[Federal Register Volume 77, Number 58 (Monday, March 26, 2012)][Rules and Regulations]
[Pages 17574-17896]
From the Federal Register Online via the Government Printing Office [www.gpo.gov]
[FR Doc No: 2012-4826]
Vol. 77
Monday,
No. 58
March 26, 2012
Part II
Department of Labor
-----------------------------------------------------------------------
Occupational Safety and Health Administration
-----------------------------------------------------------------------
29 CFR 1910, 1915 and 1926
Hazard Communication; Final Rule
Federal Register / Vol. 77 , No. 58 / Monday, March 26, 2012 / Rules
and Regulations
-----------------------------------------------------------------------
DEPARTMENT OF LABOR
Occupational Safety and Health Administration
29 CFR Parts 1910, 1915, and 1926
[Docket No. OSHA-H022K-2006-0062 (formerly Docket No. H022K)]
RIN 1218-AC20
Hazard Communication
AGENCY: Occupational Safety and Health Administration (OSHA), DOL.
ACTION: Final rule.
-----------------------------------------------------------------------
SUMMARY: In this final rule, OSHA is modifying its Hazard Communication
Standard (HCS) to conform to the United Nations' Globally Harmonized
System of Classification and Labelling of Chemicals (GHS). OSHA has
determined that the modifications will significantly reduce costs and
burdens while also improving the quality and consistency of information
provided to employers and employees regarding chemical hazards and
associated protective measures. Consistent with the requirements of
Executive Order 13563, which calls for assessment and, where
appropriate, modification and improvement of existing rules, the Agency
has concluded this improved information will enhance the effectiveness
of the HCS in ensuring that employees are apprised of the chemical
hazards to which they may be exposed, and in reducing the incidence of
chemical-related occupational illnesses and injuries.
The modifications to the standard include revised criteria for
classification of chemical hazards; revised labeling provisions that
include requirements for use of standardized signal words, pictograms,
hazard statements, and precautionary statements; a specified format for
safety data sheets; and related revisions to definitions of terms used
in the standard, and requirements for employee training on labels and
safety data sheets. OSHA is also modifying provisions of other
standards, including standards for flammable and combustible liquids,
process safety management, and most substance-specific health
standards, to ensure consistency with the modified HCS requirements.
The consequences of these modifications will be to improve safety, to
facilitate global harmonization of standards, and to produce hundreds
of millions of dollars in annual savings.
DATES: This final rule becomes effective on May 25, 2012 Affected
parties do not need to comply with the information collection
requirements in the final rule until the Department of Labor publishes
in the Federal Register the control numbers assigned by the Office of
Management and Budget (OMB). Publication of the control numbers
notifies the public that OMB has approved these information collection
requirements under the Paperwork Reduction Act of 1995.
The incorporation by reference of the specific publications listed
in this final rule is approved by the Director of the Federal Register
as of May 25, 2012.
ADDRESSES: In compliance with 28 U.S.C. 2112(a), the Agency designates
Joseph M. Woodward, Associate Solicitor for Occupational Safety and
Health, Office of the Solicitor, Room S-4004, U.S. Department of Labor;
200 Constitution Avenue NW., Washington, DC 20210, as the recipient of
petitions for review of this final standard.
FOR FURTHER INFORMATION CONTACT: For general information and press
inquiries, contact: Frank Meilinger, OSHA Office of Communications,
Room N-3647, U.S. Department of Labor, 200 Constitution Avenue NW.,
Washington, DC 20210, telephone (202) 693-1999. For technical
information, contact: Dorothy Dougherty, Director, Directorate of
Standards and Guidance, Room N-3718, OSHA, U.S. Department of Labor,
200 Constitution Avenue NW., Washington, DC 20210; telephone (202) 693-
1950.
SUPPLEMENTARY INFORMATION: This final rule modifies the Hazard
Communication standard (HCS) and aligns it with the Globally Harmonized
System of Classification and Labelling of Chemicals (GHS) as
established by the United Nations (UN). This action is consistent with
Executive Order 13563 and, in particular, with its requirement of
"retrospective analysis of rules that may be outmoded, ineffective,
insufficient, or excessively burdensome." The preamble to the final
rule provides a synopsis of the events leading up to the establishment
of the final rule, a detailed description of OSHA's rationale for the
necessity of the modification, and final economic and voluntary
flexibility analyses that support the Agency's determinations. Also
included are explanations of the specific provisions that are modified
in the HCS and other affected OSHA standards and OSHA's responses to
comments, testimony, and data submitted during the rulemaking. The
discussion follows this outline:
I. Introduction
II. Events Leading to the Revised Hazard Communication Standard
III. Overview of the Final Rule and Alternatives Considered
IV. Need and Support for the Revised Hazard Communication Standard
V. Pertinent Legal Authority
VI. Final Economic Analysis and Voluntary Regulatory Flexibility
Analysis
VII. OMB Review Under the Paperwork Reduction Act of 1995
VIII. Federalism and Consultation and Coordination With Indian
Tribal Governments
IX. State Plans
X. Unfunded Mandates
XI. Protecting Children From Environmental Health and Safety Risks
XII. Environmental Impacts
XIII. Summary and Explanation of the Modifications to the Hazard
Communication Standard
(a) Purpose
(b) Scope
(c) Definitions
(d) Hazard Classification
(e) Written Hazard Communication Program
(f) Labels and Other Forms of Warning
(g) Safety Data Sheets
(h) Employee Information and Training
(i) Trade Secrets
(j) Effective Dates
(k) Other Standards Affected
(l) Appendices
XIV. Authority and Signature
The HCS requires that chemical manufacturers and importers evaluate
the chemicals they produce or import and provide hazard information to
downstream employers and employees by putting labels on containers and
preparing safety data sheets. This final rule modifies the current HCS
to align with the provisions of the UN's GHS. The modifications to the
HCS will significantly reduce burdens and costs, and also improve the
quality and consistency of information provided to employers and
employees regarding chemical hazards by providing harmonized criteria
for classifying and labeling hazardous chemicals and for preparing
safety data sheets for these chemicals.
OSHA is required by the Occupational Safety and Health (OSH) Act of
1970 to assure, as far as possible, safe and healthful working
conditions for all working men and women. Section 3(8) of the OSH Act
(29 U.S.C. 652(8)) empowers the Secretary of Labor to promulgate
standards that are "reasonably necessary or appropriate to provide
safe or healthful employment and places of employment." This language
has been interpreted by the Supreme Court to require that an OSHA
standard address a significant risk and reduce this risk significantly.
See Industrial Union Dep't v. American Petroleum Institute, 448 U.S.
607 (1980). As discussed in Sections IV and V of this preamble, OSHA
finds that inadequate communication to employees regarding the hazards
of chemicals constitutes a significant
risk of harm and estimates that the final rule will reduce this risk
significantly.
Section 6(b)(7) of the Act (29 U.S.C. 655(b)(7)) allows OSHA to
make appropriate modifications to its hazard communication requirements
as new knowledge and techniques are developed. The GHS system is a new
approach that has been developed through international negotiations and
embodies the knowledge gained in the field of chemical hazard
communication since the current rule was first adopted in 1983. As
indicated in Section IV of this preamble, OSHA finds that modifying the
HCS to align with the GHS will enhance worker protections
significantly. As noted in Section VI of this preamble, these
modifications to HCS will also result in less expensive chemical hazard
management and communication. In this way, the modifications are in
line with the requirements of Executive Order 13563 and its call for
streamlining of regulatory burdens.
OSHA is also required to determine if its standards are
technologically and economically feasible. As discussed in Section VI
of this preamble, OSHA has determined that this final standard is
technologically and economically feasible.
The Regulatory Flexibility Act, as amended by the Small Business
Regulatory Enforcement Fairness Act (SBREFA), requires OSHA to
determine if a regulation will have a significant impact on a
substantial number of small entities. As discussed in Section VI, OSHA
has determined and certified that this rule will not have a significant
impact on a substantial number of small entities.
Executive Orders 13563 and 12866 require OSHA to assess the
benefits and costs of final rules and of available regulatory
alternatives. Executive Order 13563 emphasizes the importance of
quantifying both costs and benefits, reducing costs, harmonizing rules,
and promoting flexibility. This rule has been designated an
economically significant regulatory action under section 3(f)(1) of
Executive Order 12866. Accordingly, the rule has been reviewed by the
Office of Management and Budget, and the remainder of this section
summarizes the key findings of the analysis with respect to the costs
and benefits of the final rule.
Because this final rule modifies the current HCS to align with the
provisions of the UN's GHS, the available alternatives to the final
rule are somewhat limited. The Agency has qualitatively discussed the
two major alternatives to the proposed rule--(1) voluntary adoption of
GHS within the existing HCS framework and (2) a limited adoption of
specific GHS components--in Section III of this preamble, but
quantitative estimates of the costs and benefits of these alternatives
could not reasonably be developed. However, OSHA has determined that
both of these alternatives would eliminate significant portions of the
benefits of the rule, which can only be achieved if the system used in
the U.S. is consistently and uniformly applied throughout the nation
and in conformance with the internationally harmonized system.
Table SI-1, derived from material presented in Section VI of this
preamble, provides a summary of the costs and benefits of the final
rule. As shown, the final rule is estimated to prevent 43 fatalities
and 521 injuries and illnesses annually. Also as shown, OSHA estimates
that the monetized health and safety benefits of the final rule are
$250 million annually and that the annualized cost reductions and
productivity gains are $507 million annually. In addition, OSHA
anticipates that the final rule will generate substantial (but
unquantified) savings from simplified hazard communication training and
from expanded opportunities for international trade due to a reduction
in trade barriers.
The estimated cost of the rule is $201 million annually. As shown
in Table SI-1, the major cost elements associated with the final rule
include the classification of chemical hazards in accordance with the
GHS criteria and the corresponding revision of safety data sheets and
labels to meet new format and content requirements ($22.5 million);
training for employees to become familiar with new warning symbols and
the revised safety data sheet format ($95.4 million); management
familiarization and other management-related costs as may be necessary
($59.0 million); and costs to purchase upgraded label printing
equipment and supplies or to purchase pre-printed color labels in order
to include the hazard warning pictogram enclosed in a red-bordered
diamond on the product label ($24.1 million).
The final rule is estimated to generate net monetized benefits of
$556 million annually, using a discount rate of 7 percent to annualize
costs and benefits. Using a 3 percent discount rate instead would have
the effect of lowering the costs to $161 million per year and
increasing the gross benefits to $839 million per year. The result
would be to increase net benefits from $556 million to $678 million per
year.
These estimates are for informational purposes only and have not
been used by OSHA as the basis for its decision concerning the
requirements for this final rule.
BILLING CODE 4510-26-P
[GRAPHIC] [TIFF OMITTED] TR26MR12.000
BILLING CODE 4510-26-C
I. Introduction
In the preamble, OSHA refers to supporting materials. References to
these materials are given as "Document ID " followed by the
last four digits of the document number. The referenced materials are
posted in Docket No. OSHA-H022K-2006-0062, which is available at http://www.regulations.osha.gov;
however, some information (e.g., copyrighted
material) is not publicly available to read or download through that
Web site. All of the documents are available for inspection and, where
permissible, copying at the OSHA Docket Office, U.S. Department of
Labor, Room N-2625, 200 Constitution Avenue NW., Washington, DC 20210.
II. Events Leading to the Revised Hazard Communication Standard
The HCS was first promulgated in 1983 and covered the manufacturing
sector of industry (48 FR 53280, Nov. 25, 1983). (Please note: The
Agency's HCS (29 CFR 1910.1200; 1915.1200; 1917.28; 1918.90; and
1926.59) will be referred to as the "current HCS" throughout this
rule.) In 1987, the Agency expanded the scope of coverage to all
industries where employees are potentially exposed to hazardous
chemicals (52 FR 31852, Aug. 24, 1987). Although full implementation in
the non-manufacturing sector was delayed by various court and
administrative actions, the rule has been fully enforced in all
industries regulated by OSHA since March 17, 1989 (54 FR 6886, Feb. 15,
1989) (29 CFR 1910.1200; 1915.1200; 1917.28; 1918.90; and 1926.59). In
1994, OSHA made minor changes and technical amendments to the HCS to
help ensure full compliance and achieve better protection of employees
(59 FR 6126, Feb. 9, 1994). The development of the HCS is discussed in
detail in the preambles to the original and revised final rules (See 48
FR at 53280-53281; 52 FR at 31852-31854; and 59 FR at 6127-6131). This
discussion will focus on the sequence of events leading to the
development of the GHS and the associated modifications to the HCS
included in the final rule.
The current HCS requires chemical manufacturers and importers to
evaluate the chemicals they produce or import to determine if they are
hazardous. The standard provides definitions of health and physical
hazards to use as the criteria for determining hazards in the
evaluation process. Information about hazards and protective measures
is then required to be conveyed to downstream employers and employees
through labels on containers and through material safety data sheets,
which are now called "safety data sheets" (SDS) under the final rule
and in this preamble. All employers with hazardous chemicals in their
workplaces are required to have a hazard communication program,
including container labels, safety data sheets, and employee training.
Generally, under the final rule, these obligations on manufacturers,
importers, and employers remain, but how hazard communication is to be
accomplished has been modified.
To protect employees and members of the public who are potentially
exposed to hazardous chemicals during their production, transportation,
use, and disposal, a number of countries have developed laws that
require information about those chemicals to be prepared and
transmitted to affected parties. The laws vary on the scope of
chemicals covered, definitions of hazards, the specificity of
requirements (e.g., specification of a format for safety data sheets),
and the use of symbols and pictograms. The inconsistencies among the
laws are substantial enough that different labels and safety data
sheets must often be developed for the same product when it is marketed
in different nations.
Within the U.S., several regulatory authorities exercise
jurisdiction over chemical hazard communication. In addition to OSHA,
the Department of Transportation (DOT) regulates chemicals in
transport; the Consumer Product Safety Commission (CPSC) regulates
consumer products; and the Environmental Protection Agency (EPA)
regulates pesticides, as well as exercising other authority over the
labeling of chemicals under the Toxic Substances Control Act. Each of
these regulatory authorities operates under different statutory
mandates, and all have adopted distinct hazard communication
requirements.
Tracking and complying with the hazard communication requirements
of different regulatory authorities is a burden for manufacturers,
importers, distributors, and transporters engaged in commerce in the
domestic arena. This burden is magnified by the need to develop
multiple sets of labels and safety data sheets for each product in
international trade. Small businesses have particular difficulty in
coping with the complexities and costs involved. The problems
associated with differing national and international requirements were
recognized and discussed when the HCS was first promulgated in 1983. At
that time, OSHA committed to periodically reviewing the standard in
recognition of an interagency trade policy that supported the U.S.
pursuing international harmonization of requirements for chemical
classification and labeling. The potential benefits of harmonization
were noted in the preamble of the 1983 standard:
* * * [O]SHA acknowledges the long-term benefit of maximum
recognition of hazard warnings, especially in the case of containers
leaving the workplace which go into interstate and international
commerce. The development of internationally agreed standards would
make possible the broadest recognition of the identified hazards
while avoiding the creation of technical barriers to trade and
reducing the costs of dissemination of hazard information by
elimination of duplicative requirements which could otherwise apply
to a chemical in commerce. As noted previously, these regulations
will be reviewed on a regular basis with regard to similar
requirements which may be evolving in the United States and in
foreign countries. (48 FR at 53287)
OSHA has actively participated in many such efforts in the years
since that commitment was made, including trade-related discussions on
the need for harmonization with major U.S. trading partners. The Agency
issued a Request for Information (RFI) in the Federal Register in
January 1990, to obtain input regarding international harmonization
efforts, and on work being done at that time by the International
Labour Organization (ILO) to develop a convention and recommendations
on safety in the use of chemicals at work (55 FR 2166, Jan. 22, 1990).
On a closely related matter, OSHA published a second RFI in May 1990,
requesting comments and information on improving the effectiveness of
information transmitted under the HCS (55 FR 20580, May 17, 1990).
Possible development of a standardized format or order of information
was raised as an issue in the RFI. Nearly 600 comments were received in
response to this request. The majority of responses expressed support
for a standard safety data sheet format, and the majority of responses
that expressed an opinion on the topic favored a standardized format
for labels as well.
In June 1992, the United Nations Conference on Environment and
Development issued a mandate (Chapter 19 of Agenda 21), supported by
the U.S., calling for development of a globally harmonized chemical
classification and labeling system:
A globally harmonized hazard classification and compatible
labeling system, including material safety data sheets and easily
understandable symbols, should be available, if feasible, by the
year 2000.
This international mandate initiated a substantial effort to develop
the GHS, involving numerous international organizations, many countries, and
extensive stakeholder representation.
A coordinating group comprised of countries, stakeholder
representatives, and international organizations was established to
manage the work. This group, the Inter-Organization Programme for the
Sound Management of Chemicals Coordinating Group for the Harmonization
of Chemical Classification Systems, established overall policy for the
work and assigned tasks to other organizations. The Coordinating Group
then took the work of these organizations and integrated it to form the
GHS. OSHA served as chair of the Coordinating Group.
The work was divided into three main parts: classification criteria
for physical hazards; classification criteria for health and
environmental hazards (including criteria for mixtures); and hazard
communication elements, including requirements for labels and safety
data sheets. The criteria for physical hazards were developed by a
United Nations Sub-committee of Experts on the Transport of Dangerous
Goods/International Labour Organization working group and were based on
the already harmonized criteria for the transport sector. The criteria
for classification of health and environmental hazards were developed
under the auspices of the Organization for Economic Cooperation and
Development. The ILO developed the hazard communication elements. OSHA
participated in all of this work, and served as U.S. lead on
classification of mixtures and hazard communication.
Four major existing systems served as the primary basis for
development of the GHS. These systems were the requirements in the U.S.
for the workplace, consumers, and pesticides; the requirements of
Canada for the workplace, consumers, and pesticides; European Union
directives for classification and labeling of substances and
preparations; and the United Nations Recommendations on the Transport
of Dangerous Goods. The requirements of other systems were also
examined as appropriate, and taken into account as the GHS was
developed. The primary approach to reconciling these systems involved
identifying the relevant provisions in each system; developing
background documents that compared, contrasted, and explained the
rationale for the provisions; and undertaking negotiations to find an
agreed approach that addressed the needs of the countries and
stakeholders involved. Principles to guide the work were established,
including an agreement that protections of the existing systems would
not be reduced as a result of harmonization. Thus, countries could be
assured that the existing protections of their systems would be
maintained or enhanced in the GHS.
An interagency committee under the auspices of the Department of
State coordinated U.S. involvement in the development of the GHS. In
addition to OSHA, DOT, CPSC, and EPA, other agencies were involved that
had interests related to trade or other aspects of the GHS process.
Different agencies took the lead in various parts of the discussions.
Positions for the U.S. in these negotiations were coordinated through
the interagency committee. Interested stakeholders were kept informed
through email dissemination of information, as well as periodic public
meetings. In addition, the Department of State published a notice in
the Federal Register that described the harmonization activities, the
agencies involved, the principles of harmonization, and other
information, as well as invited public comment on these issues (62 FR
15951, Apr. 3, 1997). Stakeholders also actively participated in the
discussions at the international level and were able to present their
views directly in the negotiating process. The GHS was formally adopted
by the new United Nations Committee of Experts on the Transport of
Dangerous Goods and the Globally Harmonized System of Classification
and Labelling of Chemicals in December 2002. In 2003, the adoption was
endorsed by the Economic and Social Council of the United Nations.
Countries were encouraged to implement the GHS as soon as possible, and
have fully operational systems by 2008. This goal was adopted by
countries in the Intergovernmental Forum on Chemical Safety, and was
endorsed by the World Summit on Sustainable Development. The U.S.
participated in these groups, and agreed to work toward achieving these
goals.
OSHA published an Advance Notice of Proposed Rulemaking (ANPR) on
the GHS in September of 2006 (71 FR 53617, Sept. 12, 2006). At the same
time the ANPR was published, OSHA made available on its Web site a
document summarizing the GHS (http://www.osha.gov). The ANPR provided
information about the GHS and its potential impact on the HCS, and
sought input from the public on issues related to GHS implementation.
Over 100 responses were received, and the comments and information
provided were taken into account in the development of the
modifications to the HCS included in the September 2009 Notice of
Proposed Rulemaking (NPRM) (74 FR 50279-50549, Sept. 30, 2009). A
notice of correction was published on November 5, 2009, in order to
correct misprints in the proposal (74 FR 57278, Nov. 5, 2009). Over 100
comments were received in response to the NPRM. Commenters represented
the broad spectrum of affected parties and included government
agencies, industries, professional and trade associations, academics,
employee organizations and individuals. Public hearings were held in
Washington, DC, from March 2 through March 5, 2010, and in Pittsburgh,
PA, on March 31, 2010. Over 40 panels participated in the hearings. The
comments, testimony, and other data received regarding this rulemaking
were overwhelmingly favorable, and will be discussed in detail later in
this preamble. The final post-hearing comment period for further
submissions and briefs ended and the record was certified by
Administrative Law Judge Stephen L. Purcell and closed on May 31, 2010.
Executive Order 13563, emphasizing the importance of retrospective
analysis of rules, was issued on January 18, 2011.
This final rule is based on Revision 3 of the GHS. The adoption of
the GHS will improve OSHA's current HCS standard by providing
consistent, standardized hazard communication to downstream users.
However, even after the U.S. and other countries implement the GHS, it
will continue to be updated in the future. These updates to the GHS
will be completed as necessary to reflect new technological and
scientific developments as well as provide additional explanatory text.
Any future changes to the HCS to adopt subsequent changes to the GHS
would require OSHA's rulemaking procedures.
OSHA will remain engaged in activities related to the GHS. The U.S.
is a member of the United Nations Committee of Experts on the Transport
of Dangerous Goods and the Globally Harmonized System of Classification
and Labelling of Chemicals, as well as the Sub-committee of Experts on
the Globally Harmonized System of Classification and Labelling of
Chemicals, where OSHA is currently the Head of the U.S. Delegation.
These permanent UN bodies have international responsibility for
maintaining, updating as necessary, and overseeing the implementation
of the GHS. OSHA and other affected Federal agencies actively
participate in these UN groups. In addition, OSHA will also continue to
participate in the GHS Programme Advisory Group under the United
Nations Institute for Training and Research (UNITAR). UNITAR is
responsible for helping countries implement the GHS, and has ongoing
programs to prepare guidance documents, conduct regional workshops, and
implement pilot projects in a number of nations. OSHA will also
continue its involvement in interagency discussions related to
coordination of domestic implementation of the GHS, and in discussions
related to international work to implement and maintain the GHS.
III. Overview of the Final Rule and Alternatives Considered
Based on consideration of the record as a whole, OSHA has modified
the HCS to make it consistent with the GHS. OSHA finds that harmonizing
the HCS with the GHS will improve worker understanding of the hazardous
chemicals they encounter every day. Such harmonization will also reduce
costs for employers.
OSHA believes that adopting the GHS will result in a clearer, more
effective methodology for conveying information on hazardous chemicals
to employers and employees. Commenters overwhelmingly supported the
revision, and their submissions form a strong evidentiary basis for
this final rule. The American Health Care Association stated that the
GHS "would enhance the effectiveness of the HCS in ensuring that
employees are apprised of the chemical hazards to which they might be
exposed" (Document ID 0346). The National Institute of
Environmental Health Sciences concurred, and added that adopting the
GHS "would provide better worker health and safety protections"
(Document ID 0347). (See also Document ID 0303, 0313,
0322, 0324, 0327, 0328, 0329, 0330, 0331, 0334, 0335, 0336, 0339, 0340,
0341, 0344, 0345, 0346, 0347, 0349, 0350, 0351, 0352, 0353, 0354, 0356,
0357, 0359, 0363, 0365, 0367, 0369, 0370, 0371, 0372, 0374, 0375, 0376,
0377, 0378, 0379, 0381, 0382, 0383, 0385, 0386, 0387, 0388, 0389, 0390,
0392, 0393, 0396, 0397, 0399, 0400, 0402, 0403, 0404, 0405, 0407, 0408,
0409, 0410, 0411, 0412, 0414, 0417, 0453, 0456, 0461, and 0463.)
Consistent with Executive Order 13563, OSHA has concluded that the
revision significantly improves the current HCS standard. Moreover,
there is widespread agreement that aligning the HCS with the GHS would
establish a valuable, systematic approach for employers to evaluate
workplace hazards, and provide employees with consistent information
regarding the hazards they encounter. A member of the United Steel
Workers aptly summed up the revision by stating that "the HCS in 1983
gave the workers the 'right to know' but the GHS will give the workers
the 'right to understand' " (Document ID 0403). The American
Society of Safety Engineers (ASSE) concurred, stating that adoption of
the HCS was "necessary to help this nation's workers deal with the
increasingly difficult challenge of understanding the hazards and
precautions needed to handle and use chemicals safely in an
increasingly connected workplace" (Document ID 0336).
Phlymar, ORC, BCI, 3M, American Iron & Steel Institute, and the North
American Metals Council (NAMC) all agreed that the adoption of the GHS
would improve the quality and consistency of information and the
effectiveness of hazard communication (Documents ID 0322,
0336, 0339, 0370, 0377, 0390, 0405, and 0408). (See also Document ID
0327, 0338, 0339, 0346, 0347, 0349, 0351, 0354, 0363, 0365,
0370, 0372, 0374, 0379, 0389, 0390, 0397, 0405, 0408, and 0414.) The
evidence supporting the Agency's conclusions is discussed more
thoroughly below in Sections IV, V, and VI; the revisions to the HCS
are discussed in detail in Section XIII.
This section of the preamble provides an overview of the current
HCS and how the adoption of the GHS will change this standard.
Moreover, this section will also discuss the alternatives to mandatory
implementation and the benefits of the final rule. The specific issues
for which OSHA solicited comments in the NPRM will be discussed within
their respective sections.
1. The Hazard Communication Standard
The HCS requires a comprehensive hazard evaluation and
communication process, aimed at ensuring that the hazards of all
chemicals are evaluated, and also requires that the information
concerning chemical hazards and necessary protective measures is
properly transmitted to employees. The HCS achieves this goal by
requiring chemical manufacturers and importers to review available
scientific evidence concerning the physical and health hazards of the
chemicals they produce or import to determine if they are hazardous.
For every chemical found to be hazardous, the chemical manufacturer or
importer must develop a container label and an SDS, and provide both
documents to downstream users of the chemical. All employers with
employees exposed to hazardous chemicals must develop a hazard
communication program, and ensure that exposed employees are provided
with labels, access to SDSs, and training on the hazardous chemicals in
their workplace.
There are three information communication components in this
system--labels, SDSs, and employee training, all of which are essential
to the effective functioning of the program. Labels provide a brief,
but immediate and conspicuous, summary of hazard information at the
site where the chemical is used. SDSs provide detailed technical
information and serve as a reference source for exposed employees,
industrial hygienists, safety professionals, emergency responders,
health care professionals, and other interested parties. Training is
designed to ensure that employees understand the chemical hazards in
their workplace and are aware of protective measures to follow. Labels,
SDSs, and training are complementary parts of a comprehensive hazard
communication program--each element reinforces the knowledge necessary
for effective protection of employees. Information required by the HCS
reduces the incidence of chemical-related illnesses and injuries by
enabling employers and employees to implement protective measures in
the workplace. Employers can select less hazardous chemical
alternatives and ensure that appropriate engineering controls, work
practices, and personal protective equipment are in place. Improved
understanding of chemical hazards by supervisory personnel results in
safer handling of hazardous substances, as well as proper storage and
housekeeping measures.
Employees provided with information and training on chemical
hazards are able to fully participate in the protective measures
instituted in their workplaces. Knowledgeable employees can take the
steps required to work safely with chemicals, and are able to determine
what actions are necessary if an emergency occurs. Information on
chronic effects of exposure to hazardous chemicals helps employees
recognize signs and symptoms of chronic disease and seek early
treatment. Information provided under the HCS also enables health and
safety professionals to provide better services to exposed employees.
Medical surveillance, exposure monitoring, and other services are
enhanced by the ready availability of health and safety information.
The modifications that make up this final rule build on these core
principles by establishing a more detailed and consistent
classification system and requiring uniform labels and SDSs, which will
better ensure that workers are informed and adequately protected from
chemical exposures.
2. Current HCS Provisions for Classification, Labeling, and SDSs
The current HCS covers a broad range of health and physical
hazards. The standard is performance-oriented, providing definitions of
hazards and parameters for evaluating the evidence to determine whether
a chemical is considered hazardous. The evaluation is based upon
evidence that is currently available, and no testing of chemicals is
required.
The current standard covers every type of health effect that may
occur, including both acute and chronic effects. Definitions of a
number of adverse health effects are provided in the standard. These
definitions are indicative of the wide range of coverage, but are not
exclusive. Mandatory Appendix A of the current standard lists criteria
for specific health effects; however, it also notes that these criteria
are not intended to be an exclusive categorization scheme, but rather
any available scientific data on the chemical must be evaluated to
determine whether the chemical presents a health hazard. Any adverse
health effect that is substantiated by a study conducted according to
established scientific principles, and reporting a statistically
significant outcome, is sufficient for determining that a chemical is
hazardous under the rule.
Most chemicals in commerce are not present in the pure state (i.e.,
as individual elements or compounds), but are ingredients in mixtures
of chemicals. Evaluation of the health hazards of mixtures is based on
data for the mixture as a whole when such data are available. When data
on the mixture as a whole are not available, the mixture is considered
to present the same health hazards as any ingredients present at a
concentration of 1% or greater, or, in the case of carcinogens,
concentrations of 0.1% or greater. The current HCS also recognizes that
risk may remain at concentrations below these cut-offs, and where there
is evidence that that is the case, the mixtures are considered
hazardous under the standard.
The current HCS establishes requirements for minimum information
that must be included on labels and SDSs, but does not provide specific
language to convey the information or a format in which to provide it.
When the current HCS was issued in 1983, the public record strongly
supported this performance-oriented approach (See 48 FR at 53300-
53310). Many chemical manufacturers and importers were already
providing information voluntarily, and in the absence of specific
requirements had developed their own formats and approaches. The record
indicated that a performance-oriented approach would reduce the need
for chemical manufacturers and importers to revise these existing
documents to comply with the HCS, thus reducing the cost impact of the
standard.
3. GHS Provisions for Classification, Labeling, and SDSs
The GHS is an internationally harmonized system for classifying
chemical hazards and developing labels and safety data sheets. However,
the GHS is not a model standard that can be adopted verbatim. Rather,
it is a set of criteria and provisions that regulatory authorities can
incorporate into existing systems, or use to develop new systems.
The GHS allows a regulatory authority to choose the provisions that
are appropriate to its sphere of regulation. This is referred to as the
"building block approach." The GHS includes all of the regulatory
components, or building blocks, that might be needed for classification
and labeling requirements for chemicals in the workplace, transport,
pesticides, and consumer products. This rule only adopts those sections
of the GHS that are appropriate to OSHA's regulatory sector. For
example, while the GHS includes criteria on classifying chemicals for
aquatic toxicity, these provisions were not adopted because OSHA does
not have the regulatory authority to address environmental concerns.
The building block approach also gives regulatory agencies the
authority to select which classification criteria and provisions to
adopt. OSHA is adopting the classification criteria and provisions for
labels and SDSs, because the current HCS covers these elements. Broad
criteria were established for the GHS in order to allow regulatory
bodies to apply the same standards to a wide array of hazards. The
building block approach may also be applied to the criteria for
defining hazard categories. As a result, the GHS criteria are more
comprehensive than what was in the current HCS, and OSHA did not need
to incorporate all of the GHS hazard categories into this final rule.
Under the GHS, each hazard or endpoint (e.g., Explosives,
Carcinogenicity) is considered to be a hazard class. The classes are
generally sub-divided into categories of hazard. For example,
Carcinogenicity has two hazard categories. Category one is for known or
presumed human carcinogens while category two encompasses suspected
human carcinogens. The definitions of hazards are specific and
detailed. For example, under the current HCS, a chemical is either an
explosive or it is not. The GHS has seven categories of explosives, and
assignment to these categories is based on the classification criteria
provided. In order to determine which hazard class a mixture falls
under, the GHS generally applies a tiered approach. When evaluating
mixtures, the first step is consideration of data on the mixture as a
whole. The second step allows the use of "bridging principles" to
estimate the hazards of the mixture based on information about its
components. The third step of the tiered approach involves use of cut-
off values based on the composition of the mixture or, for acute
toxicity, a formula that is used for classification. The approach is
generally consistent with the requirements of the pre-modified HCS, but
provides more detail and specification and allows for extrapolation of
data available on the components of a mixture to a greater extent--
particularly for acute effects.
Hazard communication requirements under the GHS are directly linked
to the hazard classification. For each class and category of hazard, a
harmonized signal word (e.g., Danger), pictogram (e.g., skull and
crossbones), and hazard statement (e.g., Fatal if Swallowed) must be
specified. These specified elements are referred to as the core
information for a chemical. Thus, once a chemical is classified, the
GHS provides the specific core information to convey to users of that
chemical. The core information allocated to each category generally
reflects the degree or severity of the hazard.
Precautionary statements are also required on GHS labels. The GHS
provides precautionary statements; while they have been codified
(numbered), they are not yet considered formally harmonized. In other
words, regulatory authorities may choose to use different language for
the precautionary statements and still be considered to be harmonized
with the GHS. The GHS has codified these statements (i.e., assigned
numbers to them) as well as aligned them with the hazard classes and
categories. Codification allows the precautionary statements to be
referenced in a shorthand form and makes it easier for authorities
using them in regulatory text to organize them. In addition, there are
provisions to allow inclusion of supplementary information so that
chemical manufacturers can provide data in addition to the specified
core information.
The GHS establishes a standardized 16-section format for SDSs to
provide a consistent sequence for presentation of information to SDS
users. Items of primary interest to exposed employees and emergency responders are
presented at the beginning of the document, while more technical
information is presented in later sections. Headings for the sections
(e.g., First-aid measures, Handling and storage) are standardized to
facilitate locating information of interest. The harmonized data sheets
are consistent with the order of information included in the voluntary
industry consensus standard for safety data sheets (ANSI Z400.1).
4. Revisions to the Hazard Communication Standard
The GHS uses an integrated, comprehensive process of identifying
and communicating hazards, and the GHS modifications improve the HCS by
providing more extensive criteria for defining the hazards in a
consistent manner, as well as standardizing label elements and SDS
formats to help to ensure that the information is conveyed
consistently. The GHS does not include requirements for a written
hazard communication program, and this final rule does not make
substantive changes to the current HCS requirements for a written
hazard communication program. Nor does the GHS impose employee training
requirements; however, OSHA believes that additional training will be
necessary to ensure that employees understand the new elements,
particularly on the new pictograms. Therefore, modified training
requirements have been included in the final rule in order to address
the new label elements and SDS format required under this revised
standard.
a. Modifications
The revised HCS primarily affects manufacturers and importers of
hazardous chemicals. Pursuant to the final rule, chemical manufacturers
and importers are required to re-evaluate chemicals according to the
new criteria in order to ensure the chemicals are classified
appropriately. For health hazards, this will involve assigning the
chemical both to the appropriate hazard category and subcategory
(called hazard class). For physical hazards, these new criteria are
generally consistent with current DOT requirements for transport.
Therefore, if the chemicals are transported (i.e., they are not
produced and used in the same workplace), this classification should
already be done to comply with DOT's transport requirements. This will
minimize the work required for classifying physical hazards under the
revised rule.
Preparation and distribution of modified labels and safety data
sheets by chemical manufacturers and importers will also be required.
However, those chemical manufacturers and importers following the ANSI
Z400.1 standard for safety data sheets should already have the
appropriate format, and will only be required to make some small
modifications to the content of the sheets to be in compliance with the
final rule.
Using the revised criteria, a chemical will be classified based on
the type, the degree, and the severity of the hazard it poses. This
information will help employers and employees understand chemical
hazards and identify and implement protective measures. The detailed
criteria for classification will result in greater accuracy in hazard
classification and more consistency among classifiers. Uniformity will
be a key benefit; by following the detailed criteria, classifiers are
less likely to reach different interpretations of the same data.
b. Specific Changes From the Proposal
Based on comments from the rulemaking effort, OSHA has made some
modifications from the proposal to the final rule. These changes were
the result of OSHA's analysis of the comments and data received from
interested parties who submitted comments or participated in the public
hearings. The major changes are summarized below and are discussed in
the Summary and Explanation Section of this Preamble (Section XIII).
Safety Data Sheet
In the proposal, OSHA asked interested parties to comment on
whether OSHA's permissible exposure limits (PELs) should be included on
SDSs, as well as any other exposure limit used or recommended by the
chemical manufacturer, importer, or employer who prepares SDSs. After
reviewing and analyzing the comments and testimony, OSHA has decided
not to modify the HCS with regard to the American Conference of
Government Industrial Hygienists (ACGIH) Threshold Limit Values (TLVs)
and so will continue to require ACGIH TLVs on SDSs. We have also
retained the classification listings of the International Agency for
Research on Cancer (IARC) and the National Toxicology Program (NTP) on
SDSs. As explained more fully in the Summary and Explanation, OSHA
finds that requiring ACGIH TLVs as well as the IARC and NTP
classification listings on the SDS will provide employers and employees
with useful information to help them assess the hazards presented by
their workplaces.
Labels
As discussed in the NPRM, the GHS gives individual countries the
option of using black, rather than red, borders around pictograms for
labels used in domestic commerce. OSHA proposed requiring red frames
for all labels, domestic and international. The final rule carries
forward this requirement. As discussed in Sections IV and XIII, studies
showed that there is substantial benefit to the use of color on the
label. The color red in particular will make the warnings on labels
more noticeable, because red borders are generally perceived to reflect
the greatest degree of hazard. Further, while commenters who objected
to this requirement cited the cost of printing in red ink as a reason
to allow domestic use of black borders, OSHA was unconvinced that the
costs involved made the provision infeasible, excessively burdensome,
or warranted the diminished protection provided by black borders. (See
Sections VI and XIII below.)
One option suggested by commenters was requiring a red label but
allowing manufacturers and importers to use preprinted labels with
multiple red frames. This would save costs because the preprinted label
stock could be used for different products requiring different
pictograms. Use of this option, however, would mean that the label for
a particular chemical might have empty red frames if the chemical did
not require as many pictograms as there were red frames on the label
stock.
As explained in Sections IV and XIII, OSHA has concluded that a red
border without a pictogram can create confusion and draw worker
attention away from the appropriate hazard warnings (See Section IV for
more detail). Additionally, OSHA is concerned that empty red borders
might be inconsistent with DOT regulations (See 49 CFR 172.401).
Therefore, while OSHA is not opposed to the use of preprinted stock,
OSHA has decided not to allow the use of blank red frames on finished
labels.
Hazard Classification
Another change to the final rule is the inclusion of the IARC and
NTP as resources for determining carcinogenicity. Commenters generally
supported this modification, and OSHA believes the inclusion of this
information will assist evaluators with the classification process.
Therefore, descriptions of both the IARC and NTP classification
criteria have been added to Appendix F, and IARC and NTP
classifications may be used to determine
whether a chemical should be classified as a carcinogen.
Unclassified Hazards
OSHA has made several modifications to clarify and specify the
definition for unclassified hazards, based on the comments provided.
Executive Order 13563 states that our regulatory system "must promote
predictability and reduce uncertainty," and these efforts at
clarification are designed to achieve that goal. OSHA included this
definition to preserve existing safeguards under requirements of the
HCS for chemical manufacturers and importers to disseminate information
on hazardous chemicals to downstream employers, and for all employers
to provide such information to potentially exposed employees. Inclusion
of the definition does not create new requirements. OSHA has made
certain changes to clarify application of the definition, and to ensure
that the relevant provisions do not create confusion or impose new
burdens.
In order to minimize confusion, OSHA has renamed unclassified
hazards, "hazards not otherwise classified." More fundamentally, and
in response to the majority of the comments on this issue, OSHA has
removed from the coverage of the general definition the hazards
identified in the NPRM as not currently classified under the GHS
criteria. These hazards are: pyrophoric gases, simple asphyxiants, and
combustible dust. As described below, OSHA has added definitions to the
final rule for pyrophoric gases and simple asphyxiants, and provided
guidance on defining combustible dust for purposes of complying with
the HCS. In addition, the Agency has also provided standardized label
elements for these hazardous effects.
Precautionary/Hazard Statements
In response to concerns by commenters that, on occasion, a
specified precautionary statement might not be appropriate, OSHA
modified mandatory Appendix C to provide some added flexibility. Where
manufacturers, importers, or responsible parties can show that a
particular statement is inappropriate for the product, that
precautionary statement may be omitted from the label. This is
discussed in more detail in section XIII below.
Other Standards Affected
Changing the HCS to conform to the GHS requires modification of
other OSHA standards. For example, modifications have been made to the
standards for Flammable and Combustible Liquids in general industry (29
CFR 1910.106) and construction (29 CFR 1926.152) to align the
requirements of the standards with the GHS hazard categories for
flammable liquids. Modifications to the Process Safety Management of
Highly Hazardous Chemicals standard (29 CFR 1910.119) will ensure that
the scope of the standard is not changed by the revisions to the HCS.
In addition, modifications have been made to most of OSHA's substance-
specific health standards, ensuring that requirements for signs and
labels and SDSs are consistent with the modified HCS.
Effective Dates
In the proposal, OSHA solicited comments regarding whether it would
be feasible for employers to train employees regarding the new labels
and SDSs within two years after publication of the final rule.
Additionally, OSHA inquired as to whether chemical manufacturers,
importers, distributors, and employers would be able to comply with all
the provisions of the final rule within three years, and whether a
phase-in period was necessary.
OSHA received many comments and heard testimony regarding the
effective dates which are discussed in detail in Section XIII below.
First, after analysis of the record, the Agency has determined that
covered employers must complete all training regarding the new label
elements and SDS format by December 1, 2013 since, as supported by
record, employees will begin seeing the new style labels considerably
earlier than the compliance date for labeling. Second, OSHA is
requiring compliance with all of the provisions for preparation of new
labels and safety data sheets by June 1, 2015. However, distributors
will have an additional six months (by December 1, 2015) to distribute
containers with manufacturers' labels in order to accommodate those
they receive very close to the compliance date. Employers will also be
given an additional year (by June 1, 2016) to update their hazard
communication programs or any other workplace signs, if applicable.
Additionally, OSHA has decided not to phase in compliance based on
whether a product is a substance or a mixture. OSHA has concluded that
adequate information is available for classifiers to use to classify
substances and mixtures. Finally, as discussed in the NPRM, employers
will be considered to be in compliance with the HCS during the
transition period as long as they are complying with either the
existing HCS (as it appears in the CFR as of October 1, 2011) or this
revised HCS. A detailed discussion regarding the effective dates is in
Section XIII.
5. Alternatives of Mandatory Implementation
In the NPRM, OSHA proposed several alternatives to mandatory
implementation of the GHS in response to concerns raised by commenters
through the ANPR (74 FR at 50289). Commenters generally supported the
concept of adopting the GHS as it was proposed. However, a few
commenters indicated that they were concerned with what they saw as the
cost burden on small businesses that are not involved in international
trade. To address these concerns, OSHA solicited comments in the NPRM
on several options proposed by the Agency regarding alternatives to
mandatory harmonization. The following is a discussion of these
alternatives; the potential impact and the response from participants
in the rulemaking regarding the relative benefit, feasibility, impact
on small business; and the impact on worker safety and health.
The first alternative OSHA proposed was to facilitate voluntary
adoption of GHS within the existing HCS framework, and give
manufacturers and importers the option to use the current HCS or the
GHS system. This option would have permitted companies to decide
whether they wanted to comply with the existing standard or with the
GHS. A variation of this alternative was also proposed that would have
adopted the GHS with an exemption allowing small chemical producers to
continue to use the HCS, even after this GHS-modified HCS is
promulgated.
The second alternative was a limited adoption of specific GHS
components. Under this approach, producers could either comply with the
GHS or a modified HCS that would retain the current HCS hazard
categories, but require standardized hazard statements, signal words,
and precautionary statements. A variation of this alternative would
have omitted mandatory precautionary statements.
Commenters almost universally objected to both of the alternatives
listed above (Document ID 0324, 0328, 0329, 0330, 0335, 0338,
0339, 0341, 0344, 0351, 0352, 0355, 0365, 0370, 0377, 0381, 0382, 0385,
0387, 0389, 0393, 0495, 0403, 0404, and 0412). American Industrial
Hygiene Association (AIHA), in a representative comment, stated that
"permitting voluntary use of some of the system * * * or exempting
certain sectors based on business size or other criteria [would] defeat
the purpose of revising this standard and of the GHS" (Document ID
0365). Additionally, the Compressed Gas Association stated they "would not support any
alternative approach as it would defeat the goal of global hazard
communication coordination" (Document ID 0324).
Many commenters argued that a dual system that permitted businesses
to opt out of complying with the GHS would undermine the key benefits
of implementation. For example, Ferro Corporation stated that "for GHS
to be effective and efficient in the U.S., implementation should be
consistent and congruent" (Document ID 0363). DuPont Company
argued "dual systems would be confusing for employers" (Document ID
0329). ORC also rejected voluntary implementation, reasoning
that "consistent requirements for all manufacturers and importers of
chemicals [are] needed to maximized efficiency in the chemical supply
chain" (Document ID 0370). Additionally, the AFL-CIO cited
consistent hazard information for workers and employers as the core
objective of this rulemaking (Document ID 0340).
The commenters who supported GHS as proposed indicated that
consistency was an essential aspect of this rule. Stericycle, Inc.,
stated that SDSs which "do not follow a consistent format would cause
issues in understanding and implementing the controls to limit exposure
and protect employee safety and health," and argued that exemptions
from GHS requirements would "shift the burden from the chemical
industry to all employers" (Document ID 0338). Additionally,
commenters did not support exempting small businesses from adopting the
GHS. Ecolab argued that "large and small businesses use each others'
products" and are inextricably linked, and they indicated that
voluntary adoption "could cause confusion about product hazards if two
identical products are labeled differently due solely to the size of
the business from which [they are] obtained" (Document ID
0351).
OSHA agrees that the first alternative is unworkable as even one
business's adoption of one of the alternatives would affect other
companies. As stated in the comments above, if small businesses do not
adopt the GHS, then large businesses or distributors will either have
to generate GHS classifications for chemicals purchased, or request
that small businesses supply data and labels using GHS classifications.
Likewise, chemical producers often provide their products to
distributors who then sell them to customers who are unknown to the
original producer. This would lead to a plethora of product labels, a
situation that is bound to make hazard communication far more
difficult.
Commenters specifically cited issues with safety as their basis for
rejecting the first proposed alternative. The AIHA (Document ID
0365) stated:
If employers and employees cannot have confidence that labels
and MSDSs provide a consistent safety message superficial
standardization will not improve safety. Safety is also seriously
compromised if different hazard communication systems are present in
the work area. Effective training is not possible if pictograms and
hazard statements are not used in a consistent manner * * *. All of
the approaches discussed will create competitive pressures that can
affect classification decisions and make good and consistent hazard
communication more difficult.
North American Metal Council argued that the alternative would penalize
workers of small business, and asserted that a "worker's right to know
about chemical hazards, should not depend on the source of a chemical
or the size of the worker's employer" (Document ID 0337).
Moreover, commenters asserted that the benefits derived from the
harmonized labeling of chemicals would be significantly diluted if
employers were not uniformly required to adopt the GHS. United Steel
Workers Union aptly reiterated that the primary benefit of adopting the
GHS is not the facilitation of international trade, but rather is the
protection of workers, which is "best accomplished through a uniform
system of classification leading to comprehensible hazard information"
(Document ID 0403). (See also Document ID 0339, 0351,
0376, 0377, 0382, and 0412.)
Several commenters supported the voluntary adoption of the GHS
(Document ID 0355, 0389, and 0502). For example,
Intercontinental Chemical Corporation supported voluntary adoption for
companies not involved in international trade (Document ID
0502). Additionally, Betco supported allowing "small
businesses that market domestically" to retain the current HCS and
suggested that "voluntary adoption would not be any less protective
for employees or create confusion" (Document ID 0389).
OSHA acknowledges that small chemical manufacturers will have some
burdens associated with the adoption of GHS. However, employees who use
products produced by small employers are entitled to the same
protections as those who use products produced by companies engaged in
international trade. The confusion created by two or more competing
systems would undermine the consistency of hazard communication
achievable by a GHS-modified HCS. Moreover, whether or not a product
will wind up in international trade may not be known to the
manufacturer or even the first distributer. A producer may provide a
chemical to another company, which then formulates it into a product
that is sold internationally. Thus, the original producer is involved
in international trade without necessarily realizing it. For these
reasons, OSHA has determined that, in order to achieve a national,
consistent standard, all businesses must be required to adhere to the
revised HCS.
OSHA concludes that the rulemaking record does not support adoption
of the first alternative. The majority of private industry, unions, and
professional organizations did not support this approach, arguing
persuasively that piecemeal adoption would undermine the benefits of
harmonization. As discussed above, while improvements to international
trade are a benefit of this rulemaking; they are not the primarily
intended benefit. OSHA believes that implementation of the GHS, without
exceptions based on industry or business size, will enhance worker
safety through providing consistent hazard communication and,
consequently, safe practices in the workplace. However, as indicated
above, OSHA does recognize that there are burdens with any change and
as discussed in Section XIII, OSHA will use the input OSHA has received
to the record to develop an outreach plan for additional guidance.
The second alternative, a halfway measure allowing businesses to
adopt some of the features of a GHS-modified HCS but not requiring
adoption of others, drew little interest or comment from the
participants. OSHA has concluded that this alternative, which would
have led to even more inconsistencies in hazard communication, is not a
viable alternative. OSHA's conclusion is supported by the overwhelming
number of commenters who spoke out against the first option and
strongly supported the proposed standard. Allowing employers to adopt,
say, only the provisions for the labels or safety data sheets will
result in inconsistent use of the standardized hazard statement, signal
word, and precautionary statement without clear direction on when they
would be required, a situation that is sure to compromise safety in the
workplace. Therefore, OSHA has concluded that implementation of the GHS
is also preferable to the second alternative.
Pursuant to its analysis of the entire rulemaking record, OSHA has
decided to adopt the GHS as proposed and is not incorporating any of
the alternatives into this final rule. The adoption of any of the
alternatives would undermine the key benefits associated with the GHS.
OSHA has concluded, as discussed in Section V, that the adoption of GHS
as proposed will strengthen and refine OSHA's hazard communication
system, leading to safer workplaces.
IV. Need and Support for the Modifications to the Hazard Communication
Standard
Chemical exposure can cause or contribute to many serious adverse
health effects such as cancer, sterility, heart disease, lung damage,
and burns. Some chemicals are also physical hazards and have the
potential to cause fires, explosions, and other dangerous incidents. It
is critically important that employees and employers are apprised of
the hazards of chemicals that are used in the workplace, as well as the
associated protective measures. This knowledge is needed to understand
the precautions necessary for safe handling and use, to recognize signs
and symptoms of adverse health effects related to exposure when they do
occur, and to identify appropriate measures to be taken in an
emergency.
OSHA established the need for disclosure of chemical hazard
information when the Hazard Communication Standard (HCS) was issued in
1983 (48 FR 53282-53284, Nov. 25, 1983). As noted in the NPRM (74 FR
50291, Sept. 30, 2009), this need continues to exist. The Agency
estimates that 880,000 hazardous chemicals are currently used in the
U.S., and over 40 million employees are now potentially exposed to
hazardous chemicals in over 5 million workplaces. During the September
29, 2009, press conference announcing the publication of the HCS NPRM,
Deputy Assistant Secretary of Labor for Occupational Safety and Health,
Jordan Barab, discussed the impact that the HCS has had on reducing
injury and illness rates. Mr. Barab stated that, since the HCS's
original promulgation in 1983, "OSHA estimates that chemically-related
acute injuries and illness [have] dropped at least 42%." Reiterating
information from OSHA's preliminary economic analysis in the NPRM, Mr.
Barab also stated:
[T]here are still workers falling ill or dying from exposure to
hazardous chemicals. OSHA estimates, based on BLS data, that more
than 50,000 workers became ill and 125 workers died due to acute
chemical exposure in 2007. These numbers are dwarfed by chronic
illnesses and fatalities that are estimated in the tens of
thousands.
OSHA believes that aligning the Hazard Communication Standard
with the provisions of the GHS will improve the effectiveness of the
standard and help to substantially improve worker safety and health.
The GHS will provide a common system for classifying chemicals
according to their health and physical hazards and it will specify
hazard communication elements for labeling and safety data sheets.
Data collected and analyzed by the Agency also reflect this
critical need to improve hazard communication. Chemical exposures
result in a substantial number of serious injuries and illnesses among
exposed employees. The Bureau of Labor Statistics estimates that
employees suffered 55,400 illnesses that could be attributed to
chemical exposures in 2007, the latest year for which data are
available (BLS, 2008). In that same year, 17,340 chemical-source
injuries and illnesses involved days away from work (BLS, 2009).
The BLS data, however, do not indicate the full extent of the
problem, particularly with regard to illnesses. As noted in the
preamble to the HCS in 1983, BLS figures probably only reflect a small
percentage of the incidents occurring in exposed employees (48 FR
53284, Nov. 25, 1983). Many occupational illnesses are not reported
because they are not recognized as being related to workplace
exposures, are subject to long latency periods between exposure and the
manifestation of disease, and other factors (e.g., Herbert and
Landrigan, 2000, Document ID 0299; Leigh et al., 1997,
Document ID 0274; Landrigan and Markowitz, 1989, Document ID
0299).
While the current HCS serves to ensure that information concerning
chemical hazards and associated protective measures is provided to
employers and employees, the Agency has determined that the revisions
adopted in this final rule will substantially improve the quality and
consistency of the required information. OSHA believes these revisions
to the HCS, which align it with the GHS, will enhance workplace
protections significantly. Better information will enable employers and
employees to increase their recognition and knowledge of chemical
hazards and take measures that will reduce the number and severity of
chemical-related injuries and illnesses.
A key foundation underlying this belief relates to the
comprehensibility of information conveyed under the GHS. All hazard
communication systems deal with complicated scientific information
being transmitted to largely non-technical audiences. During the
development of the GHS, in order to construct the most effective hazard
communication system, information about and experiences with existing
systems were sought to help ensure that the best approaches would be
used. Ensuring the comprehensibility of the GHS was a key principle
during its development. As noted in a Federal Register notice published
by the U.S. Department of State (62 FR 15956, April 3, 1997): "A major
concern is to ensure that the requirements of the globally harmonized
system address issues related to the comprehensibility of the
information conveyed." This concern is also reflected in the
principles of harmonization that were used to guide the negotiations
and discussions during the development of the GHS. As described in
Section 1.1.1.6(g) of the GHS, the principles included the following:
"[T]he comprehension of chemical hazard information, by the target
audience, e.g., workers, consumers and the general public should be
addressed."
As was discussed in the proposal (74 FR 50291), to help in the
development of the GHS, OSHA had a review of the literature conducted
to identify studies on effective hazard communication, and made the
review and the analysis of the studies available to other participants
in the GHS process. One such study, prepared by researchers at the
University of Maryland, entitled "Hazard Communication: A Review of
the Science Underpinning the Art of Communication for Health and
Safety" (Sattler et al., 1997, Document ID 0191) has also
long been available to the public on OSHA's Hazard Communication web
page. Additionally, OSHA conducted an updated review of the literature
published since the 1997 review. This updated review examined the
literature relevant to specific hazard communication provisions of the
GHS (ERG, 2007, Document ID 0246).
Further work related to comprehensibility was conducted during the
GHS negotiations by researchers in South Africa at the University of
Cape Town--the result is an annex to the GHS on comprehensibility
testing (See GHS Annex 6, Comprehensibility Testing Methodology)
(United Nations, 2007, Document ID 0194). Such testing has
been conducted in some of the developing countries preparing to
implement the GHS, and has provided these countries with information
about which areas in the GHS will require more training in their
programs to ensure people understand the information. The primary purpose of these
activities was to ensure that the system developed was designed in such
a way that the messages would be effectively conveyed to the target
audiences, with the knowledge that the system would be implemented
internationally in different cultures with varying interests and
concerns.
Another principle that was established to guide development of the
GHS was the agreement that levels of protection offered by an existing
hazard communication system should not be reduced as a result of
harmonization. Following these principles, the best aspects of existing
systems were identified and included in a single, harmonized approach
to classification, labeling, and development of SDSs.
The GHS was developed by a large group of experts representing a
variety of perspectives. Over 200 experts provided technical input on
the project. The United Nations Sub-Committee of Experts on the GHS,
the body that formally adopted the GHS and is now responsible for its
maintenance, includes 35 member nations as well as 14 observer nations.
Authorities from these member states are able to convey the insight and
understanding acquired by regulatory authorities in different sectors,
and to relate their own experiences in implementation of hazard
communication requirements. In addition, over two dozen international
and intergovernmental organizations, trade associations, and unions are
represented, and their expertise serves to inform the member nations.
The GHS consequently represents a consensus recommendation of experts
with regard to best practices for effective chemical hazard
communication, reflecting the collective knowledge and experience of
regulatory authorities in many nations and in different regulatory
sectors, as well as other organizations that have expertise in this
area.
United States-based scientific and professional associations have
endorsed adoption of the GHS since publication of the Advance Notice of
Proposed Rulemaking (ANPR) in 2006 (71 FR 53617, Sept. 12, 2006). For
example, the American Chemical Society (ACS) indicated its support for
the GHS, stating: "The American Chemical Society strongly supports the
adoption of the GHS for hazard communication in general and
specifically as outlined in the ANPR" adding that "* * * ACS
anticipates that OSHA implementation of GHS in the U.S. will enhance
protection of human health and the environment through warnings and
precautionary language that are consistent across different products
and materials as well as across all workplaces" (Document ID
0165). The American Industrial Hygiene Association (AIHA)
affirmed its support for modification of the HCS to adopt the GHS. AIHA
maintained that standardized labels and safety data sheets will make
hazard information easier to use, thereby improving protection of
employees (Document ID 0034). While acknowledging that the GHS
presents a number of concerns and challenges, the Society of Toxicology
has also expressed its support for the GHS, stating that "a globally
harmonized system for the classification of chemicals is an important
step toward creating consistent communications about the hazards of
chemicals used around the world" (Document ID 0304). The
American Association of Occupational Health Nurses joined these
organizations in advocating adoption of the GHS, arguing that
standardization of chemical hazard information is critical to
protecting the safety and health of employees (Document ID
0099). Responders to the 2009 NPRM reiterated their support or, in the
case of new commenters, echoed the comments from other scientific and
professional associations to the ANPR (See, e.g., Document ID
0338, 0357, 0365, 0393, and 0410). The positions taken by
these organizations point to wide support for the GHS among the
scientific and professional communities.
Stakeholders representing a wide range of sectors and interests
agreed with OSHA that aligning the HCS with the GHS will improve
comprehensibility, and thus lead to reductions in chemical source
illnesses and injuries. American Society of Safety Engineers, Dow
Chemical, and ORC all voiced their support for the proposed rule,
citing improved comprehensibility and quality of transmitted
information as key benefits (Document ID 0336, 0353, and
0370). Representing union labor, the American Federation of State,
County and Municipal Employees (AFSCME) stated that this rulemaking
would "allow critical communication about the hazards of chemicals to
be understood by all workers, regardless of their literacy level or
primary language * * * [and] will in turn lead to safer, more
productive workplaces" (Document ID 0414). Many stakeholders
asserted that adopting the GHS would lead to safer workplaces. The
Chamber of Commerce provided its support for the rulemaking, stating
that the GHS could "improve worker safety, and facilitate business
growth and international trade" (Document ID 0397). The
American Subcontractors Association, Inc. added that consistent hazard
communication is critical to having a safe work program (Document ID
0322). Additionally, North American Metals Council (NAMC),
which represents the interests of the metals and mining industry,
stated that a single, globally harmonized classification and labeling
system is of vital interest to its members (Document ID 0233).
The position that GHS would increase worker protection was also raised
in testimony during the hearings. Elizabeth Treanor of Phylmar
Regulatory Roundtable testified that adopting the GHS would "enhance
the effectiveness of the hazard communication standard by improving the
quality and consistency of chemical hazard information that is provided
to employees and employers" (Document ID 0497 Tr. 92).
In addition to the endorsement of the GHS by a group of experts
with extensive knowledge and experience in chemical hazard
communication, support from scientific and professional associations
with expertise in this area, and support from industry and labor
stakeholders, a substantial body of evidence indicates that the
modifications to the HCS will better protect employees. Specifically,
this evidence supports OSHA's findings that: (1) Standardized label
elements--signal words, pictograms, hazard statements and precautionary
statements--will be more effective in communicating hazard information;
(2) standardized headings and a consistent order of information will
improve the utility of SDSs; and (3) training will support and enhance
the effectiveness of the new label and SDS requirements.
