uncertainty or variability). In the Clewell et al. analysis, probability distributions were specified for each PBPK model parameter in an attempt to characterize imprecision. Computer-based techniques were used to obtain pseudorandom samples from these statistical distributions, generating multiple sets of model parameter values. These sets of parameter values were then used to obtain a corresponding distribution of PBPK model predictions of various measures of internal dose for a simulated animal bioassay (e.g., GST metabolism in lungs of mice exposed to 2000 ppm and 4000 ppm for 6 hrs/day, 5 days/wk). The mean of the mouse internal dose distribution was used as the dose input to obtain the MLE and UCL on the multistage model parameters, using the tumor incidence data from the NTP bioassay. The multistage model was run a second time using the upper 95th percentile of the mouse internal dose distribution as the dose input to obtain the MLE and UCL on the multistage model parameters. This yielded a total of four estimates of the parameters q(o), q(1), and q(2) of the mouse dose-response function: 1) Mean of internal dose distribution/MLE of multistage model parameters; 2) Mean of internal dose distribution/UCL of multistage model parameters; 3) Upper 95th percentile of internal dose distribution/MLE of multistage model parameters; and 4) Upper 95th percentile of internal dose distribution/UCL of multistage model parameters.
Each set of dose-response parameters obtained from the analysis of the mouse data was then used to calculate human risk estimates. The upper 95th percentile of the human internal dose distribution was used to calculate the dose surrogate at 25 ppm, 8 hr/d exposure and then substituted into the MLE and UCL of the multistage parameters to obtain the MLE and UCL estimates of risk. Similarly the mean of the human internal dose distribution was used in conjunction with the MLE and UCL of the multistage model parameters. Therefore, four human risk estimates were generated, based on the distribution of human internal doses and the dose-response function derived from the multistage analysis of the NTP mouse bioassay. The four human risk estimates are: 1) upper 95th percentile of the human internal dose distribution/MLE of the multistage model parameters; 2) mean of human internal dose distribution/MLE of the multistage model parameters; 3) upper 95th percentile of the human internal dose distribution/UCL of the multistage model parameters; and 4) mean of the human internal dose distribution/UCL of the multistage model parameters.
A major finding of that analysis was that the mean estimate of added cancer risk for occupational exposure at the proposed PEL of 25 ppm based on the PBPK-derived GST-lung dose surrogate (PBPK(mean)/potency(MLE) = 0.39 x 10 (-3)) was 6-fold lower than the corresponding OSHA estimate (MLE = 2.32 x 10 (-3)) based on administered dose scaled to body weight. The 95 percentile upper bound estimate of risk using the same PBPK distributions and the distribution of 95%UCLs on carcinogenic potency (PBPK(95%)/potency(95%) = 1.56 x 10 (-3)), was nearly 2-fold less than OSHA's 95%UCL on risk (2.97 x 10 (-3)).
b. Clewell's PBPK model. The PBPK model used by Clewell et al. in performing their Monte Carlo analysis was slightly modified from the PBPK model developed by Andersen et al. and submitted to OSHA by HSIA [Ex. 328]. The primary modification was the addition of a separate compartment for the GI-tract. The general structure of this model has received considerable use by PBPK modelers. Nevertheless, there were several deficiencies in this model and in the subsequent statistical analysis that the Agency believed warranted major modification. These are described in the following section.
c. Prior distributions for model parameters. Truncated normals were used as the form for all probability distributions except for metabolic constants, which were described by truncated lognormals. All distributions were truncated to prevent sampling of nonsensical values (e.g., negative values). Truncation in some instances was 2 standard deviations (SDs) from mean values, in others more than 4 SDs.
A variety of sources of information were used as a basis for the probability distributions of the PBPK parameters in Clewell's model: literature summaries for most physiologic and anatomic parameters, direct laboratory measurement of partition coefficients based on vial equilibration studies, and statistical regression analyses of experimental data for fitted metabolic constants.
Clewell et al. stated that the focus of their analysis was on characterizing the effect of "uncertainty" in parameter values on uncertainty in PBPK model predictions, uncertainty being defined as the possible error in estimating the "true" value of a parameter for a representative "average" animal. To maintain consistency with a focus on investigating effects of parameter uncertainty, a logical choice would have been to center their probability distributions using estimates of mean values for all model parameters and to use the standard error of the mean (SEM) to characterize dispersion. It it unclear whether this was done for blood flows, tissue volumes, inhalation rates or cardiac output, since Clewell et al. appear to have relied extensively on an unpublished review of scientific literature performed by S. Lindstedt for the ILSI Risk Science Institute Physiological Parameter Working Group.
Based on Clewell's comments accompanying his PBPK model, it appears that standard errors were not used to characterize variability among individual replicates of measured equilibrium partition coefficients; instead, standard deviations were used. Nor does it appear that Clewell et al. consistently made use of standard errors in characterizing imprecision in their fitted metabolic constants. Inspection of the joint confidence region for their fitted estimates of mouse VmaxC and Km (for the MFO pathway), shown in Figure 6 of Ex. 399, suggest coefficients of variation (%CVs) for VmaxC of about 2%. Similarly, for KfC, the %CV in the fitted MLE appears to be about 3%. These %CVs are considerably smaller than the assumed values of 20% and 30%, respectively, used by Clewell et al. in their Monte Carlo analysis. On the other hand, their %CV for Km does coincide with that indicated by the joint confidence regions. One should also note the high degree of correlation among the fitted values for VmaxC and Km.
In assessing variability in the ratio of in vitro MFO and GST metabolism in lung versus liver tissue (i.e., the A1 and A2 parameters), Clewell et al. used the in vitro MC metabolism data of Reitz et al. (1989). Yet it appears that the %CV for these data is 24% when one uses SDs among replicates for MFO metabolism in lung and liver of mice. This is substantially less than the 50% assumed by Clewell. One obtains a %CV of 9% when using SEMs.
It appears then, that some of the probability distributions used by Clewell et al. reflect variability beyond that readily identifiable as uncertainty in estimates of sample means. It may be that Clewell made a subjective inflation of variances. Though ad hoc, inflating variances would not be unreasonable given the sparse data on certain model parameters. Another possibility is that the distributions reflect variability due to both uncertainty and intersubject heterogeneity -- another reason to inflate variances, or alternately, use SDs rather than SEMs to describe the distributions of the parameters. If so, then it might be more appropriate to view the proportion of simulated estimates of risk that fall within a specified interval as the probability that the true risk for a randomly selected individual is in that interval. Yet strictly speaking this would require that the probability distributions reflect both the full range of uncertainty and heterogeneity in the population of interest, with the latter being unlikely based on inspection. If the analysis only considered imprecision due to uncertainty, as suggested in Clewell et al., then the resulting distribution should instead be viewed as describing the uncertainty in risk for a hypothetical "average" individual.
2. OSHA's Modifications to PBPK Analysis
a. Basis for modifying approach of Clewell et al. In addition to the likelihood that Clewell et al. used broader distributions than those necessary to model uncertainty in the PBPK analysis (as opposed to modeling some hybrid of uncertainty and variability), the analytical approach they used (1992 and 1993) also has two well-known methodological limitations. Their representation of imprecision in fitted parameters (e.g., VmaxC, Km, KfC) is problematic because they estimated the variability in these parameters by optimizing the model fit to in vivo data, while assuming nominal values for all other model parameters. However, the organ volumes, blood flows, and partition coefficients for the mice used in the gas uptake studies and the humans used in the open chamber studies are clearly not known with exact precision, and are not, therefore, accurately represented by nominal values. Consequently, the variances of the fitted parameters will be underestimated with this approach, since full acknowledgment of variability in other model parameters will have been ignored. Furthermore, it is quite likely that the joint parameter space for fitted PBPK model parameters will exhibit a considerable degree of correlation. Importantly, failure to account for such covariances when performing Monte Carlo sampling may overstate variance in some model predictions by assuming independence where it does not exist. The implications of these methodological limitations on predicted risk are unclear, since they would seem to exert countervailing effects on estimating uncertainty. Thus, OSHA decided that it was important to perform an analysis that addressed these limitations. The use of a Bayesian statistical framework provided a means of overcoming the above limitations.
b. Bayesian Approach. A Bayesian analysis allows the logical combination of two forms of information: "prior knowledge" about parameter values drawn from the scientific literature, and data from experimental studies (e.g., the mouse gas uptake studies, or, for humans, the open chamber experiments performed by Dow Chemical company), all within the context of a PBPK model. Clearly, neither prior information about parameter values nor experimental data alone are capable of precisely determining all parameter values in the PBPK model. If prior information were sufficient, the additional experiments performed by Clewell et al. and Dow Chemical Co. would not have had to be done. But the available experimental data alone are insufficient to pin down all parameters of the model to reasonable values (which is why no attempt was made to simultaneously optimize all PBPK parameters to data). Fitting only two or three parameters while holding others constant so as to reduce dimensionality leads to the biases and underestimation of variance mentioned above.
A second feature of this Bayesian approach is that it yields distributions for all of the PBPK model parameters together with information about their entire joint covariance structure. Thus, the Bayesian analysis outputs distributions of parameter values that are consistent with both all the available data as well as the prior information. It is then possible to use samples from the joint posterior distribution of the parameters to simulate formation of GST metabolites in lung tissue from different species and cancer risk, therefore producing posterior distributions for these endpoints. It should be noted that if no data are available (or if the data are not informative as to the likely value of the parameter), the posterior distribution is equivalent to the prior distribution and this approach is then equivalent to the standard Monte Carlo sampling from the prior distribution, as in Clewell et al. Alternately, Bayesian updating with a uniform prior distribution (i.e., complete ignorance about plausible values) used in conjunction with data leads to a posterior distribution proportional to the distribution of the data. The most important applications of the Bayesian approach arise when informative (e.g., physiological, anatomical) prior distributions exist, in parallel with experimental metabolic data. This is now the case with PBPK modeling of MC. In this case, Bayesian modeling results in all the information content of both prior distributions of parameter values and metabolic data being incorporated in the posterior distribution of parameter values, which will have reduced variance compared to the prior distribution. Distributions of parameter values for both human and mouse PBPK models, and the multistage cancer model, were determined with this technique.
c. PBPK Model Modifications. OSHA's final risk estimates were based on the Bayesian analysis described here. The Clewell model formed the structural core of the analysis, although five additional structural modifications were made as described below. These modifications were necessary to make the PBPK model more physiologically realistic:
(1) Bone marrow was treated as a separate compartment. In the Clewell model (as in many PBPK models), bone marrow tissue was combined with other tissues into a (presumably) kinetically homologous compartment. Based on blood perfusion rates, a reasonable choice would be to place marrow in the well-perfused tissue compartment. However, if the physicochemical affinity of the compartment is considered, it makes more sense to place marrow in the adipose tissue compartment, since red marrow (at least in humans) has a fat content of about 40% and yellow marrow has a fat content of 80%. In comparison, liver, brain, kidney and heart all have fat contents (in humans) well under 20%. In addition, bone marrow accounts for a significant percentage of body weight and receives a substantial fraction of cardiac output. Therefore, a strong argument can be made for treating bone marrow as a separate compartment, as OSHA has done here.
(2) Partitioning MFO and GST metabolism between the lung and liver. Clewell made the MFO and GST metabolic constants for lung dependent on the fitted constants for the liver, so as to reduce the number of fitted parameters to be simultaneously estimated from rodent and human in vivo data. For example, A1 is defined as the ratio of lung to liver in vitro MFO enzymatic activity, normalized to microsomal protein,
| a1 | = | nmol DCM oxidized/min/mg lung microsomal protein ------------------------------------------------- nmol DCM oxidized/min/mg liver microsomal protein |
| Similarly, A2 is the ratio of lung to liver in vitro GST enzymatic activity, normalized to cytosolic protein, | ||
| a2 | = | nmol DCM conjugated/min/mg lung cytosolic protein ------------------------------------------------- nmol DCM conjugated/min/mg liver cytosolic protein |
This assumes that lung and liver have equivalent mg protein per mg tissue contents. Yet the data of Litterst et al. (1973) argue against such an assumption. Litterst et al. measured microsomal protein and soluble protein in lung and liver tissues of mice, rats, hamsters, guinea pigs and rabbits. These data indicated ratios of mg microsomal protein content of lung versus liver tissue of less than 0.3, and a similar ratio for soluble protein of about 0.7. Thus, some adjustment of the constants A1 and A2 are required.
The equations used to compute a lung Vmax for the MFO pathway and a lung Kf for the GST pathway from a liver Vmax and Kf were thus modified to include an additional proportionality factor to account for differences in microsomal and cytosolic protein content of lung and liver tissue. Specifically,
Vmax(lung.MFO) = Vmax(liver.MFO) x [V(lung)/V(liver)] x A1 x B1
where B1 is the ratio of [mg microsomal protein per mg of lung tissue] to the same measure for liver tissue. A geometric mean and geometric standard deviation for B1 were derived from the data of Litterst et al. (1973) to use as input in the Bayesian prior distribution for this parameter. Notably, accounting for this difference in protein content leads to a proportionality factor approximately four-fold less than that used by the Clewell et al. (i.e., A1 x B1 = 0.41 x 0.27 = 0.11).
Similarly, for Kf(lung.GST),
Kf(lung.GST) = Kf(liver.GST) x A2 x B2
Here too, the data of Litterst et al. (1973) were used to compute a ratio of mg soluble protein per mg lung to the same measure for liver, yielding a mean value of 0.68 for B2. For a human B2, the average of the ratios computed for mice, rats, hamsters, guinea pigs, and rabbits as per Litterst et al. (1973) was used.
(3) Linkage of alveolar ventilation to cardiac output. In recognition of OSHA's interest in occupational exposures, Clewell used values of cardiac output and alveolar ventilation rates consistent with the performance of light work. However, they did not account for the altered distribution of regional blood flows known to occur in response to increases in work intensity [Exs. 7-115, 7-120, 21-81], as was done in subsequent MC PBPK work by Dankovic and Bailer [Ex. 23-18] (1994). In the latter analysis, alveolar ventilation (QP) was made dependent on cardiac output (QC) by making QP = QC x VPR, where VPR is the ventilation-perfusion ratio. VPR was treated as a random variable with an assigned prior probability distribution.
(4) Linkage of work intensity to changes in physiology. Cardiac output, ventilation perfusion ratio, and percent of cardiac output delivered to tissues were made dependent on work intensity. Using the data of Astrand (1983) [Ex. 21-81] -- and similar to what was done by Dankovic and Bailer (1994) [Ex. 23-18] -- slope factors were derived to describe change in flows per change in work intensity as measured in watts. These slope factors were then used to modify resting flows for varying levels of work intensity. This approach was taken so that the influence of variability in work load (i.e, work load was treated as a random variable) -- with concomitant adjustments to regional blood flows and ventilation rate -- on delivered dose could be modeled.
(5) Maintaining mass balance in sampling of fractional blood flows and compartment volumes. Monte Carlo sampling of fractional quantities such as the proportion of cardiac output delivered to different compartments, or the proportion of body weight represented by a given compartment, requires the imposition of some type of constraint to prevent random sampling leading to summed proportions greater than the whole (and thus causing nonsensical departures from mass balance). The following constraint was imposed: VppC = 0.82 - deltaViC 's (0.82 is a nominal value for the fraction of body weight absent bone, blood, and stomach and intestinal contents), QwpC = 1 - deltaQiC 's (in the mouse model), and QppC = 1 - deltaQiC 's (in the human model). The use of either QwpC or QppC as the quantity to be made dependent on the other fractional flows has biological appeal -- one expects that higher fractional blood flow to the poorly-perfused compartment (i.e., muscle and skin) should be accompanied by a lower fractional flow to the well-perfused compartment, and vice versa. The choice of QwpC versus QppC as the one to be made dependent on others appeared to be unimportant in work with the mouse model. The choice was important in work with the human model. Here it was necessary to choose QppC, because of its large variance relative to QwpC (i.e., since QppC cannot be estimated precisely, it makes sense to let our greater knowledge of the other fractional flows inform us about plausible values of QppC).
The above approach modifies the approach taken by Clewell et al. [Ex. 96]. Their approach was to randomly draw from the distributions for cardiac output and all fractional flows, use the random draws to compute the absolute flows to the individual compartments, and then to sum the individual flows to make a new cardiac output value for use in the simulation. On the other hand, OSHA's final analysis avoided arbitrarily modifying the prior distribution for cardiac output (which happens to be one of the relatively well-known parameters). Furthermore, Clewell did not make the fractional flows dependent on one another.
d. Prior Probability Distributions. A skewed, lognormal-like distribution is generally observed for biological parameters. However, most, if not all, parameters are also positive and have physiological bounds. Thus, truncated lognormal distributions of the parameter values were used in this analysis. They do not differ appreciably from normal distributions for small values of the variance.
In specifying prior distributions an attempt was made to characterize the variability of the mean parameter values for small groups of rodents and humans. This focus was adopted to make the prior distribution congruent with the data sets available for Bayesian analysis. For example, the rodent gas uptake data represent the aggregate pharmacokinetic behavior of groups of 5 mice. Prior distributions were therefore constructed to reflect the degree of variability in mean physiological and anatomical PBPK parameters for small groups of mice. A similar approach was taken in defining prior distributions for human physiologic and anatomic parameters, since the available experimental data reflected the averaged pharmacokinetic behavior of 6 subjects. In practice, this meant amassing studies reporting mean values for certain PBPK parameters (e.g., tissue weights, blood flows, cardiac output, minute ventilation), and then using these means as data for computing a geometric mean (GM) and geometric standard deviation (GSD) with which to estimate the parameter values for the truncated lognormal distributions. Sampling of all lognormal distributions was truncated at 2 GSDs, with one exception. Truncation of the blood:air partition coefficient was extended to 3 GSDs based on results from preliminary runs.
Table VI-5 presents a summary of the prior probability distributions used in the Bayesian fitting of the mouse and human data sets. The prior distributions for metabolic constants to be estimated from in vivo data were made very broad (i.e., assigned a GSD of 10) to reflect our ignorance of these values before examining the data. Similarly, the prior distributions for parameters of the multistage cancer model were broad uniform distributions, constrained to be positive, as required by the standard model.
| - | Parameter | Mouse priors | |
|---|---|---|---|
| - | - | GM | GSD |
| Flows: | |||
| QCC | cardiac Output (l/hr/kg -- BW) | (a)34.8 | 1.14 |
| VPR | alveolar Ventilation Perfusion Rate | (b)1.22 | 1.95 |
| Tissue Blood Flows (fraction of cardiac output): | |||
| QgiC | GI Tract | 0.165 | 1.30 |
| QliC | Liver | 0.017 | 1.20 |
| QfatC | Fat | 0.047 | 1.60 |
| QppC | Poorly Perfused Tissues | 0.276 | 1.25 |
| QwpC | Well Perfused Tissues | (c)0.369 | 1.10 |
| QmarC | Bone Marrow | 0.089 | 1.60 |
| Tissue Volumes (fraction of body weight): | |||
| VgiC | GI Tract | 0.035 | 1.30 |
| VliC | Liver | 0.045 | 1.20 |
| VfatC | Fat | 0.077 | 1.40 |
| VppC | Poorly Perfused Tissues | (c)0.556 | 1.10 |
| VwpC | Well Perfused Tissues | 0.065 | 1.15 |
| VluC | Lung | 0.008 | 1.30 |
| VmarC | Bone Marrow | 0.033 | 1.50 |
| Equilibrium Partition Coefficients: | |||
| Pblo | Blood:Air | 13.7 | 1.80 |
| Pgi | GI Tract:Air | 10.5 | 1.20 |
| Pli | Liver:Air | 22.9 | 2.00 |
| Pfat | Fat:Air | 98.2 | 1.40 |
| Ppp | Poorly Perfused Tissues:Air | 9.5 | 1.30 |
| Pwp | Well Perfused Tissues:Air | 10.2 | 1.20 |
| Plu | Lung:Air | 10.0 | 1.30 |
| Pmar | Bone Marrow:Air | 62.0 | 1.60 |
| Metabolic Parameters: | |||
| VmaxC | Maximum metabolic velocity of MFO saturable pathway (mg/hr/ kg -- liver) | 750 | 10.00 |
| KM | affinity of MFO saturable pathway (mg/l) | 1.35 | 10.00 |
| KFC | First order rate constant for GST pathway (l/hr/kg-0.25) | 1.5 | 10.00 |
| a1 | Ratio of lung to liver in-vitro MFO metabolic velocities (nmol/min/gm -- lung -- micros.Prot)/ (nmol/ min/gm -- liver -- micros.Prot) | 0.405 | 1.67 |
| a2 | Ratio of lung to liver in-vitro GST metabolic velocities (nmol/min/gm -- lung -- cytos.Prot)/ (nmol/ min/gm -- liver -- cytos.Prot) | 0.282 | 1.67 |
| B1 | Ratio of lung and liver tissue content of microsomal protein | 0.271 | 1.25 |
| B2 | Ratio of lung and liver tissue content of cytosolic protein | 0.721 | 1.25 |
| Sp -- Kf | allometric scaling power for body weight scaling of KFC from mice to humans | ||
| ROWSPAN=2>Parameter | Human priors | ||
| GM | GSD | ||
| Flows: QCC | cardiac Output (l/hr/kg -- BW) | 4.2 | 1.10 |
| VPR | alveolar Ventilation Perfusion Rate | 1.35 | 1.15 |
| Tissue Blood Flows (fraction of cardiac output): | |||
| QgiC | GI Tract | 0.191 | 1.25 |
| QliC | Liver | 0.067 | 1.20 |
| QfatC | Fat | 0.057 | 1.45 |
| QppC | Poorly Perfused Tissues | 0.198(c) | 1.55 |
| QwpC | Well Perfused Tissues | 0.443 | 1.25 |
| QmarC | Bone Marrow | 0.044 | 1.70 |
| Tissue Volumes (fraction of body weight): | |||
| VgiC | GI Tract | 0.017 | 1.10 |
| VliC | Liver | 0.026 | 1.10 |
| VfatC | Fat | 0.204 | 1.20 |
| VppC | Poorly Perfused Tissues | 0.470(c) | 1.15 |
| VwpC | Well Perfused Tissues | 0.044 | 1.10 |
| VluC | Lung | 0.008 | 1.15 |
| VmarC | Bone Marrow | 0.050 | 1.10 |
| Equilibrium Partition Coefficients: | |||
| Pblo | Blood:Air | 8.4 | 1.30 |
| Pgi | GI Tract:Air | 8.1 | 1.60 |
| Pli | Liver:Air | 9.9 | 1.60 |
| Pfat | Fat:Air | 97.6 | 1.25 |
| Ppp | Poorly Perfused Tissues:Air | 6.8 | 1.60 |
| Pwp | Well Perfused Tissues:Air | 7.6 | 1.40 |
| Plu | Lung:Air | 7.6 | 1.50 |
| Pmar | Bone Marrow:Air | 48.8 | 1.60 |
| Metabolic Parameters: | |||
| VmaxC | Maximum metabolic velocity of MFO saturable pathway (mg/hr/ kg -- liver) | 75 | 10.00 |
| KM | affinity of MFO saturable pathway (mg/l) | 0.6 | 10.00 |
| KFC | First order rate constant for GST pathway (l/hr/kg-0.25) | Mouse | Mouse post.(d) |
| a1 | Ratio of lung to liver in-vitro MFO metabolic velocities (nmol/min/gm -- lung -- micros.Prot)/ (nmol/ min/gm -- liver -- micros.Prot) | 0.0045 | 4.50 |
| a2 | Ratio of lung to liver in-vitro GST metabolic velocities (nmol/min/gm -- lung -- cytos.Prot)/ (nmol/ min/gm -- liver -- cytos.Prot) | 0.122 | 3.60 |
| B1 | Ratio of lung and liver tissue content of microsomal protein | 0.297 | 1.10 |
| B2 | Ratio of lung and liver tissue content of cytosolic protein | 0.807 | 1.20 |
| Sp -- Kf | allometric scaling power for body weight scaling of KFC from mice to humans | -0.272(e) | 0.08(e) |
| Notes: (a) value computed for 0.025 kg mouse, 70 kg human; (b) unitless; (c) prior distribution not used, fractional flow made functionally dependent on others (see text); (d) human prior set equal to mouse posterior; (e) mean and standard deviation of a truncated normal distribution | |||
While it is desirable to separate variability into components reflecting pure uncertainty (e.g., measurement error) versus interindividual heterogeneity and to propagate them separately, it is necessary to build from the start an adequate statistical model. The problem here is complicated by the fact that both the rodent and human in vivo data used for estimating metabolic constants reflected either aggregated or averaged pharmacokinetic behavior. Thus the prior distributions and the statistical model used here aggregate variability due to both finite precision in measured values and heterogeneity among average values for small groups of rodents or humans; they do not, it must be emphasized, reflect heterogeneity among the individual humans in a large, representative population.
e. In Vivo Rodent and Human data. Bayesian updating of the distributions was performed using the same data sets used by Clewell et al. to obtain fitted estimates of mouse and human metabolic constants; namely, gas uptake studies with mice with or without pretreatment with a MFO inhibitor and the human open chamber inhalation studies. All mouse gas uptake studies were conducted with 5 female mice in a single chamber. Thus, measured observations of decline in chamber concentration of MC represent the aggregate pharmacokinetic behavior of groups of 5 animals.
The human in vivo data were obtained from Tables 2 and 3 in Andersen et al. (1991) [Ex. 21-94]. Briefly, these data represent exhaled breath and venous blood concentrations of MC for 6 male human volunteers exposed to MC concentrations of 100 or 350 ppm for a period of 6 hours. These data have only been reported as means and standard deviations of the six subjects, which is unfortunate. Thus, the available data reflect the average pharmacokinetic behavior of the 6 subjects. When simulating the human data reported in Andersen et al. (1991), the work load was assumed to be zero watts (rest) and the averaged body weight of the 6 subjects was assumed to be known without error (86 kg).
f. Simulating the Rodent Bioassay and Human Occupational Exposure. Distributions for GST metabolism in the lungs of mice exposed to 2000 ppm or 4000 ppm exposures, for 6 hrs/day and 5 days/week, were obtained by simulating these two exposures (the ones used in the NTP bioassay) with 5000 realizations drawn from the joint posterior distribution of the mouse PBPK parameters.
The quantity of metabolites formed during the 4th week (dynamic equilibrium reached) was divided by 7 to give an average measure per day. For use as an input dose to the multistage model, these posterior distributions were approximated by truncated lognormals.
The same set of 5000 parameter vectors was used to simulate both 2000 and 4000 ppm MC exposures. The control dose was always assumed to be 0. Thus, a 5000-by-3 matrix of doses was generated, where the three column vectors represent different realizations of a particular dose group (0, 2000 and 4000 ppm MC) and the row vectors represent different realizations of bioassay doses.
This method of using the joint posterior distributions for the two doses in the mouse bioassay implies certain assumptions about the uncertainties. Most importantly, this approach (referred to in this document as the "dependence case") assumes that the posterior distributions primarily reflect uncertainty about a single average value equally applicable to all groups of approximately 50 mice (i.e., it assumes groups of 50 mice will have the same "average" physiological, anatomical, physicochemical and metabolic attributes, and that these average values are simply known to us with uncertainty). An alternative would be to model the "independence case" by using a different random draw from the vector of PBPK parameters for one dose group than for the other. This approach assumes that the posterior distributions primarily reflect heterogeneity in the average attributes of groups of 50 rodents. Under the dependence case, estimates of metabolized dose for the two exposures would tend to move in tandem for a given simulation (i.e., when one dose is estimated to be low relative to its average, so is the other; likewise, when one is high, so is the other), and in principle would therefore exhibit less variability in dose-response shape (e.g., linear, sublinear, supralinear).
It appears that the dependence case is more reasonable than the independence case, by appealing to biological theory and by examining the results of the sensitivity analysis conducted as part of this risk assessment. The sensitivity analysis showed that predicted mouse GST metabolism at 2000 ppm was most sensitive to variation in the model parameter A2. Variability in A2 was primarily a consequence of uncertainty in using an in vitro ratio of enzymatic activity to make inferences about an in vivo ratio. Therefore, uncertainty rather than heterogeneity seems to dominate the distribution of mouse GST metabolism estimates. Besides, laboratory rodents have a carefully controlled genetic makeup, primarily so that they will differ little from each other physiologically; thus, groups of 50 rodents should have extremely similar average characteristics (the variance of the mean of a characteristic within a group of 50 rodents will be approximately 50 times smaller than the (already small) inter-individual variance). OSHA has determined that this reasoning supports use of the dependence case in this analysis. (Note that the excess risk estimates using the dependence case are only about a factor of 1.5 higher than those using the independence case).
Five human occupational exposures were simulated: constant exposure to 10, 25, 50, 100 or 500 ppm MC for 8-hrs per day and 5 days per week. Simulations were made up to 4 weeks of work, at which a dynamic equilibrium was reached, and as with mice, were performed using 5000 parameter human vectors drawn from their joint posterior distribution, augmented by allowing for additional variability in human body weight and work intensity (the latter linked to changes in cardiac, ventilation-perfusion and regional blood flow as described above).
g. Sensitivity Analysis. The influence of variability in mouse and human PBPK model parameters on variability in predicted mouse and human GST lung metabolism was assessed by computing pairwise correlation coefficients using each parameter vector (i.e., the marginal posterior distribution) and the corresponding vector of model predictions. For mice, the sensitivity to predicted GST -- lung metabolism in the simulated 2000 ppm bioassay dose group was evaluated. For humans, predicted GST -- lung metabolism for an occupational exposure to 25 ppm was considered. Pairwise correlation coefficients were computed using 5000 parameter vectors drawn from the joint posterior distribution and the associated model output vector.
Table VI-6 presents the results from the sensitivity analysis. The strongest pairwise correlation between predicted lung GST metabolism and any input parameter, for either mouse or human simulations, was A2. For the mouse simulation of a 2000 ppm exposure, B2 gave the next strongest pairwise correlation. The mouse parameters QlivC, VlivC, VmaxC, Pfat and QppC all exhibited more moderate (though not negligible) correlations. For the human occupational simulation, the parameters KfC, VmaxC, Sp--Kf, and B2 all exhibited moderate pairwise correlations with human lung GST metabolism. For both mice and human sensitivity analyses, there were a half-dozen or more parameters exhibiting weak (r between 0.1 and 0.2) correlations. It is important to note that all parameters are further correlated via their posterior joint distribution function. This explains why the sum of the regression coefficients (i.e., squares of the correlation coefficients) is greater than 1. Thus considerable care should be exercised in quantitatively estimating the ability of variability in any input parameter to explain variability in predicted GST metabolism, especially among parameters with similar pairwise correlation coefficients.
| Mouse 2000 PPM | Human 25 PPM | ||
|---|---|---|---|
| Parameter | correlation coefficient (r) | Parameter | correlation coefficient (r) |
| a2 | 0.860 | a2 | 0.850 |
| B2 | 0.530 | KfC | 0.315 |
| QliC | 0.335 | VmaxC | -0.291 |
| VliC | -0.248 | Sp -- Kf | 0.232 |
| VmaxC | -0.229 | B2 | 0.221 |
| Pfat | -0.203 | Pmar | -0.183 |
| QppC | -0.202 | QfatC | 0.180 |
| VPR | 0.193 | B1 | 0.179 |
| Pli | -0.173 | VliC | 0.161 |
| a1 | -0.149 | VmarC | 0.146 |
| QgiC | -0.145 | Work | 0.142 |
| Pmar | 0.144 | QwpC | 0.141 |
| VwpC | -0.121 | VfatC | 0.136 |
| KfC | 0.120 | QmarC | 0.136 |
| Pwp | -0.106 | Km | -0.095 |
| VluC | -0.120 | QC | -0.083 |
| B1 | -0.093 | QliC | -0.083 |
| QmarC | -0.083 | a1 | -0.071 |
| Ppp | -0.076 | QgiC | -0.065 |
| VgiC | 0.074 | Pfat | -0.061 |
| Pgi | 0.054 | Pwp | -0.058 |
| QC | -0.049 | VluC | -0.052 |
| BW | -0.042 | Pgi | -0.050 |
| Plu | 0.039 | VwpC | 0.041 |
| Km | -0.035 | Pblood | 0.039 |
| tVmaxC | 0.024 | dVPR/dW | 0.039 |
| QfatC | 0.020 | BW | -0.038 |
| Pblood | 0.019 | dQli/dW | -0.033 |
| VfatC | -0.013 | Plu | 0.023 |
| Vmar | -0.007 | Ppp | 0.021 |
| dQfat/dW | 0.016 | ||
| VgiC | -0.012 | ||
| Pli | -0.010 | ||
| dQgi/dW | -0.010 | ||
| dQmar/dW | -0.009 | ||
| VPR | 0.006 | ||
| dQC/dW | -0.000 | ||
| dQwp/dW | -0.000 | ||
h. Posterior PBPK Parameter Distributions. Table VI-7 lists the posterior distributions for mouse PBPK parameters obtained by Bayesian updating of the prior distributions using the available gas uptake data. Comparison of the prior and posterior probability distributions reveals that the gas uptake data retain considerable influence on the distributions of many of the important PBPK model parameters. Medians of the posterior distributions for VPR, Qfat, Pblood, Pmar, Km, A1, and A2 were all appreciably different than the medians for their corresponding prior distributions. Percent CVs for nearly all posterior distributions were considerably smaller than those of their prior distributions. As expected, the marginal variances for the metabolic constants were considerably greater than what was obtained under nonlinear maximum likelihood regression analysis with all other model parameters fixed at nominal values.
| - | Parameter | Central tendency | Maximum posterior | Variablility | ||
|---|---|---|---|---|---|---|
| Prior median | Posterior median | Prior%CV | Posterior%CV | |||
| Flows: | ||||||
| QCC | cardiac output (l/hr/kgBW) | 34.8 | 34.4 | 37.6 | 18 | 9 |
| VPR | alveolar Ventilation Perfusion Ratio | 1.22 | 1.59 | 1.49 | 75 | 14 |
| Tissue Blood Flows (fraction of cardiac output): | ||||||
| QgiC | GI Tract | 0.165 | 0.140 | 0.175 | 26 | 16 |
| QliC | Liver | 0.017 | 0.020 | 0.017 | 19 | 16 |
| QfatC | Fat | 0.047 | 0.090 | 0.098 | 43 | 19 |
| QppC | Poorly Perfused Tissues | 0.276 | 0.290 | 0.243 | 22 | 18 |
| QwpC | Well Perfused Tissues | 0.369 | (a)0.360 | 0.378 | (a) | |
| QmarC | Bone Marrow | 0.089 | 0.100 | 0.090 | 51 | 27 |
| Tissue Volumes (fraction of body weight): | ||||||
| VgiC | GI Tract | 0.035 | 0.040 | 0.038 | 26 | 22 |
| VliC | Liver | 0.045 | 0.050 | 0.050 | 18 | 12 |
| VfatC | Fat | 0.077 | 0.070 | 0.055 | 35 | 24 |
| VppC | Poorly Perfused Tissues | 0.556 | (b)0.540 | 0.569 | (b) | |
| VwpC | Well Perfused Tissues | 0.065 | 0.070 | 0.065 | 14 | 12 |
| VluC | Lung | 0.008 | 0.010 | 0.007 | 27 | 22 |
| VmarC | Bone Marrow | 0.033 | 0.040 | 0.037 | 42 | 29 |
| Equilbrium Partition Coefficicients: | ||||||
| Pblo | Blood:Air | 13.7 | 18.5 | 13.1 | 66 | 18 |
| Pgi | GI Tract:Air | 10.5 | 11.3 | 9.5 | 19 | 17 |
| Pli | Liver:Air | 22.9 | 28.2 | 23.9 | 79 | 32 |
| Pfat | Fat:Air | 98.2 | 100.5 | 106.7 | 35 | 21 |
| Ppp | Poorly Perfused Tissues:Air | 9.5 | 12.1 | 13.1 | 27 | 17 |
| Pwp | Well Perfused Tissues:Air | 10.2 | 10.4 | 10.3 | 19 | 16 |
| Plu | Lung:Air | 10.0 | 11.3 | 12.5 | 27 | 22 |
| Pmar | Bone Marrow: Air | 62.0 | 70.4 | 89.2 | 50 | 25 |
| Metabolic Parameters: | ||||||
| VmaxC | Maximum metabolic velocity of MFO saturable pathway (mg/hr/kg liver) | 750 | 718 | 661 | 1413 | 12 |
| tVmaxC | Maximum metabolic velocity of MFO saturable pathway in t-DCE pretreated mice | 8.4 | 7.2 | 11.3 | 58 | 50 |
| Km | affinity of MFO saturable pathway (mg/l) | 1.35 | 0.04 | 0.03 | 1413 | 97 |
| KfC | First order rate constant for GST pathway (l/hr/kg ^0.25) | 1.5 | 1.77 | 2.47 | 1413 | 24 |
| a1 | Ratio of lung to liver in-vitro MFO metabolic velocities (nmol /min/gmlung micros.Prot)/ (nmol/min/gm livermicros. Prot) | 0.405 | 0.28 | 0.30 | 54 | 31 |
| a2 | Ratio of lung to liver in-vitro GST metabolic velocities (nmol/min/gm lungcytos. Prot)/(nmol/min/ gmlivercytos .Prot) | 0.282 | 0.37 | 0.30 | 55 | 41 |
| B1 | Ratio of lung and liver tissue content of microsomal protein | 0.271 | 0.26 | 0.29 | 23 | 18 |
| B2 | Ratio of lung and liver tissue content of cytosolic protein | 0.721 | 0.70 | 0.84 | 22 | 17 |
| Notes:(a) functionally defined as 1sum (other fractional flows);(b) functionally defined as 0.82sum (other fractional volumes) | ||||||
Table VI-8 presents he corresponding set of results for human PBPK parameters. The human in vivo data also appeared to contain considerable information about many of the model parameters, as evidenced by shifts in medians and tightening of posterior distributions relative to priors. Fitted estimates of the metabolic constants were fairly precise, even for Km (Table VI-8); indeed, the fits were markedly superior to those shown in Andersen et al. [Ex. 21-94] and Clewell et al. [Ex. 96].
| - | Parameter | Prior distribution | ||
|---|---|---|---|---|
| GM | GSD | %CV | - | - |
| Flows: | ||||
| QCC | cardiac Output (1/hr/kgBW) | 4.2 | 1.10 | 10 |
| VPR | alveolar Ventilation Perfusion Ratio | 1.35 | 1.15 | 15 |
| Tissue Blood Flows (fraction of cardiac output) | ||||
| QgiC | GI Tract | 0.191 | 1.25 | 23 |
| QliC | Liver | 0.067 | 1.20 | 19 |
| QfatC | Fat | .057 | 1.45 | 38 |
| QppC | Poorly Perfused Tissues | 0.198 | 1.55 | (a) |
| Qwpc | Well Perfused Tissues | 0.443 | 1.25 | 23 |
| QmarC | Bone Marrow | 0.044 | 1.70 | 57 |
| Tissue Volumes (fraction of body weight): | ||||
| VgiC | GI Tract | 0.017 | 1.10 | 10 |
| VliC | Liver | 0.026 | 1.10 | 10 |
| VfatC | Fat | 0.204 | 1.20 | 18 |
| VppC | Poorly Perfused Tissues | 0.470 | 1.15 | (b) |
| VwpC | Well Perfused Tissues | 0.044 | 1.10 | 9 |
| VluC | Lung | 0.008 | 1.15 | 14 |
| VmarC | Bone Marrow | 0.050 | 1.10 | 10 |
| Equilibrium Partition Coefficients: | ||||
| PC.blood | Blood:Air | 8.4 | 1.30 | 26 |
| PC.gi | GI Tract:Air | 8.1 | 1.60 | 50 |
| PC.li | Liver:Air | 9.9 | 1.60 | 50 |
| PC.fat | Fat:Air | 97.6 | 1.25 | 22 |
| PC.pp | Poorly Perfused Tissues:Air | 6.8 | 1.60 | 48 |
| PC.wp | Well Perfused Tissue:Air | 7.6 | 1.40 | 35 |
| PC.lu | Lung:Air | 7.6 | 1.50 | 43 |
| PC.mar | Bone Marrow:Air | 48.8 | 1.60 | 49 |
| Metabolic Parameters: | ||||
| VmaxC | Maximum MFO metabolic rate (mg/mg/hr/kgliver) | 75.0 | 10.00 | 1413 |
| Km | MFO Michaelis Menton constant (mg/1) | 0.60 | 10.00 | 1413 |
| Kf | 1st order rate constant for GST pathway (1/hr) | 0.12 | 2.07 | 81 |
| a1 | [V/S]lung/[V/S] MFOliver | 0.0045 | 4.50 | 226 |
| a2 | [V/S]lung/[V/S] GSTliver | 0.236 | 2.04 | 83 |
| B1 | [mg micr.Prot/gm lung]/[mg micr. Prot/gm liver] | 0.297 | 1.10 | 10 |
| B2 | [mg cyt. Prot/gm lung]/[mg cyt. Prot/gm liver] | 0.807 | 1.20 | 18 |
| - | Parameter | Posterior distribution | |||
|---|---|---|---|---|---|
| Posteriors for Bayesian fit | Modified by exercise | ||||
| Median | %CV | Median | %CV | ||
| Flows: | |||||
| QCC | cardiac Output (1/hr/kgBW) | 4.0 | 6 | 6.2 | 17 |
| VPR | alveolar Ventilation Perfusion Ratio | 1.03 | 1 | 1.37 | 9 |
| Tissue Blood Flows (fraction of cardiac output) | |||||
| QgiC | GI Tract | 0.149 | 12 | 0.122 | 14 |
| QliC | Liver | 0.063 | 15 | 0.041 | 24 |
| QfatC | Fat | 0.045 | 10 | 0.052 | 11 |
| QppC | Poorly Perfused Tissues | 0.378 | (a)9 | (a)0.453 | 10 |
| Qwpc | Well Perfused Tissues | 0.294 | 3 | 0.258 | 7 |
| QmarC | Bone Marrow | 0.071 | 38 | 0.072 | 38 |
| Tissue Volumes (fraction of body weight): | |||||
| VgiC | GI Tract | 0.018 | 8 | 0.018 | 8 |
| VliC | Liver | 0.026 | 8 | 0.026 | 8 |
| VfatC | Fat | 0.183 | 11 | 0.183 | 11 |
| VppC | Poorly Perfused Tissues | 0.489 | (b)5 | (b)0.489 | 5 |
| VwpC | Well Perfused Tissues | 0.47 | 7 | 0.047 | 7 |
| VluC | Lung | 0.008 | 11 | 0.008 | 11 |
| VmarC | Bone Marrow | 0.049 | 8 | 0.049 | 8 |
| Equilibrium Partition Coefficients: | |||||
| PC.blood | Blood:Air | 16.5 | 2 | 16.5 | 2 |
| PC.gi | GI Tract:Air | 10.7 | 36 | 10.7 | 36 |
| PC.li | Liver:Air | 13.7 | 33 | 13.7 | 33 |
| PC.fat | Fat:Air | 84.4 | 12 | 84.4 | 12 |
| PC.pp | Poorly Perfused Tissues:Air | 13.3 | 13 | 13.3 | 13 |
| PC.wp | Well Perfused Tissue:Air | 13.1 | 14 | 13.1 | 14 |
| PC.lu | Lung:Air | 9.4 | 33 | 9.4 | 33 |
| PC.mar | Bone Marrow:Air | 47.8 | 27 | 47.8 | 27 |
| Metabolic Parameters: | |||||
| VmaxC | Maximum MFO metabolic rate (mg/mg/hr/kgliver) | 97.2 | 11 | 97.2 | 11 |
| Km | MFO Michaelis Menton constant (mg/1) | 0.52 | 39 | 0.52 | 39 |
| Kf | 1st order rate constant for GST pathway (1/hr) | 0.23 | 63 | 0.23 | 63 |
| a1 | [V/S]lung/[V/S] MFOliver | 0.024 | 77 | 0.024 | 77 |
| a2 | [V/S]lung/[V/S] GSTliver | 0.364 | 49 | 0.364 | 49 |
| B1 | [mg micr.Prot/gm lung]/[mg micr. Prot/gm liver] | 0.300 | 8 | 0.300 | 8 |
| B2 | [mg cyt. Prot/gm lung]/[mg cyt. Prot/gm liver] | 0.845 | 15 | 0.845 | 15 |
| Notes(a) operationally defined as 1 -- sum (other fractional flows) (b) functionally defined as 0.82 -- sum (other fractional volumes) | |||||
Tables VI-9 and VI-10 compare the posterior distributions for mice and human PBPK parameters with the distributions used by Clewell. For mice, there were appreciable differences in the median values for QCC, VPR, QfatC, QwpC, VwpC, VmaxC, Km, KfC, and the apparent A1 (i.e., A1 x B1). With the exception of VliC, Pblood, Pliv, Ppp and Km, the posterior distributions for all other parameters were tighter than the distributions used by Clewell. The human posterior distributions in Table VI-10 are somewhat different than those in Table VI-8, in that they reflect the influence of modeling variable work intensity on QC, VPR, and all regional blood flows. In comparing these modified posterior distributions to the distributions used by Clewell, one finds appreciable differences in median values for VPR, many of the fractional blood flows (QgiC, QliC, QppC, QwpC), VgiC, PCblood, PCliv, PCfat, VmaxC, KfC, and the apparent A2 (i.e., A2 x B2). All human posterior distributions except for VliC, Pli, and Sp--Kf, had appreciably tighter distributions than those used by Clewell et al. [Ex. 96].
| - | Parameter | central tendency | |
|---|---|---|---|
| clewell et al. median | OSHA median | ||
| Flows: | |||
| QCC | cardiac Output (1/hr/ kgBW | (a)41.5 | 34.4 |
| VPR | alveolar Ventilation Perfusion Ratio | (b)1.76 | 1.59 |
| Tissue Blood Flows (fraction of cardiac output): | |||
| QgiC | GI Tract | 0.165 | 0.14 |
| QliC | Liver | 0.035 | 0.02 |
| QfatC | Fat | 0.030 | 0.09 |
| QppC | Poorly Perfused Tissues | 0.250 | 0.29 |
| QwpC | Well Perfused Tissues | 0.520 | (c)0.36 |
| QmarC | Bone Marrow | NA | 0.10 |
| Tissue Volumes (fraction of body weight): | |||
| VgiC | GI Tract | 0.031 | 0.04 |
| VliC | Liver | 0.046 | 0.05 |
| VfatC | Fat | 0.100 | 0.07 |
| VppC | Poorly Perfused Tissues | 0.513 | (d)0.54 |
| VwpC | Well Perfused Tissues | 0.041 | 0.07 |
| VluC | Lung | 0.008 | 0.01 |
| VmarC | Bone Marrow | NA | 0.04 |
| Equilibrium Partition Coefficients: | |||
| Pblo | Blood:Air | 23.0 | 18.5 |
| Pgi | GI Tract:Air | 11.4 | 11.3 |
| Pli | Liver:Air | 38.7 | 28.2 |
| Pfat | Fat:Air | 107.0 | 100.5 |
| Ppp | Poorly Perfused Tissues:Air | 8.5 | 12.1 |
| Pwp | Well Perfused Tissues:Air | 11.4 | 10.4 |
| Plu | Lung:Air | 10.0 | 11.3 |
| Pmar | Bone Marrow:Air | NA | 70.4 |
| Metabolic Parameters: | |||
| VmaxC | Maximum metabolic velocity of MFO saturable pathway (mg/hr/ kgliver) | 970 | 718 |
| Km | affinity of MFO saturable pathway (mg/l) | 1.35 | 0.04 |
| KfC | First order rate constant for GST pathway (l/hr/ kg0.25) | 1.5 | 1.77 |
| a1 | Ratio of lung to liver in-vitor MFO metabolic velocities (nmol/min/ gmlungmicros.Prot)/ (nmol/min/gmliver micros.Prot) | 0.405 | 0.28 |
| a2 | Ratio of lung to liver in-vitro GST metabolic velocities (nmol/min/ gmlungcytos.Prot)/ (nmol/min/gmliver cytos.Prot) | 0.282 | 0.37 |
| B1 | Ratio of lung and liver tissue content of microsomal protein | 1 | 0.25 |
| B2 | Ratio of lung and liver tissue content of cytosolic protein | 1 | 0.69 |
-Continued-
| - | Parameter | Variability | |
|---|---|---|---|
| clewell et al.%CV | OSHA%CV | ||
| Flows: | |||
| QCC | cardiac Output (1/hr/kgBW | 9 | 9 |
| VPR | alveolar Ventilation Perfusion Ratio | 58 | 14 |
| Tissue Blood Flows (fraction of cardiac output): | |||
| QgiC | GI Tract | 25 | 16 |
| QliC | Liver | 96 | 16 |
| QfatC | Fat | 60 | 19 |
| QppC | Poorly Perfused Tissues | 40 | 18 |
| QwpC | Well Perfused Tissues | 50 | (c) |
| QmarC | Bone Marrow | NA | 27 |
| Tissue Volumes (fraction of body weight): | |||
| VgiC | GI Tract | 30 | 22 |
| VliC | Liver | 6 | 12 |
| VfatC | Fat | 30 | 24 |
| VppC | Poorly Perfused Tissues | 30 | (d) |
| VwpC | Well Perfused Tissues | 30 | 12 |
| VluC | Lung | 30 | 22 |
| VmarC | Bone Marrow | NA | 29 |
| Equilibrium Partition Coefficients: | |||
| Pblo | Blood:Air | 15 | 18 |
| Pgi | GI Tract:Air | 30 | 17 |
| Pli | Liver:Air | 20 | 32 |
| Pfat | Fat:Air | 30 | 21 |
| Ppp | Poorly Perfused Tissues:Air | 10 | 17 |
| Pwp | Well Perfused Tissues:Air | 20 | 16 |
| Plu | Lung:Air | 30 | 22 |
| Pmar | Bone Marrow:Air | NA | 25 |
| Metabolic Parameters: | |||
| VmaxC | Maximum metabolic velocity of MFO saturable pathway (mg/hr/ kgliver) | 20 | 12 |
| Km | affinity of MFO saturable pathway (mg/l) | 30 | 97 |
| KfC | First order rate constant for GST pathway (1/hr/ kg0.25) | 30 | 24 |
| a1 | Ratio of lung to liver in-vitor MFO metabolic velocities (nmol/min/ gmlungmicros.Prot)/ (nmol/min/gmliver micros.Prot) | 50 | 31 |
| a2 | Ratio of lung to liver in-vitro GST metabolic velocities (nmol/min/ gmlungcytos.Prot)/ (nmol/min/gmliver cytos.Prot) | 50 | 41 |
| B1 | Ratio of lung and liver tissue content of microsomal protein | 0 | 18 |
| B2 | Ratio of lung and liver tissue content of cytosolic protein | 0 | 17 |
| Note(a) value computed for 0.025 kg mouse; (b) unitless; (c) functionally defined as 1 -- sum(other fractional flows); (d) functionally defined as 0.82 -- sum(other fractional volumes); (na) not applicable | |||
| - | Parameter | Central tendency | Variability | ||
|---|---|---|---|---|---|
| clewell et al. median | OSHA median | clewell et al.%CV | OSHA%CV | ||
| Flows: | |||||
| QCC | cardiac Output (1/hr/kgBW) | (a)6.2 | (c)6.3 | 9 | (c)17 |
| VPR | alveolar Ventilation Perfusion Ratio | (b)1.95 | (c)1.36 | 18 | (c)9 |
| Tissue Blood Flows (fraction of cardiac output): | |||||
| QgiC | GI Tract | 0.195 | (c)0.12 | 10 | (c)13 |
| QliC | Liver | 0.070 | (c)0.04 | 35 | (c)23 |
| QfatC | Fat | 0.050 | (c)0.05 | 30 | (c)15 |
| QppC | Poorly Perfused Tissues | 0.240 | (c)0.46 | 15 | (c)10 |
| QwpC | Well Perfused Tissues | 0.445 | (c,d)0.26 | 20 | (c,d)7 |
| QmarC | Bone Marrow | NA | (c)0.07 | NA | (c)45 |
| Tissue Volumes (fraction of body Weight) | |||||
| VgiC | GI Tract | 0.045 | 0.017 | 10 | 8 |
| VliC | Liver | 0.023 | 0.026 | 5 | 8 |
| VfatC | Fat | 0.160 | 0.187 | 30 | 12 |
| VppC | Poorly Perfused Tissues | 0.480 | (e)0.483 | 30 | (e)5 |
| VwpC | Well Perfused Tissues | 0.033 | 0.047 | 10 | 7 |
| VluC | Lung | 0.006 | 0.008 | 10 | 12 |
| VmarC | Bone Marrow | NA | 0.050 | NA | 8 |
| Equilibrium Partition Coefficients: | |||||
| Pblo | Blood:Air | 12.9 | 16.5 | 15 | 2 |
| Pgi | GI Tract:Air | 12.0 | 13.5 | 30 | 31 |
| Pli | Liver:Air | 37.4 | 13.6 | 20 | 34 |
| Pfat | Fat:Air | 117.0 | 81.2 | 30 | 13 |
| Ppp | Poorly Perfused Tissues:Air | 10.0 | 13.3 | 10 | 14 |
| Pwp | Well Perfused Tissues:Air | 12.0 | 13.0 | 20 | 14 |
| Plu | Lung:Air | 10.6 | 9.1 | 30 | 32 |
| Pmar | Bone Marrow:Air | NA | 51.2 | NA | 35 |
| Metabolic Parameters: | |||||
| VmaxC | Maximum metabolic velocity of MFO saturable pathway (mg/hr/kgliver) | 75.2 | 94.2 | 30 | 12 |
| Km | affinity of MFO saturable pathway (mg/l) | 0.4 | 0.49 | 50 | 35 |
| KfC | First order rate constant for GST pathway (l/ hr/kg-0.25) | 1.5 | 1.82 | 50 | 24 |
| a1 | Ratio of lung to liver in-vitro MFO metabolic velocities (nmol/min/gm lungmicros. Prot)/(nmol/ min/gmliver micros.Prot) | 0.015 | 0.03 | 70 | 69 |
| a2 | Ratio of lung to liver in-vitro GST metabolic velocities (nmol/min/gm lungcytos. Prot)/(nmol/ min/gmliver cytos.Prot) | 0.18 | 0.45 | 70 | 71 |
| B1 | Ratio of lung and liver tissue content of microsomal protein | 1.0 | 0.31 | 0 | 8 |
| B2 | Ratio of lung and liver tissue content of cytosolic protein | 1.0 | 0.84 | 0 | 14 |
| SpKf | allometric scaling power for body weight scaling of KFC from mice to humans | -0.25 | -0.267 | 0 | 22 |
Notes: (a) value computed for 70 kg human; (b) unitless; (c) posterior distributions adjusted for effects fo light activity; (d) functionally defined as 1 -- sum(other fractional flows); (e) functionally defined as 0.82 -- sum(other fractional volumes); (NA) not applicable.
i. Alternative analysis using the "parallelogram" approach. Andersen et al. [Ex. 21-94] estimated a human first order rate constant (Kf) for glutathione (GST) metabolism of MC in the liver by allometric scaling of a fitted estimate of an in vivo mouse rate constant (KfC(mouse)). Specifically,
Kf(human) = KfC(mouse) x BW(spKf)
where spKf was the allometric scaling power with value -0.25. In their Monte Carlo analysis, Clewell et al. followed the approach of Andersen et al., treating KfC(mouse) as a lognormally distributed random variable and spKf as a known constant. The Bayesian analysis summarized above also made use of the allometric scaling given by the equation above, but prior probability distributions were specified for both KfC(mouse) and spKf.
Reitz et al. (1988, 1989) [Exs. 7-225 and 21-53] proposed an alternative approach for estimating an apparent in vivo human Kf. The approach, referred to as the "parallelogram method," assumes there is a constant proportionality across species between in vitro and apparent in vivo metabolic rates when normalized for substrate concentration ([S]). For example, the equation modeling the apparent in vivo rate of GSH conjugation (dM(GST/dt)) is given by:
| dM(GST) ------- dt |
= | Kf x [S] x Vol(liver) |
The constant proportionality between apparent in vivo rates of metabolism and in vitro rates implies
| dM(GST)/dt ---------- [S] |
= | k(p) x [V/S](GST) = Kf x Vol(liver) |
where [V/S](GST) denotes an in vitro enzymatic rate normalized to [S] and k(p) the in vivo -- in vitro proportionality constant. This approach assumes a common value of k(p) across species, such that knowledge of a [V/S](GST-rodent) and Kf(rodent) (sufficient to estimate a value for k(p) as the ratio of Kf(rodent) to [V/S](GST-rodent) and knowledge of [V/S](GST-human) is sufficient to estimate the remaining corner of a parallelogram, namely Kf(human). Therefore, this approach assumes,
| [V/S](GST(human)) ------------------- [V/S](GST(rodent)) |
= | Kf(human) ---------- Kf(rodent) |
|
| or: | |||
| Kf(human) | = | [V/S](GST(human)) X | Kf(rodent) ----------- [V/S](GST)(rodent) |
Reitz et al. [Ex. 21-53] obtained an estimate for Kf(human) using the parallelogram method that was very similar to the estimate obtained by Andersen et al. [Ex. 21-94] using allometric scaling. However, Reitz and coworkers estimated a mean [V/S](GST-human) based on liver samples from only four human subjects -- three of which had appreciable enzymatic activity and one with no detectable activity. More recent publications (Bogaards et al., 1993 [Ex. 127-16]; Graves et al., 1995 [Ex. 122]) and unpublished data (Green et al., 1987 [Ex. 14]) provide measured values of [V/S](GST) on another 35 human subjects. These additional data demonstrate considerable variation in rates of GST metabolism among human subjects, consistent with a known human polymorphism for GST, described earlier in this Quantitative Risk Assessment. Moreover, these data indicated that, putting aside questions of interlaboratory comparability of measurements, three of the four human samples used by Reitz et al. had GST metabolic rates among the highest reported to date. Consequently, the mean [V/S](GST-human) used by Reitz and coworkers was greater than the mean estimable from the full complement of data on human GST activity.
Since OSHA was interested in assessing the effect of accounting for the full complement of data on human GST activity on estimates of cancer risk, this additional analysis was performed, despite the Agency's reservations concerning the appropriateness of using the parallelogram approach in the MC risk assessment. Although this approach allows the use of all of the available data, the uncertainties in the ratio of in vitro to in vivo metabolic constants raise serious questions for the utility of this analysis. OSHA is presenting this analysis for purposes of comparison and notes that HSIA and Clewell used allometric adjustments in their final PBPK models.
The use of a Kf(human) derived by the parallelogram method required:
(1) modification of the human PBPK model; (2) specification of a prior probability distribution for Kf(human); (3) replication of the Bayesian analysis of the human in vivo open chamber data using the new prior for Kf(human); (4) simulation of the occupational exposure scenario using the joint posterior distributions from the new Bayesian analysis to obtain a posterior distribution for human GST lung metabolism; and (5) re-estimation of the extra cancer risk.
(1) PBPK Model Modifications. The only structural modification to the PBPK models was to replace the parameter for allometric scaling of Kf(mouse) with a direct insert of a model parameter Kf(human), having its own prior probability distribution.
(2) Prior Probability Distributions. Mouse prior probability distributions were unchanged. Prior probability distributions for human model parameters were also unchanged, with the exception of prior distributions for KfC, spKf and A2. Prior probability distributions for KfC and spKf were replaced with a prior probability distribution for Kf(human). The prior probability distribution for A2 was modified to account for additional data on human lung GST activity submitted to OSHA by HSIA [Ex. 122].
The prior probability distribution for Kf(human) was derived using the equation:
| Kf(human) | = | Kf(rodent) x | [V/S](GST)(human) ------------------- [V/S](GST)(rodent) |
x err(k)(p) |
where err(kp) is an error term added to account for uncertainty in estimating the proportionality constant k(p), as k(mouse). Thus, to derive a prior probability distribution for Kf(human), it was necessary to derive prior distributions for Kf(rodent), [V/S] (GST-rodent), [V/S](GST-human) and err(kp), which in turn were propagated using Monte Carlo techniques in accordance with the relationships specified by the equation above.
(i) Prior distribution for rodent Kf. The posterior probability distribution used in the main analysis for the apparent in vivo rodent KfC parameter was used as the basis for a prior probability distribution for Kf(rodent). The posterior distribution was well described by a truncated lognormal distribution with a mean and standard deviation of 1.8 and 0.43 l/hr/bw /(-0.25), and lower and upper truncations at 0.84 and 3.07 l/hr/bw /(-0.25), respectively. The posterior distribution was converted to units of (hour) (-1) by using Monte Carlo techniques to multiply the truncated lognormal by the scalar, (rodent body weight) (-0.25).
(ii) Prior for rodent liver GST [V/S]. A prior probability distribution for a low dose mouse [V/S](GST) was obtained as the ratio of the fitted estimates of in vitro V(max) and K(m) reported by Reitz et al. for liver glutathione conjugation of MC. The fitted estimates of V(max) and K(m) and their associated standard errors were used to set the parameters for normal distributions. Monte Carlo techniques were used to obtain the ratio of these two distributions (i.e., V(max)/K(m)), under the assumption that the joint sample space for V(max) and K(m) was correlated with a pi = 0.9. Correlation was induced because a reanalysis of the mouse in vitro reported in Reitz et al. showed that the joint parameter space for these two fitted parameters was highly correlated.
(iii) Prior distribution for human GST [V/S]. There were four data sets reporting measured values of in vitro GST activity in liver samples from 39 human subjects. These data reflect work from different laboratories using (in some cases) different assay methods and different substrate concentrations. In order to make use of all the data to estimate central tendencies and population variability, it was necessary that all measurements be placed on a common scale.
With respect to assay methods, two of the studies [Exs. 21-53 and 122] reported measured values of [V/S](GST) based on detection of [36]Cl from labelled MC. The other two studies [Exs. 14 and 127-16] reported values of [V/S](GST) based on detection of formaldehyde, a known decomposition product from GSH conjugation with MC. In a comparison of these two methods, Green et al. [Ex. 14] reported results indicating a systematic difference in measured values of [V/S](GST), with the [36]Cl detection method appearing to give estimates approximately 1.7-fold higher than the formaldehyde detection method. In this analysis, the [36]Cl method was chosen as the common scale, since the mouse [V/S](GST) data used above were based on this method. Thus, the formaldehyde assay results were multiplied by 1.7 to put them on the [36]Cl scale.
Adjustments for both substrate concentration and nonlinear metabolism were made by converting all the reported in vitro velocity data, [V](GST), to V(max)/K(m) ratios (i.e., low dose metabolic velocity), by the equation:
| V(max) -------- K(m) |
= | ([V](GST) x (K(m) + [S]))/[S] ------------------------------ K(m) |
The above equation follows from assuming in vitro kinetics can be reasonably modeled as a single-substrate Michaelis-Menton process (i.e., [V](GST) = {V(max) x [S]}/{K(m) + [S]}). In making adjustments, assay specific substrate concentrations were used (i.e., [S], which ranged from 35 to 94 mM) along with the average estimate of an in vitro Km reported by Reitz et al. [Ex. 21-53] in analysis of data from two human subjects ( 44 mM). It is noteworthy that none of the human in vitro [V/S](gst) data reported in Reitz et al. were truly reflective of linear kinetics, whereas the mice data were.
After the two above adjustments were made, a lognormal distribution was fit to the transformed data yielding a GM of 0.031 l/min/mg protein, and a GSD of 2.72. This distribution models intersubject variability in in vitro metabolic activity. However, the prior probability distribution for [V/S](gst-human) should reflect variation in means of six subjects, because the in vivo human data from Dow Chemical Company reflect the averaged pharmacokinetic behavior of tissue from six subjects. Thus, dispersion in the above distribution was adjusted to give the corresponding sampling distribution for means of n = 6.
(iv) Prior distribution for error term. The in vivo and in vitro metabolic data on the MFO metabolic pathway, reported by Reitz et al. [Ex. 21-53], were used to estimate the uncertainty in assuming a constant k(p) across species. These were the only data for which both in vivo and in vitro information was available on several species and which was directly relevant to MC. To avoid artifacts due to the very imprecise fitted estimates of apparent in vivo Km's, in vivo / in vitro comparisons were constructed based on estimates of Vmax alone. These estimates were then normalized by the ratio obtained for mice, providing a measure of the error in using a mouse ratio to estimate ratios in three other species: rats (1.42), hamsters (0.64), and humans (0.41). The GM (0.72) and GSD (1.89) of these three values were used to set parameters for a lognormal distribution used as the prior probability distribution for err(kp). Note that the human value of 0.41 reflected an average of separate estimates on four human subjects, with ratios ranging from 0.1 to 1.0.
(v) Monte Carlo simulation to obtain a prior for human Kf. The above prior probability distributions for Kf(mouse), [V/S](gst-mouse), [V/S](GST) and err(kp) were independently sampled by Monte Carlo techniques (n = 5000) and combined to give a prior distribution for Kf(human) for use in Bayesian analysis of the human open chamber data.
(vi) Revised prior distribution for A2. A2 is the ratio of in vitro GST enzymatic activity in lung tissue to the same activity in liver tissue. In the main analysis, the prior probability distribution for A2 was derived according to the equation:
| a2 | = | [V/S](GST)(lung) ------------------- [V/S](GST)(liver) |
x err(vivo/vitro) |
where err(vivo/vitro) is an error term to account for uncertainty in using a ratio of in vitro activity to make inferences about in vivo activity, and the data of Reitz et al. [Ex. 21-53] were used to estimate prior distributions for [V/S](GST-lung) and [V/ S](GST-liver). This prior distribution was revised to account for additional human [V/S](GST-lung) and [V/S](GST-liver) data.
(vii) Prior for human lung GST [V/S]. Previously, only a single measured value for [V](GST-lung) from a pooled lung sample from two human subjects was available for estimating A2. Mainwaring et al. [Ex. 124] recently submitted additional [V](GST-lung) data to OSHA, consisting of measured values on three additional human subjects (0.00, 0.06 and 0.21 nmol/min/mg protein). The value reported as 0.00 was assumed equal to one-half the detection limit for the assay. Since these new [V](GST-lung) data were obtained using the formaldehyde detection assay, it was necessary to transform the values to the [36]Cl scale. Lacking direct information, it was assumed that the same HCOOH -- ->[36]Cl correction factor derived for the liver data held for the lung data. A correction for substrate concentration was also made, under the assumption of equivalency in lung and liver in vitro Km's. The resulting transformed [V](GST-lung) data were used to construct a prior probability distribution describing uncertainty in the mean of five(1) observations (GM = 0.00082, GSD = 1.61). Note that, in this case, an attempt was made to model pure uncertainty in a low dose [V/S](GST-lung), without information indicating appreciable heterogeneity in the ratio of lung and liver enzymatic activity within an individual.
(viii) Prior probability distribution for uncertainty in human liver GST [V/S]. Because of the focus on uncertainty in A2, the prior probability distribution for [V/S](GST-liver) derived above was modified to describe uncertainty about the mean, given a sample size of 39 subjects.
(ix) Uncertainty in using an in vitro ratio of lung and liver GST activity to make an inference about the corresponding ratio for apparent in vivo GST activity. A prior probability distribution for err(vivo/vitro) was derived using data on in vivo and in vitro ratios of liver MFO enzymatic activity for different species, as a surrogate for intra-species lung versus liver GST enzymatic activity. Thus, two key assumptions are made: (i) That relative enzymatic activity for liver tissue from two species is a reasonable surrogate for relative activities of lung versus liver tissue within a single species, and (ii) that the degree of consistency in ratios of in vivo versus in vitro enzymatic activity will be the same for either MFO or GST mediated processes.
If the apparent in vivo Vmax for the MFO pathway in the lung was modeled as:
| V(max)(MFO)(lung) = V(max)(MFO)(liver) x | [V/S](MFO)(lung) ----------------- [V/S](MFO)(liver) |
x | Vol(lung) --------- Vol(liver) |
|
| it follows that, | ||||
| V(max)A(MFO)(lung) ------------------ V(max)A(MFO)(liver) |
= | [V/S](MFO)(lung) ----------------- [V/S](MFO)(liver) |
||
where VmaxA denotes normalization of Vmax to unit tissue volume. Although there were insufficient data to allow for a direct evaluation of the above equation, the data tabulated by Reitz et al. [Ex. 7-225] for MFO enzymatic activity in mice, rats and hamsters did allow an evaluation of the equality,
| V(max)A(MFO)(liver)(sp1) ----------------------------- V(max)A(MFO)(liver)(sp2) |
= | [V/S](MFO)(liver)(sp1) ----------------------------- [V/S])MFO)(liver)(sp2) |
where the subscripts sp1 and sp2 denote species 1 and 2 (e.g., mouse and rat). Using the apparent in vivo Vmax and in vitro [V/S] data reported in Reitz et al. [Exs. 7-225 and 21-53], it was possible to compute mouse:rat, hamster:mouse and rat:hamster ratios for in vivo Vmax and in vitro [V/S] as shown in table VI-11, below.
| Species ratio | Ratios of MFO enzymatic activity | ||
|---|---|---|---|
| in vivo Vmax | in vitro [V/S] | Fold-Difference(*) | |
| Rat:mouse | 0.49 | 0.36 | 1.38 |
| Mouse:hamster | 1.20 | 0.79 | 1.53 |
| Hamster:rat | 0.59 | 0.28 | 2.06 |
| Footnote(*) Ratio of values in in vivo Vmax column to values in in vitro [V/S] column | |||
The assumption was made that the use of an in vitro ratio as a surrogate for an in vivo ratio is unbiased (i.e., err(vivo/vitro) should be centered on a value of 1). The mean of the three estimates of fold-difference (1.65) is our best estimate of a GSD for err(vivo/vitro). Thus, the prior probability distribution for err(vivo/vitro) was modeled as a lognormal variate with expected value 1.0 and GSD of 1.65.
(x) Monte Carlo simulation to obtain a prior probability distribution for A2. The above prior probability distributions for [V/S]GST-lung, [V/S]GST-liver and err(vivo/vitro) were independently sampled by Monte Carlo techniques (n = 5000) and combined to give a prior probability distribution for A2 for use in Bayesian analysis with the human open chamber data. The resulting distribution was well described as a lognormal variate with a GM of 0.236 and a GSD of 2.0.
(3) Human in vivo data and simulating occupational exposure. Bayesian updating was performed with the same human in vivo data used in the main analysis. These data consisted of time serial measurements of exhaled breath and venous blood concentrations of MC for 6 human volunteers exposed to 100 and 350 ppm MC for 6 hours. Unfortunately, the data have only been reported as averages of the 6 subject-specific observations at each time point. When simulating the human data, subjects were assumed to be at rest (i.e., work load set equal to 0), and the reported average body weight for the six subjects (86 kg) was assumed to be known without error.
A single human occupational exposure was simulated: constant exposure to 25 ppm MC for 8-hours per day and 5 days per week.
(4) Distribution of human metabolized dose and sensitivity analysis. The distribution for GST metabolism in the human lung resulting from simulated occupational exposure to 25 ppm MC had a median and mean of 0.139 and 0.192 mg/day/liter lung, about 3-fold less than values obtained using the allometrically scaled Kf.
From the sensitivity analysis, Kf and A2 exhibited the strongest pairwise correlations with predicted lung GST metabolism, with all other parameters having considerably smaller correlation coefficients. Indeed, other than PC.mar (partition coefficient air:marrow), all other parameters were only weakly correlated with GST lung metabolism. These results differ somewhat from those obtained when using an allometrically scaled Kf, and reflect the effect of greater variability in a Kf based on the parallelogram method.
(5) Posterior distributions in the "parallelogram method" analysis. The posterior distributions for many model parameters were considerably tighter than their corresponding prior distributions, most notably for fractional blood flow and partition coefficient parameters. Similar results were obtained in the main analysis. In general, medians and %CVs of the posterior distributions were similar to those in the main analysis, with the exception of Kf, which was expected, given its revised prior distribution. However, differences among the posterior distributions for Kf were less than expected due to an appreciable shift toward larger values (and some tightening) in the posterior distribution for the parallelogram-based Kf relative to its prior distribution. Thus, it would appear that the data had some information about plausible values of Kf.
The results of the covariance analysis indicated that the covariance structure was fairly similar to the results from the main analysis, with moderate to high pairwise correlations among 15 pairs of parameters.
G. Results of OSHA's PBPK Risk Assessments; Discussion
Summary statistics for OSHA's main analysis modifying the other analysis and the alternative (parallelogram) analysis are reported in Table VI-12. From the main analysis, the MLE of excess cancer risk obtained using the upper 95th percentile of the human internal dose distribution was 3.62/1000, for an occupational lifetime exposure to 25 ppm MC. The MLE of cancer risk obtained using the mean of the human internal dose distribution was 1.24/1000. The alternative (parallelogram) analysis yielded slightly lower estimates of risk. In that analysis, the MLE of cancer risk using the upper 95th percentile of the human internal dose distribution was 1.23/1000. The MLE of cancer risk for the alternative analysis using the mean of the human internal dose distribution was 0.40/1000. After evaluating the methodologies and uncertainties in the two analyses, OSHA determined that the main analysis was most appropriate for the Agency's final risk assessment and the MLE of cancer risk using the upper 95th percentile of the human internal dose distribution was best supported as OSHA's final MC risk estimate. Therefore, OSHA's final risk estimate for occupational lifetime exposure to MC at 25 ppm is 3.62/1000.
| computational approach | Summary statistics for distributions of extra risk | ||||
|---|---|---|---|---|---|
| - | 95%(**) | Mean | %CV(*) | Skewness | Kurtosis |
| Maximum likelihood fitting: Dependence case | 3.62(***) per 1000 | 1.24 per 1000 | 103 | 2.2 | 10.2 |
| Maximum likelihood fitting: Independence case | 2.43 per 1000 | 0.79 per 1000 | 113 | 2.3 | 11.3 |
| Footnote(*) CV denotes coefficient of variation ([standard deviation/mean] X 100) | |||||
| Footnote(**) 95% denotes the 95h percentile value of the distribution of GST matabolites for extra cancer risk | |||||
| Footnote(***) OSHA's final risk estimate | |||||
Figure VI-1 shows the end result of the main PBPK analysis: the cumulative distribution function of excess lifetime cancer risk (log(10) scale) from exposure to 25 ppm MC, 8 hours per day, 5 days per week for 45 years, when estimated using the MLE of the dose-response parameters, GST lung metabolism as the dose surrogate, and a human Kf based on allometric scaling and Bayesian prior information. As described in the main analysis, the "dependence case" was used. Several summary statistics can be discerned from this cumulative distribution function: (1) the 95th percentile of this hybrid distribution of uncertainty and heterogeneity gives a risk estimate of 3.62 x 10(-3) (point "A" in the figure); (2) the mean value of the distribution (point "B" in the figure) gives a risk estimate of 1.24 x 10(-3).
Figure VI-1 Dependence case; Estimated cumulative distribution of human cancer risk linked to a 45 year occupational exposure to methylene chloride, at 25 ppm in the air, 8 hours/day, 5 days per year. Generated using the results described in Figure 3.
OSHA conducted the alternative analysis in order to determine the impact of basing the human GST metabolite distribution on allometry (human GST metabolic rates estimated based on the relative size of animals and humans) versus the parallelogram approach (human GST metabolic rates based on ratio of various rodent in vitro: in vivo metabolic rates applied to human in vitro rates) on risk estimates. As discussed in greater detail above, allometry predicts that one would expect that humans have approximately seven-fold less GST activity than mice. The parallelogram approach, on the other hand, predicts approximately 18-fold less GST activity in humans than in mice. After analyzing the available data, OSHA has determined that the allometric assumptions are best supported by the scientific literature, primarily because of the lack of human in vivo GST data and the lack of validation of the parallelogram approach. The Agency has therefore used that approach in its final (main) estimate of risk, but has also presented an alternative analysis using the parallelogram methodology.
During the rulemaking, studies were submitted to the Agency by HSIA challenging the relevance of the mouse data for estimating human cancer risks. However, as described in detail previously, if one examines the HSIA data critically, it is clear that the studies most likely could not detect differences in metabolic activity (and hence in risk) between mice and humans of the magnitude predicted by allometry. For example, the lack of detection of an increase in DNA ss breaks in human cells compared to mouse cells could be explained because the methodology used could not detect an increase in ss breaks 7-fold smaller than that observed in mice. Clearly, an 18-fold difference, as predicted by the parallelogram method, would be even harder to detect.
Moreover, if the human in vitro data are examined more closely, it becomes apparent that the in vitro: in vivo ratios calculated for the 35 individual humans who have been studied were as low as 4.6 (the median value in this series was 24). Therefore, the use of allometry (ratio = 7) or the parallelogram approach (ratio = 18) would lead to risk estimates that clearly underestimate the risks for some individuals. In addition, RNA adduct data [Ex. 126-25] indicate that exposure of human cells to MC results in only a 3-fold lower amount of RNA adducts than formed in mouse cells. This ratio may not be a close surrogate for the GST ratio, but it does heighten concern that both PBPK approaches may be underestimating cancer risks from occupational exposure to MC, because humans may be appreciably less sensitive than mice.
The distribution of risk presented in either the main or the alternative analysis most closely reflects uncertainty about risk for some randomly chosen worker (with respect to work intensity and body weight), chosen among the population of workers with physiologic, anatomic, and metabolic attributes similar to those of the average subject from the Dow human study group. The Dow pharmacokinetic data did not contain individual data on the 6 subjects, so the results obtained and the predictions made are conditioned by the use of averages. This means that the model is truly only applicable to people who physiologically and biochemically resemble the Dow group of six subjects. Although six subjects do not represent a large data base from which to draw a representative PBPK sample, this is much more human data than is usually available to base a risk assessment on. In fact, in OSHA's preliminary quantitative risk assessment, point estimates were used for body weight, breathing rates, etc. to represent the entire working population with a single "average" number. Therefore, this sample, although small, represents a significant improvement over the point estimates of human parameter values for PBPK modeling. Although these are the best data available, the small number of individuals upon which the human parameter values are based increases concern that the Agency may be underestimating risks for a significant portion of the working population by relying upon these values and using PBPK modeling to estimate human internal doses. OSHA considered making an ad hoc inflation of the variance of the distributions of human GST enzyme kinetics parameters in order to account for some of this unmeasured heterogeneity (as recommended by the NAS Committee report discussed above), but decided not to make this "conservative" choice but instead to rely on the unadjusted analyses.
OSHA has chosen for its final risk estimate to couple one measure of central tendency (the MLE of the dose-response parameters) with a somewhat "conservative" measure (the 95th percentile of the distribution of human GST metabolites (internal dose)). Congress and the courts have permitted -- indeed, encouraged -- OSHA to consider "conservative" responses to both uncertainty and human variability. The OSH Act addresses the latter when it refers, for example, to OSHA's responsibility to set standards such that "no employee shall suffer material impairment of health* * *;" a standard that only considered risk to the average employee clearly would not be responsive to the statute. Similarly, the 1980 "Benzene decision" affirmed that "the Agency is free to use conservative assumptions in interpreting the data with respect to carcinogens, risking error on the side of over-protection rather than under-protection."
In past rulemakings, OSHA has frequently estimated carcinogenic potency via the MLE of the multistage model parameters. The Agency has recently received comments, particularly in a public meeting in February 1996 on risk assessment issues surrounding the first phase of its "PEL Update" process, critical of the MLE on the grounds that this estimator can be highly unstable with respect to small fluctuations in the observed bioassay response rates. Although OSHA may in the future move to a different estimator, such as the mean value of the likelihood function of the multistage model parameters, such a change would have neglible practical impact in the case of MC. The observed data in the NTP mouse bioassay follow a nearly precisely linear trend, so the MLE, mean and UCL estimates are all very nearly equivalent to each other.
However, OSHA needs to take particular care not to underestimate risk when it departs from a relatively simple methodology (in this case, the assumption that administered dose is the most relevant measure of exposure) in favor of a relatively more complex and computationally-intensive methodology (in this case, that the human lung GST metabolite, calculated via a PBPK model, is the most relevant measure of exposure). This is even more important in this particular PBPK analysis, because the variance of the output distributions represents an unknown hybrid of uncertainty in the various parameters and true heterogeneity among the humans exposed to MC. As Clewell stated with respect to his own PBPK analysis (see discussion above), the 95th percentile estimator provides a modicum of assurance that the risk to the average human -- and hence the population risk -- is not underestimated.
Moreover, it is critical to use an estimator other than the central tendency here so that it will not be inevitable that the risk to a human of above-average susceptibility (due to enzyme kinetics that produce relatively more reactive metabolite per unit of administered dose, or due to other attributes related to body weight, organ volumes, partition coefficients, etc.) is not underestimated, potentially by a substantial amount. Any "conservatism" introduced by using the 95th percentile of the PBPK output distribution is further attenuated by the unmeasured model uncertainty inherent in this more complex model structure. Several aspects of the model itself are known to be oversimplifications (e.g., assuming the lung is the only tissue at risk); therefore, the resulting risk distributions could be biased downward.
Finally, it is important to note that there is no risk of "cascading conservatism" with this 95th percentile estimator; the individual model parameters are permitted to vary over their entire ranges, and the selected percentile is only applied to the distribution resulting from the combined influence of all parameters. Furthermore, the newest refinements to the model ensure that the 95th percentile is not affected by any probability assigned to impossible combinations of parameters. The attention paid to issues of mass balance, covariance structure and truncation ensures that this percentile represents a fully plausible set of input parameters. In sum, the combination of the MLE of the multistage parameters and the 95th percentile of the PBPK output distribution represents a reasonable attempt to account for uncertainty and variability without unduly exacerbating the magnitude or the probability of underestimation of errors.
H. Comparison of Animal-Based Risk Estimates With "Non-Positive" Epidemiology Data
Direct comparisons between animal bioassays and human epidemiological studies are difficult to make because experimental protocols between animal and human studies differ substantially. Animals are generally exposed to a fixed dose of a chemical, for several hours per day, from approximately 6-8 weeks of age until study termination, which is usually at 2 years. This would be chronologically equivalent to a human exposure that starts when a human is approximately 4-5 years old and continuing until the human is approximately 74 years old (assuming a 74 year average life-span for humans) [Ex. 89]. This clearly differs from the typical pattern of occupational exposure encountered in epidemiological studies of worker populations. For example, in the Kodak cohort, the workers were never exposed to a constant level of MC; exposure to MC for these workers did not start until their adult life; and most of them were exposed to the chemical for less than one third of their life-span.
Exposure to MC has been found to induce lung and liver cancer in mice and mammary tumors in rats. As discussed above, there are positive epidemiology studies which suggest an association between MC exposure and cancer risk. Because exposure data are inadequate or unavailable, it is not possible to quantify the risks in these studies. OSHA acknowledges that there are also non-positive epidemiology studies.
In 1986, Crump analyzed the preliminary results from the 1964-70 Kodak cohort followed through 1984 and compared them to the rodent bioassay results. The results from the Kodak epidemiological study have also been used by Tollefson et al. [Ex. 7-249], Hearne [Ex. 91-D], and NIOSH to compare the predictions of excess cancer risk from the animal risk assessment models. In addition, Hearne used data from the cellulose triacetate fiber study in Cumberland, Maryland, and a different analytical approach, to validate the excess cancer risk predicted by the animal data [Ex. 91-D]. The details of these analyses can be found in the cited exhibits. OSHA has analyzed the different approaches to assessing the mouse bioassay in light of the epidemiology data and has determined that the approach taken by NIOSH (summarized below) represents the most comprehensive and clearest way to examine those data. OSHA also agrees with the conclusions reached by NIOSH, that the epidemiology results and the mouse bioassay data are not inconsistent with each other.
NIOSH compared the confidence intervals for the standardized mortality ratios (SMRs) from the Kodak study with the predicted confidence intervals derived from OSHA's risk assessment models from the NPRM [Ex. 89]. To estimate predicted SMRs using the multistage model, NIOSH used the following approach:
1. The expected excess number of deaths in each of the exposure groups was derived by multiplying the number of workers in each exposure group by the excess risk as determined by the multistage model (after correcting for dose equivalence between animals and humans, and differences in length of follow-up).
2. This number of expected deaths, derived from the animal data, was then added to the expected (denoted E(p)) number of deaths which were derived from the Kodak study, after correcting for the HWE, (this can be viewed as the background risk) to estimate the number of "observed" deaths that would have been predicted by the multistage model assuming it was valid for humans (denoted O(p)).
3. O(p) was then divided by E(p) to calculate predicted SMRs and 95% confidence intervals, where calculated.
NIOSH's results indicated that the non-positive findings from the Kodak study were not inconsistent with the predicted risk estimates in OSHA's risk assessment. The predicted confidence intervals from the animal multistage model were completely nested within the observed confidence intervals from the Kodak study. This is not to suggest that results from this non-positive epidemiology study are equivalent to the positive results from the animal inhalation study. Rather, based on these findings, one can conclude that the non-positive results from the Kodak epidemiologic study were not of sufficient power to contradict risk predictions of the multistage model developed from the animal bioassay data (when appropriate adjustments for differences in study protocol were taken into account).
Basically, the Kodak study examined approximately 1000 workers whose average MC exposure was 26 ppm. Therefore, the animal-based potency estimates would predict only about 3 excess cancer deaths in that cohort (the risk at 26 ppm is approximately 3 per 1000), even if they were followed for many decades after exposure ceased. This small predicted excess is clearly too small an increment to be observable with statistical confidence, considering the much larger background of cancer present in the human population. The differences between the NIOSH and Hearne analyses essentially represent different ways to estimate the "signal-to-noise" ratio for the Kodak study; OSHA believes that any reasonable method of estimating this ratio would conclude that the Kodak study has insufficient power to rule out a "signal" of significant human risk.
NIOSH's approach for adjusting for the healthy worker effect (HWE) was criticized in the comments to the record submitted by Hearne. Hearne stated that the HWE is unlikely to be present in long term cancer studies and therefore an adjustment for the HWE is not necessary [Ex. 91-D]. Hearne argued that since the HWE diminishes with time, the healthy worker effect would have been minimal in the 1946-70 Kodak cohort because the median follow-up period was 32 years and that only 20% of the cohort members were still actively employed [Tr. 10/15/92].
There is evidence in the literature showing that the HWE can be weaker for some types of cancer than for other causes of death; however, in this case NIOSH believed and OSHA agreed that the difference between control and exposed populations reflected an HWE for cancer. In addition, results from a similar analysis done by NIOSH without the HWE adjustment did not contradict the results including the HWE adjustment. NIOSH testified [Tr. 985-6, 9/21/92] that there would be a difference in the results obtained when adjusting for HWE and the unadjusted results. However, the conclusions reached would not be different. In other words, the analysis still supported the conclusion that the epidemiologic and mouse bioassay results were not inconsistent with each other. OSHA supports NIOSH's position on the use of an adjustment factor for HWE in this cohort. Other criticisms of NIOSH's approach can be found in the hearing transcripts and post-hearing comments. OSHA has evaluated these methodological criticisms and has determined that NIOSH used the best available methodology in analyzing this issue and that their conclusions are supported by those arrived at independently by Crump and by Tollefson et al.
Specifically, NIOSH predicted 23.25 deaths from cancers (at all sites) in the full cohort, after adjusting for the HWE. This value is closer to the observed number (22) than is the unadjusted expected number of deaths (29.61). Looking at lung cancer deaths separately, NIOSH predicted 22.36 deaths for the entire cohort (adjusted for HWE) compared with 22 observed and 28.67 expected by Hearne. Hearne observed no deaths from liver cancer in the entire cohort (1.14 deaths were expected). NIOSH predicted 0.88 deaths from liver cancer when they adjusted for the HWE.
OSHA believes that NIOSH's approach in comparing results from an animal bioassay to those of an epidemiological study is the most reasonable comparison between data sets because it is more accurate and better addresses computational and experimental issues inherent in the data sets. The Agency has evaluated the extent to which the cancer risk calculated using the human data is consistent with the cancer risk calculated using animal data. Based on its review of those studies, OSHA concluded that the human epidemiology results are not inconsistent with the animal bioassays and has determined that the bioassays are the appropriate basis for its quantitative risk assessment.
I. Conclusions
OSHA has determined that MC is a potential occupational carcinogen and has conducted a quantitative risk assessment in order to estimate human risks of cancer after occupational exposure to MC. The Agency reviewed all of the human and animal data on MC and determined that MC is carcinogenic in mice and in rats, causing tumors at multiple sites, in both species, and in both sexes of animals. Some epidemiologic data also indicate an association between MC exposure and excess cancer in exposed workers (statistically significant increases in biliary cancers in textile workers and astrocytic brain cancer in workers exposed to MC in solvent applications). Mechanistic data indicate that MC is likely to be metabolized to a genotoxic carcinogen. MC has been clearly shown to be metabolized by similar enzymatic pathways in rodents and humans, indicating that the metabolic processes which produce cancer in mice and rats are also present in humans. Finally, no data have been presented which demonstrate that the mouse is an inappropriate model for humans because of a physiological or biochemical component or process. Therefore, the Agency has determined that it is appropriate to assess the carcinogenic risks of MC using the NTP mouse bioassay dose-response.
The NTP mouse MC bioassays demonstrated a clear dose-tumor response relationship. OSHA determined that the NTP female mouse lung tumor response was the best data set on which to base a quantitative analysis because there was a clear dose-response, low background tumor incidence and it represented the most sensitive tumor site/sex combination.
After examining the PBPK models submitted to the Agency, OSHA concluded that PBPK modeling estimates of the amount of GST metabolites produced are reasonable dose surrogates for MC and are supported by substantial scientific evidence in the record. For that reason, OSHA has used PBPK modeling in its final risk assessment. OSHA reviewed methodologies used in PBPK models submitted to the Agency and decided to modify and expand an existing model. Specifically, a Bayesian analysis was conducted as described above. Use of the Bayesian model analysis was a logical next step in development and use of pharmacokinetic models for MC. It has great advantages in accounting for the covariance of the PBPK parameters and incorporating distributions of physiological parameters obtained from the scientific literature. OSHA's final estimates of risk use the PBPK analysis described above and are based on the MLE of the dose-response parameters using the upper 95th percentile of the human internal dose distribution. For an occupational lifetime exposure to 25 ppm MC, OSHA estimates an excess risk of 3.6 MC-induced cancer deaths per 1000 workers.
VII. Significance of Risk
A. Introduction.
In the 1980 Benzene decision, the Supreme Court, in its discussion of the level of risk that Congress authorized OSHA to regulate, indicated its view of the boundaries of acceptable and unacceptable risk. The Court stated:
It is the Agency's responsibility to determine in the first instance what it considers to be a "significant" risk. Some risks are plainly acceptable and others are plainly unacceptable. If for example, the odds are one in a billion that a person will die from cancer by taking a drink of chlorinated water, the risk clearly could not be considered significant. On the other hand, if the odds are one in a thousand that regular inhalation of gasoline vapors that are 2 percent benzene will be fatal, a reasonable person might well consider the risk significant and take the appropriate steps to decrease or eliminate it. (I.U.D. v. A.P.I., 448 U.S. 607, 655).
So a risk of 1/1000 (10(-3)) is clearly significant. It represents the uppermost end of a million-fold range suggested by the Court, somewhere below which the boundary of acceptable versus unacceptable risk must fall.
The Court further stated that "while the Agency must support its findings that a certain level of risk exists with substantial evidence, we recognize that its determination that a particular level of risk is significant will be based largely on policy considerations." The Court added that the significant risk determination required by the OSH Act is "not a mathematical straitjacket," and that "OSHA is not required to support its findings with anything approaching scientific certainty." The Court ruled that "a reviewing court [is] to give OSHA some leeway where its findings must be made on the frontiers of scientific knowledge [and that] . . . the Agency is free to use conservative assumptions in interpreting the data with respect to carcinogens, risking error on the side of overprotection rather than underprotection" (448 U.S. at 655, 656).
Nonetheless, OSHA has taken various steps that make it fairly confident its risk assessment methodology is not "conservative" (in the sense of erring on the side of overprotection). For example, there are several options for extrapolating human risks from animal data via interspecies scaling factors. The plausible factors range from body weight extrapolation (risks equivalent at equivalent body weights) to (body weight)(2/3) (risks equivalent at equivalent surface areas). Intermediate values have also been used, and the value of (body weight)(3/4), which is supported by physiological theory and empirical evidence, is generally considered to be the midpoint of the plausible values. (Body weight)(2/3) is the most conservative value in this series. Body weight extrapolation is the least conservative. OSHA has generally used body weight extrapolation in assessing risks from animal data, our approach which tends to be significantly less conservative than the other methodologies and most likely is less conservative even than the central tendency of the plausible values.
Other examples in OSHA's risk assessment methodology where the Agency does not use a conservative approach are selection of the maximum likelihood estimator to parameterize the dose-response function rather than the upper 95% confidence limit, and the use of site-specific tumor incidence rather than pooled tumor response in determining the dose-response function for a chemical agent.
OSHA's overall analytic approach to regulating occupational exposure to particular substances is a four-step process consistent with recent court interpretations of the OSH Act, such as the Benzene decision, and rational, objective policy formulation. In the first step, OSHA quantifies the pertinent health risks, to the extent possible, performing quantitative risk assessments. The Agency considers a number of factors to determine whether the substance to be regulated poses a significant risk to workers. These factors include the type of risk posed, the quality of the underlying data, the plausibility and precision of the risk assessment, the statistical significance of the findings and the magnitude of risk [48 FR 1864, January 14, 1983]. In the second step, OSHA considers which, if any, of the regulatory options being considered will substantially reduce the identified risks. In the third step, OSHA looks at the best available data to set permissible exposure limits that, to the extent possible, both protect employees from significant risks and are also technologically and economically feasible. In the fourth and final step, OSHA considers the most cost-effective way to fulfill its statutory mandate by crafting regulations that allow employers to reach the feasible PEL as efficiently as possible.