This evidence was obtained from sources predating the ANPR and from
more recent data. OSHA commissioned several studies to examine the
quality of information on SDSs (Karstadt, 1988, Document ID
0296; Kearney/Centaur 1991a, 1991b, Document ID 0309
and 0310; Lexington Group, 1999, Document ID 0257); the
General Accounting Office (GAO) has issued two reports based on its
evaluation of certain aspects of the HCS (GAO 1991 and 1992, Document
ID 0271 and 0272); a National Advisory Committee on
Occupational Safety and Health (NACOSH) workgroup conducted a review of
hazard communication and published a report of its findings (NACOSH,
1996, Document ID 0260); and a substantial amount of
scientific literature relating to hazard communication has been
published. As mentioned previously, OSHA
commissioned a review of the literature, and a report based on that
review was published in 1997 (Sattler et al., 1997, Document ID
0191). An updated review was conducted in 2007 (ERG, 2007,
Document ID 0246). In addition, OSHA conducted a review of the
requirements of the HCS and published its findings in March of 2004
(OSHA, 2004, Document ID 0224). Key findings derived from
these sources are discussed below.
No commenters questioned the validity of studies presented in the
NPRM. Similarly, commenters did not question OSHA's analysis or
interpretation of the study findings. Only one commenter suggested that
OSHA should adopt more "conservative expectations for the effects that
warning format changes can have on the behavior of end users," adding
that "real-world conditions" must be accounted for when determining
the actual responses of users (Document ID 0396). However, the
commenter did not disagree with OSHA's overall conclusion that this
final rule would improve safety. OSHA agrees that external factors may
influence the overall benefits of label elements (this will be
addressed in Section VI).
The studies discussed in the NPRM formed the evidentiary basis for
the revised HCS. As such, OSHA infers that commenters generally found
the studies, as well as OSHA's analysis, to be sound. OSHA's rationale
for adopting the GHS is tied to anticipated improvements in the quality
and consistency of the information that would be provided to employers
and employees. Hazard classification is the foundation for development
of this improved information. Indeed, hazard classification is the
procedure of identifying and evaluating available scientific evidence
in order to determine if a chemical is hazardous, and the degree of
hazard, pursuant to the criteria for health and physical hazards set
forth in the standard. Hazard classification provides the basis for the
hazard information that is provided in labels, SDSs, and employee
training. As such, it is critically important that classification be
performed accurately and consistently.
The GHS provides detailed scientific criteria to direct the
evaluation process. The specificity and detail provided help ensure
that different evaluators would reach the same conclusions when
evaluating the same chemical. Moreover, the GHS refines the
classification process by establishing categories of hazard within most
hazard classes. These categories indicate the relative degree of
hazard, and thereby provide a basis for determining precise hazard
information that is tailored to the level of hazard posed by the
chemical. The classification criteria established in the GHS thus
provide the necessary basis for development of the specific, detailed
hazard information that would enhance the protection of employees.
Labels
Labels serve as immediate visual reminders of chemical hazards, and
complement the information presented in training and on SDSs. The
current HCS requires that labels on hazardous chemical containers
include the identity of the hazardous chemical; appropriate hazard
warnings that convey the specific physical and health hazards,
including target organ effects; and the name and address of the
chemical manufacturer, importer, or other responsible party. The HCS
does not specify a standard format or design elements for labels.
In the NPRM, OSHA proposed to improve the HCS by changing the
performance requirements for labels to the GHS-specific requirements
that labels include four standardized elements: a signal word; hazard
statement(s); pictogram(s); and precautionary statement(s) (See Section
XV for a detailed discussion of the requirements). The appropriate
label elements for a chemical are to be determined by the hazard
classification. OSHA has concluded that these standardized label
elements better convey critically important hazard warnings, and
provide useful information regarding precautionary measures that will
serve to better protect employees than the performance-oriented
approach of the current rule.
This requirement is different from the current HCS in that it will
require consistent and detailed information regarding a chemical based
on the hazard classification. The current rule does not specify a
standard format or design elements for labels. Rather, all that is
required in the current HCS is that the label of the hazardous chemical
containers include the identity of the hazardous chemical; appropriate
hazard warnings that convey the specific physical and health hazards,
including target organ effects; and the name and address of the
chemical manufacturer, importer, or other responsible party.
Additionally, as discussed in the proposal (74 FR 50291, Sept. 30,
2009), a great deal of literature has been developed that examines the
effectiveness of warnings on labels. These studies support OSHA's
adoption of standardized warnings on the labels of hazardous chemicals.
Although the studies discussed below pertain to prescription and non-
prescription medications, alcoholic beverages, or consumer products
rather than hazardous chemicals, it does not diminish the importance or
relevance of the data. This literature provides a substantial body of
information directly applicable and analogous to workplace chemical
labels. In spite of the differences in affected populations, workplace
chemical labels have many characteristics that are comparable to those
found in other sectors. Pharmaceutical labels, for example, are similar
to chemical labels in that they often have explicit instructions for
use which, if not followed, can cause adverse health effects or death.
Designers of pharmaceutical labels also encounter many of the same
challenges faced by those who design chemical labels, such as container
space limitations and the need to convey information to low-literate or
non-English-literate users. In addition, some of the research is not
directly related to any particular sector or type of product. Some
findings related to use of color, for example, could reasonably be
applied to a wide variety of label applications. The studies are
discussed below in the specific labeling sections.
Signal Words
A signal word is a word that typically appears near the top of a
warning, sometimes in all capital letters. Common examples include
DANGER, WARNING, CAUTION, and NOTICE. The signal word is generally
understood to serve a dual purpose: Alerting the user to a hazard and
indicating a particular level of hazard. For example, users generally
perceive the word DEADLY to indicate a far greater degree of hazard
than a term like NOTICE.
This final rule requires the use of one of two signal words for
labels--DANGER or WARNING--depending on the hazard classification of
the substance in question. These are the same two signal words used in
the GHS. DANGER is used for the more severe hazard categories, while
WARNING denotes a less serious hazard. These signal words are similar
to those in other established hazard communication systems, except that
some other systems have three or more tiers. For example, ANSI Z129.1
(the American National Standard for Hazardous Industrial Chemicals--
Precautionary Labeling) uses DANGER, WARNING, and CAUTION, in
descending order of severity (ANSI, 2006, Document ID 0280).
A number of studies have examined how people perceive signal words
and, in particular, how they perceive signal words to be different from one
another. Overall, this research supports the use of signal words on
labels, demonstrating that they can attract attention and help people
clearly distinguish between levels of hazard. The research also
supports the decision to use only two tiers, as many recent studies
have found clear differences between DANGER and WARNING, but little
perceived difference between WARNING and CAUTION.
Wogalter et al. investigated the influence of signal words on
perceptions of hazard for consumer products (Wogalter et al., 1992,
Document ID 0300). Under the pretext of a marketing research
study, 90 high school and college students rated product labels on
variables such as product familiarity, frequency of use, and perceived
hazard. Results showed that the presence of a signal word increased
perceived hazard compared to its absence. Between extreme terms (e.g.,
NOTE and DANGER), significant differences were noted.
Seeking to test warning signs in realistic settings, Adams et al.
tested five industrial warning signs on a group of 40 blue-collar
workers employed in heavy industry, as well as a group of students
(Adams et al., 1998, Document ID 0235). Signs were manipulated
to include four key elements (signal word, hazard statement,
consequences statement, and instructions statement) or a subset of
those elements. Participants were asked questions to gauge their
reaction and behavioral intentions. Overall, 77 percent (66 percent of
the worker group) recognized DANGER as the key word when it appeared,
and more than 80 percent recognized BEWARE and CAUTION, suggesting that
the signal word was generally noticed, and it was recognized as the key
alerting element. DANGER was significantly more likely than other words
to influence behavioral intentions.
Laughery et al. also demonstrated the usefulness of signal words.
The authors tested the warnings on alcoholic beverage containers in the
U.S., and found that a signal word (WARNING) was one of several factors
that decreased the amount of time it took for participants to locate
the warning (Laughery et al., 1993, Document ID 0281).
Several studies have tested the arousal strength or perceived
hazard of different signal words. Arousal strength is a term used to
indicate the overall importance of the warning, and incorporates both
the likelihood and severity of the potential threat. Silver and
Wogalter tested the arousal strength of signal words on college
students and found that DANGER connoted greater strength than WARNING
and CAUTION (Silver and Wogalter, 1993, Document ID 0308). The
results failed to show a difference between WARNING and CAUTION. Among
other words tested, DEADLY was seen as having the strongest arousal
connotation, and NOTE the least.
Griffith and Leonard asked 80 female undergraduates (who were
unlikely to have already received industrial safety training) to rate
signal words. Results included a list of terms in order of
"meaningfulness," representing conceptual "distance" from the
neutral term NOTICE (Griffith and Leonard, 1997, Document ID
0250). From most to least meaningful, these terms were
reported to be DANGER, URGENT, BEWARE, WARNING, STOP, CAUTION, and
IMPORTANT.
Wogalter et al. asked over 100 undergraduates and community
volunteers to rank signal words (Wogalter et al., 1998, Document ID
0286). DEADLY was perceived as most hazardous, followed by
DANGER, WARNING, and CAUTION. All differences were statistically
significant. In a follow-up experiment using labels produced in the
ANSI Z535.2 (American National Standard for Environmental and Facility
Safety Signs), ANSI Z535.4 (American National Standard for Product
Safety Signs and Labels), and alternative formats, the authors found a
similar rank order for signal words with all labeling systems. Finally,
the authors tested the same terms on employees from manufacturing and
assembly plants and found the same general order: DEADLY, then DANGER,
then WARNING and CAUTION with no significant difference between the
last two terms.
In more of a free-form experiment, Young asked 30 subjects to
produce warning signs for a set of scenarios, using different sign
components available on a computer screen (Young, 1998, Document ID
0289). In roughly 80 percent of the signs, the participant
chose to use a signal word. DANGER, DEADLY, and LETHAL were more likely
to be used for scenarios with severe hazards; CAUTION and NOTICE for
non-severe scenarios. WARNING was used equally in both types of
scenarios. The author suggests that these results support a two-tiered
system of signal words. In a separate task, users ranked the perceived
hazard of signal words, resulting in the following list from most to
least severe: DEADLY, LETHAL, DANGER, WARNING, CAUTION, and NOTICE.
While these studies have focused on the relative perceptions of
signal words, others have sought to evaluate how the absolute meaning
of common signal words is perceived. Drake et al. asked a group of
students and community volunteers to match signal words with
definitions borrowed from consensus standards and other sources (Drake
et al., 1998, Document ID 0244). Participants matched DANGER
to a correct definition 64 percent of the time, while NOTICE was
matched correctly 68 percent of the time. WARNING and CAUTION were
matched correctly less than half of the time, suggesting confusion. The
authors recommended using WARNING and CAUTION interchangeably. The
authors also suggested that a standard set of signal words (but not
synonyms) is helpful for users with limited English skills, who can be
trained to recognize a few key words.
Signal word perceptions are reported to be consistent among some
non-U.S. populations, as well. Hellier et al. asked 984 adults in the
UK to rate DANGER, WARNING, and CAUTION on a hazard scale from 1 (low)
to 10 (high) (Hellier et al., 2000a, Document ID 0252). DANGER
was ranked as 8.5, WARNING was ranked as 7.8, while CAUTION was rated
as 7.25. These results are consistent with the findings of studies on
subjects in the U.S. In a second study published in 2000, Hellier et
al. asked a mixed-age group of participants in the UK to rate the
arousal strength of 84 signal words commonly used in the U.S. (Hellier
et al., 2000b, Document ID 0253). The authors found that
DANGER is stronger than WARNING, while WARNING and CAUTION are not
significantly different from each other.
Similar results were found among workers in Zambia. Banda and
Sichilongo tested GHS-style labels using four different signal words
(as well as other variables) (Banda and Sichilongo, 2006, Document ID
0237). Among workers in the industrial and transport sectors,
DANGER was generally perceived as the most hazardous signal word.
WARNING was one of a group of terms that were largely indistinguishable
from one another, but distinct from DANGER. The authors support
adoption of the GHS, suggesting that having just two possible signal
words will lead to "more impact and less confusion about the extent of
hazard."
In addition, comparable results were found in South Africa (London,
2003, Document ID 0311). In a large study on SDS and label
comprehensibility conducted for South Africa's National Economic
Development and Labour
Council (NEDLAC), DANGER was generally ranked as more hazardous than
WARNING by participants in the four sectors tested: industry,
transport, agriculture, and consumers.
Cumulatively, these studies provide a clear indication that signal
words are effective in alerting readers that a hazard exists, and in
conveying the existence of a particular level of hazard. The studies
found a generally consistent hierarchy of signal words with respect to
perceived hazard. DANGER and WARNING appear to connote different levels
of hazard, while the perceived difference between WARNING and CAUTION
is often insignificant.
In response to the NPRM, OSHA received a comment from Croplife
America about the impact of using a two-tiered signal word system on
pesticide labels (Document ID 0387). Croplife America
explained that they believe a three-tiered system (DANGER, WARNING and
CAUTION) provides "a little more distinction in the relative toxicity
of a compound" and "if everything says 'warning,' we run the risk of
diluting the effectiveness of the signal word" (Document ID
0495 Tr. 251). During the informal public hearings, OSHA
requested that Croplife America support their position on why a three-
tiered warning system is better than a two-tiered system. To support
this assertion, Croplife America submitted a late comment containing an
additional paper by Hellier et al. which analyzed how signal words are
interpreted (Hellier et al., 2007, Document ID 0646).
This paper discusses two studies performed in 2007 to analyze if
alternative information is communicated with signal words (Hellier et
al., 2007, Document ID 0646). Using 17 signal words, 30
undergraduate students were asked to rate the similarities of paired
signal words. In the first study, the result ratings revealed that
signal words were interpreted by the participants along three
dimensions; dimension one: the level of hazard implied by the signal
words, dimension two: the extent to which they explicitly implied a
risk, and dimension three': the clarity of the instruction given by the
signal word. Using the same signal words as in the first study, the
second study explored how these signal words were interpreted by the
study participants. Using statistical analysis, the analysis confirmed
that the participants were able to discern the levels of hazard implied
by the signal words and how it to relates to the explicitness of the
implied risk (dimensions one and two). The results of the third
dimension were unclear. The studies indicate that the extent to which
signal words imply risk is important--people may not respond when
repeatedly exposed to warnings that do not explicitly imply a risk. The
results support using signal words to denote the level of hazard
implied by the situation, and that there might be utility in using
signal words to convey both information about a potential risk and the
level of hazard.
Even if it had been timely submitted, OSHA is not convinced that
this study supplies sufficient evidence that using a two-tiered signal
word approach will diminish the chemical user's ability to distinguish
hazard severity. In OSHA's opinion, if anything, the Hellier study
provides additional support for the use of signal words on labels to
attract attention and to identify levels of hazard. Indeed, its results
show that the signal word "caution" was substantially less connected
by participants with communicating hazards than "warning" and
"danger," which supports OSHA's decision not to use "caution" as a
signal word. The record supports OSHA's determination that using the
signal word in combination with the hazard statement alerts the
chemical user to the hazard and allows him or her to distinguish the
level of hazard severity posed by hazardous chemicals in the workplace.
Commenting on the studies presented in the proposal, Applied Safety
and Ergonomics (ASE) agreed that there are benefits associated with the
standardization of warning elements. However, they also urged "OSHA to
adopt more conservative expectations for the effect that warning format
changes can have on the behavior of end users" (Document ID
0396). See Section VI of this final rule for a detailed
discussion of the benefits of standardized warning elements. OSHA does
not disagree with these comments and has determined that requiring the
use of the combined labeling elements (pictograms, signal words, hazard
statements, and precautionary statements) will result in a uniform and
consistent system of identifying and communicating chemical hazards in
the workplace. No other comments were received on the studies OSHA used
in its discussion of the need for signal words in this revised HCS.
Comments received from stakeholders support the revision of the HCS
to include the use of standardized signal words (Document ID
0321, 0338, 0339, and 0349). For example, the Communications
Workers of America (CWA) stated: "Clearly, the Rule's requirements
regarding revised SDSs and labeling provisions requiring the use of
standardized signal words, pictograms, and hazard and precautionary
statements would prove invaluable to affected CWA members whom have
been exposed to hazardous chemicals and chemical products that have
produced negative health effects and medical problems" (Document ID
0349). These comments support OSHA's conclusion that signal
words alert chemical users to a hazard and indicate a particular level
of hazard.
After reviewing the comments received and the evidence presented in
the record, OSHA has determined that, in this revised rule, use of the
signal words "DANGER" and "WARNING" is appropriate.
Pictograms
A pictogram is a graphical composition that may include a symbol
along with other graphical elements, such as a border or background
color. A pictogram is a communication tool and is intended to convey
specific information. The proposed rule included requirements for use
of eight different pictograms. Each of these pictograms consists of a
different symbol in black on a white background within a red square
frame set on a point (i.e., a red diamond). The specific pictograms on
a label were to be determined based on the hazard classification of the
substance in question. OSHA has found ample evidence to support the
requirement for pictograms.
A study by Kalsher et al. reported that users preferred labels with
pictorials. The authors concluded that pictorials focused the attention
of the user, helped users who were unable to read the small font size
or print on the labels, and were useful for individuals who did not
understand English (Kalsher et al., 1996, Document ID 0256).
The presence of the symbol can attract attention to the warnings and
are more memorable than written warnings (Parsons et al., 1999,
Document ID 0262). Symbols serve several important functions
in warning labels. As Wogalter et al. explained (Wogalter et al., 2006,
Document ID 0275), symbols may alert the user to a hazard more
effectively than text alone:
Symbols may be more salient than text because of visual
differentiations of shape, size, and color. Usually symbols have
unique details and possess more differences in appearance than do
the letters of the alphabet. Letters are highly familiar and are
more similar to one another than most graphical symbols.
Other investigators have examined the benefits of pictograms for
those with low literacy levels and those who do not understand the
language in which the label text is written. A study by Parsons et al. concluded that
nonverbal graphics are especially helpful for ensuring that
individuals, who do not speak English or who have limited understanding
of English, understand the meaning of the intended warning (Parsons et
al., 1999, Document ID 0262). Another study has shown that
people with low literacy skills can, with the help of pictographs,
recall large amounts of medical information over significant periods of
time (Houts et al., 2001, Documents ID 0254).
Several researchers have sought to evaluate how people comprehend
symbols, including the symbols that were proposed to be required.
Several studies have found that the skull and crossbones icon--one of
the symbols proposed and included in the final rule--is among the most
recognizable of safety symbols. For example, Wogalter et al. asked 112
undergraduates and community volunteers to rank various label elements
(Wogalter et al., 1998, Document ID 0244). Among shapes and
icons, the skull symbol (in this case, without the crossbones) was
rated most hazardous and most noticeable. The skull connoted the
greatest hazard among industrial employees as well. Smith-Jackson and
Wogalter asked 48 English-speaking workers to rate the perceived
hazards of six alerting symbols (Smith-Jackson and Wogalter, 2000,
Document ID 0196). The skull was rated significantly higher
than all other symbols.
Several studies have examined other pictograms included in the
final rule. As part of an experiment to see how individuals comprehend
warnings on household chemical labels, Akerboom and Trommelen asked 60
university students whether they understood the meaning of several
pictograms, including four that are included in the final rule
(Akerboom and Trommelen, 1998, Document ID 0236). The authors
reported the following levels of comprehension for these pictograms:
[ssquf] Flame: 93 percent comprehension;
[ssquf] Skull and crossbones: 85 percent comprehension;
[ssquf] Corrosion: 20 percent comprehension; and
[ssquf] Flame over circle: 13 percent comprehension.
Only the flame and skull and crossbones pictograms met the 85
percent comprehension criteria suggested by ANSI Z535.3 (the American
National Standard Criteria for Safety Symbols) (ANSI, 2002a, Document
ID 0276). The authors recommend that labels present the hazard
phrase [statement] and symbol together, along with corresponding
precautions, as has been included as a requirement in the final rule.
Banda and Sichilongo tested comprehension of labels among 364
workers in four sectors in Zambia (transport, agriculture, industrial,
and household consumers) (Banda and Sichilongo, 2006, Document ID
0237). Within this population, the skull and crossbones symbol
was widely understood, as was the "flame" symbol. Based on these
results, the authors suggest a preference for symbols that depict
familiar, meaningful, and recognizable images.
London performed a similar study among the same four sectors in
South Africa, finding that the skull and crossbones was understood by
at least 96 percent of each sector and "flame" by at least 89 percent
(London, 2003, Document ID 0311). "Exploding bomb" was
correctly comprehended by 44 to 71 percent of each sector. On the other
hand, many health-related symbols did not fare well, and six symbols
had less than 50 percent comprehension across all four sectors. Outside
the transport sector, "Gas cylinder" was the least comprehended
symbol.
These findings indicate that some of the pictograms included in the
final rule are already widely recognized by a general audience. Others,
however, are not commonly understood. Therefore, simply adding some of
the pictograms on labels will not provide useful information unless
efforts are also undertaken to ensure that employees understand the
meaning of the pictograms. As Wogalter et al. noted, some studies have
found slower processing, poorer recognition, and greater learning
difficulties with symbols versus with text--particularly if the symbols
are complex or non-intuitive (Wogalter et al., 2006, Document ID
0275). These results emphasize the need to train employees on
the meaning of the pictograms that will be included on chemical labels.
Where pictograms are used and understood, communication of hazards
can be improved. Houts et al. studied long-term recall of spoken
medical instructions when accompanied by a handout with pictograms
(Houts et al., 2001, Document ID 0254). Nearly 200 pictograms
were tested with 21 low-literate adults (less than grade 5 reading
level). Immediately after training, participants recalled the meaning
of 85 percent of the pictograms, and they recalled 71 percent after 4
weeks. This study found that recall was better for simple pictograms
where there is a direct relationship between the image and its
meaning--that is, where no inference is required.
Another body of literature focuses on the utility of symbols in
general. Ganier found that people generally construct mental
representations faster with pictures than they do with text, supporting
earlier findings on the usefulness of symbols (Ganier, 2001, Document
ID 0275). Evans et al. found similar results with a task in
which undergraduates were asked to sort items into categories using
either text clues, visual clues, or a combination of pictures and text
(Evans et al., 2002; Document ID 0192). When categories were
fixed (i.e., sorting instructions were specific), people sorted the
cards more consistently with one another when presented with pictures
than when presented with text alone.
In a follow-up article on the South African study mentioned
previously, Dowse and Ehlers found that patients receiving antibiotics
adhered to instructions much better when the instructions included
pictograms--(54 percent with high adherence, versus 2 percent when
given text-only instructions) (Dowse and Ehlers, 2005, Document ID
0243).
Pictograms also serve to attract attention to the hazard warnings
on a label. To examine factors that influence the effectiveness of
pharmaceutical labels, Kalsher et al. asked subjects to rate the
noticeability, ease of reading, and overall appeal of labels with or
without pictorials (Kalsher et al., 1996, Document ID 0256). A
group of 84 undergraduates gave consistently higher ratings to labels
with pictorials. A group of elderly subjects had similar preferences,
rating labels with pictorials as significantly more noticeable and
likely to be read.
Laughery et al. found similar results with a timed test on
alcoholic beverage labels (Laughery et al., 1993, Document ID
0281). When a pictorial was present to the left of the warning
showing what not to do when drinking, the amount of time it took to
find the label was significantly reduced. An icon consisting of the
alert symbol (an exclamation mark set within a triangle) and the signal
word WARNING also decreased response time. The fastest response time
came when four different enhancements (including the pictorial and the
icon) were included. In a follow-up exercise, an eye scan test found
that the pictorial had a particularly strong influence on reaction
time, compared with other enhancements.
Where chemical labels are concerned, London found that symbols tend
to be the most easily recalled label elements (London, 2003, Document
ID 0311). In the comprehensibility test of labels
among South African workers mentioned previously, symbols were the most
commonly recalled elements--particularly the skull and crossbones--and
people recalled looking at symbols first. Symbols were also cited as by
far the most important factor in determining hazard perception. The
author concludes that "Symbols are therefore key to attracting
attention and informing risk perception regarding a chemical" (London,
2003, Document ID 0311).
Wogalter et al. found factors other than pictorials influenced
workers (Wogalter et al., 1993, Document ID 0285). The authors
tested the influence of various warning variables on whether subjects
wore proper protective equipment during a task involving measuring and
mixing chemicals. Warning location and the amount of clutter around the
warning had significant effects on compliance, but the presence or
absence of pictorials did not.
Meingast asked subjects to recall warning content after viewing
labels that were considered either high quality (with color signal
icons, pictorials, and organized text conforming to ANSI Z535.4, the
American National Standard for Product Safety Signs and Labels) or low
quality (text only) (Meingast, 2001, Document ID 0210).
Pictorials were the items remembered most often, accounting for 48
percent of what viewers of high-quality labels recalled. The author
suggests that these pictorials also served the role of dual coding,
meaning that they help to improve the retention of corresponding text.
Other studies support this dual-coding function of pictorials,
finding that symbols tend to be most effective when paired with
redundant or reinforcing text. For example, Sojourner and Wogalter
asked 35 participants to rate several prescription label formats in
terms of ease of reading, ease of understanding, overall effectiveness,
likelihood of reading, overall preference, pictorial understanding, and
how helpful pictorials are in helping to remember the instructions
(Sojourner and Wogalter, 1997, Document ID 0288). The authors
found that people prefer fully redundant text and pictorials, which
they judged easiest to read, most effective, and preferred overall.
Dual-coded pictorials aided understanding and memory more than labels
with pictorials only (no text).
In a follow-up study, Sojourner and Wogalter gave undergraduates,
young adults, and older adults a free recall test after viewing
medication labels (Sojourner and Wogalter, 1998, Document ID
0288). Fully redundant text and pictorials led to
significantly greater recall than other formats, and were rated most
effective by all age groups.
Similarly, Sansgiry et al. found that pictograms on over-the-
counter drug labels improved comprehension, but only when they were
congruent with the corresponding text (Sansgiry et al., 1997, Document
ID 0264). The 96 adults who were tested were less confused,
were more satisfied, were more certain about their knowledge, and
understood more when shown labels that contained congruent pictures and
verbal instructions, versus verbal instructions alone. The results were
significantly better with congruent pictures and text than with either
pictures alone or incongruent pictures and text.
Some evidence links use of pictograms directly to safer behavior.
Jaynes and Boles investigated whether different warning designs,
specifically those with symbols, affect compliance rates (Jaynes and
Boles, 1990, Document ID 0290). Five conditions were tested: a
verbal warning, a pictograph warning with a circle enclosing each
graphic, a pictograph warning with a triangle on its vertex enclosing
each graphic, a warning with both words and pictographs, and a control
(no warning). Participants performed a chemistry laboratory task using
a set of instructions that contained one of the five conditions. The
warnings instructed them to wear safety goggles, mask, and gloves. All
four warning conditions had significantly greater compliance than the
no-warning condition. A significant effect was also found for the
"presence of pictographs" variable, suggesting that the addition of
pictographs will increase compliance rates.
NIOSH submitted an additional study at the informal public hearings
that analyzed the use of pictograms on labels. In 1997, Wilkinson et
al. (Document ID 0480.6), interviewed 206 farmers in Victoria
Australia. Two widely used agricultural herbicides were used for the
basis of the research. The researchers developed three "mocked-up"
labels for each herbicide--one containing existing warning text, one
containing existing text with pictograms of appropriate safety
precautions, and one containing text with pictograms that had been
tested for recognition and comprehension across a variety of cultures
and literacy levels. The interviewees answered questions using a rating
scale, which was subjected to a statistical analysis to determine the
significance of the responses. The authors concluded that "the labels
with added pictograms were perceived by pesticide users as
significantly easier to obtain information from than labels containing
text only" (Document ID 0480.6).
Stakeholders on the whole supported the inclusion of pictograms on
the labels of hazardous substances. During the hearings, Chris Trahan
of the AFL-CIO voiced support for including pictograms on the labels of
hazardous chemicals, and cited construction workers as a group whose
safety and health conditions would be greatly improved by OSHA's
adoption of "a system of symbols [workers] can then readily use to
make decisions on a daily basis" (Document ID 0494 Tr. 8).
As discussed in the proposal, a considerable amount of evidence
shows that pictograms can serve as useful and effective communication
tools. In the final rule, OSHA has decided to adopt the eight GHS
pictograms initially proposed in the NPRM. Each of these pictograms
consists of a different symbol in black on a white background within a
red square frame set on a point (i.e., a red diamond). The specific
pictograms that are required on a particular label are to be determined
based on the hazard classification of the substance in question.
OSHA finds, based on scores of supporting studies and persuasive
testimony that the pictograms will make warnings on labels more
noticeable and easier for employees to understand. In particular,
symbols will improve comprehension among people with low literacy
levels and those who are not literate in the English language.
Moreover, pictograms will be used not only in conjunction with other
label elements, but also in the context of the hazard communication
program as a whole. Training that includes an explanation of labels
(included in the final rule) will ensure that the pictograms are
understood by employees.
Red Borders
GHS allows regulatory authorities the option of permitting black
pictogram borders for labels on domestic products, and in the proposal
OSHA requested comment on this issue. Mandating the use of red borders
was supported by stakeholders, who argued persuasively that red borders
would make labels more noticeable and would make the warnings appear to
be more important (Document ID 0339, 0341, 0365, 0383, 0408,
0410, 0412, and 0456). The National Association of Chemical
Distributers, in supporting the use of red borders, reasoned that they
would be consistent with the overall goal of the
GHS (Document ID 0341). Additionally, the AIHA stated that
requiring red borders would promote the safe use of chemicals (Document
ID 0365).
Several commenters raised economic concerns, suggesting that
because red ink is more expensive, the use of black borders should be
permitted (Document ID 0318, 0328, 0370, 0377, 0382, 0393, and
0411). Dow Chemical, Troy Corporation, and several other commenters
recommended that red borders should only be required on products that
were being exported (Document ID 0352, 0353, 0399, 0405, and
0389). Similarly, API argued that in order to remain consistent with
the GHS, OSHA should only require exported chemicals to have a red
border (Document ID 0376).
OSHA finds this argument to be unpersuasive. In order to reap the
benefits of consistency in warnings, labels must have a degree of
sameness and that includes the colors used. Moreover, OSHA analyzed the
impact that the use of red borders would have on production costs.
While the use of red borders may increase the cost of printing, OSHA
has determined that the cost does not render the rule infeasible. This
issue is discussed in greater detail in Section VI. Finally, the GHS
does not even state a preference for black borders on labels of
domestic products; it simply gives the competent authority discretion
to allow black borders when the product will not enter into
international commence.
Numerous studies have found that substantial benefits exist when
color is used on labels. Due to the extensive amount of information
that needs to be displayed, warning labels can become cluttered.
Swindell found that searching for needed information on a cluttered
label is very challenging for the user (Swindell, 1999, Document ID
0284). Her study concluded that minor changes to an extensive
warning label, such as the addition of color, can greatly improve the
noticeability of the warning, grab the attention of the user faster,
and produce quicker reaction times.
Swindell also researched the effect that different colors (red,
blue, and black) had on the time it took users to locate and respond to
a warning. Red was perceived to indicate the highest degree of hazard
and was shown to increase the perceived hazard of a word presented in
that color (e.g., DANGER in blue is perceived as less hazardous than
WARNING in red).
Swindell's findings echo the results reported by Laughery et al.,
who found that alcoholic beverage labels were located significantly
faster when the text was red instead of black (Laughery et al., 1993,
Document ID 0281). These studies involve color on label
elements other than the pictogram borders, but the presence of color
and the particular color is germane to the red borders of labels.
The primacy of red as an understandable color denoting danger is
also supported by these studies.
Smith-Jackson and Wogalter asked English-speaking
community members to rate the perceived hazard of ten ANSI safety
colors (Smith-Jackson and Wogalter, 2000, Document ID 0196).
Red, yellow, black, and orange were rated the highest (in descending
order). Differences were statistically significant except the
difference between yellow and black.
Among 80 college students asked to rate colors by Griffith
and Leonard, red was rated the most "meaningful" color (i.e., most
distinct in meaning from neutral gray), followed by green, orange,
black, white, blue, and yellow (Griffith and Leonard, 1997, Document ID
0250).
Wogalter et al. asked Spanish speakers to rank the
perceived hazard of ANSI safety colors (Wogalter et al., 1997b,
Document ID 0266). Red was ranked highest, followed by orange,
black, and yellow.
Dunlap et al. surveyed 1169 subjects across several
different language groups including English, German, and Spanish
speakers (Dunlap et al., 1986, Document ID 0191). Subjects
rated the color words red, orange, yellow, blue, green, and white
according to the level of perceived hazard. The results demonstrated
that the hazard information communicated by different colors followed a
consistent pattern across language groups, with red having the highest
hazard ratings.
Wogalter et al. asked undergraduates and community
volunteers to rank various warning components (Wogalter et al., 1998,
Document ID 0286). Red connoted a significantly greater hazard
than other colors, followed by yellow, orange, and black (in that
order). A group of industrial workers ranked the colors from greatest
to least hazard as follows: red, yellow, black, orange.
London asked workers in four sectors in South Africa to
rank the colors red, yellow, green, and blue in terns of perceived
hazard; 95 percent said red represents the greatest hazard, and 58
percent said yellow is the second greatest hazard (London, 2003;
Document ID 0311).
Banda and Sichilongo asked workers in Zambia to rate the
perceived hazard of various colors used in chemical labels (Banda and
Sichilongo, 2006, Document ID 0237). Red was associated with
the greatest hazard, followed by yellow.
Among a sample of 30 undergraduates who rated the
perceived hazard of 105 signal word/color combinations, Braun et al.
reported that red conveyed the highest level of perceived hazard
followed by orange, black, green, and blue (Braun et al., 1994,
Document ID 0298).
These reports are consistent in showing that red is commonly
understood to be associated with a high level of hazard--the highest of
any color.
After reviewing stakeholder comments and studies investigating the
benefits of using the color red to signal a hazard, OSHA has decided to
require all pictograms to have red borders. OSHA finds that these
labels will be more effective in communicating hazards to employees--
both by drawing the attention of employees to the label and by
indicating the presence of a hazard through non-verbal means.
Consistently applying red borders to all labels, regardless of the
final destination, will ensure that workers are protected. OSHA has
determined that red pictogram borders will maximize recognition of the
warning label and ensure consistency; therefore the final rule requires
red borders for both domestic and international labeling.
Blank Diamonds
The final rule requires that all red diamonds printed on a label
have one of the eight pictograms printed inside the diamond. The
prohibition of blank diamonds on labels will ensure that users do not
get desensitized to warnings placed on labels. Two commenters proposed
alternatives to the prohibition of blank diamonds. The American
Chemical Council (ACC) suggested that, because the red diamond border
for pictograms are often pre-printed on shipping labels, OSHA allow
printing the word "BLANK" on, or writing "pictogram intentionally
left blank" in, the unused diamond (Document ID 0393).
Additionally, Michelle Sullivan also suggested writing "intentionally
left blank" in the empty diamonds (Document ID 0382).
OSHA acknowledges that prohibiting blank diamonds on labels may
require an adjustment in practice for entities that use pre-printed
labels or require businesses to inventory additional blank stock. OSHA
analyzed the impact that prohibiting the use of blank diamonds on
labels would have on production costs. While this requirement may
increase costs associated with labeling, OSHA has determined that the
costs do not render the rule infeasible. This issue is discussed in
greater detail in Section VI.
Including diamonds on labels only when a pictogram is required will
ensure that such warnings stand out to users. Prohibiting the use of
blank diamonds will improve the likelihood that users will notice and
react to the warning on the label. Therefore, OSHA has determined that
prohibiting the use of blank diamonds on labels is necessary to provide
the maximum recognition and impact of warning labels and to ensure that
users do not get desensitized to the warnings placed on labels.
Hazard Statements and Precautionary Statements
Hazard statements describe the hazards associated with a chemical.
Precautionary statements describe recommended measures that should be
taken to protect against hazardous exposures, or improper storage or
handling of a chemical. This revised rule replaces the current
performance-oriented requirement for "appropriate hazard warnings" on
labels with a requirement for specific hazard and precautionary
statements on labels. The statements are prescribed, based on the
hazard classification of the chemical.
Standardized requirements for hazard and precautionary statements
provide a degree of consistency that is lacking among current chemical
labels. This lack of consistency among current labels makes it
difficult for users to understand the nature and degree of hazard
associated with a chemical, and to compare chemical hazards. For
example, in an article reviewed for the record, Dr. Beach relates
experiences from the perspective of a doctor treating occupationally
exposed patients (Beach, 2002, Document ID 0238). The author
noted that different suppliers use different risk phrases for the same
chemical, making it difficult for users to compare relative risks.
ANSI standard Z129.1, Hazardous Industrial Chemicals--Precautionary
Labeling (Document ID 0610), was developed to provide a
consistent approach to labeling of hazardous chemicals. This standard
gives manufacturers and importers guidance on how to provide
information on a label, including standardized phrases and other
information that can improve the quality of labels. Because it is a
voluntary standard, however, not all chemical manufacturers and
importers have adopted the ANSI approach. As a result of the diverse
formats and language used in the past, a consistent and understandable
presentation of information was not fully achieved.
A preference for hazard statements was shown in EPA's Consumer
Labeling Initiative (Abt Associates, 1999, Document ID 0209).
This study asked consumers about their attitudes toward labels on
household chemical products. Overall, consumers indicated that they
like to have information that clearly connects consequences with
actions, and they prefer to know why they are being instructed to take
a particular precaution. A clear hazard statement provides this
information.
In some cases, clear and concise precautionary information is
necessary to enable employees to identify appropriate protective
measures. For example, Frantz et al. examined the impact of flame and
poison warning symbols prescribed in certain regulations by the
Canadian government (Frantz et al., 1994, Document ID 0191).
The results suggest that although the generic meanings of these two
symbols are well understood, people may have difficulty inferring the
specific safety precautions necessary for a particular product.
Other reports indicate that users prefer information that includes
both an indication of the hazard and the recommended action (i.e., the
precautionary statement). Braun et al. examined statements in product
instructions for a pool treatment chemical and a polyvinyl chloride
(PVC) adhesive, asking subjects to rate the injury risk posed by each
product (Braun et al., 1995, Document ID 0246). The
experimenters manipulated the instructions to include either
recommended actions only, actions followed by consequences,
consequences followed by actions, or a simple restatement of the
product label. The authors found that actions paired with consequences
led to significantly higher risk perception than a restatement of the
label or actions alone. Although the preferred wording was longer than
the alternatives, subjects did not feel that the instructions were too
complex, suggesting that they appreciate having actions and
consequences paired together. Freeman echoed these findings in a
discussion on communicating health risks to fishermen and farmers,
noting that to be useful, risk statements should be balanced with
equally strong statements of ways to reduce or avoid the risk (Freeman,
2001, Document ID 0249).
Explicit precautionary statements make it more likely that
employees will take appropriate precautions. Bowles et al. asked
subjects to review product warnings, then either decide what actions
they should take or evaluate whether someone else's actions were safe,
based on the warning (Bowles et al., 2002, Document ID 0246).
In general, situations that required the user to make inferences about
a hazard--particularly when they had to come up with their own ideas
for protective actions--led to decreased intent to comply. By providing
clear precautionary instructions on the label, the revised rule
eliminates the need for users to infer protective actions.
Evidence indicates that using key label elements together improves
warning performance, compared with labels that only contain a subset of
these elements. This is the approach taken in the revised rule, which
requires the signal word, pictogram(s), hazard statement(s), and
precautionary statement(s) together on the label. In one study,
Meingast asked students to recall information from two variations of
warning labels: Enhanced warnings with color, signal icons, pictorials,
and organized text (following the ANSI Z535.4 standard, American
National Standard for Product Safety Signs and Labels); and warnings
with text only (Meingast, 2001, Document ID 0246). The authors
reported that the enhanced warnings were more noticeable, led to
significantly greater recall, and made people report a higher
likelihood of compliance.
Other findings agree that improving all label elements can improve
warning performance. For example, Lehto tested information retrieval
from three chemical label formats and found that subjects generally did
best with an "extensive" format that included pictograms, paragraphs,
and horizontal bars indicating the degree of hazard (Lehto, 1998,
Document ID 0258). Subjects were able to answer more questions
correctly when the label included a range of content--particularly
information on first aid and spill procedures.
Wogalter et al. reported similar results in a test of four
different signs that discouraged people from using an elevator for
short trips (Wogalter et al., 1997a, Document ID 0287). Three
signs were text-only. The fourth sign had a signal word panel, icons, a
pictorial, and more explicit wording indicating the desired behavior
(i.e., "use the stairs"). Subjects rated the enhanced sign as more
understandable, and a field test found that it significantly increased
compliance over the other options.
The effectiveness of a combination of elements was also
investigated in a study of warnings on alcoholic beverage containers
(Laughery et al., 1993, Document ID 0281). Laughery et al. tested warnings to
determine which elements influenced notice ability. The authors
manipulated labels by adding a pictorial, adding an alert symbol with a
signal word, making the text red, and/or adding a border around the
warning. The warning was located fastest when all four of these
modifications were present, suggesting that the best designs include a
combination of enhancements.
The findings of these reports support OSHA's belief that the
combined label elements, i.e., pictogram, signal word, hazard and
precautionary statements, is more effective in communicating hazard
information than the individual elements would be if presented alone.
Although the warnings examined in these studies are different than
those warnings required in this final rule, they indicate that
enhancements such as color and symbols can increase the effectiveness
of a label, and that presenting hazard information and corresponding
precautions together improves understanding.
Overall, the record shows that the presentation of information on
labels through standardized signal words, hazard statements,
pictograms, and precautionary statements would provide clearer, more
consistent, and more complete information to chemical users. Comments
received in response to the ANPR support this view (e.g., Document ID
0032, 0054, 0124, and 0158). For example, the Refractory
Ceramic Fibers Coalition (Document ID 0030) pointed to the
benefits of this approach, stating:
Employers and employees would be given the same information on a
chemical regardless of the supplier. This consistency should improve
communication of the hazards. It may also improve communication for
those who are not functionally literate, or who are not literate in
the language written on the label. In addition, having the core
information developed already, translated into multiple languages,
and readily available to whomever wishes to access it, should
eliminate the burden on manufacturers and users to develop and
maintain their own such systems. Thus the specification approach
should be beneficial both to the producers and the users of
chemicals.
The majority of comments received in response to the proposal
support the use of hazard and precautionary statements on labels (See,
e.g., Document ID 0313, 0324, 0327, 0328, 0329, 0330, 0335,
0336, 0338, 0339, 0344, 0347, 0349, 0351, 0352, 0353, 0365, 0370, 0372,
0376, 0377, 0379, 0381, 0382, 0383, 0389, 0393, 0399, 0402, 0405, 0408,
0410, 0412, 0453, 0456, and 0461). No comments or testimony were
received that opposed the use of hazard or precautionary statements on
labels or safety data sheets.
In response to the proposal, stakeholders commented on the
importance of being able to comprehend hazard and precautionary
statements (See, e.g., Document ID 0321, 0339, 0349, 0410, and
0412). Morganite Industries, Inc. and Morgan Technical Ceramics USA
stated: "Hazard Statements, by and large, convey fact in simple
language" (Document ID 0321). Commenting on the use of
precautionary statements, the Phylmar Group noted that "clear, concise
use of key labeling elements can improve warning performance"
(Document ID 0339). The American Industrial Hygiene
Association also supports the use of precautionary statements, stating
that they "should improve comprehensibility and compliance" (Document
ID 0410).
Labels are intended to provide an immediate visual reminder of
chemical hazards. Whereas labels in the past could be presented in a
variety of formats using inconsistent terminology and visual elements,
labels prepared in accordance with the requirements in this final rule
will be consistent. Standardized signal words and hazard statements
attract attention and communicate the degree of hazard. Pictograms
reinforce the message presented in text and enhance communication for
low-literacy populations. Precautionary statements provide useful
instructions for protecting against chemical-source injuries and
illnesses.
A number of stakeholders submitted comments in support of
standardized labeling for hazardous chemical containers. Several
commenters stated that standardized label elements would better convey
critically important hazard warnings, and provide useful information
regarding precautionary measures that would serve to better protect
employees (Document ID 0313, 0341, 0344, 0365, 0381, 0382,
0402, and 0405). The studies contained in the record reinforce OSHA's
position on the use standardized label elements--including the use of
standardized pictograms, signal words, and hazard and precautionary
statements--to alert and inform chemical users of the hazards posed by
hazardous chemicals in the workplace.
OSHA concludes, based on the studies discussed above and supported
by the comments submitted to the record that standardizing the labels
for hazardous chemicals is an essential step in harmonizing the HCS
with the GHS. In addition, OSHA concludes that the labeling
requirements in this revised final rule will result in more effective
transmittal of information to employees. Therefore, OSHA has adopted
the labeling requirements set forth in the NPRM in this final rule.
Safety Data Sheets
The HCS requires chemical manufacturers and importers to develop an
SDS for each hazardous chemical they produce or import. SDSs serve as a
source of detailed information on chemical hazards and protective
measures. Each SDS must indicate the identity of the chemical used on
the label; the chemical and common name(s) of hazardous ingredients;
physical and chemical characteristics; physical and health hazards; the
primary route(s) of entry; exposure limits; generally applicable
precautions for safe handling and use; generally applicable control
measures; emergency and first aid procedures; the date of preparation
of the SDS; and the name, address and telephone number of the party
preparing or distributing the SDS. Prior to this final standard, the
information was not required to be presented in any particular order or
to follow a specific format.
While the effectiveness of SDSs is evident, there are concerns
regarding the quality of information provided. In particular, concerns
have been raised regarding the accuracy (i.e., the correctness and
completeness of the information provided) and comprehensibility (i.e.,
the ability of users to understand the information presented) of
information provided on SDSs. In the NPRM, OSHA proposed requiring the
information on SDSs to be presented using consistent headings in the
sequence specified in the GHS (See Section XV for a detailed discussion
of the requirements). The Agency has determined that a standardized
order of information will improve the utility of SDSs by making it
easier for users to locate and understand the information they are
seeking. A standardized format is also expected to improve the accuracy
of the information presented on SDSs.
Since the HCS was promulgated in 1983, access to chemical
information has improved dramatically due to the availability of SDSs.
OSHA believes that adopting a standardized format will build on the
demonstrated benefits that have already clearly been established from
the use of SDSs. As discussed in the proposal, the General Accounting
Office (GAO) issued a report in May 1992 that addressed issues
employers had with complying with the HCS (GAO, 1992, Document ID
0292). The findings were based on the results of a national
survey of construction, manufacturing, and personal services
providers. A total of 1,120 responses were received from employers.
One very important finding of the GAO survey was that almost 30% of
employers reported that they had replaced a hazardous chemical with a
less hazardous substitute because of information presented on an SDS.
With regard to the HCS as a whole, GAO found that over 56% of employers
reported "great" or "very great" improvement in the availability of
hazard information in the workplace and in management's awareness of
workplace hazards. Forty-five percent of those in compliance with the
HCS considered the standard to have a positive effect on employees,
compared with only 9% who viewed the effect as negative. The results
indicate that when chemical hazard information is provided, the result
is generally recognized as beneficial to employees. A number of other
studies support this conclusion.
Conklin demonstrated the utility of SDSs among employees of a
multinational petrochemical company (Conklin, 2003; Document ID
0245). Across three countries (the U.S., Canada, and the
United Kingdom), 98 percent felt that the SDS is a satisfactory
information source (the percentage was similar across all three
countries). Seventy-two percent said they would request an SDS all or
most of the time when introduced to a new chemical, although 46 percent
of workers said that SDSs are too long. The author notes that this
sample did not include any workers with low literacy.
However, while these studies show a clear benefit related to the
use of SDS in the workplace, a number of investigations raise concerns
that the information on SDSs is not comprehensible to employees. In
1991, OSHA commissioned a study that evaluated the comprehensibility of
SDSs by a group of unionized employees in manufacturing industries
located in the state of Maryland (Kearney/Centaur, 1991a, 1991b,
Document ID 0309 and 0310). The study assessed the ability of
these employees to understand information regarding the route of entry
of the substance, the type of health hazard present, appropriate
protective measures, and sources of additional help.
Each of the 91 participating workers was provided with and tested
on four different SDSs. The workers answered the test questions based
on information supplied on each of the SDSs. It should be noted that
the employees who volunteered for this study understood that it relied
on reading comprehension. This created a selection bias, as employees
with reading difficulties would not be likely to volunteer for the
study.
The results of the tests indicated that workers on average
understood about two-thirds of the health and safety information on the
SDSs. The best comprehension was associated with information providing
straightforward procedures to follow (e.g., in furnishing first aid,
dealing with a fire, or in using personal protective equipment) or
descriptions of how a chemical substance can enter the body. Workers
had greater difficulty understanding health information addressing
different target organs, particularly when more technical language was
used. Workers also reportedly had difficulty distinguishing acute from
chronic effects based on information presented in the SDSs.
Conklin reported a similar result in a study involving employees of
a multinational petrochemical company (Conklin, 2003, Document ID
0245). After viewing information on an unfamiliar chemical in
a variety of SDS formats, a questionnaire was administered to workers
to gauge their comprehension of the material presented. The workers
reportedly answered 65 percent of the questions correctly.
The Printing Industries of America reported a study that examined
the comprehensibility of SDSs to master printers in 1990 (PIA, 1990,
Document ID 0295). The subjects had an average of 13.9 years
of formal education, or approximately two years beyond high school. In
this study, 27 SDSs were selected and analyzed for reading levels using
a software program, finding an average reading grade level of 14. The
investigators found that employees with 15 years of education or more
understood 66.2% of the information presented.
Some of the difficulty workers experience in understanding
information presented on SDSs may be due to the vocabulary used in the
document. Information presented at a reading level that exceeds the
capability of the user is unlikely to be well understood. An example of
this situation was reported by Frazier et al. (Frazier et al., 2001,
Document ID 0212). The authors evaluated a sample of SDSs from
30 manufacturers of toluene diisocyanate, a chemical known to cause
asthma. Half of the SDSs indicated that asthma was a potential health
effect. One SDS made no mention of any respiratory effects, while
others used language (e.g., allergic respiratory sensitization) that
the authors believed may not clearly communicate that asthma is a risk.
However, the more technical language meets the requirements of the HCS.
Other reports substantiate the belief that many SDS users have
difficulty understanding the information on the documents. For example,
in a study evaluating the comprehensibility of SDSs at a large research
laboratory, 39 percent of the workers found SDSs "difficult to
understand" (Phillips, 1997, Document ID 0263). The study
also indicated that a third of the information provided on SDSs was not
understood. These results were obtained from a study population of
literate, trained workers who spoke English as their first language.
Smith-Jackson and Wogalter corroborated this finding in a study
involving 60 undergraduates and community volunteers (Smith-Jackson and
Wogalter, 1998, Document ID 0188). The subjects were asked to
sort SDS data into a logical order. After completing the task, subjects
were asked for their opinions on the difficulty of the content.
Overall, 43 percent found the information easy to understand, 42
percent said it was not easy, and the remaining 15 percent felt that
only scientists, experts, or very experienced workers would be able to
understand the information.
These studies are consistent in reporting that workers have
difficulty understanding a substantial portion of the information
presented on SDSs. This finding can be explained at least in part by
the fact that not all of the information on SDSs is intended for
workers. SDSs are intended to provide detailed technical information on
a hazardous chemical. While they serve as a reference source for
exposed employees, SDSs are meant for other audiences as well. SDSs
provide information for the benefit of emergency responders, industrial
hygienists, safety professionals, and health care providers. Much of
this information may be of a technical nature and would not be readily
understood by individuals who do not have training or experience in
these areas. For example, language that may be readily understood by a
population of firefighters may be poorly understood by chemical
workers.
In addition, Title III of the Superfund Amendments and
Reauthorization Act (SARA, also known as the Emergency Response and
Community Right-to-Know Act of 1986) mandated that SDSs be made
available to state emergency response commissions, local emergency
planning committees, and fire departments in order to assist in
planning and response to emergencies, as well as to provide members of
the general public with information about chemicals used in their
communities. It is difficult, if not impossible, for a document to meet
the informational needs of all of these audiences while being
comprehensible to all as well.
Product liability concerns also play a role in the
comprehensibility of SDSs. Producers of chemicals may be subject to
"failure to warn" lawsuits that can have significant financial
implications. Attempts to protect themselves against lawsuits can
affect the length and complexity of SDSs, as well as the way in which
information is presented. In some cases the length and complexity of
SDSs reportedly make it difficult to locate desired information on the
documents. For example, in testimony before the U.S. Senate
Subcommittee on Employment, Safety, and Training, one hospital safety
director described a situation in which an employee was unable to find
critical information on an SDS in an emergency situation (Hanson, 2004,
Document ID 0200):
* * * two gallons of the chemical xylene spilled in the lab of
my hospital. By the time an employee had noticed the spill, the
ventilation had already sucked most of the vapors into the HVAC.
This, in turn, became suspended in the ceiling tile over our
radiology department. Twelve employees were sent to the emergency
room. To make the matter worse, the lab employee was frantically
searching through the MSDS binder in her area for the xylene MSDS.
Once she found it, she had difficulty locating the spill response
section. After notifying our engineering department, she began to
clean up the spill with solid waste rags, known for spontaneous
combustion, and placing the rags into a clear plastic bag for
disposal. She did not know that xylene has a flash point of 75
degrees Fahrenheit. She then walked the bag down to our incinerator
room and left it there, basically creating a live bomb. Twelve
people were treated from this exposure. The lab employee was very
upset and concerned about the safety of the affected employees and
visitors, and hysterically kept stating that she could not find the
necessary spill response information.
SDSs at this particular hospital were reported to range from one page
to 65 pages in length.
To accommodate the needs of the diverse groups who rely on SDSs, a
standardized format has been viewed as a way to make the information on
SDSs easier for users to find, and to segregate technical sections of
the document from more basic elements. A standardized format was also
thought to facilitate computerized information retrieval systems and to
simplify employee training.
The first attempt to establish a format for SDS was made in 1985,
when OSHA established a voluntary format to assist manufacturers and
importers who desired some guidance in organizing SDS information. This
two-page form (OSHA Form 174) includes spaces for each of the items
included in the SDS requirements of the standard, to be filled in with
the appropriate information as determined by the manufacturer or
importer. However, some members of the regulated community desired a
more comprehensive, structured approach for developing clear, complete,
and consistent SDSs.
In order to develop this structure, the Chemical Manufacturers
Association (now known as the American Chemistry Council) formed a
committee to establish guidelines for the preparation of SDSs. This
effort resulted in the development of American National Standards
Institute (ANSI) standard Z400.1, a voluntary consensus standard for
the preparation of SDSs. Employers, workers, health care professionals,
emergency responders, and other SDS users participated in the
development process. The standard established a 16-section format for
presenting information as well as standardized headings for sections of
the SDS. In 2004, an updated version of the ANSI standard that was
consistent with the GHS format was published. This ANSI standard has
since been combined with the ANSI Z129 consensus standard on
precautionary labeling preparation. The ANSI Z400.1/Z129.1 standard was
issued in 2010.
By following the recommended format, the information of greatest
concern to employees is featured at the beginning of the document,
including information on ingredients and first aid measures. More
technical information that addresses topics such as the physical and
chemical properties of the material and toxicological data appears
later in the document. The ANSI standard also includes guidance on the
appearance and reading level of the text in order to provide a document
that can be easily understood by readers.
OSHA currently allows the ANSI format to be used as long as the SDS
includes all of the information required by the HCS. Because it is a
voluntary standard, however, the ANSI format has not been adopted by
all chemical manufacturers and importers. As a result, different
formats are still used on many SDSs.
The International Organization for Standardization (ISO) has
published its own standard for SDS preparation. This standard, ISO
11014-1, has been revised for consistency with the GHS (new version
issued in 2009). The standard includes the same 16 sections as the GHS,
as well as similar data requirements in each section. These two
consensus standards, ANSI Z400.1-2004 and ISO 11014-1 (2009), have
essentially the same provisions and are consistent with GHS. There are
minor differences, such as units of measure recommended in the national
ANSI standard versus the international ISO standard.
Another development has been the creation of International Chemical
Safety Cards (ICSCs). The documents, developed by the International
Programme on Chemical Safety, summarize essential health and safety
information on chemicals for use at the "shop floor" level by workers
and employers (Niemeier, 1997, Document ID 0191). ICSCs are
intended to present information in a concise and simple manner, and
they follow a standardized format that is shorter (one double-sided
page) and less complex than the ANSI approach. The ICSCs were field
tested in their initial stages of development, and new ICSCs are
verified and peer reviewed by internationally recognized experts (id.).
ICSCs have been developed in English for 1,646 chemicals, and are also
available in 16 other languages. The ICSCs are being updated to be
consistent with the GHS.
A study by Phillips compared the effectiveness of different SDS
formats as well as ICSCs among workers at a large national laboratory
(Phillips, 1997, Document ID 0191). The employees represented
a variety of trades, including painters, carpenters, truck drivers, and
general laborers. Each worker was tested for knowledge regarding a
hazardous chemical before and after viewing an SDS or ICSC. Three
designs were tested: a 9-section OSHA form, the 16-section ANSI Z400.1
format (an earlier and slightly different version of the current ANSI
Z400.1 format), and the 9-section ICSC. A subsequent paper described
the final results of this study (Phillips, 1999, Document ID
0263). All three formats led to significant improvements in
subjects' knowledge, and there was no statistically significant
difference among the three formats in terms of total test score.
However, there were a few significant differences in how well readers
of each SDS format answered specific types of questions:
[ssquf] The ICSC performed better than the OSHA form regarding
chronic and immediate health effects.
[ssquf] The other two formats performed better than the ANSI format
on fire-related questions.
[ssquf] The OSHA form performed better than the other two formats
on spill response questions.
[ssquf] The OSHA form performed better than the ANSI format
regarding carcinogenic potential.
The ANSI Z400.1 template has been used by a wide number of
employers for creating SDSs. By following the recommended format, the
information of greatest concern to employees is featured at the
beginning of the document, including information on ingredients and
first aid measures. More technical information that addresses topics
such as the physical and chemical properties of the material and
toxicological data appears later in the document. The ANSI standard
also includes guidance on the appearance and reading level of the text
in order to provide a document that can be easily understood by
readers.
The ANSI format is commonly used. However, because it is a
voluntary standard, not all chemical manufacturers and importers have
adopted it. As a result, different formats are still used on many SDSs.
Of the comments received regarding SDS, none were in favor of allowing
voluntary adoption of the SDS format. The California Industrial Hygiene
Council (CIHC) (Document ID 0463) reiterated its support for a
uniform format, and specifically the implementation of the ANSI format
for SDSs. The CIHC also stated that a mandatory format would establish
a harmonized structure for all "global target audiences" (Document ID
0463).
In a separate comparison, Conklin also found similarities in the
overall performance of several standard SDS formats (Conklin, 2003,
Document ID 0245). In this study, employees of a multinational
petrochemical company were given one of three versions of an SDS for an
unfamiliar chemical: A U.S. version (OSHA's required content within an
ANSI Z400.1-1998 16-part structure); a Canadian version following the
9-part structure prescribed by Canada's Workplace Hazardous Materials
Information System (WHMIS); and a version following the European
Union's content and 16-part structure. SDSs were controlled for font,
layout, and reading level. Overall, Conklin found no statistically
significant difference in mean post-test scores using the three
different formats, although there were significant differences on 5 out
of 10 questions (no one format was consistently better).
OSHA also examined several studies addressing what sequence of
information would prove to be most beneficial for users. Because
extensive searching can be a barrier to SDS use, researchers have
examined whether there is a preferred order of information that more
closely matches users' cognitive expectations. Smith-Jackson and
Wogalter asked 60 undergraduates and community volunteers to arrange
portions of six SDSs in the order they considered most usable (Smith-
Jackson and Wogalter, 1998; Document ID 0188). The authors
found a few consistent results:
[ssquf] Information about health hazards, protective equipment, and
fire and explosion data tended to be placed toward the beginning.
[ssquf] Physical and reactivity data tended to be placed near the
end.
[ssquf] Spill or leak procedures were placed near the beginning or
the middle, depending on the type of chemical.
A majority of subjects reported that they had attempted to
prioritize the hazard information that needed to be communicated. The
participants' suggested order of information generally did not match
either the original SDS order or the order listed in the HCS--
particularly the subjects' emphasis on health hazard information near
the beginning.
In the previously discussed 1991 study that evaluated the
comprehensibility of SDSs by a group of 91 unionized workers in
manufacturing industries in the state of Maryland, a subset of the
group (18 workers) was also tested on an ICSC (Kearney/Centaur, 1991a,
1991b, Document ID 0309 and 0310). While the results indicated
that workers on average understood about two-thirds of the health and
safety information on SDSs, ICSCs provided better results. The average
ICSC test score ranged from 6% to 23% higher than the average test
score on the four SDSs evaluated. This finding was considered by the
authors to suggest that an improved format for SDSs may serve to
increase user comprehension of the information presented.