B. Review of Data Quality and Statistical Significance
The former OSHA standard for MC was designed to prevent irritation and injury to the neurological system of the employees exposed to MC. In 1985, the National Toxicology Program (NTP) released the results of their MC rodent lifetime bioassays. Those results indicated that MC is carcinogenic to rats and mice. As discussed in the Events Leading to the Final Standard section, based on the NTP findings, EPA now considers MC a probable human carcinogen, and NIOSH regards MC as a potential occupational carcinogen and recommends controlling the exposure to MC to the lowest feasible level. In 1988, ACGIH classified MC as an industrial substance suspected of carcinogenic potential for humans.
As discussed in the Health Effects section, OSHA has determined, based on the NTP data, that MC is a potential occupational carcinogen. This conclusion is supported by high-quality data in both rodent species. Having determined, as discussed in the Quantitative Risk Assessment section, that the NTP study provided suitable data for quantitative analysis, OSHA performed quantitative risk assessments to determine if MC exposure at the current PEL presents a significant risk.
As discussed in the Health Effects and Quantitative Risk Assessment sections, OSHA evaluated four MC rodent bioassays [Exs. 4-35, 4-25, 7-29, 7-30, 7-31] to select the most appropriate bioassay as the basis for a quantitative risk assessment. These bioassays were conducted in three rodent species (rat, mouse, and hamster) using two routes of administration (oral and inhalation). The NTP study (rat and mouse, inhalation) was chosen for a quantitative risk assessment because it provides the clearest toxicological and statistical evidence of the carcinogenicity of MC [Exs. 12, 7-127] and because the studies were of the highest data quality. In the NTP study, MC induced significant increases both in the incidence and multiplicity of alveolar/ bronchiolar and hepatocellular neoplasms in male and female mice. In rats, dose-related, statistically significant increases in mammary tumors were also observed. OSHA chose the female mouse tumor response as the basis of its quantitative risk assessment, because of the high quality of data, the clear dose response of liver and lung tumors and the low background tumor incidence. OSHA chose female mouse lung tumors as the specific tumor site for its final quantitative risk assessment. There is no a priori reason to prefer the mouse lung tumor response over the liver tumor response because both data sets were of high quality, showed a clear dose-response relationship and had low background tumor incidence. In fact, in the NPRM, the Agency reported estimates of risk generated using both sites. However, to reduce the complexity of the final PBPK analysis, which required highly intensive computations, OSHA chose one site (the female mouse lung tumor response) for its final risk estimates. The risks calculated using the female mouse liver response would likely be only slightly lower than those calculated using the lung tumor response. On the other hand, pooling the total number of tumor-bearing animals having either a lung or liver tumor (or both) would have yielded risk estimates higher than OSHA's final values.
Once the alveolar/bronchiolar neoplasms in female mice were chosen as the most appropriate data set, the multistage model of carcinogenesis was used to predict a lifetime excess risk of cancer from occupational exposure to MC at several concentration levels. The multistage model is a mechanistic model based on the biological assumption that cancer is induced by carcinogens through a series of stages. The model may be conservative, in the sense that it risks error on the side of overprotection rather than underprotection, because it assumes no threshold for carcinogenesis and because it is approximately linear at low doses, although there are other plausible models of carcinogenesis which are more conservative. The Agency believes that this model conforms most closely to what we know of the etiology of cancer. There is no evidence that the multistage model is biologically incorrect, especially for genotoxic carcinogens, which MC most likely is. OSHA's preference is consistent with the position of the Office of Science and Technology Policy which recommends that "when data and information are limited, and when much uncertainty exists regarding the mechanisms of carcinogenic action, models or procedures that incorporate low-dose linearity are preferred when compatible with limited information" [Ex. 7-227].
In the NPRM, OSHA solicited comment and testimony on the application of physiologically-based pharmacokinetic (PBPK) modeling to refine the MC risk assessment. There was an intensive discussion of pharmacokinetic issues during the hearings and in comments and briefs submitted to OSHA. PBPK modeling is used to account for metabolic and pharmacokinetic differences between rodents and humans and when extrapolating from high experimental doses to lower occupational exposures. OSHA has evaluated several risk assessments produced using pharmacokinetic models. Discussion of the major issues surrounding the use of PBPK in risk assessment can be found in the Quantitative Risk Assessment section. Although serious questions remain concerning the application of these models in the MC risk assessment, the Agency has used the estimates generated via PBPK modeling as its final estimate of the carcinogenic risk of MC exposure.
In accepting PBPK analysis, the Agency wanted to be able to utilize all of the data available and appropriate for the analysis. OSHA was also concerned that the uncertainties and inter-individual variabilities in PBPK models were insufficiently quantified to allow analysis of the impact of those uncertainties on the risk. Several rulemaking participants have conducted sensitivity and uncertainty analyses, the most extensive of which was that submitted by Mr. Harvey Clewell on behalf of the U.S. Navy. These analyses show the impact of the variability and uncertainty of the parameters which are used in the PBPK model and suggest methods of quantifying the impact of that uncertainty on the risk estimates.
OSHA has determined that the PBPK data are of sufficient weight to warrant reliance on PBPK modeling to develop a risk estimate in the specific case of MC, a chemical with more extensive information on metabolism than exists for most other substances. To that end, OSHA adopted a Bayesian approach in which all of the physiological and MC-specific data could be used to generate a distribution of estimates of the carcinogenic risks of MC. OSHA used the mean and the upper 95th percentile estimator of the distribution of human PBPK parameters, coupled with the maximum likelihood estimator of cancer potency, to generate its final estimates of risks.
As discussed in more detail in the Health Effects Section above, human data concerning the carcinogenicity of MC were presented in several epidemiology studies. In a study of cellulose triacetate fiber production (MC used as solvent) workers, an increased incidence of liver/biliary cancer [Ex. 7-260] was noted. Although the case numbers were small and the exposure information limited, this epidemiological evidence is consistent with findings from animal studies and indicates that there may be an association between human cancer risk and MC exposure. A study of workers in photographic film production was non-positive [7-163]. However, the exposures experienced by these workers were likely to have been much less than those in the cellulose triacetate fiber plant and, as discussed in the quantitative risk assessment section, the study lacked the power to detect the magnitude of the increase in cancer deaths that would have been predicted given only the bioassay results. A case-control study conducted by the National Cancer Institute showed a statistically significant association between occupational MC exposure and development of astrocytic brain cancer. Exposure levels could not be determined in this study. The results of the epidemiological studies summarized here were not inconsistent with the results of the animal-based cancer potency estimate.
C. Material Impairment of Health
MC is a potential occupational carcinogen. Cancer is a material impairment of health. OSHA has set the 8-hour TWA PEL primarily to reduce the risk to employees of developing cancer.
The STEL of 125 ppm averaged over 15 minutes is primarily designed to protect against MC's non-cancer risks. As discussed in the Health Effects section, there are substantial risks of CNS effects and cardiac toxicity resulting from acute exposure to MC and its metabolites. CNS effects have been demonstrated in workers at concentrations as low as 175 ppm [Ex. 7-153] and a STEL of 125 ppm for 15 minutes would thus be protective against the CNS effects described. Metabolism of MC to CO increases the body burden of COHb in exposed workers. Levels of COHb above 3% COHb may exacerbate angina symptoms and reduce exercise tolerance in workers with silent or symptomatic heart disease. Smokers are at higher risk for these effects because of the already increased COHb associated with smoking (COHb ranges from 2 to 10% in most smokers). Limiting short term exposure to 125 ppm for 15 minutes will keep COHb levels due to MC exposure below the 3% level, protecting the sub-population of workers with silent or symptomatic heart disease and also limiting the additional COHb burden in smokers.
In addition to protecting against CNS and cardiac effects, there is evidence that reducing the GST metabolite production by reducing short term exposure to high concentrations of MC may also lower the cancer risk. This is because metabolism by the MFO pathway (not generally believed to be associated with carcinogenesis) appears to saturate beginning around 100 ppm. This means that exposure to higher concentrations of MC would lead to increased metabolism by the GST pathway (the putative carcinogenic pathway) and therefore, greater than proportionally increased risk.
All of the health effects averted by reducing MC exposure are potentially or likely to be fatal, and this clearly represents "material impairment of health" as defined by the OSH Act and case law.
D. Risk Estimates
OSHA's final estimate of excess cancer risks at the current PEL of 500 ppm (8-hour TWA) is 126 per 1000. The risk at the new PEL of 25 ppm is 3.62 per 1000. The risk at 25 ppm is similar to the risk estimated in OSHA's preliminary quantitative risk assessment based on applied dose of MC on a mg/kg/day basis (2.3 per 1000 workers) and clearly supports a PEL of 25 ppm. Risks greater than or equal to 10(-3) are clearly significant and the Agency deems them unacceptably high. However, OSHA did not collect the data necessary to document the feasibility of a PEL below 25 ppm across all affected industry sectors, and so the Agency has set the PEL at 25 ppm in the final rule. OSHA intends in the future to gather more information pertaining to the feasibility of lower PELs.
E. "Significant Risk" Policy Issues
Further guidance for the Agency in evaluating significant risk and narrowing the million-fold range provided in the "Benzene decision" is provided by an examination of occupational risk rates, legislative intent, and the academic literature on "acceptable risk" issues. For example, in the high risk occupations of mining and quarrying, the average risk of death from an occupational injury or an acute occupationally-related illness over a lifetime of employment (45 years) is 15.1 per 1,000 workers. The typical occupational risk of deaths for all manufacturing industries is 1.98 per 1,000. Typical lifetime occupational risk of death in an occupation of relatively low risk, like retail trade, is 0.82 per 1,000. (These rates are averages derived from 1984-1986 Bureau of Labor Statistics data for employers with 11 or more employees, adjusted to 45 years of employment, for 50 weeks per year).
Congress passed the Occupational Safety and Health Act of 1970 because of a determination that occupational safety and health risks were too high. Congress therefore gave OSHA authority to reduce significant risks when it is feasible to do so. Within this context, OSHA's final estimate of risk from occupational exposure to MC at the current 8-hour TWA PEL (126 per 1000) is substantially higher than other risks that OSHA has concluded are significant, is substantially higher than the risk of fatality in some high-risk occupations, and is substantially higher than the example presented by the Supreme Court. Moreover, a risk of 3.62 per 1000 at 25 ppm is also clearly significant; therefore, the PEL must be set at least as low as the level of 25 ppm documented as feasible across all industries.
Further, applying the rationale of the Benzene decision, the other risk assessments presented by OSHA and the risk estimates presented by rulemaking participants, including the HSIA (see Table VII-1, below), all support OSHA's conclusion that the human cancer risk for employees exposed to MC above 25 ppm as an 8-hour TWA is significant.
| Model | MLE (UCL) (**) | ||
|---|---|---|---|
| 25 ppm | 50 ppm | 500 ppm | |
| OSHA NPRM Risk Assessment (mg/kg/d, BW extrapolation) without PBPK Adjustment | 2.32(2.97) | 4.64(5.92) | 45.5(57.7) |
| PPM to PPM extrapolation without PBPK Adjustment | 11.3(14.4) | 22.4(28.5) | 203(251) |
| PBPK Reitz female mouse lung -- Reitz human (HSIA assumptions) | 0.43(0.53) | 0.93(1.17) | 14.3(17.9) |
| PBPK Reitz female mouse lung -- Dankovic average human (NIOSH assumptions) | 0.81(1.02) | 1.69(2.12) | 15.0(18.7) |
| PBPK Clewell female mouse lung -- Clewell human (Navy assuptions)(*) | 0.91(1.14) | 1.88(2.36) | 27.5(34.2) |
| OSHA Final Risk Assessment (female mouse lung with PBPK) | 3.62 | 7.47 | 125.8 |
| Footnote(*) Upper 95th percentile of the GST metabolites distribution was used as input in the multistage model | |||
| Footnote(**) Maximum likelihood estimates are 95th percentile upper confidence limit (in parentheses) of the multistage dose-response function | |||
In addition to being 100 to 1000 times higher than the risk levels generally regarded by other Federal Agencies as on the boundary between significant and insignificant risk (see, e.g., Travis et al., 1987), and 1000 times higher than the "acceptable risk" level Congress set in the 1990 Clean Air Act Amendments, the level of 10(-3) is within the range where economic studies document a marked nonlinearity. In other words, individuals regard risks this high as qualitatively different from "smaller" risks. Although risks below 10(-3) are not unambiguously significant, depending on the size of the affected population, the benefits associated with the risky activity, and other factors, this policy determination is not relevant to this regulation, since OSHA's final risk estimate is substantially greater than 1 per 1000. Risks at or above 10(-3) are always significant by any empirical, legal or economic argument available.(2)
Because of the lack of documented feasibility data for potential PELs of less than 25 ppm, OSHA has concluded that there is not enough information available to support lowering the 8-hour TWA PEL or STEL further at this time. However, OSHA has integrated other protective provisions into the final standard to further reduce the risk of developing cancer among employees exposed to MC. Employees exposed to MC at the 8-hour TWA PEL limit without the supplementary provisions would remain at risk of developing adverse health effects, so that inclusion of other protective provisions, such as medical surveillance and employee training, is both necessary and appropriate. The action level will encourage those employers for whom it is feasible to do so to lower exposures below 12.5 ppm to further reduce significant risk. consequently, the programs triggered by the action level will further decrease the incidence of disease beyond the predicted reductions attributable merely to a lower PEL. As a result, OSHA concludes that its 8-hour TWA PEL of 25 ppm and associated action level (12.5 ppm) and STEL (125 ppm) will reduce significant risk and that employers who comply with the provisions of the standard will be taking reasonable steps to protect their employees from the hazards of MC.
The Agency notes that even at the final PELs, the risks to workers remain clearly significant. OSHA will be gathering information on the risks of, and feasibility of compliance with, PELs less than 25 ppm, to determine whether future rulemaking is appropriate in order to further reduce the MC risks to employees.
VIII. Summary of the Final Economic Analysis
In its Final Economic and Regulatory Flexibility Analysis document, OSHA addresses the significant issues related to technological and economic feasibility and small business impacts raised in the rulemaking process. The Final Economic Analysis is also OSHA's most comprehensive explanation of the standard's practical impact on the regulated community; in the Final Economic Analysis, OSHA explains in detail the Agency's findings and conclusions concerning pre-standard (baseline) conditions, such as exposure levels, in establishments in the regulated community, and discusses how and why the requirements of the standard are expected to eliminate significant risk to the extent feasible. This document also sets forth OSHA's Final Regulatory Flexibility Analysis and the analyses required by Executive Order 12866. This Federal Registerpreamble and the Final Economic Analysis are integrally related and together present the fullest statement of OSHA's reasoning concerning this standard. The Final Economic and Regulatory Flexibility Analysis, together with supporting appendix material, has been placed in the rulemaking docket for methylene chloride (Ex. 129).
The purpose of the Final Economic Analysis is to:
- Describe the need for a standard governing occupational exposure to methylene chloride;
- Identify the establishments and industries potentially affected by the standard;
- Evaluate the costs, benefits, economic impacts and small business impacts of the standard on affected firms;
- Assess the technological and economic feasibility of the standard for affected establishments, industries, and small businesses;
- Evaluate the availability of effective non-regulatory approaches to the problem of occupational exposure to methylene chloride; and
- Present changes designed to reduce the impact of the standard on small firms while meeting the objectives of the OSH Act.
Need for the Standard
OSHA's final methylene chloride (MC) standard covers occupational exposures to this substance, one of the most widely used of all organic solvents, in general industry, construction, and shipyard employment. In all, about 237,000 employees are estimated to be exposed to MC. These workers are exposed to MC in many different ways, including the manufacturing, formulation, distribution, and use of MC-containing products. The most common uses of MC are in paint stripping, metal cleaning, and furniture stripping.
Workers exposed to MC are at significant risk of developing cancer, heart and liver effects, and central nervous system impairments, as well as eye, skin, and mucous membrane irritation. Animal bioassays have shown MC to be carcinogenic in mice and rats of both sexes, and epidemiologic studies in workers have produced suggestive evidence of its carcinogenicity in humans. Acute overexposure to the vapors of MC can lead to central nervous system depression, respiratory paralysis, and death: OSHA receives fatality reports every year involving workers who have died using MC to perform such tasks as stripping floors and removing paint. To protect all MC-exposed workers from these adverse health effects, the final standard lowers the airborne concentration of MC to which workers may be exposed from the current permissible exposure limit (PEL) of 500 ppm as an 8-hour time-weighted average (8-hour TWA) to 25 ppm, and from the Agency's current short-term limit of 1000 ppm as an acceptable ceiling, or 2000 ppm as an acceptable peak above the acceptable ceiling for 5 minutes in any 2-hour period, to a short-term exposure limit (STEL) of 125 ppm, averaged over 15 minutes. (For a detailed discussion of the risks posed to workers by exposure to MC, see the Quantitative Risk Assessment and Significance of Risk sections of the preamble, above.) OSHA's final MC standard is similar in format and content to other health standards issued under Section (6)(b)(5) of the Act. In addition to setting PELs, the standard requires employers to monitor the exposures of workers; establish regulated areas when exposures may reasonably be expected to exceed one of these PELs; implement engineering and work practice controls to reduce employee exposures to MC; provide respiratory protection to supplement engineering controls where these are not feasible, are insufficient to meet the PELs, or in emergencies; provide other protective clothing and equipment as necessary for employee protection; make industrial hygiene facilities (such as eyewash and emergency showers) available in certain circumstances; provide medical surveillance; train workers about the hazards of MC (as required by OSHA's Hazard Communication Standard); and keep records relating to the standard. The contents of the standard are explained briefly in Chapter I of the Final Economic Analysis and in detail in the Summary and Explanation (Section X of the preamble, below).
Chapter II of the economic analysis describes the uses of methylene chloride and the industries in which such use occurs. Employee exposures to MC are analyzed on the basis of "application groups," i.e., groups of firms that use MC to perform a particular function, such as metal cleaning or industrial paint stripping, regardless of the particular industry in which the use takes place. The methodology used by OSHA in the analysis is appropriate when a ubiquitous chemical like MC is used to perform the same function in many kinds of firms in many industries, because the processes used, employee exposures generated, and controls in place or needed to achieve compliance are the same, whether the process takes place in a machine shop, on board ship, or on a construction site. For example, because the process of using MC to strip paint or coatings from an object is essentially the same whether the object being stripped is a spray paint booth, boat, church pew, or automobile, and the exposures generated during the process are similar in important respects, it is appropriate to analyze such activities as a group. However, OSHA's technological feasibility and cost analyses reflect the fact that job classifications and work processes may differ within a given application group. Table VIII-1 shows the application groups analyzed in the economic analysis, and the numbers of MC-using establishments, MC-exposed workers, and estimated volume of MC handled annually by establishments in each application group.
| application group | Estimated number of MC-using establishments(*) | Estimated total employment(*) | Estimated number of exposed workers(*) | Estimated MC handled (millions of lbs) |
|---|---|---|---|---|
| Methylene Chloride Manufacturing | 4 | 1,664 | 84 | 469.20 |
| Distribution/Formulation of Solvents | 320 | 84,004 | 1,701 | 189.65 |
| Metal Cleaning: | ||||
| cold Degreasing and Other Cold Cleaning: | 23,717 | 901,232 | 94,537 | 32.56 |
| Open-Top Vapor Degreasing | 278 | 27,105 | 608 | 14.87 |
| conveyorized Vapor Degreasing | 45 | 2,920 | 75 | 1.13 |
| Semiconductors | 239 | 217,960 | 1,392 | 0.40 |
| Printed Circuit Boards | 141 | 77,795 | 298 | 13.98 |
| aerosol Packaging | 52 | 4,142 | 520 | 25.21 |
| Paint Remover Manufacturing | 80 | 6,134 | 200 | 136.85 |
| Paint Manufacturing | 49 | 8,909 | 229 | 3.54 |
| Paint Stripping: Aircraft Stripping | 300 | 266,826 | 2,470 | 13.17 |
| Furniture Stripping | 6,152 | 23,592 | 7,872 | 23.26 |
| Other Industrial Paint Stripping | 35,041 | 2,312,721 | 46,605 | 59.36 |
| Flexible Polyurethane Foam Manufacturing | 100 | 9,800 | 600 | 50.32 |
| Plastics and Adhesives | ||||
| Manufacturing and Use | 3,487 | 1,186,040 | 10,481 | 41.90 |
| adhesive Production | 165 | 56,254 | 497 | |
| adhesive Use | 1,753 | 596,291 | 5,269 | |
| Injection Molding | 80 | 27,211 | 240 | |
| Lamination | 1,323 | 450,031 | 4,070 | |
| Mold Release | 165 | 56,254 | 497 | |
| width="600" colspan=5>Ink Use: | ||||
| Ink and Ink Solvent Manufacturing | 15 | 2,010 | 58 | 3.68 |
| Ink Solvent Use in Printing | 11,869 | 197,619 | 39,481 | 3.68 |
| Pesticide Manufacturing and Formulation | 60 | 1,440 | 120 | 9.58 |
| Pharmaceutical Manufacturing | 108 | 70,223 | 1,431 | 39.53 |
| Solvent Recovery | 34 | 932 | 137 | 32.10 |
| Film Base Manufacturing | 1 | 45,000 | 500 | 8.90 |
| Polycarbonate Manufacturing | 4 | 1,898 | 67 | 6.70 |
| construction | 9,504 | 63,115 | 24,896 | 2.44 |
| Shipyards | 25 | 85,212 | 3,040 | 0.47 |
| Total, all application groups | 91,624 | 5,598,293 | 237,496 | (**) |
| Footnote(*) In most cases, the estimated number of establishments in each application group was based on the volume flow of MC in 1990 divided by the estimated MC use per facility. The estimated number of establishments was multiplied by the total number of employees per establishment and exposed employees per establishment as reported in CONSAD's survey | ||||
| Footnote(**) Netting out rehandling, estimated total consumption equals 469.2 million pounds manufactured, minus 129.1 million pounds exported, + 19.3 million pounds imported, + 32.10 million pounds recovered from used solvent. The column does not sum to 391.5 million pounds because non-consumptive uses such as production, distribution and formulation, and solvent recovery are included | ||||
| Sources: CONSAD, HSIA, PRMA, Office of Regulatory Analysis | ||||
In all, OSHA analyzed 28 application groups. These application groups include, among others, methylene chloride manufacturing, paint manufacturing, metal cleaning, polyurethane foam manufacturing, plastics and adhesives manufacturing, ink use, pharmaceuticals, and construction and shipyards. A total of 91,624 establishments are estimated to be potentially affected by the standard. These establishments employ a total of 5.6 million employees, of whom 237,496 are estimated to be exposed to MC in the course of their work. The application groups with the largest numbers of directly exposed employees are the Metal Cleaning, All Other Industrial Paint Stripping, and Ink Solvent Use groups. In many facilities, MC is used only by a small number of employees; the average number of MC-exposed employees per establishment covered by the final rule is only 2.6 employees.
Chapter III of the analysis assesses the technological feasibility of the final standard's requirements, and particularly its PELs, for firms in the 28 application groups identified in the Industry Profile. OSHA finds, based on an analysis of exposure data taken on workers performing the MC-related tasks identified for each application group, that compliance with the standard is technologically feasible for establishments in every application group studied. With few exceptions, employers will be able to achieve compliance with both PELs through the use of engineering controls and work practices. The few exceptions are certain maintenance activities, such as vessel cleaning, which have traditionally involved the use of respiratory protection, and operations in two applications where the supplemental use of respirators may be necessary. These operations are centrifuge unloading and dryer loading at one bulk pharmaceutical manufacturing facility operated by Abbott Laboratories, and operations involving access to and entering of the roll coating machine used by the Eastman Kodak Company to make film base.
The exposure data relied on by OSHA in making its technological feasibility determinations have been compiled in a database that contains thousands of MC exposure results (see Appendix B of this analysis) taken by OSHA compliance officers, consultation program consultants, MC-using companies, and interested parties. These data show that many facilities in many of the affected application groups have already achieved the reductions in employee exposures required by the final rule. In addition, the exposures of many employees in many job categories in a number of the application groups have been reduced to levels that are close to those required by the standard. OSHA's analysis of technological feasibility analyzes employee exposures at the operation or task level to the extent that such data are available. In other words, the analysis identifies relevant exposure data on a job-category-by-job category basis to permit the Agency to pinpoint those MC-exposed workers and job operations that are not yet under good process control and will thus need additional controls (including improved housekeeping, maintenance procedures, and employee work practices) to achieve compliance. Costs are then developed (see Chapter V of the economic analysis) for the improved controls needed to reach the new levels.
The benefits that will accrue to MC-exposed employees and their employers are substantial and take a number of forms. Chapter IV of the analysis describes these benefits, both in quantitative and qualitative form. First, based on a physiologically-based pharmacokinetic (PBPK) model, OSHA estimated that, if all 237,000 employees were exposed at the existing 8-hour TWA exposure limit of 500 ppm for an occupational lifetime of 45 years, a total of 29,862 excess cancer deaths would occur, or 126 excess cancer deaths per 1,000 workers. If, however, the 237,000 employees were exposed to the final standard's PEL of 25 ppm for 45 years, 8533 excess cancer deaths would be expected (3.6 per thousand workers). However, few workers are currently being exposed to 500 ppm of MC as an 8-hour TWA. The actual exposure levels of most affected workers are considerably lower, and, when these exposure levels, rather than 500 ppm, are used as the baseline, the PBPK model estimates that 1405 cancer deaths will be averted over a 45-year period. By reducing the total number of MC-related cancer deaths from 1,804 deaths to 399 deaths over 45 years, the standard will save an average of 31 cancer deaths per year. Table VIII-2 shows these risk estimates.
| 0-12.5 | 12.5-25 | 25 | 25-50 | 50-100 | |
| Lifetime Excess Cancer Risk (per thousand workers)(*) | 0.91 | 2.71 | 3.60 | 5.53 | 11.98 |
| Baseline Number of Workers Ex- posed | 141,323 | 26,464 | 162 | 22,839 | 23,903 |
| Estimated Excess Deaths in Base- line (Existing PEL)(**) | 129 | 72 | 1 | 126 | 286 |
| Predicted Number of Workers Ex- posed at New PEL | 159,825 | 28,441 | 49,229 | 0 | 0 |
| Predicted Excess Deaths at New PEL(**) | 146 | 77 | 176 | 0 | 0 |
| -Continued- | |||||
| 100-200 | 200-350 | 350-500 | 500+(***) | Total | |
| Lifetime Excess Cancer Risk (per thousand workers)(*) | 28.45 | 61.75 | 104.44 | 125.78 | |
| Baseline Number of Workers Exposed | 14,803 | 3,281 | 1,297 | 3,422 | 237,495 |
| Estimated Excess Deaths in Base- line (Existing PEL)(**). Predicted Number | 421 | 203 | 135 | 430 | 1,804 |
| of Workers Ex- posed at New PEL | 0 | 0 | 0 | 0 | 237,495 |
| Predicted Excess Deaths at New PEL(**) | 0 | 0 | 0 | 0 | 399 |
| Footnote(*) Based on OSHA's final estimate using the PBPK model, as presented in the Quantitative Risk Assessment section of the Preamble | |||||
| Footnote(**) Computed as level of lifetime risk times the number of exposed workers | |||||
| Footnote(***) For workers exposed to levels of greater than the current PEL of 500 ppm, the risk estimate is that associated with a lifetime esposure to 500 ppm | |||||
| Source: Office of Regulatory Analysis; OSHA; Department of Labor | |||||
In addition to cancer deaths, the standard is estimated to prevent 3 deaths per year from MC's acute central nervous system and carboxyhemoglobinemic effects. (Carboxy-hemoglobinemia is the inability of the blood to carry sufficient oxygen to supply the heart muscle; because methylene chloride interferes with the blood's ability to carry oxygen, exposure to it places susceptible individuals, such as those with silent cardiovascular disease, pregnant women, and smokers, at greater risk.) OSHA receives reports every year of workers who have succumbed to MC's acute CNS toxicity while they were engaged in such tasks as floor stripping. For example, the Agency recently received a fatality report on two young workers who died after pouring 14 gallons of MC on a squash court they were refinishing. Both of these employees lost consciousness, collapsed, and subsequently died of respiratory failure. In addition, MC exposures above the level at which the final rule's STEL is set -- 125 ppm -- are also associated with acute central nervous system effects, such as dizziness, staggered gait, and diminished alertness, all effects that can lead to workplace accidents. OSHA estimates that as many as 30,000 to 54,000 workers will be protected by the final rule's STEL from experiencing CNS effects and episodes of carboxyhemoglobinemia every year. Moreover, exposure to the liquid or vapor forms of MC can lead to eye, skin, and mucous membrane irritation, and these material impairments will also be averted by compliance with the final rule. Finally, contact of the skin with MC can lead to percutaneous absorption and systemic toxicity and thus lead to additional cases of cancer that have not been taken into account in the benefits assessment presented in Chapter IV of the Final Economic Analysis.
The costs employers in the affected application groups are estimated to incur to comply with the standard total $101 million in 1994 dollars. These costs, which are presented in Chapter V of the full economic analysis, are annualized over a 10-year horizon at a discount rate of 7 percent. Table VIII-3 shows annualized costs by provision of the standard; the most costly provisions are those requiring engineering controls, protective clothing and eye protection, and medical surveillance for MC-exposed workers. These three provisions together account for approximately 75 percent of the standard's compliance costs.
| Provision | annualized Costs |
| Engineering Controls | $38,773,642 |
| Respirators | 6,374,083 |
| Monitoring | 9,849,577 |
| Protective Clothing and Eye Protection | 29,578,340 |
| Emergency Eyewash and Shower | 3,183,486 |
| Medical Surveillance | 7,986,493 |
| Leak and Spill Detection Program | 3,703,286 |
| Regulated Areas | 150,884 |
| Recordkeeping | 652,121 |
| Training | 196,656 |
| Understanding Regulation and Developing Training | 777,132 |
| Subtotal | 101,225,701 |
| costs of Substitution | 237,336 |
| Total | 101,463,037 |
| Source: Office of Regulatory Analysis; OSHA; Department of Labor | |
Table VIII-4 analyzes compliance costs by application group and shows that the Cold Cleaning application group, which is in the larger Metal Cleaning grouping, and the Furniture Stripping application group, which is in the larger Paint Stripping category, will incur the largest costs of compliance (though not necessarily the largest economic impacts). These costs reflect the high exposures and relative lack of control measures currently existing in many establishments in these two application groups. In other words, because MC exposures are poorly controlled in so many cold cleaning and furniture stripping facilities, employers in these industries will be required by the standard to implement control measures to protect their employees from the significant risk of MC exposure.
| application group | annualized costs |
|---|---|
| Methylene Chloride Manufacturing | 8,150 |
| Distribution/Formulation of Solvents | 794,099 |
| Metal Cleaning: | |
| Cold Degreasing and Other Cold Cleaning | 26,950,869 |
| Open-Top Vapor Degreasing | 371,096 |
| Conveyorized Vapor Degreasing | 97,253 |
| Semiconductors | 247,666 |
| Printed Circuit Boards | 217,479 |
| aerosol Packaging | 297,999 |
| Paint Remover Manufacturing | 229,724 |
| Paint Manufacturing | 89,697 |
| Paint Stripping: | |
| Aircraft Stripping | 8,148,754 |
| Furniture Stripping | 10,689,840 |
| All Other Industrial Paint Stripping | 24,413,924 |
| Flexible Polyurethane Foam Manufacturing | 4,252,861 |
| Plastics and Adhesives Manufacturing and use | 5,417,950 |
| Adhesive Production | |
| Adhesive Use | |
| Injection Molding | |
| Lamination | |
| Mold Release | |
| Ink and Ink Solvent Manufacturing | 23,518 |
| Ink Solvent Use | 3,360,723 |
| Pesticide Manufacturing and Formulation | 106,060 |
| Pharmaceutical Manufacturing | 311,708 |
| Solvent Recovery | 49,829 |
| Film Base Manufacturing | 47,454 |
| Polycarbonate Manufacturing | 4,651 |
| construction | 14,922,000 |
| Shipyards | 518,544 |
| Total, all application groups | 101,463,037 |
| Source: Office of Regulatory Analysis; OSHA; Department of Labor | |
Chapter VI of the economic analysis analyzes the impacts of compliance costs on firms in affected application groups. The standard is clearly economically feasible: on average, annualized compliance costs amount only to 0.18 percent of estimated sales and 3.79 percent of profits. For all but three application groups -- polyurethane foam blowing, furniture stripping, and construction -- compliance costs are less than 3 percent of profits, and for all but one application group -- furniture stripping -- annualized compliance costs are less than 0.5 percent of the value of sales. Table VIII-5 shows average compliance cost impacts across the many Standard Industrial Classification (SIC) codes potentially involved in the application groups studied.
| application group | Number of establishments complying | annualized costs of compliance | |
|---|---|---|---|
| as percent of sales | as percent of profit | ||
| Manufacture of MC | 4 | (*) | 0.04 |
| Distribution/Formulation of Solvents | 320 | 0.04 | 0.55 |
| Metal Cleaning: | |||
| Cold Degreasing and Other Cold Cleaning | 23,717 | 0.01 | 0.18 |
| Open-Top Vapor Degreasing | 278 | 0.01 | 0.22 |
| Conveyorized Vapor Degreasing | 45 | 0.02 | 0.35 |
| Semiconductors | 239 | (*) | 0.05 |
| Printed Circuit Boards | 141 | 0.02 | 0.41 |
| aerosol Packaging | 50 | 0.01 | 0.13 |
| Paint Remover Manufacturing | 80 | 0.02 | 0.06 |
| Paint Manufacturing | 49 | 0.01 | 0.04 |
| Paint Remover Use (Paint Stripping): | |||
| Aircraft Stripping (Large Firms) | 75 | 0.07 | 1.34 |
| Aircraft Stripping (Small Firms) | 225 | 0.08 | 2.12 |
| Furniture Stripping | 6,152 | 2.04 | (**)39.40 |
| All Other Industrial Paint Stripping | 35,041 | 0.01 | 0.11 |
| Flexible Polyurethane Foam Manufacturing | 100 | 0.32 | (**)9.23 |
| Plastics and Adhesives Manufacturing and Use | 3,487 | 0.03 | 0.52 |
| Ink and Ink Solvent Manufacturing | 15 | (*) | 0.03 |
| Ink Solvent Use | 11,869 | 0.03 | 0.05 |
| Pesticide Manufacturing and Formulation | 60 | 0.01 | 0.35 |
| Pharmaceutical Manufacturing | 108 | (*) | 0.03 |
| Solvent Recovery | 37 | 0.05 | 0.85 |
| Film Base | 1 | (*) | 0.01 |
| Polycarbonates | 4 | (*) | (*) |
| construction | 9,504 | 0.35 | (**)9.67 |
| Shipyards | 25 | 0.07 | 1.72 |
| all Application groups | 91,625 | 0.18 | 3.79 |
| Footnote(*) = less than .005% | |||
| Footnote(**) These relatively high impacts on profits assume that no price increase is possible. In all three cases, price increases of 2.1 percent or less would fully restore profits. In all of these application groups, most firms will be able to increase prices to offset their regulatory costs. In furniture stripping, a substantial portion of the market is for antique refinishing that involves MC use, a service which is relatively price insenstive. Soft flexible foam of the kind MC is used to make is an essential material in the construction of cushions of all types. In the construction sector, MC based paint stripping and foam blowing are essential operations of many of the jobs in which they are used | |||
| Sources: CONSAD; Dun & Bradstreet; Office of Regulatory Analysis, OSHA, Department of Labor | |||
It is important to understand that OSHA's methodology tends to overestimate the economic impacts of the standard, for a number of reasons. For example, OSHA's cost methodology does not take into account the many simple and virtually cost-less improvements in employee work practices and housekeeping procedures that would enable many employers to achieve compliance with the final rule's PELs. In flexible polyurethane foam manufacturing, for example, OSHA's costs may be overestimated because it was assumed that no firms would substitute away from MC entirely, even though some firms have already done so (as described in Chapter III, Technological Feasibility). Despite the fact that OSHA's cost estimates are likely to be overestimates, OSHA decided to examine in greater detail the three application groups shown by the economic analysis to have the highest costs as a percentage of profits, i.e., furniture stripping, polyurethane foam manufacturing, and construction.
In the furniture refinishing application group, compliance costs are 2.0 percent of the value of revenues and 39 percent of the value of before-tax profits. Approximately half of all furniture refinishing sales derive from antique refinishing, a market niche that is unlikely to be sensitive to a 2.0 percent change in price. Even in the area of used furniture refinishing, which constitutes the remaining half of the furniture refinishing market, a 2.0 percent price increase would be unlikely to significantly alter the amount of furniture being refinished. In general, price increases of this magnitude would be expected to result only in a very small drop in the demand for furniture refinishing. If this were not the case, normal business fluctuations, such as drops in the relative cost of new furniture or a major increase in the price of methylene chloride (such as has occurred in recent years) would also have had major impacts on the industry.
In construction and polyurethane foam manufacturing, compliance costs for the average firm are 9.2 and 9.7 percent of profits, respectively. However, to offset these costs, construction firms would need only to increase their revenues by 0.35 percent and foam blowing operations would need only to increase the price of their products by 0.32 percent. In construction, such price increases are unlikely to present a problem, since the use of MC is essential on many larger construction projects. For example, it is difficult to believe that demand for remodeling or renovation projects would be seriously altered by a 0.35 percent increase in the cost of the paint stripping portion of the job. In flexible polyurethane foam manufacturing, either MC or an appropriate substitute is essential to the production of low density, or soft, foam, and foam, in turn, is essential to the production of many kinds of furniture. Demand for such products is unlikely to change as a result of an 0.32 percent increase in the price of flexible foam. OSHA therefore concludes that even marginal firms in these three sectors -- furniture stripping, construction, and flexible foam blowing -- are unlikely to close as a result of the compliance costs of this standard.
To ensure that the analysis of average impacts presented in the economic analysis did not obscure potentially significant economic impacts at the 4-digit SIC level, OSHA performed an in-depth analysis of the 4-digit SICs potentially involved in the Cold Cleaning and All Other Industrial Paint Stripping application groups. The results of this in-depth analysis are presented in Appendix D of the full economic analysis. In all, a total of 162 4-digit SICs potentially impacted by the standard in the Cold Cleaning group and more than 200 4-digit SICs in the Other Industrial Paint Stripping group were analyzed. Across all of the Cold Cleaning SICs, the average impact of the costs of compliance is 0.06 percent of revenues and 1.12 percent of profits. The largest impacts on profits occur in SIC 3412, Metal Barrels, Drums, and Pails, and SIC 3494, Valves and Pipe Fittings not elsewhere classified; in these cases, impacts on profits are 13.3 and 15.1 percent, respectively. In both of these cases, however, these impacts are explained by extremely low profit margins (less than .02 percent of sales, i.e., less than $2 per $10,000 in sales, in 1994). As a result, a price increase of less than one cent per $100 of revenue would leave profits unchanged. Such a price increase is feasible because an increase of this magnitude is unlikely to lead to significant changes in the demand for metal barrels or valves and pipe fittings. In no other 4-digit Cold Cleaning SIC did impacts reach even 5 percent of profits.
Across all 200-plus Industrial Paint Stripping SICs, the average impact of the costs of compliance on revenues is 0.03 percent. The largest impact of costs on sales is 0.33 percent and occurs in SIC 7532, Auto Top, Body Repair, and Paint Shops (discussed further below). The average impacts of costs on profits across these SICs is 0.17 percent. The largest impacts on profits occur in SIC 3412, SIC 3494 (both discussed above), and in SIC 7532, Auto Tops, Body Repair and Paint Shops; in all three of these SICs, cost impacts are between 6 and 8 percent of profits. Again, the explanation for these impacts in SICs 3412 and 3494 is that their profit margin in 1994 was vanishingly low. The resulting price increases required to maintain profits are also extremely small, and OSHA concludes that such an increase is likely to take place in these cases. In SIC 7532, the other relatively high impact SIC, profit margins are relatively high (approximately 4.4 percent), and thus a small decline of this magnitude would have relatively little impact.
Summary of the Regulatory Flexibility Analysis
In its 1991 proposal, OSHA requested comments and information that would assist the Agency in identifying small-business users of MC and in structuring the final standard so that these users would be able to achieve the standard's worker protection goals in ways that would be technologically and economically feasible for them (56 FR 57041 to 57043). OSHA anticipated that, as stated in the proposal, the standard might have a significant economic impact on small entities in at least two application groups: firms with fewer than 20 employees that engage in stripping of paint from aircraft, and firms with fewer than 20 employees that engage in furniture stripping.(3) OSHA also requested comment concerning the standard's impact on small employers in light of the Regulatory Flexibility Act's mandate to consider and minimize impacts on small businesses, consistent with the purposes and criteria of the standard's enabling legislation (56 FR 57115 to 57121).
Many commenters identified additional application groups that include small establishments likely to have difficulty achieving all of the standard's protective goals if the requirements of the standard were structured in a one-size-fits-all manner. These commenters provided considerable data and identified many possible modifications and alternatives to the proposed standard that they believed would facilitate compliance and mitigate the standard's impact on MC-using establishments with fewer than 20 employees.
None of the comments concerning small employer issues, whether in the context of economic or technological feasibility or the Regulatory Flexibility Act, disagreed with OSHA's basic premise that the fewer-than-20-employee cut-off was appropriate to distinguish between large and small MC-using businesses, was a useful way of characterizing the compliance abilities and limitations of affected employers and is an appropriate definition for purposes of the Regulatory Flexibility Act. Use of this numerical cut-off point captures 61 percent of all establishments potentially affected by the final rule. MC-users with fewer than 20 workers tend to have the characteristics of "mom-and-pop" businesses, whereas establishments with 20 or more workers are generally more sophisticated in terms of the technology they use and their management resources. The 20-employee threshold has also proved to be an agreed-on and useful cut-off point in past OSHA rulemakings (see, for example, the permit-required confined spaces standard (58 FR 4547) and the process safety management standard (57 FR 6402)).
During Executive Order 12866 review, the Office of Advocacy of the Small Business Administration expressed its views concerning OSHA's small business definition. In a letter to OMB, the SBA's Chief Counsel for Advocacy stated in a letter dated August 16, 1996, that "[t]he regulatory alternatives developed, using OSHA's size standard of less than 20 employees, were somewhat beneficial to two of the three industries [furniture stripping, polyurethane foam blowing, and construction]. These industries, i.e., furniture stripping and construction, are predominantly micro businesses that fall into OSHA's definition of small" (Ex. 130). The Office of Advocacy was concerned, however, that the 20-employee cut-off did not adequately deal with the MC-using polyurethane foam manufacturing sector. (In this application group, the majority of establishments likely to experience significant economic impacts fall into the 20 to 99-employee size category.) "[T]he characteristics of the manufacturing sector indicate that the [20 employee] size standard was not appropriate in that industry for the purposes of regulatory flexibility." Id. The SBA concluded that OSHA should consider taking additional steps to address implementation burdens and the needs of the polyurethane foam manufacturing sector.
Working with OMB and the SBA's Office of Advocacy to resolve this concern, OSHA reexamined the potential impacts of the standard on polyurethane foam manufacturing establishments in the 20 to 99 employee size category in the context of economic impact issues. As explained more fully in the Final Economic and Regulatory Flexibility Analysis, OSHA concluded that, even though members of this group were not small employers, some accommodation would be necessary to assure that employees working in establishments of this size in this industry would not receive less protection than all other MC-exposed employees. Accordingly, OSHA extended the engineering control implementation date for this group of establishments by one year. This extended phase-in is designed to enable this group of employers to plan for and accumulate the capital to finance needed controls, install them, and ensure their effective and consistent operation before the compliance deadline.
OSHA's extensive feasibility studies and focus on small business issues resulted in a number of modifications that have made the standard more cost-effective for business while maintaining protection for workers. In addition, OSHA conducted an alternative screening analysis to measure the final rule's potential impacts on establishments in the regulated community using the SBA's size standards. For most application groups, this meant that OSHA examined the standard's economic impacts on firms at the 500 employee level. (Financial data are not available for cut-off points higher than 500 employees; thus, OSHA used that cut-off for all application groups.) In some cases, the SBA size standards are defined in terms of annual revenues, and for SICs so defined, OSHA translated these revenue figures into the appropriate employee size category. This SBA-based alternative screening analysis enabled the Agency to determine whether, by failing to look at potential impacts among firms in other size classes, significant impacts had been overlooked. The analysis conducted using the SBA size standards confirmed that any potentially significant economic impacts associated with the final rule occur among firms in the fewer-than-20-employee category, with one exception, i.e., firms in the 20-99 employee size category in the polyurethane foam manufacturing industry. (See the full Final Economic Analysis for additional detail.) For the final rule, OSHA has analyzed the costs of compliance as a percentage of profits, and costs as a percentage of revenues, for firms with fewer than 20 employees in every application group. This analysis identified significant economic impacts on a substantial number of small entities, and the Agency has accordingly conducted a full Final Regulatory Flexibility Analysis in accordance with the Regulatory Flexibility Act, as amended in 1996. The three application groups for which such impacts were identified were Furniture Stripping, Polyurethane Foam Blowing, and Construction. Table VIII-6 shows the results of this analysis in detail.
The full regulatory flexibility analysis is presented in Chapter VI of the Final Economic and Regulatory Flexibility Analysis. The remainder of this section briefly summarizes that analysis.