OSHA believes that a standardized format will improve the
effectiveness of SDSs for the following reasons: A consistent format
makes it easier for users to find information on an SDS. Headings for
SDS sections are standardized, so SDS users know which section to
consult for the information they desire. The sections are presented in
a consistent, logical sequence to further facilitate locating
information of interest. Information commonly desired by exposed
employees and of greatest interest to emergency responders (e.g.,
Hazards Identification; First Aid Measures) is presented in the
beginning of the document for easy reference. More technical
information (e.g., Stability and Reactivity; Toxicological Information)
is presented later.
Specifically, the revised SDS format now segregate more complex
information from information that is generally easier to understand.
This order of information places basic information in the first
sections, allowing SDS users to find basic information about hazardous
chemicals without having to sift through a great deal of technical
information that may have little meaning to them. In emergency
situations, rapid access to information such as first-aid measures,
fire-fighting measures, and accidental release measures can be
critically important.
Several stakeholders expressed dissatisfaction with the degree that
current SDSs vary from manufacturer to manufacturer (Document ID
0330 and 0351). The International Brotherhood of Teamsters
stated that the quality and usefulness of SDSs has been grossly
inconsistent in terms of content and format, adding that such
discrepancies ultimately result "in a failure to achieve the objective
of the standard" (Document ID 0357). John Schriefer, head of
Local 9477, indicated that workers often didn't bother to request SDSs,
because they are so complicated (Document ID 0494 Tr. 54-55).
He suggested that a simplified, standard format for SDSs would go a
long way toward improving worker safety (Document ID 0494 Tr.
63).
Commenters supported putting information targeted to the employees
first on the SDS in order to improve how emergency situations are
addressed (Document ID 0332, 0386 and 0414). Stericycle, Inc.
supported placing hazard identification information in one location
rather than "sprinkling it through the documents, as is sometimes the
case with [SDSs]" (Document ID 0338). United Steelworkers
stated that the difficulty in locating information on current SDSs "is
bad enough with routine assessments, but in an emergency situation like
a spill, splash or fire it can be deadly" (Document ID 0402).
Additionally, the American Wind Energy Association argued that
requiring hazard identification and first aid information to be placed
in the first sections of the SDS would serve to "better assist
emergency response teams to more efficiently recognize hazards during
incidents" (Document ID 0386). American Federation of State,
County and Municipal Employees (AFSCME) also supported the adoption of
a standardized SDS, reasoning that it would enable workers to better
understand SDSs, and could ultimately lead to faster responses as well
as a reduction in the number of incidents altogether (Document ID
0386).
A standardized format does not address all issues affecting SDS
comprehensibility. Reading level and some design elements would
continue to vary. In many respects, this is inevitable given the
different target audiences that SDSs have, and the varying
qualifications of those who prepare SDSs. Nevertheless, OSHA believes
that the revisions will result in a substantial improvement in the
quality and ease of comprehension of information provided on SDSs.
In addition to the issues regarding comprehensibility, researchers
raised concerns that some SDSs may be incomplete or contain erroneous
information. The magnitude of the problem is unclear, because only very
limited numbers of SDSs have been evaluated in these studies, and in
some cases the investigations were performed so long ago that the
results may not reflect current practices. Nevertheless, the evidence
appears to indicate that a substantial number of SDSs may not contain
complete and correct information.
An initial examination of the accuracy of SDSs was commissioned by
OSHA shortly after the scope of the rule was expanded to cover all
industries in 1987 (Karstadt, 1988, Document ID 0296). The
report, which analyzed the content of 196 SDSs for products used in
auto repair and body shops, provided a general indication that the
content and presentation of information was inconsistent on the SDSs
examined. In 1991, OSHA commissioned an additional study that examined
the accuracy of SDSs (Kearney/Centaur, 1991a, 1991b, Document ID
0309 and 0310). The study examined information presented in
five areas considered crucial to the health of workers potentially
exposed to hazardous substances. The five areas assessed were: Chemical
identification of ingredients; reported health effects of ingredients;
recommended first aid procedures; use of personal protective equipment;
and exposure level regulations and guidelines. The evaluation indicated
that 37% of the SDSs examined accurately identified health effects
data, 76% provided complete and correct first aid procedures, 47%
accurately identified proper personal protective equipment, and 47%
correctly noted all relevant occupational exposure limits. Only 11% of
the SDSs were accurate in all four information areas, but more (51%)
were judged accurate, or considered to include both accurate and
partially accurate information, than were judged inaccurate (10%). The
study also concluded that the more recent SDSs examined (those prepared
between 1988 and 1990) appeared to be more accurate than those prepared
earlier.
This belief that some SDSs are not complete and correct was
corroborated by an examination of SDSs for lead and ethylene glycol
ethers (Paul and Kurtz, 1994, Document ID 0302). Although
these substances are known reproductive and developmental toxicants,
researchers found that 421 of 678 SDSs examined (62%) made no mention
of effects on the reproductive system. OSHA also commissioned a study,
completed in 1999, focusing specifically on the accuracy of first aid
information provided on SDSs (Lexington Group, 1999, Document ID
0257). A total of 56 SDSs for seven chemicals were examined.
First aid information on the SDSs was compared with information from
established references. The researchers reported that nearly all of the
SDSs reviewed had at least minor inaccuracies.
A standardized format does not directly address the concerns that
have been raised regarding the accuracy of information present on SDSs.
However, standardization would improve the accuracy of chemical hazard
information indirectly. With consistent presentation of information,
the task of reviewing SDSs and labels to ensure accuracy will be
simplified. Individuals preparing and reviewing these documents should
find it easier to identify any missing elements and compare information
presented on an SDS to reference sources and other SDSs. OSHA
enforcement personnel will be able to more efficiently examine SDSs
when conducting inspections. The detailed entries for SDSs are
particularly noteworthy in this regard. The sub-headings provide an
organized and detailed list of pertinent information to be included
under the headings on the SDS. For example, while the HCS currently
requires physical and chemical characteristics of a hazardous chemical
to be included on the SDS, the final rule provides a list of 18
properties for Section 9 of the SDS. The party preparing the SDS must
either include the relevant information for these entries, or indicate
that the information is not available or not applicable. This approach
provides both a reminder to the party preparing the SDS regarding the
information required and a convenient means of reviewing the section to
ensure that relevant information is included and is accurate.
Additionally, several stakeholders agreed that standardization
would result in improved accuracy of the information on SDSs. For
example, Ecolab, Inc. stated that a uniform approach to hazard
classification and labeling would improve the accuracy of the
information presented on labels and SDSs and reduce "the currently
observed variability among suppliers in chemical classification and
presentation of that information" (Document ID 0351).
Additionally, American Iron and Steel Works noted that "standardized
criteria to evaluate and communicate hazards via SDSs * * * should
assure consistent communication and lower the likelihood of
miscommunication and misinterpretation" (Document ID 0408).
Alliance for Hazardous Materials Professionals also indicated that the
standardization of SDSs is likely to "resolve language and content
inconsistencies among similar product providers" (Document ID
0327).
OSHA concludes that the classification criteria included in the
final rule will also improve the accuracy and precision of information
on SDSs. The detailed criteria provided will direct evaluators to the
appropriate classification for a chemical. For example, while directing
the evaluator to use expert judgment in taking all existing hazard
information into account, the criteria for serious eye damage/eye
irritation is tied to specific results found in animal testing. In
addition, assignment to hazard categories would lead to provision of
detailed information that would be specific to the degree of hazard
presented by the chemical.
Classification of hazards will play an important role in increasing
the usefulness of SDSs under the final rule. By including the
classification of the substance on the SDS, employers will be in a much
better position to compare the hazards of different chemicals. Hazard
categories generally give an indication of the severity of the hazard
associated with a chemical. For example, all other things being equal,
a chemical classified for skin corrosion/irritation in category 1 as a
skin corrosive would be more hazardous than a chemical classified in
category 2 as a skin irritant. If chemicals are classified into hazard
categories, this information can be used to simplify the process of
comparing chemicals. As noted previously, employers use SDSs as a means
of comparing chemical hazards to select less hazardous alternatives.
Thus, it is reasonable to conclude that this final rule will result in
more effective use of the SDS as an instrument for identifying less
hazardous substitutes for hazardous chemicals.
Stakeholders have expressed support for a standard SDS format. The
development of an industry consensus standard for preparation of SDSs,
ANSI Z400.1, in itself, shows a desire on the part of many parties for a
consistent approach to SDSs. The final rule follows the same section
and sequence as the ANSI Z400.1, which was updated in 2004 and combined
with the ANSI 129 standard in 2010.
A report drafted by the GAO recommended that OSHA clearly specify
the language and presentation of information on SDSs (GAO, 1991,
Document ID 0292). In addition, the report of the National
Advisory Committee for Occupational Safety and Health Review of Hazard
Communication (September 12, 1996) indicated that during the public
presentations and workgroup discussions, there was general agreement
that a uniform format should be encouraged, and most workgroup members
agreed that OSHA should endorse use of the ANSI Z400.1 format (NACOSH,
1996, Document ID 0260).
Comments received in response to the ANPR indicated widespread
support for a standard format for SDS (See, e.g., Document ID
0030, 0054, 0064, 0124, and 0158). The American Foundry
Society, for example, said that consistent SDSs make it easier for
users to find information and compare products (Document ID
0158). The Jefferson County Local Emergency Planning Committee
maintained that critical information can be missed by first responders
due to the current lack of consistency in presentation of information
on SDSs, stating: "It is not overreaching for us to say that lives
will be saved through harmonization" (Document ID 0037).
Moreover, stakeholder response to the NPRM also overwhelmingly
supported requiring a consistent, standardized format for SDSs
(Document ID 0307, 0313, 0321, 0322, 0328, 0329, 0330, 0335,
0341, 0344, 0349, 0352, 0357, 0365, 0372, 0374, 0381, 0382, 0383, 0386,
0389, 0392, 0393, 0403, 0404, 0405, 0410, 0415, 0456, and 0463).
American Subcontractors of America stated that a standardized format
would make SDSs a more effective resource and better educational tool
(Document ID 0322). Additionally, the Communications Workers
of America asserted that standardizing SDSs would be an invaluable
solution for addressing current inconsistencies and quality issues on
SDSs (Document ID 0349).
Based on the studies and comments in the record, OSHA has concluded
that not only will the standardized SDS format indirectly improve the
quality of information provided on SDSs, but that it is in the format
that stakeholders already know and overwhelmingly prefer.
Training
Along with labels on containers and SDSs, employee training is one
of three core components of a comprehensive hazard communication
program. Training is needed to explain and reinforce the information
presented on labels and SDSs, to ensure that employees understand the
chemical hazards in their workplace and are aware of the protective
measures they need to follow. The final rule includes a relatively
minor revision to the existing HCS training requirements for employers
to train employees on the label elements and SDS format. This revision
is intended to ensure that labels and SDSs are adequately explained to
employees (See Section XIII for a detailed discussion of the training
requirements). In light of the evidence discussed and new information
submitted to the record related to label and SDS comprehension, the
importance of training should not be underestimated.
Training is necessary to ensure that employees understand the
standardized headings and sequence of information on SDSs. Likewise,
employees must be able to understand the meaning of the standardized
label elements in order for them to be effective. In certain instances,
label elements already appear to be fairly well understood. For
example, "Danger" appears to be generally recognized to represent a
higher degree of hazard than "Warning." Other label elements,
particularly some pictograms, are less well understood. This finding is
not surprising given the limited amount of exposure that most of the
population has had to some of these pictograms.
A relatively high level of understanding is generally recommended
for pictograms. For example, ANSI Z535.3, the American National
Standard that addresses criteria for safety symbols (Document ID
0276), contains a test method for determining the
effectiveness of a pictogram. The criterion for a successful, effective
pictogram is 85% correct responses, with no more than 5% critical
confusion. (Critical confusion refers to when the message conveyed is
the opposite of the intended message.) A score below 85% does not mean
the pictogram should not be used, but rather that it should not be used
without some additional element, such as written text. The
International Standards Organization has similar criteria in ISO 9186,
Procedures for the Development and Testing of Public Information
Symbols (Document ID 0255). This standard recommends testing
methodologies to evaluate symbols intended to be used internationally.
It sets a somewhat lower level of acceptability (66%) than the ANSI
standard.
While initial understanding of some pictograms may not be
satisfactory, research shows that training can improve comprehension.
In one study, Wogalter et al. tested how well undergraduate subjects
comprehended a set of 40 pharmaceutical and industrial safety
pictorials before and after training (Wogalter et al., 1997c, Document
ID 0288). Training led to a significant increase in pictorial
comprehension. The improvement was greatest for the most complex
symbols. Training was equally effective whether the subject was given a
simple printed label (e.g., "Danger, cancer-causing substance") or a
label with additional explanatory text.
Lesch conducted a similar study, testing how well workers
recognized a set of 31 chemical and physical safety symbols before and
after training (Lesch, 2002, Document ID 0246; Lesch, 2003,
Document ID 0282). Training significantly improved
comprehension, which remained higher up to 8 weeks later. As in the
Wogalter et al. study described above, Lesch found little difference in
performance whether training took the form of a written label assigned
to each symbol, a label plus explanatory text, or an accident scenario.
Training also improved response speed.
In a survey of South African workers, London examined the impact of
brief training on the meaning of symbols and hazard phrases (London,
2003, Document ID 0311). Here, the author found no statistical
difference in comprehensibility of four familiar hazard symbols, but
did find that training improved comprehension of one symbol (the GHS
health hazard symbol), and it also reduced the overall incidence of
critical confusion. This study also found that workers with previous
workplace training were more likely to understand label text and some
pictograms, and were better able to identify the active ingredient.
Banda and Sichilongo reported a similar result in their evaluation of
GHS labels in Zambia. The authors found that "correct responses to
label elements were not a result of social class and/or age but
appeared to be influenced by extent of duration of exposure either
through specialized training or acquaintance" (Banda and Sichilongo,
2006, Document ID 0237). Recognizing that symbols are the
items most often recalled from a label, London advised a strong
emphasis on training for GHS symbols, particularly the "flame over
circle" and "flame" symbols--which were reported to be easily confused--and other
symbols that may generate critical confusion (London, 2003, Document ID
0311).
NIOSH, in its post-hearing comments, provided the following
additional studies. These studies support OSHA's position that training
ensures the understanding of standardized label elements (pictograms,
signal words, hazard statement, and precautionary statements) and is an
essential part of an effective hazard communication program.
Burt et al. (1999, Document ID 0480.1) conducted an
ergonomic study of correct lifting posture. The project included three
separate studies: using 135 undergraduate students, Study 1 consisted
of a questionnaire to evaluate nine symbols to select the most
appropriate symbols to encourage correct lifting posture. Four of the
symbols used in Study 1 met the appropriateness criteria and were used
in Study 2 by 21 city council workers to test their understanding of
each symbol. Using 100 random subjects, Study 3 was a field test that
examined the effect of the best performing symbol (from Study 2) on
subjects when asked to lift a box. Burt et al. found that once trained
on the meaning of a label, the presence of a standard recognized label
prompted the test subject to take the proper action. The author also
found significant increases in correct lifting posture when a symbol
was present compared with a control condition in which people were
trained in correct lifting techniques, but did not see the symbol as a
reminder.
In 2007, Lesch (Document ID 0480.3) conducted a study
looking at different training conditions. During the training, warning
symbols with labels (to better explain the meaning of the symbol) were
paired with accident scenarios. The accident scenarios illustrated the
nature of the hazard, the required or prohibited actions, and the
possible consequences of failing to comply with the warning. The
participants were tested before and following the training (immediately
after and two weeks later). The results showed the benefits of
training--improved comprehension, reduced reaction times, and an
improved confidence in their responses--and illustrated that, by
strengthening the connections between the warning symbol and its
associated meaning, accident scenario training can be used to prevent
accidents and injuries.
In 2007, Su and Hsu (Document ID 0480.5) tested 1,000
college students on their perception of GHS labels and traffic safety
signs. The study found that students who had taken training did better
in perceiving various traffic safety signs than those who did not. With
regards to chemical labeling, students who had taken hazard
communication training had better perception ratings than those without
training. Analysis showed that 17 out of 27 hazards had perception
ratings lower than 66%, the ISO suggested acceptable rate for a good
sign. The statistical analysis used in the study indicated that
pictograms should not be used alone but accompanied by warning
statements or other kinds of textual materials. The study also
suggested that training on pictograms and warning statements should be
integrated into school curriculum.
Rother (2008, Document ID 0480.4) conducted a study to
assess how South African farm workers interpret the pictograms used in
the pesticide industry. Administered to 115 farm workers from
commercial vineyards in Western Cape, South Africa, this study used a
questionnaire designed to interpret the workers' understanding of 10
pictograms commonly used in the pesticide industry. Fifty percent or
more of the study participants had misleading, incorrect, or critically
confused interpretations of the label pictograms. The study identified
a response as critically confused when a farm worker incorrectly
interpreted a pictogram to require an action or behavior that would
increase his or her health risks. OSHA agrees with NIOSH's
interpretation that the study "found that lack of training severely
affected farm worker's abilities to correctly interpret pesticide
pictogram warning labels" (Document ID 0470).
These reports reinforce OSHA's longstanding belief that labels,
SDSs, and training are complementary parts of a comprehensive hazard
communication program--each element reinforces the knowledge necessary
for effective protection of employees. The need for training to ensure
comprehension of hazard information is widely recognized. Annex A of
ANSI Z535.2 (the American National Standard for Environmental and
Facility Safety Signs) (Document ID 0277), for example,
recommends training on the meaning of standard safety symbols and
signal words, and ANSI Z535.4 (Document ID 0278) contains
similar guidance.
OSHA received many comments supporting the importance of training
(See, e.g., Document ID 0329, 0331, 0347, 0370, 0382, 0387,
0412, 0527, 0640, 0644, and 0647). The National Institute of
Occupational Safety and Health (NIOSH) (Document ID 0412)
stated:
Training is key to ensuring effective hazard communication.
Although written information is important, training is an
opportunity to explain the data and helps to ensure that the
messages are being received accurately so they can be acted on
appropriately.
The USW stated that "there is no question good training greatly
improves the ability to understand chemical labeling and safety data
sheets. Unfortunately, the OSHA standard is vague * * *" (Document ID
0403). Several organizations, including Western Region
Universities Consortium, ORC Worldwide, SOCMA, NIOSH, Building &
Construction Trades Department of AFL-CIO, NIEHS, and USW (e.g.,
Document ID 0331, 0370, 0402, 0412, 0527, 0640, and 0647)
stated that training, though essential, is often not done well, and
urged OSHA to "strengthen training requirements and worker
protection" (Document ID 0331).
Others, such as DuPont, API, Michelle Sullivan, ACC, and American
Iron and Steel Institute/American Coke & Coal Chemicals Institute,
stated that the standardized SDS and label format should facilitate
training efforts and the overall effectiveness of hazard communication
in industry (Document ID 0329, 0376, 0382, 0393, and 0408).
The American Iron and Steel Institute stated: "Standardized criteria
to evaluate chemicals should facilitate training. With a single
teaching format for SDSs and Labels, understanding, regardless of an
employee's educational background, should be improved" (Document ID
0408).
OSHA not only received many comments indicating that the training
requirements in the HCS are not adequate, several organizations
requested that OSHA either add regulatory text or a mandatory appendix
specifying training content, frequency, and methods of evaluation
(Document ID 0331, 0340, 0347, 0349, 0357, 0403, 0414, 0456,
0640, and 0647). For example, the National Institute of Environmental
Health Sciences Worker Education and Training Program (NIEHS WETP)
(Document ID 0347 and, 0516) provided training information,
including a training program guidance manual, and an outline detailing
specific training topics for the HCS.
OSHA agrees that training is important for ensuring effective
hazard communication. However, OSHA did not propose to change the
training provisions in the HCS other than initial training on the new
GHS elements.
Similarly, the GHS discusses the importance of training, but does not
contain specific training requirements. Since the purpose of this
rulemaking is to align with the requirements of the GHS, OSHA did not
propose modifications that were outside of those necessary to maintain
alignment with the GHS. OSHA has decided to stay within the scope of
the rulemaking and retain the proposed training provisions in the HCS
final rule. See Section XIII for a more detailed discussion on
training.
Conclusion
It is a longstanding Agency position that employees have the
"right to know" and understand the hazards of chemicals they are
exposed to in the workplace (53 FR 29826, Aug. 8, 1988; 59 FR 6126,
Feb. 9, 1994). This knowledge is needed in order to take the
precautions necessary for safe handling and use, to recognize adverse
health effects associated with chemical exposure, and to respond
appropriately in emergency situations.
Equally important in terms of employee protection is that employers
have access to chemical hazard information as well. Chemical
information is the foundation of workplace chemical safety programs--
without it, sound management of chemicals is impossible. By ensuring
that emergency responders, physicians, nurses, industrial hygienists,
safety engineers and other professionals have the information they
need, the HCS reduces the likelihood of chemical source illnesses and
injuries. Selection of appropriate engineering controls, work
practices, and personal protective equipment is predicated upon knowing
the chemicals that are present, the form they are present in, and their
hazardous properties.
In his testimony at the informal public hearings, Mr. David Irby, a
union safety representative at the Severstal Steel Plant in Sparrows
Point, Maryland, expressed the importance of the right to understand
SDSs, stating that employees "need an easy-to read format written in a
clear, precise and understandable manner in our workplace" (Document
ID 0494 Tr. 55-57). OSHA agrees that employees must be able to
read and comprehend the information presented on both labels and SDSs
so that they can respond accordingly. Therefore, OSHA has determined
that the provisions in this final rule--the standardized label elements
(including pictograms, signal words, and hazard and precautionary
statements), a standardized 16-section SDS, and the requisite training
provisions--provide the necessary conventions to support understanding
the hazards posed by chemicals in the workplace and that this final
rule provides employees not only with the "right to know" but also
the "right to understand."
OSHA concludes that aligning the HCS with the GHS will improve the
quality and consistency of the chemical hazard information provided to
employers and employees. A combination of label elements--signal word,
hazard statement(s), pictogram(s), and precautionary statement(s)--is
expected to make label warnings more noticeable and easier to
understand, and will better communicate hazard and precautionary
information. Standardized headings and a consistent order of
information are anticipated to make it easier for users to find
information on SDSs, improve their accuracy, and better enable users to
compare the relative hazards of different substances. Along with
effective training in the context of a comprehensive chemical hazard
communication program, OSHA has determined that these revisions will
more adequately inform employees of chemical hazards, and lead to
better protections in the workplace.
V. Pertinent Legal Authority
The primary purpose of the Occupational Safety and Health Act (the
"OSH Act" or "Act") (29 U.S.C. 651 et seq.) is to assure, so far as
possible, safe and healthful working conditions for every American
employee over the period of his or her working lifetime. One means
prescribed by Congress to achieve this goal is the mandate given to,
and the authority vested in, the Secretary of Labor to "promulgate,
modify, or revoke" mandatory occupational safety and health standards.
OSH Act Sec. 6(b), 29 U.S.C. 655(b).
An occupational safety and health standard is defined under the Act
as:
[A] standard which requires conditions, or the adoption or use
of one or more practices, means, methods, operations, or processes,
reasonably necessary or appropriate to provide safe or healthful
employment and places of employment.
OSH Act Sec. 3(8), 29 U.S.C. 652(8). The Supreme Court has
interpreted this provision as requiring OSHA to determine, before
promulgating a permanent standard under section 6(b) of the Act, that
the standard is reasonably necessary and appropriate to remedy a
significant risk of material health impairment. Indus. Union Dep't v.
Am. Petroleum Inst., 448 U.S. 607, 642 (1980) ("Benzene"). This
"significant risk" determination constitutes a finding that, absent
the change in practices mandated by the standard, the workplace in
question would be "unsafe" in the sense that employees would be
threatened with a significant risk of harm. Id.
Section 6(b)(5) provides that:
The Secretary, in promulgating standards dealing with toxic
materials or harmful physical agents under this subsection, shall
set the standard which most adequately assures, to the extent
feasible, on the basis of the best available evidence, that no
employee will suffer material impairment of health or functional
capacity even if such employee has regular exposure to the hazard
dealt with by such standard for the period of his working life.
Development of standards under this subsection shall be based upon
research, demonstrations, experiments, and such other information as
may be appropriate. In addition to the attainment of the highest
degree of health and safety protection for the employee, other
considerations shall be the latest available scientific data in the
field, the feasibility of the standards, and experience gained under
this and other health and safety laws. Whenever practicable, the
standard promulgated shall be expressed in terms of objective
criteria and of the performance desired.
29 U.S.C. 655(b)(5).
Thus, once OSHA determines that a significant risk due to a health
hazard is present and that such risk can be reduced or eliminated by a
proposed standard, section 6(b)(5) requires it to issue the standard,
based on the best available evidence, that "most adequately assures"
employee protection, subject only to feasibility considerations. As the
Supreme Court has explained, in passing section 6(b)(5) "Congress * *
* plac[ed] the 'benefit' of worker health above all other
considerations save those making attainment of this 'benefit'
unachievable." Am. Textile Mfrs. Inst. Inc. v. Donovan, 452 U.S. 490,
509 (1981) ("Cotton Dust"). Where, however, there are two equally
effective methods of reducing significant risk to the most protective
feasible level, OSHA must choose the less costly method. See Cotton
Dust, 452 U.S. 490, 513 n.32; Int'l Union, UAW v. OSHA, 37 F.3d 665,
668 (D.C. Cir. 1994).
In addition, section 6(b)(7) of the Act provides in part that:
Any standard promulgated under this subsection shall prescribe
the use of labels or other appropriate forms of warning as are
necessary to insure that employees are apprised of all hazards to
which they are exposed, relevant symptoms and appropriate emergency
treatment, and proper conditions and precautions of safe use or
exposure.
29 U.S.C. 655(b)(7). Section 6(b)(7)'s labeling and employee warning
requirements provide basic protections for employees in the absence of
specific permissible exposure limits, particularly by providing
employers and employees with information necessary to design work
processes that protect employees against exposure to hazardous
chemicals in the first instance. The Supreme Court has recognized such
protective measures that may be imposed in workplaces where chemical
exposure levels are below that for which OSHA has found a significant
risk. Benzene, 448 U.S. at 657-58 & n.66. In Benzene, the Court relied
on section 6(b)(7) to sanction OSHA's requirements for monitoring and
medical testing when it sets a permissible exposure limit "in reliance
on less-than-perfect methods." Id. These requirements serve as a
"backstop," the Court said, allowing OSHA to check the validity of
its assumptions in developing the PEL, and employers to remove
particularly susceptible workers before they suffered any permanent
damage. Id. at 657-58; See also Nat'l Cottonseed Products Ass'n v.
Brock, 825 F.2d 482, 485-87 (D.C. Cir. 1987) (upholding decision to
retain medical monitoring requirement while revoking PEL to "provide a
backstop if that judgment is incorrect and this surveillance will
protect the health of the employees").
In promulgating a standard under the Act, OSHA's determinations
will be deemed conclusive if they are "supported by substantial
evidence in the record considered as a whole." OSH Act Sec. 6(f), 29
U.S.C. 655(f). When the standard deals with toxic materials or harmful
physical agents, OSHA must use the "best available evidence." Such
evidence includes "the latest scientific data in the field,"
"research, demonstrations, experiments, and such other information as
may be appropriate," and "experience gained under this and other
health and safety laws." OSH Act Sec. 6(b)(5), 29 U.S.C. 655(b)(5).
The Supreme Court has held that OSHA is not required to support its
finding of significant risk of material health impairment "with
anything approaching scientific certainty" and that the determination
of whether a level of particular risk is "'significant' will be based
largely on policy considerations." Benzene, 448 U.S. at 655-56 & n.62.
The OSH Act allows the Secretary to "modify" and "revoke"
existing occupational safety or health standards. OSH Act Sec.
6(b)(2); 29 U.S.C. 655(b)(2). In passing the Act, Congress recognized
that OSHA should revise and replace its standards as "new knowledge
and techniques are developed." S. Rep. 91-1282 at 6 (1970). The
Supreme Court has observed that administrative agencies "do not
establish rules of conduct to last forever, and * * * must be given
ample latitude to adapt their rules and policies to the demands of
changing circumstances." Motor Vehicle Mfrs. Ass'n v. State Farm Mut.
Auto. Ins. Co., 463 U.S. 29, 42 (1983) (internal quotation marks and
citations omitted).
A. Legal Authority for the Current HCS
OSHA's Hazard Communication Standard ("HCS") is a standard
promulgated under the authority of sections 6(b)(5) and 6(b)(7) of the
Act (29 U.S.C. 655(b)(5) and 655(b)(7)). See Associated Builders and
Contractors, Inc. v. Brock, 862 F.2d 63, 67-68 (3rd Cir. 1988); United
Steelworkers of Am. v. Auchter, 763 F.2d 728, 738 (3rd Cir. 1985);
United Steelworkers of Am. v. Auchter, 819 F.2d 1263, 1267 (3rd Cir.
1987). Authority for the HCS may also be found in section 8(c) and 8(g)
of the Act, 29 U.S.C. 657(c) and 657(g). Section 8(c)(1) of the Act
requires employers to make, keep, and preserve records regarding
activities related to the Act and to make such records available to the
Secretary pursuant to regulations that the Secretary may prescribe. 29
U.S.C. 657(c)(1). Section 8(g)(2) of the Act authorizes the Secretary
to "prescribe such rules and regulations as [she] may deem necessary
to carry out [her] responsibilities under this Act * * *." 29 U.S.C.
657(g)(2).
As a 6(b)(5) standard, OSHA was required to establish that the HCS
would substantially reduce a significant risk of material harm. Some
OSHA standards protect employees from exposure to a concentration of a
hazardous substance that OSHA has found to create a significant risk of
material health impairment. Thus, in making the significant risk
determination in these cases, OSHA is concerned with determining the
level at which a significant risk arises.
OSHA took a different approach to its significant risk
determinations in promulgating the HCS in 1983 and revising it in 1994.
The agency relied on NIOSH data showing that about 25 million, or about
25% of, American employees were potentially exposed to one or more of
8,000 NIOSH-identified chemical hazards and that, for the years 1977
and 1978, more than 174,000 illnesses were likely caused by workplace
exposure to hazardous chemicals. 48 FR 53280, 53282 (Nov. 25, 1983). It
then noted the consensus evident in the record among labor, industry,
health professionals, and government that an "effective federal
standard requiring employers to identify workplace hazards, communicate
hazard information to employees, and train employees in recognizing and
avoiding those hazards" was necessary to protect employee health. Id.
at 53283.
Thus, OSHA found that because:
* * * inadequate communication about serious chemical hazards
endangers workers and that the practices required by this standard
are necessary or appropriate to the elimination or mitigation of
these hazards, the Secretary is hereby able to make the threshold
"significant risk" determination that is an essential attribute of
all permanent standards.
Id. at 53321. The U.S. Court of Appeals for the Third Circuit agreed
that "inadequate communication is itself a hazard, which the standard
can eliminate or mitigate." United Steelworkers v. Auchter, 763 F.2d
at 735. The Third Circuit has upheld OSHA's finding of significant risk
as sufficient to justify the HCS on several occasions. See Associated
Builders and Contractors, 862 F.2d at 67 (discussing the history of its
review of the issue). OSHA reaffirmed its finding of significant risk
in adopting revisions to the HCS in 1994. 59 FR 6126, 6136-40 (Feb. 9,
1994).
A characteristic of hazard communication that OSHA confronted in
adopting the HCS is that information about the hazards associated with
a particular chemical, and the exposures associated with its use, is
not uniformly distributed across industry. That is, chemical
manufacturers and importers tend to have greater knowledge and
scientific expertise with respect to the composition of the chemicals
they make or import than do downstream employers. See 48 FR at 53322
(Nov. 25, 1983). Therefore, manufacturers and importers are usually in
the best position to assess the inherent hazards associated with them.
Id. However, it is the downstream users and their employees who tend to
have the best information about the means and methods of exposure, and
are therefore usually in the best position to determine the risk
arising from the use of the chemical in their workplaces. See 48 FR at
53307 (Nov. 25, 1983); 59 FR at 6132-33 (Feb. 9, 1994).
OSHA's approach in promulgating the HCS reflects this reality. It
places the duty to ascertain and disclose chemical hazards on
manufacturers and importers, so that downstream users can use this
information to avoid harmful exposures to chemical hazards. But because
manufacturers and importers will often have less information about the
particular exposures of downstream users, their hazard assessment and
communication obligations are imposed only for all normal conditions of
use of their chemicals and foreseeable emergencies associated with those
chemicals. 29 CFR 1910.1200(b)(2).
In previous rulemakings, OSHA rejected suggestions that the hazard
assessment and communication obligations should arise only where the
downstream use creates a significant risk because it is difficult, if
not impossible, for OSHA or manufacturers and importers to know where
these risks might occur before the fact. 48 FR at 53295, 53296, 53307
(Nov. 25, 1983; 59 FR at 6132 (Feb. 9, 1994). Further, it is only by
the provision of hazard information that downstream employers and
employees can determine how to use the chemical so that exposure and
risk may be minimized. Id. Thus, the HCS protects employees from
significant risk by requiring communications about all chemicals that
may present a hazard to employees, regardless of the exposure or risk
levels any particular downstream user might actually experience. See
Durez Div. of Occidental Chem. Corp. v. OSHA, 906 F.2d 1, 3-4 (D.C.
Cir. 1990); General Carbon Co. v. OSHRC, 860 F.2d 479, 484-85 (D.C.
Cir. 1988).
For these reasons, hazard communication--as opposed to risk
communication--"most adequately assures" employee protection from the
significant risk of material impairment of health arising from the use
of hazardous chemicals in the workplace for purposes of OSHA's
authority under section 6(b)(5) of the Act. In addition, the HCS is
authorized under section 6(b)(7), which requires OSHA to prescribe
"labels or other appropriate forms of warning as are necessary to
insure that employees are apprised of all hazards to which they are
exposed, relevant symptoms and appropriate emergency treatment, and
proper conditions and precautions of safe use or exposure." 29 U.S.C.
655(b)(7). As noted above, the Benzene case recognizes that the
"backstop" provisions of section 6(b)(7) allow OSHA to impose
information requirements even before the employee is exposed to the
significant risk. In this way, the HCS ensures that employers and
employees have the information they need to avoid situations of
exposure in the workplace even before the employee is exposed to a
hazardous chemical. As OSHA explained in the preamble to the 1994 HCS
amendments: "OSHA has concluded that imposing informational
requirements is necessary and appropriate to protect workers even when
OSHA has not determined that the level of risk at a particular worksite
warrants a substance-specific standard that would employ more elaborate
types of controls." 59 FR at 6132 (Feb. 9, 1994).
B. Authority for the Final Rule
1. Section 6(b)(7) Authority. OSHA has authority to adopt the
revisions to the HCS made in the final rule under the last sentence of
section 6(b)(7) of the Act, which provides that:
The Secretary, in consultation with the Secretary of Health and
Human Services, may by rule promulgated pursuant to section 553 of
title 5, United States Code, make appropriate modifications in the
foregoing requirements relating to the use of labels or other forms
of warning, monitoring or measuring, and medical examinations as may
be warranted by experience, information, or medical or technological
developments acquired subsequent to the promulgation of the relevant
standard.
29 U.S.C. 655(b)(7).
This provision exempts modifications to hazard communication,
monitoring, and medical examination requirements from the standard-
setting requirements of section 6(b), and so evidences Congress's
intent to provide OSHA with an expedited procedure to update these
requirements. OSHA believes that exercise of this authority does not
require a new finding of significant risk. As noted above, the
"backstop" 6(b)(7) requirements of hazard communication, exposure
monitoring, and medical surveillance may be imposed even in the absence
of a significant risk finding. See Benzene, 448 U.S. at 657-58; Nat'l
Cottonseed Products Ass'n, 825 F.2d at 485-87. The last sentence of
section 6(b)(7) merely allows these requirements to be updated to
reflect the latest knowledge available. The authorization to use
Administrative Procedure Act notice and comment procedures rather than
the more elaborate framework established by section 6(b) demonstrates
congressional intent to treat such modifications differently from
rulemakings to adopt standards. Congress envisaged a simple, expedited
process that is inconsistent with the idea that OSHA must undertake
additional significant risk analyses before exercising this authority.
Rather than requiring a finding of significant risk, the last
sentence of section 6(b)(7) provides other assurances that OSHA is
exercising its authority appropriately: by requiring the involvement of
the Secretary of Health and Human Services, and by limiting the
authority only to modifications that are based on "experience,
information, or medical or technological developments" acquired since
the promulgation of the standard in the limited areas of hazard
communication, monitoring, and medical examinations. Therefore, OSHA
need not make any new significant risk findings; rather, the final rule
is supported by the significant risk findings that OSHA made when it
adopted the current HCS.
OSHA has used the authority of section 6(b)(7) in the past to
revise its standards. See, e.g., Standards Improvement Project-Phase
II, 70 FR 1112 (Jan. 5, 2005); Standards Improvement (Miscellaneous
Changes) for General Industry and Construction Standards, 63 FR 33450,
33458 (June 18, 1998). For example, it used this authority to revise
the inorganic arsenic and coke oven emissions standards to eliminate
the requirement of sputum cytology testing and to reduce the required
frequency of mandatory chest x-rays from semi-annual to annual. 63 FR
at 33458 (June 18, 1998). OSHA justified these changes on the grounds
that studies reported after the promulgation of the relevant standards
showed that sputum cytology did not improve employee survival rates and
that the survival rates when semi-annual x-rays were used were not
higher than when annual exams were administered. 63 FR at 33458-59
(June 18, 1998). In addition, OSHA has used its section 6(b)(7)
authority to authorize new respirator fit protocols under its
respiratory protection standard. 69 FR 46986 (Aug. 4, 2004); See
generally 29 CFR 1910.134 App. A, Pt. II. On neither occasion has OSHA
made new findings about significant risk.
The final rule fits well within the authority granted by the last
sentence of section 6(b)(7). Adoption of GHS provisions constitutes a
"modification[]" of the HCS regarding "the use of labels or other
forms of employee warning." For the reasons summarized above and
explained more fully elsewhere in this preamble, OSHA believes that the
adoption of GHS is "appropriate" based on "experience, information,
or medical or technological developments acquired subsequent to the
promulgation of the relevant standard." The formulation of GHS may
also be considered a "technological development" that has occurred
since the promulgation of the original standard in 1983. GHS was
negotiated and drafted through the involvement of labor, industry, and
governmental agencies, and thus represents the collective experience
and information on hazard communication gathered by the participants in
these sectors over the last several decades. See Parts III and XIII of
this preamble; 74 FR 50280, 2085-86 (Sept. 30, 2009); 71 FR 53617,
53618-19 (Sept. 12, 2006). Indeed, OSHA noted the possibility of a
future internationally harmonized standard in the preamble accompanying the
original HCS rule. See 48 FR at 53287 (Nov. 25, 1983).
The last sentence of section 6(b)(7) also requires consultation
with the Secretary of Health and Human Services. As detailed in the
NPRM, NIOSH was involved in the development of the proposal through
briefings and review of the proposed rule before publication. See 74 FR
at 50306 (Sept. 30, 2009). NIOSH strongly supported the proposal in
comments and hearing testimony (Document ID 0412, 0470, 0472,
and 0497) and has actively supported the development of the GHS. See 74
FR at 50306 (Sept. 30, 2009).
Paul A. Shulte, Ph.D., testified on behalf of NIOSH that:
[A] significant advantage of the proposed standard is the
detailed technically sound criteria for classification that will
improve accuracy and consistency in the information provided to
employers and employees on chemical hazards and protective measures
* * *. In summary, the proposed standard will serve as a powerful
tool for the protection of working people.
(Document ID 0497 Tr. 36-37). OSHA has consulted with HHS in
accordance with section 6(b)(7). For all the reasons set forth above,
revision of the HCS through adoption of the GHS as proposed by OSHA is
authorized by section 6(b)(7) of the OSH Act, 29 U.S.C. 655(b)(7).
2. Section 6(b)(5) Authority. OSHA also has authority to adopt the
proposal under section 6(b)(5) of the Act, 29 U.S.C. 655(b)(5). As
noted above, section 6(b) explicitly allows OSHA to "modify"
standards, and adoption of the GHS is justified because it "most
adequately assures" employee protection for purposes of section
6(b)(5) for the reasons detailed in parts IV and XIII of this preamble.
HCS is a 6(b)(5) standard since it acts to mitigate the significant
health risk of using dangerous chemicals without adequate hazard
communication. See Int'l Union, UAW v. OSHA, 938 F.2d 1310, 1313 (D.C.
Cir. 1991). The Society of the Plastics Industry, Inc. (SPI), however,
argues that because the rule also addresses physical hazards, "the
agency must comply with the more demanding burden of proof at least
with respect to the safety hazards," and that some form of cost-
benefit analysis is required (Document ID 0392). OSHA
disagrees. Safety standards must be "highly protective," which means
OSHA may "deviate only slightly from the stringency required by
section 6(b)(5)." Int'l Union, UAW v. OSHA, 37 F.3d 665, 669 (D.C.
Cir. 1994). The burden of proof for safety standards is therefore not
more demanding than that required for 6(b)(5) standards, as SPI argues.
Nor does OSHA believe that the OSH Act requires a cost-benefit analysis
in setting safety standards. See Control of Hazardous Energy Sources,
Supplemental Statement of Reasons, 58 FR 16612, 16621-23 (Mar. 30,
1993). However, as discussed in Section VI, Final Economic Analysis,
OSHA has examined the costs and benefits of the final rule, and found
that the benefits exceed costs by a large margin. In any event, OSHA
believes that the more protective requirements of section 6(b)(5) apply
to this standard because the standard addresses health hazards.
Standards adopted under the authority of section 6(b)(5) must be
supported by a finding of significant risk. However, as explained
elsewhere, the GHS is an improved method of communicating chemical
hazards to employers and employees over the current standard, and
therefore the final rule, which incorporates the GHS, is now the
"standard that most adequately assures" worker protection. OSH Act
Sec. 6(b)(5); 29 U.S.C. 655(b)(5). Adoption of GHS will substantially
reduce the significant risk of inadequate communication workers face.
As discussed above, OSHA supported the current rule with a finding,
affirmed by the Third Circuit, that "inadequate communication about
serious chemical hazards endangers workers" and that the HCS will
mitigate this risk. 48 FR 53321 (Nov. 25, 1983); United Steelworkers v.
Auchter, 763 F.2d at 735; See also 59 FR 6126, 6127, 6129, 6132-38
(Feb. 9, 1994). The record shows that this significant risk of
inadequate communication was not eliminated by the current standard.
As discussed in Section IV, several studies show that employees do
not understand approximately one-third of the safety and health
information listed on SDSs prepared in accordance with the current
standard (Document ID 0245, 0263, 0295, 0309, and 0310).
Studies also report that roughly 40% of persons reviewing SDSs found
them difficult to understand (Document ID 0188 and 0262). The
results from these studies probably overstate the level of
comprehension in the workforce, because the studies had a selection
bias towards employees who have stronger English reading skills. These
findings are corroborated by worker testimony stating that they and
their coworkers find SDSs "difficult and confusing," "inadequate and
incomprehensible," and a "nightmare." One witness stated that
employees he works with would not ask to see SDSs because they were too
complicated, and as a result, the employees unwittingly expose
themselves to chemical hazards (Document ID 0494 Tr. 50, 54-
55; and 0499 Tr. 134, 147-48, 151, 162, 165-66, and 167).
Moreover, the evidence in the record shows workers who read SDSs
prepared in a standardized format have substantially improved
comprehension of the information they present (Document ID
0191, 0263, 0309, and 0310). Indeed, standards specifying
uniform formats for SDSs have been adopted by ANSI and other standards
bodies, indicating a consensus that standardized SDSs will more
effectively communicate chemical hazards to workers and employers.
Moreover, commenters overwhelmingly agreed that standardizing SDSs
would improve hazard communication. (See, e.g., Document ID
0330, 0335, 0336, 0341, 0344, 0348, 0357, 0370, 0372, 0376,
0381, 0410, 0414, and 0415).
Likewise, the record shows that the current HCS's performance-
oriented labeling requirements result in inadequate communication.
Research conducted over the last twenty years and summarized in section
IV of this preamble shows that use of the signal words "Danger" and
"Warning," pictograms, red borders, and standardized hazard warnings
and precautionary statements better convey information about chemical
hazards. Studies show that the information conveyed by these techniques
is better understood, especially among low literacy populations, better
remembered, and more likely to be acted upon. Again, commenters agreed
that the current performance-oriented labeling requirement leads to
worker confusion, and that the standardized GHS labeling requirements
would minimize that confusion. (See, e.g., Document ID 0313,
0327, 0335, 0336, 0341, 0344, 0348, 0351, 0365, 0370, 0410, 0412, and
0644.)
Finally, employees still continue to suffer chemical-related
injuries, illnesses and deaths. As discussed in more detail in Section
VI, Final Economic Analysis, of the preamble, OSHA estimates that over
40 million employees are potentially exposed to hazardous chemicals.
BLS data show that in 2007, there were approximately 55,400 illnesses
related to hazardous chemical exposures and 125 chemical-related
fatalities. These statistics probably represent only a small portion of
the illnesses experienced by exposed employees; most occupational
illnesses are not reported because they are not recognized as being
related to workplace exposures and are subject to long
latency periods between exposure and the manifestation of disease. The
most recent nationwide study of chronic illness estimated that in 1992,
there were between 46,900 to 73,700 fatalities from chronic illnesses
related to occupational exposures to chemicals (Document ID
0274). In addition, a 2004 study of chronic occupational
illness in California reported that more than 200,000 workers were
diagnosed with serious chronic diseases attributable to chemical
exposures in the workplace, and that an additional 4,400 workers in
California died during that year from chemical exposures in the
workplace (Document ID 0269).
These data corroborate the idea that currently there is inadequate
communication of chemical hazards in the workplace. Further, they show
that the use of chemical hazards in the workplace creates a significant
risk to employees. For the reasons explained above and in sections IV
and XIII of the preamble, OSHA believes that the final rule will reduce
the risk to employees by providing better and more easily understood
information to employees and employers about the hazards of the
chemicals they use, which in turn will allow precautionary measures to
be taken.
In its post-hearing comment, the Styrene Information and Research
Council (SIRC) argued that OSHA should also have examined injury and
illness rates in the EU. It states that "the GHS is substantially the
system that has been in place in the EU for the last 40 years" for
substances covered by the EU Dangerous Substances Directive and for the
10 years for mixtures covered by the EU Dangerous Preparations
Directive (Document ID 0642). OSHA disagrees with SIRC's
premise. There are significant differences between the GHS and the
relevant EU directives. These differences include the criteria for
classifying hazards, as well as the label elements used to communicate
the hazardous effects. In addition, even if the EU's hazard
communications obligations were substantially similar to the GHS, there
are technical hurdles that would have to be overcome before such a
study could yield useful information. There are significant differences
in the way that statistics for occupational illness and injuries
collected by the US and the EU (and its members) that make direct
comparisons difficult. Furthermore, the regulatory structure for
mitigating the hazards identified and communicated in varying systems
also differ significantly, and this would confound any effort to
compare illness and injury rates in the two jurisdictions. In any
event, OSHA need not wait for scientific certainty to update its
regulations, but rather it must rely on the best available evidence,
and may use conservative assumptions in interpreting the evidence. OSH
Act Sec. 6(b)(5), 29 U.S.C. 655(b)(5); Benzene, 448 U.S. at 655-56 &
n.62. As discussed above and in Sections IV and XIII, the best
available evidence indicates that a significant risk continues to exist
under the current standard and that the final rule will improve
chemical hazard communications, thereby reducing the risk of injury,
illness or death associated with the use of hazardous chemicals in the
workplace.
C. Feasibility
OSHA standards must be feasible, which means "capable of being
done, executed or effected." Cotton Dust, 452 U.S. at 508-09.
Feasibility has two aspects, economic and technological. United
Steelworkers of Am. v. Marshall, 647 F.2d 1189, 1264 (D.C. Cir. 1981)
("Lead I"). A standard is technologically feasible if the protective
measures it requires already exist, can be brought into existence with
available technology, or can be created with technology that can
reasonably be expected to be developed. Id. at 1272. A standard is
economically feasible if industry can absorb or pass on the cost of
compliance without threatening its longer term profitability or
competitive structure. (See Cotton Dust, 452 U.S. at 530 n.55; Lead I,
647 F.2d at 1265.)
In addressing feasibility in the 1994 HCS revisions, OSHA found
that:
The feasibility question raised by the HCS is not difficult to
resolve. This standard does not relate to activities on the
frontiers of scientific knowledge; the requirements are not the
sorts of obligations that approach the limits of feasibility.
Associated Builders & Contractors, 862 F.2d at 68. The record on
which the original and expanded HCS's were based did not contain
credible evidence that the HCS would be technologically or
economically infeasible for any industrial sector, id., and there
was substantial evidence of feasibility, 52 FR 31855-58.
59 FR at 6133 (Feb. 9, 1994). OSHA has repeatedly found that the
requirements of the HCS are technologically feasible. See 52 FR at
31855-57 (Aug. 24, 1987); 59 FR at 6133 (Feb. 9, 1994). While the GHS
modifications to HCS impose more specific requirements for hazard
classification, labeling, and safety data sheets, employers may use the
same expertise and methods to meet these requirements as they are
already utilizing to comply with the requirements of HCS.
As discussed below and in section VI.E of this preamble, OSHA
believes the final rule poses no technological feasibility issues. The
most important resource employers will need in order to comply with the
GHS modifications to HCS is technical expertise in hazard
classification and the communication of those hazards. OSHA found that
such expertise was already available in promulgating the initial HCS
rule in 1983. 48 FR at 53296-99 (Nov. 25, 1983). OSHA believes that the
availability of professionals with this expertise has only increased in
the intervening time. The GHS has already been implemented, in whole or
in part, by a number of major U.S. trading partners, including Japan
and the EU. Companies that export to these jurisdictions should already
have developed expertise in the GHS, and there are a number of GHS
training resources developed on the international level (Document ID
0405, 0410, and 0514). At least one professional organization
currently provides GHS training in hazard communication to
professionals and businesses in the United States (Document ID
0021 and 0145). Through OSHA's Alliance with the Society for
Chemical Hazard Communication, training to small businesses in the
requirements of hazard communication and information about the GHS
modifications has been made available. See http://www.osha.gov/dcsp/alliances/schc/schc.html.
NIOSH is preparing a program for employers to
use in training their employees in the new labeling scheme (Document ID
0412). OSHA received numerous comments discussing the
professionals and tools (both manual and electronic) that employers
have available to comply with current hazard communication
requirements. (See, e.g., Document ID 0015, 0024, 0026, 0036,
0038, 0042, 0046, 0050, 0053, 0072, 0077, 0107, 0108, 0116, 0123, 0128,
0141, 0144, 0145, 0154, 0155, 0163, 0330, 0352, and 0389.) The Agency
has been engaged on several fronts to facilitate the transition from
the current standard to the GHS modifications. For instance, the United
Nations Institute for Training and Research is developing basic and
more advanced training courses for the GHS, and OSHA has been involved
with and committed resources to this effort. As discussed in more
detail below in the Summary and Explanation, OSHA plans to issue a
number of outreach and compliance assistance materials. Additionally,
NIOSH testified that the World Health Organization has started the
process to convert International Safety Cards to GHS and as of March
2010; approximately 249 (15%) have
already been converted (Document ID 0497 Tr. 46). OSHA
believes that adopting the GHS modifications poses no technological
feasibility issues.
Likewise, for the reasons more fully discussed in Section VI, Final
Economic Analysis, OSHA believes that the adoption of GHS will not pose
economic feasibility issues. Again, OSHA previously found that the
implementation of HCS would have no such effect. See 52 FR at 31855-57
(Aug. 24, 1987); 59 FR at 6133 (Feb. 9, 1994). As discussed in Section
VI, OSHA has found that, once conversion to the new system is
completed, compliance with the GHS-modified HCS will not be more
expensive than compliance with the current HCS and will result in
savings for employers. While industry will incur the cost of converting
to the new system, OSHA does not believe that this cost is so
substantial as to threaten long term profitability or the competitive
structure of any industry.
VI. Final Economic Analysis and Voluntary Regulatory Flexibility
Analysis
A. Introduction and Summary
Introduction
OSHA is required by the Occupational Safety and Health (OSH) Act of
1970 to ensure and demonstrate that standards promulgated under the Act
are reasonably necessary and appropriate, as well as technologically
and economically feasible. Executive Orders 12866 and 13563, the
Regulatory Flexibility Act, and the Unfunded Mandates Reform Act also
require OSHA to estimate the costs, assess the benefits, and analyze
the impacts of certain rules that the Agency promulgates. Executive
Orders 12866 and 13563 direct agencies to assess all costs and benefits
of available regulatory alternatives and, if regulation is necessary,
to select regulatory approaches that maximize net benefits (including
potential economic, environmental, public health and safety effects,
distributive impacts, and equity). Executive Order 13563 emphasizes the
importance of quantifying both costs and benefits, of reducing costs,
of harmonizing rules, and of promoting flexibility. OSHA has determined
that this action is "economically significant" within the meaning of
3(f)(1) of the executive order because it is likely to have an effect
on the economy of $100 million or more in any one year. Accordingly,
the rule has been reviewed by OMB.
Accordingly, OSHA has prepared this Final Economic Analysis (FEA),
including a Final Regulatory Flexibility Screening Analysis (FRFSA),
for the modifications to the Hazard Communication Standard (HCS). The
OSHA FEA is based largely on research conducted for the Preliminary
Economic Analysis (PEA) by Policy, Planning, and Evaluation, Inc.
(PP&E), as presented in its revised final report, "Data and Analysis
in Support of an Economic Analysis of Proposed Changes to the OSHA
Hazard Communication Standard," prepared under contract to OSHA, and
on research conducted for purposes of completing this FEA by Eastern
Research Group (ERG). ERG and OSHA analyses updated both costs and
benefits. The materials prepared by PP&E, 2009 (Document ID
0273) and ERG (2010, 2011, and 2012) \1\ are available in the
public docket for this rulemaking, OSHA-H022K-2006-0062, through
www.regulations.gov.
---------------------------------------------------------------------------
\1\ Eastern Research Group (ERG, 2010). Harmonization of Hazard
Communication: Labeling Costs. Final Report. Submitted to
Occupational Safety And Health Administration, Directorate of
Evaluation and Analysis, Office of Regulatory Analysis, Contract No.
GS-10-F-0125P. April 28, 2010. Eastern Research Group (ERG, 2011).
Harmonization of Hazard Communication: Summary of Labeling Costs.
Final Report. Submitted to Occupational Safety And Health
Administration, Directorate of Evaluation and Analysis, Office of
Regulatory Analysis, Contract No. GS-10-F-0125P. March 23, 2011.
Eastern Research Group (ERG, 2012). Excel Spreadsheets in
Support of OSHA Final Economic Analysis for GHS Rule. Submitted to
Occupational Safety And Health Administration, Directorate of
Evaluation and Analysis, Office of Regulatory Analysis, Contract No.
GS-10-F-0125P. January 20, 2012.
---------------------------------------------------------------------------
Need for Regulation
Employees in work environments covered by the HCS are exposed to a
variety of significant hazards that can and do cause serious injury and
death. The HCS serves to ensure that both employers and employees are
provided needed information about chemical hazards that was not
provided by markets in the absence of such a standard. The HCS also
facilitates interstate commerce by promoting consistency among federal
and individual state requirements.
The changes to the HCS will create a uniformity standard for the
presentation of hazard information and, as such, will serve to improve
the efficiency and effectiveness of the existing hazard communication
system in the U.S., and to reduce unnecessary barriers to trade. Hazard
communication is currently addressed by many different international,
national, and State authorities. As described in Section IV of this
preamble, these existing requirements are not always consistent and
often contain different definitions of hazards and varying provisions
for what information is required on labels and safety data sheets.
Complying with these different rules results in increased costs for
employers with hazardous chemicals in their workplace and for chemical
manufacturers, distributors, and transporters involved in international
trade. In addition to these effects on businesses, the different
existing requirements result in workplaces receiving chemicals with
varying information, with potential adverse impacts on the safety and
health of employees. The revisions to the OSHA HCS will standardize the
hazard communication requirements for products used in U.S. workplaces,
and thus provide employees with uniform and consistent hazard
communication information. Secondarily, because these revisions will
harmonize the U.S. system with international norms, they will
facilitate international trade.
Affected Industries
The revisions would affect employers and employees in many
different industries across the economy. Based on ERG (2012), OSHA
estimates that the HCS covers over five million workplaces in which
employees are potentially exposed to hazardous chemicals (see Table VI-
3).
For establishments with employees whose only exposures to hazardous
chemicals result from their use of the chemical products, the revisions
to the HCS would generally involve minor effects, such as
familiarization with new warning labels. For establishments producing
hazardous chemicals, which are generally part of the chemical
manufacturing industry, the revisions to the standard would involve
reclassifying chemicals in accordance with the new classification
system and revising safety data sheets (SDSs) and labels associated
with hazardous chemicals. OSHA has judged that SDSs for imported
chemicals would normally be produced in the country of origin, and thus
would not represent expenses for importers. OSHA solicited comment on
this judgment in the PEA and did not receive any contrary testimony or
evidence.
Benefits
There is ample evidence of the substantial risks of chemical
exposure in the workplace. In 2007, according to the Bureau of Labor
Statistics, employees suffered an estimated 55,400 illnesses
attributable to chemical exposures (BLS, 2008), and some 17,340
chemical-source injuries and illnesses involved days away from work
(BLS, 2009). However, as noted in the preamble to the HCS in 1983, BLS
estimates probably only reflect a small percentage of occupational
illnesses (48 FR 53284, Nov. 25, 1983) because most occupational
illnesses are not reported. The principal reasons are that they are not
recognized as being related to workplace exposures and are subject to
long latency periods between exposure and the manifestation of disease.
The key study of the issue of the number of fatalities from chronic
illnesses, not recorded in any way by BLS, is Leigh et al., 1997
(Document ID0274). That study found that in 1992, there were
from 46,900 to 73,700 fatalities from chronic illnesses related to
occupational exposures to chemicals. This critical category dwarfs all
acute injuries and illnesses due to chemicals recorded by BLS.\2\
---------------------------------------------------------------------------
\2\ A more recent study prepared by the University of California
Centers for Occupational and Environmental Health, and commissioned
by the California Environmental Protection Agency, suggests that
fatalities from chronic illnesses remain an important problem
(University of California COEH, 2008 p. 18). That study estimated
that, in 2004, more than 200,000 workers, in California alone, were
diagnosed with serious chronic diseases (encompassing cancer, COPD,
asthma, pneumoconiosis, chronic renal failure, and Parkinson's
disease) attributable to chemical exposures in the workplace, and
that an additional 4,400 workers in California died during that year
from chemical exposures in the workplace.
---------------------------------------------------------------------------
Section IV of this preamble describes some of the incidents that
may have been related to the non-standardized approach to SDSs in the
current HCS, including xylene exposure at a hospital when an employee
was unable to find critical information on an SDS in an emergency spill
situation (Document ID 0251). As a result, twelve employees
required emergency room treatment. Were the information on SDSs more
uniformly formatted and comprehensible, as required under the
modifications to HCS, incidents such as this would be less likely to
occur.
In general, the modifications to the HCS are expected to result in
increased safety and health for the affected employees and to reduce
the numbers of accidents, fatalities, injuries, and illnesses
associated with exposures to hazardous chemicals.
It is difficult to quantify precisely how many injuries, illnesses,
and fatalities would be prevented due to the revisions to the HCS.\3\
The benefits associated with the current HCS may indirectly help
provide a general sense of the potential magnitude of the benefits of
the revisions to the HCS. OSHA estimates that if the rule could capture
one percent of the benefits estimated for the original 1983 and 1987
HCS rules, the revisions would result in the prevention of 318 non-
lost-workday injuries and illnesses, 203 lost-workday injuries and
illnesses, 64 chronic illnesses, and 43 fatalities annually. The
monetized value of the corresponding reduction in occupational risks
among the affected employees is an estimated $250 million on an
annualized basis.
---------------------------------------------------------------------------
\3\ While comments in the record did not attempt to estimate the
magnitude of these safety and health benefits, they largely
supported the conclusion that these revisions would yield increased
protection for workers. For additional discussion of the comments
regarding OSHA's estimate of benefits, see Section VI:D Benefits in
this preamble.