This rule is needed to prevent cancer deaths and other illnesses, as discussed in greater detail in the Health Effects Section (Section V of this Preamble). Section III of this preamble, Events Leading to the Final Standard, summarizes OSHA's efforts to assure input to this rulemaking by affected small firms. Table VIII-6 identifies the affected small firms by sector. OSHA estimates that a total of 56,000 small firms will be affected by this standard.
| application group | Number of small establishments affected | costs as a percentage of profits for small firms | costs as a percentage of sales for small firms |
|---|---|---|---|
| Manufacture of MC | 0 | NA | NA |
| Distribution/Formulation of Solvents | 139 | 3.0% | 0.2 |
| Metal Cleaning: | |||
| cold Degreasing and Other Cold Cleaning | 9,223 | 0.9 | 0.0 |
| Open-Top Vapor Degreasing | 0 | NA | NA |
| Conveyorized Vapor Degreasing | 11 | 2.4 | 0.1 |
| Semiconductors | 0 | NA | NA |
| Printed Circuit Boards | 20 | 2.0 | 0.1 |
| aerosol Packaging | 10 | 0.7 | 0.1 |
| Paint Remover Manufacturing | 34 | 0.3 | 0.1 |
| Paint Manufacturing | 7 | 0.1 | 0.0 |
| Paint Remover Use (Paint Stripping): | |||
| Aircraft Stripping (Large Firms) | 0 | NA | NA |
| Aircraft Stripping (Small Firms) | 75 | 4.5 | 0.1 |
| Furniture Stripping | 5,901 | 41.5(*) | 2.2 |
| All Other Industrial Paint Stripping | 25,441 | 0.8 | 0.0 |
| Flexible Polyurethane Foam Manufacturing | 8 | 60.3(*) | 1.7 |
| Plastics and Adhesives | |||
| Manufacturing and Use | 498 | 1.8 | 0.1 |
| Ink and Ink Solvent Manufacturing | 3 | NA | NA |
| Ink Solvent Use | 5,395 | 0.1 | 0.1 |
| Pesticide Manufacturing and Formulation | 40 | 6.6 | 0.2 |
| Pharmaceutical Manufacturing | 0 | NA | NA |
| Solvent Recovery | 17 | 2.7 | 0.1 |
| Film Base | 0 | NA | NA |
| Polycarbonates | 0 | NA | NA |
| construction | 9,085 | 19.9(*) | 0.5 |
| Shipyards | 0 | NA | NA |
| all Application groups | 55,908 | 8.2 | 0.3 |
| NA=No small firms in this application group | |||
| Footnote(*) These relatively high impacts on profits assume that no price increase is possible. In all three cases, price increases of 2.1 percent or less would fully restore profits. In all of these application groups, most firms will be able to increase prices to offset their regulatory costs. In furniture stripping, a susbtantial portion of the market is for antique refinishing that involves MC use, a service which is relatively price insensitive. Soft flexible foam of the kind MC is used to make is an essential material in the construction of cushions of all types. In the construction sector, MC based paint stripping and foam blowing are essential operations of many of the jobs in which they are used | |||
| Sources: CONSAD; Dun & Bradstreet; Office of Regulatory Analysis, OSHA, Department of Labor | |||
The Summary and Explanation section of this preamble provides a description of the compliance requirements associated with this rule, and a paperwork burden analysis of the record keeping requirements is provided in the Collection of Information Request for Comment section at the beginning of this preamble. Based on comments regarding anticipated effects on small businesses, OSHA has reduced the final rule's overall paperwork requirements from those proposed and has refined some paperwork requirements to simplify compliance for small entities.
OSHA considered numerous regulatory alternatives and modifications to the requirements of the proposed standard (ranging from higher PELs, to 40-hour rather than 8-hour time weighted average exposure limits, to delayed implementation dates) that commenters believed might minimize significant economic impacts on small businesses. OSHA rejected those alternatives that clearly decreased the safety of workers in small establishments, but the Agency also adopted many regulatory changes that will improve small employers' ability to provide their employees with the same level of protection as that afforded workers in larger establishments. As explained more fully in the Final Economic Analysis and summarized in Table VIII-7, the final standard contains delayed implementation dates, reduced paperwork requirements, streamlined medical surveillance provisions and other accommodations that, in the Agency's judgment, will minimize any significant economic impacts of the standard on small employers to the extent necessary to enable them to meet the standard's protective goals.
| change to proposed regulation | Impact on small businesses |
|---|---|
| Firms with fewer than 20 employees given 3 years (rather than 1) to achieve PEL using engineering controls | More performance oriented and flexible, reduces costs to small businesses in first two years by 30 to 40%, allows small businesses time to plan major expenditures |
| allows the use of licensed health care professionals in addition to physicians for medical surveillance | Provides greater flexibility |
| Laboratory tests are at the discretion of physician rather than automatically required | Reduces costs of medical surveillance by more than 14 percent, more performance oriented |
| Employees under 45 are required to have a physical every three years rather than annually | Reduces costs of medical surveillance by 30 percent |
| Respirators required in regulated areas only when PEL is likely to be exceeded | Decreases respirator use and costs for small business |
| If MC is used less than 30 days per year, monitoring may be conducted with direct reading instruments | Significantly reduces costs of monitoring for establishments making limited use of MC; this provision will be especially helpful in construction |
| Written compliance plans are no longer required | Reduces paperwork |
| Hazard communication requirements do not go beyond what is already required by hazard communication standard | Reduces paperwork and costs |
| Employee re-training only as needed rather than annually | More performance oriented, reduces costs of training 80 percent |
| Simplified recordkeeping for small businesses for exposure monitoring data | Reduces paperwork |
IX. Environmental Impact
This section analyzes the impact on the environment of changing the standard for methylene chloride (MC) to an eight-hour time weighted average (TWA8) permissible exposure limit (PEL) of 25 parts per million (ppm), with a 125 ppm 15-minute short-term exposure limit (STEL) and ancillary requirements. It is based principally on information collected for OSHA by CONSAD Research Corporation and its subcontractor, PEI Associates Inc., and reported in Economic Analysis of Draft Regulatory Standard for Methylene Chloride, 1990, OSHA Docket, Ex. 15, and also draws upon other materials in the OSHA docket.
Current uses of methylene chloride involve releases to the air through venting of storage tanks or drums and through evaporation of MC during the performance of various activities such as paint stripping and cold cleaning indoors or outdoors. The volume of MC emitted as a percentage of MC used varies greatly among industries. Some processes, such as polyurethane foam manufacturing and paint stripping, typically release 100 percent of the MC to the atmosphere (Ex. 15). Other uses, such as solvent recovery and the manufacture of methylene chloride, involve less than 1 percent of the MC used being emitted to the atmosphere (Ex. 15). In addition, air, water, or solid waste pollution may occur as a result of the disposal of waste residues containing MC. Additional details by application group are presented in CONSAD's report [Ex. 15].
Future environmental releases of methylene chloride resulting from the final standard will largely be a function of how it affects the demand for methylene chloride and for its substitutes. The demand for methylene chloride has been declining (e.g., generally, it is no longer being used in formulating hairsprays). Any regulatory action by OSHA is expected to further reduce the demand for MC and thus the extent of its environmental releases.
Although it is technically possible to substitute chlorofluorocarbons (CFCs) for methylene chloride in electronics and foam blowing, OSHA does not expect the revision of the MC standard to have any such effect. CFC products are significantly more expensive than MC products and are themselves being phased out or banned because of their effects on the environment.
To the extent that firms might have to use greater quantities of substitute chemicals to get the same effects formerly obtained with MC, waste residues and disposal costs would increase. On the other hand, increases in MC leak prevention and recycling would improve the environment.
The Paint Remover Manufacturers Association (PRMA) has charged that the standard would cause "massive amounts" of methylene chloride to be emitted into the atmosphere (Ex. 19-11). In Chapter III, OSHA noted that it could find no convincing argument by PRMA as to why the total amount emitted after installation of exhaust ventilation would differ significantly from the amount now simply leaking into the atmosphere.
At informal public hearings, PRMA stated that "an exposure level of 25 PPM is so low that it brings into the issue the formation of vapor clouds with levels of greater than 25 PPM that could move in and around the neighborhood," allegedly through decomposition of the MC [Tr. 245, 9/17/92]. There is no evidence that this hypothetical situation has ever occurred. PRMA may have confused decomposition with diffusion [Tr. 940-941, 9/21/92]. At Eastman Kodak Company, which currently emits more methylene chloride into the atmosphere than any furniture stripper possibly could, the chemical has diffused so rapidly that no clouds of MC have been formed [Tr. 1237-1238, 9/22/92].
Generally, it is not expected that any significant environmental impact will result from revision of the methylene chloride standard.
X. Summary and Explanation of the Final Standard
Introduction
The final standard for occupational exposure to methylene chloride (MC) is different in several important respects from the proposed MC standard published in the Federal Registerin 1991 (56 FR 57036). For example, the standard has been written in plain language, is more performance-oriented than the proposal, and substantially reduces the amount of paperwork employers will have to complete. Employers will thus find compliance with the standard easier, their paperwork less extensive, and their obligations clearer and less burdensome. These changes are discussed in greater detail in the appropriate sections of this Summary and Explanation. OSHA seeks input from users of the standard on whether these changes are helpful and what other changes could be made to future standards to increase their user-friendliness. OSHA will also be conducting a number of compliance assistance and outreach projects in connection with this standard to assist employers and employees to comply.
As part of the Agency's new approach to standards writing, OSHA has included an introductory paragraph in the standard to provide readers with information on MC, its health effects and principal uses, and the reasons OSHA is regulating this toxic substance. This introductory language is non-mandatory and is intended only to provide information and enhance compliance.
This final rule is an occupational health standard that establishes requirements to control employee exposure to MC, a chemical compound found in many different types of industries. OSHA has determined that this standard is necessary because exposure to MC places employees at significant risk of developing exposure-related adverse health effects. These effects include cancer, effects on the heart and central nervous system, and skin and eye irritation. Employee exposure to MC can occur through inhalation or through skin absorption or contact with the skin. This substance is frequently used as a solvent in many different kinds of jobs, including furniture stripping, foam blowing, film manufacturing and metal degreasing.
Although the final rule covers many different types of workplaces where MC is used, the extent of coverage depends on the magnitude of employee exposure. Although all covered employers, i.e., those with MC in the workplace, must determine initially the extent to which their employees are exposed to MC, those with exposures at or below the action level will only have to document the results of this initial determination, provide employee information and training, and provide means of protecting employees from contact with liquid MC. The standard's other requirements, such as those for engineering controls, medical surveillance, etc. apply only to workplaces where employee exposures to MC exceed the action level.
Paragraph (a) Scope and application
This standard applies to all occupational exposures in workplaces covered by OSHA in general industry, construction and shipyards where MC is produced, released, stored, handled, or used.
As discussed in the Health Effects and Significance of Risk sections of this preamble, OSHA has determined that exposure to MC at the former PEL creates a significant risk that employees' health will be materially impaired. Possible adverse health effects include cancer, cardiac effects, central nervous system effects, and skin or eye irritation. Exposures to MC are found in various general industry, construction, and shipyard facilities, and OSHA has determined that there are feasible measures to control them in each of these types of employment.
In the proposal's Authority section, OSHA preliminarily determined, under Section 4(b)(2) of the OSH Act, that it would be appropriate for the MC standard to supersede any corresponding longshoring standards in 1910.16 and 29 CFR part 1918. The Agency therefore proposed to add a new paragraph (m) to 1910.19. In addition, in questions raised by the Agency in its Notice of Public Hearing, OSHA requested input regarding the use of MC in longshoring. However, OSHA has subsequently proposed (59 FR 28594, June 2, 1994) to revise its marine terminal (part 1917) and longshoring (part 1918) standards. Those proposed standards (proposed Secs. 1910.16(b)(2), 1917.1(b)(2)(xiv), and 1918.1(b)(1)) would apply OSHA's toxic substance standards (part 1910, subpart Z) only when the packaging in which a substance is being transported in the maritime environment has broken open. This language, based on the existing marine terminal standard (1910.16(b)(2)(ii)), reflects the view that hazardous substances, when properly packaged, do not pose significant exposure risks for the shipyard employees transporting them in closed packages.
Therefore, as revised, final rule 1910.19(m) states that 1910.1052 will address MC exposure in marine terminal and longshore employment only where leaking or broken packages allow MC exposure that is not addressed through compliance with 29 CFR parts 1917 and 1918. Given the promulgation of 1910.19(m), the Agency has determined that it is unnecessary to mention marine terminals and longshoring in final rule 1910.1052(a), Scope and application.
OSHA has not learned of any circumstances in which marine terminal or longshore employees have been exposed to MC because of damage to packaging. The Agency, accordingly, anticipates that the MC final rule will have little or no impact on the marine terminal and longshoring industries.
In developing this rule, OSHA has consulted with its Shipyard Employment Standards Advisory Committee (SESAC) to obtain information on MC use and exposure in shipyards and has taken the Committee's input into consideration in developing the standard. In particular, OSHA has relied on data provided by SESAC in assessing the technological feasibility and costs of compliance of the standard for shipyards covered by the rule.
Since the construction industry is also included in the scope of the final rule, OSHA is required to consult the Advisory Committee on Construction Safety and Health (ACCSH) in accordance with section 107 of the Contract Work Hours and Safety Standards Act (40 U.S.C. 333) (the Construction Safety Act) and 29 CFR 1911.10. On July 28, 1992, OSHA formally consulted with ACCSH regarding the construction-specific aspects of occupational exposure to MC. The Agency solicited comment and testimony regarding ACCSH's recommendations through a Federal Registernotice (57 FR 36964, August 17, 1992). One of ACCSH's suggestions was that the rule specifically require originators of contract bids to stipulate a requirement for compliance with the MC standard in their bids. OSHA has not adopted this suggestion in the final rule because construction contracts already require compliance with all relevant Federal regulations. The specific suggestions made by ACCSH and OSHA's responses to ACCSH's input are discussed below in the relevant paragraphs of the Summary and Explanation.
In the proposal, the scope and application paragraph included an exemption for employers with workplaces where MC products were present but objective data were available to demonstrate that the product could not release MC above the action level or STEL under those foreseeable conditions of processing, use, and handling that would cause the greatest possible release. This concept remains in the final standard, although the provision has been moved to the exposure monitoring section (paragraph (d)), because this provision constitutes, in effect, an exception to the standard's requirement for initial monitoring.
The Air Transport Association [Ex. 19-75] requested that airlines be excluded from the general industry standard, and that a separate standard covering MC use in the airline industry be developed. OSHA has specifically determined that the exposures, work operations, and means of compliance for aircraft-related MC uses are similar to those in many other establishments and thus that there is no substantive basis for the requested exemption. Consequently, OSHA has concluded that no industry-specific standard for airlines is warranted. MC uses in the airline industry are discussed in the section of the final economic analysis entitled "Aircraft Stripping."
Paragraph (b) Definitions
This paragraph includes definitions of a number of terms used in the regulatory text of the final standard. Although some of these terms are in common use, OSHA believes that these definitions will help to ensure that their meaning in the context of the standard is clear.
Action level means an airborne concentration of MC of 12.5 ppm, measured as an 8-hour time-weighted average. One purpose of the action level is to relieve the burden on employers by providing a cut-off point below which many of the compliance activities in the standard are not required. In addition, due to the variable nature of employee exposures to airborne concentrations of MC, compliance with an action level provides employers with greater assurance that their employees will not be exposed to MC concentrations above the permissible exposure limits.
The action level also increases the cost-effectiveness and performance orientation of the standard while improving employee protection. The standard will encourage employers who can, in a cost-effective manner, identify approaches or innovative methodologies to reduce their employees' exposures to levels below the action level, because this will eliminate the costs associated with exposure monitoring and medical surveillance, two provisions of the standard that are triggered by exposure exceeding the action level. At the same time, the employees of such employers will be protected because their MC exposures will be less than half of those permitted by the permissible exposure limit. Employees of those employers who are not able to lower exposures below the action level will have the additional protection provided by medical surveillance, exposure monitoring, and the other provisions of the standard that are triggered by the action level.
The statistical basis for using an "action level" has been discussed in connection with several other OSHA health standards [see, for example, acrylonitrile (29 CFR 1910.1045) and ethylene oxide (29 CFR 1910.1047)]. In brief, although all employee exposure measurements on a given day may fall below the permissible exposure limit, some probability exists that on unmeasured days the employee's actual exposure may exceed the permissible exposure limit. Where exposure measurements are above the action level, the employer cannot reasonably be confident that the employee may not be overexposed on a given day. Therefore, requiring periodic employee exposure measurements to begin at the action level provides the employer with a reasonable degree of confidence in the results of his or her exposure measurement program [Ex. 7-248]. OSHA's decision to set the action level at one-half the PEL is based on its successful experience using this fraction as the action level in many standards, such as arsenic, ethylene oxide, vinyl chloride and benzene.
OSHA received comments from a number of rulemaking participants [Exs. 19-16, 19-20, 19-22, 19-31, 19-47, 19-75] suggesting that the proposed PELs and, by association, the action level, be revised. For instance, Hukill Chemical Corporation [Ex. 19-47] argued that the action level should be set at 100 ppm because it believes that: 1) CNS effects from MC are not observed in humans until 300 ppm; and 2) there is no evidence of excess cancer mortality in humans up to a level of 475 ppm. As explained in the Health Effects and Quantitative Risk Assessment sections of this preamble, OSHA disagrees with this commenter because the Agency has determined that significant risks exist at levels substantially below those referred to by the commenter and therefore that the suggested levels would not be adequately protective.
The Pharmaceutical Manufacturers Association (PMA) [Ex. 19-25] commented that the action level of 12.5 ppm is appropriate, but requested an exemption from "various requirements of the standard" if exposure occurs on fewer than 30 days a year. In particular, PMA suggested that periodic monitoring be required only when there is exposure above the PEL or STEL for at least 10 days a year or at or above the action level for at least 30 days a year. OSHA has considered this issue, along with similar concerns raised by ACCSH, and agreed that in cases where exposure occurs only on a few days per year, it was appropriate to alter the exposure monitoring requirements. Specifically, paragraph (d)(2)(iii) would permit employers whose employees are exposed to MC on fewer than 30 days per year to forego the initial monitoring required by paragraph (d)(2), provided that the employer has taken measurements that give immediate results (such as those taken by detector tube) and that provide sufficient information about exposures to determine what (if any) control measures are necessary. In addition, the medical surveillance requirement (paragraph (j)), with the exceptions described in the final rule, applies only where employees are exposed above the action level on at least 30 days within a year or above the PELs on at least 10 days within a year.
Newport News Shipbuilding [Ex. 19-37] suggested that the action level be set at 15 ppm. However, adopting this suggestion would not be consistent with the statistical basis for establishing the action level at one-half the PEL, as described above. In addition, Markey Restoration Company [Tr. 2671-72, 10/16/92] recommended that the action level be eliminated based on the costs of medical surveillance triggered by that level. As noted above, an action level is based on the probability of exceeding the PEL and is designed to enhance both employee protection and the standard's cost-effectiveness, and OSHA does not believe it would serve either employers or employees to eliminate this concept from the final rule.
The UAW [Tr. 1885-86, 9/24/92] questioned the statistical arguments underpinning the action level that OSHA has used for some years. According to the UAW's calculations, the action level should actually be set at one-tenth the PEL to accomplish the purpose OSHA intended. Accordingly, the UAW argued that: "[I]f you leave it [the action level] at 1/2, [there is] almost the virtual certainty that workers are overexposed on that job." In response, OSHA notes that its experience with action levels set at one-half the 8-hour TWA PEL has been favorable and that employers and employees have benefitted from the use of the action level concept. In particular, it is OSHA's experience that, for most workplaces, variability is normally such that an action level set at one-half the TWA PEL is appropriate. The final standard thus continues this practice.
Emergency means any occurrence, such as but not limited to, equipment failure, rupture of containers, or failure of control equipment, which results, or is likely to result in an uncontrolled release of MC. The word "uncontrolled" was changed from "unexpected" in the proposal to be more descriptive and to be consistent with the Hazard Communication Standard (29 CFR 1910.1200) and the Hazardous Waste Operations and Emergency Response Standard (29 CFR 1910.120). Incidental releases of MC -- i.e., those where the substance can be absorbed, neutralized, or otherwise controlled at the time of release by maintenance personnel or other employees working in the immediate release area -- are not considered to be emergencies within the scope of this standard. Dow Chemical Company [Ex. 19-31] indicated that the examples of emergencies provided in the proposal (purging lines and cleaning sludge from tanks) should not be included in the final rule. Other commenters [Exs. 19-25, 19-28, 19-57] agreed with Dow that the examples provided with the definition in the proposal were inappropriate. In particular, Eli Lilly and Company [Ex. 19-28, p. 7] stated
Lilly agrees with the concept that an emergency should be tied to unexpected releases. It is therefore curious and illogical that the examples given -- purging of lines and cleaning tanks -- are not unexpected events. To the contrary, in the pharmaceutical industry these are planned events which could even occur daily.
On the other hand, the Upjohn Company [Ex. 19-49] commented as follows:
The language "unexpected significant release" is very vague and will not result in any consistent interpretation as to what type of a release meets this definition. We would recommend that the language be changed to "* * * which may lead to employee exposure at or above the eight hour, timed-weighted average (TWA) or at or above the short-term exposure limit (STEL)."
OSHA acknowledges that the language in question could be misunderstood and has deleted the parenthetical listing of some examples of emergency situations. Furthermore, the Agency recognizes that emergency situations, by their very nature, are difficult to anticipate and describe. Therefore, OSHA has not provided examples of emergency situations in the final rule. Instead, the final rule lists situations that OSHA does not consider emergencies, because these will help employers to identify situations in their workplaces that do constitute emergencies. OSHA recognizes that emergencies have certain aspects in common but that other aspects are specific to a given workplace. For example, employee exposure must be uncontrolled for an emergency to exist. Provisions of the standard that include requirements that employers must meet in case of an emergency include Methods of Compliance, Respiratory Protection, Medical Surveillance, and Employee Information and Training.
Employee exposure is defined as that exposure to airborne MC which occurs or which would occur if the employee were not using respiratory protective equipment. This definition is consistent with OSHA's previous use of the term "employee exposure" in other health standards.
Methylene chloride (MC), or dichloromethane, means an organic compound with the chemical formula, CH(2)Cl(2). Its Chemical Abstracts Registry Number is 75-09-2. Its molecular weight is 84.9 g/mole. Other information regarding the characteristics of MC may be found in the appendices to the final standard. MC is a colorless, volatile, liquid with a chloroform-like odor and is not flammable by standard tests in air, but will burn under extreme conditions. It has a boiling point of 39.85 C (104 F) at standard atmospheric pressure, a lower explosive limit of 12% and an upper explosive limit of 19.5% in air. It is completely miscible with most organic solvents but is sparingly soluble in water (1.3% by weight at room temperature). It has an extensive oil and fat solubility. Decomposition products during combustion or fire include phosgene, hydrochloric acid and carbon monoxide.
Physician or other licensed health care professional is defined as a person whose legally permitted scope of practice allows him or her to independently provide or be delegated the responsibility to provide some or all of the health care services required by final rule paragraph (j), Medical Surveillance. Use of this phrase is designed to increase the flexibility of the standard; the proposal used the more restrictive term "physician." OSHA intends that employers should consider the opinion of the applicable state licensing board, which defines the scope of practice for licensed health care professionals, when they are determining the appropriate provider to supply some or all of the medical services required by the standard. The new terminology recognizes that there are many services that non-physicians can provide, that some non-physicians have particular expertise in diagnosing and treating occupationally related diseases, and that the use of these providers is often a cost-effective and protective approach to the provision of medical care.
Regulated area means an area, demarcated by the employer, where an employee's exposure to airborne concentrations of MC exceeds or can reasonably be expected to exceed either the eight (8)-hour time-weighted average limit or the short-term exposure limit. The wording of this definition has been changed slightly from that in the proposal for clarity. The requirements for regulated areas are discussed below in relation to paragraph (e).
OSHA has added a definition for symptom to the final rule to clarify what is meant by that term when it is referred to in the regulatory text. MC has a wide range of possible adverse health effects. This definition clarifies what portion of that range would be considered a symptom for purposes of the standard. The covered symptoms would include indications of central nervous system effects, such as headaches, disorientation, dizziness, fatigue, and decreased attention span; cardiac effects, such as chest pain and shortness of breath; and skin effects, such as chapping, erythema, or skin burns.
The definitions of "Assistant Secretary," "Authorized Person,"
"Director" and "This section" are consistent with OSHA's previous uses of these terms in other health standards.
The Boeing Company [Ex. 19-26] suggested that a definition be added for "work area" to preclude unnecessary monitoring in areas that do not contain MC. OSHA does not believe that this is necessary. If there is no MC present in an area, no monitoring needs to be performed for MC. In addition, the focus of this standard is employee exposure, as measured by personal monitoring, and not particular locations.
Paragraph (c) Permissible Exposure Limits
OSHA is promulgating an 8-hour time-weighted average (TWA) permissible exposure limit (PEL) of 25 ppm, and a short-term exposure limit (STEL) of 125 ppm averaged over 15 minutes, as proposed. OSHA has determined, based on evidence in the record, that occupational exposure to MC at the current 500 ppm 8-hour TWA PEL presents a significant risk of material health impairment, and particularly of cancer, to exposed employees and that compliance with the new standard will substantially reduce that risk. In combination with the STEL, the 8-hour TWA PEL and the other industrial hygiene provisions of the standard will also protect exposed employees from the other health effects caused by exposure to MC.
The basis for the 8-hour permissible exposure limit is discussed above in the sections on Health Effects and Significance of Risk, as well as in the economic analysis. OSHA believes that compliance with the new 25 ppm 8-hour TWA PEL is feasible and necessary to protect exposed employees from this significant risk of material health impairment.
OSHA received comments from a number of rulemaking participants suggesting that the proposed PELs and, by association, the action level be revised. The arguments for revising the proposed PELs were based on interpretations of the scientific support for given PELs and the feasibility of particular PELs in certain situations. Some commenters felt that the current level of 500 ppm does not provide adequate protection for employees and agreed that the PEL should be set at 25 ppm [Exs. 19-15, 19-49]. Specifically, Striptech International, Inc. [Ex. 19-15] stated:
The OSHA proposed 25 ppm standard for MC does substantially eliminate significant risk and it is feasible and definitely appropriate. The technology exists to enable the industries using MC to comply or to use an alternate method.
However, a number of rulemaking participants [Exs. 19-22, 19-23, 19-36, 19-38, Tr. 530, 9/18/92, Tr. 1776, 9/24/92, Tr. 1869, 9/24/92] suggested that OSHA set the 8-hour TWA PEL below 25 ppm, because they believe that the proposed 25 ppm limit would not adequately protect workers. For example, the UAW stated that setting a PEL at 25 ppm "will permit too much exposure to methylene chloride, therefore placing workers at great risk, contrary to the requirements of the OSHA Act" [Tr. 1869, 9/24/92]. The UAW stated that the proposed limit "would permit 2 deaths per thousand workers," and therefore suggested setting a PEL of 10 ppm, which the union felt would be feasible through specified engineering and work practice controls [Ex. 19-22, Tr. 1869, 9/24/92]. Scott Schneider, representing the IUE, also suggested that "because of the evidence of health effects from low level exposures" to MC, the PEL should be lowered below 25 ppm [Ex. 19-38]. The IUE and the ACTWU both supported the UAW recommendation of 10 ppm [Tr. 530, 9/18/92, Tr. 1776, 9/24/92].
The Laborers' Safety and Health Fund of North America [Ex. 19-36] suggested that worker exposure should be controlled to the lowest feasible level, which is consistent with NIOSH's position. NIOSH recommended "that occupational exposure to methylene chloride, which is a potential occupational carcinogen and may induce ischemic heart disease, be reduced below the proposed PEL to the lowest feasible level" [Tr. 868, 9/21/94]. OSHA agrees with these commenters that a significant risk remains at 25 ppm, but believes that this level is the lowest level for which OSHA can currently document feasibility across the affected application groups and industries.
OSHA's primary justification for the new standard is the risk of cancer associated with exposure to MC. Some commenters stated that the carcinogenicity of MC has not been proven and therefore that carcinogenicity should not be the basis for setting the PEL [Exs. 19-18, 19-29, 19-31, 19-45]. In particular, Kodak [Ex. 19-18] stated that it "does not believe that the human or animal data demonstrate a need to establish methylene chloride exposure limits at the levels proposed by OSHA in order to adequately protect employee health." Mr. Bixenman, representing Benco Sales, testified [Tr. 2638, 10/16/92] "And surely with our current level of technology, if methylene chloride were a human carcinogen, it could be established without question with actual diagnosed cases." Also, the Air Transport Association stated [Ex. 19-75]:
[T]he limited findings regarding cancer in mice at high MC dosage is weak justification for the proposed regulatory action. None of our members have found permanent health symptoms related to the use of MC, while usage at some facilities goes back at least 30 years. We have no data or experience connecting heart disease with MC use.
As discussed more extensively in the Quantitative Risk Assessment section, above, OSHA has based its assessment of MC cancer risk on the determination (supported by the NTP, EPA, and other agencies) that there is clear evidence of MC carcinogenicity in mice and rats. Although there are a few substances for which clear evidence of carcinogenicity in rodents has been deemed to be irrelevant to humans due to compelling evidence of mechanisms of action unique to the species tested, no such evidence exists for MC. In fact, as discussed in the Risk Assessment section, mechanistic evidence adds to the weight-of-the-evidence suggesting that MC is also carcinogenic in humans.
OSHA's final risk estimate indicates a risk of 7.5 deaths per 1000 workers exposed to MC at 50 ppm over a working lifetime and a risk of 3.6 deaths per thousand workers exposed to MC at 25 ppm over a working lifetime. OSHA has determined, using quantitative risk assessment, that the estimated risk of developing cancer warrants setting the 8-hour TWA PEL at 25 ppm and a 15-minute STEL at 125 ppm; in fact, at the 25 ppm PEL the residual risk still greatly exceeds any significant risk threshold, and only the lack of documentation of the feasibility of lower PELs across the affected industries has convinced the Agency not to reduce the PEL even further at this time.
OSHA disputes the contention of Mr. Bixenman that "actual diagnosed cases" are a precondition for establishing that a particular substance is carcinogenic to humans. Due to the natural background rate of all cancers, epidemiologic studies of groups are the only way to analyze human cause-effect relationships. As discussed in the Quantitative Risk Assessment section, OSHA has concluded that some of the available epidemiologic studies suggest a positive association between MC exposure and human cancer and that no epidemiologic studies of sufficient power exist to cast serious doubt on such conclusions.
Several commenters preferred a PEL of 50 ppm, which is the current ACGIH threshold limit value for MC, because they felt that a 25 ppm PEL would be either too costly to implement or the technology to achieve such a level of control was not available [Exs. 19-2, 19-3, 19-12, 19-14, 19-15, 19-29, 19-31, 19-35, 19-37, 19-39, 19-48, 19-50, 19-56, 19-57]. For example, Abbott Laboratories [Ex. 19-29] commented that specific processes in the pharmaceutical industry "cannot be controlled through existing conventional engineering controls." Also, AMETEK [Ex. 19-12] stated that "It will be hard for many industries to reach the 50 ppm level and extremely difficult, if not, impossible, for most to reach the 25 ppm level." Therefore, this commenter proposed "that OSHA set the PEL for methylene chloride at 50 ppm (8-hour TWA) with no AL [action level] and leave the STEL at 125 ppm (15-minute average) as originally written." AMETEK contended that this approach "combines aspects of both ACGIH guidelines and OSHA's proposed standard into a regulation which would be both protective of worker health and economically feasible for industry" [Ex 19-12].
Many other commenters argued for a PEL of at least 100 ppm [Exs. 19-1, 19-4, 19-10, 19-11, 19-16, 19-24, 19-47, 19-51, 19-52, 19-53, 19-54, 19-67, 19-75, 19-79, 98, 115-3, Tr. 397, 9/17/92, Tr. 2216, 10/14/ 92, Tr. 2627, 10/16/92, Tr. 2671, 10/16/92, Tr. 2702, 10/16/92]. For example, Besway Systems, Inc., testified [Tr. 397, 9/17/92]: "We would like to see a PEL for these companies of 200 ppm, which we've been able to show is safe and economically attainable in our real life experience. We believe that the absolute maximum PEL for our industry should be set at 100 ppm eight hour time weighted average. . . ." Also, Benco Sales [Tr. 2627, 10/16/92] stated "We feel the American workers would receive more benefit by implementation of an exposure level of 100 parts per million, which is achievable, and the subsequent enforcement of that level." ChemDesign Corporation [Ex. 19-24] believes that the "sharp reduction in the exposure limit is unjustified based on lack of credible data that this chemical has the potential to cause cancer in humans." This commenter therefore suggested that the PEL be "lowered by a factor of five to 100 parts per million" [Ex. 19-24].
Other commenters supported a variety of PEL values. One suggested that a lower PEL be phased in over time, with 75 ppm for two years, then 50 ppm for two years, and finally 30 ppm [Ex. 19-20]. The reasoning behind this suggestion was that, during this period, alternative options to best fit specific operations could be evaluated and implemented and sufficient time provided to gather the funds necessary to implement the entire system [Ex. 19-20]. OSHA holds, however, that the types of engineering controls required under this standard are relatively simple and that engineering to 75 ppm, then 50 ppm, then 30 ppm is likely to be more costly in time and money than engineering to or below 25 ppm initially. The suggested phase-in would also be administratively burdensome for employers, who would be subject to changing OSHA requirements over the years, with no clear advantage in reducing the costs of compliance. In addition, if OSHA allowed such a phase-in period, workers would be exposed to MC at higher levels than would occur if OSHA required no phase-in period. Therefore, the Agency sees no advantage to using the phased-in approach described. Moreover, the Agency notes that the time-frames for compliance with the provisions of the standard, including implementation of engineering controls, have been tailored to the size of the establishments, in order to give all employers a reasonable amount of time to gather resources and information necessary to comply with this regulation. See the discussion of start-up dates later in this document.
Smith Fiberglass Products, Inc. suggested that the PEL should remain at 500 ppm because there is no evidence of human harm at the present PEL and STEL, since "studies with rats and mice show that only a serious overdose far above the present STEL can cause carcinogenic effects" [Ex. 19-82]. Another commenter [Ex. 19-86] stated that "The present PEL of 500 parts per million (ppm) is not protective enough of employees based on toxicological data developed since the PEL was established." This commenter therefore suggested that the PEL should be lower than 500 ppm but higher than 25 ppm (no specific value identified). As discussed above, however, OSHA has determined that exposure to MC above 25 ppm poses significant cancer risks and that it is feasible to protect affected employees from those risks (see the Significance of Risk section of the preamble).
A number of commenters addressed the availability of suitable substitutes for MC in their concerns about feasibility [see, e.g., Exs. 19-6, 19-8, 19-37, 19-43, 19-55, 19-74, 19-79, 19-84, 115-3; Tr. 433, 9/17/92; Tr. 1591, 9/23/92; Tr. 1712-13, 9/24/92; Tr. 2636-38, 10/16/ 92]. Substitution is often a valid means of controlling exposures to a particular hazardous chemical when a less hazardous substitute is available that can be used to perform a similar function. In particular, some commenters stated that there are no viable substitutes for MC products used to perform particular tasks. These participants argued that companies would go out of business because they would be unable to comply with the final standard in a feasible way [Exs. 19-6 and 19-8]. In addition, one commenter [Ex. 19-8] expressed concern that substitute products would pose fire hazards. The National Tank Truck Carriers, Inc. testified [Tr. 1712, 9/24/92]:
One company which discontinued the use of methylene chloride found it necessary to supplement the methylene chloride substitute with even more hazardous acetone and toluene in order to remove the residues from the trailers and containers and properly service the industry by providing clean trailers.
OSHA has determined that for all application groups, compliance with this regulation can generally be achieved through the use of engineering controls and work practices. The Agency's Final Economic Analysis estimated the cost of compliance assuming that almost all firms would continue using MC and that only a small fraction of firms would substitute away from MC. OSHA agrees that, in an individual establishment, the potential use of substitution as a means of control must be evaluated carefully to ensure that the magnitude of the hazard posed is not the same or increased as a result of the substitution. For some applications described in this regulation, many substitutes for MC are available for specific applications that do not pose increased health or safety hazards. In general, however, OSHA has based it findings of feasibility not on the ability of companies in the affected sectors to substitute away from MC but on their ability to implement conventional engineering and work practice controls.
In addition to the 8-hour TWA PEL, OSHA is promulgating a short-term exposure limit (STEL) of 125 ppm, measured over a 15-minute period, to protect employees from the acute toxicity of MC and its metabolites. The acute toxicity of MC is characterized primarily by CNS effects, such as decreased alertness and coordination, headaches, and dizziness, which may lead, in turn, to accidents on the job as well as material impairment of health. Absence of a STEL would mean that employees could be exposed to up to 800 ppm for 15 minutes. Such levels are clearly associated with central nervous system effects.
MC is also metabolized to carbon monoxide (CO). CO produced from MC exposure has the same toxic effects in the body as direct exposure to CO does. The primary toxic effect of CO is reduction of the ability of the blood to carry oxygen to the tissues of the body.
In the body, carbon monoxide is converted to carboxyhemoglobin. Background levels of carboxyhemoglobin in the non-smoking U.S. population vary from approximately 0.5% to 2.0%. Carboxyhemoglobin in smokers ranges from approximately 3% to 10%. Additional body burden of CO (carboxyhemoglobin) due to MC or direct CO exposure can have adverse health effects on affected individuals. For example, exposure to relatively low levels of carbon monoxide (for example, levels which increase carboxyhemoglobin by 2%) reduced time to angina in patients with pre-existing heart disease exposed to occupational levels of CO [Ex. 21-93]. Exposure of pregnant women to CO has been shown to produce adverse health effects on the developing fetus. Workers with anemia or other blood abnormalities may be at increased risk of material impairment to health because of an already decreased oxygen-carrying capacity.
The carbon monoxide-mediated cardiac effects of MC exposure are of particular concern in the occupational setting because a significant fraction of the U.S. working population (some investigators estimate 30% of the U.S. population) has silent or symptomatic heart disease. NIOSH has expressed concern that the STEL proposed by OSHA is not low enough to protect workers from the adverse central nervous system and cardiac effects of MC.
In addition to reducing risks of cardiac and CNS effects, the STEL will also enhance employee protection from MC-induced carcinogenesis by reducing total exposure to MC and by limiting the metabolism of MC by the GST pathway (the putative carcinogenic metabolic process). Metabolic evidence suggests that the GST pathway produces more than proportionately greater quantities of the putative carcinogenic metabolite when MC concentrations reach levels of about 100 ppm. For this reason, it is important to limit high concentration, short duration exposures to MC. Thus the STEL will reduce the exposure-related risks of acute CNS effects, episodes of carboxyhemoglobinemia, and cancer.
Another advantage in requiring a STEL is that it focuses attention on sources of MC exposure in the workplace. General industrial hygiene principles state that a well-controlled process should have peaks no higher than five times the 8-hour TWA. Measurement of STEL exposures can indicate point sources which have unacceptably high MC emissions and help the employer target those processes for abatement. This can be an efficient mechanism to concentrate industrial hygiene resources on those emission sources which, when controlled, will reduce total employee MC exposure.
In addition, it has been established that "[i]f in fact a STEL would further reduce a significant health risk and is feasible to implement, then the OSH Act [section 6(b)(5)] compels the agency to adopt it barring alternative avenues to the same result." (emphasis in the original) Public Citizen Health Research Group v. Tyson, 796 F.2d 1479, 1505 (D.C. Cir. 1986) (Ethylene oxide). See also Building and Construction Trades Department, AFL-CIO v. Brock, 838 F.2d 1258, 1271 (D.C. Cir. 1988) (Asbestos).
In summary, many commenters questioned the need for a reduced PEL, for a PEL of 25 ppm, and for the particular 8-hour TWA PEL-STEL combination proposed by OSHA, citing concerns about the feasibility of these limits and the ability of companies to identify controls and/or substitutes to comply with them. However, as discussed in the final economic analysis, OSHA has determined that it is both technologically and economically feasible for facilities in all affected sectors to comply with the final rule. In almost every case, companies will be able to use conventional engineering controls and work practices to reduce their employees" exposures to these levels. In addition, many employers will find that substitution is a viable approach to eliminating the significant risk posed to workers by MC. As the economic analysis points out, many firms in many of the covered industries have already substituted away from MC, and have enjoyed considerable cost savings in the process. Finally, it is important not to lose sight of the reasons for regulating MC in the first place: this substance poses a significant risk of cancer, central nervous system and cardiac effects, and sensory irritation to the quarter of a million workers who manufacture, formulate, use, or transport this substance in the workplace.
As the Quantitative Risk Assessment and Significance of Risk sections of the preamble demonstrate, the cancer risk remaining at an 8-hour TWA PEL of 25 ppm is clearly of great concern, in that it exceeds the 1/1000 level indicated by the Supreme Court to be clearly significant. OSHA therefore encourages employers to further reduce the MC exposures of their employees wherever it is feasible to do so. Because the residual risk remaining at 25 ppm is great, the Agency intends to gather data and information on the feasibility of reducing the 8-hour TWA PEL to reduce remaining significant risk in a future rulemaking action. The priority assigned to any future rulemaking activity will depend in large measure on the prevailing exposure levels, feasibility, scientific advances and other information, at the time OSHA considers further proposals; to the extent prevailing levels are significantly below 25 ppm, the need for subsequent proposals will diminish.
Paragraph (d) Exposure Monitoring
Paragraph (d) addresses the employee exposure monitoring requirements for workplaces where employees are exposed to MC. As discussed in the preamble to the proposed rule (57 FR 57118-20), OSHA requires employee monitoring to facilitate compliance with the PELs. As a general matter, exposure monitoring of employee exposure to toxic substances is a well-recognized and accepted risk management tool. The monitoring provisions of this final MC standard are consistent with the monitoring provisions of other OSHA standards. Section 6(b)(7) of the OSH Act, which addresses rulemaking requirements for hazardous chemicals, requires health standards to include provisions for monitoring employee exposures. In the final rule, the exposure monitoring provisions have been reorganized and rewritten to improve their clarity and readability. The substance of the requirements is essentially the same, with the few exceptions noted below.
The provisions of proposed paragraph (d) elicited a considerable amount of comment and testimony. Several rulemaking participants [Ex. 19-57; Tr. 249, 9/17/92; Tr. 458, 9/17/92; Tr. 1711, 9/24/92] stated that the proposed requirements for exposure monitoring would impose excessive economic burdens on some employers (e.g., paint strippers, tank cleaners). However, in the final rule OSHA has structured the exposure monitoring requirements to minimize the burden for employers whose employees have lower exposures and for workplaces where groups of employees have similar exposures. In addition, the Agency has included some alternatives to the initial monitoring provisions that will reduce the amount of monitoring required for some workplaces. Ultimately, however, the Agency has determined that it is essential to the protection of exposed employees that exposure levels be quantified in order to select and implement the proper measures to reduce employee exposures to MC.
The overall rulemaking record supports the need for exposure monitoring to ascertain exposure levels for the purpose of designing appropriate protective measures for employees. In addition, evidence in the record indicates that the exposure monitoring requirements are economically and technologically feasible for firms in all of the affected industry sectors. (See the discussion in the Final Economic Analysis [Ex. 129].) Paragraph (d)(1) sets forth the general requirements that apply to all monitoring provisions. Paragraph (d)(1)(i) states that employers must characterize the MC exposure of each employee. Employers may chose one of two ways to determine an employee's MC exposure level. First, the employer can take a personal air sample in the breathing zone of each affected employee. This approach is the most precise method of exposure monitoring because it allows each employee's exposure to be individually ascertained. However, OSHA recognizes that this approach may be burdensome for employers with many employees. Therefore, paragraph (d)(1)(ii) permits employers to establish a representative monitoring scheme.
Under this option, a personal breathing zone air sample may be considered representative of another employee's 8-hour TWA or STEL exposure if the following conditions are met. First, the sampled employee must be that employee who is likely to have the highest MC exposure among the employees included in the group that is to be represented by the sample. Second, if the employer wishes a sample taken on an employee in a given job on one work shift to represent the exposure of another employee in the same job classification on another shift, the employer must sample at least one employee in each job classification in each work area during every work shift. Paragraph (d)(1)(ii) also contains an exception under which a personal breathing zone sample taken on one employee in one job classification in a given work area and on a particular shift will be considered representative of the exposure of employees on other shifts, where the employer documents that the tasks performed and conditions in the workplace are similar for all employees whose exposures are represented.
The provision for representative sampling, which is very similar to the corresponding provision of the proposed rule, eliminates unnecessary monitoring and thus further improves the cost-effectiveness of the standard. In a change from the proposal, the final standard also allows employers to use representative monitoring to comply with the standard's requirement for initial monitoring. OSHA believes that representative initial monitoring is appropriate in those cases where the employer can accurately determine which employees are likely to have similar exposures.
The accuracy of the methods used to perform exposure monitoring is addressed under paragraph (d)(1)(iii). For monitoring of airborne concentrations above the 8-hour TWA PEL or the STEL, the results must be accurate within plus or minus 25 percent at a confidence level of 95 percent. Where concentrations are above the action level but at or below the PEL, the accuracy must be within plus or minus 35 percent at a confidence level of 95 percent.
Methods of measurement are presently available that can detect MC within these limits. One such method is OSHA method 80, which has a limit of detection of 0.201 ppm. Copies of this method are available from OSHA and can be downloaded from OSHA's World Wide Web site on the Internet at "http://www.osha.gov/." Sampling and analysis may also be performed by portable direct reading instruments, real-time continuous monitoring systems, passive dosimeters or other methods that meet the accuracy and precision requirements of the standard under the particular conditions which exist at the employer's worksite.
Paragraph (d)(2) requires employers to make an initial determination of affected employees' exposure to MC. OSHA anticipates that most employers will need to perform monitoring in order to characterize employee exposure and has framed the rule accordingly. The standard allows employers to characterize their employee exposures using other means, providing that they can meet the requirements for such other means presented in the standard. For example, as discussed above, some employers may have objective data that establishes that employees will not be exposed above the action level or the STEL under reasonably foreseeable circumstances. Some employers generate such data themselves, while others rely on information provided by the manufacturer or supplier. Accordingly, paragraph (d)(2)(i) provides that employers can rely on objective data in certain circumstances in lieu of performing initial monitoring. The objective data must represent the highest MC exposures likely to occur under reasonably foreseeable conditions of proccessing, use, or handling in the workplace, and the employer must document the objective data relied on (see paragraph (m)). This provision corresponds to proposed paragraph (a)(2), which was the subject of several comments [Exs. 19-14. 19-31, 19-57].
Occidental Chemical testified [Tr. 2010 and 2023, 10/14/92] that OSHA should expand the proposed objective data exemption so that mixtures with less than one percent MC would be excluded from the scope of the MC standard. The Hazard Communication Standard (HCS) addresses mixture composition for the purpose of identifying those constituents and concentrations that impart their hazardous characteristics to the mixture as a whole. According to the HCS, carcinogenic substances such as MC are considered to impart their carcinogenic characteristics to the mixture if they are present in concentrations of more than one-tenth of one percent or can be released in concentrations that exceed an existing PEL. This is a much more protective requirement than that suggested by Occidental, and the Agency believes it would be inappropriate to lessen the protections provided to employees under the HCS in this substance-specific MC standard. Therefore, OSHA has not made the suggested change.
In addition, OSHA recognizes that it would be unreasonable to require initial monitoring under this standard where employers have already performed the monitoring needed to characterize employee exposure. Paragraph (d)(2)(ii) allows employers who have monitored their employees' exposures to MC within one year prior to April 10, 1997 and that monitoring complies with the accuracy and other requirements for monitoring contained in the final rule, to designate such monitoring results as sufficient in lieu of performing the initial monitoring.
Dow Chemical Co. [Ex. 19-31] commented that OSHA should allow monitoring data collected as much as two years prior to the effective date of the final rule to qualify as initial monitoring data. The Agency believes that data more than a year old would be unlikely to provide a reliable basis for characterizing employee exposure, because workplace conditions may well have changed since such data were collected. Accordingly, the Agency has not made the suggested change.
Addressing this point, Scott Schneider of the International Union of Electronic, Electrical, Salaried, Machine and Furniture Workers (IUE) testified [Tr. 531, 9/18/92] as follows:
While we support the requirements for exposure monitoring that were proposed, we have reservations about section (d)(2)(ii) regarding the use of "earlier monitoring results" to satisfy the initial monitoring requirements. OSHA must specify exactly which requirements the data must meet, in terms of both quality and quantity. Otherwise, it will be an enormous loophole for companies to avoid monitoring.
The International Brotherhood of Painters & Allied Trades (IBPAT) agreed with Mr. Schneider; the union stated that the use of "historical monitoring data to characterize exposures for similar processes * * * may lead to erroneous estimates of actual exposures" [Ex. 19-23]. OSHA believes that the concerns of these commenters have been addressed in the final rule because, to be acceptable under the standard, any previously gathered exposure data must meet the analytical, sampling, and other requirements specified for initial monitoring.
A number of commenters addressed the application of monitoring requirements in construction [Ex. 19-23; Tr. 544-45, 9/18/92; Tr. 814-17, 9/21/92; and Tr. 1377-80, 9/23/92]. OSHA agrees that conditions on construction sites often present special industrial hygiene and monitoring problems, particularly since the job may be completed before sampling results taken by conventional personal monitoring methods have been returned from the laboratory. For example, IBPAT [Ex. 19-23] pointed to the exposure variability that typifies construction sites, noting that weather, a highly transient workforce, and other factors often complicate accurate characterization of construction worker exposures. OSHA's Advisory Committee for Construction Safety and Health (ACCSH) and other participants suggested that OSHA allow the use of direct-reading instruments to address this problem [ACCSH Tr. 100-103, 7/28/ 92; Workgroup report, pp. 3-4; Tr. 814-818, 9/21/92; Tr. 1377-1382, 9/23/92].
In response to these comments, the final rule has been revised to allow the use of such instruments where employees are exposed to MC on fewer than 30 days within a given year. This means that construction employers who are involved in short-term construction projects will be able to use these instruments to characterize the MC exposures of their employees. Paragraph (d)(2)(iii), which addresses transient workplaces or work operations where employees are exposed on fewer than 30 days a year, permits employers to use direct reading instruments such as detector tubes to estimate exposure and determine what protective measures to provide to their MC-exposed employees. Although these simple measurement tools often do not meet the accuracy requirements that other types of monitoring methods do, they have the advantage of immediate results and thus allow employers to provide protection immediately. OSHA believes that this provision is responsive to the comments discussed above and represents an effective solution to a difficult worker protection problem.
Paragraph (d)(3) addresses periodic monitoring. Table X-1, below, which corresponds to Table 1 of paragraph (d)(3), displays the various monitoring scenarios possible under the final rule's periodic monitoring requirements. When the initial determination shows employee exposures to be at or above the action level or above the STEL, the employer is required to establish a periodic monitoring program. The 8-hour TWA monitoring is to be done every six months if exposures are at or above the action level but at or below the 8-hour TWA PEL and the STEL. The 8-hour TWA or STEL monitoring must be done every three months if the initial determination or subsequent monitoring shows results that are above the 8-hour TWA PEL or the STEL, respectively. If two consecutive subsequent monitoring results taken at least seven days apart show that exposures have decreased to or below the 8-hour TWA PEL, but above the action level, the frequency may be decreased to every six months. Eight-hour TWA monitoring may be terminated when two consecutive monitoring results taken at least seven days apart show that exposures are below the action level. STEL monitoring may be terminated when two consecutive monitoring results taken at least seven days apart show that exposures are at or below the STEL (See note to paragraph (d)(3)).
There are six possible initial determination exposure scenarios, or combinations of 8-hour TWA and short-term exposures, that determine the frequency of required monitoring. Table X-1 below lists these six exposure scenarios, along with their monitoring frequencies. As shown by Table X-1, the action level trigger largely determines whether employers must monitor employee exposure to MC. The only exception is the scenario in which 8-hour TWA exposures are below the action level and short-term exposures are above the STEL. In this case, exceeding the STEL obligates employers to monitor short-term exposures four times per year at those job locations where the STEL was exceeded, but employers are not required to monitor 8-hour TWA exposures at those job locations.
| Exposure Scenario | Required Monitoring Activity |
|---|---|
| Below the action level and at or below the STEL | No 8-hour TWA or STEL monitoring required |
| Below the action level and above the STEL | No 8-hour TWA monitoring required; monitor STEL exposures every three months |
| at or above the action level, at or below the TWA, and at or below the STEL | Monitor 8-hour TWA exposures every six months |
| at or above the action level, at or below the TWA, and above the STEL | Monitor 8-hour TWA exposures every six months and monitor STEL exposures ever three months |
| above the TWA and at or below the STEL | Monitor 8-hour TWA exposures every three months |
| above the TWA and above the STEL | Monitor 8-hour TWA exposures and STEL exposures every three months |
Several commenters stated that the proposal required unnecessarily frequent monitoring [Exs. 19-25, 19-26, 19-28, 19-30, 19-31, and 19-57]. Some commenters [Exs. 19-30, 19-31] said that the frequency of monitoring should be the same as that in the benzene standard (29 CFR 1910.1028 (e)(3)), since frequent monitoring does nothing to reduce or control exposures. The benzene standard requires monitoring at least every six months if employee exposure exceeds the 8-hour TWA, at least every year if exposure is at or above the action level but at or below the 8-hour TWA, and "as necessary" to evaluate short-term exposures. OSHA believes that MC exposure is highly variable due to the substance's volatility (vapor pressure = 350 mmHg at 20 C, compared with a vapor pressure for benzene of 75 mmHg at the same temperature) and the way that it is commonly used (e.g., in manual applications), and that reducing the frequency of exposure monitoring could therefore result in inadequate employee protection. The frequency of monitoring required by this MC standard is similar to that in other OSHA standards such as Ethylene Oxide (29 CFR 1910.1047), and is sufficient to characterize employee exposure and to evaluate the effectiveness of exposure control strategies.
The Advisory Committee on Construction Safety and Health suggested that OSHA trigger exposure monitoring by frequency of use as well as the exposure level. OSHA believes, however, that the magnitude of an employee's exposure is the appropriate determinant of monitoring frequency (and the selection of protective measures based on the results of that monitoring) because it is cumulative MC dose, not frequency of use, that determines the significance of the risk to which employees are exposed. Therefore, the Agency has not made the suggested change.
The Polyurethane Foam Association (PFA) [Ex. 19-39] questioned the necessity of requiring exposure monitoring at the action level. According to the PFA [Ex. 19-39], "An action level of 12.5 ppm would require that workers be monitored at a level that has only a remote health risk associated with it. The costs of such monitoring, however, would be significant." OSHA disagrees strongly with the PFA's analysis of the significance of the risk remaining at the action level. As discussed in the Significance of Risk and Economic Analysis sections of this preamble, only feasibility has constrained the Agency from reducing the 8-hour TWA PEL in the final rule to levels below the action level, because even at 10 ppm, the risk remaining is significant. That is, an employee exposed to an MC concentration of 10 ppm as an 8-hour TWA over a working lifetime would still be at significant risk of dying of MC-induced cancer.
Under paragraph (d)(4)(i), employers are required to perform additional monitoring when workplace conditions change or there is an indication that employee exposures may have increased. Paragraph (d)(4)(ii) requires that, where exposure monitoring is performed due to a spill, leak, rupture or equipment breakdown, the employer must clean up the MC and perform repairs and then monitor MC levels. The changes referred to in these provisions would include deliberate changes, such as a process or production change, or unexpected changes, such as a leak, rupture, or other breakdown. In the case of the latter, the employer is to perform the monitoring after taking whatever immediate action is required to clean-up or repair the equipment or source of exposure. OSHA recognizes that such occurrences can result in very high exposures. Several rulemaking participants [Exs. 19-31, 19-57, Tr. 2035, 10/14/92] stated that remonitoring is not necessary after a spill or leak since MC has a high vapor pressure, there would be no visible residual MC and no opportunity for significant exposure. However, OSHA believes that such remonitoring is an appropriate way to ascertain if proper corrective methods have been instituted and if the magnitude of an employee's exposure has changed significantly as a result of the leak or spill.
Employees are to be notified in writing of the results of exposure monitoring under paragraph (d)(5). This is to be done within 15 working days of the time the employer receives the monitoring results, and can be done either individually or by posting. When the results show that the 8-hour TWA PEL or the STEL has been exceeded, the employer must also notify employees of the corrective action being taken, and the schedule for completion of the action. This provision is effectively identical to the corresponding provision of the proposed rule.
One commenter [Ex. 19-49] argued that 15 working days is not enough time to develop corrective actions, especially where engineering controls are involved. OSHA believes that this comment misunderstands the requirement, which merely states that employers are required to "describe the corrective action being taken * * * and the schedule for completion of this action." The Agency believes that 15 working days is adequate time for the employer to make a preliminary assessment that includes the immediate steps being taken to reduce employee exposure, such as utilization of air-supplied respirators, and the employer's plan for implementing permanent controls and/or work practices. This requirement is necessary to assure employees that the employer is making efforts to furnish them with a safe and healthful work environment, in accordance with section 8(c)(3) of the Act. OSHA would expect employers to update the notification when plans for permanent controls are made.
Employees or their designated representatives are provided by paragraph (d)(6) with the opportunity to observe any required monitoring of employee exposure to MC. This provision is required by section 8(c)(3) of the Act (29 U.S.C. 657(c)(3)). It was relocated to paragraph (d)(6) of the final rule from proposed paragraph (l) to consolidate all of the exposure monitoring requirements in one place. The observer, whether an employee or a designated representative, must be provided (at no cost to the observer) with any personal protective clothing or equipment required to be worn by employees working in the area that is being monitored, and must additionally comply with all other applicable safety and health procedures. These provisions of the final rule are identical to those of the proposed rule.
As noted above, OSHA received a number of comments on the monitoring provisions proposed in the NPRM. For example, Occidental Chemical Corporation requested that OSHA consider using what they termed "exposure assessment" rather than monitoring, testifying [Tr. 2012-2013, 10/14/92] as follows:
[I]nstead of just looking at monitoring, which is in the middle of the process, exposure assessment looks at a basic * * * characterization: What is the characterization of the work force? What is the characterization of the workplace? What is the characterization of the contaminants in the workplace? All of that is weighed together; it's a collection of information.
The next step, then, is to interpret that information and determine what are the actual exposure levels, what category would they fit into * * *. If, at that point, and this is still just a paper exercise based on that information, you * * * conclude that exposures [are] unacceptable * * * you act. You may conclude that you have insufficient data and you'd like to monitor. Or you may conclude the data are acceptable; in this case, you would act and * * * change something and go through the process again. Or, in the case they [employee exposures] are acceptable, * * * you would document that it is acceptable and then reevaluate at some regular frequency, say annually or something like that.
In response to this comment, OSHA notes that nothing in the standard prevents employers from conducting exposure assessments. Indeed, the fact that the final standard allows employers to use objective data and recent (within the past year) exposure data are both examples of the kinds of evaluation made by industrial hygienists performing exposure assessments. An employer unable to avail himself or herself of the exclusions to initial monitoring offered by the standard would logically move to the next step in the exposure assessment process: the direct monitoring of employees' exposures to MC. Thus the final rule, far from interfering with exposure assessment, actually both reflects this process and encourages employers to engage in such assessments themselves.
Paragraph (e) Regulated Areas
Paragraph (e)(1) requires employers to establish a regulated area wherever an employee's exposure to airborne concentrations of MC exceeds or can be reasonably expected to exceed either the 8-hour TWA PEL or the STEL. This paragraph was changed slightly from the proposal to clarify that OSHA is concerned with employee exposures that can reasonably be anticipated to exceed one of the PELs, rather than excessive exposures that "may" occur. Regulated areas can be either temporary or permanent, depending on the characteristics of a given workplace. Such areas are required by the standard to reduce employee exposures and to alert employees to those areas in the workplace that present the greatest danger of MC overexposures.
Paragraph (e)(2) limits access to regulated areas to authorized persons (a term which is defined in the definitions paragraph (b)). This provision applies when either the TWA PEL or STEL is exceeded or can reasonably be expected to be exceeded. OSHA believes that the establishment of a regulated area will help to ensure that employees are aware of areas in the workplace where MC levels are above the 8-hour TWA PEL or STEL. OSHA believes that regulated areas are an effective means of limiting the risks of high exposures to substances suspected of being carcinogenic to humans to as few employees as possible.
Comments from Bristol-Myers Squibb [Ex. 19-14] suggested that OSHA delete the regulated area concept from the standard and replace it with a "regulated job classification" for jobs exceeding the PEL and a "regulated procedure" for procedures exceeding the STEL. This commenter's rationale was that since airborne concentrations are measured by personal monitoring and by job classification, it does not make sense to define an "area" of exposure. OSHA does not agree, for a number of reasons. First, in many workplaces, specific areas, such as quality control monitoring stations, mixing tanks, cutoff saw stations, spray booths, etc., are known to be associated with high levels of MC on a routine basis, and demarcating these areas protects employees by making them aware of the potential for these exposures in these locations. Second, it is standard industrial hygiene practice to use area monitoring to identify areas of exceptionally high exposures so that all non-authorized employees can be protected from overexposure. Finally, OSHA does not believe that the approach suggested by Bristol-Myers has the same potential to alert employees to the presence of high airborne concentrations that a demarcated area does, and therefore believes that the suggested change would not provide equivalent protection from overexposure.
The Laborers' Safety and Health Fund of North America [Tr. 1378-79, 9/23/92] testified that, in construction, a regulated area should be established wherever MC is used. Although there are many uses of MC on construction sites that may warrant establishing regulated areas, there are also engineering controls available (for example, portable ventilation) which may reduce employee exposures so that a regulated area would be unneccessary. OSHA believes that employers should not be required to establish regulated areas unless potential exposure levels warrant them. The Agency also believes that the employer is in the best position to determine whether the exposures from a particular MC application will warrant establishing regulated areas at a particular work site. The Advisory Committee on Construction Safety and Health also suggested that the establishment of regulated areas could replace some of the standard's monitoring requirements [Ex. 21-69]. As discussed previously, however, OSHA believes that both employers and employees benefit from knowing what exposures to MC are in a given workplace or on a specific job assignment. OSHA has therefore not revised the final rule's requirement for regulated areas in locations where exposures exceed or can reasonably be expected to exceed either or both of the PELs.
The proposal would have required that employers supply employees entering regulated areas with appropriate respiratory protection and ensure its use in such areas at all times. Several commenters [Exs. 19-25, 19-31 and 19-49] argued that respirator use in such areas should be required only if occupational exposures in such areas either exceeded the 8-hour TWA PEL or the STEL or could reasonably be expected to exceed one or both of these limits. OSHA agrees with these commenters and has revised the final rule accordingly. Paragraph (e)(3) states that employers must supply a respirator to each person who enters a regulated area, but shall require each affected employee to use that respirator only if MC exposures are likely to exceed the 8-hour TWA PEL or STEL. Thus, not all workers in regulated areas will be required to wear respirators in regulated areas at all times.
For example, under the final rule, an employer would be required to demarcate the area around a cutoff saw operator's work station in a foam blowing plant as a regulated area and to train the operator to recognize the area as regulated; however, the operator would only be required to wear a respirator in the area at times when the foam "bun" was coming out of the tunnel for cutting. The employer would demarcate the area because he or she recognizes, based on monitoring results for the cutoff saw operator, that this work station is one where the 8-hour TWA PEL is regularly exceeded during foam blowing operations. Because of the intermittent nature of many foam blowing operations, however, respirators would need to be worn by the operator (or other workers assisting the operator) only when foam was actually being blown. This example assumes that foam blowing operations are intermittent and that exposures at the cutoff saw would exceed the PELs only during foam blowing, although this may not be the case in all plants or at all times. In facilities where foam is blown continually and the saw operator is stationed at the end of the tunnel over the full shift, respiratory protection would likely be required to be worn in the regulated area at all times because exposures would routinely exceed the PEL in that area.
Under paragraph (e)(4), which has been added to the final rule, the employer shall ensure that, within a regulated area, employees do not engage in non-work activities which may increase dermal or oral MC exposure. This provision indicates that such non-work activities as eating, drinking, smoking, taking medication, applying lotions or cosmetics or storing such products in regulated areas are prohibited. Proposed paragraph (e)(4) has been promulgated as final rule paragraph (e)(6), as discussed below.
In addition, under paragraph (e)(5), which has been added to the final rule, the employer shall ensure that employees who are wearing respirators do not engage in activities (such as taking medication or chewing gum or tobacco) which interfere with respirator seal or performance. Proposed paragraph (e)(5) has been promulgated as final rule paragraph (e)(7), as discussed below.
Final rule paragraphs (e)(4) and (e)(5) are based on the response to NPRM Issue 41 (56 FR 57043) which indicated that OSHA was considering a provision to prohibit activities such as eating, drinking, smoking, etc. in regulated areas and asked for comments on this subject. This prohibition was supported by some rulemaking participants [Ex. 19-36, Tr. 1379, 9/23/92]. OSHA notes that it is standard industrial hygiene practice to limit such activities in regulated areas, both because employees should be aware at all times that they are working in a high-exposure area and because of health concerns. Among other things, since respirators are generally (although not always) required to be worn in regulated areas, engaging in the prohibited activities while wearing respirators might interfere with the respirator seal, placement or performance, thus reducing the effectiveness of the respirator. Furthermore, in the case of MC, smoking while being exposed to high MC concentrations (such as those prevailing in regulated areas) is particularly hazardous because MC is metabolized to CO in the body and leads to carboxyhemoglobinemia, a potentially life-threatening condition for some individuals, e.g., those with silent or symptomatic heart disease. Other OSHA health standards (e.g., asbestos, cadmium, ethylene oxide) have included similar prohibitions, and OSHA has concluded, based on the reasons discussed above and the Agency's experience with other standards, that including these provisions in the final MC standard is appropriate.
OSHA has broadened the language and separated it into two provisions (paragraphs (e)(4) and (e)(5)) to differentiate the types of activities which would generally not be allowed in a regulated area and those which would interfere with the effective use of respiratory protection. This is consistent with OSHA's intent in this rule to allow establishment of regulated areas, but require respirator use only when the 8-hour TWA PEL or STEL is likely to be exceeded.
Paragraph (e)(6), which is essentially unchanged from the proposed provision, requires employers to demarcate their regulated areas, but it does not specify how this is to be done as long as employees are aware of the location of the area and access to it is thus minimized. Factors that the Agency believes are appropriate for employers to consider in determining how to demarcate their areas include the configuration of the area, whether the regulated area is permanent, the airborne MC concentration present in the area, the number of employees in adjacent areas, and the period of time the area is expected to have exposure levels above the PEL or STEL. Permitting employers to choose how to identify and limit access to regulated areas is consistent with OSHA's belief that employers are in the best position to make such determinations, based on the specific conditions of their workplaces. This performance-oriented approach gives employers compliance flexibility without compromising employee health.
Paragraph (e)(7), proposed as paragraph (e)(5), requires employers at multi-employer worksites who establish a regulated area to communicate information to other potentially affected employers at the worksite about the location and access restrictions pertaining to the regulated area. OSHA believes that such communication will reduce the likelihood that unauthorized persons will enter the area or that workers not involved in MC-related operations will be exposed inadvertently. Those employers whose employees are exposed to MC at concentrations above either or both of the PELs must coordinate their operations with other employers whose employees could suffer excessive exposure because of their proximity to a regulated area where MC is being used. Compliance with this provision will ensure that only those employees at multi-employer worksites who are properly authorized, trained, and equipped enter regulated areas. This provision also recognizes OSHA's awareness that, although multi-employer worksites are common in construction, they are also increasingly found in other industry sectors.
Paragraph (f) Methods of Compliance
Paragraph (f) addresses the means by which employers are to reduce employee exposures to or below the 8-hour time-weighted average (TWA) PEL or the STEL. Under paragraph (f)(1), employers are required to institute and maintain the effectiveness of engineering controls and work practices to reduce employee exposure to or below the PEL and STEL, except to the extent the employer can demonstrate such controls are not feasible. Where these measures cannot reduce the concentration of airborne MC to or below the TWA PEL and STEL, the employer is nevertheless required to implement them to achieve the lowest feasible level. The employer is required to supplement these controls with respirators where necessary to ensure that employees are not exposed to MC at levels above either the 8-hour TWA PEL or the 15-minute STEL. Section 1910.134(a)(1) of the respiratory protection standard requires respirators to be used where effective engineering controls are not feasible.
One commenter [Ex. 19-57] indicated that it should be left to professional judgment to determine whether engineering controls or respirators are the best method for protecting employees. OSHA does not agree with this comment because it fails to acknowledge the industrial hygiene hierarchy of controls, which places engineering controls ahead of administrative or personal protective equipment as methods of protecting employees from hazardous exposures. The hierarchy of controls has been established industrial hygiene practice since the 1950s and is based on the fact that engineering controls are the most effective method of protecting employees because they remove the hazard from the workplace. In contrast, respirators merely prevent employees from breathing the contaminant -- it remains in the workplace air. Effective respirator use also requires constant supervision, extensive employee training and fit testing, and regular (often daily) care and maintenance of the respirator. Consequently, respirators should only be used as a means of achieving the PELs where feasible engineering controls are not available (such as in some vessel cleaning and non-stationary maintenance operations) or are not sufficient to control exposures to required levels. All OSHA substance-specific health standards have recognized and required employers to observe the hierarchy of controls, and OSHA's enforcement experience with these standards has reinforced the importance of this concept to the protection of employee health.
In the Final Economic Analysis, OSHA has described feasible control technologies for each industry affected by the final MC standard. Many employers have already implemented such controls in their workplaces and are currently achieving the MC levels required by the final rule. Examples of such feasible control strategies include dilution and local exhaust ventilation, chilling coils, magnetic pumps and magnetic floating gauges, exhausted lances for drum filling, and inline quality control sampling equipment.
OSHA acknowledges that there may be a few operations where the use of engineering and work practice controls to control exposure to MC is infeasible because exposures are highly intermittent in nature and limited in duration. In particular, OSHA is aware that the use of engineering and work practice controls to comply with the PELs is infeasible for some maintenance and repair operations and during emergency situations. Where it is infeasible to reduce workplace MC levels below the PELs through engineering and work practice controls, the employer is required to protect employees from excess exposure by providing and requiring the proper use of personal protective equipment, in this case supplied-air respirators.
As discussed in the NPRM (56 FR 57120-21), OSHA asked for comments on whether employers should be allowed to place increased reliance on the use of respirators to protect employees exposed to MC. The International Brotherhood of Painters and Allied Trades [Ex. 19-23] commented that "[w]ith the exception of emergencies that require use of a SCBA respirator, engineering and work practice controls should be the sole method of compliance."
In addition, the IUE [Tr. 530, 9/18/92] testified as follows:
[R]equirements to control those exposures using engineering controls are particularly important because of the lack of adequate chemical cartridge respirators for methylene chloride. For that reason, we reject the question posed by OSHA regarding the provisions to allow greater use of respirators which came from earlier proceedings on revisions to 1910.1000. Also, NIOSH [Tr. 884, 9/21/92] testified as follows:
NIOSH supports the existing OSHA policy on methods of compliance, that is the hierarchy of controls for controlling exposures to hazardous agents. Generally, this policy states that whenever feasible, engineering controls and work practices should be used to prevent exposures, and that personal protective equipment, including respiratory protection, should be used only when engineering controls are not feasible.
As discussed above, OSHA agrees with these comments. The Agency considers the use of respirators to be the least satisfactory approach to exposure control because respirators provide adequate protection only if employers ensure, on a constant basis, that they are properly fitted and worn. Also, unlike engineering and work practice controls, respirators protect only the employees who are wearing them from a hazard, rather than reducing or eliminating the hazard from the workplace as a whole. Moreover, respirators are uncomfortable to wear, cumbersome to use, and interfere with communication in the workplace, which can often be critical to maintaining safety and health. As mentioned above, OSHA has reached similar conclusions for other standards promulgated to protect employees from exposure to toxic substances. Paragraph (g) of the final standard discusses respiratory protection requirements.
The NPRM also proposed requirements for a written compliance program that would have required employers to detail their plans for implementing engineering and other controls. However, OSHA has decided to eliminate these provisions from the final rule for MC to reduce the amount of paperwork employers would be required to complete. The Paperwork Reduction Act of 1995 (PRA 95), (44 U.S.C. 3501 et seq.), requires agencies to minimize the paperwork burdens on the public. Preparation of written compliance plans would be classified as paperwork under the new Act. OSHA believes that the lack of a written compliance plan will not substantially reduce the effectiveness of the standard; the Agency solicits comment on this point. One of the primary benefits of a written plan is that it encourages employers to consider remedial actions soon after the standard is promulgated. For MC, however, this may not be an issue because the necessary control measures are not complex and, except for the very smallest employers, the period for compliance allowed by the standard is relatively short. Nevertheless, OSHA believes that many employers will voluntarily develop these plans because they make it easier for employers and employees to monitor progress toward compliance. OSHA will be considering including compliance plans in its standards on a case-by-case basis in future rulemakings when they are appropriate. The Agency believes that employers benefit from having a plan to meet the start-up dates, and has included examples of how this might be done in Appendix B. There were very few comments about the written compliance plan requirements, other than one stating that a written plan is reasonable but annual review and update of it is not [Ex. 19-26].
Paragraph (f)(2), proposed as paragraph (f)(1)(iv), precludes use of a schedule of employee rotation as a means of compliance with the PELs. Employee rotation reduces the extent of exposure to individual employees, but increases the number of employees exposed. OSHA is regulating MC as an occupational carcinogen, and the Agency therefore prohibits practices that would place more employees at risk. No threshold has been demonstrated for the carcinogenic action of MC, and it is therefore prudent public health policy to limit the number of workers exposed. In addition, since the dose-response relationship for MC is convex, exposure to higher concentrations for shorter periods of time is riskier than exposure to the equivalent ppm-hour concentration spread over 8 hours (when rotation is used as a method of employee exposure control, employees tend to be exposed to higher concentrations for shorter durations).
Paragraph (f)(3) requires employers to address leak and spill detection in the workplace. Employers must implement procedures to detect leaks and contain spills as well as follow appropriate methods to dispose of contaminated materials and clean-up or repair the spill or leak. These requirements were addressed in proposed paragraph (f)(1)(iii), but in the final rule have been separated out and clarified to emphasize their importance. Appendix A provides examples of procedures that would meet these requirements. Liquid MC has a high vapor pressure (350 mm Hg at 20 C). Accordingly, leaks and spills of MC-containing products could generate high airborne MC levels. The leak and spill detection program reduces the possibility of worker overexposure to MC.
Bristol-Myers Squibb (BMS) [Ex. 19-14] and Dow [Ex. 19-31] supported OSHA's performance-oriented requirement for a program to detect leaks and spills. For example, BMS stated:
[T]here are many ways in which this can be done (e.g.monitoring of tank levels, walks through areas where leaks may occur). In some cases, continuous monitoring can be done to detect leaks, however, this is not always feasible. Monitoring equipment may be very difficult and expensive to maintain and may not provide the sensitivity needed for early detection. We recommend that OSHA leave this section as it is and not specify the system or the equipment which should be used for the detection program.
Proposed paragraph (h) required employers to develop emergency plans, implement those plans when necessary, equip employees correcting emergency situations with appropriate PPE, and alert and evacuate employees potentially affected by emergencies, as necessary. In reviewing the proposed rule, OSHA concluded that the proposed requirements duplicated provisions of the Hazardous Waste Operations and Emergency Response (HAZWOPER) standard (Section 1910.120). The Agency has therefore deleted the separate MC requirement for an emergency plan, and has added a note to final rule paragraph (f)(3)(ii) which refers employers to the HAZWOPER standard for the applicable requirements.
Paragraph (g) Respiratory Protection
Paragraph (g) of the final rule addresses requirements for respiratory protection allowed to be used to comply with the MC standard. Paragraph (g)(1) requires that employers provide respirators at no cost to each affected employee, and to ensure that each affected employee uses a respirator under the following conditions:
(1) Whenever an employee's exposure to MC exceeds or can reasonably be expected to exceed the 8-hour TWA PEL or the STEL;
(2) During the time interval necessary to install or implement feasible engineering and work practice controls;
(3) In a few work operations, such as some maintenance operations and repair activities, for which the employer demonstrates that engineering and work practice controls are infeasible;
(4) Where feasible engineering and work practice controls are not sufficient to reduce exposures to or below the PELs; or (5) In emergencies. These limitations on the required use of respirators are consistent with OSHA's longstanding position on the hierarchy of controls in the workplace, as reflected in the respiratory protection requirements in other OSHA health standards (e.g., asbestos, 1910.1001; ethylene oxide, 1910.1047; benzene, 1910.1028; cadmium, 1910.1027) and with good industrial hygiene practice. They reflect OSHA's determination that respirators are inherently less reliable in providing protection to exposed employees than engineering and work practice controls.
However, to reflect the changes made to the final rule's regulated area provision (paragraph (e)(1)), the final rule's respiratory protection requirements differ somewhat from those in proposed paragraph (g). In the NPRM, OSHA proposed to require that employers provide respirators in the following circumstances: (1) During the time interval necessary to install or implement feasible engineering and work practice controls; (2) in work operations, such as maintenance and repair activities, vessel cleaning, or other activities for which engineering and work practice controls are demonstrated to be infeasible, and when exposures are intermittent in nature and limited in duration; (3) in work situations where feasible engineering controls are not yet sufficient to reduce exposure to or below the PELs; and (4) in emergencies. In the final rule, another situation where respirator use is appropriate is acknowledged: whenever an employee's exposure to MC exceeds or can reasonably be expected to exceed either or both of the PELs.
The Building and Construction Trades Department, AFL-CIO, testified [Tr. 816-17, 9/21/92] that proposed paragraph (g)(1)(ii) could be interpreted by construction contractors "as an exemption from the requirement for adopting a control strategy that places engineering and work practice controls above that of the PPE." In response, OSHA has revised final rule paragraph (g)(1)(ii) to clarify OSHA's intent. OSHA recognizes that it may be infeasible to control MC exposure with engineering and work practice controls during certain maintenance and repair operations, although OSHA is also aware that portable local exhaust, "elephant trunks," and other means of providing ventilation to, and removing contaminated air from, process vessels and other difficult-to-reach work spaces are widely used in construction and elsewhere. The Agency also recognizes that there may be other MC-related activities where an employer could establish the infeasibility of controls, particularly where employee exposure is highly intermittent or of short duration. Accordingly, OSHA has revised proposed paragraph (g)(1)(ii) as described above. This change also addresses comments made by the Pharmaceutical Manufacturers Association (PMA) [Ex. 19-25; Tr. 1430, 9/23/92], which stated that it was infeasible for employers to protect employees during manual unloading of batch operated centrifuges and manual loading of dryers from MC exposure with engineering and work practice controls. The PMA suggested that OSHA revise proposed paragraph (g)(1)(ii) to include those loading and unloading activities in the list of operations allowed to protect affected employees through the use of air-supplied respirators. However, OSHA included examples in the proposal only to provide a general indication of the situations where the Agency would accept the use of air-supplied respirators in lieu of engineering and work practice controls. OSHA believes that the examples suggested by the PMA are too narrowly focused for inclusion in such a list. It would not be possible for OSHA to enumerate in the final rule all of the workplace-specific operations where engineering and work practice controls may be infeasible. Therefore, in accordance with longstanding OSHA practice, employers claiming that engineering and work practice controls are infeasible must establish infeasibility on an objective basis.
Other commenters were concerned about requiring respirators during emergency escape situations, noting the time involved in donning a respirator in an emergency. The Dow Chemical Company stated "Dow believes the respiratory protection requirements for emergency escape are excessive. For the short period of time it takes to escape a release of MC, considering the minor acute effects of the material, it is excessive to require, as a minimum, a gas mask with an organic vapor canister" [Ex. 19-86].
Similarly, comparing escaping right away or first finding a respirator and then escaping during an emergency situation, Occidental Chemical testified [Tr. 2041, 10/14/92]:
Methylene chloride is not incapacitating so the goal should be to escape as fast as possible not trying to find a device -- and it may be close, it may be further -- and then put it on, which could take a minute or so, 30 seconds or a minute, and then decide about escape. That whole process becomes much longer. So I'm not advocating we don't have escape respirators, just that the process should be, escape should be the number one priority.
OSHA agrees that escape is the first priority for employees exposed to MC in an emergency situation. Furthermore, the Agency has determined, in general, that the ready availability of escape respirators is essential to ensure that employees are able to escape safely. To that end, emergency plans must provide for fast access to escape respirators where the potential for emergency exposure situations has been identified by the employer. In addition, employees must be trained to don those respirators properly and quickly and to recognize any foreseeable situations where taking the time to obtain and put on their respirators would significantly reduce their ability to escape or where they can safely escape an emergency situation without using respirators. OSHA recognizes that immediate escape is not always possible, so respirators are needed to protect those employees while they are still in the exposure area.
Paragraph (g)(2), proposed as paragraph (i)(1)(ii), requires employers to determine that any employee required by this standard to wear a supplied-air respirator in the negative pressure mode or a negative-pressure respirator for escape purposes is medically fit to use such a respirator. This provision has been changed from the proposal to recognize that medical fitness for respirator users under this standard is appropriate only for negative-pressure respirators or those operated in that mode. This change will assist employers to direct their medical surveillance resources effectively. In addition, in keeping with the greater flexibility provided by this standard to employers in selecting an appropriate health care professional, paragraph (g)(2) uses the final rule's language, "Physician or other licensed health care professional," in lieu of the proposal's exclusive use of "physician."
Paragraph (g)(3), proposed as paragraph (g)(2), requires employers to select appropriate atmosphere-supplying respirators from among those listed in Table 2 (Table 1 in the proposed rule), which sets forth the minimum requirements for respiratory protection and is unchanged from the proposal. Employers may use respirators approved for a higher level of protection in lower concentrations of MC. Employers are required to select atmosphere-supplying respirators that have been approved by NIOSH under the provisions of 42 CFR Part 84. Also, employers must select vapor canisters which have been approved by NIOSH when they provide gas masks with organic vapor canisters for use in emergency escape. The final rule differs from proposed paragraph (g)(2) in that it does not require employers to give employees who cannot wear negative pressure air-supplied respirators or who cannot wear a negative pressure (organic vapor canister) during an emergency escape the option of wearing a respirator with less breathing resistance. OSHA believes that the respirators required by the final rule will not strain an employee's respiratory system during such use.
Issue 30 (56 FR 57042) asked if the proposed respirator selection table (Table 1 in the proposal) appropriately regulated the choice of respirators. Several commenters suggested changes. For example, Abbott Laboratories [Ex. 19-29] suggested that OSHA allow the use of a continuous flow air-supplied hood or helmet for exposures up to 5,000 ppm instead of 625 ppm of MC. On the other hand, the Laborers' Health & Safety Fund of North America [Ex. 19-36] suggested that OSHA require employers to provide positive pressure SCBAs or airline positive-pressure full facepieces with auxiliary escape for all exposures over 25 ppm, instead of allowing any flexibility, in keeping with NIOSH recommendations for respiratory protection against carcinogens. The Advisory Committee on Construction Safety and Health [Ex. 21-69] recommended that respirators, when used, be pressure-demand, supplied air respirators with an auxiliary self-contained breathing apparatus, because of MC's fast cartridge/canister breakthrough and the lack of effective end-of-service-life indicators.
OSHA is currently in the process of developing a final standard to revise its general respiratory protection provisions in 29 CFR 1910.134. Until that rulemaking is completed the Agency will continue to rely on NIOSH's Assigned Protection Factors (APF) for determining the types of respirators required for protection to airborne concentrations of MC. The APF for continuous flow hoods/helmets is 25 in the NIOSH Respirator Decision Logic. The maximum specified use concentration for a respirator is generally determined by multiplying the exposure limit, in this case 25 ppm, by the protection factor, which is 25; therefore, these hood/helmets could be used only up to 625 ppm of MC. Using the same decision logic, OSHA believes that adequate protection can be provided by the respirators described in Table 2 when they are used under appropriate exposure conditions.
Some commenters questioned the reliability of atmosphere-supplying respirators. For example, in the furniture stripping industry commenters noted that MC could cause damage or potential damage to the hoses, the plastic lens, and the gasket of the facepiece of air line respirators or other kind of respirators, resulting in inadequate protection. [Ex. 19-11; Tr. 348-9, 9/17/92; Tr. 2146-7, 10/14/92; Tr. 2505-2506, 10/15/92]. In addition, the Occidental Chemical Corporation [Tr. 2115, 10/14/92] noted that none of the manufacturers contacted had hoses resistant to MC-induced corrosion. The Agency acknowledges that MC may damage respirator components, if the MC is left on them for extended periods of time. However, existing 1910.134 (f) already requires employers to inspect respirators frequently and to maintain respirators at their original effectiveness. In addition, MC does not damage rubber components which are available. Most importantly, if feasible engineering controls and work practices are not available, properly utilized air-supplied respirators are the only way to protect employee health from significant risk.
Issue 30 also requested information on the circumstances under which air-purifying respirators may be used. Dr. Morton Corn of Johns Hopkins University testified [Tr. 2352, 10/15/92] that "* * * with the current state of knowledge and the breakthroughs I indicated, [allowing gas masks with organic canisters for emergency escape only] is a prudent restriction at this time."
Several commenters disagreed with Dr. Corn and remarked that there are some situations where air-purifying respirators may be appropriate in addition to emergency situations, and recommended that OSHA expand the provision to allow the use of air-purifying (filter) respirators. For example, Occidental Chemical testified [Tr. 2113-4, 10/14/92] as follows:
Transportation workers who make deliveries in trucks can have intermittent exposure to methylene chloride inside the truck and, if you set the PEL too low, and in that emergency situation * * * you can't have engineering controls on some types of trucks, especially if they are rented. You ought to allow the use of respirators in that case; it's a very short type exposure, goes in, takes the drum out, and then gets back in the truck. Now it may be possible to schedule operations in certain industries where the PEL is exceeded for short periods of time. Filter cartridge respirators could be used to protect the worker during the short periods of time without the use of cumbersome supplied-air respirators. Of course, you have to have changes in the regulated areas in the rules also if you're going to allow the use of respirators where you have intermittent exposures above the PEL.
And a short breakthrough time does not mean a respirator is useless. If you use the NIOSH calculations, at 200 parts per million which might be typical of paint stripping, you ought to have about 118 minutes worth of time before you get breakthrough; and that may be enough in paint stripping operations.
Similarly, Bristol-Myers Squibb stated that air-purifying respirators may be appropriate in certain circumstances [Ex. 19-14]:
Based upon the scientific information now in the record, BMS requested that OSHA consider allowing chemical cartridge air-purifying respirators for specific types of activities (lower MC concentrations, shorter durations).
Organic vapor cartridges can be used for protecting employees against exposures to MC where using an air-supplied respirator would not be feasible due to costs or process (e.g.multiple working areas). Only air-supplied respirators should be used for operations involving the need for extended wear (e.g.greater than several hours).
The Eastman Kodak Company [Ex. 102] also requested that OSHA allow air-purifying respirators "in circumstances where their effectiveness can be adequately demonstrated, engineering controls are not feasible and supplied-air respirators are impractical or potentially unsafe. OSHA also should permit the use of half mask respirators" [Tr. 1196-7, 9/22/92]. In addition, Kodak described specific situations where it believed the use of air-purifying respirators was appropriate:
The use of air-supplied respirators must be an essential component of the exposure-control strategies for both the Roll Coating Division and the Dope Department. Moreover, the evidence demonstrates that air-purifying canister or cartridge-type respirators may appropriately be used in some operations, such as certain dope maintenance tasks. The use of air-purifying respirators is appropriate where: (1) air-supplied respirators or other controls are impractical or potentially unsafe, (2) personal monitoring of employees is conducted regularly, (3) the extremes and conditions of the exposure potential are well characterized, and (4) used cartridges are tested after use to verify the absence of unacceptable breakthrough. It is essential that OSHA permit the use of air-purifying respirators under these circumstances so that Kodak can control employee exposure when engineering and work practice controls and air-supplied respirators are infeasible, ineffective or potentially unsafe.
OSHA considered including a provision in the final rule to allow exceptions for the use of air-purifying respirators in limited circumstances where very tight control of the respirator program is implemented. However, the Agency has rejected this alternative for several reasons. First, the record strongly supports the inadequacy of such respirators for employee protection. Consequently, the use of air-purifying respirators should only be considered when the use of air-supplied respirators presents major disadvantages. Second, a program to use air-purifying respirators would have to be very detailed and be tailored to a specific workplace. It would be difficult, if not impossible, to list all of the relevant factors and criteria for such a program in the regulatory text, which must necessarily be appropriate to apply to many workplaces. (Below, OSHA discusses the Agency's variance procedures, which employers wishing to use air-purifying respirators may use to apply for a variance.) While there may be circumstances when the use of filter respirators may seem preferable to the use of atmosphere-supplied respirators, OSHA has concluded, as a general matter, that air-purifying respirators do not provide sufficient, consistent, and reliable protection to employees exposed to MC. In support of this conclusion, NIOSH testified as follows [Tr. 887-89, 9/21/92]:
At the request of OSHA, NIOSH has completed an in-depth study of the breakthrough characteristics of MC for organic vapor respirator cartridges and canisters under a variety of test conditions. This work was undertaken to determine MC breakthrough time for commercially available, organic vapor respirator cartridges and canisters. Several MC challenge concentrations were studied, ranging from 50 ppm to 1,000 ppm. As received cartridges and canisters were tested at equivalent flow rates of 64 Lpm through the respirator and at both 50% and 80% relative humidities (RHs). Breakthrough times were determined for individual cartridges and canisters, as well as stacked cartridges. The results of this study show rapid breakthrough of MC for organic vapor cartridges even for low concentrations of MC (e.g., 5 ppm breakthrough at approximately 30 minutes for 50 ppm challenge concentration and 80% RH). Appendix D is a detailed report of this study. At 125 ppm challenge concentration, 5 ppm breakthrough, and 80% RH, one brand of cartridge showed breakthrough times of approximately 40 minutes. The same brand of chin-style canister, that contains approximately 2 and 1/2 to 3 times more sorbent than two cartridges (i.e., two cartridges per respirator) showed breakthrough times of approximately 100 minutes when tested at the same conditions. The same brand of front-or back-mounted canister, that contains approximately 10 times more sorbent than two cartridges, showed breakthrough times of approximately 600 minutes. Based on the results of this study, NIOSH supports the OSHA proposal to require the use of air-supplied respirators in lieu of air-purifying respirators. However, because of the potential carcinogenicity of MC, NIOSH continues to recommend only the most protective positive-pressure respirators as noted previously.
The NIOSH study indicated that MC quickly penetrates organic vapor cartridges (in a fraction of a typical work shift), contrary to the assertions of Occidental Chemical and the other commenters mentioned above. Larger canisters, which contain greater amounts of absorbent, last longer, but are still effective for less than a work shift (except for very large canisters). Another problem with organic vapor cartridges and canisters is that MC migrates through the absorbent even when the respirator is not being used. This further decreases the breakthrough time and raises the possibility that the employee will be exposed to significant concentrations of MC. Also, humidity decreases the amount of MC collected by the absorbent.
Another problem with air-purifying respirators in the case of MC is this substance's poor warning properties, which mean that workers will not be able to smell or sense the presence of MC when breakthrough occurs. OSHA believes that employees wearing air-purifying respirators could easily have a false sense of security and be lulled into believing that they were being protected against MC when it could already have broken through the absorbent. Accordingly, OSHA has concluded that it would be inappropriate to allow broad-scale use of air-purifying respirators because of MC's quick breakthrough time and its carcinogenic health effects.
Employers who believe that the use of filter respirators is appropriate for their operations may apply for a permanent variance from the requirements of paragraph (g)(3) of this section, pursuant to the authority granted by § 6(d) of the Occupational Safety and Health Act and the procedures set out in 29 CFR part 1905. In particular, an applicant would need to establish that the use of filter respirators in a specific workplace would provide employee protection equivalent to that which would be provided through compliance with final rule paragraph (g)(3). As discussed below, the respirator program, procedures, and data needed to support the use of such respirators under a variance are extensive.
A successful variance application for an exception that would allow air-purifying respirators would have to address a number of the characteristics that employers such as Eastman-Kodak [Ex. 102] indicate they have undertaken with regard to the use of such equipment. For example, extensive exposure monitoring would have to be done to accurately characterize employee MC exposure levels. Furthermore, the breakthrough time for MC when used in the airborne concentrations expected in the workplace would have to be known, and cartridges would have to be changed before employees are unacceptably exposed. The program would have to be carefully monitored by a trained and experienced individual such as a certified industrial hygienist or the equivalent. Finally, the respirators would have to be appropriately fit tested for each affected employee. For all of the reasons stated above, OSHA has determined that the interests of employee protection will be best served by requiring all employers, except those whose respiratory program, procedures, and exposure data can support a variance request, to provide their employees with the respirators shown in Table 2.
Paragraph (g)(4), which is identical to the proposed (g)(3), requires employers to implement a respiratory protection program in accordance with 29 CFR 1910.134 whenever respirator use is required by this standard. The respiratory protection program must include basic requirements for proper selection, fit, use, training of employees, cleaning, and maintenance of respirators. For employers to ensure that employees use respirators properly, OSHA has found that the employees need to understand the respirator's limits and the hazard against which it is providing protection in order to appreciate why specific requirements must be followed.
Paragraph (g)(5) (effectively identical to proposed paragraph (g)(4)) requires that employers allow employees wearing respirators to leave the regulated area to readjust the respirator facepiece to their faces for proper fit. In addition, employers must permit employees who wear respirators to leave the regulated area to wash their faces as necessary to prevent skin irritation associated with respirator use. These requirements encourage the proper use of respirators by authorizing employees to take specific actions that ensure the effective functioning of respirators and reduce the likelihood that employees will experience adverse side effects from wearing respirators.
Paragraph (g)(6), which is essentially the same as the corresponding proposed paragraph, addresses situations where employers provide gas masks with organic vapor cartridges for purposes of emergency escape. If gas masks are used, the canisters are to be replaced before the gas masks are returned to service. This requirement is necessary because actual MC exposures during emergencies are generally not known, so the expected service life of the canister cannot be determined. In addition, the migration of MC within the canister after emergency exposure further reduces the amount of useful life remaining, posing exposure risks for subsequent users.
Paragraph (g)(7) addresses respirator fit and is essentially identical to the corresponding provision of the proposal. It requires the employer to ensure that each respirator issued is properly fitted and has the least possible facepiece leakage.
Under paragraph (g)(7)(ii), the employer must perform qualitative or quantitative fit testing initially and at least annually thereafter for each employee wearing a negative pressure respirator, including those employees for whom emergency escape respirators of this type are provided. A note has been added to this provision to indicate clearly that the only supplied-air respirators to which this provision would apply are SCBAs operated in the negative pressure mode and full facepiece supplied-air respirators operated in negative pressure mode. Quantitative fit testing relies on objective data generated by measurements of facepiece seal leakage, in contrast to qualitative fit testing, which is based on subjective observations made by the respirator wearer. Many commenters expressed a preference for quantitative fit testing over qualitative fit testing. For example, Newport News Shipbuilding (NNS) [Ex. 19-37, p. 2] stated: "Quantitative respirator fit testing is the method of choice. At NNS we use quantitative fit testing exclusively, as this method is more definitive than qualitative fit testing and provides a record of the fit test." The Shipbuilders Council of America [Ex. 19-56, p. 11] took the same view.