---------------------------------------------------------------------------
The harmonization of hazard classifications, safety data sheet
formats, and warning labels for affected chemicals and products would
also yield substantial savings to businesses. Fewer different SDSs
would have to be produced for affected chemicals, and many SDSs would
be able to be produced at lower cost due to harmonization and
standardization. The benefits represented by these cost reductions
would primarily affect businesses involved in chemical manufacturing.
In addition, businesses that purchase or use hazardous chemicals can
expect reductions in operating costs as a result of the promulgation
and implementation of the modifications to the HCS due the
standardization of SDSs, which will make it easier to locate
information and determine handling requirements, and other factors
related to simplification and uniformity which will improve workplace
efficiency.
In 2008, in preparation for OSHA's Notice of Proposed Rulemaking,
PP&E conducted extensive research on the processes that companies use
to classify chemical hazards, to develop SDSs and labels, and to
handle, store, and use hazardous chemicals. PP&E evaluated how these
processes would be affected by the revisions to the HCS and analyzed
the potential savings that would be realized as a result of adopting
these revisions. Using the parameters estimated by PP&E through its
research and employing updated data on wages and the number of affected
establishments and employees, OSHA has concluded that the annual cost
savings for these companies would be an estimated $507.4 million.
OSHA also expects the revised HCS will reduce the costs of
providing hazard communication training to employees in future periods.
Stakeholders largely corroborated that expectation. Standardized SDS
and label formats will reduce the amount of time needed to familiarize
employees with the HCS, which will reduce the training time for all
employees once the final rule is fully implemented. OSHA did not
monetize these estimated cost savings, but anticipates that they will
be substantial.
As an additional benefit, the modification of the HCS by the
inclusion of the globally harmonized system (GHS) of classification and
labeling of chemicals would be expected to facilitate international
trade, increasing competition, increasing export opportunities for U.S.
businesses, reducing costs for imported products, and generally
expanding the selection of chemicals and products available to U.S.
businesses and consumers. As a result of both the direct savings
resulting from harmonization and the increased competitiveness, prices
for the affected chemicals and products, and the corresponding goods
and services using them, would be lowered.
Finally, the GHS modifications to the OSHA HCS would meet the
international goals for adoption and implementation of the GHS that
have been supported by the U.S. government. Implementing GHS in U.S.
federal laws and policies through appropriate legislative and
regulatory action was anticipated by the U.S. support of international
mandates regarding the GHS in the Intergovernmental Forum on Chemical
Safety, the World Summit on Sustainable Development, and the United
Nations. It is also consistent with the established goals of the
Strategic Approach to International Chemicals Management, a policy
framework that the U.S. helped to craft (See http://www.chem.unep.ch/saicm/).
Compliance Costs
The estimated compliance costs for the revisions to the HCS
represent the additional costs necessary for employers to achieve full
compliance. They do not include costs associated with current
compliance that has already been achieved; nor do they include costs
necessary to achieve compliance with existing requirements, to the
extent that some employers may currently not be fully complying with
applicable regulatory requirements.
The majority of the costs associated with compliance with the
revisions to the HCS would generally be incurred by the affected
industries as one-time transitional costs over the phase-in period of
four years including the costs to reclassify chemical hazards and
revise SDSs and labels, to train workers, and for management to
familiarize itself with the requirements of the final rule. There will
be additional ongoing annual compliance costs associated with the
revisions to the HCS due to the cost to purchase and maintain color
printing ink or cartridges or to purchase pre-printed color labels in
order to comply
with the requirement that the GHS hazard warning pictogram be presented
with a red border. However, OSHA's analysis has found that these costs
will not be substantial relative to the other costs of the rule.
The compliance costs are expressed as an annualized cost for
purposes of assessing the cost-effectiveness of the revisions, in order
to be able to compare the economic impact of the rulemaking with other
regulatory actions, and to be able to add and track federal regulatory
compliance costs and economic impacts in a consistent manner.
Annualized costs also represent a better measure for assessing the
longer-term potential impacts of the rulemaking. A seven percent
discount rate was applied to costs incurred in future years to
calculate the present value of these costs for the base year in which
the standard becomes effective, and the same discount rate was then
applied to the total present value costs, over a 20-year period,\4\ to
calculate the annualized cost.
---------------------------------------------------------------------------
\4\ OSHA annualized costs for this rule over a 20-year period in
accordance with Executive Order 13563, which directs agencies "to
use the best available techniques to quantify anticipated present
and future benefits and costs as accurately as possible." In
addition, OMB Circular A-4 states that analysis should include all
future costs and benefits using a "rule of reason" to consider for
how long it can reasonably predict the future and should limit its
analysis to this time period. The choice of a 20-year period is
designed to capture out-year benefits given a 4-year phase-in
period. A shorter period would place too much emphasis on the phase-
in period, where benefits would not be accruing. A longer discount
period might over-emphasize the long-term benefits since net
benefits increase with the length of the annualization period. As a
comparison, the life of OSHA's original hazard communication rule
was 1987 to 2011, a 24-year period, suggesting that 20 years is a
reasonable estimate.
---------------------------------------------------------------------------
The total annualized cost of compliance with the final rule is
estimated to be about $201 million. The major cost elements associated
with the revisions to the standard include the classification of
chemical hazards in accordance with the GHS criteria and the
corresponding revision of safety data sheets and labels to meet new
format and content requirements ($22.5 million); training for employees
to become familiar with new warning symbols and the revised safety data
sheet format ($95.4 million); management familiarization and other
management-related costs as may be necessary ($59.0 million); and costs
to purchase upgraded label printing equipment and supplies or to
purchase pre-printed color labels in order to include the hazard
warning pictogram enclosed in a red-bordered diamond on the product
label ($24.1 million).
Net Benefits, Cost-Effectiveness, and Regulatory Alternatives
Table VI-1 provides a summary of the costs and benefits of the
modifications to the OSHA HCS, and it shows the net benefits of the
modifications to the standard are estimated to be $556 million
annually, using a discount rate of 7 percent to annualize costs and
benefits. (Using a 3 percent discount rate instead would have the
effect of lowering the costs to $161 million per year and increasing
the gross benefits to $839 million per year. The result would be to
increase net benefits from $556 million to $674 million per year.)
Because compliance with the standard would result in cost savings that
exceed costs, OSHA has not provided estimates of costs per life saved
or other metrics of cost-effectiveness. However, it should be noted
that the estimated benefits exceed costs by more than a factor of
three.
In response to comments on the proposed rule, OSHA has made the
following changes to the economic analysis from the PEA to the FEA:
(1) Increased by 100 percent the amount of training time necessary
to train employees on the revised HCS during the transition period--
from 30 minutes to 60 minutes;
(2) Increased by over 60 percent the number of SDSs (with
corresponding labels) covered by the rule--from approximately 0.9
million to over 1.4 million;
(3) Added annualized costs of $24.1 million to print product labels
in color; and
(4) Incorporated updated economic data on the number of
establishments, number of employees, annual revenues, annual profits,
etc. and adjusted estimates from 2007 dollars to 2010 dollars.
The change from 2007 to 2010 dollars using the GDP deflator (for
non-wage-related costs and benefits) increased affected costs and
benefits by about 4 percent. The rule changes that increased the phase-
in period reduced the annualization factors and the associated costs
and benefits by about 9.6 percent. All other changes to costs and
benefits were the result of updated economic data, including wages, and
revised cost factors (e.g., number of SDSs, number of affected
employees) in response to comments on the proposed rule.
BILLING CODE 4510-26-P
[GRAPHIC] [TIFF OMITTED] TR26MR12.001
BILLING CODE 4510-26-C
As discussed in Section III of this preamble, the available
alternatives to the final rule are somewhat limited since this final
rule modifies the current HCS in order to align with the provisions of
the UN's GHS. In Section III, the Agency qualitatively discussed the
two major alternatives presented during this rulemaking process--(1)
voluntary adoption of GHS within the existing HCS framework and (2) a
limited adoption of specific GHS components and a variation on (1) that
would require compliance with GHS but allow an exemption for small
businesses to comply with either the current HCS or with the GHS-
compliant HCS. All of these alternatives were soundly rejected by
stakeholders. To allow certain parties to follow an alternative system
or to allow voluntary adoption of the elements of a uniformity standard
does nothing to reduce confusion, improve efficiency, or simplify
processes. In order for those benefits to be realized, all elements
must apply to all affected parties. OSHA has determined that both of
the alternatives presented above would eliminate significant portions
of the benefits of the rule.
OSHA did not attempt to evaluate the costs and benefits for the
regulatory alternatives that involved partial or voluntary adoption of
the GHS. The Agency did evaluate two alternatives where the effective
dates were altered. In the first alternative considered, all elements
of the revised HCS would be required to be implemented within two
years. Under this alternative, all transitional costs would be incurred
in two years and benefits would be realized beginning in the third
year. The second alternative that OSHA evaluated extended the timeline
for training to be completed. For this alternative, all elements of the
revised HCS (including training) would be required to be implemented by
June 1, 2016. Under this alternative, training costs would not be
realized for four and a half years (as opposed to the two year
requirement for training in the final version of this rule) while
benefits would not be realized for five years (unchanged from the final
rule). The results of these evaluations are presented in Table VI-2
below and are discussed in further detail, including significant
qualifications, in Section VI:G Net Benefits, Cost Effectiveness, and
Regulatory Alternatives in this preamble. Although both alternatives
show greater net benefits, the Agency concludes that the timing of the
final rule is preferable because of additional (but unquantified)
compliance costs and reduced (but unquantified) benefits under the
first alternative and because of reduced (but unquantified) worker
health and safety benefits under the second alternative. In addition,
OSHA expects that the final rule offers coordination benefits in that
its requirements will fully take effect at the same time as the EU
completes its transition.
[GRAPHIC] [TIFF OMITTED] TR26MR12.002
Economic Impacts
To assess the nature and magnitude of the economic impacts
associated with compliance with the final rule, OSHA developed
quantitative estimates of the potential economic impact of the new
requirements on entities in each of the affected industry sectors. The
estimated compliance costs were compared with industry revenues and
profits to provide an assessment of the economic feasibility of
complying with the final rule and an evaluation of the potential
economic impacts.
Only the compliance costs were considered for purposes of assessing
the potential economic impacts and economic feasibility of the
revisions. As described in Section VI.G: Net Benefits, Cost-
effectiveness, and Regulatory Alternatives, in this preamble, the
overall economic impacts associated with this rulemaking are expected
to result in significant net benefits to employers, employees, and the
economy generally.
As described in greater detail in Section VI.F: Costs of Compliance
in this preamble, the costs of compliance with the rulemaking are not
large in relation to the corresponding annual financial flows
associated with each of the affected industry sectors. The estimated
costs of compliance represent about 0.001 percent of revenues and about
0.011 percent of profits, on average, across all entities; compliance
costs represent less than 0.09 percent of revenues or, with the
exception of three chemical manufacturing industries, less than 0.9
percent of profits in any individual industry sector. These three
chemical manufacturing industries are NAICS 325181 Alkalies & chlorine
manufacturing, NAICS 325191 Gum & wood chemical manufacturing, and
NAICS 325992 Photographic film, paper, plate, & chemical manufacturing,
and their compliance costs as a percentage of profits are 4.3 percent,
2.1 percent, and 2.4 percent, respectively. The higher percentage of
profits for these three industries are mainly the result of low profit
margins, low baseline estimates of the number of color printers
currently employed in these industries (causing higher costs of
compliance with the color printing requirements), and a large estimated
number of labels produced by these industries.
The economic impact of achieving compliance with the final rule,
without considering the associated benefits, is most likely to consist
of an extremely small increase in prices of about 0.001 percent, on
average, for affected hazardous chemicals. It is highly unlikely that a
price increase of this magnitude would significantly alter the types or
amounts of goods and services demanded by the public or any other
affected customers or intermediaries. If the compliance costs of the
final rule can be substantially recouped with a minimal increase in
prices, there may be little or no effect on profits.
In general, for most establishments, it would be very unlikely that
none of the compliance costs could be passed along in the form of
increased prices. In the event that a price increase of 0.001 percent
were not possible, profits in the affected industries would be reduced
by an average of about 0.011 percent.
Given the minimal potential impact on prices or profits in the
affected industries, OSHA has concluded that compliance with the
requirements of the rulemaking would be economically feasible in every
affected industry sector.
In addition, based on an analysis of the costs and economic impacts
associated with this rulemaking, OSHA concludes that the effect of the
final rule on employment, wages, and economic growth for the United
States would be negligible. The effect on international trade is likely
to be beneficial and similar to the effect of a small reduction in non-
tariff trade barriers.
Final Regulatory Flexibility Screening Analysis
OSHA has analyzed the potential impact of the final rule on small
entities, and has prepared a Final Regulatory Flexibility Screening
Analysis (FRFSA) in conjunction with this rulemaking to describe the
potential effects on small entities. The FRFSA is included as a part of
this preamble in Section VI:I.
As a result of the analysis of the potential impact on small
entities, OSHA concludes and certifies that the rulemaking would not
have a significant impact on a substantial number of small entities.
Therefore, a Final Regulatory Flexibility Analysis (FRFA) is not
required for this rulemaking. Nevertheless, OSHA has voluntarily
provided the elements of the FRFA as part of the FRFSA presented in
Section VI:I: Final Regulatory Flexibility Screening Analysis in this
preamble. As part of this rulemaking, OSHA has fulfilled its
requirements under the Regulatory Flexibility Act and under the Small
Business Regulatory Enforcement Fairness Act, as applicable, to ensure
that no unnecessary burdens are imposed on small businesses.
The remainder of this FEA includes the following sections:
B. Need for Regulation
C. Profile of Affected Industries
D. Benefits
E. Technological Feasibility
F. Costs of Compliance
G. Net Benefits, Cost-Effectiveness, and Regulatory Alternatives
H. Economic Feasibility and Impacts
I. Final Regulatory Flexibility Screening Analysis
J. Environmental Impacts
K. Unfunded Mandates Reform Act Analysis
L. Sensitivity Analysis
B. Market Failure and the Need for Regulation
Employees in work environments addressed by OSHA's hazard
communication standard (HCS) are exposed to a variety of significant
hazards associated with chemicals used in the workplace that can and do
cause serious injury and death. OSHA's HCS was designed to ensure that
employers and employees are provided the information they need about
the hazards in chemical products both to make informed purchases and to
provide for safe use. The current HCS contains a set of requirements
for chemical products, including mandatory hazard determination,
labeling, and detailed information (in safety data sheets). Based on
evidence presented in the record,\5\ OSHA determined that the revisions
to the HCS will make employers' hazard communication programs more
worker-protective, efficient, and effective. In addition, the revisions
will have the effect of harmonizing hazard communication to facilitate
international trade by replacing a plethora of national rules with a
single international system.
---------------------------------------------------------------------------
\5\ See Document ID 0303, 0313, 0322, 0324, 0327, 0328,
0329, 0330, 0331, 0334, 0335, 0336, 0339, 0340, 0341, 0344, 0345,
0346, 0347, 0349, 0350, 0351, 0352, 0353, 0354, 0356, 0357, 0359,
0363, 0365, 0367, 0369, 0370, 0371, 0372, 0374, 0375, 0376, 0377,
0378, 0379, 0381, 0382, 0383, 0385, 0386, 0387, 0388, 0389, 0390,
0392, 0393, 0396, 0397, 0399, 0400, 0402, 0403, 0404, 0405, 0407,
0408, 0409, 0410, 0411, 0412, 0414, 0417, 0453, 0456, 0461, and 0463
and additional discussion in Section III of this preamble.
---------------------------------------------------------------------------
The standard, through conformance with GHS (as explained in Section
IV and XIII of this preamble), contains a number of changes to improve
the performance of the U.S. hazard communication system:
Revised criteria for more consistent classification of
chemical hazards;
Standardized signal words, pictograms, hazard statements,
and precautionary statements on labels; and
A standardized format for SDSs.
In short, GHS is a "uniformity standard" for the presentation of
hazard information (Hemenway, 1975, Document ID 0293, Tr. 8).
And much like other uniformity standards, such as driving on the right side of
the road (in the U.S.), screw threads for fire hose connectors,
"handshake" protocols for communication between computers, and, for
that matter, language, GHS will provide significant efficiencies and
economies.\6\ In the case of GHS, manufacturers will be able to produce
SDSs at lower cost, and users of SDSs will be able to more fully and
quickly utilize the information contained in the SDSs, thereby reducing
costs and, more importantly, better protect workers against chemical
hazards.\7\
---------------------------------------------------------------------------
\6\ In contrast to a uniformity standard, a specification
standard, such as an engineering standard, would spell out, in
detail, the equipment or technology that must be used to achieve
compliance. The usual rationale for a specification standard is that
compliance would be difficult to verify under a performance
standard; hence, only a specification standard would guarantee that
employees are protected against the risk in question. A
specification standard would generally not provide the efficiencies
or economies (such as easier, less expensive training on uniform
pictograms and a uniform SDS format made possible by this rule) to
the regulated community that a uniformity standard would. On the
contrary, a specification standard could impose additional costs on
some firms that may be able to effectively protect workers using a
cheaper alternative approach if such flexibility were permitted.
It is also worth noting that, for uniformity standards with
technological implications, the benefits of reduced information
costs, economies of uniformity, and facilitation of exchange may
need to be weighed against possible losses of flexibility,
experimentation, and innovation. However, because GHS is limited to
the presentation of hazard information and does not involve other
than incidental technological or strategic considerations, the
possible costs of uniformity here would be non-existent or
minuscule.
\7\ On the ability of individuals to more fully and effectively
utilize knowledge when uniformity requirements are present, see
Hemenway, 1975 (Document ID 0293), pp. 34-35.
---------------------------------------------------------------------------
Since publication of the current HCS, there has been some movement
by industry toward standardization, consistent with the revisions.
However, OSHA does not believe that full and comprehensive
standardization as required under the revisions, or the goal of
harmonizing the U.S. system with the international one, can be achieved
voluntarily in the absence of regulation.
First, in a basic sense, GHS cannot simply be implemented by the
market. Some aspects of GHS, such as the reorganization of SDSs, would
be allowed under the current OSHA standard, but other aspects, such as
the classifications system, would not be. Use of differing
classification criteria would lead to label warnings that are not
consistent with current HCS requirements in some situations. Thus, at a
minimum, OSHA would need to modify HCS to allow the use of GHS in the
U.S. OSHA cannot simply provide a compliance interpretation that labels
and safety data sheets prepared in accordance with the GHS meet the HCS
requirements because the requirements of a standard cannot be changed
through a compliance interpretation. While there is considerable
overlap between the HCS and the GHS in terms of coverage, there are
differences in the criteria used to classify both substances and
mixtures that can result in different hazards being covered in some
situations. This is particularly true in the area of acute toxicity,
where OSHA is covering more substances under the modified rule than the
current HCS, but potentially fewer mixtures.\8\
---------------------------------------------------------------------------
\8\ The coverage of fewer mixtures is due to the bridging
principles and formula being applied to the mixtures'
classification, rather than being based strictly on a 1 percent cut-
off.
---------------------------------------------------------------------------
Second, it is important to understand that while the costs of
creating SDSs and labels under GHS are borne directly by the chemical
producers, the bulk of the benefits of adopting GHS accrue to the
users. The set of all users includes employers who are direct customers
of a chemical manufacturer, employees who use or are exposed to
workplace chemicals, and emergency responders who typically have no
market relationship with the producers of the chemical. Even if one
thought that market forces might ensure the socially optimal approach
to SDSs between manufacturers of chemicals and their customers, there
are limited market forces at work between the chemical manufacturer and
these two other sets of users--the employees and the emergency response
community. Therefore, the benefits achieved by a uniformity standard,
such as GHS, cannot be obtained in the private market, without
regulation.
OSHA does anticipate that there will be some increased market
pressure to comply with GHS that will affect some firms that may think
that they have no need to switch to the GHS system because they do not
ship their products internationally. Many small firms do not realize
the extent to which they are involved in international trade. There are
probably few companies who have products that are never involved in
international trade, or who never import chemical products and need
hazard communication information for them. Many chemical producers ship
their products to distributors and are unaware of where their products
are ultimately used. OSHA can envision a likely scenario in which these
distributors put pressure on their suppliers to become GHS-compliant.
Further, small companies sell products to larger companies. The larger
companies may use those products to prepare goods that are exported.
These larger companies might also be expected to pressure their small-
firm suppliers to be GHS-compliant. Nevertheless, such an approach
would surely involve a long transition period, with attendant losses in
worker protection and production efficiencies, and it is doubtful that
market pressure alone would achieve full compliance.
The changes made by GHS will involve costs for all parties.
Producers of chemicals will incur substantial costs, but will also
achieve benefits--in part because they themselves benefit as both
producers and users, and in part, as a result of foreign trade benefits
that OSHA has not quantified. Some producers may not see these types of
trade benefits unless they engage in chemical export. However, many
small companies are currently prevented from engaging in international
trade because of the substantial burdens of complying with many
different countries' requirements. International harmonization of
hazard communication requirements would enable these small companies to
become involved in international trade if they so desire.
Of more significance to the concerns of the OSH Act, the changes
also provide substantial benefits to users, including:
Fewer worker illnesses, injuries, fatalities, and
accidents due to a more consistent and comprehensible system that does
not require English literacy to obtain some minimal hazard information;
Greater ease of use of SDSs; and
Less time needed to train workers due to a clearer and
more uniform system.
Because many of these benefits require uniformity, and the benefits
are dispersed throughout a network of producers and users, only some of
which have direct market relationships with each other, OSHA believes
that only a single, uniform standard can achieve the full net benefits
available to a hazard communications system.
C. Profile of Affected Industries
The revisions to the HCS would affect establishments in a variety
of different industries in which employees are exposed to hazardous
chemicals or in which hazardous chemicals are produced. Every workplace
in OSHA's jurisdiction in which employees are exposed to hazardous
chemicals is covered by the HCS and is required to have a hazard
communication program.
The revisions to the HCS are not anticipated to either increase or
decrease the scope of affected industries or establishments. The
revisions define and revise specific classifications and categories of
hazards, but the scope of the requirements under which a chemical,
whether a substance or mixture of substances, becomes subject to the
requirements of the standard is not substantially different from the
previous version of HCS. Therefore, the revisions should have little or
no effect on whether an entire establishment falls within the scope of
the standard. OSHA solicited comment on this determination and received
no comment in the record presenting contrary evidence.
For establishments with employees exposed to hazardous chemicals,
the revisions to the HCS will generally involve management becoming
familiar with and employees receiving training on the new warning
labels and the new format of the SDSs. For establishments producing or
importing hazardous chemicals, generally as part of the chemical
manufacturing industry, these revisions to the standard will involve
reclassifying chemicals in accordance with the new classification
system and revising safety data sheets and labels associated with
hazardous chemicals.
OSHA's estimates of the number of employees covered by the standard
are based on the determination that all production employees in
manufacturing will be covered, and that, in addition, employees in
other industries working in any of the occupations specified in the
PP&E (2009) report would also be exposed to hazardous chemicals.
Table VI-3 provides an overview of the industries and estimated
numbers of employees potentially affected by the HCS. The data in this
table update the estimates provided in the PEA in support of the
proposed rule. They rely on the most recent data from the U.S. Census
Bureau (2007a, 2007b).\9\
---------------------------------------------------------------------------
\9\ U.S. Census Bureau (2007a). County Business Patterns, 2007.
U.S. Department of Commerce. Available at: http://www.census.gov/econ/cbp/.
U.S. Census Bureau (2007b). 2007 Economic Census. U.S.
Department of Commerce. Available at: http://www.census.gov/econ/census07/.
---------------------------------------------------------------------------
The industries and establishments affected by the revisions can be
divided into two categories. The first category contains establishments
that are required to produce labels and SDSs; the second category
contains establishments that do not produce labels or SDSs but are
required to provide employee access to labels and SDSs, supplied by
others, for the chemicals to which their employees may be exposed in
the workplace. As noted in the introduction to this FEA, OSHA has
judged that SDSs and labels for imported chemicals would normally be
produced in the country of origin, and thus would not represent
expenses for importers or other US firms.
BILLING CODE 4510-26-P
[GRAPHIC] [TIFF OMITTED] TR26MR12.003
[GRAPHIC] [TIFF OMITTED] TR26MR12.004
[GRAPHIC] [TIFF OMITTED] TR26MR12.005
[GRAPHIC] [TIFF OMITTED] TR26MR12.006
[GRAPHIC] [TIFF OMITTED] TR26MR12.007
[GRAPHIC] [TIFF OMITTED] TR26MR12.008
BILLING CODE 4510-26-c
As shown in Table VI-3, approximately 75,000 firms, in over 90,000
establishments, create hazardous chemicals (i.e., products, substances,
or mixtures) for which a label and SDS are required in accordance with
the OSHA HCS. In response to testimony presented on the proposed rule,
OSHA has revised its estimate of the number of SDSs (and corresponding
container labels) potentially affected by the revisions to the HCS from
approximately 0.9 million SDSs to approximately 1.4 million SDSs.\10\
OSHA estimates that the adoption of GHS will not significantly change
the numbers of labels and SDSs produced.
---------------------------------------------------------------------------
\10\ A representative from the Independent Lubricant
Manufacturers Association suggested that OSHA had underestimated the
number of SDSs produced per firm in the lubricating oils industry
and that the average firm in the industry produces approximately
1,700 lubricating products requiring an SDS. OSHA has considered
this testimony and accepted the estimate of 1,700 SDSs produced per
firm in NAICS 324191: Petroleum lubricating oil & grease
manufacturing. With 329 affected establishments in this industry,
OSHA's estimate of the number of affected SDSs has increased by
approximately 0.4 million SDSs in the FEA (as compared to the PEA).
The industry profile has been revised accordingly (Document ID
0495 Tr. 296-7).
---------------------------------------------------------------------------
In many instances, firms may be already producing several different
versions of SDSs and labels for the same product to satisfy different
regulatory requirements in different jurisdictions, including SDSs and
labels consistent with GHS criteria. For these products, the revisions
to the OSHA HCS will be satisfied relatively easily and may result in a
reduction in overall compliance costs by reducing the number of
different labels and SDSs needed for each affected product.
The second category of industries and establishments affected by
the revisions contains those that do not produce labels or SDSs but are
required to provide their employees with access to SDSs supplied by
others as part of a hazard communication program covering chemicals to
which employees may be exposed in the workplace. The effects on these
establishments will generally involve promoting employee awareness of
and management familiarization with the revisions to SDSs and labels.
As shown in Table VI-3, an estimated 41 million employees are
potentially exposed to hazardous chemicals in these workplaces and are
covered by the OSHA HCS. Including employees working in establishments
that produce labels and SDSs, a total of 44 million employees would
potentially need to become familiar with the revisions to SDSs and
labels. The estimated number of employees to be trained, as shown in
Table VI-3, is equal to the number of production employees in all
affected industries. As also shown in Table VI-3, OSHA estimates that
there are over five million workplaces where employees may be
potentially exposed to hazardous chemicals.
OSHA received comment from the American Wind Energy Association and
Duke Energy Business Services, LLC that asserted that the Agency had
underestimated the number of employees that would need to be trained in
the electric power generation industry (Document ID 0386 and
0453). OSHA estimated that approximately 49 percent of employees were
production employees in this industry who would need to be trained to
familiarize them with the revisions to the HCS and that an additional
11,000 managers and logistic personnel would receive training as well.
The commenters felt that 60 to 70 percent of employees would need to be
trained. OSHA evaluated the concerns of the AWEA and Duke Energy and
has decided to defer to their expertise on the subject and adopt their
recommendation (by changing the percentage of employees who would need
to be trained in NAICS 2211 Electric power generation, transmission and
distribution to 65 percent). The change from 49 percent of employees to
65 percent of employees to be trained results in a negligible change to
the costs to this industry. Increasing the number of production
employees needing training from 245,715 to 315,623 results in an
increase of about $39 per firm in annualized costs to this industry,
and the costs as a percent of revenues would increase from 0.0052
percent to 0.0060 percent.
D. Benefits
OSHA estimates that the promulgation of the revisions to the HCS
will result in substantial benefits from a variety of sources. OSHA's
estimates of the benefits include improvements in occupational safety
and health and a corresponding reduction in the annual number of
injuries, illnesses, and fatalities sustained by employees from
exposure to hazardous chemicals; cost reductions for producers of
hazardous chemicals; increased efficiencies in the handling and use of
hazardous chemicals; reduced costs to provide HCS training to new
employees; and other benefits as described in this section.
OSHA expects the revisions to the HCS will result in an increased
degree of safety and health for affected employees and a reduction in
the numbers of accidents, fatalities, injuries, and illnesses
associated with exposures to hazardous chemicals.
As explained in detail in Sections IV and XIII of this preamble,
the design of GHS was based on years of extensive research that
demonstrated the effectiveness of pictograms, specific signal words,
and a standardized format.\11\ As a result of this research, OSHA is
confident that the GHS revisions to the HCS for labeling and safety
data sheets will enable employees exposed to workplace chemicals to
more quickly obtain and more easily understand information about the
hazards associated with those chemicals. Warning labels on products
covered by the standard, which provide an immediate visual reminder of
the chemical hazards involved, would be made more intuitive, self-
explanatory, and logical, and the nature and extent of any associated
hazards would be more readily understood as a result of the training
required under the standard. Relatedly, the revisions are expected to
improve the use of appropriate exposure controls and work practices
that can reduce the safety and health risks associated with exposure to
hazardous chemicals.
---------------------------------------------------------------------------
\11\ See Sections IV and XIII of this preamble for a discussion
of the studies related to these issues.
---------------------------------------------------------------------------
In addition, the standardized format of the safety data sheets
would enable critical information to be accessed more easily and
quickly during emergencies. This can reduce the risk of injury,
illness, and death to exposed employees and to rescue personnel and can
also reduce property damage.
It is difficult to quantify precisely how many injuries, illnesses,
and fatalities will be prevented due to the revisions to the HCS. The
benefits associated with the current HCS may help provide a general
sense of the potential magnitude of the benefits of these revisions. A
discussion and analysis of the benefits that would result from the
implementation of the current OSHA HCS were included as part of the
rulemaking process for the promulgation of the current standard in the
1980s.
The current HCS was originally promulgated in two parts. First, a
final rule covering the manufacturing industry was published in the
Federal Register in 1983 (48 FR 53280, Nov. 25, 1983); a second final
rule covering other general industries, maritime industries,
construction industries, and agricultural industries was published in
the Federal Register in 1987 (52 FR 31852, Aug. 24, 1987).
For both of these final rules, OSHA conducted research specifically
regarding the benefits that could be expected from the promulgation of
these standards, as described in the preambles to the final rules. In
addition, through the rulemaking process, OSHA evaluated the best
available evidence, including the data and comments submitted by the
public.
The information, data sources, analyses, and findings related to
the estimation of the benefits associated with these standards are
included in the public records for the rulemakings. The complete
rulemaking records for these standards can be found in OSHA public
dockets H-022B and H-022D.
The estimated benefits associated with the Hazard Communication
Standards were published in the Federal Register with the promulgation
of the final standards (48 FR 53329, Nov. 25, 1983 and 52 FR 31872,
Aug. 24, 1987). OSHA estimated that compliance with the various Hazard
Communication Standards would produce annual benefits that would
include the prevention of 31,841 non-lost-workday injuries and
illnesses, 20,263 lost-workday injuries and illnesses, 6,410 chronic
illnesses, and 4,260 fatalities.
Using a willingness-to-pay approach for valuing these benefits,
OSHA determined that the annual safety and health benefits would be
over $18.2 billion annually, expressed in 1985 dollars. Applying the
BLS inflation calculator, the $18.2 billion of benefits in 1985 is
equivalent to $36.7 billion of benefits in 2010 after adjusting for
inflation of 102 percent of the period.12 13
---------------------------------------------------------------------------
\12\ http://data.bls.gov/cgi-bin/cpicalc.pl. The BLS inflation
calculator was used on January 18, 2011.
\13\ Using OSHA's current willingness-to-pay estimates of $8.7
million per life saved and $62,000 per injury avoided, those
benefits are equivalent to about $38.7 billion worth of benefits in
2010 dollars. OSHA decided to use the lower benefits estimate in the
text ($36.7 billion), which is consistent with the estimation
procedure used for the proposed rule.
---------------------------------------------------------------------------
Based on the material presented in this preamble, OSHA expects that
the revisions to the HCS will result in incremental improvements in
employee health and safety above that already achieved under the
current HCS. In the PEA, OSHA estimated that compliance with the
revisions to the HCS would result in benefits equal to 1 percent of the
health and safety benefits attributed to the current HCS. It is
conceivable that actual benefits might be somewhat lower, but because
GHS is expected to result, in some situations, in more timely and
appropriate treatment of exposed workers, OSHA expects that actual
benefits may be larger, perhaps several times larger.\14\ OSHA
solicited comment on the anticipated health and safety benefits of the
revisions to the HCS and received numerous comments indicating that
stakeholders anticipate increased worker protection as a result of the
revisions. The Alliance of Hazardous Materials Professionals responded
that they believed that these revisions to the HCS would yield
"benefits in preventing injuries and illnesses" (Document ID
0327) and DuPont Company reported that they "believe domestic
implementation of the GHS will serve to further enhance worker
protection through a more standardized approach to hazard
classification and communication" (Document ID 0329). The
National Association of Chemical Distributors said that their
association members "believe that there are benefits associated with
preventing injuries, illnesses and fatalities through clearer and more
accessible information" (Document ID 0341) and likewise, the
Communications Workers of America reported that they believed that
application of the elements of the revised HCS "would lead to a
reduction in the incidence of workplace injuries, illnesses, and
fatalities" (Document ID 0349). This sentiment was echoed by
the American Health Care Association, National Center for Assisted
Living who felt that the revised HCS will "reduce incidence of
chemical-related illnesses and injuries" (Document ID 0346),
and the Associated General Contractors of America who felt that the
revisions "will allow employees to easier understand hazard
information and will assist in better job planning and injury
prevention" and that they "should reduce eye and skin contact
injuries" (Document ID 0404). The U.S. Chamber of Commerce
stated that they "(b)elieve * * * the new rule will improve workplace
safety" (Document ID 0397). One commenter (Document ID
0033), representing an organization whose membership includes
first responders and emergency management, wrote the following in
response to the Advance Notice of Proposed Rulemaking (ANPR):
---------------------------------------------------------------------------
\14\ OSHA believes that a reasonable range for the magnitude of
the health and safety benefits resulting from the proposed revisions
would be between 0.5 percent and 5 percent of the benefits
associated with the current HCS. These ranges are considered in the
sensitivity analysis presented in Section VI.L of this preamble.
The emergency planning and first responder community depends
upon MSDS information for life and safety. The ability to
immediately examine an MSDS and glean hazard and response
information at the scene of an incident is critically important. The
lives of first responders, employees of the facility and the public
---------------------------------------------------------------------------
depend upon the accuracy and ease of use of the MSDS.
Some stakeholders questioned whether the revisions would result in any
health and safety benefits. For example, the Society of Plastics
Industries, Inc. felt that there was a "serious question as to what
improvements to workplace safety and health can reasonably be
expected" (Document ID 0392), and the U.S. Chamber of
Commerce was concerned that OSHA "overestimated the utility and
benefits of this proposed revision to the HCS" (Document ID
0397). However, even this commenter suggested the rule " "*
* * will promote consistency in the identification, classification, and
labeling of chemicals, improve workplace safety, and facilitate
business growth and international trade." (Document ID 0392).
The Agency feels that the record supports that these revisions to the
HCS will reduce confusion and lead to better hazard communication,
which will translate into fewer accidents, illness, injuries, and
fatalities. OSHA's estimate that these revisions will provide one
percent of the benefits attributed to the original HCS rulemaking
represents a very small and easily realized improvement of workplace
safety and health. The Agency did not receive additional comments on
what level of benefits commenters believed would be more reasonable or
accurate and therefore OSHA has retained the estimated health and
safety benefits as part of the FEA. OSHA is confident that its initial
estimates of the reductions in injuries, illnesses, and fatalities is a
minimal estimate given the general agreement by almost all parties that
the rule will have safety and health benefits.
OSHA prepared a sensitivity analysis to test the effect of
variations in its estimates and found that, even if the estimated
health and safety benefits were overstated by a factor of 2 (or even if
the health and safety benefits were omitted altogether--see Table VI-
1), the benefits would still exceed the costs of the final rule. Those
results can be seen in Section VI.L: Sensitivity Analysis in this
preamble.
Using the 1 percent estimate, OSHA anticipates that once all
requirements take effect for the final rule, they would result in the
prevention of an additional 318 non-lost-workday injuries and
illnesses, 203 lost-workday injuries and illnesses, 64 chronic
illnesses, and 43 fatalities annually. The monetized value of these
health and safety benefits is an estimated $367 million annually in
2010 dollars.
In order to obtain a sense of how realistic these estimated safety
and health benefits are in light of the current level of occupational
injuries, illnesses, and fatalities that are chemically related, OSHA
reviewed relevant BLS data for the periods 1992-2007. OSHA's
examination of these data shows a 42 percent decline in chemically
related acute injuries and illnesses over the period, but both remain
significant problems--55,400 chemically related illnesses and 125
chemically related fatalities in 2007. However these readily measurable
reported acute illnesses and fatalities are dwarfed by chronic
illnesses and fatalities. For chronic illness fatalities, there is
little information available, and certainly no annual time-series data.
The most recent estimate is that there were 46,900 to 73,700 fatalities
due to occupational illnesses in 1992 (Document ID 0274). OSHA
believes these more recent data from 1992-2007 suggest that the HCS has
had a desirable effect on chemically related illnesses and injuries,
but there remains a very significant role for further and better hazard
information, as would be provided by aligning the current HCS with the
GHS.
The annual health and safety benefits associated with the revisions
to the OSHA HCS are estimated to begin after full implementation of the
changes and associated employee training. The phase-in period for the
main provisions of the final rule is approximately four years from the
date of publication. Thus, in order to calculate the estimated
annualized health and safety benefits over a twenty-year period
associated with this rule in a manner that would be comparable to the
corresponding annualized costs, the delay in the realization of the
benefits was incorporated into the calculation. Using a discount rate
of 7 percent, the estimated annual benefits of $367 million, beginning
four years after the effective date of the final rule, were multiplied
by 0.6803 to calculate the annualized benefits over a twenty-year
period beginning with the effective date of the final rule.\15\ Thus,
the annualized monetized benefits associated with the reduction in
safety and health risks attributable to the revisions to the HCS are an
estimated $250 million.
---------------------------------------------------------------------------
\15\ The formula for annualizing the benefits is equal to:
[(1.07)-4] * [ (1--(1.07)-16)/0.07] * [0.07/
(1--(1.07)-20)],where the first term in brackets reflects
the four year delay until annual benefits are realized; the second
term in brackets reflects the present value of sixteen years of
annual benefits (from years 5 through 20), and the third term in
brackets annualizes the present value of benefits over a 20-year
period.
Other substantial benefits, in addition to the improved
occupational safety and health of affected employees, are also expected
to result from this rulemaking, as discussed in the following
paragraphs.
The harmonization of hazard classifications, safety data sheet
formats, and warning labels for affected chemicals and products would
yield substantial savings to the businesses involved in these
activities. Fewer different SDSs would have to be produced for affected
chemicals, and many SDSs would be able to be produced at lower cost due
to harmonization and standardization. The record supports these savings
with comment from Stericycle, Inc. stating that they anticipate that
"less time will be spent in reviewing new chemicals due to the changed
format and better characterizations of the hazard" (Document ID
0338), from the Ecological and Toxicological Association of
Dyes and Organic Pigments Manufacturers (ETAD), which felt that these
revisions to the HCS would "ultimately increase efficiency and reduce
time needed to prepare labels and SDSs" (Document ID 0374),
and from ORC Worldwide, which said that the "use of one harmonized
classification system is expected to significantly reduce the time
needed to classify global products" (Document ID 0123). The
American Chemistry Council reported that they would "expect a positive
economic and time impact on developing and reviewing SDSs" (Document
ID 0393) as a result of these revisions to the HCS. Troy
Corporation reported that they believed that "providing harmonized
SDSs will reduce development and maintenance time" (Document ID
0352) and that there "will be tangible savings when materials
only have to be classified once instead of multiple times" (Document
ID 0128). Two commenters suggested that harmonization could
lead to a 50 percent time savings in classification (Document ID
0313 and 0327). The benefits represented by these cost
reductions would primarily affect businesses involved in chemical
manufacturing.
In addition, reductions in operating costs are also expected as a
result of the promulgation of the revisions to the HCS for many
businesses that purchase or use hazardous chemicals. The current non-
uniformity of SDSs and labels received by establishments in many
industries requires employees and managers to spend additional time on
a daily basis to ascertain the appropriate way to handle and store the
hazardous chemicals in their workplaces. Under the revised standard,
the presence of uniform and consistent information would help employers
and employees to make decisions more efficiently and save substantial
time. There is ample evidence in the record that stakeholders
anticipate that the revisions to the HCS will improve the quality of
the SDSs and labels and that the standardization of the SDS and label
elements will increase the consistency of the hazard information and
better communicate the hazards to users (See Document ID 0313,
0327, 0329, 0334, 0335, 0336, 0339, 0341, 0344, 0347, 0351, 0352, 0354,
0357, 0363, 0365, 0370, 0372, 0374, 0377, 0379, 0382, 0386, 0389, 0390,
0399, 0404, 0405, 0408, 0409, 0410, and 0414). Stakeholders reported
that they expected that simplification and reduction in "the number of
documents that we manage * * * will reduce expenses" (Document ID
0018), and Tom Duffy testified on behalf of the United
Steelworkers of America at the Pittsburgh, PA, public hearing that a
uniform system for SDSs would result in time savings (Document ID
0499 Tr. 171-72). These sentiments were echoed by Gary
Valasek, who represented the Intercontinental Chemical Corporation
(Document ID 0499 Tr. 63-64), the National Association of
Chemical Distributors, which stated that standardized SDSs and labels
would "create a more efficient process for chemical distributors"
(Document ID 0341), and Wacker Chemical Company, which
reported "that uniformity in SDS and labels will help employees and
customers * * * find needed information" (Document ID 0335).
The International Brotherhood of Teamsters reported that the
"standardized, specific approach to labels and SDSs with a set format,
content, and order will help with consistency and comprehensibility,
and improve the SDSs ability to communicate hazard info to workers"
(Document ID 0357). The American Industrial Hygiene
Association felt that "standardized label elements will make hazard
identification easier" (Document ID 0365). The American
Petroleum Institute commented that the revisions to the HCS would
"improve downstream hazard assessments" (Document ID 0376).
OSHA solicited comment on its estimated monetized benefits in the PEA
arising from increased efficiency in handling hazardous materials.
While a few stakeholders questioned OSHA's benefits estimates, they did
not offer an alternative methodology for estimating potential time
savings; nor did they offer quantitative alternatives for OSHA
to evaluate. As demonstrated throughout this preamble, stakeholders
were largely supportive of OSHA's estimates.
For the benefits estimated in the PEA, PP&E worked closely with
stakeholders, conducting multiple interviews and extensive research on
the processes that companies use to classify chemical hazards, to
develop SDSs and labels, and to handle, store, and use hazardous
chemicals. Based on interviews with hazardous materials professionals
in more than a dozen affected establishments, PP&E evaluated how these
processes would be affected by the proposed revisions to the HCS and
analyzed the potential savings that could reasonably be expected as a
result of adopting these revisions.
For the PEA, OSHA used the PP&E 2009 report (Document ID
0273) to develop estimates of the cost reductions that the
affected companies would expect to obtain as a result of the revisions
to the OSHA HCS.\16\ Among the various benefits expected to be realized
as a result of the implementation of the revisions, as described in
this section, OSHA quantified two general categories of cost savings in
the PEA and has maintained the methodology employed to create those
estimates \17\ but used the most recent available economic data in
arriving at the estimates of costs presented in this final analysis.
---------------------------------------------------------------------------
\16\ The full final report from PP&E detailing the extensive
process by which these estimates were derived is available on the
rulemaking docket. See Document ID 0550.
\17\ There is no indication that two years would have been
sufficient time to affect the processes involved with handling
hazardous chemicals, and therefore OSHA did not feel it necessary to
re-estimate the savings parameters established through PP&E's
research.
---------------------------------------------------------------------------
In the PEA (74 FR 50280, 50322, Sept. 30, 2009), OSHA estimated the
number of hours that each industry would save by improving the
efficiency and productivity of personnel who use SDSs in performing
their job functions. OSHA estimated that the amount of time spent
during affected activities in the manufacturing sector could be reduced
by 3 percent for health and safety supervisors and by 15 percent for
logistics personnel specializing in handling hazardous chemicals.\18\
The Agency updated the number of health and safety supervisors and
logistics personnel for this FEA to reflect the most recent data and
estimated that the time reductions for handling hazardous chemicals,
and the associated cost savings, would apply to about 7,000 health and
safety supervisors and 49,000 logistics personnel in the manufacturing
sector and would yield annualized benefits of approximately $475
million.\19\ Similar potential time and cost savings as a result of the
revisions to the OSHA HCS were not quantified for the non-manufacturing
sectors.
---------------------------------------------------------------------------
\18\ For example, as described by PP&E (2009, Document ID
0273), the job of a logistics person, depending on the
company, consists of the following tasks: (1) Receive hazardous
chemicals; (2) gather the associated SDSs--either those that are
attached to the shipment or those that are attached to the invoice;
(3) extract the relevant information from the SDSs and enter it in
the plant's SDS management system; (4) insert paper copies of the
SDSs into the (hard copy) SDS management folder; (5) if the
information is not available (particularly in the older 9-section
SDSs), then look for 12-section SDSs prepared by some other
manufacturer; (6) prepare in-plant labels; (7) determine special
storage and use requirements, make appropriate arrangements for
short-term and long-term storage, and distribute information to
different process lines or field offices; (9) participate in the
training of line supervisors and production workers; (10) train new
employees; and (11) carry out other logistics duties at the plant.
The GHS standard, by making the structure and content of SDS
uniform, would help to reduce the time it takes to perform each of
the above tasks.
\19\ These estimates assume 2,000 hours of work a year for 7,070
health and safety supervisors and 49,486 logistics personnel
specializing in handling hazardous chemicals in the manufacturing
sector; an hourly wage of $66.01 and $45.17, respectively; and a
time savings of 3 percent and 15 percent, respectively, for health
and safety supervisors and logistics personnel. The resulting annual
savings of $699 million was multiplied by 0.6803 to annualize the
savings over a twenty-year period with savings not accruing until
four years after the effective date of the revisions (Document ID
0273).
---------------------------------------------------------------------------
As part of the PEA (Id. at 50322-23), OSHA also estimated that, for
the manufacturing sectors, the costs associated with the creation and
revision of SDSs in future years would be reduced as a result of the
revisions to the HCS. The methodology for creating this estimate has
been retained for the FEA but new economic data were incorporated where
available. The creation and revision of individual SDSs will be less
burdensome, and, in addition, fewer different versions of SDSs would
need to be produced for affected chemicals and products. OSHA estimated
that, depending on firm size, the combination of these two effects
would result in annual savings equivalent to between 2.5 and 4 hours of
a professional's time per existing SDS and a total annualized savings
of $32 million.\20\
---------------------------------------------------------------------------
\20\ These estimates assume \1/3\ of the estimated 1,414,636
SDSs are reviewed each year; savings per SDS is between 2 [frac12]
and 4 hours, depending on firm size (with an average per SDS of
about 3.2 hours); personnel reviewing the SDSs receive an hourly
wage of $66; and existing compliance rates are between 1 percent and
75 percent, depending on firm size (with an average per SDS of about
53 percent). The resulting annual savings of $47 million was
multiplied by 0.6803 to annualize the savings over a twenty-year
period with savings not accruing until four years after the
effective date of the revisions.
---------------------------------------------------------------------------
Combining the improved productivity of personnel who use SDSs and
the improved efficiency of those who revise SDSs and labels, OSHA
concluded that the annualized productivity savings for companies would
be an estimated $507 million.
Another area in which the final rule is likely to provide cost
savings to industry is in the provision of hazard communication
training to new employees after the transition period. Both the current
HCS and the revised HCS require employers to provide training on the
safe handling of chemicals, on understanding SDSs and labels, and on
being familiar with other information crucial to worker safety.
Employers are permitted to offer training for categories of hazards
(such as flammability or carcinogenicity) rather than training
individually on each chemical. The primary sources of information for
this training are the SDSs supplied by manufacturers, and the primary
method for employees to determine the hazard associated with a specific
chemical they are using is through the manufacturer's HCS-compliant
label.
Under the revised HCS, SDSs and labels produced in the United
States will all be formatted in the same way. As more countries and
regions adopt the GHS, fewer variations of SDSs and labels will be seen
in the workplace. Information will be located in the same place on
every SDS and label an employee will encounter. Employers will no
longer have to train on as many SDS formats; nor will they need to
devote as many resources to gather information on work practices, PPE,
etc. SDSs and labels will be required to provide complete hazard
information, and the language that the hazard information is presented
in will be uniform across labels and section 2 of the SDSs. The
inclusion of the pictograms and standardized hazard statement removes
or, at least reduces, training time spent on interpreting various--and
in some cases ambiguous--hazard warnings that current SDSs and labels
may bear. The standardized labels and elements based on the detailed
criteria for each hazard also greatly simplify training by facilitating
training on "categories of hazard" rather than having to cover every
chemical individually where the hazard determination is based on broad
definitions. All of these changes can be expected to reduce the costs
of training employees to recognize chemical hazards in the workplace.
The rulemaking record included numerous descriptions of the
difficulties for both employees andemployers associated with training
under the current HCS (see Document
ID 0307, 0499 Tr. 92-3, 0499 Tr. 167-8, 0499 Tr. 175, 0527)
and supported the idea that training would be easier--and therefore
cheaper--under the revised HCS (see Document ID 0123, 0338,
0408, 0414, 0494 Tr. 74-5, 0495 Tr. 308-9, 0497 Tr. 95-6, 0499 Tr. 93,
0499 Tr. 96, 0499 Tr. 190-91). Nevertheless, given that the annualized
benefits of the final rule already significantly exceed the costs, OSHA
did not feel it was necessary to try to develop, from the limited data
available, a quantified estimate of the monetized savings resulting
from simplified training.\21\
---------------------------------------------------------------------------
\21\ However, in the sensitivity analysis presented in Section
VI.L of this preamble, OSHA develops an estimate of monetized cost
savings from simplified hazard communication training based on one
commenter's estimate of the percentage reduction in training time
resulting from the final rule.
---------------------------------------------------------------------------
An additional benefit of the adoption of GHS is that it would
facilitate international trade, increasing competition, increasing
export opportunities for U.S. businesses, reducing costs for imported
products, and generally expanding the selection of chemicals and
products available to U.S. businesses and consumers. The Society for
Chemical Manufacturers and Affiliates, for example, stated in their
comment that while "SOCMA member companies do not foresee significant
savings from the change * * * for companies that do business globally
there will be" (Document ID 0402). While OSHA did not take
quantitative benefits for these savings, the Agency believes that firms
that operate globally may realize a cost savings as a result of the
adoption of the GHS (Document ID 0336, 0339, 0361, and 0405).
As a result of the direct savings resulting from the harmonization and
the associated increase in international competition, prices for the
affected chemicals and products, and the corresponding goods and
services using them, should decline, although perhaps only by a small
amount.
Finally, the GHS modifications to the OSHA HCS would meet the
international goals for adoption and implementation of the GHS that
have been supported by the U.S. government. Implementing GHS in U.S.
federal laws and policies through appropriate legislative and
regulatory action was anticipated by the U.S. support of international
mandates regarding the GHS in the Intergovernmental Forum on Chemical
Safety, the World Summit on Sustainable Development, and the United
Nations. It is also consistent with the established goals of the
Strategic Approach to International Chemical Management that the U.S.
helped to craft.
A number of commenters suggested that the benefits OSHA estimated
will result from this rule were incorrect or overstated. The National
Association of Homebuilders expressed a belief that OSHA's "assumption
that the proposed revisions to the HCS [would] result in cost
reductions * * * due to productivity gains is false" (Document ID
0372), while the American Composites Manufacturers Association
voiced concern that the benefits OSHA had estimated were speculative
(Document ID 0407). Southern Company submitted that "the
benefits of adopting the GHS are minimal at best" (Document ID
0378). Applied Safety and Ergonomics, Inc., urged OSHA to
adopt a more conservative view of the expected benefits as they
asserted that "it is possible that many of the implied or expected
benefits of the proposed changes to the HCS may not materialize"
(Document ID 0396). OSHA takes these comments seriously and
evaluated all concerns raised by stakeholders on the estimated benefits
of this standard. Unfortunately, most commenters did not include
adequate detail or data that would allow the Agency to evaluate
alternative benefits estimates. While future benefits (or costs) cannot
be estimated with scientific precision, OSHA believes that the
estimated benefits associated with this standard are based on sound
data and that the resulting estimates are reasonable and have largely
been supported by testimony and comment from stakeholders. It should be
noted that many commenters who raised questions or concerns over OSHA's
benefits estimates still largely supported the overall aim of the
rulemaking and wished to see OSHA proceed with promulgation. The Agency
addresses the inherent uncertainty in the economic analysis in Section
VI.L Sensitivity Analysis in this preamble. In that section, various
parameters are adjusted to evaluate the impact on the overall cost and
benefits of the rule, and OSHA finds that even if estimated benefits
were grossly overstated, this standard's benefits would still exceed
costs.
E. Technological Feasibility
In accordance with the OSH Act, OSHA is required to demonstrate
that occupational safety and health standards promulgated by the Agency
are technologically feasible. OSHA has reviewed the requirements that
would be imposed by the rule, and has assessed their technological
feasibility. As a result of this review, OSHA has determined that
compliance with the requirements of the rule is technologically
feasible for all affected industries.
The revisions to OSHA's HCS would require employers that produce
chemicals to reclassify chemicals in accordance with the new
classification criteria and revise safety data sheets and labels
associated with hazardous chemicals. Compliance with these requirements
is not expected to involve any technological obstacles. A comment in
the record indicated that "[s]ome of the work [* * *] has already been
done in order to comply with GHS implementation in Asian countries"
(Document ID 0405; see also Document ID 0352, 0377,
and 0410). In addition to stakeholder comments, a January 4, 2011 press
release from the European Chemicals Agency (ECHA) announced that the
ECHA had received 3,114,835 notifications of 24,529 substances for the
Classification and Labelling Inventory. Industry was required to notify
the classification and labeling of all chemical substances that are
hazardous or subject to registration under the Registration, Evaluation
and Authorization of Chemicals (REACH) regulation and placed on the EU
market in accordance with the GHS criteria. NIOSH is also currently
working to update its International Chemical Safety Cards and Pocket
Guide to incorporate the GHS classifications, which will further reduce
the technological burdens of reclassification borne by manufacturers.
(For a more detailed discussion of the EU implementation of the GHS and
NIOSH's classification work, see Section XIII. Summary and Explanation
of the Final Rule in this preamble.) This evidence lends support to
OSHA's assertion that the requirements of the revisions to the HCS will
not prove technologically infeasible. The rule would also require
employers whose workplaces involve potential exposure to hazardous
chemicals to train employees on the relevant aspects of the revised
approach to hazard communication. Affected employees would need
additional training to explain the new labels and safety data sheets.
Compliance with these requirements is not expected to involve any
technological obstacles.
The revisions to the HCS will require establishments that package
or label hazardous chemicals to affix labels that include hazard
warning pictograms enclosed in a red bordered diamond. While some
establishments may not currently be printing labels in colors other
than black and white, color printing technology is widely available
and printing labels with a red bordered diamond or purchasing
preprinted labels with a red bordered diamond is not expected to
involve any technological obstacles. Research conducted by ERG (2010)
under contract for OSHA found that printer technology is rapidly
evolving--resulting in lower costs for printers and printing supplies
and making better technology available to a wider range of buyers.
Combined with currently available printing technology, this clearly
demonstrates that printing product labels in color is technologically
feasible.
Compliance with all of the requirements of the rule can be achieved
with readily and widely available technologies. Businesses in the
affected industries have long been required to be in compliance with
the existing HCS, which includes similar requirements. The revised HCS
would simply require modifying the labels and SDSs for hazardous
chemicals, adding some training to ensure employees are familiar with
these changes, and upgrading printing technology with widely available
color printers or purchasing preprinted color labels. No new
technologies are required for compliance with the modifications to the
HCS. OSHA is aware that many U.S. businesses in the affected industries
have already begun implementing many of the requirements of the GHS in
order to meet the new foreign requirements for exported products.
Therefore, OSHA believes that there are no technological constraints
associated with compliance with any of the requirements of the
revisions to the HCS.
F. Costs of Compliance
Introduction
This section presents the estimated costs of compliance for the
revisions to the OSHA HCS. The estimated costs of compliance represent
the additional costs necessary for employers to achieve full compliance
with the new requirements of the final rule. They do not include costs
associated with firms whose current practices are already in compliance
with the new requirements.
The costs of compliance with the revisions to the HCS consist of
four main categories: (1) The cost of reclassification and revision of
SDSs and labels, (2) the cost of management familiarization and other
management costs associated with the administration of hazard
communication programs, (3) the cost of training employees, and (4) the
cost of printing labels for hazardous chemicals in color. The first
three categories are considered to be one-time transitional costs and
were included in the PEA in support of the proposed rule. The fourth
category is new and was developed in response to comments on the
proposed rule. It includes both one-time transitional costs and costs
that recur throughout the life of the rule.
The estimated compliance costs are based on a determination made by
the Agency that the revisions would not significantly change the number
of chemicals or products for which an SDS will be required. This also
means that there will be no change in the number of establishments that
are required to implement a hazard communication program. OSHA received
no comments as part of the rulemaking record for this standard
challenging this determination.
Other than the direct costs of reclassification and relabeling, the
estimated compliance costs do not include any further costs or impacts
that may result from the reclassification or relabeling of chemicals
and products already subject to the HCS, such as possible changes in
production or demand for products. Theoretically, such impacts, if any,
with regard to possible changes in the uses and applications of
affected chemicals, could be positive as well as negative. OSHA has
determined that such effects, if any, will not be significant, and
received no comment from stakeholders disputing this determination.
In addition to the revisions to the HCS, the rulemaking also
includes related revisions to other OSHA standards. The revisions to
the other standards generally ensure that all OSHA requirements related
to hazard communication remain consistent with each other and become
consistent with the revised HCS. OSHA has determined that the revisions
to the other standards would not impose significant costs beyond those
reflected in the compliance cost estimates for this rulemaking.
In order to have compliance costs presented on a consistent and
comparable basis across various regulatory activities, the costs of
compliance for this rule are expressed in annualized terms. Annualized
costs represent the more appropriate measure for assessing the longer-
term potential impacts of the rulemaking and for purposes of comparing
compliance costs and cost-effectiveness across diverse regulations with
a consistent metric. In addition, annualized costs are often used for
accounting purposes to assess the cumulative costs of regulations on
the economy or specific parts of the economy across different
regulatory programs or across years. Annualized costs also permit costs
and benefits to be presented in a comparable manner.
A seven percent discount rate was applied to costs incurred in
future years to calculate the present value of these costs for the base
year in which the standard becomes effective, and the same discount
rate was then applied to the total present value costs, over a 20-year
period, to calculate the annualized cost.\22\
---------------------------------------------------------------------------
\22\ OSHA annualized costs for this rule over a 20-year period
in accordance with Executive Order 13563, which directs agencies
"to use the best available techniques to quantify anticipated
present and future benefits and costs as accurately as possible."
In addition, OMB Circular A-4 states that analysis should include
all future costs and benefits using a "rule of reason" to consider
for how long it can reasonably predict the future and limit its
analysis to this time period. Annualization should not be confused
with depreciation or amortization for tax purposes. Annualization
spreads costs out evenly over the time period (similar to the
payments on a mortgage) to facilitate comparison of costs and
benefits across different years. In this analysis, OSHA estimated a
lifetime for hardware purchases (5 years for printers, for instance)
which is unrelated to the annualization period. OSHA felt that an
annualization period much shorter than 20 years (say, 10 years)
would have been inappropriate for this rule because of the lagged
phase-in of provisions (some of which will not take effect until
five years after the final rule is published).
---------------------------------------------------------------------------
Table VI-4 shows the estimated annualized compliance cost by cost
category and by industry sector. All costs are reported in 2010
dollars. As shown in Table VI-4, the total annualized cost of
compliance with the rulemaking is estimated to be about $201 million.
Of this amount, the annualized cost of chemical hazard reclassification
and revision of SDSs and labels is an estimated $22.5 million, the
annualized cost of training employees is an estimated $95.4 million,
the annualized cost of management familiarization and other management
costs is an estimated $59.0 million, and the additional annualized
label printing costs, incurred to comply with the requirement of a
black pictogram surrounded by a red-bordered diamond, is an estimated
$24.1 million.