Several commenters noted the importance of proper selection and fit testing of respirators [Exs. 19-12, p. 3; 19-31, pp. 15-17; 19-71, p. 4]. Dr. David Newcombe of the Department of Environmental and Health Sciences at The Johns Hopkins University testified as follows:
I think that's [quantitative fit testing] a very important parameter because, first of all, respiratory protection when it's required takes a reasonable amount of time to ensure that the individual is properly fitted so that the mask fits if that's the piece that's going to be used and is protective against the substance that you're protecting against and, in addition, I think it's important to note that some people may have deformities that cause a poor fit and, therefore, don't protect and so I would think that you have to have a careful assessment of the type of respiratory protection you're going to use, its fit in a single individual as well [Tr. 800, 9/18/92].
In most cases, OSHA has determined that positive pressure respirators are the respirators of choice for MC exposure, especially loose-fitting models such as hoods or helmets; for these respirators, fit testing is generally not needed. However, for those situations where negative pressure respirators are used, fit testing is needed. Qualitative or quantitative fit testing allows the employer to test various respirators on the employee until the appropriate fit is identified and selected for the employee.
Paragraph (h) Protective Work Clothing and Equipment
Paragraph (h) requires that, where needed, employers provide and ensure the use of the appropriate protective clothing and equipment. The requirements for protective work clothing and equipment were separated from proposed paragraph (g) (respiratory protection and personal protective equipment) and moved to paragraph (h) to facilitate compliance. Proposed paragraph (g)(6) was effectively identical to this paragraph.
Protective clothing used during exposure to MC, such as gloves or aprons, must be resistant to MC. The Building and Construction Trades Department, AFL-CIO [Tr. 832, 9/21/92] suggested that OSHA codify NIOSH's recommendations for protective clothing materials suitable for use with MC. MC is a constituent of so many different products that a codification of guidance regarding appropriate protective clothing would be unwieldy and unlikely to be complete. Further, the continual formulation and reformulation of MC products virtually ensures the early obsolescence of any protective clothing guidelines.
Therefore, OSHA believes that it is appropriate for paragraph (h) to set general criteria and for the Agency to adopt the NIOSH recommendations in a nonmandatory appendix so employers will have more detailed guidance and so OSHA can update that guidance, without rulemaking, as advances in PPE technology cause existing guidance to become outdated. As discussed above, this performance-oriented approach reflects OSHA's belief that employers are in the best position to select protective measures that are tailored specifically to the needs of their workplaces.
Paragraph (h) requires the employer to provide all necessary protective clothing and equipment at no cost to the employee and to launder, repair, replace and safely dispose of that clothing and equipment. The final rule is performance-oriented so the employer has the flexibility to provide only the protective clothing and equipment necessary to protect employees in each particular work operation from MC exposure. The generic requirements for PPE in the general industry, construction, and shipyard standards also apply to PPE for MC, except where a specific provision of the MC standard applies.
Paragraph (i) Hygiene Facilities
Paragraph (i) of the final rule establishes requirements for hygiene facilities in establishments where it is reasonably foreseeable that an employee's eyes or skin may contact solutions containing 0.1 percent or greater MC. Although such provisions were not part of the proposed rule, OSHA requested comment on the appropriateness of including such requirements in Issue 38 (56 FR 57122). Specifically, the Agency requested comment on the appropriateness of including requirements for quick-drench showers and eye-wash facilities in the final rule. OSHA described quick-drench showers as," * * * showers that could drench an employee with piped-in water applied with force," and eyewash facilities as devices "that could flush the eyes repeatedly with a great amount of water." In response to comments, described below, the Agency has decided that it is not necessary to specify in the final rule when showers and eyewash facilities are required to protect employees from skin or eye contact with MC, because employers are in the best position to determine whether the MC used in their establishments meets the 0.1 percent cutoff specified in this provision and whether contact of the eyes or skin with MC can reasonably be foreseen.
Paragraph (i)(1) requires employers to provide conveniently located washing facilities appropriate to removing MC if it is reasonably foreseeable that the employee's skin may contact a solution containing 0.1 percent or greater MC through splashes or spills. MC can be absorbed into the body through skin contact (percutaneous absorption), which would add to the dose employees receive via inhalation and thus increase the risk of cancer and other adverse health effects. However, MC is not a corrosive chemical, and, if left on the skin for short periods, is not likely to cause long-term or irreversible damage. Therefore, it is important that employers make provisions to remove MC from the skin of employees quickly, although immediate drenching is not usually required. This requirement has been stated in performance-oriented language in the final rule to allow employers to determine what type of washing facilities are needed and at what distance from affected employees. This provision thus recognizes that employers in some facilities, such as furniture stripping shops where a thick MC gel is used that may burn the skin on contact, employers need to position washing facilities in closer proximity to affected employees than is the case where less hazardous solutions of MC are used. OSHA believes that this requirement of the final rule strikes the right balance between employee protection and employer flexibility by ensuring that washing facilities for the skin will be available and appropriately placed in workplaces where such contact is likely.
MC splashed into the eyes will cause irritation if the MC is not promptly washed out, and immediate flushing is therefore required. Paragraph (i)(2) requires employers to provide appropriate eyewash facilities within the immediate work area for emergency use if it is reasonably foreseeable that an employee's eyes will contact solutions containing 0.1 percent or greater MC through splashes or spills.
Existing OSHA requirements at 1910.141 and § 1926.51 establish generic provisions for hygiene facilities but do not focus on MC-specific situations. Existing § 1910.151(c) and § 1926.50 (g) require employers to provide suitable facilities for quick-drenching or flushing of body and eyes within the immediate work area for immediate emergency use, when the body or eyes may be exposed to injurious corrosive materials. However, because MC is not classified as a corrosive material, these existing requirements would not apply. Thus the final rule's performance-oriented requirements will provide guidance to employers about what facilities and access distances are appropriate for conditions in their workplaces. In addition, Appendix A provides examples of both washing facilities and eyewash facilities that would satisfy this requirement.
The response to Issue 38 emphasized the need for eyewash and shower facilities [Exs. 19-37, 19-56; Tr. 2644-2645, 10/16/92; Tr. 1942-1943, 9/24/92]. For example, PRMA testified [Tr. 348, 9/17/92] that MC splashes happen "almost every day" in furniture stripping workplaces.
Commenters also addressed the health effects associated with such accidental exposures. The Amalgamated Clothing and Textile Workers Union testified [Tr. 1825, 9/24/92]:
I would advocate including it [the provisions for showers and eyewash facilities]. It [methylene chloride] has skin effects. Anyone who's ever stripped paint can tell you about what it's like to get it on their skin or their eyes. So it's very important to be able to irrigate an affected area promptly.
One means to provide protection from prolonged skin or eye exposure to MC from accidents is to specifically require quick-drench showers and eyewashes. The NPRM sought comments on whether or not the final rule should require employers to provide quick-drench showers and eyewash facilities. Many commenters recommended that the final rule contain such provisions [Exs. 19-15; 19-36; Tr. 532, 9/18/92; Tr. 1380, 9/23/92; Tr. 2352-53, 10/15/92]. For example, PRMA [Ex. 19-11] favored a requirement for eyewash/ quick drench facilities, stating as follows:
An eyewash station is a safety device that should be required in any work environment where there is the possibility of splashing chemicals into ones eyes. Quick drench showers are also a safety device that should be standard equipment in every facility. MC paint removers are one of the few paint removers that are easily rinsed from one's eyes.
The Dow Chemical Company commented [Ex. 19-31]:
Washing facilities are always a good idea when working with any material, however, it is not always necessary to have quick-drench showers, etc. Incidentally, quick-drench showers do not deliver water "applied with force." They work on a deluge system delivering a large amount of water to wash off the material, not force it off. Installing showers and eyewash fountains in all workplaces may not be economically feasible. There are other systems such as water hoses, portable eye-washes, etc. that work effectively for MC. MC is a material that, in some cases, may be painful if held against the skin for a period of time, but is not eye nor skin nor life threatening. Therefore, an immediate shower is not required.
OSHA agrees that quick drench and eyewash facilities are effective means for treating employees who have been accidentally exposed to MC by spills or splashes. However, the Agency agrees with Dow Chemical that quick drench showers are not the only means to ensure proper first aid treatment for MC exposure due to accidental splashes or spills and believes that other types of washing facilities can also provide effective treatment for accidental exposure.
In some cases, the availability of a hose attached to a potable water supply would enable employers to provide effective first aid treatment. This could be an especially effective means of protection at a construction worksite. Several commenters [Ex. 19-23, 19-38; Tr. 859, 9/21/92] agreed that construction employers should have potable water at the worksite in case of accidental exposure. For example, the Building and Construction Trades Department, AFL-CIO, testified [Tr. 817, 9/21/92]:
The standard does not address the need for available hygiene facilities. Since methylene chloride can damage the skin and eyes and potable water is often in limited supply on construction sites, the requirement for potable washing areas must be clearly stated in the standard. Potable water supplies should be of sufficient volume to provide at least 15 minutes of continuous flushing.
The Occupational Health Foundation testified that the MC standard should require that hygiene facilities be provided within a reasonable distance at construction worksites [Tr. 858-859, 9/21/92]:
Unlike in a lot of other work sites where at least there's a sink nearby, in construction you really need to specifically mandate that provision to be sure that there's going to be water anywhere remote, you know, within a reasonable distance to the work site.
Issue 38 also requested information on the extent to which MC-exposed employees are already provided with quick drench showers and eye wash facilities. Several commenters described workplaces that have emergency shower or eyewash facilities in place. The United Automobile, Aerospace and Agricultural Implement Workers of America (UAW) testified [Tr. 1942-1943, 9/24/92] "[t]here are a lot of showers and eye washes in areas where you have open-top chemicals or use of chemicals." In addition, the Occidental Chemical Corporation testified [Tr. 2159, 10/14/92]:
. . . we conducted a survey of our customers that were not CMA and not NACCD members recently and asked them questions like that. We have some information on that. It doesn't necessarily mean that we hit a large percentage of our methylene chloride customers, though.
. . . we have safety shower[s] and eyewash[es] [in our plants], certainly. We have . . . recommendations on it and we certainly follow the ANSI standards on it.
Newport News Shipbuilding (NNS) and the Shipbuilders Council for America both commented [Exs. 19-37 and 19-56] that "[p]rocedures at NNS now require eyewash units. For the most part we use portable (5 gallon) units. Plumbed combination units would be better." The National Tank Truck Carriers, Inc. also indicated that their facilities are already equipped with emergency showers [Tr. 1750-51, 9/24/92].
With regard to the proximity of employees to emergency showers and eye washes, commenters and testimony indicated that, depending on the work operation, shower facilities have been installed as close as eight feet or as far away as 100 feet. For example, the J. M. Murray Center, testified [Tr. 1047-48, 9/21/92] that they have both eye washes and showers that are ten to twelve feet from the employees.
The Polyurethane Foam Association (PFA) testified [Tr. 1630, 9/23/92] that the proximity of shower facilities and eye washes depends on the plant and operation within the plant, stating as follows:
We've got methylene chloride in bulk storage area and we also use it at the foam machine. The total range from those things that you might be would be anywhere from eight feet to may be 60 feet. And I'm guessing at the 60 feet. That, again, is specific for those plants that I am responsible for. There are 80-some-odd plants out there, and I can't speak for that particular physical setup in each one of those plants.
The PFA further stated in its post hearing comment:
Eye wash and drench showers are available in the production areas. These are located within 10 to 15 feet of the work stations, such as near bulk storage tanks and the mixing head, where a higher risk of employee exposure exists. Hygiene facilities may be 50 to 75 feet away from other work areas [Ex. L-100A].
The Eastman Kodak Company testified [Tr. 1259, 9/22/92] that emergency eye-wash and quick-drench showers are available in their workplaces, and that such stations are between 50 and 100 feet from all work areas where exposure to chemicals may occur.
Striptech International, which advocated requirements for pressure showers and eyewash facilities where workers are exposed to MC [Ex. 19-15], also testified that hygiene facilities are not readily accessible in the aircraft paint stripping industry [Tr. 1834-35, 9/24/92]:
I've heard people ask about deluge in eye wash. Does it exist in aircraft maintenance hangars? Yes, it surely does; but you also have to look at where they normally are. They're normally on the walls. When a man or a lady is on top of an aircraft, on the tail of an aircraft, they may be nine stories in the air. If they get methylene chloride in their eyes or really a bad shot of it, they've got to come down nine stories and may be cross a 400 to 600-foot-long hangar to get to it. Deluge showers, yes; all aircraft people have them. Are they readily accessible? No.
It is important for the employer to evaluate the potential hazard posed by the particular use of MC and to provide appropriate washing facilities within a reasonable distance and eyewash facilities within immediate reach. In addition, employers are required to provide employees who are at risk of skin and/or eye contact with MC with appropriate protective clothing and eye protection. Portable eyewash units, which would significantly reduce any delay in irrigating the eyes, are available and can be located within easy access distance of affected employees. As described above, access to washing facilities should be quick, but immediate showering is not generally necessary to address the MC skin hazard. Therefore, an employee stripping an airplane would likely have time to get to the showers located along the walls of the hangar to wash MC from the skin. (Note: Some paint stripping compounds do contain corrosives, and immediate access to quick-drench facilities is essential in such cases.) Based on a review of the rulemaking record, the Agency has determined that performance-oriented provisions for hygiene facilities are reasonably necessary to supplement the other requirements of the final rule and has promulgated paragraph (i) accordingly.
Paragraph (j) Medical Surveillance
Section 6(b)(7) of the OSH Act requires that, where appropriate, occupational health standards shall prescribe the type and frequency of medical exams or other tests to be made available, by the employer or at the employer's cost, to exposed employees in order to determine if the employee's health is being adversely affected by exposure to workplace hazards.
A medical surveillance program that complies with paragraph (j) enables the employer to:
(1) Determine if an employee has an underlying health condition that places the employee at increased risk from the effects of exposure to MC;
(2) detect, insofar as possible, early or mild clinical conditions arising as a result of MC exposure, so that appropriate preventive measures can be taken;
(3) identify any occupational diseases that occur as a result of MC exposure; and
(4) help to evaluate possible trends in the incidence of these diseases.
The most serious health effect that may result from MC exposure is cancer. Although a medical surveillance program cannot detect MC-induced cancer at a preneoplastic stage, OSHA anticipates that, as in the past, methods for early detection and treatments leading to increased survival rates will continue to evolve. Moreover, the cardiovascular disease, central nervous sytem and dermal irritation effects caused by MC exposure can already be detected at early or mild stages by medical surveillance provisions such as a medical history and a medical exam. MC has not been tested adequately for the full range of possible health effects that may result from exposure, so it is also not presently possible to identify all diseases that may be associated with exposure to MC. The specific level of protection afforded the worker by the final standard cannot be predicted with certainty, although the risk of exposure for those effects that have been identified are significant, and the record shows that reducing the exposure of employees will significantly reduce that risk. An important goal of the medical surveillance program is to provide information related to the adequacy of the PELs for MC by documenting the health condition of exposed employees, particularly in the area of carcinogenicity.
Several rulemaking participants [Exs. 19-31, 19-83, Tr. 1802-3, 9/24/92] stated that the proposed medical surveillance provision should be deleted from the final rule because it would not detect employee exposure to harmful levels of MC. In addition participants contended [Ex. 19-83, Tr. 458, 9/17/92] that the medical surveillance provision is too expensive and burdensome. OSHA has determined that the medical surveillance program required by the final rule is reasonably necessary for the protection of workers. In particular, medical surveillance will directly benefit workers with cardiovascular disease, central nervous system effects, and dermal irritation. These conditions can be detected by the medical surveillance program required by this paragraph of the final rule, and the detection of such conditions can, in turn, alert the employer to potential overexposures to MC in the workplace and to the need to limit MC exposures for certain employees with underlying heart disease or other conditions.
In addition, by increasing the performance orientation of the rule, OSHA has minimized the costs of medical surveillance while maintaining its effectiveness. For example, the final rule leaves the content of laboratory surveillance for individual employees to the discretion of the physician or other licensed health care professional. Also, the requirement for a physical examination has been tailored to the age of the employee, so that employees younger than 45 generally receive an exam only every three years, instead of annually. The medical surveillance program also will aid in the evaluation of cancer incidence in the workplace and temporal trends therein.
Paragraph (j)(1) specifies the circumstances under which employers must provide medical surveillance for employees who are or may be exposed to MC. Under paragraph (j)(1)(i), employers must make medical surveillance available to all employees who are exposed to MC at or above the action level for 30 days or more in any year or above either of the PELs for at least 10 days in any year. This provision is effectively identical to the corresponding provision of the proposed rule. Also, this requirement is consistent with the approach taken by OSHA in the benzene standard (29 CFR 1910.1028). OSHA recognizes that the health effects associated with MC exposure are, in general, the result of chronic exposures to MC. Accordingly, employees exposed only for a few days in any year will be at relatively low risk of developing MC-induced disease. The exposure duration thresholds in the final rule will thus enable employers to focus valuable medical resources on high-risk employees.
Some commenters were concerned about the use of the PELs and action level as triggers for medical surveillance. The Building and Construction Trades Department, AFL-CIO [Tr. 817, 9/21/92] was concerned that this provision would preclude medical surveillance for some employees with MC exposures that exceeded the PELs on fewer than 10 days in a given year but who might nonetheless be at risk of adverse health effects. OSHA has determined that employees who have been identified by a physician or other licensed health care professional as being at risk for cardiac disease or some other serious MC-related health condition and who are exposed to MC at levels that exceed the PELs on fewer than 10 days in any year should have the option of participating in a medical surveillance program. Accordingly, paragraph (j)(1)(ii) has been added to the final rule. This provision states that medical surveillance must be provided to any employee (1) who is exposed above the 8-hour TWA PEL or STEL for any time period, and (2) who has been identified by a physician or other licensed health care professional as being at risk from cardiac disease or from some other serious MC-related health condition, and (3) who requests inclusion in the medical surveillance program. As noted in the Health Effects section, above, OSHA is concerned that any MC exposure above either of the PELs could exacerbate cardiac problems. This paragraph enables such high-risk employees to participate in a medical surveillance program.
Under paragraph (j)(1)(iii), appropriate surveillance is required to be made available to employees exposed in an emergency regardless of the airborne concentrations of MC normally present in the workplace. Where very large amounts of materials are kept in a sealed system, routine exposure may be very low. However, rupture of the container might result in extremely high MC exposures. Thus, it is appropriate for employers who have identified operations where there is a potential for an emergency involving MC to plan ahead so that emergency medical surveillance would be available if needed. This provision is effectively identical to proposed paragraph (i)(1)(iii).
Proposed paragraph (i)(1)(ii) would have required that the employer have the examining physician or other licensed health care professional determine if affected employees are physically fit to wear respirators. OSHA has placed this requirement with the other respiratory protection provisions in paragraph (g) of this final rule.
Paragraph (j)(2) requires that employers offer examinations without cost to employees, at a reasonable time and place, and without loss of pay. OSHA believes that this provision is necessary to encourage employees to participate in the medical surveillance program. Final rule paragraph (j)(2), which is essentially identical to proposed paragraph (i)(2), is also consistent with other OSHA health standards and with provisions contained in the OSH Act.
Paragraph (j)(3) requires that all medical procedures be performed by or under the supervision of a physician or other licensed health care professional, defined as "an individual whose legally permitted scope of practice (i.e., license, registration, or certification) allows him or her to independently provide or be delegated the responsibility to provide some or all of the health care services required by paragraph (j) of the standard." The proposal required that all medical procedures be performed only by or under the supervision of a physician. Only one commenter [Ex. 19-31] specifically supported this provision.
OSHA has long considered the issue of whether and how to identify the particular professionals who are to perform the medical surveillance required by its health standards. The Agency has determined that other professionals who are licensed under state laws to provide medical surveillance services would also be appropriate providers of such services for the purposes of the MC standard. The Agency recognizes that the personnel able to provide the required medical surveillance may vary from state to state, depending on state licensing laws. Under the final rule, an employer has the flexibility to retain the services of a range of qualified licensed health care professionals, thus potentially reducing costs, increasing flexibility, and allowing employers to identify those professionals, who may not necessarily be physicians, with the greatest expertise in diagnosing and treating occupational diseases. In future rulemakings, OSHA may attempt, with the cooperation of interested stakeholders, to specify which licensed health care professionals are the most appropriate to perform each of the diagnostic, therapeutic, medical management and other services required by the Agency's standards.
Paragraph (j)(4) of the final standard addresses when medical examinations and consultations are to be provided.
Initial surveillance. Under paragraph (j)(4)(i), initial medical surveillance must be provided before an employee's initial assignment to work in an area where they would be exposed to MC or by the start-up dates described in paragraph (n)(2)(iii) of the final MC standard, whichever is later. The employer need not repeat equivalent medical surveillance if it has already been provided within the past 12 months. OSHA's requirement for a preplacement examination is intended to determine if an individual is at increased risk of adverse effects from exposure to MC. It also establishes a general baseline for future reference. The provisions of final rule paragraph (j)(4) are effectively identical to those in proposed paragraph (i)(3), except that the proposed rule did not take into account medical surveillance provided prior to the effective date of this section. In the preamble to the NPRM (56 FR 57124), OSHA stated that it was considering a provision that would give employers credit for medical examinations provided within one year of the standard's effective date. The Agency requested comment on the usefulness of such a provision. Commenters [Exs. 19-31, 19-55b, 19-83] supported such a provision. In particular, Dow Chemical [Ex. 19-31] stated "[i]f this is not done this section will be unfair to those employers who have on-going health surveillance programs." OSHA agrees with these commenters and has promulgated the final rule accordingly.
Periodic surveillance. Paragraph (j)(4)(ii) addresses periodic medical surveillance. OSHA proposed to require annual medical surveillance for all affected employees. In the final rule, this has been changed so that the employer is required to update the medical and work history for each affected employee every year but must only provide physical examinations on a schedule that varies with the age of the employee. For affected employees 45 years of age or older, the physical examination must be conducted every year. For employees less than 45 years of age, the examination need only be done every three years.
OSHA differentiated these groups of employees in an effort to target surveillance resources effectively. The probability of developing heart disease (which can be exacerbated by MC exposure) increases as employees age. Age 45 is a rough approximation of the point at which medical professionals would have heightened concern for cardiac effects. In other words, it is generally more likely that employees 45 years and older would experience the adverse cardiac effects of MC exposure. Three-year intervals between physical examinations for workers younger than 45 seemed the proper interval to balance the conservation of valuable medical resources and the provision of a medical surveillance program that is useful for detecting adverse MC health effects. The annual updates on medical and work history will enable the physician or other licensed health care professional to identify those individuals for whom more frequent examinations would be appropriate.
To a lesser extent, this would be true for the detection of MC-induced cancer as well. Although MC-induced cancer cannot currently be detected at the pre-neoplastic stage, early detection of cancer generally increases the survival rate, so it is important to include employees exposed to MC in a medical surveillance program that may detect tumors. Since any cancers caused by MC are more likely to be found in older employees and employees exposed to MC for longer durations, it is reasonable to concentrate medical surveillance resources on older employees.
The main goal of periodic medical surveillance for workers is to detect adverse health effects at an early, and potentially still reversible, stage. The intervals chosen based on the age of the employee are consistent with this purpose and with other OSHA health standards. The Agency believes that these periodic surveillance requirements strike a proper balance between the need to diagnose health effects, such as cancer, at an early stage, thus increasing the effectiveness of medical intervention, and the expectation that a limited number of cases will be identified through the surveillance program. This approach decreases the cost burden of surveillance by lengthening the period of time between examinations for younger employees who have fewer years of exposure and thus have a lower risk of adverse health effects.
Termination of employment or reassignment. Paragraph (j)(4)(iii) requires the employer to provide medical surveillance when an employee terminates employment or is reassigned to an area where exposure is consistently at or below the action level and the STEL. The termination examination need not be conducted if medical surveillance has been performed within the past six months. This requirement reduces the likelihood that an employee who terminates employment has an active, but undiagnosed, disease related to his or her MC exposure. In the NPRM, OSHA had proposed that the termination examination be performed unless medical surveillance had been conducted on that employee within the past three months. The Motor Vehicle Manufacturers Association [Ex. 19-42] requested that the exam should only be required if the employee has not had a medical exam within six months of termination or reassignment, instead of three months as had been proposed. The MVMA stated that "six months is adequate and consistent with other OSHA health standards (Cadmium, 1910.1027(l)(8)). We see no contribution to reducing employee risk from examining such employees at an earlier date, especially since the exposure to methylene chloride has been removed." Upon reconsideration of the issue, OSHA has adopted this suggestion in the final rule.
The Agency requested public comment on whether continued annual surveillance should be offered to employees who have left employment, retired, or transferred to other areas within the employer's operations. Such an approach would be consistent with the requirement in the Benzene standard (29 CFR 1910.1028), which makes medical surveillance available to certain employees who have been exposed to benzene during their employment with their current employer. Several commenters [Exs. 19-31, 19-38, 19-42, 19-48, 19-55b, 19-58] stated that there should be no medical surveillance after an employee leaves a job in an exposure area or for employees previously exposed to MC. In particular, Dow Chemical [Ex. 19-31] stated: "[W]e do not believe that the employer should be responsible for continued medical surveillance for employees who leave MC exposure areas * * *. [T]he continued surveillance does nothing more than divert occupational medical resources from more important work." Taking a different view, the IUE [Tr. 533, 9/18/92] testified that formerly exposed retirees should be included in the medical surveillance program. They also stated that retirees, presently employed workers formerly exposed to MC in previous jobs, and workers relocated to nonexposed areas should be included in the medical surveillance program. The ACTWU agreed, testifying [Tr. 1763-1764, 9/24/92] that employees who continue to work for the same employer after their exposure to MC is terminated should be entitled to participate in the medical surveillance program.
OSHA has decided that it would be inappropriate to include retirees and other formerly exposed employees in the medical surveillance program. A major value of medical surveillance is to detect the acute heart disease and CNS effects associated with MC exposure. Workers no longer exposed to MC, or retirees, would be at much less risk of experiencing these effects.
Additional surveillance. Paragraph (j)(4)(iv) requires employers to provide additional surveillance when the physician or other licensed health care professional recommends that it be provided. This may be warranted, for example, for an employee who is under 45 years of age but has a health condition that requires surveillance more frequently than every 3 years. Inclusion of this provision in the final rule will ensure that all employees receive the most appropriate level of surveillance for their particular health situation. The proposed provision was essentially identical.
Paragraph (j)(5) of the final rule, like paragraph (i)(4) of the proposal, establishes the requirements for the content of medical exams. This provision requires a comprehensive medical and work history, a physical examination, laboratory surveillance, and any additional information determined to be necessary by the physician or other licensed health care professional. The language in the proposed rule, which was similar, has been revised for clarity and to provide guidance about what constitutes adequate medical surveillance. For example, the final rule addresses medical and work history in greater detail than the proposal because, in some cases, three years may elapse before a subsequent physical examination is provided. On the other hand, the specific content of the physical examination and laboratory surveillance has been left largely to the discretion of the physician or other licensed health care professional.
Paragraph (j)(5)(i) requires that a comprehensive medical and work history be obtained from each participating employee. This paragraph requires a medical evaluation that includes a comprehensive medical and work history with special emphasis on neurological symptoms, skin conditions, history of hematologic or liver disease, signs or symptoms suggestive of heart disease (angina, coronary artery disease), risk factors for heart disease, MC exposures, and the work practices and personal protective equipment used to control exposures. OSHA has included an example of a medical and work history format that would satisfy this requirement in non-mandatory Appendix B of the standard. The proposed provision required a comprehensive or interim medical and work history with emphasis on neurological symptoms, mental status, and cardiac health. Final rule paragraph (j)(5)(i) has been revised to indicate clearly what is required.
The medical and work history component of the initial medical evaluation will assist the physician or licensed health care professional in identifying pre-existing conditions that might place the employee at increased risk when exposed to MC. It also establishes a health baseline for future monitoring. The subsequent annual updates will identify changes in neurological symptoms, skin conditions or cardiac health, and, in combination with laboratory analyses and information on exposure history, may provide early warnings of MC toxicity. The information derived from a medical evaluation assists the physician or other licensed health care professional in distinguishing between MC-related effects and those effects that are unrelated to MC exposure. This information is particularly important because the health effects associated with MC exposure are not unique to such exposure. For example, the proposed requirement to assess mental health status has been eliminated from the final rule because no specific correlation has been demonstrated between mental health status and MC exposure.
Paragraph (j)(5)(ii) requires that the extent and nature of the required physical examinations be determined by the physician or licensed health care professional based on the health status of the employee and analysis of the medical and work history for that employee. The standard also requires that the examiner give particular attention to the lungs, cardiovascular system (including blood pressure and pulse), liver, nervous system and skin. Proposed paragraph (i)(4)(ii) specifically would have required that the examination address the lungs, liver, nervous system and breast. OSHA has determined that, in order to indicate clearly that the physician or licensed health care professional should assess the potential cardiac health impacts of MC, the medical exam should give attention to the cardiovascular system, blood pressure and pulse. In addition, the Agency has decided that, because of the skin irritation effects of MC, it is necessary to include evaluation of the skin in the medical exam.
Two hearing participants [Tr. 803, 9/18/92; Tr. 2434-35, 10/15/92] testified that men over 40 years old should be given electrocardiograms (ECGs), which should be repeated every 1 to 3 years. OSHA is not requiring ECGs because there is no evidence in the record that associates specific changes in ECGs with MC exposures. However, the physician or licensed health care professional has the discretion to order an ECG for any employee where it is deemed appropriate.
Proposed paragraph (i)(4)(iv) also required the physician to make a determination of any reproductive difficulties of the employee. Vulcan Chemicals [Ex. 19-48] and Organization Resources Counselors (ORC) [Ex. 19-51] commented that the evidence for a relationship between reproductive effects and MC exposure did not warrant inclusion of such a provision in the final rule. OSHA agrees with these commenters that the evidence associating MC exposure and specific reproductive health effects is sparse. Therefore, the Agency has not included reproductive effects in the list of effects the physician or other licensed health care professional should focus on. However, the Agency will continue to monitor the literature to determine if future evidence indicates that inclusion of this provision is warranted.
Two commenters [Exs. 19-28, 19-42] stated that the breast examination requirement should be eliminated from the final rule because breast exams would be highly unlikely to identify effects related to exposure to MC. In the proposal OSHA placed attention on the breast because of concern raised by the increased number of breast tumors in the rat bioassay. Upon further consideration, OSHA has dropped the requirement for breast exams. The Agency notes that rats are particularly sensitive to mammary tumors and it is unclear that humans have similar risks of developing breast cancer after exposure to MC. The Agency remains concerned about the potential for MC carcinogenicity evidenced by the rat mammary tumors, however, and has relied, in part, on mammary tumor data in identifying MC as a cancer hazard.
In final rule paragraph (j)(5)(iii), laboratory surveillance of employees is to be conducted as the examining physician or licensed health care professional determines to be necessary and appropriate, based on the employee's health status and the medical and work history. This is a more performance-oriented provision than the corresponding provision of the proposed rule. The proposal would have required several specific laboratory tests, while the final rule leaves laboratory test requirements to the discretion of the physician or other licensed health care professional. Non-mandatory Appendix B includes guidance regarding the types of tests that may be appropriate.
Some commenters [Exs. 19-28, 19-42, 19-48, 19-49] stated that COHb levels, which had been included among the tests in the NPRM, are not a good measure of toxic exposure to MC. In particular, the MVMA [Ex. 19-42] stated that it is difficult to determine the COHb level attributable to MC exposure for employees who are smokers or who may have other exposures to CO. Several other participants [Exs. 19-25, 19-57, 19-83 and Tr. 1438, 9/23/92] suggested that COHb testing should be done only after over-exposure to MC, such as after an emergency. The Laborers Health and Safety Fund [Tr. 1386, 9/23/92] testified,
[W]e're not convinced that that's [COHb monitoring] an appropriate and accurate measure of exposures, given other sources of carbon monoxide on construction sites as well as the issue of smokers versus non-smokers.
However, the Department of the Army [Ex. 19-55b] suggested that COHb levels are a more cost-effective measurement of the oxygen-carrying capacity of blood than a complete blood count. Similarly, the California Department of Health Services [Ex. 19-17] requested that references to COHb testing be moved from the appendix to the regulatory text.
COHb levels greater than 3% can exacerbate angina symptoms, decrease exercise tolerance and increase risks for myocardial infarctions (heart attacks) in susceptible individuals. COHb concentrations can also be used as a rough estimate of worker exposure to MC (taking into consideration smoking behavior, time since exposure, and exposure to other CO sources) to calibrate personal MC monitoring measurements. Before-and after-shift COHb determinations can be useful in correlating recent MC exposures with COHb levels. The Agency is not requiring COHb testing, however, because confounding factors, such as smoking or exposure to a CO source, can reduce the usefulness of the results of the tests and, in addition, COHb does not measure a health effect per se but is instead a surrogate measure of MC exposure. However, COHb testing may be clinically important in the evaluation of a symptomatic worker and therefore remains an option for the physician or other licensed health care professional to pursue. Exposure monitoring (see paragraph (d) of the final rule) must be performed to quantify an employee's exposure to MC.
In the comments received subsequent to publication of the ANPR for MC [Exs. 10-3, 10-10, 10-28], several industry commenters indicated that urine analysis, liver function tests and chest X-rays are commonly performed as part of the medical surveillance programs of these companies. OSHA believes that annual urine analysis or chest X-ray would not be relevant to detection of MC-related health effects. Liver function tests have also been evaluated for inclusion as a requirement in the medical surveillance provision. As discussed above in the Health Effects section, animal studies and human clinical studies show an association between chronic MC exposure and some changes in liver enzymes, particularly after high exposures or doses of MC for prolonged periods of time. The changes in liver enzyme levels after MC exposure are not consistent in the human clinical studies, however, and in general, changes in liver enzymes are not specific or unique to MC exposure. Therefore, the Agency believes that it should be left to the physician's or other licensed health care professional's discretion to determine if laboratory analysis of liver enzymes is warranted.
Several commenters [Exs. 19-11, 19-26, 19-42, 19-48, 19-55b] agreed that routine use of all of the tests included in the proposal would not be appropriate or necessary for the detection of MC-related health effects. The Agency also sought comments on the inclusion of other medical tests in the final MC rule. Two commenters [Exs. 19-31, 19-48] stated that a complete blood count was not necessary because the results of this test may not correlate with MC overexposure. In particular, the Dow Chemical Co. [Ex. 19-31] commented that a complete blood count is not necessary because blood cell volume and hemoglobin findings would suffice. OSHA has reevaluated the utility of the proposed tests and has decided that leaving laboratory surveillance to the discretion of the physician or licensed health care professional is more cost-effective than the approach taken in the proposal and will not negatively impact worker health.
In paragraph (j)(5)(iv), the final rule requires the medical surveillance program of the employer to include any other information or reports the physician or other licensed health care professional determines are necessary. This is to ensure that a complete medical profile is available to the physician or licensed health care professional to make decisions regarding the employee's health and exposure status. This provision is essentially identical to that proposed.
Paragraph (j)(6) of the final rule describes the required contents of emergency medical surveillance. The proposed rule did not specify what elements should be included in an emergency medical exam. The final rule clarifies that emergency medical surveillance should include any appropriate emergency treatment and decontamination of the exposed employee, a comprehensive physical exam, an updated medical and work history, and laboratory surveillance, if needed.
The Dow Chemical Company [Ex. 19-31] commented that employees exposed to MC during an emergency should not automatically be included in the regular medical surveillance program. Instead, this commenter argued that only those components of a medical examination that are appropriate in a given situation should be conducted. OSHA believes that it is important for an employer to provide medical examinations and appropriate follow-up to employees exposed to MC during an emergency. After considering the issue and comments raised during the rulemaking, the Agency agrees with Dow that employees exposed to MC during an emergency should not necessarily be enrolled in the continuing medical surveillance program provided to employees routinely exposed to MC. To that end, OSHA has added language to the final rule that clearly indicates what emergency medical surveillance is required. OSHA believes that final rule paragraph (j)(6) allows the employer appropriate flexibility, while at the same time ensuring that those employees exposed to MC during an emergency receive appropriate medical surveillance.
Paragraph (j)(7) requires the employer to provide medical surveillance services, in addition to those specified in final rule paragraphs (j)(5) and (j)(6), when the physician or other licensed health care professional determines that they are necessary. Compliance with this requirement will ensure that the information needed to evaluate the effects of MC exposure on employees is available. This provision is essentially the same as proposed paragraph (i)(5).
Paragraph (j)(8) requires that the employer provide the physician or other licensed health care professional with (1) a copy of the standard, including the relevant appendices; (2) a description of the affected employee's past, current, and anticipated future duties as they relate to the employee's MC exposure; (3) a description of former, current or anticipated exposure levels (including the frequency and exposure levels anticipated to be associated with emergencies), as applicable; (4) a description of any PPE that the employee must use or will use, such as respirators; and (5) information from any previous medical examinations that would not otherwise be available to the examining physician or other licensed health care professional. OSHA has determined that the physician or other licensed health care professional needs the above-listed background information in order to place the information derived from medical surveillance in the proper context. For example, a well-documented exposure history assists the physician or other licensed health care professional in determining whether an observed health condition may be related to MC exposure. It also helps this individual to determine if the results of medical surveillance indicate a need to limit an employee's occupational exposure to MC. This paragraph is essentially the same as proposed paragraph (i)(6).
Paragraph (j)(9) of the final rule requires employers to ensure that the examining physician or other licensed health care professional provides the employer and the affected employee with a written opinion that addresses (1) the physician's or other licensed health care professional's opinion as to whether the employee has any detected medical condition that would place the employee at increased risk of material health impairment as a result of exposure to MC; (2) any recommended limitations on the employee's exposure or use of personal protective clothing or equipment and respirators; (3) a statement that the employee has been informed of the potential carcinogenicity of MC, the risk factors for heart disease, and the potential for exacerbation of underlying heart disease associated with exposure to MC; and (4) a statement that the employee has been informed of the results of the medical examination and any medical conditions related to MC exposure that require further explanation or treatment.
The physician or other licensed health care professional must provide copies of the written medical opinion to the employee and the employer within 15 days after completion of the evaluation of medical and laboratory findings, but no later than 30 days after the medical examination. This requirement was included to ensure that the employee and the employer have been informed of the above-mentioned results of the medical examination in a timely manner. This requirement differs slightly from that in proposed paragraph (i)(7)(i). Instead of the physician providing a copy of the written medical opinion to the employer, who then provides a copy to the employee, the final rule requires the physician or other licensed health care professional to supply a copy of the written medical opinion directly to both the employer and the employee. In addition, the time allowed for providing the opinion has been changed to recognize that time may be needed to receive and evaluate laboratory or other medical findings. The Agency believes that notifying both the employer and affected employees of the MC-related results of the medical surveillance at the same time is an efficient approach to disseminating this information to the appropriate parties. Providing copies of the same written opinion both to the employer and the employee ensures that the employer is aware of any factors that may influence work assignments or choice of personal protective equipment.
OSHA has added a requirement to the final rule that the physician or other licensed health care professional inform the employee of the carcinogenic and cardiac effects of MC to reinforce the information on MC's serious health effects that was transmitted during training. The Agency believes that this reinforcement will help to ensure that employees are aware of the potential effects of MC and take appropriate precautions when using this toxic substance.
OSHA received several comments on different aspects of paragraph (j)(9). For example, the UAW [Tr. 1884, 9/24/92] testified that the written opinion transmitted to the employer by the physician or other licensed health care professional should only state the limitations on the employee's exposure or use of respiratory or other personal protective equipment recommended by the physician or other health care professional, and should not include the medical or other reasons behind the recommended limitations.
OSHA agrees with the UAW that it is important to protect the privacy of employees enrolled in medical surveillance programs. Consequently, OSHA health standards have traditionally included a statement to the effect that no findings or diagnoses should be included in the physician's written opinion that are unrelated to occupational exposure. This requirement is intended both to protect the employee's privacy and to encourage employees to participate in the employer's medical surveillance program. The restriction on what may be revealed in the written opinion appears in the final rule as paragraph (j)(9)(ii), and is intended to apply to all of the information provided in the physician's or other licensed health care professional's written opinion, including that related to recommended limitations.
The MVMA [Ex. 19-42] and ORC [Ex. 19-57] stated that the proposed 15-day requirement for providing the employer with a copy of the written opinion should be 15 days from the physician's or other licensed health care professional's receipt of the test results rather than 15 days from the date of the examination. The Agency agrees and, as described above, has changed the requirement so that the written opinion must be provided within 15 days of completion of evaluation of medical findings, but not more than 30 days after the examination. OSHA believes that this strikes the proper balance between allowing sufficient time for the physician or other licensed health care professional to evaluate any laboratory findings while still providing the information to the employer and the employee in a timely manner.
Newport News Shipbuilding [Ex. 19-37] and the Shipbuilders Council of America [Ex. 19-56] stated that the written opinion should require only that employees be notified of abnormal test results, not normal results. In response to these comments, OSHA notes that such a provision would actually require many physicians and other licensed health care professionals to change their current practice because it would require them specifically to delete normal results from printouts of laboratory and other findings. Such reports routinely display all results, both normal and abnormal, for a given individual. In addition, OSHA believes that employees benefit from knowing which of their blood parameters and other test results are normal and which are abnormal. OSHA does not believe that requiring medical personnel to increase the amount of paperwork they perform is a good use of medical resources, and has therefore not revised the final rule to respond to these comments.
Under paragraph (j)(9)(ii) of the final rule, the physician or other licensed health care professional must exclude findings or diagnoses that are unrelated to MC exposure from the written opinion provided to the employer. As discussed above, OSHA has included this provision in the final rule to reassure employees participating in medical surveillance that they will not be penalized or embarrassed by the employer's obtaining information about them that is not directly pertinent to MC exposure. The above provisions are identical to those in proposed paragraph (i)(7)(ii). A note has been added to the final rule that states that the written opinion developed to comply with the MC standard may also contain information related to other OSHA standards. For example, an employer whose employees are enrolled in medical surveillance due to their exposure to benzene, formaldehyde and MC could receive a single, consolidated written opinion that addressed findings related to all three substances. This performance-oriented provision could result in reduced paperwork burdens for employers.
NPRM Issue 3 solicited input regarding whether the Agency should add a provision for Medical Removal Protection (MRP). Medical removal protection encourages employee participation in (and therefore increases the effectiveness of) the medical surveillance program by ensuring that reporting symptoms or health conditions to the physician or licensed health care professional will not result in loss of job or pay. Several rulemaking participants expressed support for the inclusion of MRP in the final rule [Exs. 19-23, 19-38; Tr. 1787, 9/24/92; Tr. 1802, 9/24/92; Tr. 1869, 9/24/92; and Tr. 1883, 9/24/92]. For example, the Amalgamated Clothing and Textile Workers (ACTWU) [Tr. 1793, 9/24/92] testified that OSHA should require MRP based on clinical judgment, as OSHA allowed in the final rule for formaldehyde (29 CFR 1910.1048). They also stated that they believed it was critical to have a medical removal protection provision in the MC standard in order to ensure worker participation. Mr. Frumin of the ACTWU testified as follows [Tr. 1792-1793, 9/24/92]:
As I say, the problems that employers, physicians and, for that matter, OSHA confront in trying to assure the integrity of medical surveillance programs are not limited to a particular substance. They deal with the general perception -- these problems arise from the general perception of workers, which is widespread through industry, that if they submit to a medical examination and it's not confidential, and employers could get the results of the medical findings, that health problems may result in some negative action. You have a symptom-based medical surveillance program, at least for the non-cancer effects. And if workers are supposed to report the types of symptoms, for instance, that Dr. Soden was looking for, shortness of breath, things of that nature -- and they're concerned that reporting that might involve some negative action against them: either their job security or their pay. You know, they will be discouraged from participating in medical surveillance, and the whole structure of the program is undermined. So the fact that these health effects are symptom-based rather than, say, based on laboratory tests alone, makes it all the more important to include medical removal protection and multiple physician review in the final rule.
Two commenters [Exs. 19-23, 19-38] suggested that MRP should be based on COHb levels. However, Dr. Mirer of the UAW [Tr. 1940, 9/24/92] disagreed with this idea and concurred with Mr. Frumin's remarks that medical removal protection should be based on symptoms and professional discretion. He stated,
* * * the guidance for the physicians, once the physician decides this employee is at increased risk, if they continue in this exposure and I want to remove him or her from the job, that's the trigger. At this moment, I would leave it that way. Increased carboxyhemoglobin is more an index of exposure than an adverse clinical effect, so I don't have any particular guidance. If the doctor wants to pull that man or woman out of a job, that's where I am now.
He continued,
* * * the other benefit of protecting the disclosure of symptoms is that it's going to identify sources of exposure, because one of the ways of determining exposure is by the presentation of symptoms. So the benefit of having them disclose symptoms is it will lead to lower exposure.
I can't think of anything much else that you would need to get out of MRP than improved participation, although at least our experience in lead is that MRP has been the driving force to reduce exposures independent of that.
OSHA considered the issues raised during the MC rulemaking and in general agrees with these worker representatives that MRP increases employee participation in medical surveillance. OSHA remains concerned about several issues, however. The Agency recognizes that employees may hesitate to participate in medical surveillance if they have reason to expect that the results may adversely affect them economically. However, OSHA has determined that there is no substantive guidance that it could give a physician or other licensed health care professional to indicate when it might be appropriate to remove an employee temporarily from the workplace, or what an appropriate trigger for return to work might be. Accordingly, OSHA has decided to promulgate the final rule for MC without including MRP provisions. The Agency will continue to monitor compliance with the medical surveillance and PPE provisions of this standard and the experience in industries subject to standards with medical removal protection provisions to determine whether any further action is warranted.
Paragraph (k) Hazard Communication
The requirements for hazard communication have been changed from proposed paragraph (j) (Communication of MC hazards to employees) and promulgated in paragraph (k) of the final rule. The paragraph addressing hazard communication in the final MC rule is consistent with the requirements of OSHA's Hazard Communication Standard (HCS). The HCS requires all chemical manufacturers and importers to assess the hazards of the chemicals they produce or import. It also requires all employers to provide information concerning the hazards of such chemicals to their employees. The transmittal of hazard information to employees is to be accomplished by such means as container labeling and other forms of warning, material safety data sheets and employee training.
Since the HCS "is intended to address comprehensively the issue of evaluating the potential hazard of chemicals and communicating information concerning hazards and appropriate protective measures to employees" (52 FR 31877), OSHA is including paragraph (k) in the final rule only to reference the HCS requirements for labels and material safety data sheets, and to indicate specifically the MC health effects that are required to be addressed under that rule. This additional guidance to employers simply reiterates the requirements of the HCS to convey information to affected employees about all health hazards to which they are potentially exposed. The health effects addressed by the final MC rule are cancer, cardiac effects (including elevation of carboxyhemoglobin), central nervous system effects, and skin and eye irritation. There may also be other health hazards or physical hazards associated with MC that meet the definitions of coverage under the HCS. These should be addressed appropriately on the label and MSDS as well.
Employers who have already met their longstanding requirements to comply with the HCS will have no additional duties with regard to labels and MSDSs under the MC final rule. This is consistent with the suggestions of some commenters that no requirements should be mandated beyond those listed in the HCS [Exs. 19-25, 19-31, 19-42]. OSHA agrees that the HCS addresses the issue comprehensively, and additional requirements are not necessary to protect MC-exposed employees specifically. As a result, the Agency has deleted the proposed requirement for warning signs. Such signs are not required under the HCS, although they may be useful in some situations and employers may choose to use them. The Organization Resources Counselors [Ex. 19-57] commented that the required signs should say "warning" and not "danger" as proposed, and suggested consistency with the benzene and ethylene oxide standards. It should be noted that the terms "warning" and "danger" have specific meaning in the context of labels, and there are criteria for their application under voluntary consensus standards such as the ANSI Z129.1 standard for precautionary labeling. ORC's comment is otherwise moot at this point since the relevant requirement has been deleted.
Paragraph (l) Employee Information and Training
The requirements for employee information and training, which were part of proposed paragraph (j) (Communication of MC hazards to employees), have been separated from the hazard communication requirements for labels and data sheets described above, and promulgated as paragraph (l) in the final MC rule. Some of the training provisions that were proposed duplicated requirements of the HCS. These have been removed, and a reference to the information and training required under the HCS has been added to simply remind employers of their longstanding obligations under that rule to ensure that employees are apprised of the hazards of the chemicals in their workplaces, as well as appropriate protective measures. The information and training requirements in the final MC rule build upon those requirements with additional information specific to MC that will help employees understand the risks of exposure and the means to prevent adverse health effects from occurring in their particular workplaces.
It should be noted that the information and training requirements in the final rule have been separated from each other rather than being addressed together, because they deal with different ways of conveying information. "Information" transmittal is simply that -- a passive process of making information available to employees should they choose to use it. In some cases, this may be done in writing or some other simple manner of information transfer. "Training," on the other hand, is not a passive process. The information provided to employees in training requires them to comprehend it and subsequently to use it in the performance of their duties in the workplace. There are many different ways to accomplish training effectively, but it cannot be a simple transfer of information such as handing someone a written document. OSHA's voluntary training guidelines, which are found in OSHA Publication No. 2252, are available to provide employers additional guidance in setting up and implementing an appropriate employee training program. An effective training program is a critical component of any safety and health program in the workplace. Workers who are fully informed and engaged in the protective measures established by the employer will play a significant role in the prevention of adverse health effects. Ineffective training will not serve the purpose of making workers full participants in the program, and the likelihood of a successful program for safety and health in the absence of an effectively trained workforce is remote.
Paragraph (l)(1) requires employers to provide all employees who are potentially exposed to MC with information and training on MC prior to or at the time of initial assignment to a job involving MC exposure. Thus employees will have the information they need to protect themselves before they are actually subject to exposure. The final rule further indicates in paragraph (l)(2) that employers shall ensure that the information and training is presented in a manner that is understandable to employees and that employees have received the information and training required under the HCS.
Paragraph (l)(3) addresses the information to be provided to affected employees. This includes the requirements of the final MC standard and information available in its appendices, as well as how the employee can access or obtain a copy of it in the workplace. This will ensure that MC-exposed employees are aware that specific requirements have been established to protect them from adverse health effects, and give them an opportunity to review those requirements themselves if they so desire. Wherever employee exposures exceed or can reasonably be expected to exceed the action level, the employer is required to inform employees about the location of MC in the workplace, what operations may be affected, particularly noting where in the workplace there may be exposures above the permissible exposure limits.
Paragraph (l)(4) requires each employer to train each affected employee as required under the Hazard Communication Standard (29 CFR 1910.1200, 29 CFR 1915.1200 or 29 CFR 1926.59, as appropiate). This provision simply reminds employers of their obligation to train employees regarding the hazards of MC under the Hazard Communication Standard.
The final rule does not provide a specific time period for updating the training, whereas the proposed standard included a requirement for annual retraining. Instead, the final rule indicates in paragraph (l)(5) that the employer shall re-train each affected employee as necessary to ensure that employees exposed above the action level or the STEL maintain a good understanding of the principles of safe use and handling of MC in the workplace. Employers can assess whether this understanding is generally present in exposed employees in various ways, such as by observing their actions in the workplace. For example, if an employee is not using appropriate protective equipment or following safe work practices routinely, this may be an indication that additional training is required. This provision of the final rule is a performance-oriented requirement that allows each employer to determine how much or how often training is needed.
Paragraph (l)(6) requires that the employer do additional training when the workplace is modified or changed in such a way that employees are subject to greater exposures and those exposures exceed or can reasonably be expected to exceed the action level and those employees need information and training to understand how to implement the modifications or training successfully. This provision was not in the proposal, but the Agency considers it necessary to further protect employees from the hazards of MC when significant changes in workplace conditions occur.
Paragraph (l)(7) requires the employer whose employees are exposed to MC at a multi-employer worksite to notify the other employers with work operations at that site regarding the use of MC-containing materials, the hazards associated with the use of those materials and the control measures implemented to protect affected employees from MC exposure, in accordance with the requirements of the Hazard Communication Standard (HCS). The HCS addresses sharing information at multi-employer worksites, and since this final rule covers construction where most of the sites are multi-employer, this provision was added to remind such employers of these requirements. OSHA is also aware that an increasing number of manufacturing worksites involve more than one employer.
In paragraph (l)(8) of the final rule, OSHA has indicated that the Assistant Secretary or the Director may access all materials relating to employee information and training in the workplace. This would be done in conjunction with an inspection to ascertain compliance with the rule, or in the event of a NIOSH health hazard evaluation. Review of the available materials regarding information and training will help assess whether the program has been properly conducted, as well as evaluate what could be improved if employees do not appear to be effectively trained.
The information and training provisions of this standard are performance-oriented, because employees are exposed to MC in a wide variety of circumstances and the best method of conveying the necessary data may vary from site-to-site. The standard lists the categories of information to be transmitted to employees but does not specify the ways in which it is to be transmitted.
Some commenters [Tr. 531-32, 9/18/92; Tr. 545-49, 9/18/92; Tr. 828-32, 9/21/92; Tr. 1380, 1384-85, 9/23/92] suggested that OSHA make the proposed training provisions more specific, such as by including requirements for length of training, qualifications of instructors, or requirements for interactive training. In addition, hearing participants and commenters suggested that OSHA require employers to monitor the effectiveness of training [Ex. 19-38, Tr. 531-32, 9/18/92]. These participants suggested that provisions be made, as well, for training of workers in languages other than English and for training of workers with limited literacy [Ex. 19-38, Tr. 531-32, 9/18/92; Tr. 831-32, 9/21/92].
The International Brotherhood of Painters and Allied Trades, AFL-CIO, testified [Tr. 830-831, 9/21/92]:
We urge OSHA to promulgate a standard that requires that workers receive a minimum of 16 hours training. Such training would include at the minimum information on the hazards of methylene chloride and how it harms the body. Engineering controls that can be implemented in the field should be described and demonstrated. We will submit information on one such control to the record. Training should also include information on work practices associated with specific job assignments, methods by which workers can protect themselves, the limits of respirators use, appropriate procedures for work in confined spaces, employee rights under the standard, the purpose of medical surveillance and other elements of training as enumerated in Section (j)(4).
OSHA does not agree that specifying a time frame for training ensures that it will be complete, appropriate, or effective. The amount of training required will depend to a large extent on the conditions of use in a given workplace. It will also be related to the extent of training on MC that has already been done by the employer under the HCS. Therefore, the final rule provisions remain performance-oriented with regard to the time needed to convey the information and training.
With regard to the issues of literacy and language, these remain a significant consideration in the proper design and implementation of any training program. Because working safely with MC is such a significant concern, the employer must make every effort to ensure that the training is presented in such a way that employees can understand and act on the information.
OSHA expects that employers will ensure that the information and training is effective. Any good training program should include an evaluation component to help ensure effectiveness. The voluntary training guidelines previously recommended can provide additional guidance in this respect.
OSHA received comments that indicated that the MC standard should simply refer to the HCS rather than having separate requirements [Exs. 19-25; 19-49]. While the Agency agrees with these comments in reference to the label and MSDS requirements, it does not appear that this is the appropriate approach to training. While the HCS addresses training about the hazards of a chemical and appropriate precautionary measures, there are other items of training that are specific to the MC standard requirements and the determinations made in this rulemaking regarding MC. As such, it is important to ensure that the already-required HCS training is supplemented with information and training specific to MC.
Paragraph (m) Recordkeeping
Paragraph (m) of the final rule addresses requirements for employers to create and maintain records of their compliance with some of the provisions of this section. Section 8(c)(1) of the OSH Act authorizes the Agency to promulgate regulations requiring employers to keep necessary and appropriate records regarding activities to permit the enforcement of the Act or to develop information regarding the causes and prevention of occupational accidents and illnesses. Section 8(c)(3) of the Act specifically addresses the promulgation of "regulations requiring employers to maintain accurate records of employee exposures to potentially toxic materials or harmful physical agents which are required to be monitored or measured under section 6."
Paragraph (m)(1) requires that employers who rely on objective data to characterize potential exposures to MC, rather than conducting initial monitoring under paragraph (d) of this section, maintain records that show the information and methodology used in reaching their conclusion that exposures are at or below the action level and no additional monitoring is required. The record must include the MC-containing material evaluated; the source of the objective data; the testing protocol, and the results or analysis of the testing; a description of the operation(s) exempted from monitoring, and how the data support the exemption; and other relevant data.
Since the use of objective data exempts the employer from conducting monitoring, as well as establishing that most of the other provisions need not be complied with due to the low level of potential exposure, it is critical that this determination be carefully documented. Compliance with the requirement to maintain a record of objective data protects the employer at later dates from the contention that initial monitoring was improperly omitted. The record will also be available to employees so that they can examine the determination made by the employer. The employer is required to maintain the record for the duration of the employer's reliance upon objective data. This provision is effectively identical to proposed paragraph (k)(1).
Paragraph (m)(2) requires that employers establish and keep an accurate record of all measurements taken to monitor employee exposure to MC. For employers with 20 or more employees, the record must include at least: the date of measurement for each sample taken; the operation involving exposure to MC which is being monitored; sampling and analytical methods used and evidence of their accuracy; number, duration and results of samples taken; the type of personal protective equipment, such as respiratory protective devices worn (if any); and name, social security number, and job classification and exposure of all the employees deemed to be represented by such monitoring, indicating which employees were actually monitored. For employers with fewer than 20 employees, the record shall include, at a minimum: the date of measurement for each sample; the number, duration and results of samples taken; and name, social security number, job classification and exposure of all the employees deemed to be represented by such monitoring, indicating which employees were actually monitored. OSHA believes it is necessary to maintain these records so that employers, employees and OSHA can determine the extent to which MC exposure has been identified and subsequently controlled. Over time, the exposure records can help determine if additional measures are needed for employee protection. OSHA has reduced the amount of information required for small businesses in recognition of the more limited variety of operations and exposure levels there. This should ease these employers' recordkeeping burden without compromising employee safety and health in these types of facilities.
Two commenters [Exs. 19-25, 19-49] suggested that such documentation should only be required for each person actually monitored (paragraph (d)(1) provides for representative monitoring). However, OSHA believes that it is necessary for records to be kept for each employee represented by the exposure monitoring so that individual employees can access information that characterizes their own exposures to MC. If records were kept only for those actually monitored, it would be unreasonably difficult for an employee to identify the exposure measurement that is intended to represent his or her experience. Accordingly, OSHA has not made the suggested change.
Paragraph (m)(3) requires that the employer keep accurate medical records for each employee subject to medical surveillance. The information to be included in the record addresses identification of the employee; the physician's or other licensed health care professional's written opinions; and documentation of any employee medical conditions that are found to be related to MC exposure. Maintenance of employee medical records is necessary for the proper evaluation of the employee's health, as well as for appropriate followup.
Proposed paragraph (k)(3)(ii)(D) required that a copy of the information provided to the physician or other licensed health care professional be included in the employee record. The Dow Chemical Company [Ex. 19-31] requested that, because many larger companies have company medical facilities, some provision be made so that records do not have to be maintained in medical department records and duplicated in the personnel record of every employee potentially exposed to MC. The information required under paragraph (j)(8) of this section includes a copy of this section including its appendices, a description of duties involving MC exposure, exposure levels, personal protective equipment, and previous medical surveillance information. Since this information is available to the employee through other means, OSHA believes that the requirements under proposed paragraph (k)(3)(ii)(D) were unnecessarily burdensome, and OSHA has therefore deleted this paragraph from the final rule. OSHA has also deleted proposed requirements for maintaining records of employee fit testing as being unnecessarily burdensome. Dow also suggested that an employee identification number be permitted in lieu of social security number [Ex. 19-31]. OSHA does not agree with this suggestion. Social security numbers have much wider application, and are correlated to employee identity in other types of records. These numbers are a more useful differentiation among employees since each number is unique to an individual for a lifetime and does not change as an employee changes employers.
Paragraph (m)(4) of the final rule specifies that access to exposure and medical records by employees, employees" designated representatives, NIOSH and OSHA shall be provided in accordance with 29 CFR 1910.1020. OSHA promulgated 29 CFR 1910.1020 as the generic rule for access to employee exposure and medical records on May 23, 1980 (45 FR 35212). It applies to records created under specific OSHA standards and to records that are voluntarily created by employers. OSHA retains unrestricted access to medical and exposure records but its access to personally identifiable records is subject to the Agency's rules of practice and procedure concerning OSHA access to employee medical records, which have been published at 29 CFR 1913.10.
The time periods required for retention of exposure records and medical records is thirty years and the period of employment plus thirty years, respectively. These retention requirements are consistent with those in the OSHA records access standard and with pertinent sections of the Toxic Substances Control Act. It is necessary to keep records for extended periods of time because of the long latency periods commonly observed for the induction of cancer caused by exposures to carcinogens. Cancer often cannot be detected until 20 or more years after onset of exposure. The extended record retention period is therefore needed for two purposes. First, possession of past and present exposure data and medical records furthers the diagnosis of workers' ailments. In addition, retaining records for extended periods makes possible a review at some future date of the effectiveness and adequacy of the standard.
Paragraph (m)(5) requires employers to comply with the requirements of 29 CFR 1910.1020(h). That provision requires the employer to notify the Director of NIOSH in writing at least 90 days prior to the disposal of records and to transfer those records to NIOSH unless told not to do so by NIOSH. The employer is required to comply with any other applicable requirements set forth in the records retention standard.
Paragraph (n) Dates
This paragraph establishes the effective date for the MC final rule, and the start-up dates for the various provisions of the standard. The start-up dates allow employers additional time to comply with some of the provisions of the standard that require more effort to accomplish. It is expected that such work will commence by the effective date, and be completed as soon as possible but in no case later than the compliance deadline established by the effective date. All other obligations imposed by the standard become effective on the effective date unless otherwise indicated.
Paragraph (n)(1) of the final rule provides that this standard will become effective on April 10, 1997. This date is 90 days from the date of publication in the Federal Register. Proposed paragraph (m)(1) had provided that the final rule would become effective 60 days after publication in the Federal Register. OSHA stated in the preamble to the proposed rule [56 FR 57128] that the proposed effective date, in conjunction with the proposed start-up dates, would allow sufficient time for employers to achieve compliance with the substantive requirements of the proposed rule.
Although no commenters directly addressed the 60-day period proposed in paragraph (m)(1), several commenters addressed the reasonableness of the start-up dates in proposed paragraph (m)(2). Those comments, discussed below, indicated that some employers would need more time to comply than the proposed rule would have allowed.
The Agency sets the effective date to allow sufficient time for employers to obtain the standard, read and understand its requirements, and undertake the necessary planning and preparation for compliance. Section 6(b)(4) of the OSHA Act provides that the effective date of an OSHA standard may be delayed for up to 90 days from the date of publication in the Federal Register. Given the concerns expressed by commenters, OSHA's interest in having employers implement effective compliance efforts, and the minimal effect of the additional 30 day delay, the Agency has decided that it is appropriate to set the effective date at 90 days from publication, rather than at 60 days.
Paragraph (n)(2) of the final rule establishes the start-up dates for compliance with the provisions of the MC standard. The start-up dates are based on information in the record about the state of the art with regard to the types of provisions employers are expected to implement, such as available control measures, their complexity, and the time that is reasonably necessary to complete their installation and implementation. In the case of MC, the types of provisions included in the rule, such as requirements that will require conventional controls, are identical to the elements included in all OSHA health standards.
Proposed paragraphs (m)(2)(i), (ii) and (iii) required that initial monitoring be completed by all employers within 120 days of the effective date of the MC standard, engineering controls within one year of the effective date and all other requirements within 180 days of the effective date. As described below, OSHA received numerous comments on the appropriateness of the start-up dates, especially for small businesses. Given the large number of small employers covered by the requirements, and the special problems of many of those employers in identifying and implementing appropriate control measures, OSHA has decided to phase-in compliance and to permit these employers a longer time period in which to comply with the requirements of the standard. The schedule for compliance with the provisions of the standard are described below.
OSHA received a number of comments on the proposed periods for compliance with the control requirements. In 1992, Kodak [Exs. 19-18 and 19-102] described circumstances at its film base production facility that would prevent compliance with the PELs through engineering controls before mid-1995. Kodak stated "[it] is essential that OSHA be responsive to these considerations in promulgating the final rule. OSHA should permit adequate time for Kodak to implement feasible engineering controls in an orderly and minimally disruptive schedule." Considering the effective date and start-up dates in this regulation, OSHA has determined that affected parties will have sufficient time to comply with the standard.
Similar requests for longer time periods for compliance were also received from a variety of other commenters [Exs. 19-55, 19-57, 19-67, 19-72, 19-75, 115-3, 115-28, 115-33, 115-37, Tr. 1422, 1427-29, 9/23/92, Tr. 2103, 10/14/92, Tr. 2291-92, 2300, 10/15/92]. However, OSHA's Final Economic Analysis for this rulemaking indicates that readily available control measures can be used to control exposure in many of the operations where MC is present. In general, compliance will not require the development of new or novel control technology. Accordingly, OSHA believes that more extended time periods for compliance are not necessary for all affected industries. However, as discussed below, small businesses (for example, those with fewer than 20 employees and polyurethane foam manufacturers with 20 to 99 employees) have been granted additional time to comply.
As discussed above in Section VIII, several commenters [Exs. 19-14, 19-25, 19-28 and 19-29] stated that engineering controls to achieve compliance were not available. These commenters further stated that the development and implementation of the process changes and engineering controls needed to achieve compliance would take four years from the effective date, not the single year proposed. For example, the Pharmaceutical Manufacturers Association and Abbott Laboratories [Exs. 19-25 and 19-29] stated as follows:
[I]f the agency should rule that the exposure level to MC be reduced to 25 ppm for an 8-hour TWA and a 125 ppm STEL, a minimum of 1 year from the effective date must be allowed for identification of the engineering controls. A minimum of 3 years from the effective date must be allowed for compliance with paragraph (f)(1) of the proposed rule.
Those commenters and the HSIA [Ex. 19-45] also indicated that FDA approval is needed in the pharmaceutical industry for any alteration of manufacturing processes, substitution for MC, or modification of work practices to achieve compliance with OSHA's MC standard, and requested that OSHA consider the FDA's regulatory requirements when establishing start-up dates. In particular, Abbott Laboratories described how it took three years to obtain FDA approval for the substitution of hydroalcoholic or aqueous solutions for MC in tablet coating operations, stating "[p]resently, completion of required testing and obtaining FDA approval for production of a single product can take 3 months to three years, depending upon the extent of the change."
Abbott also commented as follows [Ex. 19-29]:
As stated previously, feasible engineering controls do not exist for the present bulk pharmaceutical centrifugal separation and drying equipment. Implementation of engineering controls would therefore require the use of a different process or a different production method. Changes of that degree require Abbott Laboratories to complete development work on an alternative process and/or identify new production equipment; erect a building to house the equipment; purchase, receive and install the equipment; train employees; and validate the process. This cannot be accomplished in one year.
OSHA is aware that pharmaceutical manufacturers must comply with other regulatory requirements, including those set by the FDA. The Agency has considered how affected employers, in general, need to coordinate their OSHA compliance efforts with their other regulatory compliance activities, that this regulation does not require implementation of particularly complicated or novel control technologies, and that the compliance time frames are in keeping with those in other OSHA standards. OSHA views the coordination of OSHA compliance with other regulatory compliance activities as an ongoing employer effort, not just an ad hoc response to a particular OSHA action (such as the revision of a PEL). For example, a pharmaceutical manufacturer would need to consider the implications for OSHA compliance of process changes undertaken due to FDA requirements or for other reasons, whether those changes were to be made during the MC standard's "start-up" period or subsequently.
Accordingly, the Agency has determined that the commenters have not established a need for the requested extension of the start-up dates. OSHA believes that the proposed one-year period in which to implement controls will, in general, be adequate and, therefore, has not made the suggested change. However, as discussed elsewhere, OSHA has tailored the compliance schedule to the size of the establishment and anticipated impact of the standard on those businesses.
Dow [Ex. 19-31] also expressed concern that many employers would be unable to meet the start-up dates, focusing on the time and resources that would be required to conduct initial monitoring. In addition, Dow stated as follows "OSHA should require that certain actions be completed within the stated time periods and that if the actions can not be completed, the employer should have a written plan and corresponding actions to show a good faith effort to meet the requirements." OSHA agrees that there may be circumstances where, despite good faith efforts, employers cannot achieve compliance within the time periods specified by paragraph (n)(2). OSHA further agrees that developing a written plan and taking other "good faith" actions towards compliance would be appropriate measures to mitigate any circumstances of non-compliance with the regulation. Indeed, the suggested procedure closely resembles the temporary variance process already established by OSHA.
Under section 6(b)(6) of the OSH Act, an employer can obtain a temporary variance from compliance with an OSHA standard if it shows that it cannot achieve compliance by the effective date; is taking all available steps to safeguard its employees from the pertinent hazard; and has an effective program for coming into compliance with the standard. The implementing regulations for the temporary variance process appear at 29 CFR part 1905. Employers who experience difficulties in meeting the start-up dates should contact OSHA and apply for a temporary variance.
The HSIA [Ex. 19-45] recommended that OSHA "provide a compliance schedule similar to that provided in the generic PEL update * * * [which] in some circumstances allows employers until December 31, 1993 to comply (a total of 4 years and 10 months)." In addition to mentioning the lengthy FDA approval process, the HSIA noted that "DCM users, particularly many of the smaller companies, will find compliance technologically and economically difficult at best."
As stated above, OSHA believes that the sort of extended compliance schedule set through the generic PEL update is unnecessary for the MC standard. Based on its review of the rulemaking record, the Agency has reached the general conclusion that employers will be able to achieve compliance within the time frames established in paragraph (n).
However, OSHA is concerned that some small facilities affected by this rulemaking, such as many of those in the furniture refinishing industry and the polyurethane foam manufacturing industry, may have difficulties determining the appropriate control measures to use and also may not be able to absorb the costs of compliance, particularly those associated with implementing the appropriate engineering controls within the time frames initially proposed. The Agency has estimated (see Section VIII, Summary of the Final Economic Analysis) that allowing a variable schedule of compliance, based upon size of establishment, will enable firms in all impacted sectors to absorb many of the compliance costs without endangering their financial health.
Based on these considerations, OSHA has determined that the following implementation schedule is reasonable and appropriate for businesses of all sizes:
| Establishment size | Initial monitoring provisions must be complied with | Implementation of engineering controls must be completed within | all other provisons must be complied with within |
|---|---|---|---|
| Fewer than 20 employees | within 300 days of the effective date | 3 years of the effective date | 1 year of the effective date |
| Polyurethane foam manufactureers with 20 to 99 employees | 210 days of the effective date | 2 years of the effective date | 270 days of the effective date |
| all other employers | 120 days or the effective date | 1 year of the effective date | 180 days of the effective date |
The Agency is promulgating paragraph (n) accordingly. The schedule of intermediate start-up dates (210 d, 270 d and 2 years) for polyurethane foam manufacturers with 20 to 99 employees was limited to this application group because this group has the highest potential economic impacts except for the furniture stripping and construction groups. In both of the latter groups, most firms have fewer than 20 employees, and thus would already be allowed additional time to comply with the final rule's start-up dates. In contrast, in the flexible polyurethane foam manufacturing group, even firms with fewer than 100 employees will need to install several types of engineering controls and are likely to have unusually high capital expenditures in order to meet the requirements of the regulation. This extension of compliance deadlines will allow those firms that need extensive engineering controls time to adequately plan for and implement their system of controls. This modification will thus also help to ensure adequate protection for workers.
Paragraph (o) Appendices
The final paragraph of the standard simply states that the appendices which follow are not intended to create any additional obligations beyond those already specified in the standard. They are basically intended as non-mandatory guidance documents to supplement and complement the regulatory requirements in the standard, and to provide additional information about MC and its safe handling and use to exposed employees, employers, and health care professionals.
A few comments were received by OSHA regarding the text of the appendices as proposed. These addressed the need for additional information [Ex. 57, Tr. 832, 9/21/92, Tr. 1380 and 1384-85, 9/23/92], or whether information should appear in an appendix or in the regulatory text itself [see, e.g., Tr. 2435-36 and 2448-49, 10/15/92]. OSHA has reviewed and updated the text in the appendices to address these comments and ensure that they are consistent with the new regulatory text in the final standard.
Also, proposed Non-mandatory Appendix C, which addressed respirator fit testing, has not been included in the final rule, because OSHA has determined that very few of the respirators used to comply with this standard will require fit testing. In addition, OSHA's revision of the generic respirator standard (29 CFR 1910.134) will contain an up-to-date appendix that addresses fit testing for all respirators.
XI. Authority and Signature
This document was prepared under the direction of Joseph A. Dear, Assistant Secretary of Labor for Occupational Safety and Health, U.S. Department of Labor, 200 Constitution Avenue, NW., Washington, DC 20210.
Pursuant to sections 4, 6(b), 8(c) and 8(g) of the Occupational Safety and Health Act (29 U.S.C. 653, 655, 657), section 107 of the Contract Work Hours and Safety Standards Act (the Construction Safety Act) (40 U.S.C. 333); the Longshore and Harbor Workers' Compensation Act (33 U.S.C. 941); the Secretary of Labor's Order No. 1-90 (55 FR 9033); and 29 CFR part 1911; 29 CFR parts 1910, 1915 and 1926 are amended as set forth below.
List of Subjects in 29 CFR Part 1910, 1915 and 1926
Chemicals, Cancer, Health risk-assessment, Methylene chloride, Occupational safety and health.
Signed at Washington, D.C., this 31st day of December 1996.
Joseph A. Dear,
Assistant Secretary of Labor.
XII. Final Standard Regulatory Text
Parts 1910, 1915, and 1926 of Title 29 of the Code of Federal Regulations are amended as follows:
PART 1910 -- [AMENDED]
Subpart B -- [Amended]
1. The authority citation for subpart B of part 1910 continues to read as follows:
Authority: Secs. 4, 6 and 8 of the Occupational Safety and Health Act, 29 U.S.C. 653, 655, 657; Walsh-Healey Act, 29 U.S.C. 35 et seq; Service Contract Act of 1965, 41 U.S.C. 351 et seq; Contract Work Hours and Safety Standards Act (Construction Safety Act), 40 U.S.C. 333; Sec 41 Longshore and Harbor Worker's Compensation Act, 33 U.S.C. 941; National Foundation on Arts and Humanities, 20 U.S.C. 951 et seq; Secretary of Labor's Order No, 12-71 (36 FR 8754); 8-76 (41 FR 25059); 9-83 (48 FR 35736); 1-90 (55 FR 9033); and 29 CFR part 1911.
2. By adding a new paragraph (m) to 1910.19 to read as follows:
1910.19 Special provisions for air contaminants.
* * * * *
(m) Methylene Chloride (MC): Section 1910.1052 shall apply to the exposure of every employee to MC in every employment and place of employment covered by 1910.16 in lieu of any different standard on exposure to MC which would otherwise be applicable by virtue of that section when it is not present in sealed, intact containers.
Subpart Z -- [Amended]
3. The authority citation for subpart Z of 29 CFR part 1910 continues to read, in part, as follows:
Authority: Secs. 6 and 8 Occupational Safety and Health Act, 29 U.S.C. 655, 657; Secretary of Labor's Orders 12-71 (36 FR 8754), 8-76 (41 FR 25059), 9-83 (48 FR 35736) or 1-90 (55 FR 9033), as applicable; and 29 CFR part 1911.
* * * * *
1910.1000 [Amended]
4. By removing the entire entry for Methylene Chloride (Z37.23-1969) in Table Z-2 of 1910.1000 and adding the following entry in its place in the substance column: "Methylene chloride: see 1910.1052".
5. By adding a new 1910.1052 to read as follows:
1910.1052 Methylene Chloride.
This occupational health standard establishes requirements for employers to control occupational exposure to methylene chloride (MC). Employees exposed to MC are at increased risk of developing cancer, adverse effects on the heart, central nervous system and liver, and skin or eye irritation. Exposure may occur through inhalation, by absorption through the skin, or through contact with the skin. MC is a solvent which is used in many different types of work activities, such as paint stripping, polyurethane foam manufacturing, and cleaning and degreasing. Under the requirements of paragraph (d) of this section, each covered employer must make an initial determination of each employee's exposure to MC. If the employer determines that employees are exposed below the action level, the only other provisions of this section that apply are that a record must be made of the determination, the employees must receive information and training under paragraph (l) of this section and, where appropriate, employees must be protected from contact with liquid MC under paragraph (h) of this section. The provisions of the MC standard are as follows:
(a) Scope and application. This section applies to all occupational exposures to methylene chloride (MC), Chemical Abstracts Service Registry Number 75-09-2, in general industry, construction and shipyard employment.
(b) Definitions. For the purposes of this section, the following definitions shall apply:
Action level means a concentration of airborne MC of 12.5 parts per million (ppm) calculated as an eight (8)-hour time-weighted average (TWA).
Assistant Secretary means the Assistant Secretary of Labor for Occupational Safety and Health, U.S. Department of Labor, or designee.
Authorized person means any person specifically authorized by the employer and required by work duties to be present in regulated areas, or any person entering such an area as a designated representative of employees for the purpose of exercising the right to observe monitoring and measuring procedures under paragraph (d) of this section, or any other person authorized by the OSH Act or regulations issued under the Act.
Director means the Director of the National Institute for Occupational Safety and Health, U.S. Department of Health and Human Services, or designee.
Emergency means any occurrence, such as, but not limited to, equipment failure, rupture of containers, or failure of control equipment, which results, or is likely to result in an uncontrolled release of MC. If an incidental release of MC can be controlled by employees such as maintenance personnel at the time of release and in accordance with the leak/spill provisions required by paragraph (f) of this section, it is not considered an emergency as defined by this standard.
Employee exposure means exposure to airborne MC which occurs or would occur if the employee were not using respiratory protection.
Methylene chloride (MC) means an organic compound with chemical formula, CH(2Cl)(2). Its Chemical Abstracts Service Registry Number is 75-09-2. Its molecular weight is 84.9 g/mole.
Physician or other licensed health care professional is an individual whose legally permitted scope of practice (i.e., license, registration, or certification) allows him or her to independently provide or be delegated the responsibility to provide some or all of the health care services required by paragraph (j) of this section.
Regulated area means an area, demarcated by the employer, where an employee's exposure to airborne concentrations of MC exceeds or can reasonably be expected to exceed either the 8-hour TWA PEL or the STEL.
Symptom means central nervous system effects such as headaches, disorientation, dizziness, fatigue, and decreased attention span; skin effects such as chapping, erythema, cracked skin, or skin burns; and cardiac effects such as chest pain or shortness of breath.
This section means this methylene chloride standard. (c) Permissible exposure limits (PELs). (1) Eight-hour time-weighted average (TWA) PEL. The employer shall ensure that no employee is exposed to an airborne concentration of MC in excess of twenty-five parts of MC per million parts of air (25 ppm) as an 8-hour TWA.
(2) Short-term exposure limit (STEL). The employer shall ensure that no employee is exposed to an airborne concentration of MC in excess of one hundred and twenty-five parts of MC per million parts of air (125 ppm) as determined over a sampling period of fifteen minutes.
(d) Exposure monitoring. (1) Characterization of employee exposure. (i) Where MC is present in the workplace, the employer shall determine each employee's exposure by either:
(A) Taking a personal breathing zone air sample of each employee's exposure; or (B) Taking personal breathing zone air samples that are representative of each employee's exposure.
(ii) Representative samples. The employer may consider personal breathing zone air samples to be representative of employee exposures when they are taken as follows:
(A) 8-hour TWA PEL. The employer has taken one or more personal breathing zone air samples for at least one employee in each job classification in a work area during every work shift, and the employee sampled is expected to have the highest MC exposure.
(B) Short-term exposure limits. The employer has taken one or more personal breathing zone air samples which indicate the highest likely 15-minute exposures during such operations for at least one employee in each job classification in the work area during every work shift, and the employee sampled is expected to have the highest MC exposure.
(C) Exception. Personal breathing zone air samples taken during one work shift may be used to represent employee exposures on other work shifts where the employer can document that the tasks performed and conditions in the workplace are similar across shifts.
(iii) Accuracy of monitoring. The employer shall ensure that the methods used to perform exposure monitoring produce results that are accurate to a confidence level of 95 percent, and are:
(A) Within plus or minus 25 percent for airborne concentrations of MC above the 8-hour TWA PEL or the STEL; or (B) Within plus or minus 35 percent for airborne concentrations of MC at or above the action level but at or below the 8-hour TWA PEL.
(2) Initial determination. Each employer whose employees are exposed to MC shall perform initial exposure monitoring to determine each affected employee's exposure, except under the following conditions:
(i) Where objective data demonstrate that MC cannot be released in the workplace in airborne concentrations at or above the action level or above the STEL. The objective data shall represent the highest MC exposures likely to occur under reasonably foreseeable conditions of processing, use, or handling. The employer shall document the objective data exemption as specified in paragraph (m) of this section;
(ii) Where the employer has performed exposure monitoring within 12 months prior to April 10, 1997 and that exposure monitoring meets all other requirements of this section, and was conducted under conditions substantially equivalent to existing conditions; or (iii) Where employees are exposed to MC on fewer than 30 days per year (e.g., on a construction site), and the employer has measurements by direct-reading instruments which give immediate results (such as a detector tube) and which provide sufficient information regarding employee exposures to determine what control measures are necessary to reduce exposures to acceptable levels.
(3) Periodic monitoring. Where the initial determination shows employee exposures at or above the action level or above the STEL, the employer shall establish an exposure monitoring program for periodic monitoring of employee exposure to MC in accordance with Table 1:
| Exposure scenario | Required monitoring activity |
|---|---|
| Below the action level and at or below the STEL | No 8-hour TWA or STEL monitoring required |
| Below the action level and above the STEL | No 8-hour TWA monitoring required; monitor STEL exposures every three months |
| at or above the action level, at or below the TWA, and at or below the STEL | Monitor 8-hour TWA exposures every six months |
| at or above the action level, at or below the TWA, and above the STEL | Monitor 8-hour TWA exposures every six months and monitor STEL exposures every three months |
| above the TWA and at or below the STEL | Monitor 8-hour TWA exposures every three months |
| above the TWA and above the STEL | Monitor 8-hour TWA exposures and STEL exposures every three months |
[Note to paragraph (d)(3): The employer may decrease the frequency of exposure monitoring to every six months when at least 2 consecutive measurements taken at least 7 days apart show exposures to be at or below the 8-hour TWA PEL. The employer may discontinue the periodic 8-hour TWA monitoring for employees where at least two consecutive measurements taken at least 7 days apart are below the action level. The employer may discontinue the periodic STEL monitoring for employees where at least two consecutive measurements taken at least 7 days apart are at or below the STEL.] (4) Additional monitoring. (i) The employer shall perform exposure monitoring when a change in workplace conditions indicates that employee exposure may have increased. Examples of situations that may require additional monitoring include changes in production, process, control equipment, or work practices, or a leak, rupture, or other breakdown.
(ii) Where exposure monitoring is performed due to a spill, leak, rupture or equipment breakdown, the employer shall clean-up the MC and perform the appropriate repairs before monitoring.
(5) Employee notification of monitoring results. (i) The employer shall, within 15 working days after the receipt of the results of any monitoring performed under this section, notify each affected employee of these results in writing, either individually or by posting of results in an appropriate location that is accessible to affected employees.
(ii) Whenever monitoring results indicate that employee exposure is above the 8-hour TWA PEL or the STEL, the employer shall describe in the written notification the corrective action being taken to reduce employee exposure to or below the 8-hour TWA PEL or STEL and the schedule for completion of this action.
(6) Observation of monitoring. (i) Employee observation. The employer shall provide affected employees or their designated representatives an opportunity to observe any monitoring of employee exposure to MC conducted in accordance with this section.
(ii) Observation procedures. When observation of the monitoring of employee exposure to MC requires entry into an area where the use of protective clothing or equipment is required, the employer shall provide, at no cost to the observer(s), and the observer(s) shall be required to use such clothing and equipment and shall comply with all other applicable safety and health procedures.
(e) Regulated areas. (1) The employer shall establish a regulated area wherever an employee's exposure to airborne concentrations of MC exceeds or can reasonably be expected to exceed either the 8-hour TWA PEL or the STEL.
(2) The employer shall limit access to regulated areas to authorized persons.
(3) The employer shall supply a respirator, selected in accordance with paragraph (h)(3) of this section, to each person who enters a regulated area and shall require each affected employee to use that respirator whenever MC exposures are likely to exceed the 8-hour TWA PEL or STEL.
[Note to paragraph (e)(3): An employer who has implemented all feasible engineering, work practice and administrative controls (as required in paragraph (f) of this section), and who has established a regulated area (as required by paragraph (e)(1) of this section) where MC exposure can be reliably predicted to exceed the 8-hour TWA PEL or the STEL only on certain days (for example, because of work or process schedule) would need to have affected employees use respirators in that regulated area only on those days.] (4) The employer shall ensure that, within a regulated area, employees do not engage in non-work activities which may increase dermal or oral MC exposure.
(5) The employer shall ensure that while employees are wearing respirators, they do not engage in activities (such as taking medication or chewing gum or tobacco) which interfere with respirator seal or performance.
(6) The employer shall demarcate regulated areas from the rest of the workplace in any manner that adequately establishes and alerts employees to the boundaries of the area and minimizes the number of authorized employees exposed to MC within the regulated area.
(7) An employer at a multi-employer worksite who establishes a regulated area shall communicate the access restrictions and locations of these areas to all other employers with work operations at that worksite.
(f) Methods of compliance. (1) Engineering and work practice controls. The employer shall institute and maintain the effectiveness of engineering controls and work practices to reduce employee exposure to or below the PELs except to the extent that the employer can demonstrate that such controls are not feasible. Wherever the feasible engineering controls and work practices which can be instituted are not sufficient to reduce employee exposure to or below the 8-TWA PEL or STEL, the employer shall use them to reduce employee exposure to the lowest levels achievable by these controls and shall supplement them by the use of respiratory protection that complies with the requirements of paragraph (g) of this section.
(2) Prohibition of rotation. The employer shall not implement a schedule of employee rotation as a means of compliance with the PELs.
(3) Leak and spill detection. (i) The employer shall implement procedures to detect leaks of MC in the workplace. In work areas where spills may occur, the employer shall make provisions to contain any spills and to safely dispose of any MC-contaminated waste materials.
(ii) The employer shall ensure that all incidental leaks are repaired and that incidental spills are cleaned promptly by employees who use the appropriate personal protective equipment and are trained in proper methods of cleanup. [Note to paragraph (f)(3)(ii): See Appendix A of this section for examples of procedures that satisfy this requirement. Employers covered by this standard may also be subject to the hazardous waste and emergency response provisions contained in 29 CFR 1910.120 (q).] (g) Respiratory protection. (1) General requirements. The employer shall provide a respirator which complies with the requirement of this paragraph, at no cost to each affected employee, and ensure that each affected employee uses such respirator where appropriate. Respirators shall be used in the following circumstances:
(i) Whenever an employee's exposure to MC exceeds or can reasonably be expected to exceed the 8-hour TWA PEL or the STEL (such as where an employee is using MC in a regulated area);
(ii) During the time interval necessary to install or implement feasible engineering and work practice controls;
(iii) In a few work operations, such as some maintenance operations and repair activities, for which the employer demonstrates that engineering and work practice controls are infeasible;
(iv) Where feasible engineering and work practice controls are not sufficient to reduce exposures to or below the PELs; or (v) In emergencies. (2) Medical Evaluation. Before having any employee use a supplied-air respirator in the negative pressure mode, or a gas mask with organic vapor canister for emergency escape, the employer shall have a physician or other licensed health care professional ascertain each affected employee's ability to use such respiratory protection. The physician or other licensed health care professional shall provide his or her findings to the affected employee and the employer in a written opinion.
(3) Respirator selection. The appropriate atmosphere-supplying respirators, as specified in Table 2, shall be selected from those approved by the National Institute for Occupational Safety and Health (NIOSH) under the provisions of 42 CFR Part 84, "Respiratory Protective Devices." When employers elect to provide gas masks with organic vapor canisters for use in emergency escape, the organic vapor canisters shall bear the approval of NIOSH.
| Methylene chloride airborne concentration (ppm) or condition of use | Minimum respirator required(1) |
|---|---|
| Up to 625 ppm (25 X PEL) | (1) Continuous flow supplied-air respirator, hood or helmet |
| Up to 1250 ppm (50 X 8-TWA PEL) | (1) Full facepiece supplied-air respirator operated in negative pressure (demand) mode. (2) Full facepiece self-contained breathing apparatus (SCBA) operated in negative pressure (demand) mode |
| Up to 5000 ppm (200 X 8-TWA PEL) | (1) Continuous flow supplied-air respirator, full facepiece. (2) Pressure demand supplied-air respirator, full facepiece. (3) Positive pressure full facepiece SCBA |
| Unknown concentration, or above 5000 ppm (Greater than 200 X 8- TWA PEL) | (1) Positive pressure full facepiece SCBA. (2) Full facepiece pressure demand supplied-air respirator with an auxiliary self-contained air supply |
| Fire fighting | Positive pressure full facepiece SCBA |
| Emergency escape | (1) Any continuous flow or pressure demand SCBA. (2) Gas mask with organic vapor canister |
| Footnote(1) Respirators assigned for higher airborne concentrations may be used at lower concentrations | |
(4) Respirator program. Where respiratory protection is required by this section, the employer shall institute a respirator program in accordance with 29 CFR 1910.134.
(5) Permission to leave area. The employer shall permit employees who wear respirators to leave the regulated area to readjust the facepieces to their faces to achieve a proper fit, and to wash their faces and respirator facepieces as necessary in order to prevent skin irritation associated with respirator use.
(6) Filter respirators. Employers who provide gas masks with organic vapor canisters for the purpose of emergency escape shall replace those canisters after any emergency use before those gas masks are returned to service.
(7) Respirator fit testing. (i) The employer shall ensure that each respirator issued to the employee is properly fitted and exhibits the least possible facepiece leakage from among the facepieces tested.
(ii) The employer shall perform qualitative or quantitative fit tests at the time of initial fitting and at least annually thereafter for each employee wearing a negative pressure respirator, including those employees for whom emergency escape respirators are provided.
[Note to paragraph (g)(7)(ii): The only supplied-air respirators to which this provision would apply are SCBA in negative pressure mode and full facepiece supplied-air respirators operated in negative pressure mode. The small business compliance guides will contain examples of protocols for qualitative and quantitative fit testing.] (h) Protective Work Clothing and Equipment. (1) Where needed to prevent MC-induced skin or eye irritation, the employer shall provide clean protective clothing and equipment which is resistant to MC, at no cost to the employee, and shall ensure that each affected employee uses it. Eye and face protection shall meet the requirements of 29 CFR 1910.133 or 29 CFR 1915.153, as applicable.
(2) The employer shall clean, launder, repair and replace all protective clothing and equipment required by this paragraph as needed to maintain their effectiveness.
(3) The employer shall be responsible for the safe disposal of such clothing and equipment. [Note to paragraph (h)(4): See Appendix A for examples of disposal procedures that will satisfy this requirement.] (i) Hygiene facilities. (1) If it is reasonably foreseeable that employees' skin may contact solutions containing 0.1 percent or greater MC (for example, through splashes, spills or improper work practices), the employer shall provide conveniently located washing facilities capable of removing the MC, and shall ensure that affected employees use these facilities as needed.
(2) If it is reasonably foreseeable that an employee's eyes may contact solutions containing 0.1 percent or greater MC (for example through splashes, spills or improper work practices), the employer shall provide appropriate eyewash facilities within the immediate work area for emergency use, and shall ensure that affected employees use those facilities when necessary.
(j) Medical surveillance. (1) Affected employees. The employer shall make medical surveillance available for employees who are or may be exposed to MC as follows:
(i) At or above the action level on 30 or more days per year, or above the 8-hour TWA PEL or the STEL on 10 or more days per year;
(ii) Above the 8-TWA PEL or STEL for any time period where an employee has been identified by a physician or other licensed health care professional as being at risk from cardiac disease or from some other serious MC-related health condition and such employee requests inclusion in the medical surveillance program;
(iii) During an emergency. (2) Costs. The employer shall provide all required medical surveillance at no cost to affected employees, without loss of pay and at a reasonable time and place.
(3) Medical personnel. The employer shall ensure that all medical surveillance procedures are performed by a physician or other licensed health care professional, as defined in paragraph (b) of this section.
(4) Frequency of medical surveillance. The employer shall make medical surveillance available to each affected employee as follows:
(i) Initial surveillance. The employer shall provide initial medical surveillance under the schedule provided by paragraph (n)(2)(iii) of this section, or before the time of initial assignment of the employee, whichever is later. The employer need not provide the initial surveillance if medical records show that an affected employee has been provided with medical surveillance that complies with this section within 12 months before April 10, 1997.
(ii) Periodic medical surveillance. The employer shall update the medical and work history for each affected employee annually. The employer shall provide periodic physical examinations, including appropriate laboratory surveillance, as follows:
(A) For employees 45 years of age or older, within 12 months of the initial surveillance or any subsequent medical surveillance; and
(B) For employees younger than 45 years of age, within 36 months of the initial surveillance or any subsequent medical surveillance.
(iii) Termination of employment or reassignment. When an employee leaves the employer's workplace, or is reassigned to an area where exposure to MC is consistently at or below the action level and STEL, medical surveillance shall be made available if six months or more have elapsed since the last medical surveillance.