As shown at the bottom of Table VI-4, most of the compliance cost
associated with chemical hazard reclassification and revision of SDSs
and labels would be borne by the chemical manufacturing industry (shown
as the total for industries that produce SDSs and labels). Table VI-4
also shows that compliance costs are spread across all industries in
the U.S. economy subject to OSHA jurisdiction, reflecting the fact that
employee exposures to hazardous chemicals occur in almost every
industry sector.
Other than the costs of printing labels in color, OSHA expects that
all compliance costs would be incurred over a period of four years, as
the rule would incorporate a four-year transition
period into the compliance schedule for the standard. Specifically, for
purposes of estimating the annualized compliance costs, OSHA assumed
that the compliance costs associated with employee training and
management familiarization would be incurred in the two-year period
following the effective date of the final standard, and that other one-
time compliance costs would be incurred in the four-year period
following the effective date of the final standard. Initial printer
costs to facilitate color printing would also be incurred during the
four-year period following the effective date of the final standard,
but all other color-printing costs would occur subsequent to the four-
year transition period on a recurring annual basis.
BILLING CODE 4510-26-P
[GRAPHIC] [TIFF OMITTED] TR26MR12.009
[GRAPHIC] [TIFF OMITTED] TR26MR12.010
[GRAPHIC] [TIFF OMITTED] TR26MR12.011
[GRAPHIC] [TIFF OMITTED] TR26MR12.012
[GRAPHIC] [TIFF OMITTED] TR26MR12.013
[GRAPHIC] [TIFF OMITTED] TR26MR12.014
BILLING CODE 4510-26-C
In the appendix to this cost section, Table VI-8 shows, by industry
and by cost element, total non-annualized (non-discounted) compliance
costs of about $2.1 billion estimated to be incurred during the four-
year phase-in of the revisions to the HCS.
OSHA received numerous comments on additional costs that had not
been considered as part of the PEA. OSHA has carefully evaluated those
comments on costs and prepared the following responses.
Stakeholders were concerned about the costs associated with
relabeling current inventory. Procter & Gamble reported that they felt
"the largest economic impact of GHS compliance to our business will be
in the area of re-labeling" (Document ID 0381) and numerous
other commenters echoed those concerns (Document ID 0386,
0392, 0393, 0400, and 0402). OSHA anticipates that the four-year phase-
in for the revisions to the OSHA HCS (increased from three years in the
proposed rule) will provide adequate time for companies to deplete
inventory and replace in-house containers that are labeled in
accordance with the original OSHA HCS and therefore will mitigate any
costs associated with relabeling in-house containers or products in
inventory.
The Society of Chemical Manufacturers and Affiliates was concerned
that OSHA had not considered the costs associated with mailing revised
labels, stating that "a large portion of label revisions will go via
the mail service. If a chemical manufacturer produces 75 chemicals and
has 50 customers at 70 cents a mailing, it could cost the company as
much as $2625.00" (Document ID 0402). The revisions to the
HCS do not require that establishments mail revised labels to
customers. Manufacturers are only required to provide products labeled
in accordance with the GHS criteria by the effective date. OSHA did
consider the costs associated with mailing updated SDSs and determined
that manufacturers are currently providing updated paper or electronic
SDSs to customers as they are revised and would not incur additional
costs associated with this standard.
Some comments felt that OSHA had overlooked the time and costs
associated with relabeling in-house containers with GHS compliant
labels (Document ID 0378 and 0386). The phase-in period for
the revisions to the HCS provides adequate time for firms to deplete
products in inventory that are not labeled with GHS-compliant labels
and to replace workplace containers or signs/permanent labels (such as
regulated area signs) in the course of the normal cycle for wear-and-
tear replacement. OSHA believes that any costs incurred that are
outside the costs that would normally be incurred to replace in-house
containers would be negligible and has not estimated a cost for this
activity.
Some stakeholders anticipated costs associated with translating
labels and SDSs into Spanish (Document ID 0381 and 0393).
While some companies may find it necessary, based on customer demand,
to provide products with labels and SDSs printed in Spanish, the
revisions to the OSHA HCS do not contain any requirement for
translating labels or SDSs into Spanish. OSHA has not taken costs
related to translating labels and SDSs as part of this FEA.
OSHA received comment that firms will incur costs associated with
managing multiple SDSs during the transition period. For example, the
Society of Plastics Industry, Inc., reported that "multiple suppliers
of the same chemical [may] switch over to the GHS on different
schedules" and that "additional time will be required for personnel
to sort out and implement appropriate measures for managing this
situation" (Document ID 0392, 0402, 0415, and 0452). OSHA
appreciates that there may be some time during the transition period
where some SDSs are GHS-compliant while others are not. However, given
the non-uniformity of SDSs currently circulating to firms, the Agency
feels that users will already have a system in place for managing
multiple SDSs for identical products and that no additional costs will
be incurred as a result of the transition to new SDSs.
The U.S. Chamber of Commerce expressed concern that "employers
will also incur legal costs for counsel to review and analyze the
revised SDSs to make sure the SDSs provide appropriate explanations and
protection from liability" (Document ID 0397). However, the
final rule primarily changes the format of SDSs, and generally does not
make substantial changes to the categories of information that must be
included in the SDS. OSHA does not see why a new legal review to
protect against tort liability would be necessary in such
circumstances. In addition, the Agency believes that such legal costs
would be relatively rare and not representative of the vast majority of
employers. Furthermore, such legal costs as occur may simply be an
alternative to other in-house professional review services that OSHA
has already included in the costs. Finally, employers incurring such
legal costs for SDS review arguably have been regularly incurring these
costs under the existing HCS as part of periodic SDS changes; in that
case, they are costs not attributable to this final rule.
The Society of Chemical Manufacturers and Affiliates felt that
costs would be incurred because "someone will have to inventory all of
the MSDSs, make the required changes and then communicate those changes
to customers and other affected personnel" (Document ID
0402). The revisions to the OSHA HCS do not require
manufacturers to provide new SDSs to customers who have purchased a
product and received an SDS in the past. This final rule also includes
a four-year phase-in period for firms to update their SDSs and requires
only that those updated, GHS-compliant SDSs be provided to users who
purchase a company's product after the effective date. OSHA realizes
that some firms may choose to provide updated SDSs to past purchasers
of their products, but the updates to the OSHA HCS do not require that
they do so. Subsequently, OSHA has not taken any costs related to this
activity.
Ferro Corporation's comment in the rulemaking record expressed
concern that OSHA did not take into account conversion costs for
"MSDSs and labels for experimental products that are being resampled"
(Document ID 0363). OSHA's analysis does not make a
distinction between commercial and experimental products, but it does
not exclude costs associated with experimental products. The Agency
feels that this economic analysis captures those costs as well as the
transitional costs for products that are sold commercially.
The Society of Plastics Industry, Inc. expressed concern that the
revisions to the OSHA HCS would require employers "to perform new
personal protective equipment (PPE) hazard assessments, select new PPE
or select PPE for workers who did not previously use it" or "to add
or modify ventilation systems or to have their employees use
respiratory protection to address newly discovered hazards, and to
implement respiratory protection programs" (Document ID
0392). The scope of hazards covered by the GHS is very similar
to what is covered by the current HCS as discussed in Section XIII
Summary and Explanation. While the revisions to the OSHA HCS could,
theoretically, result in some chemicals that were not considered
hazardous being classified as such now, OSHA does not expect any
significant change in chemicals covered under this final rule and did
not receive any specific examples from stakeholders, despite
repeated requests for them. For this reason, OSHA has concluded that
there will be no additional costs related to PPE for this standard.
Multiple stakeholders questioned whether OSHA had taken into
account the cost to update workplace signs to come into compliance with
the revised OSHA HCS. Southern Company reported that the cost to
purchase signs for their 29 affected plants would be $58,000 plus the
cost of employee time to install the signs (Document ID 0378),
and API reported that one of its member companies recently updated the
signs at its small refinery at a cost of $200,000 (Document ID
0376). OSHA feels that the four-year phase-in time for these
revisions to the HCS, combined with the limited number of affected
workplace signs, will minimize any cost that firms may incur. The
phase-in period will allow firms to update their signs during the
normal replacement lifecycle of three to five years for those signs and
will result in minimal costs.
Commenters felt that "costs for re-classification and modification
of SDS and labels would need to include substantial consulting fees"
(Document ID 0392). OSHA maintains that any firm preparing
labels and SDSs under the current OSHA HCS will not find it
significantly burdensome to prepare labels and SDSs under the revised
HCS. On the contrary, OSHA expects that the revisions to the HCS would
be able to prepare SDSs and labels at lower cost in the future (for
which the Agency earlier, in Section VI.D: Benefits, estimated
productivity savings). In addition, much reclassification work has
already been done by firms that sell to the EU or to Asian markets.
Estimation of Compliance Costs
The remainder of this section explains how the compliance costs
arising from the final rule were calculated by describing the data and
methodology used to estimate each of the major cost elements. A more
complete and detailed description of the estimation of compliance costs
can be found in the revised final version of the PP&E 2009 report
(Document ID 0273), the ERG (2010, 2011) reports focusing on
the costs of printing labels in color, and the updated cost estimates
for the final rule in ERG (2012).
The major elements of the revisions to the HCS that involve
compliance costs include (1) the classification of chemicals in
accordance with the GHS criteria, and the revisions to the safety data
sheets and labels corresponding to the affected hazardous chemicals;
(2) even though it is not directly a result of any specific requirement
included in the revisions to the HCS, the cost for managers and
administrators of hazard communication programs to become familiar with
the revisions to the standard and to manage, update, and revise their
programs as may be necessary to ensure compliance with the revised
standard; (3) incremental training for employees already trained under
the existing OSHA hazard communication programs to ensure their
familiarization with the new formats, information, and symbols that
would be introduced into the workplace as a result of the revisions to
the HCS; and (4) costs to upgrade label printing technology or purchase
labels preprinted in multiple colors in order to comply with the
requirement that the pictogram on the label be enclosed in a red-
bordered diamond.
The estimated compliance costs presented in this analysis of the
revisions to the HCS are largely based on research conducted by PP&E
(2009), which was expanded and updated for the FEA by ERG (2010, 2011,
and 2012). Both PP&E and ERG performed this research under contract to
the Department of Labor specifically for the purpose of developing
estimates of compliance costs for, and assessing the potential impacts
that may be associated with, revisions to the OSHA HCS in order to
implement the GHS.
The estimated costs of compliance with many of the provisions of
the final rule involve wages paid for the labor hours required to
fulfill the requirements. In some cases, compliance could be achieved
by purchasing services or products in lieu of paying employees
directly. The estimated compliance costs are intended to capture the
resources required for compliance, regardless of how individual
establishments may choose to achieve compliance.
Costs Associated With Chemical Classifications and Revisions to Safety
Data Sheets and Labels
The revisions to the OSHA HCS continue to require firms that sell
hazardous chemicals to employers to provide information about the
associated hazards. Information is required to be presented in a safety
data sheet (SDS) in the format specified in the revised standard, and
some information is also required to be presented on product labels.
The existing OSHA HCS already requires information about hazardous
chemicals to be provided in SDSs and on labels. In addition, under the
existing standard, SDSs are to be revised within three months after a
manufacturer or employer becomes aware of any significant new
information about a chemical hazard.
The final rule requires chemicals to be classified into the
appropriate hazard classes and categories based on the information
about the chemicals that the manufacturers currently have. This
information would have been assembled for purposes of conducting a
hazard determination under the current HCS. In addition, the current
HCS requires chemical manufacturers and importers to remain aware of
developments regarding the hazards of the chemicals they produce or
import in order to update the labels and SDSs for the chemicals in a
timely manner. The classification of the chemicals into the hazard
classes and categories under the revised provisions does not require
any additional testing, studies, or research to be conducted.
Manufacturers would be able to rely on the information they already
have in determining how to properly classify their chemicals.
Generally, chemical manufacturers and importers periodically
review, revise, and update SDSs and labels. Changes are made as
necessary as information regarding specific hazards develops, new
information about protective measures is ascertained, or changes are
made to product information and marketing materials. Labels and SDSs
must also be produced or modified when products are introduced or
changed. Therefore, there is a regular cycle of change for these
documents for a variety of reasons. The final rule may require more
extensive change than would normally occur, but the phase-in period is
such that the chemical manufacturers and importers can take advantage
of the normal cycle of change to phase in the revisions for all their
products over a reasonable time period. This should have less impact on
normal operations than a short time period that would require all SDSs
and labels to be revised at the same time.
The transition period that would be allowed by the delayed
effective date for the requirement to adopt the new format should help
ensure that the transition can be completed in conjunction with
revisions and updates that would normally be expected to occur even
without the implementation of the final rule. In addition, the format
for SDSs required by the final rule is consistent with the format
adopted by the American National Standards Institute (ANSI) and
therefore has already been implemented by many of the affected
businesses.
Based on ERG (2012), OSHA developed estimates of the costs that
would be associated with the
classification of chemicals in accordance with the final rule and with
the revisions to the corresponding SDSs and labels for those chemicals.
The estimated compliance costs represent the incremental costs that
would be incurred to achieve compliance with the final rule. These
estimated costs would be in addition to the costs that would already be
incurred to continue to remain in compliance with applicable
requirements of the existing HCS.
The revisions to the HCS would allow for a transition period of
four years following the publication of a final rule. During this
period, even in the absence of any pertinent OSHA rulemaking, producers
of affected chemicals would presumably be ensuring that the information
provided in their SDSs and labels remains accurate and current.
Producers of hazardous chemicals are generally expected to regularly
review the available information regarding any hazards that may be
associated with their products and to revise SDSs and labels
accordingly.
In addition, for every affected product that is newly created,
reformulated, mixed with new ingredients, modified with new or
different types of additives, or has any changes made in the
proportions of the ingredients used, the chemical producer would be
required under existing OSHA and other applicable standards to review
the available hazard information, to classify the chemical in
accordance with applicable hazard criteria, and to develop
corresponding SDSs and labels.
The estimated costs of compliance with the final rule do not
include the costs associated with activities such as those described in
the above paragraphs, but rather reflect only the additional costs that
chemical producers would not already be expected to incur.
The estimated compliance costs associated with the reclassification
of hazards and changes to SDSs and labels are directly related to the
numbers of SDSs affected. Based on ERG (2012), OSHA developed estimates
of the number of potentially affected SDSs by industry, for each of the
industries producing the corresponding chemicals and products (as shown
in Table VI-3). Downstream users, distributors, and wholesalers are
generally expected to continue to rely on SDSs provided by
manufacturers to fulfill their obligations under the OSHA standard, as
has been the practice for decades.
The costs of compliance associated with the classification of
chemicals in accordance with the criteria specified in the final rule
and with the revisions to the corresponding SDSs and labels for those
chemicals were based on PP&E industry interviews and, as described
below, are based on the same time and software estimates as those
presented in the proposed rule.
Generally, for smaller establishments with relatively few chemicals
affected, OSHA estimated the incremental compliance costs to be the
equivalent of the cost of seven hours of time of a professional with
the requisite expertise for each affected chemical, on average. Based
on ERG's (2012) updates to the PP&E 2009 report (Document ID
0273), OSHA estimated the cost of hourly compensation for a
professional for this purpose to be $66. As a result, a small
establishment (with fewer than 100 employees) with 20 SDSs for 20
chemicals, for example, would have estimated incremental compliance
costs of $9,240 (7 hours times 20 SDSs times $66).
In larger establishments with more affected chemicals, the
incremental compliance costs were estimated to consist of two parts.
First, labor costs were estimated according to the size of the
establishment. OSHA, based on PP&E interviews with stakeholders,
estimated that entities with 100 to 499 employees would incur, on
average, the equivalent of five hours of time of a professional with
the requisite expertise for each affected chemical, and that entities
with 500 or more employees would incur the equivalent of three hours of
professional time per chemical. Again, OSHA estimated the hourly
compensation for a professional for this purpose to be $66.
The rulemaking record presented a wide range of estimates for the
time required to update SDSs with a low estimate of four hours per SDS
(Document ID 0119 and 0123), a few estimates in the range of
25-30 hours per SDS (Document ID 0134 and 0402), and upper
bound estimates as high as 150 hours per SDS (Document ID
0341). OSHA evaluated these estimates and felt that the upper
estimates are not defensible for the following reasons: (1) Firms will
not be required to gather or evaluate additional data; (2) firms
currently must update their SDSs periodically, and there was no
evidence presented in the record that suggested that updates under the
current HCS take anywhere near 150 hours per SDS; and (3) the Agency
does not feel that it is clear that these estimates account for only
the incremental time needed to prepare an updated SDS, taking into
account any time that would be spent updating SDSs during the
transition period in the absence of any revisions to the OSHA HCS. The
Agency acknowledges that some SDS updates may take longer than the
average listed above, but also feels that many chemicals--especially
pure substances which will likely already have been classified
according to the GHS for the EU or Asian markets--will take less than
the estimated time used in the economic analysis. Therefore, OSHA feels
that the estimated time to update SDSs used in this analysis represents
a reasonable average for most chemicals.
The labor cost per SDS was estimated to be lower for larger
companies based on the determination that larger companies produce more
SDSs, and would therefore experience efficiencies associated with
producing them. These efficiencies include economies of scale, the use
of software specifically designed to classify hazards and produce SDSs,
and the generally lower cost per SDS associated with many mixtures.
In addition to labor costs, many of these larger establishments may
incur additional expenditures to purchase or modify software that can
be used to classify chemicals and to produce corresponding SDSs and
labels. Such software is available from a variety of vendors; the
software can be purchased or used on a subscription basis. Publicly
available information about the products and services being offered and
sold to businesses for purposes of complying with hazard communication
requirements indicates that most of the relevant vendors are aware of
and prepared for an upcoming alignment with the GHS. Therefore, their
products and services are or will be adapted to enable compliance with
the revisions to the HCS. In addition, some firms may purchase custom
or proprietary software from private vendors to achieve compliance with
existing requirements or future revisions to hazard communication
requirements or for other purposes.
Regardless of the particular approach individual companies may
choose to most efficiently fulfill their obligations under the existing
HCS, OSHA expects that a part of the costs associated with achieving
compliance with the final rule would involve costs attributable to
software modifications. Based on industry data obtained by PP&E, OSHA
apportioned these costs on a per-SDS basis and estimated the cost per
SDS to be $208, on average. Numerous stakeholders raised the issue of
software updates and modifications in their comments submitted to the
rulemaking record (Document ID 0018, 0105, 0114, 0363, 0371,
and 0389). In response to the ANPR, the American Chemistry Council
reported that their members estimated anticipated software update and
conversion costs of up to $70,000. The ACC also reported that their
members typically have hundreds, if not thousands, of SDSs (Document ID
0105). Using OSHA's per-SDS cost of $208, a firm that produced
336 SDSs (which would fall within the typical range for ACC members)
could expect to incur costs of $70,000. This example suggests that
OSHA's estimated cost-per-SDS is a reasonable one.
Based on ERG's (2012) updates to the PP&E 2009 report (Document ID
0273), OSHA estimated the numbers of SDSs produced in each
industry that would potentially need to be revised under the final
rule. As shown in Table VI-3, a total of about 1.4 million SDSs, one
for each type of chemical produced by an individual manufacturer in the
United States, were estimated to be in potential need of revision.
In developing estimates of the compliance costs associated with the
rule, PP&E also considered the extent to which many firms have already
performed the necessary reclassifications of chemical hazards and
revisions to SDSs. Some chemical hazards have already been reclassified
as would be required by the OSHA final rule because the U.S. Department
of Transportation has required such classifications as part of their
regulations for the transportation of hazardous chemicals (49 CFR Parts
171-180). The criteria for physical hazard classifications for purposes
of transport have been internationally harmonized for some years, and
these criteria formed the basis for the physical hazard criteria in the
GHS. Therefore, many products intended for transport have already been
classified under the new physical hazard criteria as well as the
existing criteria in the HCS.
Many current SDSs are already produced to varying degrees in
accordance with the requirements of the OSHA final rule because the
widely followed ANSI industry consensus standard already reflects many
of these requirements in its relevant criteria. In addition, many firms
have implemented or are beginning to implement hazard
reclassifications, SDS revisions, software modifications, and other
changes in accordance with the requirements of the final rule, because
these provisions are generally anticipated to be adopted as part of the
implementation of the GHS in countries and regions around the world.
Since some other countries are already implementing the GHS, companies
in the U.S. that ship to those countries are already having to comply
with the GHS for products being exported. Stakeholder comment in the
docket suggested that some of the work related to reclassification has
already been done (e.g., Document ID 0352, 0377, 0405, and
0410), lending support to OSHA's baseline estimates of current
compliance rates.
Research conducted by PP&E indicates that all of these factors
contribute to a substantial degree of current compliance with the
requirements of the final rule, even if the existing OSHA HCS standard
remains unchanged.\23\ Based on the ERG (2012) updates to the PP&E
(2009) report (Document ID 0273), OSHA estimates that, on
average, about 53 percent of the gross costs that would otherwise be
associated with the revisions to the HCS have already been incurred by
firms. However, this average is a result of very different levels of
current compliance for different sizes of firms. PP&E estimated that
the percentage of firms in current compliance with the final rule--with
the exception of employee training--is 75 percent for firms with over
500 employees; 25 percent for firms with 100 to 500 employees; 5
percent for firms with 20 to 99 employees; and 1 percent for firms with
fewer than 20 employees. OSHA used these percentages to reduce the
number of affected firms reported in Table VI-3, for purposes of
estimating the costs for affected firms to comply with the final rule
(again, with the exception of employee training).
---------------------------------------------------------------------------
\23\ By current compliance, OSHA means firms that have already
reclassified chemicals and prepared SDSs and labels in accordance
with GHS requirements specified in the final rule and would
therefore be ready to introduce these modifications at negligible
additional cost when GHS becomes effective.
---------------------------------------------------------------------------
Based on the preceding analysis, OSHA estimates an annualized cost
of approximately $22.5 million for the classification of chemicals in
accordance with the criteria specified in the final rule and for
revisions to the corresponding SDSs and labels for those chemicals.\24\
---------------------------------------------------------------------------
\24\ This annualized estimate of $22.5 million reflects software
costs of $55 million and labor costs of $226 million, both
multiplied by 0.079932 to annualize these costs (incurred over the
first four years) over a 20-year period. The $55 million in software
costs is the result of about 264,000 modified SDSs [(929,000 SDSs
for large establishments x 25% not in existing compliance x 95%
requiring modification) + (233,000 SDSs for establishments with 100-
500 employees x 75% not in existing compliance x 25% requiring
modification)] at a cost of $208 per SDS. The $226 million in labor
cost is the result of about 666,000 affected SDSs multiplied by an
average of 5.14 hours of professional time per SDS (from 3 to 7
hours per SDS) multiplied by $66 per hour. The annualization factor,
0.079932, is equal to:
[(\1/4\] * [ (1-(1.07)-4)/0.07] * [0.07/((1-
(1.07)-20)],
where the first term in brackets reflects the fact that these
costs are assumed to be spread equally over the first four years;
the second term in brackets calculates the present value of the
costs, and the third term in brackets annualizes the present value
of the costs over a 20-year period.
---------------------------------------------------------------------------
As discussed below, OSHA received some comments from the public
regarding the estimated costs associated with chemical classifications
and revisions to safety data sheets in response to the ANPR published
by OSHA in the Federal Register on September 12, 2006 (71 FR 53617) and
the Proposed Rulemaking published by OSHA in the Federal Register on
September 30, 2009 (74 FR 50280). The comments received are publicly
available as part of the rulemaking record, accessible through
regulations.gov, in docket OSHA-H022K-2006-0062. Relevant information
submitted by the public was incorporated into the development of the
methodology and estimates presented in this economic analysis.
Some commenters provided examples of cost estimates that generally
support the estimates of the preliminary economic analysis. Information
from other commenters provided a wide range of cost estimates. The
figures presented in some comments appeared to correspond to gross
costs of creating SDSs, and in other cases it was not clear whether
gross or incremental costs were being presented. In general, commenters
did not provide the rationale underlying their cost estimates.
Comment from the Fragrance Materials Association of the United
States (Document ID 0061) and the Flavor and Extract
Manufacturers Association of the United States (Document ID
0062) stated that these Associations' best assessment is that
it would take anywhere from two to eight hours to review information
and prepare new labels and safety data sheets for each hazardous
chemical
One company that produces and distributes about 4,000 different
hazardous chemicals estimated that it will take four to six hours per
product to prepare a GHS SDS. (Document ID 0026).
The National Paint and Coatings Association stated that it would
take approximately five hours to research the information for a product
SDS/label at a small company, at a cost of about $300 per product; it
also estimated that, at a medium-sized company, this same task would
take from 3-5 days to 3 weeks at a cost of approximately $1,000 to
$1,800, and that at a larger company, the task would be even more
expensive (Document ID 0050).
The National Association of Chemical Distributors estimated that
converting an existing SDS to the new GHS format would require about
150 hours as compared to about 100 hours currently to revise an MSDS
(Document ID 0060 and 0341).
Another commenter, Merck, which produces, imports, or distributes
about 500 hazardous chemicals annually, estimated that, on average, it
takes approximately 3 weeks to generate a single safety data sheet at
an average cost of $1,500. Merck also stated that with a sufficient
transition period of three to six years, the costs of moving to GHS
would be minimal. Merck noted that the time and cost for additional
changes to the GHS format should be minimal because it had already
converted its SDSs to the 16-section ANSI/GHS format several years ago
(Document ID 0072).
One trade association estimated that the costs associated with
revising SDSs and labels for the 1,600 firms in the cleaning product
formulator industry would total $575 million, not including the time
needed to review changes to hazard classifications. The total numbers
of SDSs per establishment are generally higher for the establishments
represented by the trade association than the OSHA estimates for the
industry category as a whole (Document ID 0032).
This trade association also provided some of the details underlying
its cost estimates for individual companies. Cost estimates provided by
the trade association for individual companies included costs per SDS
as low as $30 and $80, and as high as $600 or more. One company
(identified as Company 11) estimated the cost to revise the
label and SDS would be $120 per product; another company (Company
2) estimated that this cost would be $2,600 per product. Some
of the higher compliance cost estimates appear to be unrealistically
high; for example, the estimated costs associated only with revising
labels for company 3 appear to represent about 3 percent of
total annual sales. While acknowledging that some firms may incur
higher costs than others to revise SDSs and labels, these data
generally appear to support that, at least for several firms in the
industry, the costs minimally necessary to achieve compliance would be
close to or less than the costs estimated by OSHA.
Ameren, an electric and gas services provider, estimated that all
9,000 of their employees would need one hour of training initially at a
total cost of $450,000. The company estimated that it would take 100
hours to update their SDSs (fewer than 25) at a total cost of $6,500
and that updating the 25,000 SDSs in their database would take five
minutes per SDS for a total cost of $102,700 (Document ID
0330).
The Independent Lubricant Manufacturers Association surveyed their
members and reported that, with one SDS per product, their members
could be expected to incur costs of $340,000 to $559,000 ($329 or $200
per SDS multiplied by 1700 SDSs per firm) to update SDSs. One member
company estimated costs associated with update software at $200,000 in
the first year and $1,000 per SDS in subsequent years to maintain the
software and SDSs. Another company estimated that software would cost
$50,000 and would include an additional $300,000 in staff time
(Document ID 0371).
Another trade organization, The Society of Chemical Manufacturers
and Affiliates, felt that it would take ten hours to revise a label or
an SDS (Document ID 0402).
Several other commenters provided cost estimates related to the
adoption of GHS requirements for chemical classifications and revisions
to safety data sheets and labels. (See, for example, Document ID
0015, 0018, 0024, 0036, 0079, 0105, 0107, 0116, 0128, 0141,
0145, 0327, 0341, and 0377, among others.) Many estimates are broadly
consistent with OSHA's estimates; in addition, some estimates appear to
be similar to, but may actually be substantially lower than, OSHA's
estimates to the extent they include costs attributable to the existing
standard rather than just the incremental costs associated with the
revisions to the HCS. Other estimates are substantially higher, but
many of these also appear to represent gross costs associated with
fulfilling hazard communication requirements without consideration of
the incremental nature of the compliance costs for the revisions to the
HCS, as discussed above.
Management Familiarization and Other Management-Related Costs
The implementation of GHS as part of the OSHA HCS would require
that employees currently covered by the standard become familiar with
the new system. The nature and extent of the familiarization required
would vary depending on an employee's job and business. OSHA considered
separately various training needs that may be imposed by the revisions.
Although it would not be explicitly required by the final rule,
some establishments may choose to provide training to managers and
other employees that are not directly covered by the training
requirements of the HCS. Other management-related costs may include
making revisions, if necessary, to existing hazard communication
programs; promoting awareness of and providing information about the
revisions to hazard communication programs; coordinating and
integrating changes to hazard communication programs with other
programs, processes, and functions; serving as an in-house resource for
supporting the general adoption of the revised HCS; creating
supplemental capacity for providing training and assistance to affected
employees; and other ancillary costs for company-specific changes and
general hazard communication program administration that may be
incurred at some establishments.
These management costs could be considered discretionary since they
are not explicitly required by the regulatory provisions. However, OSHA
recognizes that these costs may be incurred in practice due to the
manner in which some companies have implemented and integrated hazard
communication programs in their facilities. These costs reflect the
fact that hazard communications programs often are not implemented
solely for purposes of complying with the OSHA standard, but may serve
a variety of other purposes that are part of and that benefit the
overall production process.
In some cases, health and safety supervisors, logistics personnel,
and other personnel involved in administering, implementing, and
ensuring compliance with the requirements of the HCS in affected
establishments would be expected by company managers to become familiar
with the revisions to the HCS. The responsibilities of these employees
may include modifying written hazard communication programs as
necessary, reviewing and preparing training materials, and training new
and existing employees regarding the changes. A commenter asserted that
OSHA had overlooked the cost to train the employees who would be
providing training to production workers (Document ID 0392),
and the American Chemistry Council also questioned whether OSHA had
considered the necessary training for fire, EMS, or other emergency
workers (Document ID 0393). The Agency has included these
occupations in the cost estimates, allocating eight hours for training
on the revised HCS elements, and included employees responsible for
providing training as part of the management training and
familiarization costs and has continued to include them in estimated
the costs of the rule for this FEA.
In the PEA, OSHA estimated 8 hours of time, or an equivalent cost,
would be associated with the necessary familiarization and
implementation of revisions to hazard communication programs in affected establishments
in the manufacturing sector. Comments received on the topic of
management familiarization yielded a wide range of time needed for this
task. Some estimates were what OSHA considers to be unreasonably high
(ranging from 16 to 56 hours (Document ID 0372)) and may not
represent incremental costs only. OSHA did receive a comment that
"eight hours * * * [may be enough to gain] a basic understanding" of
the revisions to the OSHA HCS but went on to say that "as much as a
week * * * [may be needed to gain an] understanding of the details"
(Document ID 0392). OSHA believes that under the current HCS,
managers spend some time each year reviewing and updating their hazard
communication program. So, while a manager may spend more than 8 hours
total reviewing and familiarizing themselves with the revised HCS, a
portion of that time would not fall under new costs resulting from the
promulgation of the rule. OSHA did not feel that commenters presented a
strong case for changing the estimate of incremental time needed for
familiarization with the revised HCS and has therefore maintained the
estimate of 8 hours.
In many potentially affected establishments that do not produce
SDSs, and that have few affected chemicals or few affected employees, a
very basic hazard communication program may achieve compliance with the
OSHA standard. For these establishments, outside of the manufacturing
sector, that have a health and safety supervisor, the incremental
management and administrative costs associated with the revisions to
the OSHA standard were estimated to be two hours per establishment. For
establishments outside of the manufacturing sector that do not have a
health and safety supervisor, OSHA estimated that these costs would be
negligible.
Based on the preceding analysis, OSHA estimates an annualized cost
of approximately $59 million for management familiarization and other
related management activities in response to GHS.\25\
---------------------------------------------------------------------------
\25\ This annualized estimate of $59 million reflects total
costs of $692 million multiplied by 0.085332 to annualize these
costs (incurred over the first two years) over a 20-year period. The
$692 million is equal to $6 million for health and safety managers
(7,070 affected managers x $1039 per manager (the estimated cost of
one day training per manager) x 83% not currently in compliance)
plus $15 million for logistics personnel in manufacturing (49,100
affected logistics persons x 8 hours x $66 per hour x 83% not
currently in compliance) plus $163 million for health and safety
supervisors in manufacturing (370,000 affected health and safety
supervisors in manufacturing x 8 hours x $66 per hour x 83% not
currently in compliance) plus $508 million for health and safety
supervisors in non-manufacturing (3,848,000 affected H&S supervisors
in non-manufacturing x 2 hours x $66 per hour x 100% not currently
in compliance).
The annualization factor, 0.085332, is equal to:
[(\1/2\] * [ (1-(1.07)-2)/0.07] * [0.07/((1-
(1.07)-20)],
where the first term in brackets reflects the fact that these
costs are assumed to be spread equally over the first two years; the
second term in brackets calculates the present value of the costs,
and the third term in brackets annualizes the present value of the
costs over a 20-year period.
---------------------------------------------------------------------------
Costs Associated With Training Employees
Production employees who are currently covered by and trained under
the provisions of the existing HCS would need to receive some
additional training to become familiar with the changes to SDSs and
labels.
In many potentially affected establishments that do not produce
SDSs, and that have few affected chemicals or few affected employees, a
very basic hazard communication program may achieve compliance with the
OSHA final rule. In these establishments, the incremental employee
training costs associated with the revisions to the HCS may be
relatively small. In other cases, employers may be able to integrate
the necessary training into existing training programs and other
methods of distributing safety and health information to employees, and
thus may not incur much additional cost. Nevertheless, in general,
employers will need to devote real time and resources to provide the
necessary training in order to ensure that workers are familiar with
the new hazard communication system.
In response to comments in the rulemaking record, the training time
associated with the revisions to the OSHA HCS has been increased from
those presented in the PEA. OSHA increased the estimated training time
from 30 minutes to 60 minutes for most employees; from 15 minutes to 30
minutes for employees with minimal contact with hazardous chemicals;
and from 5 to 10 minutes for employees in certain occupations in the
transportation sector, where GHS pictograms are already in use. A
complete occupation-by-occupation summary of OSHA's estimates is
provided in the ERG (2012) revisions to the PP&E (2009) report.
The United Parcel Service, Inc. submitted comment supporting this
increase, reporting that "[i]nitial training takes about 15 minutes
currently but will [* * *] double during the phase-in process" and
that "training time (\1/2\ hr) will double to one hour [* * *] for
employees who are 'users' " (Document ID 0369). Other
stakeholders also felt that training time was underestimated (Document
ID 0330, 0345, 0347, 0363, 0392, 0397, 0400, 0402, 0404, and
0440), with the estimates of additional time needed over and above
OSHA's estimates ranging from 15 minutes (Document ID 0330,
0369, and 0378) to 15 hours (Document ID 0400). OSHA's
increase of training time by 100 percent over the estimated training
time in the PEA represents a significant increase in response to
comments, and the Agency believes that these estimates of training
times are reasonable. The extra time OSHA has incorporated also
addresses concerns of some stakeholders that firms will have to offer
two iterations of training --one before the two-year familiarization
deadline set forth in the regulatory text, and one closer to the
effective date when all products have been converted to GHS-compliant
SDSs and labels (Document ID 0339). However, for costing
purposes, all training costs for workers to become familiar with GHS
requirements were assumed to be incurred within the first two years
after the effective date of the final rule. OSHA received comment that
additional training time would be required to train employees
responsible for reclassifying chemicals under the revised HCS (Document
ID 0392). OSHA believes that the changes to the HCS are such
that an employer who was capable of classifying chemical hazards under
the current HCS would be able to become familiar with the GHS criteria
in a relatively short period of time. The Agency has also allocated 3
to 7 hours per product to complete the reclassification and produce an
updated SDS, which should allow for additional familiarization time if
necessary. OSHA has not included additional training time for training
on new hazards disclosed as a part of the transition. This concern was
raised by a commenter (Document ID 0339), because it is
theoretically possible that some chemicals could be classified with new
hazards through the GHS classification schemes that were not previously
presented in the workplace. However, the data used for classification
is the same used for the current hazard determination, and OSHA
believes that few new hazards would actually be introduced through this
process. Compliance with the final rule is not expected to impose any
additional training costs after the transition period.
Based on the preceding analysis, OSHA estimates that the annualized
cost of training employees in response to GHS would be approximately
$95.4 million.\26\
---------------------------------------------------------------------------
\26\ This annualized estimate of $95.4 million reflects total
costs of $1,118 million multiplied by 0.085332 to annualize these
costs (for costing purposes, assumed to be entirely incurred over
the first two years) over a 20-year period. The $1,118 million is
equal to $785 million in employee hours to receive training (43.8
million affected employees x 0.84 hours x $21 per hour) plus $333
million in management hours to provide the training (6.0 million
training sessions x 0.84 hours x $66 per hour). The 0.84 hours is
the average estimated training time for all affected employees, with
most receiving 60 minutes of training, some receiving 30 minutes of
training, and a very few receiving 10 minutes of training. The total
number of managers providing training (3.8 million) would, on
average, be equal to approximately 8.7 percent of the number of
employees receiving training in response to GHS.
---------------------------------------------------------------------------
The revisions to the HCS may result in reductions in the costs
associated with providing training for employees as required by the
existing OSHA HCS. Affected companies could save considerable time and
effort in training new employees in the future. The savings may be
attributable in part to reducing or eliminating the need to explain the
different types of formats used to convey hazard information and the
different types of information included in the contents of SDSs and
labels. OSHA did not quantify these potential savings in training costs
as part of this FEA but, based on stakeholder comment and testimony in
the rulemaking record, OSHA anticipates that companies will realize
cost savings in future time periods from simplified hazard
communication training facilitated by the final rule. A qualitative
discussion of these cost savings was presented in Section VI.D:
Benefits in this preamble and an estimate of the possible magnitude of
these cost savings is presented in the sensitivity analysis in Section
VI.L in this preamble.
Cost of Color Printing
The revisions to OSHA's HCS include a requirement that labels
include a pictogram enclosed in a red-bordered diamond. The rulemaking
record showed widespread (although not unanimous) support for requiring
the red-bordered diamond. One commenter felt that "the use of color to
draw attention to a potential hazard is a useful tool and is likely to
enhance the communication of safety information" (Document ID
0327), another stated that "the color red has been
universally accepted as indicating a potential danger or hazard"
(Document ID 0339), and others showed general support for
requiring red borders in order to achieve the highest level of
harmonization (Document ID 0351 and 0383). Many stakeholders
raised concerns that this requirement would result in additional costs
to firms since many do not currently print labels in multiple colors or
purchase pre-printed labels in multiple colors (Document ID
0120, 0327, 0328, 0344, 0363, 0383, 0389, and 0402). Requiring
the red-bordered diamond on the label would mean that some firms would
have to upgrade their printer technology or purchase more expensive
pre-printed label stock that included the red-bordered diamond.
OSHA estimated the cost impacts of the rule's requirement that
pictogram borders be printed in red based on a report on the subject
prepared by ERG (2011). That report is based on data provided in an
earlier report prepared by ERG (2010). The full ERG reports are
available in the rulemaking docket on regulations.gov. To estimate
costs for this provision, OSHA estimated the number of hazard labels
printed per year, the number of establishments that would incur costs
to upgrade their printing technology, and the cost to those
establishments to upgrade their printing technology. OSHA estimates
that approximately 949 million hazard labels are printed each year and
the total incremental cost for establishments to comply with this
provision of the OSHA standard is $24.1 million per year. The following
section explains how OSHA, using ERG (2010 and 2011), developed
estimates of the number of hazard labels printed per establishment, the
number of establishments that would need to upgrade printer technology,
and the cost to those establishments to comply with this provision of
the final rule.
ERG (2011) used data on Shipment Characteristics by Commodity by
Shipment Weight from the U.S. Census Bureau \27\ and DOT's jointly
produced Commodity Flow Survey (CFS) (U.S. Census Bureau, 2007).\28\http://www.dol.gov/cio/programs/infoguidelines/informationqualitytext.htm
Commodity shipments reported in this survey were classified using the
Standard Classification of Transported Goods (SCTG) commodity
codes,\29\ which ERG mapped to the relevant NAICS industries.
---------------------------------------------------------------------------
\27\ U.S. Census Bureau, 2007. Commodity Flow Survey: Shipment
Characteristics by Commodity by Shipment Weight. Available at
http://www.bts.gov/publications/commodity_flow_survey/.
\28\ U.S. Census Bureau, 2007a. American Fact Finder: Commodity
Flow Survey. Available at http://www.census.gov/econ/census07/index.html.
\29\ The following 13 commodity codes were considered as those
that would potentially contain hazardous chemicals: Alcoholic
Beverages (Commodity code 8), Gasoline, including Aviation
(Commodity code 17), Fuel Oils (Commodity code 18), Other Coal and
Petroleum Products (Commodity code 19), Basic Chemicals (Commodity
code 20), Pharmaceutical Products (Commodity code 21), Fertilizers
(Commodity code 22), Other Chemical Products & Preparations
(Commodity code 23), Plastics and rubber (Commodity code 24), Pulp,
newsprint, paper, and paperboard (Commodity code 27), Nonmetallic
mineral products (Commodity code 31), Base Metal in Primary or Semi-
Finished Forms and in Finished Basic Shapes (Commodity code 32), and
Miscellaneous Manufactured Products (Commodity code 40).
---------------------------------------------------------------------------
For each of the SCTG commodity codes, the U.S. Census data present
shipments of basic chemicals by shipment weight. In order to establish
the types of shipments that might fall into each weight class, OSHA
relied on preliminary research conducted by ERG (2010) on the weight
and capacity of various shipping container units and the weight per
gallon of various chemicals. Information was gathered on the types of
containers typically used by specific industries and whether those
containers would typically ship inside a labeled exterior container.
OSHA calculated shipment weights for various chemicals shipped in
various container types by multiplying the product weight per gallon by
container capacity and adding the weight of the shipping container. As
shown in Table VI-5, minimum, maximum, and simple average weights per
full container were estimated for the different commodities evaluated
in this test case using the Census-reported commodity shipments by
shipment weight to establish some bounds on possible shipment types.
[GRAPHIC] [TIFF OMITTED] TR26MR12.015
Based on these calculations, OSHA was able to estimate the number
of each type of container that would fall into each of the U.S. Census
weight classes. The number of containers that would require a label
under the OSHA HCS was refined by estimating the percentage of each
commodity that was comprised of nonhazardous products and the
percentage of the remaining products that would be sold to consumers.
Neither of these types of products fall under the scope of OSHA's HCS
and would not require a hazard warning label under the revised rule.
For the remaining hazardous non-consumer shipments, assuming one label
per container and one label on the outer packaging where applicable,
ERG estimated that approximately 949 million hazard labels are applied
annually to containers of all sizes.
In most cases one SCTG maps to multiple NAICS industries. In order
to divide the number of labels for each SCTG among its constituent
NAICS industries, OSHA used receipts data from the U.S. Census Bureau's
Statistics of U.S. Businesses to calculate receipts for a particular
NAICS industry as a percentage of receipts for all NAICS industries
that map to one SCTG. This percentage was used to allocate the
estimated number of labels printed for each SCTG among its constituent
NAICS industries.
The labels printed per NAICS industry were then distributed among
the various size classes based on each size class's share of receipts.
In cases where receipts data were not available from the Statistics of
U.S. Business (a situation found exclusively within the chemical
manufacturing industry in the affected industries for this rule), OSHA
calculated the average total receipts and average receipts for each
establishment size class for six-digit NAICS in the 325 (Chemical
Manufacturing) subsector and the ratio of average receipts for size
class to total receipts for six-digit NAICS in 325. This ratio was
multiplied by total receipts for the appropriate size class for each
industry where receipts data were not available.
Having estimated the number of hazard labels used per year for each
NAICS code, OSHA next estimated the costs associated with printing
those labels with red pictogram borders. Affected establishments were
assigned to one of four categories:
[ssquf] Category 1: Companies printing only in black who don't own
a color printer
[ssquf] Category 2: Companies printing in black but who own a color
printer
[ssquf] Category 3: Companies using pre-printed stock or labels
[ssquf] Category 4: Companies printing color labels
Establishments in Category 1 and Category 2 will have to buy new
color printers (although Category 2 establishments will have to buy
fewer new printers), as well as either color cartridges for laser
printers or red ribbons for thermal transfer printers. Establishments
in Category 3 will face higher costs for pre-printed stock or labels
with red pictogram borders. Establishments in Category 4 will not face
higher costs. Relying on conversations with companies and label
printers/vendors, ERG allotted establishments into these four
[GRAPHIC] [TIFF OMITTED] TR26MR12.016
Using the estimates of the percentage of establishments per
category by size and the data presented in the industry profile, OSHA
was able to estimate the number of establishments per category by size.
OSHA used the ratio of SDSs produced by size class to the ratio of
total SDSs produced and used that ratio to estimate the number of
labels produced per size class per NAICS industry. The results are
shown in Table VI-7.
[GRAPHIC] [TIFF OMITTED] TR26MR12.017
The number of establishments per category per size class and the
number of labels per establishment were then combined with the
incremental costs to print in color as opposed to black only to arrive
at an estimate of the cost of this provision.
The unit costs by category were estimated as follows.
A low-end laser printer was estimated to cost only a few hundred
dollars while a higher-end laser printer can cost upwards of $1,000 to
$5,000. OSHA estimates that on average, the incremental cost of buying
a color printer instead of a black and white printer is $50 for a low-
end laser printer, $100 for a high-end laser printer, $100 for a low-
end thermal transfer printer, and $1,000 for a high-end thermal
transfer printer. In this analysis, OSHA considers the cost of printers
to be a one-time cost that establishments will incur during the four
year transition period. The one-time, non-annualized cost to
establishments to upgrade printer technology was estimated to be $11.8
million. Printer costs were annualized using a 7 percent interest rate
over a five-year period.
The incremental cost of color cartridges for laser printers is a
significant driver of costs under the rule. Black cartridges cost
approximately $300, while printing in color requires buying four
cartridges (cyan, magenta, yellow, and black) at an estimated cost of
$1,200. Additionally, printers using black cartridges can print 20,000
labels, while color cartridges can print only 6,000 labels. This
results in a per-label cost of $0.015 for black cartridges and $0.20
for color cartridges, for an incremental cost of $0.185.
For companies using thermal transfer printers, the cost of ribbons
varies depending on the label material, but is approximately $30 per
ribbon for black ribbons and $40 per ribbon for red ribbons. Since both
black and red ribbons will be required to print labels under the final
rule, the incremental cost of printing in color is the cost of the red
ribbon or $40. Both types of ribbons will print approximately 1,000
labels, for a per-label cost of $0.034 for black ribbons and $0.04 for
red ribbons, for an incremental cost of $0.01 per label.
For companies using pre-printed stock/labels, the cost of all black
labels is estimated to be $0.10 per label while the cost of labels with
red pictograms is estimated to be $0.15 per label. This results in an
incremental cost of $0.05 per label.
For the purposes of this analysis, OSHA estimated that for those
establishments in category 1 (those currently printing labels only with
black ink who don't own a color printer) very small establishments will
purchase one low-end laser printer, small establishments will purchase
two high-end laser printers, medium establishments will purchase three
low-end thermal transfer printers, and large establishments will
purchase four high-end thermal transfer printers. For establishments in
category 2 (those currently printing labels only in black ink but who
own a color printer), OSHA estimated that very small establishments
will purchase one low-end laser printer, small establishments will
purchase one high-end laser printer, medium establishments will
purchase two low-end thermal transfer printers, and large
establishments will purchase three high-end thermal transfer printers.
OSHA estimates that establishments in categories 3 and 4 (those
purchasing preprinted black and white labels and those currently
printing labels in color) will incur no costs to procure new printers.
Using the estimates described above, OSHA was able to determine the
current costs of printing and the cost of printing labels with red-
bordered pictograms.
For establishments in Category 1 (those printing black and white
labels), the current average cost per label is $0.02 and the average
cost per establishment is $132, and for establishments in Category 2
(those printing black and white labels but who own a color printer),
the current average cost per label is $0.03 and the average cost per
establishment is $344. Establishments in Category 1 and Category 2 will
have to buy new color printers (although those in Category 2 will have
to buy fewer printers). These establishments will also face higher
costs for purchasing color cartridges and ribbons. For these
establishments, the cost of purchasing a color printer becomes
insignificant when annualized (at a 7 percent interest rate over five
years) and when considered on a per-label basis. The main driver of
overall costs is the incremental cost of purchasing color cartridges
for those establishments using laser printers (establishments that OSHA
estimates are small and very small). For very small and small
establishments using a laser printer, the cost of cartridges goes from
under $0.02 per label for a black cartridge to $0.20 per label for
color cartridges. Cost increases are more modest for medium and large
establishments using thermal transfer printers, with ribbon costs only
increasing from $0.03 to $0.04 per label.
For establishments in Category 3 (those who use pre-printed stock
or labels) the current average cost per label is $0.10 and the average
cost to purchase labels per establishment is $1,148. Establishments in
Category 3 will have to pay more for pre-printed stock or pre-printed
labels with red pictograms than for their current hazard labels. OSHA
estimates that costs will increase from $0.10 per label to $0.15 per
label, increasing printing costs by 50 percent for all establishments
in this category.
For establishments in Category 4 (those currently printing in
color) the current average cost per label is $0.15 and the average cost
per establishment is $1,880. Establishments in Category 4 will not have
to pay any more to print red borders as they are already printing color
labels.
The annualized cost of printers was calculated by finding the
present value of the incremental printer cost incurred four years after
the rule is published (to account for the compliance time for the
labeling provisions of the rule). This present value was annualized
over five years at a 7 percent interest rate to account for the life of
the printer. In the cases of printing supplies (i.e., cartridges,
ribbons, or label stock), costs are calculated as though they would be
incurred over a 20-year period, but would not begin to be incurred
until four years after the rule is published. Detailed estimates are
presented in Table VI-9 included in the appendix at the end of this
section.
For all establishments in all categories, the total costs
associated with the requirement to print red pictogram borders are
approximately $24.1 million per year, which includes the annualized
cost of new printers (approximately $2.4 million) and of 16 years'
worth of annual printing supply costs. OSHA feels this estimate is in
line with the comments received on the subject as part of the
rulemaking record. Betco Corporation estimated that requiring color
printing would increase printing costs by 25 percent (Document ID
0389), Dow Chemical estimated that black and white printing
was 40 percent less expensive than color printing (Document ID
0353), and The National Paint & Coatings Association, Inc.
estimated an increase of 15 percent to 47 percent to print in color
depending on the size of the label (Document ID 0328). The
Agency also feels that the four-year phase-in period allows adequate
time for establishments to exhaust their current stock of labels, which
will help ameliorate some cost concerns expressed by stakeholders.
Summary of Unit Cost Estimates
The following list provides a summary of the input estimates
underlying the calculation of the compliance costs. It should be noted
that these costs are intended to reflect only the incremental costs
that would be incurred in addition to the associated costs that would
be incurred in the absence of the revisions to the HCS. Except for
employee training and color printing, these costs would apply only to
those businesses not already in compliance with the revisions.
Reclassifying chemicals and modifying SDSs and labels:
Large establishments (over 500 employees): an average of 3
hours per SDS; in addition, for 95 percent of establishments, an
average of $208 per SDS for software modifications.
Medium establishments (100-499 employees): an average of 5
hours per SDS; in addition, for 25 percent of establishments, an
average of $208 per SDS for software modifications.
Small establishments (1-99 employees): an average of 7
hours per SDS. Management familiarization and other costs:
Eight hours for health and safety managers and logistics
personnel in the manufacturing sector.
Two hours for each hazard communication program manager
not in the manufacturing sector.
Employee training:
One hour per production employee in most industries;
30 minutes in occupations exposed to few hazardous
chemicals and types of hazards;
10 minutes per employee in some occupations where GHS-type
pictograms are already in use.
Color Printing
Category 1 establishments (those currently printing only
in black & white who do not own color printers): Large establishments
$0.02 per label, medium establishments $0.01 per label, small
establishments $0.13 per label, and very small establishments $0.14 per
label.
Category 2 establishments (those currently printing only
in black & white but who own color printers): large establishments
$0.02 per label, medium establishments $0.01 per label, small
establishments $0.13 per label, and very small establishments $0.14 per
label.
Category 3 establishments (those currently purchasing pre-
printed label stock): large establishments $0.03 per label, medium
establishments $0.03 per label, small and very small establishments
$0.03 per label.
Category 4 establishments (those currently producing
labels printed in multiple colors): No additional costs related to this
provision.
Appendix to Section F: Total Non-Annualized Costs of Compliance
Table VI-8 shows the total non-annualized (non-discounted)
compliance costs by industry and by cost element that are estimated to
be incurred during the four-year phase-in of the revisions. Except for
employee training and color printing, these estimates include no costs
for businesses already in compliance with the revisions.
As shown in Table VI-8, the total cost of compliance with the
rulemaking over the course of the transition period of four years is
estimated to be about $2.1 billion. Of this amount, the cost of
chemical hazard reclassification and revision of SDSs and labels is an
estimated $281 million, the cost of training employees is an estimated
$1,118 million, the cost of management familiarization and other costs
such as updates to hazard communication programs is an estimated $692
million, and the one-time printer costs for companies needing to
upgrade printing technology to print labels in color is an estimated
$12 million.
Table VI-9 summarizes OSHA's estimates for printing costs. It shows
annualized per-label costs by category and establishment size ranging
from $0.01 to $0.14 and total annualized costs by category and
establishment size. Total annualized costs include the cost of printers
annualized over five years and the cost of printing supplies incurred
over a 20-year period beginning four years after the rule is published.
BILLING CODE 4510-26-P
[GRAPHIC] [TIFF OMITTED] TR26MR12.018
[GRAPHIC] [TIFF OMITTED] TR26MR12.019
[GRAPHIC] [TIFF OMITTED] TR26MR12.020
[GRAPHIC] [TIFF OMITTED] TR26MR12.021
[GRAPHIC] [TIFF OMITTED] TR26MR12.022
[GRAPHIC] [TIFF OMITTED] TR26MR12.023
[GRAPHIC] [TIFF OMITTED] TR26MR12.024
BILLING CODE 4510-26-C
G. Net Benefits, Cost-Effectiveness, and Regulatory Alternatives
Table VI-1 provides a summary of the costs and benefits of the
revisions to the OSHA HCS, and it shows the net benefits and cost-
effectiveness of the revisions to the standard. Net monetized benefits
are estimated to be $556 million annually, expressed in 2010 dollars
and using a 7 percent discount rate. (Using a 3 percent discount rate
instead would have the effect of lowering the costs to $161 million per
year and increasing the gross benefits to $839 million per year. The
result would be to increase net benefits from $556 million to $678
million per year.) The cost-effectiveness of the standard can be
expressed as more than three dollars of benefits for every dollar of
cost.
Some qualitative evidence of the cost-effectiveness of the standard
was provided by comments submitted in response to the ANPR published by
OSHA in the Federal Register on September 12, 2006 (71 FR 53617) and
the Proposed Rule published by OSHA in the Federal Register on
September 30, 2009 (74 FR 50280). There was widespread support among
the commenters for the adoption of GHS in the United States (Document
ID 0340, 0344, 0347, 0349, 0351, 0354, 0357, 0359, 0366, 0382,
0390, 0403, 0408, and 0414). Many stakeholders anticipate that the
revisions to the HCS will "achieve more effective hazard
communication" (Document ID 0344 and 0351), "enhance the
consistency and quality of hazard information for workers" (Document
ID 0347), and "serve to further enhance worker protection"
(Document ID 0329). These sentiments were echoed in many of
the comments submitted to the record and in much of the testimony
delivered at the public hearings. This voicing of support included
commenters who provided some of the largest estimates of the costs of
the revisions (Document ID 0032, 0050, 0329, 0338, and 0341).
The available alternatives to the final rule are somewhat limited
since this rule modifies the current HCS in order to align with the
provisions of the UN's GHS. In Section III, the Agency qualitatively
discussed the two major alternatives presented during this rulemaking
process--(1) voluntary adoption of GHS within the existing HCS
framework and (2) a limited adoption of specific GHS components and a
variation on (1) that would require compliance with GHS but allow an
exemption for small businesses to comply with either the current HCS or
with the GHS-compliant HCS. All of these alternatives were soundly
rejected by stakeholders. To allow certain parties to follow an
alternative system or to allow voluntary adoption of the elements of a
uniformity standard does nothing to reduce confusion, improve
efficiency, or simplify processes. In order for those benefits to be
realized, all elements must apply to all affected parties. OSHA has
determined that both of the alternatives presented above would
eliminate significant portions of the benefits of the rule.
OSHA did not attempt to evaluate the costs and benefits for the
regulatory alternatives that involved partial or voluntary adoption of
the GHS. The Agency did evaluate two alternatives where the effective
dates were altered. For both alternatives, OSHA re-estimated the costs,
benefits, and net benefits simply by adjusting the effective dates in
its formulas. The results are summarized in Table VI-10.
In the first alternative considered, all elements of the revised
HCS would be required to be implemented within two
years. Under this alternative, all transitional costs would be incurred
in two years and benefits would be realized beginning in the third
year. OSHA estimated that annualized costs under this alternative would
increase by $5 million, from $201 million to $206 million, while
annualized benefits would increase by $166 million, from $757 million
to $923 million. Estimated net benefits would therefore increase by
$161 million, from $556 million to $717 million. However, OSHA believes
that these estimates fail to capture the difficulty many firms would
encounter in meeting these tighter enforcement dates. As a result,
initial compliance rates would probably be lower and less effective,
leading to reduced benefits. In addition, some compliance costs--such
as for labels and signs--were viewed in this final rule as incremental,
reflective of taking place within a normal replacement cycle of 3 to 5
years. With implementation required within two years, these costs could
no longer be treated as incremental to existing HCS requirements, but
would have to be recalculated as total replacement costs.
The second alternative that OSHA evaluated extended the timeline
for training to be completed. For this alternative, all elements of the
revised HCS (including training) would be required to be implemented by
June 1, 2016. Under this alternative, training costs would not be
realized for four and a half years (as opposed to the two-year
requirement for training in the final version of this rule) while
benefits would not be realized for five years (unchanged from the final
rule). OSHA estimated that annualized costs under this second
alternative would decrease by $12 million, from $201 million to $189
million, while annualized benefits would be unchanged. Estimated net
benefits would therefore increase by $12 million, from $556 million to
$568 million. However, these estimates fail to recognize that workers
will be exposed to (some) GHS-compliant labels and SDS formats well
before the 4\1/2\ year training date. The Agency would therefore expect
an increase in injuries, illnesses, and fatalities as untrained workers
are unable to effectively process and respond to the revised labels and
SDS formats. As a result, benefits and net benefits would actually
decline relative to those estimated for the final rule.
In summary, although both alternatives show greater net benefits,
the Agency concludes that the timing of the final rule is preferable
because of additional (but unquantified) compliance costs and reduced
(but unquantified) benefits under the first alternative and because of
reduced (but unquantified) worker health and safety benefits under the
second alternative. In addition, OSHA expects that the final rule
offers coordination benefits in that its requirements will fully take
effect at the same time as the EU completes its transition.
[GRAPHIC] [TIFF OMITTED] TR26MR12.025
H. Economic Feasibility and Impacts
This section presents OSHA's analysis of the potential economic
impacts of the final rule and an assessment of economic feasibility. A
separate analysis of the potential economic impacts on small entities
(as defined in accordance with the criteria established by the Small
Business Administration) and on very small entities (those with fewer
than 20 employees) is presented in the following section as part of the
Final Regulatory Flexibility Screening Analysis, conducted in
accordance with the criteria laid out in the Regulatory Flexibility
Act.
To determine whether a rule is economically feasible, OSHA begins
with two screening tests to consider minimum threshold effects of the
rule under two extreme cases: (1) All costs are passed through to
customers in the form of higher prices (consistent with a price
elasticity of demand of zero), and (2) all costs are absorbed by the
firm in the form of reduced profits (consistent with an infinite price
elasticity of demand).
In the former case, the immediate impact of the rule would be
observed in increased industry revenues. While there is no hard and
fast rule, in the absence of evidence to the contrary, OSHA generally
considers a standard to be economically feasible for an industry when
the annualized costs of compliance are less than a threshold level of
one percent of annual revenues. Common-sense considerations indicate
that potential impacts of such a small magnitude are unlikely to
eliminate an industry or significantly alter its competitive structure,
particularly since most industries have at least some ability to raise
prices to reflect increased costs and normal price variations for
products typically exceed three percent a year (OSHA, 2011, Chapter
VI). Of course, OSHA recognizes that even when costs are within this
range, there could be unusual circumstances requiring further analysis.
In the latter case, the immediate impact of the rule would be
observed in reduced industry profits. OSHA uses the ratio of annualized
costs to annual profits as a second check on economic feasibility.