(iv) Additional surveillance. The employer shall provide additional medical surveillance at frequencies other than those listed above when recommended in the written medical opinion. (For example, the physician or other licensed health care professional may determine an examination is warranted in less than 36 months for employees younger than 45 years of age based upon evaluation of the results of the annual medical and work history.) (5) Content of medical surveillance. (i) Medical and work history.
The comprehensive medical and work history shall emphasize neurological symptoms, skin conditions, history of hematologic or liver disease, signs or symptoms suggestive of heart disease (angina, coronary artery disease), risk factors for cardiac disease, MC exposures, and work practices and personal protective equipment used during such exposures. [Note to paragraph (j)(5)(i): See Appendix B of this section for an example of a medical and work history format that would satisfy this requirement.] (ii) Physical examination. Where physical examinations are provided as required above, the physician or other licensed health care professional shall accord particular attention to the lungs, cardiovascular system (including blood pressure and pulse), liver, nervous system, and skin. The physician or other licensed health care professional shall determine the extent and nature of the physical examination based on the health status of the employee and analysis of the medical and work history.
(iii) Laboratory surveillance. The physician or other licensed health care professional shall determine the extent of any required laboratory surveillance based on the employee's observed health status and the medical and work history. [Note to paragraph (j)(5)(iii): See Appendix B of this section for information regarding medical tests. Laboratory surveillance may include before-and after-shift carboxyhemoglobin determinations, resting ECG, hematocrit, liver function tests and cholesterol levels.] (iv) Other information or reports. The medical surveillance shall also include any other information or reports the physician or other licensed health care professional determines are necessary to assess the employee's health in relation to MC exposure.
(6) Content of emergency medical surveillance. The employer shall ensure that medical surveillance made available when an employee has been exposed to MC in emergency situations includes, at a minimum:
(i) Appropriate emergency treatment and decontamination of the exposed employee;
(ii) Comprehensive physical examination with special emphasis on the nervous system, cardiovascular system, lungs, liver and skin, including blood pressure and pulse;
(iii) Updated medical and work history, as appropriate for the medical condition of the employee; and
(iv) Laboratory surveillance, as indicated by the employee's health status. [Note to paragraph (j)(6)(iv): See Appendix B for examples of tests which may be appropriate.] (7) Additional examinations and referrals. Where the physician or other licensed health care professional determines it is necessary, the scope of the medical examination shall be expanded and the appropriate additional medical surveillance, such as referrals for consultation or examination, shall be provided.
(8) Information provided to the physician or other licensed health care professional. The employer shall provide the following information to a physician or other licensed health care professional who is involved in the diagnosis of MC-induced health effects:
(i) A copy of this section including its applicable appendices;
(ii) A description of the affected employee's past, current and anticipated future duties as they relate to the employee's MC exposure;
(iii) The employee's former or current exposure levels or, for employees not yet occupationally exposed to MC, the employee's anticipated exposure levels and the frequency and exposure levels anticipated to be associated with emergencies;
(iv) A description of any personal protective equipment, such as respirators, used or to be used; and
(v) Information from previous employment-related medical surveillance of the affected employee which is not otherwise available to the physician or other licensed health care professional.
(9) Written medical opinions. (i) For each physical examination required by this section, the employer shall ensure that the physician or other licensed health care professional provides to the employer and to the affected employee a written opinion regarding the results of that examination within 15 days of completion of the evaluation of medical and laboratory findings, but not more than 30 days after the examination. The written medical opinion shall be limited to the following information:
(A) The physician's or other licensed health care professional's opinion concerning whether the employee has any detected medical condition(s) which would place the employee's health at increased risk of material impairment from exposure to MC;
(B) Any recommended limitations upon the employee's exposure to MC or upon the employee's use of protective clothing or equipment and respirators;
(C) A statement that the employee has been informed by the physician or other licensed health care professional that MC is a potential occupational carcinogen, of risk factors for heart disease, and the potential for exacerbation of underlying heart disease by exposure to MC through its metabolism to carbon monoxide; and
(D) A statement that the employee has been informed by the physician or other licensed health care professional of the results of the medical examination and any medical conditions resulting from MC exposure which require further explanation or treatment.
(ii) The employer shall instruct the physician or other licensed health care professional not to reveal to the employer, orally or in the written opinion, any specific records, findings, and diagnoses that have no bearing on occupational exposure to MC. [Note to paragraph (j)(9)(ii): The written medical opinion may also include information and opinions generated to comply with other OSHA health standards.] (k) Hazard communication. The employer shall communicate the following hazards associated with MC on labels and in material safety data sheets in accordance with the requirements of the Hazard Communication Standard, 29 CFR 1910.1200, 29 CFR 1915.1200, or 29 CFR 1926.59, as appropiate: cancer, cardiac effects (including elevation of carboxyhemoglobin), central nervous system effects, liver effects, and skin and eye irritation.
(l) Employee information and training. (1) The employer shall provide information and training for each affected employee prior to or at the time of initial assignment to a job involving potential exposure to MC.
(2) The employer shall ensure that information and training is presented in a manner that is understandable to the employees.
(3) In addition to the information required under the Hazard Communication Standard at 29 CFR 1910.1200, 29 CFR 1915.1200, or 29 CFR 1926.59, as appropiate:
(i) The employer shall inform each affected employee of the requirements of this section and information available in its appendices, as well as how to access or obtain a copy of it in the workplace;
(ii) Wherever an employee's exposure to airborne concentrations of MC exceeds or can reasonably be expected to exceed the action level, the employer shall inform each affected employee of the quantity, location, manner of use, release, and storage of MC and the specific operations in the workplace that could result in exposure to MC, particularly noting where exposures may be above the 8-hour TWA PEL or STEL;
(4) The employer shall train each affected employee as required under the Hazard Communication standard at 29 CFR 1910.1200, 29 CFR 1915.1200, or 29 CFR 1926.59, as appropiate.
(5) The employer shall re-train each affected employee as necessary to ensure that each employee exposed above the action level or the STEL maintains the requisite understanding of the principles of safe use and handling of MC in the workplace.
(6) Whenever there are workplace changes, such as modifications of tasks or procedures or the institution of new tasks or procedures, which increase employee exposure, and where those exposures exceed or can reasonably be expected to exceed the action level, the employer shall update the training as necessary to ensure that each affected employee has the requisite proficiency.
(7) An employer whose employees are exposed to MC at a multi-
employer worksite shall notify the other employers with work operations at that site in accordance with the requirements of the Hazard Communication Standard, 29 CFR 1910.1200, 29 CFR 1915.1200, or 29 CFR 1926.59, as appropiate.
(8) The employer shall provide to the Assistant Secretary or the Director, upon request, all available materials relating to employee information and training.
(m) Recordkeeping. (1) Objective data. (i) Where an employer seeks to demonstrate that initial monitoring is unnecessary through reasonable reliance on objective data showing that any materials in the workplace containing MC will not release MC at levels which exceed the action level or the STEL under foreseeable conditions of exposure, the employer shall establish and maintain an accurate record of the objective data relied upon in support of the exemption.
(ii) This record shall include at least the following information:
(A) The MC-containing material in question;
(B) The source of the objective data;
(C) The testing protocol, results of testing, and/or analysis of the material for the release of MC;
(D) A description of the operation exempted under paragraph (d)(2)(i) of this section and how the data support the exemption; and
(E) Other data relevant to the operations, materials, processing, or employee exposures covered by the exemption.
(iii) The employer shall maintain this record for the duration of the employer's reliance upon such objective data.
(2) Exposure measurements. (i) The employer shall establish and keep an accurate record of all measurements taken to monitor employee exposure to MC as prescribed in paragraph (d) of this section.
(ii) Where the employer has 20 or more employees, this record shall include at least the following information:
(A) The date of measurement for each sample taken;
(B) The operation involving exposure to MC which is being monitored;
(C) Sampling and analytical methods used and evidence of their accuracy;
(D) Number, duration, and results of samples taken;
(E) Type of personal protective equipment, such as respiratory protective devices, worn, if any; and
(F) Name, social security number, job classification and exposure of all of the employees represented by monitoring, indicating which employees were actually monitored.
(iii) Where the employer has fewer than 20 employees, the record shall include at least the following information: (A) The date of measurement for each sample taken; (B) Number, duration, and results of samples taken; and
(C) Name, social security number, job classification and exposure of all of the employees represented by monitoring, indicating which employees were actually monitored.
(iv) The employer shall maintain this record for at least thirty (30) years, in accordance with 29 CFR 1910.1020.
(3) Medical surveillance. (i) The employer shall establish and maintain an accurate record for each employee subject to medical surveillance under paragraph (j) of this section.
(ii) The record shall include at least the following information:
(A) The name, social security number and description of the duties of the employee;
(B) Written medical opinions; and
(C) Any employee medical conditions related to exposure to MC. (iii) The employer shall ensure that this record is maintained for the duration of employment plus thirty (30) years, in accordance with 29 CFR 1910.1020.
(4) Availability. (i) The employer, upon written request, shall make all records required to be maintained by this section available to the Assistant Secretary and the Director for examination and copying in accordance with 29 CFR 1910.1020. [Note to paragraph (m)(4)(i): All records required to be maintained by this section may be kept in the most administratively convenient form (for example, electronic or computer records would satisfy this requirement).] (ii) The employer, upon request, shall make any employee exposure and objective data records required by this section available for examination and copying by affected employees, former employees, and designated representatives in accordance with 29 CFR 1910.1020.
(iii) The employer, upon request, shall make employee medical records required to be kept by this section available for examination and copying by the subject employee and by anyone having the specific written consent of the subject employee in accordance with 29 CFR 1910.1020.
(5) Transfer of records. The employer shall comply with the requirements concerning transfer of records set forth in 29 CFR 1910.1020(h).
(n) Dates. (1) Effective date. This section shall become effective April 10, 1997.
(2) Start-up dates. (i) Initial monitoring required by paragraph (d)(2) of this section shall be completed according to the following schedule:
(A) For employers with fewer than 20 employees, within 300 days after the effective date of this section.
(B) For polyurethane foam manufacturers with 20 to 99 employees, within 210 days after the effective date of this section.
(C) For all other employers, within 120 days after the effective date of this section.
(ii) Engineering controls required under paragraph (f)(1) of this section shall be implemented according to the following schedule:
(A) For employers with fewer than 20 employees, within three (3) years after the effective date of this section.
(B) For polyurethane foam manufacturers with 20 to 99 employees, within two (2) years after the effective date of this section.
(C) For all other employers, within one (1) year after the effective date of this section.
(iii) All other requirements of this section shall be complied with according to the following schedule:
(A) For employers with fewer than 20 employees, within one (1) year after the effective date of this section.
(B) For polyurethane foam manufacturers with 20 to 99 employees, within 270 days after the effective date of this section.
(C) For all other employers, within 180 days after the effective date of this section.
(3) Transitional dates. The exposure limits for MC specified in 29 CFR 1910.1000 (1996), Table Z-2, shall remain in effect until the start-up dates for the exposure limits specified in paragraph (n) of this section, or if the exposure limits in this section are stayed or vacated.
(o) Appendices. The information contained in the appendices does not, by itself, create any additional obligations not otherwise imposed or detract from any existing obligation.
Appendix A to Section 1910.1052: Substance Safety Data Sheet and Technical Guidelines for Methylene Chloride
I. Substance Identification
A. Substance: Methylene chloride (CH(2)Cl(2).
B. Synonyms: MC, Dichloromethane (DCM); Methylene dichloride;
Methylene bichloride; Methane dichloride; CAS: 75-09-2; NCI-C50102.
C. Physical data:
1. Molecular weight: 84.9. 2. Boiling point (760 mm Hg): 39.8 deg.C (104 deg. F). 3. Specific gravity (water=1): 1.3. 4. Vapor density (air=1 at boiling point): 2.9. 5. Vapor pressure at 20 deg. C (68 deg. F): 350 mm Hg. 6. Solubility in water, g/100 g water at 20 deg. C (68 deg. F)=1.32. 7. Appearance and odor: colorless liquid with a chloroform-like odor.
D. Uses:
MC is used as a solvent, especially where high volatility is required. It is a good solvent for oils, fats, waxes, resins, bitumen, rubber and cellulose acetate and is a useful paint stripper and degreaser. It is used in paint removers, in propellant mixtures for aerosol containers, as a solvent for plastics, as a degreasing agent, as an extracting agent in the pharmaceutical industry and as a blowing agent in polyurethane foams. Its solvent property is sometimes increased by mixing with methanol, petroleum naphtha or tetrachloroethylene.
E. Appearance and odor:
MC is a clear colorless liquid with a chloroform-like odor. It is slightly soluble in water and completely miscible with most organic solvents.
F. Permissible exposure:
Exposure may not exceed 25 parts MC per million parts of air (25 ppm) as an eight-hour time-weighted average (8-hour TWA PEL) or 125 parts of MC per million parts of air (125 ppm) averaged over a 15-minute period (STEL).
II. Health Hazard Data
A. MC can affect the body if it is inhaled or if the liquid comes in contact with the eyes or skin. It can also affect the body if it is swallowed.
B. Effects of overexposure:
1. Short-term Exposure:
MC is an anesthetic. Inhaling the vapor may cause mental confusion, light-headedness, nausea, vomiting, and headache. Continued exposure may cause increased light-headedness, staggering, unconsciousness, and even death. High vapor concentrations may also cause irritation of the eyes and respiratory tract. Exposure to MC may make the symptoms of angina (chest pains) worse. Skin exposure to liquid MC may cause irritation. If liquid MC remains on the skin, it may cause skin burns. Splashes of the liquid into the eyes may cause irritation.
2. Long-term (chronic) exposure:
The best evidence that MC causes cancer is from laboratory studies in which rats, mice and hamsters inhaled MC 6 hours per day, 5 days per week for 2 years. MC exposure produced lung and liver tumors in mice and mammary tumors in rats. No carcinogenic effects of MC were found in hamsters.
There are also some human epidemiological studies which show an association between occupational exposure to MC and increases in biliary (bile duct) cancer and a type of brain cancer. Other epidemiological studies have not observed a relationship between MC exposure and cancer. OSHA interprets these results to mean that there is suggestive (but not absolute) evidence that MC is a human carcinogen.
C. Reporting signs and symptoms:
You should inform your employer if you develop any signs or symptoms and suspect that they are caused by exposure to MC. D. Warning Properties:
1. Odor Threshold:
Different authors have reported varying odor thresholds for MC. Kirk-Othmer and Sax both reported 25 to 50 ppm; Summer and May both reported 150 ppm; Spector reports 320 ppm. Patty, however, states that since one can become adapted to the odor, MC should not be considered to have adequate warning properties.
2. Eye Irritation Level:
Kirk-Othmer reports that "MC vapor is seriously damaging to the eyes." Sax agrees with Kirk-Othmer's statement. The ACGIH Documentation of TLVs states that irritation of the eyes has been observed in workers exposed to concentrations up to 5000 ppm.
3. Evaluation of Warning Properties:
Since a wide range of MC odor thresholds are reported (25-320 ppm), and human adaptation to the odor occurs, MC is considered to be a material with poor warning properties.
III. Emergency First Aid Procedures
In the event of emergency, institute first aid procedures and send for first aid or medical assistance.
A. Eye and Skin Exposures:
If there is a potential for liquid MC to come in contact with eye or skin, face shields and skin protective equipment must be provided and used. If liquid MC comes in contact with the eye, get medical attention. Contact lenses should not be worn when working with this chemical.
B. Breathing:
If a person breathes in large amounts of MC, move the exposed person to fresh air at once. If breathing has stopped, perform cardiopulmorary resuscitation. Keep the affected person warm and at rest. Get medical attention as soon as possible.
C. Rescue:
Move the affected person from the hazardous exposure immediately. If the exposed person has been overcome, notify someone else and put into effect the established emergency rescue procedures. Understand the facility's emergency rescue procedures and know the locations of rescue equipment before the need arises. Do not become a casualty yourself.
IV. Respirators, Protective Clothing, and Eye Protection
A. Respirators:
Good industrial hygiene practices recommend that engineering controls be used to reduce environmental concentrations to the permissible exposure level. However, there are some exceptions where respirators may be used to control exposure. Respirators may be used when engineering and work practice controls are not feasible, when such controls are in the process of being installed, or when these controls fail and need to be supplemented. Respirators may also be used for operations which require entry into tanks or closed vessels, and in emergency situations.
If the use of respirators is necessary, the only respirators permitted are those that have been approved by the Mine Safety and Health Administration (MSHA) or the National Institute for Occupational Safety and Health (NIOSH). Supplied-air respirators are required because air-purifying respirators do not provide adequate respiratory protection against MC.
In addition to respirator selection, a complete written respiratory protection program should be instituted which includes regular training, maintenance, inspection, cleaning, and evaluation. If you can smell MC while wearing a respirator, proceed immediately to fresh air. If you experience difficulty in breathing while wearing a respirator, tell your employer.
B. Protective Clothing:
Employees must be provided with and required to use impervious clothing, gloves, face shields (eight-inch minimum), and other appropriate protective clothing necessary to prevent repeated or prolonged skin contact with liquid MC or contact with vessels containing liquid MC. Any clothing which becomes wet with liquid MC should be removed immediately and not reworn until the employer has ensured that the protective clothing is fit for reuse. Contaminated protective clothing should be placed in a regulated area designated by the employer for removal of MC before the clothing is laundered or disposed of. Clothing and equipment should remain in the regulated area until all of the MC contamination has evaporated; clothing and equipment should then be laundered or disposed of as appropriate.
C. Eye Protection:
Employees should be provided with and required to use splash-proof safety goggles where liquid MC may contact the eyes.
V. Housekeeping and Hygiene Facilities
For purposes of complying with 29 CFR 1910.141, the following items should be emphasized:
A. The workplace should be kept clean, orderly, and in a sanitary condition. The employer should institute a leak and spill detection program for operations involving liquid MC in order to detect sources of fugitive MC emissions.
B. Emergency drench showers and eyewash facilities are recommended. These should be maintained in a sanitary condition. Suitable cleansing agents should also be provided to assure the effective removal of MC from the skin.
C. Because of the hazardous nature of MC, contaminated protective clothing should be placed in a regulated area designated by the employer for removal of MC before the clothing is laundered or disposed of.
VI. Precautions for Safe Use, Handling, and Storage
A. Fire and Explosion Hazards:
MC has no flash point in a conventional closed tester, but it forms flammable vapor-air mixtures at approximately 100 deg.C (212 deg. F), or higher. It has a lower explosion limit of 12%, and an upper explosion limit of 19% in air. It has an autoignition temperature of 556.1 deg.C (1033 deg. F), and a boiling point of 39.8 deg.C (104 deg. F). It is heavier than water with a specific gravity of 1.3. It is slightly soluble in water.
B. Reactivity Hazards:
Conditions contributing to the instability of MC are heat and moisture. Contact with strong oxidizers, caustics, and chemically active metals such as aluminum or magnesium powder, sodium and potassium may cause fires and explosions.
Special precautions: Liquid MC will attack some forms of plastics, rubber, and coatings.
C. Toxicity:
Liquid MC is painful and irritating if splashed in the eyes or if confined on the skin by gloves, clothing, or shoes. Vapors in high concentrations may cause narcosis and death. Prolonged exposure to vapors may cause cancer or exacerbate cardiac disease.
D. Storage:
Protect against physical damage. Because of its corrosive properties, and its high vapor pressure, MC should be stored in plain, galvanized or lead lined, mild steel containers in a cool, dry, well ventilated area away from direct sunlight, heat source and acute fire hazards.
E. Piping Material:
All piping and valves at the loading or unloading station should be of material that is resistant to MC and should be carefully inspected prior to connection to the transport vehicle and periodically during the operation.
F. Usual Shipping Containers:
Glass bottles, 5- and 55-gallon steel drums, tank cars, and tank trucks.
Note: This section addresses MC exposure in marine terminal and longshore employment only where leaking or broken packages allow MC exposure that is not addressed through compliance with 29 CFR parts 1917 and 1918, respectively.
G. Electrical Equipment:
Electrical installations in Class I hazardous locations as defined in Article 500 of the National Electrical Code, should be installed according to Article 501 of the code; and electrical equipment should be suitable for use in atmospheres containing MC vapors. See Flammable and Combustible Liquids Code (NFPA No. 325M), Chemical Safety Data Sheet SD-86 (Manufacturing Chemists' Association, Inc.).
H. Fire Fighting:
When involved in fire, MC emits highly toxic and irritating fumes such as phosgene, hydrogen chloride and carbon monoxide. Wear breathing apparatus and use water spray to keep fire-exposed containers cool. Water spray may be used to flush spills away from exposures. Extinguishing media are dry chemical, carbon dioxide, foam. For purposes of compliance with 29 CFR 1910.307, locations classified as hazardous due to the presence of MC shall be Class I.
I. Spills and Leaks:
Persons not wearing protective equipment and clothing should be restricted from areas of spills or leaks until cleanup has been completed. If MC has spilled or leaked, the following steps should be taken:
1. Remove all ignition sources. 2. Ventilate area of spill or leak. 3. Collect for reclamation or absorb in vermiculite, dry sand, earth, or a similar material.
J. Methods of Waste Disposal:
Small spills should be absorbed onto sand and taken to a safe area for atmospheric evaporation. Incineration is the preferred method for disposal of large quantities by mixing with a combustible solvent and spraying into an incinerator equipped with acid scrubbers to remove hydrogen chloride gases formed. Complete combustion will convert carbon monoxide to carbon dioxide. Care should be taken for the presence of phosgene.
K. You should not keep food, beverage, or smoking materials, or eat or smoke in regulated areas where MC concentrations are above the permissible exposure limits.
L. Portable heating units should not be used in confined areas where MC is used.
M. Ask your supervisor where MC is used in your work area and for any additional plant safety and health rules.
VII. Medical Requirements
Your employer is required to offer you the opportunity to participate in a medical surveillance program if you are exposed to MC at concentrations at or above the action level (12.5 ppm 8-hour TWA) for more than 30 days a year or at concentrations exceeding the PELs (25 ppm 8-hour TWA or 125 ppm 15-minute STEL) for more than 10 days a year. If you are exposed to MC at concentrations over either of the PELs, your employer will also be required to have a physician or other licensed health care professional ensure that you are able to wear the respirator that you are assigned. Your employer must provide all medical examinations relating to your MC exposure at a reasonable time and place and at no cost to you.
VIII. Monitoring and Measurement Procedures
A. Exposure above the Permissible Exposure Limit:
1. Eight-hour exposure evaluation: Measurements taken for the purpose of determining employee exposure under this section are best taken with consecutive samples covering the full shift. Air samples must be taken in the employee's breathing zone.
2. Monitoring techniques: The sampling and analysis under this section may be performed by collection of the MC vapor on two charcoal adsorption tubes in series or other composition adsorption tubes, with subsequent chemical analysis. Sampling and analysis may also be performed by instruments such as real-time continuous monitoring systems, portable direct reading instruments, or passive dosimeters as long as measurements taken using these methods accurately evaluate the concentration of MC in employees" breathing zones.
OSHA method 80 is an example of a validated method of sampling and analysis of MC. Copies of this method are available from OSHA or can be downloaded from the Internet at http://www.osha.gov/. The employer has the obligation of selecting a monitoring method which meets the accuracy and precision requirements of the standard under his or her unique field conditions. The standard requires that the method of monitoring must be accurate, to a 95 percent confidence level, to plus or minus 25 percent for concentrations of MC at or above 25 ppm, and to plus or minus 35 percent for concentrations at or below 25 ppm. In addition to OSHA method 80, there are numerous other methods available for monitoring for MC in the workplace.
B. Since many of the duties relating to employee exposure are dependent on the results of measurement procedures, employers must assure that the evaluation of employee exposure is performed by a technically qualified person.
IX. Observation of Monitoring
Your employer is required to perform measurements that are representative of your exposure to MC and you or your designated representative are entitled to observe the monitoring procedure. You are entitled to observe the steps taken in the measurement procedure, and to record the results obtained. When the monitoring procedure is taking place in an area where respirators or personal protective clothing and equipment are required to be worn, you or your representative must also be provided with, and must wear, protective clothing and equipment.
X. Access To Information
A. Your employer is required to inform you of the information contained in this Appendix. In addition, your employer must instruct you in the proper work practices for using MC, emergency procedures, and the correct use of protective equipment.
B. Your employer is required to determine whether you are being exposed to MC. You or your representative has the right to observe employee measurements and to record the results obtained. Your employer is required to inform you of your exposure. If your employer determines that you are being over exposed, he or she is required to inform you of the actions which are being taken to reduce your exposure to within permissible exposure limits.
C. Your employer is required to keep records of your exposures and medical examinations. These records must be kept by the employer for at least thirty (30) years.
D. Your employer is required to release your exposure and medical records to you or your representative upon your request.
E. Your employee is required to provide labels and material safety data sheets (MSDS) for all materials, mixtures or solutions composed of greater than 0.1 percent MC. An example of a label that would satisfy these requirements would be:
Danger Contains Methylene Chloride Potential Cancer Hazard
May worsen heart disease because methylene chloride is converted to carbon monoxide in the body.
May cause dizziness, headache, irritation of the throat and lungs, loss of consciousness and death at high concentrations (for example, if used in a poorly ventilated room).
Avoid Skin Contact. Contact with liquid causes skin and eye irritation.
XI. Common Operations and Controls
The following list includes some common operations in which exposure to MC may occur and control methods which may be effective in each case:
| Operations | controls |
| Use as solvent in paint and varnish removers; manufacture of aerosols; cold cleaning and ultrasonic cleaning; and as a solvent in furniture stripping | General dilution ventilation; local exhaust ventilation; personal protective equipment; substitution |
| Use as solvent in vapor degreasing | Process enclosure; local exhaust ventilation; chilling coils; substitution |
| Use as a secondary refrigerant in air conditioning and scientific testing | General dilution ventilation; local exhaust ventilation; personal protective equipment |
Appendix B to Section 1910.1052: Medical Surveillance for Methylene Chloride
I. Primary Route of Entry
Inhalation.
II. Toxicology
Methylene Chloride (MC) is primarily an inhalation hazard. The principal acute hazardous effects are the depressant action on the central nervous system, possible cardiac toxicity and possible liver toxicity. The range of CNS effects are from decreased eye/hand coordination and decreased performance in vigilance tasks to narcosis and even death of individuals exposed at very high doses. Cardiac toxicity is due to the metabolism of MC to carbon monoxide, and the effects of carbon monoxide on heart tissue. Carbon monoxide displaces oxygen in the blood, decreases the oxygen available to heart tissue, increasing the risk of damage to the heart, which may result in heart attacks in susceptible individuals. Susceptible individuals include persons with heart disease and those with risk factors for heart disease.
Elevated liver enzymes and irritation to the respiratory passages and eyes have also been reported for both humans and experimental animals exposed to MC vapors.
MC is metabolized to carbon monoxide and carbon dioxide via two separate pathways. Through the first pathway, MC is metabolized to carbon monoxide as an end-product via the P-450 mixed function oxidase pathway located in the microsomal fraction of the cell. This biotransformation of MC to carbon monoxide occurs through the process of microsomal oxidative dechlorination which takes place primarily in the liver. The amount of conversion to carbon monoxide is significant as measured by the concentration of carboxyhemoglobin, up to 12% measured in the blood following occupational exposure of up to 610 ppm. Through the second pathway, MC is metabolized to carbon dioxide as an end product (with formaldehyde and formic acid as metabolic intermediates) via the glutathione dependent enzyme found in the cytosolic fraction of the liver cell. Metabolites along this pathway are believed to be associated with the carcinogenic activity of MC.
MC has been tested for carcinogenicity in several laboratory rodents. These rodent studies indicate that there is clear evidence that MC is carcinogenic to male and female mice and female rats. Based on epidemiologic studies, OSHA has concluded that there is suggestive evidence of increased cancer risk in MC-related worker populations. The epidemiological evidence is consistent with the finding of excess cancer in the experimental animal studies. NIOSH regards MC as a potential occupational carcinogen and the International Agency for Research Cancer (IARC) classifies MC as an animal carcinogen. OSHA considers MC as a suspected human carcinogen.
III. Medical Signs and Symptoms of Acute Exposure
Skin exposure to liquid MC may cause irritation or skin burns. Liquid MC can also be irritating to the eyes. MC is also absorbed through the skin and may contribute to the MC exposure by inhalation.
At high concentrations in air, MC may cause nausea, vomiting, light-headedness, numbness of the extremities, changes in blood enzyme levels, and breathing problems, leading to bronchitis and pulmonary edema, unconsciousness and even death.
At lower concentrations in air, MC may cause irritation to the skin, eye, and respiratory tract and occasionally headache and nausea. Perhaps the greatest problem from exposure to low concentrations of MC is the CNS effects on coordination and alertness that may cause unsafe operations of machinery and equipment, leading to self-injury or accidents.
Low levels and short duration exposures do not seem to produce permanent disability, but chronic exposures to MC have been demonstrated to produce liver toxicity in animals, and therefore, the evidence is suggestive for liver toxicity in humans after chronic exposure.
Chronic exposure to MC may also cause cancer.
IV. Surveillance and Preventive Considerations
As discussed above, MC is classified as a suspect or potential human carcinogen. It is a central nervous system (CNS) depressant and a skin, eye and respiratory tract irritant. At extremely high concentrations, MC has caused liver damage in animals.
MC principally affects the CNS, where it acts as a narcotic. The observation of the symptoms characteristic of CNS depression, along with a physical examination, provides the best detection of early neurological disorders. Since exposure to MC also increases the carboxyhemoglobin level in the blood, ambient carbon monoxide levels would have an additive effect on that carboxyhemoglobin level. Based on such information, a periodic post-shift carboxyhemoglobin test as an index of the presence of carbon monoxide in the blood is recommended, but not required, for medical surveillance.
Based on the animal evidence and three epidemiologic studies previously mentioned, OSHA concludes that MC is a suspect human carcinogen. The medical surveillance program is designed to observe exposed workers on a regular basis. While the medical surveillance program cannot detect MC-induced cancer at a preneoplastic stage, OSHA anticipates that, as in the past, early detection and treatments of cancers leading to enhanced survival rates will continue to evolve.
A. Medical and Occupational History:
The medical and occupational work history plays an important role in the initial evaluation of workers exposed to MC. It is therefore extremely important for the examining physician or other licensed health care professional to evaluate the MC-exposed worker carefully and completely and to focus the examination on MC's potentially associated health hazards. The medical evaluation must include an annual detailed work and medical history with special emphasis on cardiac history and neurological symptoms.
An important goal of the medical history is to elicit information from the worker regarding potential signs or symptoms associated with increased levels of carboxyhemoglobin due to the presence of carbon monoxide in the blood. Physicians or other licensed health care professionals should ensure that the smoking history of all MC exposed employees is known. Exposure to MC may cause a significant increase in carboxyhemoglobin level in all exposed persons. However, smokers as well as workers with anemia or heart disease and those concurrently exposed to carbon monoxide are at especially high risk of toxic effects because of an already reduced oxygen carrying capacity of the blood.
A comprehensive or interim medical and work history should also include occurrence of headache, dizziness, fatigue, chest pain, shortness of breath, pain in the limbs, and irritation of the skin and eyes.
In addition, it is important for the physician or other licensed health care professional to become familiar with the operating conditions in which exposure to MC is likely to occur. The physician or other licensed health care professional also must become familiar with the signs and symptoms that may indicate that a worker is receiving otherwise unrecognized and exceptionally high exposure levels of MC.
An example of a medical and work history that would satisfy the requirement for a comprehensive or interim work history is represented by the following:
The following is a list of recommended questions and issues for the self-administered questionnaire for methylene chloride exposure.
Questionnaire For Methylene Chloride Exposure
I. Demographic Information
1. Name 2. Social Security Number 3. Date 4. Date of Birth 5. Age 6. Present occupation 7. Sex 8. Race
II. Occupational History
1. Have you ever worked with methylene chloride, dichloromethane, methylene dichloride, or CH(INF>2Cl(INF>2 (all are different names for the same chemical)? Please list which on the occupational history form if you have not already.
2. If you have worked in any of the following industries and have not listed them on the occupational history form, please do so. Furniture stripping Polyurethane foam manufacturing Chemical manufacturing or formulation Pharmaceutical manufacturing Any industry in which you used solvents to clean and degrease equipment or parts Construction, especially painting and refinishing Aerosol manufacturing Any industry in which you used aerosol adhesives
3. If you have not listed hobbies or household projects on the occupational history form, especially furniture refinishing, spray painting, or paint stripping, please do so.
III. Medical History
A. General
1. Do you consider yourself to be in good health? If no, state reason(s).
2. Do you or have you ever had:
a. Persistent thirst b. Frequent urination (three times or more at night) c. Dermatitis or irritated skin d. Non-healing wounds 3. What prescription or non-prescription medications do you take, and for what reasons? 4. Are you allergic to any medications, and what type of reaction do you have?
B. Respiratory
1. Do you have or have you ever had any chest illnesses or diseases? Explain.
2. Do you have or have you ever had any of the following:
a. Asthma
b. Wheezing
c. Shortness of breath
3. Have you ever had an abnormal chest X-ray? If so, when, where, and what were the findings?
4. Have you ever had difficulty using a respirator or breathing apparatus? Explain.
5. Do any chest or lung diseases run in your family? Explain.
6. Have you ever smoked cigarettes, cigars, or a pipe? Age started:
7. Do you now smoke?
8. If you have stopped smoking completely, how old were you when you stopped?
9. On the average of the entire time you smoked, how many packs of cigarettes, cigars, or bowls of tobacco did you smoke per day?
C. Cardiovascular
1. Have you ever been diagnosed with any of the following: Which of the following apply to you now or did apply to you at some time in the past, even if the problem is controlled by medication? Please explain any yes answers (i.e., when problem was diagnosed, length of time on medication).
a. High cholesterol or triglyceride level
b. Hypertension (high blood pressure)
c. Diabetes
d. Family history of heart attack, stroke, or blocked arteries
2. Have you ever had chest pain? If so, answer the next five questions.
a. What was the quality of the pain (i.e., crushing, stabbing, squeezing)?
b. Did the pain go anywhere (i.e., into jaw, left arm)?
c. What brought the pain out?
d. How long did it last? e. What made the pain go away?
3. Have you ever had heart disease, a heart attack, stroke, aneurysm, or blocked arteries anywhere in you body? Explain (when, treatment).
4. Have you ever had bypass surgery for blocked arteries in your heart or anywhere else? Explain.
5. Have you ever had any other procedures done to open up a blocked artery (balloon angioplasty, carotid endarterectomy, clot-dissolving drug)? 6. Do you have or have you ever had (explain each):
a. Heart murmur
b. Irregular heartbeat
c. Shortness of breath while lying flat
d. Congestive heart failure
e. Ankle swelling
f. Recurrent pain anywhere below the waist while walking
7. Have you ever had an electrocardiogram (EKG)? When?
8. Have you ever had an abnormal EKG? If so, when, where, and what were the findings?
9. Do any heart diseases, high blood pressure, diabetes, high cholesterol, or high triglycerides run in your family? Explain.
D. Hepatobiliary and Pancreas
1. Do you now or have you ever drunk alcoholic beverages? Age started: -------- Age stopped: --------.
2. Average numbers per week:
a. Beers: --------, ounces in usual container:
b. Glasses of wine: --------, ounces per glass:
c. Drinks: --------, ounces in usual container:
3. Do you have or have you ever had (explain each):
a. Hepatitis (infectious, autoimmune, drug-induced, or chemical)
b. Jaundice
c. Elevated liver enzymes or elevated bilirubin d. Liver disease or cancer
E. Central Nervous System
1. Do you or have you ever had (explain each):
a. Headache
b. Dizziness
c. Fainting
d. Loss of consciousness
e. Garbled speech
f. Lack of balance
g. Mental/psychiatric illness
h. Forgetfulness
F. Hematologic
1. Do you have, or have you ever had (explain each):
a. Anemia
b. Sickle cell disease or trait
c. Glucose-6-phosphate dehydrogenase deficiency
d. Bleeding tendency disorder
2. If not already mentioned previously, have you ever had a reaction to sulfa drugs or to drugs used to prevent or treat malaria? What was the drug? Describe the reaction.
B. Physical Examination
The complete physical examination, when coupled with the medical and occupational history, assists the physician or other licensed health care professional in detecting pre-existing conditions that might place the employee at increased risk, and establishes a baseline for future health monitoring. These examinations should include:
1. Clinical impressions of the nervous system, cardiovascular function and pulmonary function, with additional tests conducted where indicated or determined by the examining physician or other licensed health care professional to be necessary.
2. An evaluation of the advisability of the worker using a respirator, because the use of certain respirators places an additional burden on the cardiopulmonary system. It is necessary for the attending physician or other licensed health care professional to evaluate the cardiopulmonary function of these workers, in order to inform the employer in a written medical opinion of the worker's ability or fitness to work in an area requiring the use of certain types of respiratory protective equipment. The presence of facial hair or scars that might interfere with the worker's ability to wear certain types of respirators should also be noted during the examination and in the written medical opinion.
Because of the importance of lung function to workers required to wear certain types of respirators to protect themselves from MC exposure, these workers must receive an assessment of pulmonary function before they begin to wear a negative pressure respirator and at least annually thereafter. The recommended pulmonary function tests include measurement of the employee's forced vital capacity (FVC), forced expiratory volume at one second (FEV(1)), as well as calculation of the ratios of FEV(1) to FVC, and the ratios of measured FVC and measured FEV(1) to expected respective values corrected for variation due to age, sex, race, and height. Pulmonary function evaluation must be conducted by a physician or other licensed health care professional experienced in pulmonary function tests.
The following is a summary of the elements of a physical exam which would fulfill the requirements under the MC standard:
Physical Exam
I. Skin and appendages
1. Irritated or broken skin 2. Jaundice 3. Clubbing cyanosis, edema 4. Capillary refill time 5. Pallor
II. Head
1. Facial deformities 2. Scars 3. Hair growth
III. Eyes
1. Scleral icterus 2. Corneal arcus 3. Pupillary size and response 4. Fundoscopic exam
IV. Chest
1. Standard exam
V. Heart
1. Standard exam 2. Jugular vein distension 3. Peripheral pulses
VI. Abdomen
1. Liver span
VII. Nervous System
1. Complete standard neurologic exam
VIII. Laboratory
1. Hemoglobin and hematocrit 2. Alanine aminotransferase (ALT, SGPT) 3. Post-shift carboxyhemoglobin
IX. Studies
1. Pulmonary function testing 2. Electrocardiogram
An evaluation of the oxygen carrying capacity of the blood of employees (for example by measured red blood cell volume) is considered useful, especially for workers acutely exposed to MC.
It is also recommended, but not required, that end of shift carboxyhemoglobin levels be determined periodically, and any level above 3% for non-smokers and above 10% for smokers should prompt an investigation of the worker and his workplace. This test is recommended because MC is metabolized to CO, which combines strongly with hemoglobin, resulting in a reduced capacity of the blood to transport oxygen in the body. This is of particular concern for cigarette smokers because they already have a diminished hemoglobin capacity due to the presence of CO in cigarette smoke.
C. Additional Examinations and Referrals
1. Examination by a Specialist
When a worker examination reveals unexplained symptoms or signs (i.e. in the physical examination or in the laboratory tests), follow-up medical examinations are necessary to assure that MC exposure is not adversely affecting the worker's health. When the examining physician or other licensed health care professional finds it necessary, additional tests should be included to determine the nature of the medical problem and the underlying cause. Where relevant, the worker should be sent to a specialist for further testing and treatment as deemed necessary.
The final rule requires additional investigations to be covered and it also permits physicians or other licensed health care professionals to add appropriate or necessary tests to improve the diagnosis of disease should such tests become available in the future.
2. Emergencies
The examination of workers exposed to MC in an emergency should be directed at the organ systems most likely to be affected. If the worker has received a severe acute exposure, hospitalization may be required to assure proper medical intervention. It is not possible to precisely define "severe," but the physician or other licensed health care professional's judgement should not merely rest on hospitalization. If the worker has suffered significant conjunctival, oral, or nasal irritation, respiratory distress, or discomfort, the physician or other licensed health care professional should instigate appropriate follow-up procedures. These include attention to the eyes, lungs and the neurological system. The frequency of follow-up examinations should be determined by the attending physician or other licensed health care professional. This testing permits the early identification essential to proper medical management of such workers.
D. Employer Obligations
The employer is required to provide the responsible physician or other licensed health care professional and any specialists involved in a diagnosis with the following information: a copy of the MC standard including relevant appendices, a description of the affected employee's duties as they relate to his or her exposure to MC; an estimate of the employee's exposure including duration (e.g., 15hr/wk, three 8-hour shifts/wk, full time); a description of any personal protective equipment used by the employee, including respirators; and the results of any previous medical determinations for the affected employee related to MC exposure to the extent that this information is within the employer's control.
E. Physicians' or Other Licensed Health Care Professionals' Obligations
The standard requires the employer to ensure that the physician or other licensed health care professional provides a written statement to the employee and the employer. This statement should contain the physician's or licensed health care professional's opinion as to whether the employee has any medical condition placing him or her at increased risk of impaired health from exposure to MC or use of respirators, as appropriate. The physician or other licensed health care professional should also state his or her opinion regarding any restrictions that should be placed on the employee's exposure to MC or upon the use of protective clothing or equipment such as respirators. If the employee wears a respirator as a result of his or her exposure to MC, the physician or other licensed health care professional's opinion should also contain a statement regarding the suitability of the employee to wear the type of respirator assigned. Furthermore, the employee should be informed by the physician or other licensed health care professional about the cancer risk of MC and about risk factors for heart disease, and the potential for exacerbation of underlying heart disease by exposure to MC through its metabolism to carbon monoxide. Finally, the physician or other licensed health care professional should inform the employer that the employee has been told the results of the medical examination and of any medical conditions which require further explanation or treatment. This written opinion must not contain any information on specific findings or diagnosis unrelated to employee's occupational exposures.
The purpose in requiring the examining physician or other licensed health care professional to supply the employer with a written opinion is to provide the employer with a medical basis to assist the employer in placing employees initially, in assuring that their health is not being impaired by exposure to MC, and to assess the employee's ability to use any required protective equipment.


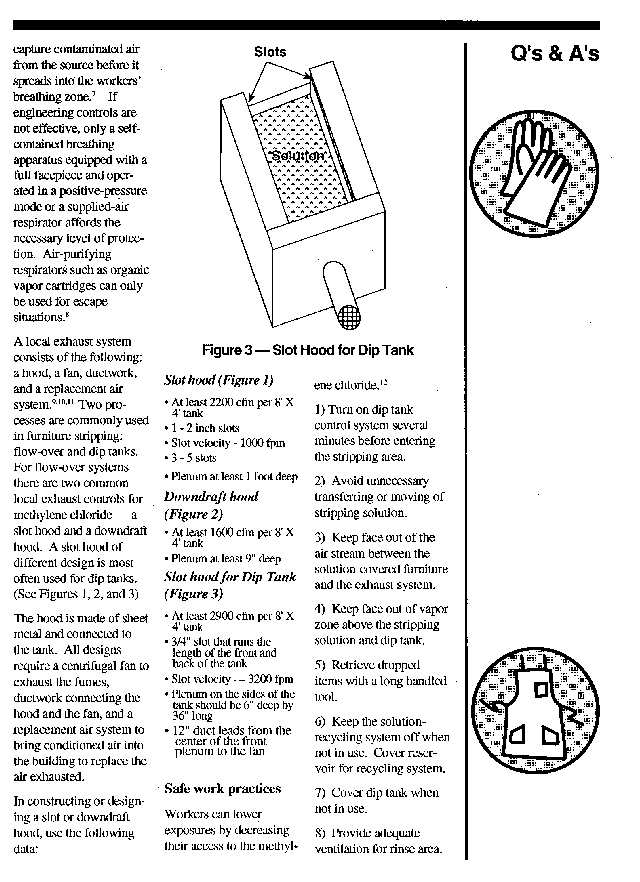


PART 1915 -- [AMENDED]
6. The authority citation for 29 CFR part 1915 continues to read as follows:
Authority: § 41, Longshore and Harbor Workers Compensation Act (33 U.S.C. 941); secs. 4, 6, 8, Occupational Safety and Health Act of 1970 (29 U.S.C. 653, 655, 657); Secretary of Labor's Order No. 12-71 (36 FR 8754), 8-76 (41 FR 25059), 9-83 (48 FR 35736) or 1-90 (55 FR 9033), as applicable; 29 CFR part 1911.
7. In Table Z of section 1915.1000, Air Contaminants, the entire entry for methylene chloride is removed and replaced with the following entry added in the substance column: "Methylene chloride: see 1910.1052".
8. Subpart Z of part 1915 is amended by adding 1915.1052, as follows:
1915.1052 Methylene chloride.
Note: The requirements applicable to shipyard employment under this section are identical to those set forth at 29 CFR 1910.1052.
PART 1926 -- [AMENDED]
Subpart D -- [Amended]
9. The authority citation for subpart D of part 1926 continues to read as follows:
Authority: § 107, Contract Work Hours and Safety Standards Act (40 U.S.C. 333), secs. 4, 6, and 8, Occupational Safety and Health Act of 1970 (29 U.S.C. 653, 655, 657); Secretary of Labor's Orders No. 12-71 (36 FR 8754), 8-76 (41 FR 25059), 9-83 (48 FR 35736), or 1-90 (55 FR 9033), as applicable.
10. In Appendix A of section 1926.55, Gases, vapors, fumes, dusts and mists, the entire entry for methylene chloride is removed and replaced by the following entry added in the substance column: "Methylene chloride: see 1910.1052".
Subpart Z -- [Amended]
11. The authority citation for subpart Z of part 1926 continues to read as follows:
Authority: Secs. 6 and 8, Occupational Safety and Health Act (29 U.S.C. 655, 657); section 41, Secretary of Labor's Orders Nos. 12-71 (36 FR 8754), 8-76 (41 FR 25059), 9-83 (48 FR 35736), or 1-90 (55 FR 9033), as applicable; and 29 CFR part 1911.
12. Subpart Z of part 1926 is amended by adding 1926.1152, as follows:
1926.1152 Methylene chloride.
Note: The requirements applicable to construction employment under this section are identical to those set forth at 29 CFR 1910.1052.
[FR Doc. 97-198 Filed 1-9-97; 8:45 am]
Footnote(1) Since the single observation of [V](GST-lung) reported by Reitz et al. (1988) was from a pooled sample of lung tissue from two human subjects, the data point was treated as two observations with the same value. (Back to Text)
Footnote(2) OSHA also conducted an alternative PBPK analysis that uses all of the available human data on MC metabolism, despite the very limited quantity of data available and the additional bias introduced by adopting the "parallelogram" assumptions for interspecies scaling (see Quantitative Risk Assessment for a discussion of this analysis and the uncertainties and biases therein). The risk estimate using this alternative method, 1.2 per 1000, is also unambiguously significant. (Back to Text)
Footnote(3) As a result of data and information received from commenters and other information in the record, the Final Economic Analysis does not identify significant impacts or technologic or economic feasibility problems for aircraft stripping operations of any size. (Back to Text)