Again, while there is no hard and fast rule, in the absence of evidence
to the contrary, OSHA generally considers a standard to be economically
feasible for an industry when the annualized costs of compliance are
less than a threshold level of ten percent of annual profits. This is a
fairly modest threshold level, given that normal year-to-year
variations in profit rates in an industry can exceed 40 percent or more
(OSHA, 2011, Chapter VI).
For this final rule, all hazardous chemicals distributed in the
United States have to be in compliance with the SDS and labeling
revisions to the HCS, and chemical producers and users in most advanced
economies will be under comparable GHS requirements (encompassing
training, etc.) specific to their own country or economic union. For
this reason, affected domestic establishments should not be susceptible
to foreign competitors not bound by the requirements of the revisions
to the HCS or similar GHS requirements. As a result, OSHA expects that
the costs of this final rule will be passed on in higher prices rather
than absorbed in lost profits, and therefore the Agency will tend to be
primarily concerned with the ratio of industry costs to industry
revenues rather than with the ratio of industry costs to industry
profits.
In order to assess the nature and magnitude of the economic impacts
associated with compliance with the final rule, OSHA developed
quantitative estimates of the potential economic impact of the
requirements on each of the affected industry sectors. The estimated
costs of compliance presented in Section VI.F of this preamble were
compared with industry revenues and profits to provide a measure of
potential economic impacts. Although Section VI.G also contains
estimates of substantial productivity benefits arising from this final
rule that more than offset the estimated costs, these cost savings have
not been included in estimating the economic impacts of the final rule.
Table VI-11 presents data on revenues and profits for each affected
industry sector at the six digit NAICS industry level, along with the
corresponding estimated annualized costs of compliance in each sector.
Potential impacts in the table are represented by the ratios of
compliance costs to revenues and compliance costs to profits.
As is evident from the data and estimates presented in Table VI-6,
the costs of compliance for the final rule are not large in relation to
the corresponding revenues and profits in each of the industry sectors.
The estimated costs of compliance represent about 0.001 percent of
revenues and about 0.011 percent of profits on average across all
entities; compliance costs represent less than 0.09 percent of revenues
or, with the exception of three chemical manufacturing industries, less
than 0.9 percent of profits in any individual industry sector. These
three chemical manufacturing industries are NAICS 325181 Alkalies &
chlorine manufacturing, NAICS 325191 Gum & wood chemical manufacturing,
and NAICS 325992 Photographic film, paper, plate, & chemical
manufacturing, and their compliance costs as a percentage of profits
are 4.3 percent, 2.1 percent, and 2.4 percent, respectively. The cost
of printing labels in color is the main cost driver for these
industries.
Based on the Agency's two screening tests to determine if the
economic impacts of the final rule exceed some minimum threshold level
(i.e., costs equal to one percent of revenue or ten percent of
profits), OSHA concludes that the rule is economically feasible for the
affected industries. In general, the courts have held that a standard
is economically feasible if there is a reasonable likelihood that the
estimated costs of compliance "will not threaten the existence or
competitive structure of an industry, even if it does portend disaster
for some marginal firms" (United Steelworkers of America v. Marshall,
647 F.2d 1189, 1272 (DC Cir. 1980)). The potential impacts of employer
costs associated with achieving compliance with the final rule fall
well within the bounds of economic feasibility in each industry sector.
OSHA does not expect compliance with the requirements of the final rule
to threaten the viability of employers or the competitive structure of
any of the affected industry sectors.
The economic impact of the final rule is most likely to consist of
a very small increase in prices for affected hazardous chemicals, of
about 0.001 percent on average. Chemical manufacturing companies, all
of whom must incur the costs of compliance unless they are already
doing so, should be able to pass through costs to customers. The
additional costs of a one-time revision to SDS and labeling criteria
and one-time investments in printing technology are extremely small in
relation to the value of the corresponding products, and there are
generally no economic substitutes, or alternatives, that would not be
subject to the same requirements. It is unlikely that a price increase
of this magnitude would significantly alter the types or amounts of
goods and services demanded by the public or any other affected
customers or intermediaries. If the compliance costs of the final rule
can be substantially recouped with a minimal increase in prices, there
would be little or no effect on profits.
BILLING CODE 4510-26-P
[GRAPHIC] [TIFF OMITTED] TR26MR12.026
[GRAPHIC] [TIFF OMITTED] TR26MR12.027
[GRAPHIC] [TIFF OMITTED] TR26MR12.028
[GRAPHIC] [TIFF OMITTED] TR26MR12.029
[GRAPHIC] [TIFF OMITTED] TR26MR12.030
[GRAPHIC] [TIFF OMITTED] TR26MR12.031
BILLING CODE 4510-26-C
In profit-earning entities, compliance costs can generally be
expected to be absorbed through a combination of increases in prices
and reductions in profits. The extent to which the impacts of cost
increases affect prices or profits depend on the price elasticity of
demand for the products or services produced and sold by the entity.
The price elasticity of demand refers to the relationship between
changes in the price charged for a product and the resulting changes in
the demand for that product. A larger price elasticity of demand
implies that an entity or industry is less able to pass increases in
costs through to its customers in the form of a price increase and must
absorb more of the cost increase through a reduction in profits.
In the case of cost increases that may be incurred due to the
requirements of the final rule, all businesses within each of the
covered industry sectors would be subject to the same requirements.
Thus, to the extent potential price increases correspond to costs
associated with achieving compliance with the standards, the elasticity
of demand for each entity will approach that faced by the industry as a
whole.
Given the small increases in prices potentially resulting from
compliance with the final rule and the lack of readily available
substitutes for the products and services provided by the covered
industry sectors, demand is expected to be sufficiently inelastic in
each affected industry to enable entities to substantially offset
compliance costs through minor price increases without experiencing any
significant reduction in revenues or profits.
OSHA expects the overall economic impact of the final rule to be
both an increase in the efficiency of production of goods and services
and an improvement in the welfare of society.
First, as demonstrated by the analysis of costs and benefits
associated with compliance with the requirements of the final rule,
OSHA expects that societal welfare will increase as a result of the
revisions to the HCS, as the benefits far exceed compliance costs. The
final rule is estimated to yield net annualized benefits of over $800
million.
Second, until now, many of the costs associated with the injuries,
illnesses, and fatalities resulting from the risks addressed by the
final rule have been externalized. For example, the costs incurred by
society to supply certain products and services that are accompanied by
injuries, illnesses, or fatalities from employee exposure to hazardous
chemicals have not been fully reflected in the prices of those products
and services. To the extent that fewer of these costs are externalized
because of improved employer and employee information about hazardous
chemicals in the workplace, the price mechanism will enable the market
to produce a more efficient allocation of resources. However,
reductions in externalities by themselves do not necessarily increase
efficiency or social welfare unless the costs of achieving the
reductions (including indirect and unintended consequences of
regulatory approaches) are outweighed by the associated benefits, as
they are in this instance.
In addition, based on an analysis of the costs and economic impacts
associated with this rulemaking, OSHA concludes that the effects of the
final rule on employment, wages, and economic growth for the United
States would be negligible. This final rule is expected to result in
increased import and export opportunities with U.S. trading partners
due to the harmonization of the U.S. system with GHS. Hence, the
primary effect on international trade, for businesses of all size, is
likely to be favorable. This determination was supported by comment in
the rulemaking record. For example, the Society of Chemical
Manufacturers and Affiliates reported that companies that do business
globally would see benefits related to the revisions to the OSHA HCS
(Document ID 0402). Other stakeholders anticipate benefits
related to global harmonization (Document ID 0382, 0388, 0393,
and 0405) and mention that the standardization of the HCS will benefit
those who are involved in international trade (Document ID
0410).
Statement of Energy Effects
As required by Executive Order 13211, and in accordance with the
guidance for implementing Executive Order 13211 and with the
definitions provided therein as prescribed by the Office of Management
and Budget (OMB), OSHA has analyzed the standard with regard to its
potential to have a significant adverse effect on the supply,
distribution, or use of energy.
As a result of this analysis, OSHA has determined that this action
is not a significant energy action as defined by the relevant OMB
guidance.
I. Final Regulatory Flexibility Screening Analysis
The Regulatory Flexibility Act (5 U.S.C. 601-612), as amended in
1996, requires the preparation of a Final Regulatory Flexibility
Analysis (FRFA) for rules where there would be a significant economic
impact on a substantial number of small firms. Under the provisions of
the law, each such analysis shall contain:
1. A description of the impact of the rule on small entities;
2. A statement of the need for, and objectives of, the rule;
3. The response of the agency to any comments filed by the Chief
Counsel for Advocacy of the Small Business Administration in response
to the proposed rule, and a detailed statement of any change made to
the proposed rule in the final rule as a result of the comments;
4. A statement of the significant issues raised by the public
comments in response to the initial regulatory flexibility analysis, a
statement of the assessment of the agency of such issues, and a
statement of any changes made in the proposed rule as a result of such
comments;
5. A description of and an estimate of the number of small entities
to which the rule will apply or an explanation of why no such estimate
is available;
6. A description of the projected reporting, recordkeeping and
other compliance requirements of the rule, including an estimate of the
classes of small entities which will be subject to the requirements and
the type of professional skills necessary for preparation of the report
or record; and
7. A description of the steps the agency has taken to minimize the
significant economic impact on small entities consistent with the
stated objectives of the applicable statutes, including a statement of
the factual, policy, and legal reasons for selecting the alternative
adopted in the final rule and why each one of the other significant
alternatives to the rule considered by the agency which affect the
impact on small entities was rejected.
The Regulatory Flexibility Act further states that the required
elements of the FRFA may be performed in conjunction with or as part of
any other agenda or analysis required by any other law if such other
analysis satisfies the relevant provisions (5 U.S.C. 605(a)).
As explained below, OSHA believes that the final rule will not have
a significant economic impact on a substantial number of small
entities, and therefore a FRFA is not required by the Regulatory
Flexibility Act. Nonetheless, OSHA has prepared this voluntary FRFA to
assure the regulated community that the agency has considered the
impacts of the final rule on small entities. While a full understanding
of OSHA's analysis and conclusions with respect to costs and economic
impacts on small businesses requires a reading of the complete FEA
and its supporting materials, this voluntary FRFA will summarize the
key aspects of OSHA's analysis as they affect small businesses.
1. A Description of the Impact of the Final Rule on Small Entities
The final regulation requires classification of chemicals,
especially chemical mixtures, somewhat different from current hazard
determination methods; a standardized format for the organization of
MSDSs (now called SDSs); standardized labels and standardized
pictograms; and training for affected employees on these changes. (Some
commenters argued that GHS would also impose more stringent testing
requirements, but as explained in Section III: Need and Support in this
preamble, the HCS does not currently require testing of chemicals, and
will not require testing with adoption of the GHS.\30\)
---------------------------------------------------------------------------
\30\ OSHA's estimation methodology assumes that firms will
undertake the most cost effective method of complying with an OSHA
requirement. Therefore, if firms choose to perform testing or to
incur other costs not required by an OSHA rule they do so only
because they feel there is some benefit to be gained.
---------------------------------------------------------------------------
For the purpose of its cost analysis, OSHA estimated four types of
cost:
(1) Costs to chemical producers of classifying chemicals,
reformatting SDSs, and developing new labels;
(2) Costs for safety and health managers and logistics personnel to
familiarize themselves with the standard (although not required by the
regulation, this is a necessary step in its implementation);
(3) Costs of training affected employees on how to find the
information they need on SDSs and to comprehend pictograms and standard
labels; and
(4) Costs to upgrade printing technology or purchase multi-colored
labels to comply with the requirement that the pictograms be presented
in a red-bordered diamond.
OSHA believes that, with the exception of the cost of color
printing ink or printing cartridges or the cost of purchasing color
pre-printed labels, these costs are a one-time cost that would be
incurred during the four-year transition period after the final rule is
published. OSHA anticipates that, once the final rule is implemented,
the costs under the revised OSHA HCS will be only marginally higher
than the costs under the existing HCS system and consist solely of the
costs associated with color printing supplies. Once chemical producers,
distributors, and users set up for and shift to the GHS system, OSHA
expects there will be no additional costs arising from the final rule
for classification, SDSs, and labeling.
OSHA also anticipates that, after the four-year transition period,
the revisions to the HCS--resulting in more consistent chemical
classifications and more uniform SDSs and labels--will yield production
efficiencies for health and safety managers, logistics personnel, and
others who handle hazardous chemicals. These cost savings (in addition
to the health benefits for affected workers arising from this final
rule) are considered in Section VI.D: Benefits in this preamble.
OSHA's criteria for determining whether there are significant
economic impacts on a substantial number of small firms are that, for
small entities in any given industry, the annualized costs exceed 1
percent of revenues or 5 percent of profits. All of OSHA's calculations
of the economic impacts on small firms totally ignore any offsetting
benefits of any kind, even though OSHA estimates that, for most small
firms, the benefits of this rule will actually exceed the costs.
OSHA's industry-by-industry analysis, both for small firms (as
defined by SBA) and for very small firms (defined by OSHA as those with
fewer than 20 employees), shows that in no industry size class do the
annualized costs exceed 0.28 percent of revenues or 3.3 percent of
profits, and in almost all cases the annualized costs for small and
very small firms are below 0.01 percent of revenues and 0.1 percent of
profits. For affected small firms as defined by SBA, the average
annualized cost per firm of the final rule would be $52 per year, which
is equal to 0.001 percent of annual revenue and 0.03 percent of annual
profit for the average firm. In terms of chemical-producing industries
only, the average annualized cost per small firm as defined by SBA
would be $544 per year, which is equal to 0.004 percent of annual
revenue and 0.03 percent of annual profit for such a firm. For affected
firms with fewer than 20 employees, the average annualized cost per
firm of the final rule would be $35 per year (or 0.002 percent of
annual revenue and 0.04 percent of annual profit), and the average
annualized cost per firm that produces chemicals would be $255 per year
(or 0.02 percent of annual revenue and 0.2 percent of annual profit).
Given these results, OSHA concludes that the final rule will not
have a significant economic impact on a substantial number of small
entities. Thus, a FRFA is not required for this rulemaking. However,
recognizing the possible value that such an analysis may provide, OSHA
has voluntarily included the elements of the FRFA as part of this
Regulatory Flexibility Analysis (RFA) and has analyzed the potential
impact of the revisions to OSHA's HCS on small entities. As described
in Section VI.D Benefits in this preamble, the revisions to the HCS, on
the whole, are expected to result in significant net benefits to
employers, as the associated cost savings outweigh the corresponding
compliance costs. This same conclusion generally applies to the small
entities affected by the final rule.
In order to ensure that any potential significant adverse impact on
a substantial number of small entities would be appropriately
considered, OSHA also specifically evaluated the impact on small
entities of the costs of compliance alone, without regard to the
associated cost savings and health and safety benefits.
The total annualized cost of compliance with the final rule for
small entities is estimated to be approximately $119 million, as shown
by industry in Table VI-12.
To assess the potential economic impact of the final rule on small
entities, OSHA calculated the ratios of compliance costs to profits and
to revenues. These ratios are presented for each affected industry in
Table VI-12. OSHA expects that among small entities potentially
affected by the final rule, the average increase in prices necessary to
completely offset the compliance costs would be 0.0013 percent. The
average price increase necessary to completely offset compliance costs
would not exceed 0.18 percent among small entities in any single
affected industry sector.
In the event that no costs could be passed through, the compliance
costs could be completely absorbed through an average reduction in
profits of less than 0.03 percent for affected small entities. For
small entities in most affected industries, the compliance costs could
be completely absorbed through an average reduction in profits of less
than 0.3 percent; the reduction in profits would be no more than 3.3
percent among small entities in any of the affected industries.
BILLING CODE 4510-26-P
[GRAPHIC] [TIFF OMITTED] TR26MR12.032
[GRAPHIC] [TIFF OMITTED] TR26MR12.033
[GRAPHIC] [TIFF OMITTED] TR26MR12.034
[GRAPHIC] [TIFF OMITTED] TR26MR12.035
[GRAPHIC] [TIFF OMITTED] TR26MR12.036
[GRAPHIC] [TIFF OMITTED] TR26MR12.037
BILLING CODE 4510-26-C
To further evaluate the potential for any adverse effects on small
entities resulting from the final rule, OSHA assessed the short-term
impacts that may be associated with the compliance costs during the
transition period.
The total non-annualized compliance costs for small entities during
the four-year transition period are estimated to be $1,330 million, or
about $333 million per year for four years. Thus, the potential
temporary impact would be about 0.004 percent of revenues or about 0.07
percent of profits, on average, per year for four years for affected
small entities.
In order to further ensure that potential impacts on small entities
were fully analyzed and considered, OSHA also separately examined the
potential impacts of the final rule on very small entities, defined as
those with fewer than 20 employees. As shown in Table VI-13, the total
annualized costs for entities in this size class would be an estimated
$67 million. The annualized costs represent about 0.002 percent of
revenues and 0.04 percent of profits, on average, for affected very
small entities. The annualized costs did not exceed 0.3 percent of
revenues or 3.3 percent of profits for very small entities in any
affected industry.
The total non-annualized compliance costs for very small entities
during the four-year transition period are estimated to be $789
million, or about $197 million per year for four years. Thus, the
potential temporary impact on very small entities would be about 0.005
percent of revenues or 0.1 percent of profits, on average, per year for
four years.
In order to more carefully focus on the industry sectors most
likely to have significant economic impacts, OSHA carefully examined
those industries in the chemical manufacturing and petroleum and coal
products manufacturing sectors ("chemical and petroleum producers")
that produce chemicals and SDSs. OSHA examined the extent to which
these firms might have significant economic impacts if they produced an
unusually high number of chemical products requiring SDSs.
To examine this issue, OSHA examined all small chemical and
petroleum producers with respect to their costs as a percentage of
revenues and profits. Using the same cost estimation methods as the
base analysis, OSHA estimated how many separate chemical products a
small firm would have to produce for its annualized costs of compliance
with the final rule to exceed 5 percent of profits. OSHA found that the
firm would have to produce 7,065 distinct chemical products, each
requiring its own SDS. OSHA thinks it very unlikely that there are
substantial numbers of small firms (with an average of 27 employees)
that produce 7,065 or more distinct chemical products. Swedish data
show that less than 0.1 percent of all firms (including large firms) in
Sweden produce more than 500 distinct chemical products. (Swedish
Chemical Agency, http://www.kemi.se/templates/Page____2859.aspx)
OSHA conducted a similar analysis for very small firms with fewer
than twenty employees. This analysis found that such firms, with an
average of 4.7 employees, would need to produce more than 310 distinct
chemical products for costs to exceed 5 percent of profits. OSHA
estimates that this would be a very rare situation.
Further, even if small firms could be found that produce more than
7,065 chemical products and very small firms that produce more than 310
chemical products, the costs would probably be much lower than OSHA
estimates. First, firms producing this many distinct products probably
would not produce SDSs and labels without the assistance of specialized
computer software, which OSHA assumes most small firms do not use, but
would instead invest in appropriate software to lower their costs, as
most larger firms do. Second, firms producing large numbers of chemical
products commonly do so because they sell a variety of different
mixtures with similar ingredients. Once appropriate data for the
ingredients of these mixtures had been developed, using the bridging
principles outlined in Appendix A of this preamble, small firms
developing SDSs and labels for each mixture would take far less than
the 7 hours per chemical product that OSHA has estimated for small
firms to convert to the GHS system.
OSHA therefore concludes that there are not a substantial number of
small entities or very small entities that would have significant
economic impacts from this rule as a result of producing a very large
number of distinct chemical products.
BILLING CODE 4510-26-P
[GRAPHIC] [TIFF OMITTED] TR26MR12.038
[GRAPHIC] [TIFF OMITTED] TR26MR12.039
[GRAPHIC] [TIFF OMITTED] TR26MR12.040
[GRAPHIC] [TIFF OMITTED] TR26MR12.041
[GRAPHIC] [TIFF OMITTED] TR26MR12.042
[GRAPHIC] [TIFF OMITTED] TR26MR12.043
BILLING CODE 4510-26-C
2. A Statement of the Need for, and Objectives of, the Rule
OSHA's HCS was first adopted in 1983 for manufacturing (48 FR
53280, Nov. 25, 1983). Later the Agency expanded the scope of coverage
to include all industries where employees are potentially exposed to
hazardous chemicals (52 FR 31852, Aug. 24, 1987).
The HCS requires chemical manufacturers and importers to evaluate
the hazards of the chemicals they produce or import. The current rule
provides definitions of health and physical hazards to use as the
criteria for determining hazards in the evaluation process. Information
about chemical hazards and appropriate protective measures is then
required to be conveyed to downstream employers and employees by
putting labels on containers and preparing and distributing safety data
sheets. All employers with hazardous chemicals in their workplaces are
required to have a hazard communication program, including container
labels, safety data sheets, and employee training.
Ensuring that this information is available in workplaces helps
employers design and implement appropriate controls for chemical
exposures, provides employees the knowledge of the hazards and
identities of the chemicals, and gives employees the opportunity to
participate actively in the successful control of exposures. Together
employers and employees can use this information to reduce the
potential for adverse effects to occur. The information transmitted
under the HCS requirements provides the foundation upon which a
workplace chemical safety and health program is built. Without this
information, appropriate controls could not be identified and
implemented.
OSHA's HCS is designed to disseminate information on chemicals,
which will precipitate changes in handling methods and thus protect
those potentially exposed to the chemical from experiencing adverse
effects. To protect employees and members of the public who are
potentially exposed to chemicals during their production,
transportation, use, and disposal, a number of countries have developed
laws that require information about those chemicals to be prepared and
transmitted to affected parties. These laws vary with regard to the
scope of chemicals covered, definitions of hazards, the specificity of
requirements (e.g., specification of a format for safety data sheets),
and the use of symbols and pictograms. The inconsistencies between the
various laws are substantial enough that different labels and safety
data sheets must often be used for the same product when it is marketed
in different nations. For example, Canada has established requirements
for labels under its Workplace Hazardous Materials Information System
(WHMIS). WHMIS requires that labels include specified symbols within a
defined circle. U.S. chemical manufacturers must label their chemicals
accordingly for marketing in Canada.
Development of multiple sets of labels and safety data sheets for
each product shipped to different countries is a major compliance
burden for chemical manufacturers, distributors, and transporters
involved in international trade. Small businesses may have particular
difficulty in coping with the complexities and costs involved, and it
has been argued that these differing requirements may be a technical
(non-tariff) barrier to trade.
These concerns led, in June 1992, to a mandate from the United
Nations Conference on Environment and Development (UNCED)(Chapter 19 of
Agenda 21), supported by the U.S., calling for development of a
globally harmonized chemical classification and labeling system. The
negotiations were extensive and spanned a number of years. The product
resulting from this effort, the Globally Harmonized System of
Classification and Labeling of Chemicals, was formally adopted by the
new United Nations Committee of Experts on the Transport of Dangerous
Goods and the Globally Harmonized System of Classification and
Labelling of Chemicals in December 2002.
The final rule incorporates the GHS's requirements into the HCS.
They require chemical manufacturers to apply new hazard classification
criteria to their chemicals and to prepare and distribute new labels
and safety data sheets. Further, these SDSs and labels will be
standardized in a way that they are not under the existing HCS. OSHA's
current performance-based approach to SDSs and labeling can create
confusion among those who seek to use hazard information effectively.
For example, labels and safety data sheets may include symbols and
hazard statements that are unfamiliar to readers or not well
understood. This lack of standardization and the absence of pictograms
are particularly a problem for U.S. workers not literate in English.
Containers may be labeled with such a large volume of information that
important statements are not easily recognized.
OSHA believes that adoption of these new requirements will benefit
employers and enhance employee safety. Employers who use chemicals and
employees exposed to those chemicals will benefit from receiving the
revised labels and safety data sheets prepared in a consistent format.
OSHA believes that the information will be easier to comprehend and
access in the new approach, allowing it to be used more effectively for
the protection of employees. The primary effect in workplaces where
chemicals are used but not produced will be to integrate the new
approach into the workplace hazard communication program, including
ensuring that both employers and employees understand the pictograms
and other information provided on the chemicals' labels and SDSs.
OSHA believes that adoption of the GHS will improve labels and SDS
comprehensibility through implementation of a uniform approach. The
current regulatory system includes a performance-oriented approach to
labels and SDSs, allowing the producers to use whatever language or
format they choose to provide the necessary information. This result in
a lack of consistency makes it difficult for users of chemicals to
properly identify their hazards and recommended protective measures,
particularly when purchasing the same product from multiple suppliers.
Having the information provided in the same words and pictograms on
labels, as well as having a standardized order of information on SDSs,
will help all users, including employers, employees, and emergency
responders, to more easily identify the critical information necessary
to protect employees.
In addition, OSHA believes that American employees and employers
will receive benefits from the international adoption of GHS.
Development of the GHS system required extensive work by a great number
of people and resources from many countries and organizations. The
reason it received such support is the belief that there are
significant benefits associated with implementation of a globally
harmonized approach to hazard communication. Countries, international
organizations, chemical producers, users of chemicals, and employees
working with chemicals would all benefit. There are at least four
reasons to expect that GHS will be adopted globally.
First and foremost, the GHS modifications of the HCS will enhance
protection of workers and the environment. Occupationally related
injuries, illnesses, and fatalities remain a serious problem in the
U.S. For example, although likely to contain very
significant underreporting, data from the Bureau of Labor Statistics
indicate that, in 2007, employees suffered an estimated 55,400
illnesses attributable to chemical exposures (BLS, 2008), and that some
17,340 chemical-source injuries and illnesses involved days away from
work (BLS, 2009). As shown in this FEA, the adoption of the revisions
to OSHA's HCS is expected to result in a significant reduction in
injuries, illnesses, and fatalities among U.S. employees exposed to
hazardous chemicals. In addition, while some countries, such as ours,
already have the benefits of protection under existing systems, many do
not have such comprehensive approaches. Thus, implementation of the GHS
would provide these countries with the important protections that
result from dissemination of information about chemical hazards and
protective measures. The U.S. expects to improve and build on worker
protections it already has.
Second, OSHA believes that the final rule will facilitate
international trade in chemicals. It will reduce the burdens caused by
having to comply with differing requirements for the same product and
facilitate small business participation in international trade.
Third, one of the initial reasons this system was pursued
internationally involved concerns about animal welfare and the
proliferation of requirements for animal testing and evaluation.
Existing systems with different definitions of hazards often result in
duplicative testing to produce data related to the varying cut-offs in
the different systems. Having one agreed definition will reduce the
need for this duplicative testing. It should be noted, however, that
OSHA's HCS has never had testing requirements. The HCS is based on
collecting and evaluating the best available existing evidence on the
hazards of each chemical.
Fourth, information transmittal systems provide the underlying
infrastructure for the sound management of chemicals in a country.
Those countries that do not have the resources to develop and maintain
such a system can use the GHS to build their chemical safety and health
programs. Since it has been developed, and will be maintained, through
an international approach, national resources used to achieve chemical
safety and health can be streamlined. Unlike some other issues, a
country's approach to the sound management of chemicals definitely
affects others countries. In some cases, bordering countries may
experience their neighbors' pollution and other effects of uncontrolled
chemical exposures. In all countries, there is a need to acquire
sufficient information to properly handle chemicals when they are
imported from other countries. Thus having a coordinated and harmonized
approach to the development and dissemination of information about
chemicals would be mutually beneficial to importing and exporting
countries.
In the U.S., there are four primary regulatory agencies that
exercise jurisdiction over chemical hazard communication: OSHA; the
Department of Transportation, which regulates chemicals in transport;
the Consumer Product Safety Commission, which regulates consumer
products; and the Environmental Protection Agency, which regulates
pesticides and has other labeling authority under the Toxic Substances
Control Act. These agencies are not domestically harmonized in terms of
definitions of hazards and other requirements. If all four agencies
adopt the GHS, the U.S. will have the additional benefit of harmonizing
the overall U.S. approach to classification and labeling. Since most
chemicals are produced in a workplace and shipped elsewhere, many
employers deal with at least two sets of federal requirements. Thus
these employers would be likely to obtain some benefits from domestic
harmonization.
OSHA has made a determination that the revisions to the HCS will
improve the quality and consistency of information provided to
employers and employees regarding chemical hazards and associated
protective measures. The Agency anticipates this improved information
will enhance the effectiveness of the HCS in ensuring that employees
are apprised of the chemical hazards to which they are exposed, and in
reducing the incidence of chemical-related occupational illnesses and
injuries. OSHA estimates that (1) savings in benefits from improved
employee health and safety exceed the costs of the final rule, and (2)
cost savings to chemical users exceed the costs of the final rule.
An additional and more complete discussion of the reasons why this
standard is being promulgated by the Agency is provided in other
sections of this preamble.
The primary objective of aligning the HCS with the GHS is to
achieve the benefits of the OSHA HCS in a more comprehensive,
efficient, and effective manner. The revisions are expected to provide
an increased degree of occupational safety and health for employees
potentially exposed to hazardous chemicals in the workplace and to
provide updated, clear, and comprehensive standards regarding the
classification of chemical hazards and the manner in which relevant
information about chemical hazards is disseminated to affected
employees.
The intent of the HCS is to ensure that all chemical hazards are
properly evaluated and that information concerning chemical hazards and
associated protective measures is transmitted to employers and
employees. The standard achieves this goal by requiring chemical
manufacturers and importers to review available scientific evidence
concerning the physical and health effects of the chemicals they
produce or import to determine if they are hazardous.
For every chemical found to be hazardous, the chemical manufacturer
or importer must develop a container label and an SDS and provide both
to downstream users of the chemical. All employers with employees
exposed to hazardous chemicals must develop a hazard communication
program and ensure that exposed employees are provided with labels,
access to SDSs, and training on the hazardous chemicals in their
workplace.
The three information components in this system--labels, SDSs, and
employee training--are all essential to the effective functioning of
the program. Labels provide a brief, conspicuous summary of hazard
information at the site where the chemical is used. SDSs provide
detailed technical information and serve as a reference source for
exposed employees, industrial hygienists, safety professionals,
emergency responders, health care professionals, and other interested
parties. Training is designed to ensure that employees understand the
chemical hazards in their workplace and are aware of recommended
protective measures. Labels, SDSs, and training are complementary parts
of a comprehensive hazard communication program--each element
reinforces the knowledge necessary for effective protection of
employees.
Information provided in accordance with the HCS serves to reduce
the incidence of chemical-related illnesses and injuries in the
workplace. This is accomplished by modifying the behavior of both
employers and employees. For example, the information contained in the
HCS enables employers to implement protective measures in the
workplace. Employers will also have information to choose less
hazardous alternatives or select appropriate engineering controls, work
practices, and personal protective equipment. Improved understanding of
chemical hazards by supervisory
personnel results in safer handling of hazardous substances, as well as
proper storage and housekeeping measures.
Employees provided with information and training on chemical
hazards are able to fully participate in the protective measures
instituted in their workplaces. Knowledgeable employees can take the
steps required to work safely with chemicals in their workplace and are
able to determine what actions are necessary if an emergency occurs.
Information on chronic effects of exposure to hazardous chemicals helps
employees recognize signs and symptoms of chronic disease and seek
early treatment. Information provided under the HCS also enables health
and safety professionals to provide better services to exposed
employees. Medical surveillance, exposure monitoring, and other
services are enhanced by the ready availability of health and safety
information.
OSHA believes that the comprehensive approach adopted in the HCS,
which includes requiring evaluation of chemicals and the transmittal of
information through labels, SDSs, and training, is sound. This final
rule does not alter that approach. Rather, the final rule is intended
to improve the effectiveness of the HCS by enhancing the quality and
consistency of the information provided to employers and employees.
OSHA believes this can be accomplished by revising the requirements of
the standard to conform to the more specific and detailed provisions of
the GHS for classification, labeling, and SDSs.
3. The response of the agency to any comments filed by the chief
counsel for advocacy of the small business administration in response
to the proposed rule, and a detailed statement of any change made to
the proposed rule in the final rule as a result of the comments.
The Office of Advocacy in the SBA did not submit any comments to
OSHA in response to the proposed rule.
4. A statement of the significant issues raised by the public
comments in response to the initial regulatory flexibility analysis, a
statement of the assessment of the agency of such issues, and a
statement of any changes made in the proposed rule as a result of such
comments.
OSHA received numerous comments in the record about the impact of
this rulemaking on small entities. There were concerns about OSHA's
preliminary cost estimates and concerns that this rule would have a
substantial impact on small manufacturers. OSHA carefully evaluated
these concerns and has addressed them below as well as in Section VI.F:
Costs of Compliance in this preamble.
Some stakeholders felt that OSHA should convene a Small Business
Regulatory Enforcement Fairness Act (SBREFA) panel for this rulemaking
(Document ID 0361, 0372, 0397, 0407, and 0411). OSHA evaluated
this rule under the provisions of the Regulatory Flexibility Act, which
requires that OSHA hold a SBREFA (or SBAR--Small Business Advocacy
Review) panel when a rule is expected to have a significant impact on a
substantial number of small entities. The modifications to the hazard
communication standard do affect a substantial number of small
entities, but the costs per firm do not rise to the level where they
would impose a significant economic impact on a substantial number of
small entities. OSHA defines a significant economic impact on small
entities as costs that exceed one percent of revenues or five percent
of profits for small entities in any affected industry. The Regulatory
Flexibility Act does not define the term "significant economic
impact." Instead, as noted in the RFA's legislative history, Congress
suggested that agencies refer to SBA guidelines for measuring the
impact of rules on small businesses. See 126 Cong. Rec. S10,942 (Aug.
6, 1980). In relevant guidance, the SBA's Office of Advocacy states
that the impact of a regulation "could be significant if the cost of
the proposed regulation (a) eliminates more than 10 percent of the
businesses' profits; (b) exceeds 1 percent of the gross revenues of the
entities in a particular sector or (c) exceeds 5 percent of the labor
costs of the entities in the sector." See "A Guide for Government
Agencies: How to Comply with the Regulatory Flexibility Act"
(http://archive.sba.gov/advo/laws/rfaguide.pdf). Notably, OSHA's threshold of 5
percent of profits is significantly more protective of small businesses
than the Office of Advocacy's suggested threshold of 10 percent.
OSHA's two thresholds have long been a part of the Agency's
published SBREFA procedures (See http://www.dol.gov/dol/regs/appendix.htm,
prepared pursuant to Section 212 of the SBREFA) and were
originally developed in close cooperation with the Office of Advocacy
(See SBA Office of Advocacy, 2003, p. 18).
Furthermore, in employing a dual threshold, based on either revenue
or profit impacts, OSHA has taken special pains to identify potentially
significant impacts on small entities.\31\
---------------------------------------------------------------------------
\31\ By comparison, many other agencies, such as EPA and the
Department of Homeland Security, rely only on revenue impacts. See
also Aeronautical Repair Station Ass'n, Inc. v. F.A.A., 494 F.3d
161, 175 (D.C. Cir. 2007). (Federal Aviation Administration made
determination that proposed regulation would not have significant
economic impact on substantial number of small entities based on its
calculation of annualized costs of less than 1 percent of annual
median revenue); Washington v. Daley, 173 F.3d 1158, 1171 (9th Cir.
1999) (parties agreed that economic impact of Department of Commerce
regulation would be considered significant if regulation resulted in
more than 5 percent reduction in annual gross revenues). It should
also be noted that, in OSHA's experience, the 5-percent
profitability threshold is much more likely than the 1-percent
revenue threshold to trigger a significant impact on a substantial
number of small entities. This is supported by the fact that, with
profit rates in the United States equal to approximately 6 percent
of revenues (as it is, on average, for all firms affected by this
final rule), for a firm with profits of 6 percent of revenues, 5
percent of profits will be approximately equivalent to 0.3 percent
of revenues.
---------------------------------------------------------------------------
While this rule will be costly in the aggregate, it is not
aggregate costs but the significance of impacts on small entities that
triggers the need for a SBREFA panel. No panel was or is needed for
this rulemaking because costs per small entity do not meet the
threshold that OSHA uses to define a significant economic impact on a
substantial number of small entities.
Stakeholders also expressed concerns that costs were underestimated
and that costs to small entities would be considerable. The U.S.
Chamber of Commerce asserted that "the imposition of a completely new
system of classification of chemicals represents huge burdens on small
employers with significant costs" (Document ID 0397). OSHA
acknowledges that there will be transitional costs for small businesses
but feels that the additional transition time OSHA has incorporated
into the final rule and discussed in more detail elsewhere in the FEA,
combined with OSHA compliance assistance and the fact that many firms
have already made the transition to GHS, should allow small employers
to adopt the GHS criteria without overwhelming challenges. The U.S.
Chamber of Commerce did not provide additional details, which were
solicited as part of both the ANPR and the NPRM, on what types of costs
small businesses would incur or the possible magnitude of those costs.
Without detailed estimates, OSHA cannot fully evaluate alternative
costs for small businesses; nor can OSHA adopt alternative cost
estimates without persuasive evidence in the record.
Wacker Chemical Company felt that the changes to the HCS would have
a large impact on small businesses "result[ing] from the lack of
personnel and financial resources to implement changes of this
magnitude which may involve reclassification of the companies'
products, reauthoring SDSs
and labels, and training personnel" (Document ID 0335), and
IBM Corporation expressed concern that small businesses "may not have
the technical resources and skill to generate safety data sheets for [*
* *] mixtures" (Document ID 0334). The Agency believes that
small firms have the expertise to make the hazard determinations and
meet the other transitional requirements of the revised HCS and, other
than comments on the possibility of technical expertise being an issue
for small firms asserted by a few firms who do not qualify as small,
OSHA did not receive solid evidence that a lack of technical expertise
among small firms would actually be a significant issue. Chemical
manufacturers and users have been able to comply with the current HCS,
and manufacturers have been able to make the classification
determinations and label their products in the appropriate manner. In
addition, some small firms are likely already complying with the
requirements of GHS in order to facilitate international trade. The
revised HCS will not be considerably more technical or require
considerably more expertise in order to comply than the current HCS.
There is also no evidence, from the experiences of firms in the EU or
in Asian markets where the GHS criteria for classification of
chemicals, label elements, and SDS formats have already been adopted
into practice, that small firms are not able to comply due to either
overwhelming costs or to a lack of technical expertise required to make
the changes.
Many comments expressed general concern that OSHA underestimated
the compliance burden on small businesses (Document ID 0336,
0372, 0397, and 0407), and OSHA has increased some costs (for instance,
doubling the time required for training) in response to these comments.
The comments, while appreciated and insightful, did not contain the
level of detail that OSHA would need in order to make a case for
changing many of the estimates in the PEA. For the most part, comments
received on the issue of costs to and impacts on small businesses
simply stated that (in general) costs to small businesses were
understated in the PEA or asserted that impacts would be significant
without providing data to support alternative estimates. In order to
assess the impacts on the cost effectiveness of this standard of
possible underestimation of cost parameters, the Agency has included a
sensitivity analysis in Section VI.L: Sensitivity Analysis in this
preamble. Additional concerns about costs that are not specific to
small businesses are addressed further in Section VI.F: Costs of
Compliance in this preamble.
Many commenters, including some who voiced concerns about costs,
did not support a voluntary adoption approach or any other exemption or
modified system for small businesses (Document ID 0324, 0327,
0328, 0329, 0335, 0338, 0351, 0352, 0370, 0376, 0377, 0381, 0382, 0393,
and 0410). DuPont felt that dual systems would "undermine the goal of
harmonization [* * * and] be very confusing for employees" (Document
ID 0329). Ferro Corporation expressed the view that "failure
to implement [the requirements of the rule] across-the-board will cause
confusion; negate main benefits; and potentially be less protective"
(Document ID 0363).
Many of the commenters who addressed small business issues felt
that the benefits to small businesses would be negligible (Document ID
0372, 0378, 0385, 0396, 0397, 0400, 0402, and 0407).
Commenters who viewed the primary benefits of adopting the GHS as
facilitating international trade were likely to favor an alternative of
less than full compliance with GHS. As has been addressed throughout
the FEA, however, OSHA's estimates of the benefits of this final rule
reflect fewer worker injuries and illnesses, efficiency improvements in
the safe handling of hazardous chemicals, and less costly and more
effective hazard communication training of new workers. While OSHA
recognizes the significant potential trade benefits of this final rule,
the Agency did not quantify or monetize these benefits.
In response to numerous comments received in the record, OSHA has
extended the phase-in period for this rulemaking and aligned the phase-
in of this rule to correspond to the EU's deadline for classification
of mixtures. Some of these comments asserted that more time would be
especially beneficial to small businesses, reducing the compliance
burden significantly (Document ID 0399, 0405, and 0408). For
example, the National Association of Chemical Distributors suggested a
timeline of 3 years plus 18 months for distributors and downstream
users (Document ID 0341). The effective dates in the final
rule take these (and other suggestions) into account and provide
substantial additional time for implementation. Where the proposal
required all labels and SDSs to be in compliance with the new
requirements in three years after publication (or August 2014), the
final rule requires manufacturers and importers to modify labels and
SDSs by June 1, 2015. The final rule also gives distributors an
additional six months, until December 1, 2015, to sell stock labeled
under the current standard. In addition, employers are given another
six months, until June 1, 2016, to update their training and their
hazard communication program with any new hazard information received
because of the final rule. Finally, the proposal required that exposed
employees receive initial training two years after adoption (or August
2013), whereas the final rule gives employers until December 1, 2013 to
complete this training.
5. Description of and estimate of the number of small entities to
which the rule will apply.
OSHA has completed an analysis of the economic impacts associated
with this final rule, including an analysis of the type and number of
small entities to which the final rule applies. In order to determine
the number of small entities potentially affected by this rulemaking,
OSHA used the definitions of small entities developed by the Small
Business Administration (SBA) for each industry.
The final standard impacts firms that are the primary producers or
distributors of hazardous chemicals, and firms whose employees are
exposed to hazardous chemicals. Based on the definitions of small
entities developed by SBA for each industry, the final rule is
estimated to potentially affect a total of 4,093,543 small entities, as
shown in Table VI-12. The rule has its greatest impacts on the 72,040
small firms that produce chemicals that require SDSs and labels.
6. Description of the projected reporting, recordkeeping, and other
compliance requirements of the rule.
The final standard includes revised criteria for classification of
chemical hazards; revised labeling provisions that include requirements
for use of standardized signal words, pictograms, and hazard
statements; a specified format for safety data sheets; and related
revisions to definitions of terms used in the standard, employee
information and training requirements, and other sections of HCS. The
final rule also modifies other OSHA standards that contain hazard
communication requirements to harmonize them with the requirements of
GHS. In addition, certain OSHA standards use HCS terms, and OSHA is
making changes to ensure that the scope of those standards is not
changed by the GHS revisions.
The preamble to the final standard provides a comprehensive
description of, and further detail regarding, the compliance
requirements of the rulemaking. A description of the types
of entities which would be subject to the new and revised requirements,
and the types of professional skills necessary for compliance with the
requirements, is presented in the relevant sections of this economic
analysis and the corresponding supporting research, and is summarized
below with a summary of unit costs. Except for employee training and
color printing, these costs would apply only to those small businesses
not already in compliance with the revisions.
Reclassifying chemicals and modifying SDSs and labels:
Medium establishments (100-499 employees): An average of 5
hours per SDS; in addition, for 25 percent of establishments, an
average of $208 per SDS for software modifications.
Small establishments (1-99 employees): An average of 7
hours per SDS. Management familiarization and other costs:
Eight hours for health and safety managers and logistics
personnel in the manufacturing sector;
Two hours for each hazard communication program manager
not in the manufacturing sector.
Employee training:
One hour per production employee in most industries;
Thirty minutes in occupations exposed to few hazardous
chemicals and types of hazards;
Ten minutes per employee in some occupations where GHS-
type pictograms are already in use.
Color Printing
Category 1 establishments (those currently printing only
in black & white who do not own color printers): Medium establishments
$0.01 per label, small establishments $0.13 per label, and very small
establishments $0.14 per label.
Category 2 establishments (those currently printing only
in black & white but who own color printers): Medium establishments
$0.01 per label, small establishments $0.13 per label, and very small
establishments $0.14 per label.
Category 3 establishments (those currently purchasing pre-
printed label stock): Medium establishments $0.03 per label, small and
very small establishments $0.03 per label.
Category 4 establishments (those currently producing
labels printed in multiple colors): No additional costs related to this
provision.
7. A description of the steps the Agency has taken to minimize the
significant economic impact on small entities.
OSHA has extended the phase-in period for this rulemaking in
response to stakeholder concern. The Agency believes that the
additional time granted to manufacturers, distributors, and users of
chemicals will serve to reduce the transitional costs associated with
this rule. Chemical manufacturers currently revise SDSs and labels
periodically to include new or updated hazard information, and the
extended time frame will allow firms to adopt the GHS criteria into
their hazard communication program and to modify SDSs, warning labels,
and workplace signs within the normal flow of their operations.
OSHA will be offering guidance materials such as quick cards and
fact sheets to aid firms in developing and implementing the training
requirements of this rule. OSHA will also be releasing a small business
compliance guide to provide additional guidance to small businesses,
which will ease the economic impact and compliance burden. The Agency
solicited comment from stakeholders as part of the ANPR and NPRM on
what compliance assistance tools would be most helpful and has
incorporated the suggestions received in the record in the development
of guidance materials.
J. Environmental Impacts
OSHA has reviewed the provisions of this final rule in accordance
with the requirements of the National Environmental Policy Act (NEPA)
of 1969 (42 U.S.C. 4321 et seq.), the Council on Environmental Quality
(CEQ) NEPA regulations (40 CFR Parts 1500-1508), and the Department of
Labor's NEPA Procedures (29 CFR Part 11). As a result of this review,
OSHA has determined that the final rule will have no significant
adverse effect on air, water, or soil quality, plant or animal life,
use of land, or other aspects of the environment. OSHA anticipates that
the more complete and easier-to-understand SDSs resulting from this
rule will, in addition to increasing employee health and safety, have
positive effects on the environment.
K. Unfunded Mandates Reform Act Analysis
Section 3 of the Occupational Safety and Health Act makes clear
that OSHA cannot enforce compliance with its regulations or standards
on the U.S. government "or any State or political subdivision of a
State." Under voluntary agreement with OSHA, some States enforce
compliance with their State standards on public sector entities, and
these agreements specify that these State standards must be equivalent
to OSHA standards. Thus, although OSHA may include compliance costs for
affected public sector entities in its analysis of the expected impacts
associated with the final HCS rule, the rule does not involve any
unfunded mandates being imposed on any State or local government
entity.
Based on the analysis presented in this economic analysis, OSHA
concludes that the final rule would impose a Federal mandate on the
private sector in excess of $100 million in expenditures in any one
year. Accordingly, this economic analysis of the final rule, concerning
revisions to the HCS, constitutes the written statement containing a
qualitative and quantitative assessment of the anticipated costs and
benefits of the Federal mandate, as required under Section 202(a) of
the Unfunded Mandates Reform Act of 1995 (2 U.S.C. 1532(a)).
L. Sensitivity Analysis
In this section, OSHA provides a sensitivity analysis of the major
assumptions underlying the Agency's estimates of the annualized costs
and annualized benefits of the final rule. The purpose is to determine
whether OSHA's conclusion that the final rule yields net benefits is
vulnerable to a reasonable change in any one of these assumptions.
OSHA's choice of how much to increase unit cost parameters in the
sensitivity analysis was intended to reflect an upper bounds (or more)
of reasonableness, based on comments, as well as on professional
experience and common sense. (As a result, there are almost no
estimates provided by commenters of higher unit costs than we used in
the sensitivity analysis, and we rejected those few outliers as being
unrealistically large and certainly not representative of the average
establishment covered by this rule.) OSHA's choice of how much to
decrease unit benefit parameters was more subjective and reflected the
fact that few commenters provided alternative quantitative estimates.
Broadly, the Agency cut unit benefit parameters by at least half in all
cases for the sensitivity analysis, which OSHA believes is consistent
with the spirit of comments that either supported OSHA's estimates of
benefits or thought benefits were somewhat overestimated--the exception
being those few commenters who disputed the existence of health and
safety benefits or productivity benefits arising from the proposed
rule. However, it should be carefully noted that any given benefit
category could be reduced to zero and the net benefits would still be
positive. This can be seen in Table VI-1, which shows that the
estimated net positive annualized benefits of the final rule ($556
million)significantly exceed the estimated annualized benefits for any
individual category of benefits--Reduction in Safety and Health Risks
($250 million); Productivity Improvements for Health and Safety
Managers and Logistics Personnel ($475 million); and Savings during
Periodic Updating of SDSs and Labels ($32 million).
The sensitivity analysis below shows that OSHA's conclusion that
the final rule produces net benefits is not dependent on any particular
assumption. In fact, the estimated annualized health and safety
benefits of the rule alone, independent of any productivity benefits,
exceed the estimated annualized cost of the rule. Further, the broad
support from industry for this rule, even from those commenters
critical of some of OSHA's estimates of costs and benefits, suggests
that industry believes the productivity benefits of the rule exceed the
costs.
The methodology and calculations underlying the estimation of the
compliance costs, benefits, and economic impacts associated with this
rulemaking are generally linear and additive in nature. Thus, the
sensitivity of the results and conclusions of the analysis will
generally be proportional to variations in the relevant input
parameters.
For example, if the estimated time that companies need to
reclassify chemical hazards and revise SDSs and labels were doubled,
the corresponding labor costs (but not software costs) of
reclassification and revision of SDSs and labels would double as well.
OSHA evaluated a series of such changes in input parameters to test
whether and to what extent the general conclusions of the economic
analysis held up. On the whole, OSHA found that the conclusions of the
analysis are reasonably robust, as changes in any of the input
parameters tend not to produce disproportionately large changes in the
results. The results also show significant net annualized benefits for
the rule regardless of the individual revisions to costs, benefits, or
discount rate. The results of the individual sensitivity tests are
summarized in Table VI-14 and are described in more detail below.
BILLING CODE 4510-26-P
[GRAPHIC] [TIFF OMITTED] TR26MR12.044
BILLING CODE 4510-26-C
In the sensitivity test on costs where OSHA doubled the estimated
time that companies need to reclassify chemical hazards and revise SDSs
and labels, and estimates of other input parameters remained unchanged,
as shown in Table VI-14, the estimated total costs of compliance would
increase by $18 million annually, or by about 9 percent, while net
benefits would also decline by $18 million, from $556 million to $538
million annually.
In a second sensitivity test, OSHA doubled the estimated total
number of affected SDSs addressed by this rulemaking, which increased
the estimated total cost of reclassification and revision of SDSs and
labels. As shown in Table VI-14, if OSHA's estimates of other input
parameters remained unchanged, the total estimated costs of compliance
would increase by $23 million annually, or by about 11 percent, while
net benefits would also decline by $23 million annually, from $556
million to $533 million annually.\32\
---------------------------------------------------------------------------
\32\ For this sensitivity analysis, OSHA calculated only the
impact on costs of an increase in the number of SDSs. However, in
principle, each additional SDS would yield future benefits due to
improved efficiencies in creating and revising SDSs under GHS.
Although not shown in Table VI-8, this effect would increase
benefits by $32 million annually, more than offsetting the $23
million annual cost increase.
---------------------------------------------------------------------------
In a third sensitivity test, when OSHA increased by 50 percent the
estimated number of employees required to be covered by hazard
communication programs and to be trained on GHS, the corresponding
estimate of the total costs associated with training employees
increased by 50 percent. As shown in Table VI-14, if OSHA's estimates
of other input parameters remained unchanged, the total estimated costs
of compliance would increase by $48 million annually, or by about 24
percent, while net benefits would also decline by $48 million annually,
from $556 million to $508 million annually.
In a fourth sensitivity test, when OSHA doubled the estimated
incremental amount of time necessary for training employees on GHS, the
corresponding estimate of the total costs associated with training
employees also doubled. As shown in Table VI-14, if OSHA's estimates of
other input parameters remained unchanged, the total estimated costs of
compliance would increase by $96 million annually, or by about 48
percent, while net benefits would also decline by $96 million annually,
from $556 million to $460 million annually.
OSHA performed a fifth sensitivity test where the estimated
incremental per-label cost of printing labels in color was doubled. As
shown in Table VI-14, if OSHA's estimates of other input parameters
remained unchanged, the total estimated costs of compliance would
increase by $24 million annually, or by about 12 percent, while net
benefits would also decline by $24 million annually, from $556 million
to $532 million annually
OSHA also performed sensitivity tests on several input parameters
used to estimate the benefits of the final rule. In one sensitivity
test on benefits, OSHA reduced its estimate of health and safety
benefits of the final rule from 1 percent to 0.5 percent of the
benefits estimated for the existing HCS. As shown in Table VI-14, if
OSHA's estimates of other input parameters remained unchanged, the
total estimated benefits of the final rule would decline by $125
million annually, or by about 17 percent, while net benefits would also
decline by $125 million annually, from $556 million to $431 million
annually.
In a second, parallel sensitivity test on benefits, OSHA increased
its estimate of health and safety benefits of the final rule from 1
percent to 5 percent of the benefits estimated for the existing HCS. As
shown in Table VI-14, if OSHA's estimates of other input parameters
remained unchanged, the total estimated benefits of the final rule
would increase by $1,000 million annually, or by about 132 percent,
while net benefits would also increase by $1,000 million annually, from
$556 million to $1,556 million annually.
In a third sensitivity test on benefits, OSHA reduced its estimate
of savings due to the improved efficiency in creating and revising SDSs
under GHS by 50 percent. As shown in Table VI-14, if OSHA's estimates
of other input parameters remained unchanged, the total estimated
benefits of the final rule would decline by $17 million annually, or by
about 2 percent, while net benefits would also decrease by $17 million
annually, from $556 million to $539 million annually.
In a fourth sensitivity test on benefits, OSHA reduced its estimate
of savings due to the improved efficiency of safety and health managers
and logistics personnel by 67 percent. As shown in Table VI-14, if
OSHA's estimates of other input parameters remained unchanged, the
total estimated benefits of the final rule would decline by $315
million annually, or by about 42 percent, while net benefits would also
decrease by $315 million annually, from $556 million to $241 million
annually.
And finally, in the fifth sensitivity test on benefits, OSHA tested
the effect of including cost savings from simplified hazard
communication training in future periods made possible by the final
rule.\33\ For this sensitivity test, OSHA added a cost savings of a
half hour, on average, in training time per new employee once the
transition period ends and the final rule is fully implemented. OSHA
chose a half-hour time savings based on the testimony of the one
commenter who provided an estimate of the time savings from simplified
hazard communication training.\34\ As shown in Table VI-14, as a result
of adding the half-hour savings in training time, assuming OSHA's
estimates of other parameters remain unchanged, the total benefits of
the final rule would increase by $285 million annually,\35\ or by about
38 percent, while net benefits would also increase by $285 million
annually, from $556 million to $841 million annually.
---------------------------------------------------------------------------
\33\ As noted in the earlier discussion on benefit, in Section
VI.D of this preamble, comments on the proposed rule contained
extensive qualitative support for the proposition that the revisions
to the HCS rule will make training easier and therefore less time-
consuming and less costly.
\34\ Printing Industries of America testified at the OSHA public
hearing held in Pittsburgh that training for an employee who would
be responsible for working with hazardous materials is
"approximately an hour to an hour and a half" and that training
would be less time-consuming under the revised HCS and might be
reduced "possibly by a third simply because [the revised HCS will]
be removing a number of types [of MSDS and labeling systems]"
(Document ID 0499, Tr. 96-7). This estimate would be
consistent with a saving in training time of one-third to one-half
of an hour relative to current training time of one to one and a
half hours. OSHA chose the one-half-hour estimate because a
representative training time for all the commenters would be at
least an hour and a half (and arguably more like 3 hours).
Furthermore, in its final economic analysis for the original hazard
communication rule, OSHA estimated that the rule would require an
average of 3 hours of training per employee (48 FR 53280, Nov. 25,
1983).
\35\ This estimate uses the BLS turnover rate to arrive at the
number of new employees per year per establishment and assumes from
one to ten employees per training session, depending on
establishment size. The cost savings due to simplified training take
into account one half hour of managerial time to deliver the
training plus one half hour of time for each of 17.5 million new
employees a year to receive the training. The annualized cost
savings of $285 million is equal to annual cost savings of $465.5
million multiplied by an annualization factor of 0.6130 to reflect
the fact that these cost savings would not begin to be realized
until five years after the effective date of the final rule.
---------------------------------------------------------------------------
OSHA also examined the effect of a change in the discount rate on
the annualized costs and benefits. Changing the discount rate from 7
percent, used in the base case, to 3 percent would have the effect of
lowering the costs to $161 million per year and increasing the gross
benefits to $839 million per year. The result, as shown in Table VI-14,
would be to increase net benefits by $122 million per year, from $556
million to $678 million per year.
OSHA also considered the sensitivity of its findings that the final
rule is economically feasible and does not have a significant economic
impact on a substantial number of small entities. For example, even if
all of the estimated annualized costs of compliance were to increase by
50 percent, these costs would still represent less than 0.005 percent
of annual revenues and less than 0.1 percent of annual profit for the
average establishment, small entity, or very small entity, and no small
entity or very small entity would have costs in excess of 1 percent of
revenues or 5 percent of profits.
In conclusion, the sensitivity analysis demonstrates that even with
relatively large variations in the input parameters, there would not be
any disproportionately large changes in the estimates of compliance
cost or benefits. Further, even if there were a 50 percent increase in
all of the compliance cost estimates, there would still be a relatively
high confidence in OSHA's finding concerning economic feasibility, the
certification that the standard will not have significant economic
impacts on a substantial number of small entities, and the conclusion
that the benefits of the final rule exceed the costs.
VII. OMB Review Under the Paperwork Reduction Act of 1995
The final rule revises existing Hazard Communication collection of
information (paperwork) requirements that are currently approved by the
Office of Management and Budget (OMB) under the Paperwork Reduction Act
of 1995 (PRA-95), 44 U.S.C. 3501 et seq., and OMB's regulations at 5
CFR part 1320. On October 30, 2009, the Department of Labor submitted
Hazard Communication collection of information requirements identified
in the NPRM to OMB for review in accordance with 44 U.S.C. 3507(d). In
accordance with 44 U.S.C. 3506(c)(2), the proposed regulation solicited
public comments on the revision of the Hazard Communication Standard's
(HCS) Information Collection Request (ICR) (paperwork burden hour and
cost analysis) for the proposal. OSHA received no public comments on
the Hazard Communication Standard's ICR. On November 18, 2009, OMB
filed a comment on the Hazard Communication Standard NPRM ICR in
accordance with 44 U.S.C. 3507(d). OMB stated, "This OMB action is not
an approval to conduct or sponsor an information collection request
under the Paperwork Reduction Act of 1995." The final Standard
modifies existing information collection requirements that are
currently approved under OMB Control Number 1218-0072. This ICR has
been revised and submitted to OMB. OSHA will publish a separate notice
in the Federal Register that will announce the result of OMB's reviews.
The Department of Labor notes that a Federal agency cannot conduct or
sponsor a collection of information unless OMB approves it under the
PRA- 95, and the agency displays a currently valid OMB control number.
Also, notwithstanding any other provision of law, no employer shall be
subject to penalty for failing to comply with a collection of
information if the collection of information does not display a
currently valid OMB control number.
The final rule standardizes the hazard communication requirements
for hazardous chemical products used in U.S. workplaces, and thus
provides employees with consistent hazard communication information.
Hazard communication is currently addressed by many different
international, national, and State authorities. These existing
requirements are not always consistent and often contain different
definitions of hazards and varying provisions for what information is
required on labels and safety data sheets (SDSs). The final standard
harmonizes the U.S. system with international norms and as a result
would enhance worker safety and facilitate international trade. The
final rule's modifications to the Hazard Communication Standard's
collection of information requirements include: (1) Revised criteria
for classification of chemical hazards; (2) revised labeling provisions
that include requirements for use of standardized signal words,
pictograms, hazard statements, and precautionary statements; (3) a
specified format for SDSs; and (4) related revisions to definitions of
terms used in the Standard and to requirements for employee training on
labels and SDSs.
Paragraph (d), "hazard classification," requires chemical
manufacturers and importers to evaluate chemicals produced in their
workplaces or imported by them to classify the chemicals' health and
physical hazards in accordance with the Standard. For each chemical,
the chemical manufacturer or importer must determine the hazard
classes, and the category of each hazard class, that apply to the
chemical being classified. Employers are not required to classify
chemicals unless they choose not to rely on the classification
performed by the chemical manufacturer or importer for the chemical.
Chemical manufacturers, importers or employers classifying chemicals
must identify and consider the full range of available scientific
literature and other evidence concerning the potential hazards. There
is no requirement to test the chemical to determine how to classify its
hazards. Mandatory Appendix A to Sec. 1910.1200 shall be consulted for
classification of health hazards, and Mandatory Appendix B to Sec.
1910.1200 shall be consulted for the classification of physical
hazards.
For mixtures, chemical manufacturers, importers, or employers
evaluating chemicals also must follow the procedures described in
Appendixes A and B to Sec. 1910.1200 to classify the hazards of the
chemicals, including determinations regarding when mixtures of the
classified chemicals are covered by the Standard. When classifying
mixtures they produce or import, chemical manufacturers and importers
of mixtures may rely on the information provided on current SDSs of the
individual ingredients except where the chemical manufacturer or
importer knows, or in the exercise of reasonable diligence should know,
that the SDS misstates or omits information required by the provisions
in the final HCS.
Pursuant to paragraph (e), employers are required to develop,
implement, and maintain at each workplace a written hazard
communication program which at least describes how the criteria
specified in paragraphs (f), (g), and (h) of the standard on labels and
other forms of warning, SDSs, and employee information and training
will be met, and which also includes the following: (i) a list of the
hazardous chemicals known to be present using a product identifier that
is referenced on the appropriate SDS (the list may be compiled for the
workplace as a whole or for individual work areas); and (ii) the
methods the employer will use to inform employees of the hazards of
non-routine tasks (for example, the cleaning of reactor vessels) and
the hazards associated with chemicals contained in unlabeled pipes in
their work areas. The final rule makes no changes to this requirement.
Paragraph (f) modifies existing label requirements by requiring
more specific information. Paragraph (f)(1) requires chemical
manufacturers, importers, or distributors to ensure that each shipped
container of classified hazardous chemicals leaving the workplace is
labeled, tagged, or marked with the following information:
(i) Product identifier;
(ii) Signal word;
(iii) Hazard statement(s);
(iv) Pictogram(s);
(v) Precautionary statement(s); and
(vi) Name, address, and telephone number of the chemical
manufacturer,
importer, or other responsible party.
The chemical manufacturer, importer, or distributor must ensure
that the information provided under (i) through (v) above must be in
accordance with the mandatory Appendix C, Allocation of Label Elements,
for each hazard class and associated hazard category for the hazardous
chemical; prominently displayed; and in English (other languages may
also be included if appropriate). In addition, the information in (ii)
through (iv) must be located together on the label, tag, or mark.
For labels in the workplace, except as provided in paragraphs
(f)(7) and (f)(8) of the Standard, employers must ensure that each
container of hazardous chemicals in the workplace is labeled, tagged,
or marked with either (i) the information specified under (f)(1)(i)
through (v) for labels on shipped containers; or (ii) product
identifier and words, pictures, symbols, or combination thereof, which
provide at least general information regarding the hazards of the
chemicals, and which, in conjunction with the other information
immediately available to employees under the hazard communication
program, will provide employees with the specific information regarding
the physical and health hazards of the hazardous chemical.
OSHA has also updated the language for workplace signs and labels
to incorporate the GHS hazard statement and the applicable
precautionary statement(s), where required. Most OSHA substance-
specific heath standards require hazard warning signs, usually for
regulated areas, and the language required on the signs varies. With
the GHS revision, these standards retain the requirements for specific
warning language for specific signs; however, OSHA has modified the
language to be compatible with GHS and consistent throughout the OSHA
standards. The GHS classification process for a specific substance
dictates the hazard warnings and the precautionary statements that will
be required on the new GHS-compliant product labels. OSHA believes that
having signs and labels in the same formats and containing identical
warnings for the same health effects will make it far easier for
employers and employees to quickly recognize the hazard and the degree
of danger of a hazard, thus enhancing communication.
The final rule modifies the language requirements for signs and
labels found in the Agency's health standards listed below in Table
VII-1. Since the final rule provides specific language for signs and
for labels on containers of contaminated clothing, waste and debris,
the Agency is exempted from taking burden hours and costs for these
provisions. (See 5 CFR 1320.2(c)(2) ("Controlling paperwork burden on
the public")). The Agency is taking burden hours and costs for
employers to label, tag, or mark each container of hazardous chemicals
with either (i) the information specified under (f)(1)(i) through (v)
for labels on shipped containers; or (ii) the product identifier and
words, pictures, symbols, or combination thereof, which provide at
least general information regarding the hazards of the chemicals.
BILLING CODE 4510-26-P
Table VII-1
[GRAPHIC] [TIFF OMITTED] TR26MR12.045
[GRAPHIC] [TIFF OMITTED] TR26MR12.046
BILLING CODE 4510-26-C
Pursuant to paragraph (f)(11), chemical manufacturers, importers,
distributors, or employers who become newly aware of any significant
information regarding the hazards of a chemical shall revise the labels
for the chemical within six months of becoming aware of the new
information, and shall ensure that labels on containers of hazardous
chemicals shipped after that time contain the new information. If the
chemical is not currently produced or imported, the chemical
manufacturer, importer, distributor, or employer shall add the
information to the label before the chemical is shipped or introduced
into the workplace again.
Paragraph (g)(2) requires the chemical manufacturer or importer
preparing the SDS to ensure that it is in English (although the
employer may maintain copies in other languages as well), and include
the following section numbers and headings, and associated information
under each heading, in the order listed (See Appendix D to Sec.
1910.1200--Safety Data Sheets, for the specific content of each section
of the safety data sheet).
Section 1, Identification;
Section 2, Hazard(s) identification;
Section 3, Composition/information on ingredients;
Section 4, First-aid measures;
Section 5, Fire-fighting measures;
Section 6, Accidental release measures;
Section 7, Handling and storage;
Section 8, Exposure controls/personal protection;
Section 9, Physical and chemical properties;
Section 10, Stability and reactivity;
Section 11, Toxicological information; and
Section 16, Other information, including date of preparation or
last revision.
Although not required by the final rule, an employer may include
the following sections to be consistent with the GHS:
Section 12, Ecological information;
Section 13, Disposal considerations;
Section 14, Transport information; and
Section 15, Regulatory information.
Paragraph (g)(5) requires the chemical manufacturer, importer or
employer preparing the SDS to ensure that the information provided
accurately reflects the scientific evidence used in making the hazard
classification. If the chemical manufacturer, importer or employer
preparing the SDS becomes newly aware of any significant information
regarding the hazards of a chemical, or ways to protect against the
hazards, this new information must be added to the SDS within three
months. If the chemical is not currently being produced or imported,
the chemical manufacturer or importer must add the information to the
SDS before the chemical is introduced into the workplace again.
Paragraph (g)(11) requires that employers ensure the SDSs are
readily available, upon request, to designated representatives, the
Assistant Secretary, and the Director, in accordance with the
requirements of 29 CFR 1910.1020(e).
OMB Control Number: 1218-0072.
Affected Public: Business or other for-profit.
Number of Respondents: 5,514,697.
Frequency: On Occasion.
Average Time per Response: The average time per response ranges
from twelve seconds for employers to label portable in-plant containers
to seven hours for employers to reclassify chemicals and revise SDSs
and labels.
Estimated Total Burden Hours: 11.3 million hours.
Estimated Cost: $34.7 million.
VIII. Federalism and Consultation and Coordination With Indian Tribal
Governments
The Agency reviewed this final rule according to the most recent
Executive Order ("E.O.") on Federalism (E.O. 13132, 64 FR 43255,
August 10, 1999). This E.O. requires that Federal agencies, to the
extent possible, refrain from limiting State policy or local
policymaking discretion, consult with States and local officials prior
to taking any actions that restrict their policy options, and take such
actions only where there is constitutional and statutory authority to
do so and the problem is of national significance. The E.O. generally
allows Federal agencies to preempt State law only where there is clear
evidence of Congressional intent to allow it, or where the exercise of
State authority would conflict with the exercise of Federal authority
under a statute; in such cases, Federal agencies must limit preemption
of State law to the extent possible.
In Section 18 of the Occupational Safety and Health Act (the OSH
Act), Congress expressly provides that States may adopt, with Federal
OSHA approval, a plan for the development and enforcement of
occupational safety and health standards. States that obtain Federal
approval for such plans are referred to as "State Plan States" (29
U.S.C. 667). Occupational safety and health standards developed by such
State Plan States, among other things, must be at least as effective in
providing safe and healthful employment and places of employment as
Federal OSHA standards.
OSHA intends to closely scrutinize amendments to previously
approved State hazard communication standards submitted under current
or future State plans to ensure equal or greater effectiveness,
including assurance that any additional requirements do not conflict
with, or adversely affect, the effectiveness of the national
application of OSHA's standard. OSHA must also determine in its review
whether any State plan standard provisions that differ from the Federal
provisions, when applicable to products distributed or used in
interstate commerce, are "required by compelling local conditions and
do not unduly burden interstate commerce." OSH Act section 18(c), 29
U.S.C. 667(c).
This final rule complies with E.O. 13132. In States that do not
have OSHA-approved State Plans, this rule limits State policy options
in the same manner as all OSHA standards.
OSHA also reviewed this final rule in accordance with E.O. 13,175
on Consultation and Coordination with Indian Tribal Governments (65 FR
67,249 (Nov. 9, 2000)), and determined that it does not have "tribal
implications" as defined in that order. The final rule does not have
substantial direct effects on one or more Indian tribes, on the
relationship between the Federal government and Indian tribes, or on
the distribution of power and responsibilities between the Federal
government and Indian tribes.
IX. State Plans
When federal OSHA promulgates a new standard or more stringent
amendment to an existing standard, the 27 States or U.S. territories
with their own OSHA-approved occupational safety and health plans must
revise their standards to reflect the new standard or amendment, or
show OSHA why there is no need for action, e.g., because an existing
state standard covering this area is already "at least as effective"
as the new federal standard or amendment. 29 CFR 1953.5(a). The state
standard must be at least as effective as the final federal rule, must
be applicable to both the private and public (state and local
government employees) sectors, and must be completed within six months
of the publication date of the final federal rule. When OSHA
promulgates a new standard or a standards amendment which does not
impose additional or more stringent requirements than an existing
standard, states are not required to revise their standards, although
OSHA may encourage them to do so.
The 27 States and U.S. territories with OSHA-approved occupational
safety and health plans are: Alaska, Arizona, California, Hawaii,
Indiana, Iowa, Kentucky, Maryland, Michigan, Minnesota, Nevada, New
Mexico, North Carolina, Oregon, Puerto Rico, South Carolina, Tennessee,
Utah, Vermont, Virginia, Washington, and Wyoming. Connecticut,
Illinois, New Jersey, New York and the Virgin Islands have OSHA
approved State Plans that apply to public-sector employees only.
This final rule modifies OSHA's hazard communication standard to
conform to the United Nations' Globally Harmonized System of
Classification and Labelling of Chemicals (GHS). It requires chemical
manufacturers to use revised criteria for classification of chemical
hazards, revised labeling provisions, and a specified format for safety
data sheets. There are also revised requirements for employers to train
their employees regarding labels and safety data sheets for hazardous
chemicals. This GHS rule will also increase worker protection by
improving the quality and consistency of information provided to
employers and employees regarding chemical hazards and protective
measures. Therefore, State Plan States must adopt comparable provisions
within six months of publication of the final rule. Each State's
existing requirements will continue to be in effect until it adopts the
required revisions.
X. Unfunded Mandates
OSHA reviewed this final rule according to the Unfunded Mandates
Reform Act of 1995 ("UMRA"; 2 U.S.C. 1501 et seq.) and Executive
Order ("E.O.") 12875 (58 FR 58093, Oct. 28, 1993).
Under Section 202 of the UMRA, an agency must prepare a written
"qualitative and quantitative assessment" of the anticipated costs
and benefits of any Federal regulation creating a mandate that "may
result in the expenditure by State, local, and tribal governments, in
the aggregate, or by the private sector, of $100,000,000 or more" in
any one year. 2 U.S.C. 1532(a). As discussed in section VI of this
preamble ("Final Economic and Voluntary Regulatory Flexibility
Analysis"), the Agency estimates that this final rule will require
private sector employers annualized expenditures of $201 million per
year. However, OSHA's final rule does not place a mandate on State or
local governments, for purposes of the UMRA, because OSHA cannot
enforce its regulations or standards on State or local governments.
(See 29 U.S.C. 652(5).) Under voluntary agreement with OSHA, some
States enforce compliance with their State standards on public sector
entities, and these agreements specify that these State standards must
be equivalent to OSHA standards. The OSH Act also does not cover tribal
governments in the performance of traditional governmental functions,
though it does when tribal governments engage in commercial activity.
However, this final rule does not require tribal governments to expend,
in the aggregate, $100,000,000 or more in any one year for their
commercial activities. Thus, although OSHA may include compliance costs
for affected governmental entities in its analysis, this rulemaking did
not trigger the requirements of UMRA based on its impact on State,
local, or tribal governments.
Based on the analysis presented in the Final Economic Analysis
(section VI above), OSHA has determined that this final rule will
impose a Federal mandate on the private sector in excess of $100
million in expenditures in any one year, and is thus subject to the
requirements under UMRA for review of private sector costs. The Final
Economic Analysis in section VI, satisfies these requirements, and
provides a written statement containing the qualitative and
quantitative assessment of costs and benefits as is required under
Section 202(a) of UMRA (2 U.S.C. 1532).
XI. Protecting Children From Environmental Health and Safety Risks
E.O.13045 requires that Federal agencies submitting covered
regulatory actions to OMB's Office of Information and Regulatory
Affairs (OIRA) for review pursuant to E.O.12866 must provide OIRA with
(1) an evaluation of the environmental health or safety effects that
the planned regulation may have on children, and (2) an explanation of
why the planned regulation is preferable to other potentially effective
and reasonably feasible alternatives considered by the agency.
E.O.13045 defines "covered regulatory actions" as rules that may (1)
be economically significant under E.O.12866 (i.e., a rulemaking that
has an annual effect on the economy of $100 million or more, or would
adversely effect in a material way the economy, a sector of the
economy, productivity, competition, jobs, the environment, public
health or safety, or State, local, or tribal governments or
communities), and (2) concern an environmental health risk or safety
risk that an agency has reason to believe may disproportionately affect
children. In this context, the term "environmental health risks and
safety risks" means risks to health or safety that are attributable to
products or substances that children are likely to come in contact with
or ingest (e.g., through air, food, water, soil, product use). This
final rule is economically significant under E.O.12866 (See section VI
of this preamble). However, after reviewing this final rule, OSHA has
determined that the standard would not impose environmental health or
safety risks to children as set forth in E.O.13045.
XII. Environmental Impacts
The Agency reviewed this final rule according to the National
Environmental Policy Act (NEPA) of 1969 (42 U.S.C. 4321 et seq.), the
regulations of the Council on Environmental Quality (40 CFR part 1500),
and the Department of Labor's NEPA procedures (29 CFR part 11).
As a result of this review, OSHA has determined that this final
rule will have no impact on air, water, or soil quality; plant or
animal life; or the use of land or aspects of the external environment.
Therefore, OSHA concludes that this final rule will have no significant
environmental impacts.
XIII. Summary and Explanation of the Final Rule
This final rule is based on the public record developed during the
rulemaking. As described in Section II, an advance notice of proposed
rulemaking (ANPR) was published by OSHA on September 12, 2006 (71 FR
53617). The ANPR included a series of questions to solicit information
on a number of specific topics. The responses from more than 100
commenters were used by the Agency to help prepare the required
analyses for the proposed rulemaking, as well as to make determinations
regarding the proposed text. The notice of proposed rulemaking (NPRM)
was published by OSHA on September 29, 2009 (74 FR 50280). Public
comments were received during a 90-day comment period that ended on
December 29, 2009. Subsequently, public hearings were convened in March
2010 in Washington, DC, and Pittsburgh, PA, for the Agency to receive
oral testimony from interested parties. Following completion of the
hearings, participants were given an opportunity to provide additional
information to OSHA during a post-hearing comment period, as well as
submit briefs summarizing their views for the record. The public record
upon which OSHA is basing the final standard includes all of the
comments, testimony, and supporting information submitted by rulemaking
participants, as well as by OSHA.
Support for the rulemaking. Many of those who responded to the ANPR
expressed their support for adoption and implementation of the GHS. The
supporters far outnumbered those who opposed or questioned adoption
(See, e.g., Document ID 0003, 0007, 0011, 0033, 0038, 0047,
0050, 0052, 0062, 0106, 0123, 0130, 0151, 0163, and 0171). The reasons
presented for this support varied, but included the belief that
adoption of the GHS will bring consistency and clarity to hazard
communication (e.g., Document ID 0038, 0046, 0059, and 0081);
will help to ensure that employees have reliable, consistent,
comprehensive, and comprehensible information (e.g., Document ID
0030, 0037, and 0124); will help to enhance human health and
the environment (improved worker safety) (e.g., Document ID
0032, 0064, 0081, and 0128); and will reduce burdens
associated with preparing multiple classifications and labels for the
same product (e.g., Document ID 0030, 0048, 0080, and 0123).
Support for implementation of the GHS by OSHA was expressed by both
users and producers of chemicals who responded to the ANPR (See, e.g.,
Document ID 0038, 0054, 0064, and 0124). While support for
implementation of the GHS was widespread in the ANPR comments, these
supporters also recognized the challenges associated with
implementation. For example, it was noted by a number of commenters
that there will be short-term costs associated with implementation, and
they urged OSHA to take steps to minimize them by providing a
reasonable time period for phase-in, coordinating with other agencies,
and providing extensive outreach (See, e.g., Document ID 0032,
0111, 0155, 0157, and 0162). Others were concerned that the GHS is not
completely harmonized because it allows countries, and agencies within
countries, to select from among a collection of building blocks when
determining the scope of their requirements (e.g., Document ID
0076).
In addition to those who supported implementation, but raised areas
of concern regarding the way in which it is pursued, there were others
who did not support implementation (Document ID 0004, 0065,
0068, and 0108). These commenters argued that it would be too
burdensome (Document ID 0004); delegates power to an
international body, which can only be accomplished through a treaty, if
at all (Document ID 0065); would change the current hazard
communication scheme and thus potentially impair safety (Document ID
0065); and should not be applied to pesticides because they
are already heavily regulated (Document ID 0108).
In the NPRM, OSHA addressed each of these concerns and concluded
that evidence, arguments, and accompanying analyses supported pursuing
the modifications to the HCS. OSHA preliminarily determined that these
modifications would enhance employee protection and facilitate
compliance for all workplaces that produce or use hazardous chemicals.
While OSHA did not include questions regarding the support of
stakeholders for adoption of the GHS, it was clear that a majority of
those responding to the ANPR supported moving forward with the
rulemaking. The arguments presented by those few who actively objected
to adoption were addressed in the NPRM and the analyses for the rule,
and were not found by OSHA to be persuasive. Other issues raised by
supporters as concerns, or suggestions for addressing concerns, were
also addressed in the proposed rule.
OSHA indicated in the NPRM (74 FR 50281, Sept. 30, 2009) that the
Agency had made a "preliminary determination that the proposed
modifications to the HCS would increase the quality and consistency of
information provided to employers and employees." OSHA also indicated
that the "standardized label elements would be more effective in
communicating hazard information;
standardized headings and a consistent order of information would
improve the utility of SDSs; and training would support and enhance the
effectiveness of the new label and SDS requirements." Participants
were asked if they agreed with this assessment, and also to provide
information that reflected on the effectiveness of the proposed
modifications in protecting employees from chemical hazards in the
workplace.
Many participants responded, and the vast majority agreed with
OSHA's preliminary determination that the proposed modifications would
be effective in protecting employees, as well as the conclusions as to
the reasons why it would be effective, and thus supported the
rulemaking (See, e.g., Document ID 0336, 0338, 0339, 0376,
0377, 0382, 0402, 0403, 0404, and 0412). These commenters reflected on
a number of different aspects regarding effectiveness when indicating
their support. For example, in comments provided on behalf of the
American Iron and Steel Institute (AISI) and the American Coke and Coal
Chemicals Institute (ACCCI), it was stated (Document ID 0360):
AISI and ACCCI support OSHA's assessment that modifications to
the Hazard Communication Standard (HCS) would increase the quality
and consistency of information provided to employers and employees.
Two improvements are expected with the changes OSHA has proposed:
a. Standardized criteria to evaluate chemicals and communicate
the hazards via Safety Data Sheets (SDSs) and labeling should assure
consistent communication and lower the likelihood of
miscommunication and misinterpretation.
b. Standardized criteria to evaluate chemicals should facilitate
training. With a single teaching format for SDSs and Labels,
understanding, regardless of an employee's educational background,
should be improved.
Comments of the Society of Chemical Manufacturers and Affiliates
(SOCMA) express support, while highlighting some of the potential
implementation challenges that will have to be addressed (Document ID
0402). SOCMA's comments are illustrative of those provided by
other commenters who qualified their support by expressing issues that
would have to be addressed in order for the benefits to occur (See
also, e.g., Document ID 0369):
SOCMA members are generally very supportive of the
implementation of GHS for workplace hazard communication in the
United States, and for over the past forty years, we have spent
millions of dollars and dedicated an insurmountable amount of time
towards evaluating potential chemical hazards, communicating hazard
information and protecting workers. The proposed rule may have a
disproportionate economic impact on small business chemical
manufacturers, particularly companies that are already struggling in
these unstable economic times. A majority of these burdens can be
mitigated, though, if the most affected entities are given adequate
time to transition and proper compliance assistance is provided.
* * * Once overcome though, the potential benefits of
implementing GHS in the United States are highly anticipated by
SOCMA members, some of which include: The harmonization of
incompatibilities and inconsistencies in labeling and
classification, more uniformity in both substance and format, the
elimination of language and reading barriers through pictograms, and
the facilitation of control banding.
OSHA addresses the suggestions of SOCMA and other commenters on ways to
mitigate implementation issues in discussions of specific provisions
below. The Agency believes it has taken the legitimate concerns of
stakeholders into consideration when determining the final provisions
of this rule.
The National Institute for Occupational Safety and Health (NIOSH)
has extensive experience in another international effort to harmonize
information on chemicals--development of International Chemical Safety
Cards under the auspices of the World Health Organization (WHO) and the
International Program on Chemical Safety (IPCS). In their comments,
they highlighted the advantages of internationally-harmonized
classification criteria (Document ID 0412):
NIOSH recognizes OSHA's Hazard Communication Standard (HCS) as
one of the most important U.S. regulations in occupational safety
and health and concurs with OSHA on the need for a revised HCS. A
significant advantage of the proposed standard is the detailed
criteria for classification will improve accuracy and consistency in
the information provided to employers and employees on chemical
hazards and protective measures. Those criteria will reduce the
likelihood of differing interpretations of the same data. In
addition, the specified hazard categories will convey the severity
of the effect, unlike the hazard classes in the current HCS.
Worker representatives also supported the proposed rulemaking. For
example, comments on behalf of the United Steel, Paper and Forestry,
Rubber, Manufacturing, Energy, Allied Industrial and Service Workers
International Union (AFL/CIO.CLC), stated (Document ID
0403.2):
The committees which designed the GHS agreed on an important
principle early in the work: The final harmonized system should not
weaken the protection afforded by any existing system. That in
itself was a significant accomplishment. However, in the United
States, adopting the GHS will go a step further--the revised, GHS-
compliant Hazard Communication rule will greatly improve the
comprehensibility of labels and safety data sheets, giving workers
and employers--especially employers in small business--information
they can more easily understand and use.
While stakeholder support for the rule was extensive, there were
some stakeholders who did not support pursuing a final rule to modify
the HCS, sought to exempt their constituents from its provisions, or
supported a different approach. For example, the American Composite
Manufacturers Association (ACMA) argued that the protections of the
current rule are sufficient, and implementation of the revisions would
be too burdensome for their industry (Document ID 0407). No
data were provided to support these contentions. The North American
Insulation Manufacturers Association (NAIMA) indicated they support
harmonization, but argued that the proposed standard will not achieve
global harmonization for a number of reasons, including conflicting
domestic requirements (See discussion below), administrative hurdles to
regularly revising the GHS to remain current with the international
version, and obstacles to keeping the GHS current (Document ID
0411). And the National Propane Gas Association (NPGA) stated
that only those who operate in an international market will benefit,
and that does not include the propane industry (Document ID
0400). Similarly, the Intercontinental Chemical Corporation
(ICC) argued that companies not involved in international trade should
be allowed to continue complying with the existing standard, and that
those who are involved can comply with the revised provisions (Document
ID 0502).
OSHA does not find any of these arguments persuasive. With regard
to NAIMA, OSHA indicated in the NPRM how it plans to maintain the
necessary consistency with the GHS through the various rulemaking
options available to the Agency, and that it continues to participate
in the international GHS activities in order to be involved in
maintenance of the system itself. We do not agree that these are
insurmountable concerns that argue against adopting the provisions, or
changing the approach in a significant way.
OSHA agrees with ACMA and ICC that the existing standard provides
extensive protections to exposed employees. However, the analyses
presented in support of the proposed and final rules demonstrate that
these protections could be improved by adopting the revised provisions. See
Sections IV and VI of this document. In addition, the argument of NPGA
that benefits only accrue to companies involved in international trade
is not accurate. The improved protections of the rule due to
standardization of classification criteria and harmonization of
communication on labels and safety data sheets apply equally to
employees of companies involved in international trade, and to those in
companies that are not involved in such trade. Workers who use
hazardous chemicals produced for the domestic market are entitled to
the same level of protection as those who use chemicals produced for
the international market, and any standard that treated them
differently might well be inconsistent with the OSH Act. As indicated
in the regulatory analyses for the proposed and final rules, the
revisions are economically and technologically feasible for all
businesses, including small businesses. See Section VI of this
document.
Other general issues. Commenters also raised a number of other
issues related to the rulemaking that were not directed to specific
paragraphs of the HCS in responses to both the ANPR and the NPRM. Some
respondents indicated that OSHA should limit changes to the HCS to
those required to align with the GHS, thus keeping the framework of the
existing HCS (See, e.g., Document ID 0047, 0080, 0104, 0123,
0145, 0163, 0167, and 0170). For example, ORC Worldwide (Document ID
0123) stated in ANPR comments:
* * * OSHA can help minimize the cost to businesses by only
modifying those sections of the OSHA Hazard Communication Standard
(HCS) that must be changed to be consistent with GHS. Therefore, we
strongly support OSHA's stated intent to maintain the current scope,
application, and interpretations of the HCS, and only modify those
sections of the standard necessary for consistency with the GHS. Not
only will this help minimize the implementation burden on industry,
it should also serve to minimize confusion among employers and
employees during the implementation period.
OSHA agreed with these commenters, and made every effort in the
NPRM to maintain the framework of the current HCS in the proposed
revisions. The modifications proposed were believed by OSHA to be those
that were required to align the current HCS with the GHS, but did not
address provisions of the current standard that are not addressed in
the GHS. Thus, for example, the scope and application paragraph
remained largely unchanged, as did the paragraph addressing trade
secret protection. The primary modifications proposed in those
paragraphs were changes in terminology required to ensure consistency.
A number of commenters addressed this issue in their NPRM comments
and testimony as well. For example, Dow Chemical Corporation indicated
(Document ID 0353) that OSHA should follow two overarching
principles as it revises the HCS. The first is to "implement the GHS
with as little US customization as possible," and the second is to
"make only those changes to the HCS that are necessary to facilitate
GHS implementation." (See also, e.g., Document ID 0370.) Both
of these principles were, in fact, followed by OSHA when preparing the
NPRM.
Others commenters recognized this was OSHA's approach, and
supported it. For example, the Defoamer Industry Trade Association
(DITA) noted (Document ID 0367):
DITA applauds the fact that OSHA did not modify the GHS
definitions to a great degree. These definitions reflect a consensus
scientific process for the review of the hazards that chemicals can
present and the toxicology data that predicts the likelihood of
hazard occurring. Accordingly, this should lead to a high level of
harmonization on the classification of chemical substances between
the EU and the US. A high degree of harmonization is desirable so
that manufacturers do not need different SDSs that satisfy the
requirements of different countries.
In the final rule, OSHA has continued to remain as consistent as
possible with the provisions of the GHS. In general, OSHA has not
changed the language of GHS provisions unless necessary to conform with
the regulatory requirements of the HCS. Country-specific deviations are
very limited, and are intended to ensure that the protections of the
current rule are maintained in the final rule. This is consistent with
the principle of the GHS developers that no country should have to
reduce protections in order to harmonize. OSHA does not believe that
any of the deviations in the final rule conflict in a substantive way
with the GHS itself.
Many commenters to the ANPR also suggested that OSHA should
coordinate implementation of the GHS with other Federal agencies. These
included primarily EPA, DOT, and CPSC (See, e.g., Document ID
0048, 0050, 0053, 0076, 0104, 0111, 0123, 0134, 0154, 0162,
and 0170). For example, the Soap and Detergent Association (Document ID
0170) stated:
SDA urges OSHA to coordinate implementation of revisions to the
HCS related to the GHS with the Environmental Protection Agency
(EPA), Department of Transportation (DOT), and the Consumer Product
Safety Commission (CPSC), which all have announced their intentions
to implement GHS provisions in their regulations. Workplace hazard
communication occurs in a stage of the overall life cycle of
chemicals and finished products. Coordination and synchronization of
implementation timing could greatly improve the efficiency of
implementation of the GHS by industry.
Others mentioned coordinating implementation with the Mine Safety and
Health Administration (MSHA) (Document ID 0049, 0101, and
0111).
Similar comments were received in responses to the NPRM (See, e.g.,
Document ID 0344, 0345, 0350, 0351, 0375, 0376, 0403, and
0411). OSHA agrees with these commenters that the U.S. government
agencies should continue to coordinate their activities with regard to
implementation of the GHS. In terms of adopting the GHS provisions, DOT
has substantially aligned the criteria for physical hazards in their
regulations with those of the GHS under the HM-215I rulemaking (71 FR
78596, Dec. 29, 2006). DOT and OSHA arguably have the greatest
interface in covered chemical products, and thus adoption of this final
rule will result in greater consistency between these two agencies. EPA
and CPSC have not initiated rulemaking on the GHS. However, as will be
discussed later in this preamble, EPA and OSHA have worked together to
develop a common position on coverage of pesticides and chemicals
covered by the hazard communication requirements of the Toxic
Substances Control Act's (TSCA's) significant new use rules. Clearly,
there is no way to coordinate timelines for adoption given that OSHA is
at the final rule stage, and neither EPA nor CPSC has started a
rulemaking process. As rulemaking develops in these Agencies,
discussions will continue to take place in the interagency committee on
this subject. With regard to MSHA, Department of Labor rulemaking
activities are coordinated through Department officials, and MSHA has
been apprised of OSHA's activities in order to determine what action
may be appropriate for them to pursue in this area.
A number of commenters to the ANPR also argued that OSHA should
coordinate implementation with major U.S. trading partners (See, e.g.,
Document ID 0042, 0048, 0101, 0116, 0128, 0141, 0155, and
0170). Similarly, several argued that countries should limit
modifications to the GHS that are country-specific, and that the UN
process should be used to control such changes (Document ID
0018, 0042,
0134, 0154, 0163, 0164, and 0171). For example, the American Petroleum
Institute (API) addressed these issues as follows (Document ID
0171):
API strongly recommends that OSHA ensure that timing and
coordination of GHS implementation schedules are in line with those
of other countries, allowing sufficient time for companies to
organize and accomplish necessary work. In order to achieve
international harmonization of hazard communication materials and to
avoid undue burden on companies, OSHA must stay engaged with all
other actors to encourage even and consistent implementation of GHS
by individual countries. Further, API recommends that OSHA work
closely with other government agencies and countries to ensure
alignment to the UN endorsed version of the GHS. As the
implementation of the GHS by countries deviates from the UN version
of GHS, the perceived benefits of harmonization substantially
decrease.
Similar comments were received by participants in the rulemaking after
the NPRM was published. For example, 3M indicated (Document ID
0405):
3M agrees that the potential benefits identified in the proposed
NPRM may be achieved through global implementation of GHS. However,
3M emphasizes that the potential benefits of GHS will depend on
countries around the world aligning as closely as possible with the
GHS. The potential benefits of GHS will be substantially undercut by
country-specific differences or additions that would require
companies to have multiple SDSs and labels for the same product.
Michele Sullivan, an independent consultant, recognized OSHA's approach
as being appropriate, and argued for coordination among trading
partners (Document ID 0382):
Consistent implementation among the major trading partners of
the world is crucial to realize the benefits of the GHS system. For
this reason, the alignment, insofar as possible, of all national and
regional GHS systems with the UN GHS system is critical. In
addition, any national or regional GHS implementation effort must
retain enough flexibility to continually adapt the system as
necessary to harmonize as closely as possible with the UN GHS
system.
OSHA agrees with these commenters that coordination among trading
partners would enhance harmonization and facilitate implementation. The
Agency remains active in the UN process, participating in the Sub-
committee of Experts on the GHS (UNSCEGHS), as well as the United
Nations Institute for Training and Research (UNITAR) Programme Advisory
Group. There is increased emphasis in the Sub-committee on
implementation issues as well as coordination. OSHA is leading a
correspondence group of interested members established by the Sub-
committee that is reviewing practical classification and hazard
communication issues, and proposing modifications to the Sub-committee
to clarify such provisions when identified. There are also other
correspondence groups that are addressing implementation issues as they
are raised to the Sub-committee. OSHA tries to participate in all of
this work in the Sub-committee to help ensure that any U.S.-identified
issues are raised and addressed. Essentially all of the countries
involved in implementation participate in the Sub-committee, so this is
OSHA's best opportunity to coordinate with them.
The Agency has also had bilateral discussions with Canada, as well
as the European Union (EU), on issues related to implementation. These
discussions continue periodically to address mutual issues of concern.
Canada has not yet proposed modifications to their system to
achieve harmonization, but they are planning to in the near future. The
EU has adopted the GHS, and according to a press release on January 4,
2011, from the European Chemicals Agency (ECHA), recently reached a
significant implementation milestone for its Classification, Labelling
and Packaging (CLP) regulation.
(http://echa.europa.eu/news/pr/201101/pr_11_01_clp_deadline_20110104_en.asp):
By 3 January 2011, ECHA received 3,114,835 notifications of
24,529 substances for the Classification and Labelling Inventory. By
this deadline, industry had to notify the classification and
labelling of all chemical substances that are hazardous or subject
to registration under the REACH regulation and placed on the EU
market. * * *
The Classification, Labelling and Packaging regulation relates
to chemical substances and mixtures. It introduces into the EU the
criteria of the United Nations' Globally Harmonised System for
classifying and labelling chemicals. One of the aims of the CLP
regulation is to improve the protection of human health and the
environment by providing criteria for defining when a substance or
mixture displays properties that lead to its classification as
hazardous.
CLP applies to manufacturers, importers, users or distributors
of chemical substances or mixtures. They must classify, label and
package any substance or mixture, regardless of its annual tonnage,
in accordance with the Regulation.
The largest number of the notifications, over 800,000, came from
Germany. Over 500,000 notifications were submitted from the United
Kingdom and nearly 300,000 from France. All together over 6,600
companies notified at least one substance.
Canada and the EU are two of the major trading partners for the
U.S. When OSHA prepared the NPRM, it examined the CLP to coordinate
where possible on approaches to implementation. However, the primary
principles followed by OSHA in developing this proposal were to ensure
that the modifications maintain or enhance the protections of the
current standard, and that the modifications are consistent with the
negotiated provisions of the GHS.
One of the issues of concern regarding implementation by some other
countries has been deviation from the GHS itself. Because GHS is
intended to be globally implemented, efforts by countries to deviate in
a collective manner from the GHS, rather than maintaining consistency,
defeats the purpose and, consequently, lessens the benefits of the GHS.
OSHA will continue to seek opportunities to ensure coordination of
implementation and promote harmonization, both internationally and
bilaterally.
It should also be noted that the GHS is a living document, and the
UN actively reviews it and considers possible changes based on
implementation experiences and other information. These changes are
made on a two-year cycle, referred to as a biennium. The OSHA proposal
and the final rule are based on Revision 3 of the GHS. Revision 3 was
adopted by the UN Committee and Sub-Committee of Experts on the GHS in
December 2008, and is available as a publication and on the UN Web
site. In December 2010, the UN Committee and Sub-committee of Experts
on the GHS adopted additional changes that will be issued as Revision
4.
It is expected that as the UNSCEGHS fulfills its mandate to ensure
that the GHS is up-to-date and relevant, further changes will be
adopted on a biennium basis. If the change(s) is substantive and
controversial, OSHA will have to engage in notice-and-comment
rulemaking in order to amend the HCS. However, for non-substantive or
clarification changes, other rulemaking options are available that can
be utilized to implement the changes more quickly than the full notice-
and-comment rulemaking process.
Two possible means are the Standards' Improvement Process (SIPs) or
a Direct Final Rule (DFR). Each of these options gives the public
notice and opportunity to comment, but has the advantage of a faster
process. Either method could be used to ensure that the HCS remains
current with the GHS.
A number of NPRM participants commented that OSHA should establish
a stakeholder process for input into U.S. government positions on
issues raised at the UN (See, e.g., Document ID 0376, 0377, 0381, 0382, and
0411). OSHA is always open to receiving suggestions from stakeholders
regarding issues raised in the UN process. The working papers are made
publicly available on the UN Web site some 12 weeks before meetings.
Public meetings are scheduled to receive input in some situations, and
stakeholders may also contact the primary OSHA delegate directly to
discuss any of the issues raised. Stakeholders can participate in the
Sub-committee discussions directly as well through organizations that
have recognized status in the Sub-committee. As already noted, changes
to the OSHA HCS as a result of modifications to the GHS in the future
will be subject to a public rulemaking process where all stakeholders
have the opportunity to participate.
In the NPRM (74 FR 50288, Sept. 30, 2009), OSHA noted that one
advantage of adopting a system with harmonized hazard statements is
that it would facilitate the use of "control banding" in the U.S.
Control banding is an approach to selecting control measures for
workplace chemical exposures. Basically, the employer can, with the use
of information readily available in the workplace, use the approach to
determine the appropriate control measures for a chemical. The
harmonized hazard statements are key to assessing the hazards, and the
degree of severity of the hazards. In combination with data about
physical and chemical characteristics, quantities used, and the types
of processing, the employer can access recommended control measures. It
is particularly helpful in situations with common operations (e.g.,
bagging operations), and chemicals with well-known hazards that are not
severe (e.g., it would not generally be applied to a carcinogen--the
control banding guidance would inform the employer that professional
assistance must be acquired to address such a hazard). Control banding
has been used successfully by small and medium-sized businesses that
don't have extensive health and safety expertise in these types of
situations.
There is considerable international interest in this approach, and
there have been a number of research studies conducted to refine the
approach and determine its applicability. Both OSHA and NIOSH have
taken part in activities to further investigate its utility in the U.S.
NIOSH has extensive information available on its Web site at
http://www.cdc.gov/niosh/topics/ctrlbanding/. As they indicated in their
comments (Document ID 0412):
The use of control banding to provide guidance for chemical
safety and health approaches in U.S. workplaces cannot be
accomplished until harmonized hazard statements are readily
available. Adoption of the GHS and its phrases would open up the
possibility that control banding guidance can be used in the United
States to help small- and medium-sized employers select and
implement appropriate control measures [NIOSH 2009].
The American Society of Safety Engineers (Document ID 0336) is
also a strong proponent of control banding. However, their position was
that OSHA should have included control banding in the NPRM, and thus in
the HCS:
* * * ASSE believes OSHA should update the HCS to incorporate
elements of control banding. Assuming that most elements of GHS will
be adopted and a national database for safety data sheets (SDSs) and
chemical classifications will be established to support the
transition to GHS from current practice, building a system that
would allow guided review of materials and processes such as control
banding would be a relatively small additional step. We encourage
OSHA to take that step now and avoid revisiting this issue when it
becomes unavoidable as control banding grows in use internationally
as well among leading employers in this nation.
While OSHA agrees with ASSE that control banding may be a very useful
approach to controlling workplace exposures to chemicals, it does not
agree that this rulemaking is the appropriate place to address this
issue. As noted by both OSHA and NIOSH, adoption of the GHS will
facilitate the use of control banding in the U.S. by making harmonized
hazard statements readily available on labels and SDSs. This will allow
the adaptation of the approach in a way that could not be readily
accomplished with the current performance orientation of the HCS.
However, it is generally viewed as a guidance approach where it is
currently used, and not a mandatory requirement. Furthermore, control
banding continues to be refined in terms of application, and is not
harmonized. Adoption of it in the HCS would also not be consistent with
the principles OSHA has followed in devising the NPRM, i.e., to limit
changes to those required to align with the GHS, and to be as
consistent as possible with the GHS provisions. Therefore, while OSHA
believes the utility of control banding should continue to be assessed
and evaluated in the U.S., it is premature to consider the approach as
a mandatory requirement and part of the revised HCS.
Outreach/compliance assistance. The ANPR included a series of
questions to solicit input from the public on what outreach or
compliance assistance materials would be appropriate and useful. OSHA
received many comments in response to these questions, with a number of
creative and interesting suggestions for outreach products. The Agency
will use this input to develop an outreach plan and prepare materials
for distribution when the rulemaking is completed. In addition, and as
suggested by a number of ANPR commenters (See, e.g., Document ID
0018, 0025, 0047, 0065, 0081, 0104, and 0154), OSHA will
continue working with interested parties to examine projects that could
be completed by them, or in coordination with them, that could be
targeted to specific industries or interest groups.
OSHA solicited additional ideas for outreach or compliance
assistance in the NPRM, and many commenters provided such information
(See, e.g., Document ID 0332, 0344, 0356, 0370, 0382, 0405,
0408, 0410, and 0414). There was a wide range of suggestions, including
training programs, workshops, web resources, and enforcement tools
addressing different aspects of the modified standard. OSHA has already
developed some compliance assistance products--or updated products
available for the existing standard--and will be developing and
distributing these and others as resources are made available. There
are also tools being developed internationally that will be available
for employers undertaking compliance, such as training materials in
preparation by the United Nations Institute for Training and Research
(UNITAR). OSHA has provided support to this activity, and expects these
materials will be made available on its Web site when completed. OSHA
encourages trade associations, professional societies, and others to
develop materials that are specific to certain interest groups or
industries, thus providing a more focused compliance assistance
approach than can be done by OSHA at the national level.
The final standard. The following is a description of the
provisions of the final standard, along with a discussion of what was
proposed and the information provided by rulemaking participants. As
noted above (and supported by rulemaking participants), OSHA's approach
has been to confine changes to the standard to those required to align
it with the GHS. Therefore, provisions that do not require changes for
that purpose have been left the way they are in the current HCS. While
participants supported this approach in general, suggestions were made
that involved changes to the current text in areas unaffected by the
GHS. Since OSHA did not propose to
open these parts of the rule in the proposed rulemaking, and the
analyses did not involve such changes, the Agency will not be adopting
them in the final rule.
Similarly, as OSHA indicated in the NPRM, the Agency's approach was
also to be as consistent as possible with the GHS itself. Editing was
limited to what was required to make the provisions mandatory in the
context of OSHA rulemaking, and using the regulatory language required
for that purpose. Additionally, as described in the NPRM, OSHA did not
propose adopting language from the GHS that was strictly provided for
guidance purposes (such as the decision logics in the chapters in the
GHS that describe the physical and health hazard criteria). There is no
question that other changes could be made to the language to make it
more readable, or to state it in American English. However, introducing
different terminology also introduces the possibility that readers will
believe that OSHA means something different than the GHS because we
have used different language. Since this is not the intent, the Agency
has avoided doing this.
Nevertheless, many such editorial changes were suggested. While
OSHA has reviewed all of them, and adopted a few that seemed
appropriate or necessary, in general the Agency did not engage in
extensive editing of agreed text for fear of changing the meaning, or
giving the impression that the meaning has changed. In particular, Dow
Chemical submitted extensive suggested edits in both its initial
comments on the NPRM and in post-hearing comments (Document ID
0353 and 0526). Most of these issues were not raised by any
other participants. Given the large number of such editorial
suggestions from Dow, OSHA does not discuss each one in this preamble,
but simply notes where changes have been made to the text. OSHA,
however, gave each of Dow's suggestions full consideration.
(a) Purpose. The HCS includes a paragraph that states the purpose
of the rule. This stated purpose is two-fold. First, the paragraph
indicates that the standard addresses assessment of the hazards of
workplace chemicals, and the transmittal of that information to
employers and employees. It also describes the contents of a
comprehensive hazard communication program as being container labeling
and other forms of warning, material safety data sheets, and employee
training.
The second part of the paragraph addresses the preemption of State
or local laws by this Federal standard. It indicates that OSHA is
addressing comprehensively the issues described, and thus the standard
preempts States, and political subdivisions of States, from addressing
these issues except under the authority of a Federally-approved State
plan under Section 18 of the OSH Act. While Section 18 applies to every
occupational safety and health standard that OSHA promulgates, the HCS
raises particular issues because of the nature of the provisions. It
requires chemical manufacturers and importers to evaluate the hazards
of the chemicals they produce or import, and to prepare labels and
safety data sheets based on those evaluations to transmit hazard
information and appropriate precautionary advice to users downstream.
This is a unique but highly appropriate approach for an OSHA standard,
as it recognizes that chemical manufacturers and importers are in the
best position to assess the hazards of their products and develop
appropriate information for labels and SDSs.
There is a national, indeed international, marketplace for
industrial chemicals, and thus chemical manufacturers and importers
affect commerce within the meaning of the OSH Act and therefore fall
under OSHA's jurisdiction. If a State, or a political subdivision of a
State, were to establish different requirements for labels and safety
data sheets, such requirements would have an impact on chemical
manufacturers and importers that are not located in that State. This is
a burden that the HCS eliminates by establishing national requirements.
The proposed revisions to the HCS had essentially the same
purposes, and thus the NPRM included only minor modifications to this
paragraph. OSHA proposed to modify paragraph (a)(1) to change the
language regarding the assessment of hazards to indicate that the
hazards will be "classified" rather than simply assessed or
evaluated. This is consistent with the approach in the GHS. In
addition, OSHA proposed to modify this paragraph to clearly indicate
that the standard is intended to be consistent with the GHS, Revision
3. That change is a reflection of the purpose of this rulemaking to
harmonize the existing requirements with the provisions of the GHS,
which is the international instrument that includes globally harmonized
provisions on hazard communication. In addition, in this paragraph and
succeeding paragraphs of the revised rule, the term "material safety
data sheet" was modified to "safety data sheet" to reflect the
terminology of the GHS.
The only modifications proposed to paragraph (a)(2) also addressed
terminology, using "classifying" instead of "evaluating", and
"safety data sheet" instead of "material safety data sheet".
There were a few comments that were related to the Purpose
paragraph provisions. One comment suggested that the standard should be
limited to a purpose of international communication so as not to
trigger hazard assessments under other OSHA standards that address
respiratory protection, personal protective equipment, or process
safety management (Document ID 0049). There were several other
comments that indicated that new assessments would have to be done for
these standards (Document ID 0111, 0134, 0164, and 0178).
Arguments were made that this would lead to extensive additional costs
for new engineering controls, respirators, or other personal protective
equipment.
As discussed above, there is no identified link to these other
standards in the stated purpose of the HCS either currently or with the
proposed modifications in the NPRM. While the current HCS and this
final standard require the provision of information on recommended
control measures, including respiratory protection, personal protective
equipment, and engineering controls, there is no requirement for
employers to implement the recommended controls. An employer should use
all available information when designing an appropriate protective
program, but a recommendation on a safety data sheet by itself would
not trigger the need to implement new controls.
Furthermore, these comments seem to imply that there will be major
changes in the classification of the hazards of chemicals as a result
of implementation of the GHS provisions. Both the HCS and the GHS are
based on identifying and communicating the inherent hazards of
chemicals. Thus the biggest change for most chemicals under the final
rule will be in categorizing the chemical's hazards. Under the current
standard, for example, a chemical either is, or is not, a carcinogen.
Under the revised HCS, if a chemical is a carcinogen, it would be
categorized as a Category 1 or a Category 2 carcinogen. Such a change
would provide additional information for the downstream user, but would
not generally result in a need to change engineering controls or
respiratory protection.
It is possible that a chemical may be classified under the final
rule as having a hazard it did not have before, but OSHA believes that
this is not likely to happen frequently given the broad coverage of the
current rule. Furthermore, the physical and chemical characteristics of
the chemical--which
affect the types of protection required--would not be changed as a
result of this proposal. OSHA believes that these revisions would
result in few, if any, changes in protective measures required under
other OSHA standards.
Several commenters to the ANPR noted what they believed to be the
continued need to address the preemption of State standards (See, e.g.,
Document ID 0036, 0048, 0056, 0080, 0123, 0135, and 0178). In
addition, commenters also noted that the impact of GHS adoption on
State and local laws should be considered in the process (for example,
California Proposition 65), and that differences between such laws and
the revised HCS should be discouraged (Document ID 0015, 0038,
0042, and 0072).
It was also indicated that changes in State laws should be
coordinated with the Federal changes to facilitate implementation
(Document ID 0146). See Section VIII and IX of this preamble
for a comprehensive discussion regarding Federalism and State plans.
There were a number of comments received in response to the NPRM
that addressed the Purpose paragraph provisions. For example, the
Styrene Information and Research Center (Document ID 0361)
indicated that OSHA should revise paragraph (a)(1) to say that it is
intended to be consistent with the GHS "with some exceptions," since
there are some deviations from the GHS. OSHA does not agree with this
suggestion. The language proposed, and in the final rule, is accurate--
it is consistent with the provisions of the GHS. The GHS is not a model
regulation, and it is not intended that countries will adopt the actual
text of the GHS. Furthermore, there is allowance for flexibility and
differences where necessary to accommodate a country's specific needs.
There was nothing in the NPRM that was inconsistent with the GHS, and
neither is the final rule inconsistent.
Dow Chemical (Document ID 0353), argued that paragraph
(a)(2) should state that OSHA is preempting personal injury suits
alleging that labels provided inadequate warnings. The Industrial
Minerals Association-North America (Document ID 0394)
indicates that the new rule must make clear that it preempts state law
tort claims alleging failure to warn. OSHA declines these invitations.
As recently explained in the Solicitor of Labor's letter to Stephen
Wodka, dated October 18, 2011, in general the HCS does not preempt
state tort failure to warn lawsuits, and OSHA does not intend to change
that position in the final rule. Indeed, the OSH Act's "savings
clause" explicitly preserves, rather than preempts, State tort law.
OSH Act Sec. 4(b)(4), 29 U.S.C. 653(b)(4); Lindsey v. Caterpiller,
Inc., 480 F.3d 202, 209 (3d Cir. 2007); Pedraza v. Shell Oil Co., 942
F.2d 48, 53-54 (1st Cir. 1991). While a limited preemption might be
possible to the extent a state tort rule directly conflicted with the
requirements of the standard, no commenter has provided any evidence of
such a conflict. For example, the record contains no evidence that a
manufacturer might be held liable under a State's tort law rules for
complying with the GHS. However, to eliminate any confusion about the
standard's preemptive effect, and to be consistent with the President's
May 20, 2009 Memorandum on Preemption, OSHA has made two small changes
to (a)(2) in the final rule, changing the words "legal requirements"
to "legislative or regulatory enactments" in the provision's first
sentence and eliminating the words "through any court or agency" in
the last sentence.
Similarly, DuPont (Document ID 0329) says OSHA should
convince States to voluntarily rescind their "right-to-know" laws, or
make them consistent with the HCS final rule. And the National Paint
and Coatings Association (NPCA) (Document ID 0328) believes
that OSHA should not allow States to promulgate a standard that is
different from the Federal rule. As indicated in paragraph (a)(2),
States with OSHA-approved State Plans will have to adopt standards that
are at least as effective as this final rule. (See, generally, 62 FR
31159, Jun. 6, 1997.) Those standards will be reviewed by Federal OSHA.
Other States are preempted from covering these areas with regard to
workplace protections. OSHA has no authority with regard to provisions
that are intended to address non-workplace situations.
Therefore, OSHA has concluded that the changes it proposed to
Paragraph (a) are appropriate, and those changes are being incorporated
into the final rule. No other revisions are being made.
(b) Scope and application. The HCS is a generic standard that has
very broad provisions in terms of chemicals addressed and workplaces
covered. It also interfaces with a number of requirements of other
Federal agencies that address labeling of chemical hazards. Paragraph
(b) thus includes all of the practical modifications the Agency has
developed to ensure that employers and employees understand how the
standard is to be applied, and to accommodate various circumstances
that potentially affect the application of the standard.
The provisions of paragraph (b)(2) in the HCS address the overall
scope of the standard as applying to "any chemical which is known to
be present in the workplace in such a manner that employees may be
exposed under normal conditions of use or in a foreseeable emergency."
This provision addresses many questions that are raised about the
application of the standard.
In general, OSHA does not expect significant changes in the
chemicals covered by the HCS under the final rule as compared to the
current standard. The scope of hazards covered by the GHS is very
similar to what is covered by the current HCS. Additional chemicals may
be considered to be acutely toxic due to the proposed adoption of
Category 4 in acute toxicity, which would expand the criteria for
inclusion from the current definition (See the discussion under
"Hazard classification"). However, these chemicals are already
covered under the voluntary national industry consensus standard on
precautionary labeling of industrial chemicals that many manufacturers
follow in their labeling programs (ANSI Z400.1/Z129.1-2010, Hazardous
Chemicals--Hazard Evaluation and Safety Data Sheet and Precautionary
Labeling Preparation), as well as being covered in the requirements
that apply to chemicals shipped to the EU. Thus many manufacturers are
already classifying and labeling these chemicals as acute toxins. The
final rule is also likely to cover fewer mixtures as acute toxins than
the current rule given the hazard classification approach in the GHS
that uses a calculation based on proportionality to determine whether a
mixture is covered, rather than the strict percentage cut-off of 1% in
the current HCS. Other definitions of health hazards would maintain the
current broad HCS scope.
In addition to the overall scope statement, the final rule, like
the current rule, provides for limited coverage in workplace situations
that have special circumstances, including laboratories (paragraph
(b)(3)) and work operations where employees only handle chemicals in
closed containers (paragraph (b)(4)).
OSHA also addresses the interface with other Federal agency
requirements by either exempting the products covered from additional
OSHA labeling (such as pesticides required to be labeled by the EPA)
(paragraph (b)(5)), or completely exempting the product (such as
hazardous waste regulated by EPA) (paragraph (b)(6)). These
accommodations help to ensure that
Federal requirements do not conflict or duplicate each other.
Under the GHS, such provisions are left under the purview of the
"competent authority." In developing the GHS, it was recognized that
countries' regulatory authorities would need to have the discretion to
address such national circumstances in ways that are suited to the
regulatory perspective of the country. Thus authorities such as OSHA
are free to make determinations about scope and application issues
while still being harmonized with the primary provisions of the GHS.
OSHA reviewed the current provisions of paragraph (b), and
determined that no significant changes were required to be consistent
with the GHS. Several minor changes to revise terminology were retained
from the proposal (i.e., adopting the terms "classifying" and
"safety data sheets"), but OSHA is not modifying any of the remaining
provisions of paragraph (b). The Agency is also deleting Appendix E of
the current HCS, which was guidance for application of the standard,
and thus is deleting the reference to it in paragraph (b)(1). The Sheet
Metal and Air Conditioning Contractors National Association (SMACNA)
(Document ID 0415) suggested in response to the NPRM that OSHA
update Appendix E and continue to include it in the standard. OSHA will
update Appendix E, and make it available as a compliance assistance
product. It was always available as a pamphlet in any event, and has
been very useful in helping small employers who are users of chemicals
comply with the standard. And as noted above, new outreach and
compliance assistance materials are being prepared as well.
Several commenters to the ANPR indicated that OSHA should adopt
exemptions included by the European Union in its requirements.
Specifically, these exemptions address non-isolated intermediates,
chemicals involved in research and development, and waste (Document ID
0049, 0134, and 0164). In response to the NPRM, the Society of
the Plastics Industry (SPI) (Document ID 0392) continued to
argue that the EU exemptions should be adopted. All of these situations
are already addressed in paragraph (b), and OSHA does not agree that it
is appropriate or necessary to change them.
In terms of non-isolated intermediates, the overall scope provision
in paragraph (b)(2) adequately addresses this situation. This was
described in the preamble to the 1983 final rule (48 FR 53335, Nov. 25,
1983):
That is, the term "known" means the employer need not analyze
intermediate process streams, for example, to determine the presence
or quantity of trace contaminants. However, where the employer knows
of such contaminants, and they are hazardous, then they fall under
the provisions of the standard.
With regard to chemicals involved in research and development,
paragraph (b)(3) limits coverage in laboratories, and partially
addresses this situation. Where there is no knowledge of the hazards of
such chemicals, the HCS does not apply at all since there is no
requirement to generate new hazard information. Where information is
available, it must be provided to exposed employees, consistent with
paragraph (b)(3) when it is in a laboratory situation. Therefore, it
appears to OSHA that this situation is also adequately addressed under
the current provisions. Hazardous waste as regulated by EPA is already
exempted under paragraphs (b)(6)(i) and (ii).
The North American Metals Council (NAMC) (Document ID
0377) argued in response to the NPRM that OSHA should use the
EU approach to exempt metals in their massive form, alloys, and other
preparations that do not present a hazard. Provisions already exist in
the current HCS, and are included in the final rule, that address these
issues (See, e.g., definition of article (paragraph (c)), special
labeling provisions for solid metals (paragraph (f)(4))).
There were commenters who suggested that OSHA maintain current
exemptions or limitations in the revised GHS, including the consumer
product exemption (Document ID 0064), guidance on byproducts
(Document ID 0064), the relative roles of manufacturers and
employers (Document ID 0064), and the article exemption
(Document ID 0160). OSHA agrees and all of these
accommodations remain the same in the revised rule. The Agency is not
changing those parts of the HCS that are not affected by the GHS.
There were also a few comments regarding the scope of the revised
rule in terms of provisions of the GHS that affect the environment or
transportation (See, e.g., Document ID 0072 and 0179). OSHA
does not have the authority to require information in these areas since
they are not directed to the protection of employees under its
jurisdiction. However, OSHA does not prohibit this type of information
on labels or safety data sheets, and is aware that it is often included
on labels and safety data sheets currently developed to comply with the
HCS. OSHA expects that chemical manufacturers will continue to
voluntarily include such data on their labels and safety data sheets to
meet the requests of their domestic and international customers.
Commenters to the NPRM continued to state that OSHA should allow
environmental information although it is not required (Document ID
0344 and 0381). OSHA maintains the position proposed that
manufacturers are free to provide additional information on labels and
safety data sheets to address environmental concerns, as well as
aspects of concern in other areas such as transportation. (74 FR 50387,
Sept. 30, 2009)
Few comments were received on this paragraph in the NPRM. Dow
Chemical (Document ID 0353) suggested that paragraph
(b)(5)(iv) be updated to reflect the changed name of the Bureau of
Alcohol, Tobacco, Firearms, and Explosives (the word "Explosives" has
been added to their name). This has been done. In addition, two
typographical errors in (b)(6)(ii) have been corrected.
The North American Insulation Manufacturers Association (NAIMA)
(Document ID 0411) states that OSHA has given unwarranted
exemption by ceding authority for products regulated by other agencies.
In particular, NAIMA is concerned about coverage by CPSC, and indicates
that CPSC addresses the fire hazards of cellulose insulation, but not
the health hazards, in its label requirements. NAIMA argues that OSHA
should not allow consumer product labels to supersede OSHA
requirements.
OSHA considered this issue at length in previous amendments to the
HCS (53 FR 29822, 29834-38, Aug. 8, 1988; 59 FR 6126, 6150-52, Feb. 9,
1994; See also 52 FR 31852, 31862-63, Aug. 24, 1987). After noting that
CPSC labels often do not contain all hazard information relevant to
worker exposures, OSHA concluded that:
OSHA nevertheless decided to permit the CPSC labels to suffice
so as not to disrupt the extensive labeling conducted in accordance
with those rules. OSHA believed that this could be justified on the
basis that some information is provided on the labels that would be
useful to workers, and that the requirement for MSDSs would provide
what information is necessary to supplement the labels. 48 FR 53289.
This additional information is critical to ensuring that training
can be properly conducted, and that adequate protective measures are
used in the workplace.
(53 FR 29834, Aug. 8, 1988; See also 59 FR 6151, Feb. 9, 1994.) Thus,
under the current HCS, SDSs and employee training are required where
employee exposure to a consumer product exceeds
the range that "could reasonably be experienced by consumers when used
for the purpose intended." 29 CFR 1910.1200(b)(6)(ix). OSHA sees no
need to revisit this issue now, and in any event it is outside the
scope of this rulemaking, which is aimed at the changes necessary to
bring the HCS in conformity with the GHS.
A few comments were received in response to the ANPR regarding EPA
labels for pesticides, noting that signal words in these labels would
change if GHS is adopted (Document ID 0178), and noting that
the requirements for these labels are dictated by the Federal
Insecticide, Fungicide, and Rodenticide Act (FIFRA), which also
controls the SDS content (Document ID 0108). A commenter also
argued that FIFRA pesticide labels are more useful because they are
risk-based rather than hazard-based (Document ID 0108). These
concerns were not related to the proposal which maintained the
exemption for additional labels on containers that are labeled in
accordance with EPA requirements. If EPA decides to adopt the GHS, then
labels for pesticides would be consistent with OSHA labels on other
types of products. With regard to SDSs, these are required by the HCS,
not FIFRA, and therefore such SDSs must be consistent with GHS
provisions as adopted in this final standard.
A number of additional comments, and oral testimony, were received
in response to the NPRM from representatives of the pesticide industry
regarding potential conflicts between OSHA and EPA requirements (See,
e.g., Document ID 0352, 0385, 0387, and 0468). OSHA does not
require additional labels on pesticides that require labels under EPA
requirements. However, OSHA does have SDS requirements that must still
be applied, and have been applied since the HCS first went into effect.
Pesticide industry representatives believe that the SDS requirements as
aligned with the GHS would conflict with the EPA-approved labels
because they may have different information on them for OSHA than what
is included in the pesticide label. For example, EPA has three signal
words for pesticides (danger, caution, and warning), while OSHA will
have the two specified by the GHS (danger and warning). There are also
other differences. For example, chronic health effects are rarely
addressed on pesticide labels as the risk mitigation measures are
intended to minimize the possibility of their occurrence. However, OSHA
would require such effects to be included when appropriate. The
commenters also argue that EPA "labels" include any information
related to the product, and thus SDSs would be preempted by the EPA
labeling requirements. Therefore, they argue that pesticides should be
exempted from the HCS. For example, the American Chemistry Council's
Biocides Panel says the reasons for exempting pesticides are as follows
(Document ID 0385):
The principal reasons for this are: (i) Requiring GHS compliant
SDS's but not pesticide labels will result in significant confusion
in workplaces in which pesticides are used; (ii) imposing GHS-based
SDS's would be inconsistent with EPA's interpretation of FIFRA,
which includes all material that may be shipped with a pesticide,
including SDS's, as part of its definition of labeling; and (iii)
applying GHS to pesticide SDS's will not provide any additional
substantive information, as EPA's evaluation of pesticides before
approving them for sale includes all aspects of potential
occupational exposures.
OSHA considered exempting pesticides from the final rule. However,
exempting pesticides would reduce protections for those workers under
OSHA's jurisdiction. For example, OSHA's jurisdiction extends to
employees in pesticide manufacture and formulation. While EPA approves
the label on the final product shipped out of these facilities, and
that label includes information needed when the products are used by
applicators, EPA does not have hazard communication requirements for
the protection of workers in production facilities. Such protection is
covered by OSHA, and OSHA requires labels on containers that are not
subject to EPA labeling, as well as SDSs and training. The workplace
exposures of these workers are of great concern. The chemicals are
generally designed to be biologically active, and the exposures can be
quite different than they would be for applicators, for example, who
may use them only on an intermittent basis.
In testimony during the public hearing, representatives from the
ACC Biocides Panel and CropLife America, Inc., agreed that EPA does not
cover workers in pesticide manufacturing or formulating facilities (See
Document ID 0495 Tr. 248-250). An exemption from the HCS would
provide reduced protection for these workers.
As a result of receiving these comments, and the concerns about
removing current protections from the final rule, OSHA considered
several options. OSHA considered allowing the SDS preparer to use the
EPA classification in section 2 of the SDS to ensure consistency with
the FIFRA label. However, in doing this the SDS would then be
inconsistent with other chemicals in the production of pesticides. In
the pesticide manufacturing workplace the pesticide chemical "active"
ingredients would bear a FIFRA label but would have an OSHA SDS,
however other chemicals in the workplace such the "inactive"
ingredients or cleaning products might still be considered hazardous
under the HCS would contain an OSHA label and an OSHA SDS. An added
complication is that an identical chemical (for example, chlorine)
could potentially be in a pesticide manufacturing workplace where in
one situation it could contain a FIFRA label and another it could bear
an OSHA style label depending on its end use (e.g., a disinfectant).
Adding a different SDS would create additional confusion not only for
the worker handling the chemicals but also the personnel in charge of
chemical management as well. Therefore, OSHA and EPA met to discuss
what would be an appropriate resolution. First, with regard to the
argument that SDSs are part of labels, and therefore preempted, EPA has
long had an interpretation that they will not apply their review
requirements to SDSs (US EPA Pesticide Registration Notices 92-04).
Based on our discussions, OSHA does not anticipate that this policy
will change. Secondly, EPA has indicated that they are committed to
working with OSHA to develop an approach that will provide both
appropriate protection for employees, as well as the environment,
through workable guidance for the pesticide industry. OSHA anticipates
that EPA will provide guidance to their regulated community (such as
through a Pesticide Registration Notice) on how to develop an OSHA GHS-
compliant SDS that will not be in conflict with the pesticide label.
Therefore, pesticides will continue to be covered in the same manner as
has been done under the HCS since its inception, and the exemption
requested by pesticides industry rulemaking participants for such
products is not granted.
Although the OSHA ICR (OMB Control No. 1218-0072) that is currently
pending review and approval by OMB addresses the information collection
activities associated with preparing the entire SDS as prescribed by
the OSHA final rule, the approach OSHA anticipates will be provided in
the EPA guidance for pesticide registrants was not considered by OSHA
at the proposed rule stage. While OSHA preliminarily believes it has
taken sufficient time in its paperwork estimate to cover compliance
with the anticipated EPA guidance, the public has not had the
opportunity to comment
on the paperwork burdens created by that guidance. As such, EPA and
OSHA are collaborating on a subsequent revision to OSHA's ICR to ensure
that it addresses the activities in the EPA guidance. EPA intends to
solicit public comment on an ICR revision that addresses the
information collection activities and related burden estimates
associated with the EPA guidance as part of its release of that
guidance. After public comments are considered by both agencies, OSHA
intends to ask OMB to revise its ICR approval, identified under OMB
Control No. 1218-0072, to capture the information collection activities
and burden adjustments, if any, related to EPA's guidance.
(c) Definitions. This paragraph in the HCS includes the terminology
used with the corresponding definitions. Comprehension of the
appropriate definitions is critical to understanding the provisions of
the standard. In some cases, terms are defined somewhat differently
than when used in other contexts, so familiarity with the standard's
definitions is important.
In the proposed revisions, OSHA retained as many definitions as
possible from the current HCS. Changes were proposed only when there
was a new term used that needed to be defined, or there is a different
definition in the GHS, and consistency with the international
definition was needed for harmonization purposes. As with the preceding
paragraphs, minor modifications were proposed to ensure terminology is
appropriate--primarily the use of terms related to classification and
safety data sheets. These modifications were retained in the final
rule. There were relatively few comments submitted on the proposed
revisions to the definitions, other than those referring to the new
definition OSHA proposed to address "unclassified hazards" and the
definition for "pictogram" that references a red border frame.
One important difference between the HCS and GHS in terminology
involves the use of the term "chemical." The HCS has used this term
since it was originally promulgated, and defines it to include
elements, chemical compounds, and mixtures of elements and/or
compounds. It has been a convenient way to describe the coverage of the
rule. The GHS, like some other international standards, uses the terms
"substance" and "mixture". OSHA has decided to retain a definition
of "chemical" in the revised standard, which minimizes the number of
terminology changes that have to be made to the regulatory text, as
well as providing a shorthand way to define the scope to include both
individual substances and mixtures of substances. This term is used in
the body of the regulatory text of the final standard, similar to its
use in the current HCS. However, the modifications also include
definitions for "substance" as well as "mixture" to align with the
GHS, and both of these terms are used as well. In particular, in the
appendixes that are adopting GHS language, the separate terms
"substance" and "mixture" are used consistent with the GHS.
"Substance" means "chemical elements and their compounds in the
natural state or obtained by any production process, including any
additive necessary to preserve the stability of the product and any
impurities deriving from the process used, but excluding any solvent
which may be separated without affecting the stability of the substance
or changing its composition." Dow Chemical (Document ID 0353)
objected to this definition, and suggested that it should be "chemical
elements and compounds in their natural state or obtained by any
production process." OSHA has concluded that it is appropriate to
maintain the GHS language for this definition to help to ensure
consistent application, and thus the revised rule includes the
definition of substance that was proposed.
A "mixture" is defined as a "combination or a solution composed
of two or more substances in which they do not react." This is
consistent with the GHS definition--and while slightly different than
the definition in the current HCS, means the same thing. Dow Chemical
(Document ID 0353) suggested that OSHA maintain part of its
current definition in order to avoid inadvertently changing the scope
of coverage by adding "if the combination is not, in whole or in part,
the result of a chemical reaction." OSHA does not believe that the
scope is changed by the GHS definition, and has retained the GHS-
consistent language that was proposed.
OSHA also proposed to maintain the term "hazardous chemical" in
this revised standard as used in the current standard (a chemical which
is a physical or health hazard), except to add the term "classified"
to indicate how it is determined that it is a physical or health
hazard. OSHA also proposed to include unclassified hazards in this
definition, but, as will be described below, has chosen a different
approach in the final rule. Instead, the definition of "hazardous
chemical" in this final rule is "any chemical which is classified as
a physical hazard or a health hazard, a simple asphyxiant, combustible
dust, pyrophoric gas, or hazard not otherwise classified." The term is
used throughout the standard to indicate that the classification
process is completed, and the chemical manufacturer has determined that
the chemical poses a hazard. Most of the substantive requirements of
the rule apply to hazardous chemicals.
Dow Chemical (Document ID 0353) indicated that OSHA should
drop the use of the word "substance" altogether, and instead use the
word "chemical." As noted in the definition of "chemical," however,
it is to be used when a reference is to both substances and mixtures.
Where a provision or statement refers only to a substance, or only to a
mixture, those terms are used in lieu of "chemical" or "hazardous
chemical." These individual designations are used most commonly in the
appendixes, particularly in the classification criteria. OSHA has
maintained consistency in the criteria with the GHS insofar as is
possible with regard to this terminology.
Another proposed modification to the definitions paragraph was to
move the specific physical hazard definitions to an appendix. In the
current HCS, health hazard definitions are addressed specifically in
Appendix A, but the physical hazard definitions were included in
paragraph (c). In the final standard, health hazard definitions
continue to be addressed in Appendix A, but a new Appendix B addresses
physical hazards. Both of these appendixes are discussed below under
the summary and explanation of paragraph (d) "Hazard Classification."
As noted in Section III above, the physical hazard definitions in
the GHS are drawn from the United Nations' Recommendations on the
Transport of Dangerous Goods. Since DOT has already adopted this
international approach, the GHS definitions are substantially
harmonized with the U.S. requirements for labeling of dangerous goods
in transport. All chemicals that are shipped in the U.S. have already
been classified according to DOT's physical hazard definitions. This
will reduce the burdens associated with classifying physical hazards
under the revised HCS. The primary differences involve exceptions that
make the definitions more applicable to workplace situations (for
example, coverage of flammable liquids that are currently defined as
combustible under the HCS). Modifying the HCS to align with the GHS
thus serves the purpose of harmonizing many of these definitions
domestically, and results in shippers only having to classify their
chemicals once for most physical hazards.
OSHA also has updated the definition of the term "classification"
to reflect the additional hazards in this final rule (simple
asphyxiant, combustible dust, and pyrophoric gas). The definition for
classification will now read: "Classification means to identify the
relevant data regarding the hazards of a chemical; review those data to
ascertain the hazards associated with the chemical; and decide whether
the chemical will be classified as hazardous according to the
definition of hazardous chemical in this section. In addition,
classification for health and physical hazards include the
determination of the degree of hazard, where appropriate, by comparing
the data with the criteria for health and physical hazards," Dow
Chemical (Document ID 0353) suggested that the language be
changed to read "for health hazards and for physical hazards." OSHA
does not find this to be a necessary revision, and has adopted the
definition as proposed. This definition is very similar to the process
of hazard determination that is currently in the HCS, with the
exception of determining the degree of hazard where appropriate. This
reflects the GHS approach of having categories for each class of
hazard. Under the current HCS, there are some definitions that have
categories in a hazard class (e.g., acute toxicity, flammability), but
other definitions are simply one category (e.g., carcinogenicity). The
additional breakdown in the GHS of classes into categories that reflect
different severities or levels of effect will provide both employers
and employees with more precise information to understand the hazards,
to consider when evaluating workplace conditions to determine the risks
in the workplace, and to respond to exposure incidents.
OSHA has also retained in the final rule the proposed definitions
for "hazard class" and "hazard category" to further explain the
approach of breaking down the hazardous effects into levels of
severity. A "hazard class" is defined as "the nature of the physical
or health hazards, e.g., flammable solid, carcinogen, oral acute
toxicity." The definition of "hazard category" is "the division of
criteria within each hazard class, e.g., oral acute toxicity and
flammable liquids include four hazard categories. These categories
compare hazard severity within a hazard class and should not be taken
as a comparison of hazard categories generally." Both of these
definitions are taken from the GHS. Dow Chemical (Document ID
0353) suggested that the last sentence of the definition of
"hazard category" should be deleted or moved to Appendix A because it
is "non-definitional information." Given that it is included in the
GHS definition, OSHA has adopted it in the final standard.
OSHA has retained the proposed definition of "health hazard" to
reflect the specific hazards defined in the GHS. While the overall
scope of what is covered is essentially the same as the current HCS,
the hazards may be identified slightly differently. For example, the
current HCS covers reproductive toxicity as a target organ effect, and
includes all aspects of the effect under that hazard. The GHS has a
separate definition for germ cell mutagenicity, which is considered
part of reproductive toxicity in the current HCS. The definition of
"health hazard" was thus proposed to be "a chemical which is
classified as posing one of the following hazardous effects: acute
toxicity (any route of exposure); skin corrosion or irritation; serious
eye damage or eye irritation; respiratory or skin sensitization; germ
cell mutagenicity; carcinogenicity; reproductive toxicity; specific
target organ toxicity (single or repeated exposure); or aspiration
hazard. The criteria for determining whether a chemical is classified
as a health hazard are detailed in Appendix A to Sec. 1910.1200--
Health Hazard Criteria."
Both the American Chemistry Council (ACC) (Document ID
0393) and Dow Chemical (Document ID 0353) suggested
that OSHA modify the phrase "any route of exposure," which refers to
"acute toxicity." ACC suggested it list the three specific routes of
exposure in the criteria, and Dow suggested that it include
"relevant" to modify routes of exposures. OSHA does not believe
either of these changes is necessary. The definition already uses the
term "classified" to refer to each of the health hazards listed, and
the acute toxicity criteria include three routes of exposure for
classification. Dow further suggested that "serious eye damage" be
modified to say "by chemical action." Again, the classification
process is for chemicals, and the definition already indicates that it
is covered as a health hazard when classified. Similarly, Dow suggested
that "aspiration hazard" be modified to say "aspiration toxicity
hazard." The proposed language is consistent with the GHS, and OSHA is
maintaining it for harmonization purposes in the final standard.
A revised definition of "physical hazard" was proposed to reflect
the physical hazards covered in the GHS. While these are similar to the
coverage of the HCS, they are in some cases described differently. The
definition proposed for "physical hazard" is "a chemical that is
classified as posing one of the following hazardous effects: Explosive;
flammable (gases, aerosols, liquids, or solids); oxidizer (liquid,
solid or gas); self-reactive; pyrophoric (liquid or solid); self-
heating; organic peroxide; corrosive to metal; gas under pressure; or
in contact with water, emits flammable gas. See Appendix B to Sec.
1910.1200--Physical Hazard Criteria." This definition has been adopted
in the final standard with one change. OSHA did not include pyrophoric
gas in the definition in the proposal. There is no definition for
pyrophoric gas in the GHS, which is covered under the current HCS, and
OSHA inadvertently left it out in the proposed standard when the
generic definition for pyrophorics was removed. This omission was
pointed out by commenters (e.g., Document ID 0382 and 0530).
OSHA is therefore returning the pyrophoric gas definition from the
current rule to paragraph (c), and making it specific to just gases
since the current rule covers all physical states. Thus, pyrophoric gas
is defined as "a chemical in a gaseous state that will ignite
spontaneously in air at a temperature of 130 degrees F (54.4 degrees C)
or below." Label elements are provided in C.4.30. The signal word will
be danger; the pictogram is the flame; and the hazard statement is
"Catches fire spontaneously if exposed to air."
Procter & Gamble (Document ID 0381) noted that the
definition for "flashpoint" was missing from the NPRM and suggested
that it should be put back into the rule. However, the meaning of the
term "flashpoint" is already addressed in the criteria for
"flammable liquid" in Appendix B by specifying the test methods to
determine it. OSHA has also included a definition for flashpoint in the
criteria chapter, rather than in the definitions paragraph.
The definition of "label" in the GHS is slightly different than
what is currently in the HCS, and OSHA proposed to modify the HCS to be
consistent with the GHS. The proposed definition of "label," which
has been retained in the final rule, is "an appropriate group of
written, printed or graphic information elements concerning a hazardous
chemical that is affixed to, printed on, or attached to the immediate
container of a hazardous chemical, or to the outside packaging." The
GHS label is more specific than what is required in the current HCS,
and includes certain core information that must be presented. Thus, a
definition for "label elements" was also proposed and adopted in the
final rule as "the specified pictogram, hazard statement,
signal word, and precautionary statement for each hazard class and
category." ACC (Document ID 0393) noted that this definition
is different from what is in the GHS. OSHA modified the definition by
making it plural to reflect the way it is used in this section to refer
to the OSHA-required label elements for each GHS label. The GHS
definition in this case defines the singular term "label element" as
"one type of information that has been harmonized for use in a label,
e.g., pictogram, signal word." OSHA has listed all of the label
elements, including precautionary statements since they are mandatory
under the revised rule. OSHA believes its definition is consistent with
the GHS but more appropriate for the revised rule, and has adopted it
in this final standard.
"Safety data sheet (SDS)" is defined in both the NPRM and the
final rule as "written or printed material concerning a hazardous
chemical which is prepared in accordance with paragraph (g) of this
section."
Definitions for terms that describe information required to be
provided on labels were also proposed to be added to the HCS and are
included in the final rule. These terms include "hazard statement,"
"pictogram," "precautionary statement," "product identifier," and
"signal word." These new definitions will help to clarify the
specific requirements for labels under the revised HCS, and are
consistent with similar definitions in the GHS.
"Hazard statement" is "a statement assigned to a hazard class
and category that describes the nature of the hazards of a chemical,
including, where appropriate, the degree of hazard." This is
essentially what is defined as a hazard warning under the current rule.
An example of a hazard statement under the GHS is: "Causes serious eye
damage." These statements have been codified, meaning that numbers
have been assigned to them. They are available in all of the official
languages of the United Nations, and thus translation will not be a
problem when shipping to countries using those languages. Having
standardized statements is expected to facilitate translation into
other languages as well. The definition for "hazard statement" is
being adopted as proposed.
There were a few comments about specific hazard statements, such as
an objection from the National Propane Gas Association (Document ID
0400) indicating the statement for flammable gas is ambiguous,
and lacks substantiation and scientific credence. They object to
labeling propane as "extremely flammable," which is the required
statement for Category 1 for flammability hazards. This objection was
also raised in a comment to the ANPR (Document ID 0068). OSHA
responded in the NPRM that it would not be making chemical-specific
changes to hazard statements (74 FR 50399, Sept. 30, 2009). The point
of having harmonized statements is that all chemicals with the same
degree of hazard have the same statement. OSHA also indicated that some
in the industry already use the "extremely flammable" terminology.
NPGA responded that not everyone is familiar with it, or uses it. That
is why OSHA is establishing a standardized approach, so everyone in an
industry with a common product like propane uses the same language to
convey the hazard. This consistency will help people understand what
the hazards are, and simplify the process of conveying them since
everyone will use the same approach. As noted previously, examples of
where the hazard statement "extremely flammable" are currently being
used for propane are readily found (e.g., Document ID 0554).
Therefore, OSHA does not agree with NPGA that the hazard statement is
inappropriate or should be modified.
A few commenters suggested that where hazard statements include two
hazards, separating them should be permitted when data indicate that
only one is applicable to the product involved (for example, it causes
infertility but not developmental hazards) (Document ID 0344,
0376, 0377, 0381, 0382, and 0393). OSHA agrees that such separation
should be permitted. The following provision has been added to Appendix
C.2.2.2: "If the chemical manufacturer, importer, or responsible party
can demonstrate that all or part of the hazard statement is
inappropriate to a specific substance or mixture, the corresponding
statement may be omitted from the label."
Additionally, OSHA permits chemical manufacturers and importers to
combine hazard statements where the information is related and the
combination can shorten the text required on the label. Appendix
C.2.2.1 states: "Hazard statements may be combined where appropriate
to reduce the information on the label and improve readability, as long
as all of the hazards are conveyed as required." OSHA also allows
additional hazard statements under supplementary information, as long
as they are accurate and do not conflict with the required statements.
"Pictogram" is defined as a "composition that may include a symbol
plus other graphic elements, such as a border, background pattern, or
color, that is intended to convey specific information about the
hazards of a chemical." This definition covers both pictograms in the
transport sector, and those in other sectors covered by the GHS. The
pictograms are required as part of the core information provided on a
label to describe the hazards of a chemical. ACC (Document ID
0393) and Procter & Gamble (Document ID 0381) noted
that the proposed definition of pictogram, which was retained in the
final rule, is slightly different than what is in the GHS: "a
graphical composition that may include a symbol plus other graphic
elements, such as a border, background pattern, or color, that is
intended to convey specific information." OSHA added "about the
hazards of a chemical" because that is the only type of information
that will be conveyed by the pictograms in the HCS. The definition is
being adopted as proposed.
The workplace pictograms proposed were a black symbol on a white
background with a red diamond border frame. Some ANPR commenters noted
that the frame should be permitted to be black for domestic shipments
as allowed under the GHS (See, e.g., Document ID 0032 and
0163). However, as described in Section IV of the proposed preamble,
there are clear safety and health benefits associated with the use of
the red frame in terms of recognition and comprehensibility. Thus OSHA
proposed to allow only the red frame to be used, whether the shipment
is domestic or international.
Many of the rulemaking participants recognized the communication
benefits of the red border, and supported the proposed requirement for
a red border frame for all shipments (See, e.g., Document ID
0313, 0324, 0330, 0335, 0336, 0339, 0341, 0365, 0383, 0408,
0410, 0412, and 0456). For example, Product Safety Solutions (Document
ID 0313) stated:
OSHA requests comment on whether pictogram borders should be
required to be in red or should be allowed to be printed in black.
While the use of a red border may increase the cost of printing some
labels, the use of color to draw attention to a potential hazard is
a useful tool and is likely to enhance the communication of safety
information. As products may also be exported to other countries,
the use of the red border would be consistent with the establishment
of a globally recognized hazard symbol. Imported products likewise,
would have to contain the red symbol border and this would have to
be made abundantly clear to Customs Agents and others responsible
for monitoring the importation of chemical products.
However, others argued that black frames should be permitted on
domestic shipments, and that the use of red borders is too costly and
burdensome in terms of printing costs in particular (See, e.g.,
Document ID 0328, 0338, 0344, 0352, 0370, 0376, 0389, 0399,
0405, and 0411). For example, ISSA (Document ID 0399) claims:
If OSHA were to require only the red frame for pictograms, it
would require those formulators that presently print single color
labels to utilize different systems for producing labels of this
nature, requiring a substantial capital investment which in turn
will add greatly to the cost of transitioning to the revised HCS.
OSHA must keep in mind, that small and medium sized formulators
handle hundreds of products, each of which in turn are sold under
multiple private labels. Thus a change in color requirements for
labels generally will literally require a formulator to revise
hundreds, if not thousands, of individual labels.
Further, we believe the use of a black frame will not present a
threat to worker health and safety. ISSA disagrees with OSHA's
conclusion that a red frame would significantly enhance the
communicative value of the label. In citing studies, OSHA does not
take into account that the use of the new labels will be the subject
of intensive employee training that will more than mitigate the use
of a black frame over a red frame.
In the NPRM regulatory analyses, OSHA did not assess the specific
costs associated with red versus black borders, but has done so in the
analyses for the final rule. See Section VI. As noted by proponents of
the black border option for domestic shipments, the costs of a red
border are greater. However, OSHA's analysis shows that they are
economically feasible. In addition, OSHA believes that it is likely
additional, cheaper printing options will be developed to comply with
this requirement in the final rule. The EU requires red frames for
pictograms: "Hazard pictograms shall be in the shape of a square set
at a point. They shall have a black symbol on a white background with a
red frame sufficiently wide to be clearly visible."
(http://europa.eu/legislation_summaries/internal_market/single_market_for_goods/chemical_products/ev0013_en.htm)
Application of this requirement in the twenty-seven (27) EU member
states is expected to lead to new printing options for compliance.
OSHA believes that the increased comprehension that will be
provided by the red border frame is compelling. The red color will
clearly delineate the hazard symbols from the other information on the
label, and the prominence will lead to increased attention and
recognition of the hazards. The transport labels and placards that have
been in use for many years have multiple colors in their pictograms,
and yet compliance has been achieved. Plus most product labels have
various colors related to their logos, brands, etc., so clearly it can
be done.
There are also some logistical issues that would make compliance
more difficult with two different colored frames. First, it is unlikely
that it would always be known whether a product would be exported at
the point of labeling it at the end of the manufacturing process. Many
containers are simply shipped to distributors, and the original
manufacturer does not know where they will be sent after that--thus
raising the question of whether a manufacturer or importer would know
when to apply a black versus a red frame. In addition, workers exposed
to chemicals purchased from different sources might have different
frames, requiring additional training to avoid potential confusion. The
final rule remains as proposed, and requires pictograms to have a red
frame, with a black symbol on a white background, for all shipped
chemicals regardless of destination.
Several commenters (Document ID 0318, 0382, and 0393) also
raised issues regarding whether pre-printed labels with blank red
frames could be used. The manufacturer would simply add the symbols to
the frames when printing the required label information. If a
manufacturer or importer took this approach, a particular label might
have one or more empty red diamonds in addition to any required
pictograms. OSHA does not believe that this would be appropriate. Blank
frames would still attract attention, but workers could be confused
about what they mean and whether something is missing from the
information. While blank frames could be marked to indicate they are
intentionally left blank, they will still contribute to clutter on the
label and distract from the primary messages (See, e.g., Document ID
0284). Blank frames are not considered acceptable by DOT. (See
49 CFR 172.401, Prohibited labeling; PHMSA Interpretation 02-0088).
OSHA does not believe this is a good alternative for compliance either,
and the final rule prohibits blank frames on the label (Appendix
C.2.3.1).
Under the GHS, a symbol is generally assigned to each hazard class
and category. There are nine agreed symbols under the GHS to convey the
health, physical and environmental hazards. Eight of these symbols were
proposed for adoption in this rulemaking, the exception being the
environmental symbol. Six of these symbols have been used for many
years in the international transport requirements, so some employers
and employees will already be familiar with them.
The symbols in the proposed rule are adopted in the final rule. Dow
Chemical (Document ID 0353) noted that the pictograms are not
entirely self-evident. While this may be true, the rule requires
training workers so they will know what the symbols mean and how to
respond.
It should be noted that in the NPRM, the pictogram for C.4.17
(oxidizing gases) was published with a "flame" symbol, rather than
the "flame over circle" symbol that was appropriate, and was
described. OSHA has corrected this error in the final rule, and has
inserted the appropriate "flame over circle" symbol in Appendix
C.4.17 for oxidizing gases.
The "precautionary statement" is "a phrase that describes
recommended measures that should be taken to minimize or prevent
adverse effects resulting from exposure to a hazardous chemical, or
improper storage or handling." The precautionary statements specified
in Appendix C will be required on containers under the final rule. An
example of a precautionary statement is: "Wear protective gloves."
The precautionary statements in the GHS are assigned to certain hazard
classes and categories.
Precautionary statements are not required under the current HCS,
although many chemical manufacturers include them on their labels for
safe handling and use. These statements are codified under the GHS,
meaning that numbers have been assigned to them. The precautionary
statements in the GHS are not harmonized like the hazard statements
are, and the regulatory authority is free to use the statements in the
GHS annex or to use alternative statements when adopting the current
version of the GHS. Using the GHS statements has the advantage of
adopting statements that have undergone expert review by the UN Sub-
committee, are assigned to the appropriate hazard class and category,
and have been translated into six languages. Work continues on them in
the Sub-committee to combine or edit the precautionary statements to
reduce repetition and the complexity of the label. The precautionary
statements may be considered harmonized in the future.
Other countries are already using them (e.g., in Europe). Since
OSHA did not previously require the use of precautionary statements,
and had no such recommended statements to provide, the Agency decided
to use those in the GHS as the mandatory
requirements. This will make it easier for compliance since chemical
manufacturers and importers will not need to develop, maintain, and
translate precautionary statements on their own. It will also help
employees since they will be seeing the same language on labels
regardless of the supplier of the chemical. Such standardization
improves comprehension, and thus the effectiveness of the information
transmitted under the standard.
While the definition of precautionary statement itself did not seem
to raise questions with rulemaking participants, there were a number of
comments on the proposal to make the GHS precautionary statements
mandatory. Many commenters agreed with OSHA that the statements should
be on the label, and should be mandatory (Document ID 0328,
0329, 0335, 0336, 0347, 0352, 0365, 0370, 0372, 0377, 0379, 0389, 0402,
0408, 0410, 0412, and 0456). Commenters mentioned increased
comprehensibility, as well as available translations, as some of the
reasons why they support this approach. It was also noted by a number
of commenters that OSHA should permit additional precautionary
statements to cover situations without an available statement in
Appendix C (Document ID 0313, 0324, 0327, 0329, 0335, 0352,
0365, 0370, 0376, and 0402). Others supported making them mandatory
when they are harmonized in the GHS (Document ID 0351 and
0405). And at least one participant argued that precautionary
statements should not appear on labels, just SDSs (Document ID
0338).
Other commenters did not support the mandatory approach, and
thought that manufacturers should be able to continue to use their own
precautionary statements (Document ID 0321, 0330, 0344, 0353,
0363, 0376, 0381, 0382, 0393, and 0399). It was also suggested that the
UN needs to provide further guidance on when precautionary statements
can be combined or omitted (Document ID 0328, 0370, and 0376),
or that the number of phrases appearing on a label should be limited
(Document ID 0329 and 0405).
In the final standard, OSHA has maintained the proposed provision
to require the precautionary statements in the GHS to be used on
labels. As noted previously, the use of prescribed precautionary
statements is consistent with the other label elements, and provides
the significant benefits of improved communication of information
through increased comprehensibility and familiarity. In terms of
flexibility, chemical manufacturers and importers are free to put
additional precautionary statements on the label from other sources in
the supplementary information area. As long as the information provided
is accurate, and does not conflict with the required information, this
is permitted.
OSHA will also permit the statements to be combined as appropriate,
and states in Appendix C.2.4.6: "Precautionary statements may be
combined or consolidated to save label space and improve readability.
For example, "Keep away from heat, sparks and open flame," "Store in
a well-ventilated place," and "Keep cool" can be combined to read
"Keep away from heat, sparks and open flame and store in a cool, well-
ventilated place."
In addition, where there are concerns, supported by evidence, about
the applicability of a statement to a particular product, the chemical
manufacturer or importer may revise the statements as appropriate for
the situation. Appendix C.2.4.8 states: "If the chemical manufacturer,
importer, or responsible party can demonstrate that a precautionary
statement is inappropriate to a specific substance or mixture, the
precautionary statement may be omitted from the label."
Thus, the final rule adopts the precautionary statements, which are
taken from the GHS. However, it allows the use of additional statements
where necessary, as long as they are accurate, do not conflict, and are
placed in supplementary information. Additionally, chemical
manufacturers and importers can use their judgment to combine related
statements to shorten the amount of information on a label, as well as
omit any statements that can be demonstrated to be inapplicable to the
particular chemical involved. OSHA believes this approach maximizes the
comprehensibility of the precautionary statements, as well as
simplifies compliance for employers. Nevertheless, there are allowances
for unique situations, and thus assurances that the information will be
accurate.
It was suggested that the precautionary statements should be
written in plain language (Document ID 0321). There were some
specific changes to particular statements that were suggested (such as
a statement regarding fighting fires near explosives, Document ID
0353). OSHA is not going to modify any of the statements as
published in the GHS in terms of technical information. These have been
reviewed by many experts. Changes should only be made to them through
the UN Sub-committee process at this point, as they are close to being
harmonized.
However, OSHA has made a few minor changes to precautionary
statements in this final rule to address clarity and related issues.
These changes were adopted by the Sub-committee of Experts on the GHS
at its December 2010 meeting, and are expected to be included in
Revision 4 of the GHS. Most changes simply amend the precautionary
statement to clarify its meaning by making the statement more concise,
or stating it in plain language. Others either provide added
flexibility in applying the precautionary statement, or provide
instructions for the classifier on the conditions relating to use of
the precautionary statement. Examples of each type are presented below.
Examples of precautionary statements for physical hazards that were
clarified in the final rule are presented below:
------------------------------------------------------------------------
Precautionary statement in
Precautionary statement in proposed rule final rule
------------------------------------------------------------------------
Keep away from any possible contact with Do not allow contact with
water. water.
In case of fire: Use * * * for extinction. In case of fire: Use * * *
to extinguish.
------------------------------------------------------------------------
An example of a precautionary statement providing instructions for
the classifier on the conditions relating to use of the precautionary
statement is provided below for the health hazard class Skin corrosion/
irritation, Category 1A to 1C (for the illustration, the instructions
for use are provided in italics). In this example, note that the
precautionary statement was clarified and the conditions relating to
use of the precautionary statement were added.
------------------------------------------------------------------------
Precautionary statement in
Precautionary statement in proposed rule final rule
------------------------------------------------------------------------
Immediately call a poison center/or doctor/ Immediately call a poison
physician. center/doctor/ * * *
Chemical manufacturer,
importer, or distributor to
specify the appropriate
source of emergency medical
advice.
------------------------------------------------------------------------
The final example of the precautionary statement changes is
provided below for instructions for the classifier on the conditions
relating to use of the precautionary statement. In certain situations,
text in a precautionary statement may not be appropriate. To address
this issue, a new paragraph C.2.4.5 has been added to explain the use
of text provided in square brackets ([ ]). Paragraph C.2.4.5 states:
"Where square brackets ([ ]) appear around text in a precautionary
statement, this indicates that the text in square brackets is not
appropriate in every case and should be used only in certain
circumstances. In these cases, conditions for use explaining when the
text should be used are provided. For example, one precautionary
statement states: "[In case of inadequate ventilation] wear
respiratory protection." This statement is given with the condition
for use: "text in square brackets may be used if additional
information is provided with the chemical at the point of use that
explains what type of ventilation would be adequate for safe use."
This means that, if additional information is provided with the
chemical explaining what type of ventilation would be adequate for safe
use, the text in square brackets should be used and the statement would
read: "In case of inadequate ventilation, wear respiratory
protection." However, if the chemical is supplied without such
ventilation information, the text in square brackets should not be
used, and the precautionary statement should read: "Wear respiratory
protection."
OSHA has included these non-substantive, minor changes approved by
the UN Sub-committee, because they make the statements more readable,
allow added flexibility, and are consistent with the latest version of
the GHS.
Container labels will also be required to include a "product
identifier." The proposed definition for this term, which was retained
in the final rule with a clarifying change (discussed below), was "the
name or number used for a hazardous chemical on a label and in the SDS.
It provides a unique means by which the user can identify the chemical.
The product identifier used shall permit cross references to be made
among the required list of hazardous chemicals, the label, and the
SDS." In other words, the product identifier is essentially the same
as the "identity" under the current HCS. The GHS allows competent
authorities for workplace requirements to choose not to require
specific chemical identities of ingredients to be listed on the label,
as long as they are on the SDS. This is the approach OSHA currently
uses in the HCS, and it has been effective. OSHA will continue to
require chemical identities only on SDSs, and has proposed a definition
for "product identifier" that is consistent with the current
definition for "identity" (which has been deleted from the final
rule) to maintain this approach. ACC (Document ID 0393) and
Procter & Gamble (Document ID 0381) suggested that OSHA should
clarify what the "required list of hazardous chemicals" refers to in
the definition. This terminology has been in the HCS since the original
standard was published in 1983, and refers to the only list of
chemicals required by the HCS, which is in the written hazard
communication program. Therefore, OSHA has modified the language in the
final rule to read: "among the list of hazardous chemicals required in
the written hazard communication program, the label and the SDS."
Another new concept in the NPRM for HCS labels is inclusion of a
"signal word" to bring attention to the hazardous effects, as well as
to contribute to the recognition of the severity of the hazard. Signal
words have been used for many years in the United States on consumer
and pesticide labels. The proposed definition is "a word used to
indicate the relative level of severity of hazard and alert the reader
to a potential hazard on the label. The signal words used in this
section are 'danger' and 'warning.' 'Danger' is used for the more
severe hazards, while 'warning' is used for the less severe." OSHA
received no objections to the proposed definition of "signal word"
and it is being carried through to the final rule.
OSHA proposed to add a definition to the HCS for "unclassified"
hazards. As has been noted, the current HCS is performance-oriented,
and takes a very broad approach to defining hazards covered by the
rule. The GHS is similarly broad in approach, but includes very
specific definitions of criteria to apply when determining whether a
chemical poses a physical or health hazard. This specification approach
has significant benefits associated with it, including providing more
guidance to help ensure a consistent approach to determining hazards.
It also allows more information to be developed that provides an
indication of the severity of effect.
OSHA proposed to add a definition to the HCS for "unclassified"
hazards. As has been noted, the current HCS is performance-oriented,
and takes a very broad approach to defining hazards covered by the
rule. The GHS is similarly broad in approach, but includes very
specific definitions of criteria to apply when determining whether a
chemical poses a physical or health hazard. This specification approach
has significant benefits associated with it, including providing more
guidance to help ensure a consistent approach to determining hazards.
It also allows more information to be developed that provides an
indication of the severity of effect.
In the ANPR, OSHA asked for comment on whether the GHS criteria are
sufficient to cover the hazards present in the workplace. While the
Agency believed the scope of coverage is similar between the two
approaches, OSHA wanted to be sure that the new approach is as
comprehensive as the existing standard. In the NPRM (74 FR 50390, Sept.
30, 2009), OSHA noted two hazards of concern--combustible dust and
simple asphyxiants. Both of these are mentioned in the GHS in the SDS
annex as examples of hazards not classified that should be addressed on
the SDS.
It is possible that there are other hazards that may not yet be
specifically defined. Rulemaking participants have mentioned several
(e.g., static accumulators) (Document ID 0382 and 0402). The
addition of the definition for unclassified hazards was intended to
address these situations. Where a classifier has identified evidence of
a hazard, but the evidence does not meet the currently specified
criteria for hazards covered by the rule, the definition for
unclassified hazards captures those effects to ensure that the final
rule is appropriately protective, and covers all of the hazards covered
by the current rule. During the negotiations for the GHS, U.S. industry
representatives often raised the issue of ensuring that they could
provide additional hazard information in order to satisfy product
liability laws in the U.S. This was the rationale for allowing such
information to be included on labels under supplementary information,
and on SDSs under Section 2. OSHA believed that addition of the
proposed definition of "unclassified hazards," and specific
recognition of the need to provide information when such effects arise,
would help U.S. industry address its product liability concerns as well
as protect exposed workers (74 FR 50390, Sept. 30, 2009).
OSHA proposed to require the chemicals posing unclassified hazards
to be treated as hazardous chemicals under the rule. The Agency
anticipated that this information would appear in Section 2 of the SDS
(Hazard Identification)--the GHS already identifies this as the
appropriate place in its guidance on the contents of SDSs (A4.3.2.3,
Other hazards which do not result in classification), and proposed
Appendix D included the requirement to list unclassified hazards. In
terms of labeling, there are no specified label elements in the GHS for
chemicals that pose unclassified hazards. OSHA proposed to require that
the label for such hazards must name the chemical, and describe the
hazardous effects under supplementary information on the label, as well as provide any
appropriate precautionary information. OSHA also expected that such
hazards would be addressed in worker training programs.
It is important to understand that the Agency anticipated that
there would be relatively few situations where there would be
scientific evidence or data indicating an effect that is not currently
classified, and merely wanted to ensure that this information is
captured and conveyed to employers and employees. OSHA also indicated
that it would be appropriate to establish a feedback mechanism, where
classifiers could inform OSHA of situations where the current criteria
are insufficient, and the Agency can then suggest to the United Nations
that appropriate criteria be developed and added to the GHS. This is
consistent with the overall approach to hazard classification in the
GHS that OSHA proposed to adopt--that specific criteria be provided to
help ensure that classification is appropriate, and information
transmittal is consistent from company to company. Therefore, the use
of the definition of unclassified hazard was to be a temporary
situation for these hazards, ensuring information is provided until
such time as the criteria are added to the rule.
There were many comments received regarding the NPRM definition and
concept of "unclassified hazards." A number of participants agreed
with OSHA that there is a need to cover some hazardous effects that
have not yet been spelled out in the GHS with criteria (Document ID
0313, 0327, 0347, 0363, 0365, 0366, 0367, 0410, and 0412).
Others suggested that it was an appropriate interim step, while working
with the UN to get criteria added to the GHS (Document ID
0329, 0330, 0335, 0339, 0352, 0370, 0376, 0383, 0405, and
0414). Some argued that these hazardous effects should have specific
criteria so employers would know with certainty what is covered
(Document ID 0327, 0361, 0366, 0377, and 0392).
With regard to the actual definition, some thought it was too broad
and ambiguous (Document ID 0344, 0379, 0381, and 0399). The
U.S. Chamber of Commerce (Document ID 0397) argued that the
definition should be withdrawn, or substantially revised, and that OSHA
was exceeding its authority. There were other commenters who thought
the effects should be called "hazards not otherwise classified" or
"additional hazards" rather than "unclassified hazards." See, e.g.,
Document ID 0328, 0344, 0363, 0370, 0376, 0393, and 0405. It
was also suggested that the approach should only cover those hazards
currently covered by the HCS (Document ID 0338).
OSHA has considered all of these comments, and the need to provide
sufficient protection for exposed employees, in devising an approach
for the final rule. First, OSHA agrees with commenters that using the
term "hazards not otherwise classified" is a better designation.
Secondly, OSHA has revised the language to clarify the intent and
address what was perceived as ambiguity. The definition in the final
rule, which replaces and amends the proposed definition of
"unclassified hazard," now reads: "'Hazard not otherwise classified
(HNOC) means an adverse physical or health effect identified through
evaluation of scientific evidence during the classification process
that does not meet the specified criteria for the physical or health
hazard classes addressed in this section. This does not extend coverage
to adverse physical and health effects for which there is a hazard
class addressed in this section, but the effect either falls below the
cut-off value/concentration limit of the hazard class or is under a GHS
hazard category that has not been adopted by OSHA (e.g., acute toxicity
Category 5)."
Additionally, and importantly, OSHA has deleted proposed paragraph
(f)(2), which specified information to include on labels for the HNOC
chemicals. Given that there are no harmonized label elements available
for these effects, it appears that this could be confusing to both the
label preparers and the users of the chemicals. However, provision of
an SDS for HNOC chemicals is required under the final rule, and
information regarding their hazards is to be included in Section 2.
The U.S. Chamber of Commerce objected to the inclusion of
"unclassified hazards" in the final rule because, in its view, the
proposed definition is "broad," "expansive," and will "impose new
requirements on employers without undertaking all of the steps in a
full OSHA rulemaking" (Document ID 0397). OSHA appreciates
the concerns and has carefully considered (and in some respects
revised) the provision with those concerns in mind. OSHA does not
intend to impose new requirements, or to bypass rulemaking, but
includes the definition to continue the longstanding requirements that
such hazards be disclosed. As finalized and clarified, the relevant
provision does not expand on those requirements or add new burdens; on
the contrary, it preserves requirements in the current rule. The
following discussion is designed to clarify these points.
As noted above, the final rule retains the proposed requirement,
using the term "hazard not otherwise classified" (HNOC) instead of
unclassified hazard. In essence, this definition requires classifiers
who find "scientific evidence" that a chemical can cause death,
illness, or injury to workers in a way not currently covered by the GHS
classification criteria to disclose that fact on the SDS. This is meant
to be a modest and narrow requirement. It is triggered only when the
classifier has objective, scientific evidence of the hazard. OSHA
believes that there are likely to be few such hazards outside those
covered by the specific criteria in the final rule, which are the
product of over thirty years of international experience in hazard
communication.
It is important to understand that the HNOC definition essentially
preserves (and does not expand) the scope of the current rule, which is
not as tightly bound to specific criteria as the GHS. The HNOC
definition should be interpreted and understood with this preservative
goal in mind. For example, under the current rule, "health hazard"
means a chemical for which there is at least one statistically
significant scientific study showing that "acute or chronic health
effects may occur to exposed employees." Indeed, while mandatory
Appendix A of the current standard lists criteria for specific health
effects, it also notes that these criteria are not intended to be an
exclusive categorization scheme, but rather any available scientific
data on the chemical must be evaluated to determine whether the
chemical presents a health hazard. Likewise, though the current
definition of physical hazard is tied to a specific list of effects,
some of these can also be quite broad. For example, under the current
rule, "flammable solid" includes a material "which can be ignited
readily and when ignited burns so vigorously and persistently as to
create serious hazard."
The essential point is that the HNOC definition is designed so as
to prevent the final rule from being less protective than the current
standard by picking up any hazards that might fall within the
definitions of the current rule, but might fall outside the GHS hazard
classes. As discussed above, it is OSHA's intent that the HNOC
classification would be an interim measure, used until harmonized
criteria for a hazard can be adopted at the UN Sub-committee level, and
subsequently incorporated into the HCS through rulemaking.
If the provision is understood in light of the foregoing points,
this rulemaking is all the OSH Act and the Administrative Procedures
Act (APA) requires of OSHA before adopting the HNOC requirement. By preserving
the requirements equivalent to those in the current rule, all the final
rule does is to require chemical manufacturers and importers with
reliable information that exposure to their chemical can cause illness,
injury or death to an employee to disclose that fact on an SDS. OSHA
has the authority to regulate hazard communication on a general level;
indeed it must if it is to provide comprehensive worker protection in
this area. See National Ass'n of Manuf. v. OSHA, 485 F.3d 1201, 1204
(D.C. Cir. 2007); Associated Bldrs & Contrs. Inc. v. Brock, 862 F.2d
63, 68 (3d Cir. 1988). Stakeholders have had a chance to comment on the
HNOC requirement, and this rulemaking proceeding satisfies OSHA's
statutory obligations.
With regard to the three hazards specifically mentioned during the
rulemaking (pyrophoric gases, simply asphyxiants, and combustible
dust), OSHA is handling them as follows in the final rule.
OSHA inadvertently removed the definition of pyrophoric gases from
the proposal when it removed the generic definition for pyrophorics.
The American Chemistry Council (ACC) correctly pointed out that
excluding the pyrophoric gases, even though there is no corresponding
definition in GHS, would mean that they would not be labeled or
classified appropriately (Document ID 0393). OSHA agrees and
has included the definition of pyrophoric gas in the current HCS in
this final rule. Pyrophoric gases must therefore be addressed both on
container labels and SDSs, and in worker training programs. Therefore,
OSHA has retained the definition for pyrophoric gases from the current
HCS and has added pyrophoric gases to the definition of "hazardous
chemical". Label elements are provided in C.4.30. The signal word will
be danger; the pictogram is the flame; and the hazard statement is
"Catches fire spontaneously if exposed to air."
For the two examples of effects not addressed in the GHS that were
raised in the proposal (simple asphyxiants and combustible dust), OSHA
is addressing them specifically in the final rule rather than covering
them under the HNOC definition. Using comments in the record, and
commonly applied voluntary industry consensus standards, the Agency has
designated chemicals with these properties under the definition of
"hazardous chemical." The chemicals posing such effects must
therefore be both labeled where appropriate, and addressed on SDSs and
in training. In addition, OSHA has added C.4.30 to Appendix C to
provide the label elements for OSHA defined hazards.
With regard to simple asphyxiants, OSHA had indicated in Issue
8 (74 FR 50282, Sept. 30, 2009) that it believed it might be
more appropriate to simply add a definition of this effect to the final
rule rather than covering it under the "unclassified hazard"
approach. A definition was proposed as follows:
"Simple asphyxiants" are substances that displace oxygen in
the ambient atmosphere, and can thus cause oxygen deprivation in
exposed workers that leads to unconsciousness and death. They are of
particular concern in confined spaces. Examples of asphyxiants
include: nitrogen, helium, argon, propane, neon, carbon dioxide, and
methane.
OSHA also solicited comments on proposed specific label elements.
No symbol would be required, but the signal word "warning" would be
used, with the hazard statement "may be harmful if inhaled." In
addition, a precautionary statement such as the following would be
required: "May displace oxygen in breathing air and lead to
suffocation and death, particularly in confined spaces."
A number of commenters agreed with the definition and the approach
(Document ID 0339, 0347, 0351, 0365, 0366, 0370, 0405, 0408,
and 0456). Others had specific comments on what was proposed, such as
arguing for simplification of the language (Document ID 0414);
proposing to replace the definition with the NFPA 704 definition of
"simple asphyxiant" (Document ID 0330); suggesting a
reference to "suffocation" (Document ID 0329 and 0335), or
indicating that the hazard statement is really a precautionary measure,
or vice versa (Document ID 0376, 0382, 0393, and 0405).
Procter & Gamble suggested it should not be covered since it is not an
inherent toxicity (Document ID 0381).
OSHA disagrees with Procter & Gamble's argument. Chemicals with
certain properties can displace oxygen and cause asphyxiation. Not
every chemical has those properties, so the asphyxiation hazard is
inherent and chemical-dependent. Moreover, OSHA has provided
longstanding interpretations that indicate simple asphyxiants are
covered under the current HCS (e.g., OSHA interpretation, March 4,
1993) and therefore industries working with these substances have
provided labels and SDSs on simple asphyxiants in accordance with HCS
requirements.
OSHA believes that coverage of simple asphyxiants is very important
to the HCS. Such substances result in fatalities in the workplace,
particularly in confined spaces, and need to be warned about
effectively. The definition has been revised based on the comments
received, and included in paragraph (c): " 'Simple asphyxiant means a
substance or mixture that displaces oxygen in the ambient atmosphere,
and can thus cause oxygen deprivation in those who are exposed, leading
to unconsciousness and death." Label elements are provided for simple
asphyxiants in Appendix C.4.30. Simple asphyxiants will require the
signal word "warning" and the hazard statement "may displace oxygen
and cause rapid suffocation." In addition, OSHA has added "simple
asphyxiant" to the definition of "hazardous chemical." Thus all of
the provisions of the rule that apply to hazardous chemicals will apply
to simple asphyxiants as well.
OSHA will continue to work with the UN to add this hazard to the
GHS. (The U.S. has raised this issue in the UN Sub-committee, but it
has not yet been resolved. Some of the Sub-committee members share the
view that it should not be covered since, according to them, it is not
an inherent hazard.) We will evaluate the need for additional
rulemaking to change the definition and label elements if the UN
incorporates simple asphyxiants into the GHS.
For combustible dust, OSHA has also already provided considerable
guidance on the nature and definition of combustible dust in a variety
of materials, including OSHA's Hazard Communication Guidance for
Combustible Dusts, OSHA (3371-08 2009), and its Combustible Dust
National Emphasis Program Directive CPL 03-00-008. As described in the
preamble to the NPRM (74 FR 50395, Sept. 30, 2009), this was an issue
that many ANPR commenters had provided information on, and is clearly a
concern in the workplace. There have been a number of workplace
incidents involving combustible dust, and the U.S. Chemical Safety and
Health Investigation Board highlighted the need to address this
specifically in the HCS (Document ID 0110):
The CSB therefore recommends that OSHA amend the HCS to
explicitly address the fire and explosion hazards of combustible
dusts, and those materials that could reasonably be expected to
produce combustible dusts, among the substances covered by the
standard, and also that the Agency require inclusion of dust fires
and explosions among the physical hazards that must be addressed in
Material Safety Data Sheets. The CSB also requests that OSHA
advocate similar changes to the GHS through appropriate
international mechanisms.
OSHA has introduced this issue to the UN Sub-committee as well, and
is leading a correspondence group on it. However, one of the problems
in pursuing this approach is that some countries' systems are limited
to supply chain requirements, and do not cover hazard communication
issues that arise in the workplace as a result of processing. OSHA's
rule does cover such workplace hazards, and requires the provision of
information to downstream customers when known processing approaches
will result in a hazard. Therefore, discussions continue, but the Sub-
committee will not resolve this for at least two years.
In light of the important nature of the issue, a number of public
comments, and the need to provide clarity sooner than the UN Sub-
committee will complete its work, OSHA is including combustible dust in
the definition of "hazardous chemical" in this final rule. We have
noted that many commenters agreed that there was a need to provide
hazard communication on combustible dust, as has been required by OSHA
under the current rule. But there were also suggestions that criteria
and greater clarity were needed in order to avoid confusion. A few
commenters argued that OSHA should not cover combustible dust since it
is not an intrinsic hazard of a product (See, e.g., Document
ID0393). However, OSHA believes that similar to the situation
with simple asphyxiants, all dusts in the workplace are not
combustible, and processing of them does not always result in
combustible atmospheres. Consistent with Executive Order 13563 and its
emphasis on reducing uncertainty, OSHA agrees with commenters noted
above that employers need certainty to properly cover it.
It is true that a separate rulemaking is ongoing on this topic in
OSHA, and some commenters suggested that the combustible dust issue
should therefore not be addressed in this rulemaking. Such an approach
would, however, eliminate safeguards that have long been in place
(since 1983). Similar to the situation with simple asphyxiants, OSHA
has provided longstanding interpretations that indicate combustible
dusts are covered under the current HCS (e.g., OSHA interpretation,
January 16, 1986). Specifically, under OSHA's existing Hazard
Communication Standard, combustible dust is addressed under the broad
definition as both a flammable solid and an explosive hazard.
Therefore, not addressing combustible dust in this rulemaking would
fail to meet the requirements--which are central to the existing
standard--that chemical manufacturers and importers provide information
on hazardous chemicals.
While OSHA is currently in the preliminary stages of developing a
proposed rule to address combustible dust, the new standard is not
expected to be completed for some time. It is also important to note
that there is a clear distinction between coverage under the HCS, and
potential provisions promulgated under a specific rulemaking for
combustible dust. The rulemaking on combustible dust is a much broader
approach to the issue, and will likely establish methods to control and
address such dusts in the workplace. The HCS is an information
transmittal standard. Provision of information to downstream employers
is critical now, as it can alert them to the need to have a protective
program. This is a fundamental purpose of the HCS--to provide employers
and employees with information about hazards so they can take steps to
protect their employees and themselves. A failure to continue to
address the combustible dust issue in the HCS at this time would
eliminate current protections. Therefore, the Agency is clarifying its
position that it will continue to regard combustible dust as a serious
hazard for which chemical manufacturers and importers must provide
information to downstream employers.
The Agency is not adding a definition for combustible dust to the
final rule given ongoing activities in the specific rulemaking, as well
as in the UN Sub-committee. However, guidance is being provided through
existing documents, including the Combustible Dust National Emphasis
Program Directive CPL 03-00-008. This directive includes an operative
definition, as well as provides information about current
responsibilities in this area. In addition, there are a number of
voluntary industry consensus standards (particularly those of the NFPA)
that address combustible dust, and were noted by commenters as
providing further guidance in this area. (See, e.g., Document ID
0379 and 0530). Chemical manufacturers and importers must be
aware of the hazards of their products, both in the shipped form, and
under normal conditions of use or foreseeable emergencies in downstream
workplaces, in order to comply with the HCS. Information about these
hazards is required to be transmitted through labels and SDSs as
specified in the standard. The protection of workers in downstream
workplaces depends on the provision of accurate information to their
employers.
Label elements are also provided for combustible dust in C.4.30
requiring, when appropriate, the signal word "warning" and the hazard
statement "May form combustible dust concentrations in air" (similar
to ANSI Z400.1/Z129.1--2010 statements).
Concerns were raised by commenters that labels with a signal word
and hazard statement may not be appropriate in some situations, because
the combustible dust is created through processing downstream, and the
product may not present a hazard in its shipped form. (See, e.g.,
Document ID 0050 and 0353.) Dow (Document ID 0353)
pointed out: "Over-warning would dilute the message."
OSHA has already addressed a similar situation under paragraph
(f)(4) of the final standard, which addresses solid metal, solid wood,
plastic, and shipments of whole grain that present no hazard in
shipping, but which are used in such a way in downstream operations
that employees can be exposed to hazards. In this situation, the
downstream employer needs label information about the hazards to
protect employees, but OSHA determined that such label information does
not need to accompany the product. Therefore, paragraph (f)(4) allows
the chemical manufacturer or importer to transmit the label to the
customer at the time of the initial shipment, but the label does not
need to be included with subsequent shipments unless it changes. This
provides the needed information to the downstream users on the
potential hazards in the workplace, while acknowledging that the solid
metal or other materials do not present the same hazards that are
produced when these materials are processed under normal conditions of
use.
Many products that are a combustible dust hazard when processed are
similar in nature, and therefore paragraph (f)(4) would apply. A
shipment of grain, for example, does not present a combustible dust
hazard in the shipped form. But when processed downstream in a plant,
such hazards are a concern, and the employer needs the label
information to properly address the hazard in the workplace. Since this
is a normal condition of use for the grain, the chemical manufacturer
or importer must provide the information at the time of the initial
shipment, and in the future if there is new information regarding the
hazards or protective measures. An SDS must always be provided.
In other situations where the material is shipped in a dust form
that is potentially combustible without further processing, the
chemical manufacturer or importer must have appropriate labels on the
containers when shipped under the requirements of paragraph (f)(1). If
the chemical manufacturer labels the product for combustible dust,
the label must use the required labeling elements in C.4.30.
Combustible dust has been added to the definition for hazardous
chemical, and thus all of the provisions of the standard as amended by
the final rule that apply to hazardous chemicals will also apply to
combustible dusts, including safety data sheets and worker training.
Employers with workplaces where combustible dusts are generated must
comply with the workplace labeling requirements in paragraph (f)(6).
As with simple asphyxiants, OSHA will continue to encourage the UN
Sub-committee to deal with combustible dusts and develop criteria to be
adopted by countries such as ours where workplace exposures are a key
part of the hazard communication system.
(d) Hazard Classification
Hazard determination under the current standard. Under the current
HCS, chemical manufacturers and importers are required to evaluate the
scientific data available regarding each chemical they produce or
import, and determine whether the chemical is hazardous within the
meaning of the standard. This requires a thorough search of the
scientific literature on both the health and physical hazards that the
chemical may pose. The identified information must be evaluated within
the parameters established in the standard to determine whether the
chemical is considered to pose a hazard. Paragraph (d), Hazard
determination, provides the regulatory approach for evaluation. This
approach is to be implemented using the definitions provided in
paragraph (c) as well as in Appendix A, which provides further
elaboration on the nature and breadth of health hazards covered.
Appendix B provides additional requirements for identifying and
evaluating data regarding hazards. Both of these appendixes are
mandatory.
In order to ensure the broadest dissemination of information, and
to reduce the number of situations where conflicting determinations may
be made for the same chemical by different suppliers, the current HCS
considers one study, conducted according to established scientific
principles and producing a statistically significant result consistent
with the definitions of hazard in the standard, to be sufficient for a
finding of health hazard under the rule. See 29 CFR 1910.1200(d)(2) and
Appendix B. This approach was the broadest among those systems that
were used as the basis for the development of the GHS.
Most of the definitions under the current HCS simply lead to a
conclusion that the chemical involved poses that hazard or it does not.
For example, a chemical might be found to be a carcinogen under the
rule based on one study indicating that it poses a carcinogenic effect.
The current standard does not generally address the degree of severity
of the hazardous effect in most of the definitions--so a chemical is
either a carcinogen, or it is not. However, while a one-study
determination leads to providing information about that hazardous
effect on a safety data sheet, it may not lead to a hazard warning on a
label. The current HCS requires such warnings to be "appropriate,"
and there are situations where the data do not support warning about
the hazard on the label because of other negative studies or
information. See 29 CFR 1910.1200 (f)(1)(ii). Thus, there is
consideration of the weight of evidence when deciding what to include
on a label. Chemical manufacturers and importers may also review the
weight of evidence in preparing SDSs, and are permitted to discuss
negative evidence and other constraints when reporting the information.
Under the current standard, OSHA expects the hazard evaluation process
to go beyond simply identifying one study, and include a complete
evaluation of all of the information available when determining what
information to transmit to users of the chemical.
This hazard evaluation process is consistent with product
stewardship processes that have evolved in the chemical industry. (See,
e.g., the Responsible Care[supreg] program implemented by chemical
manufacturers.) Under such processes, chemical manufacturers develop
and maintain thorough knowledge of their chemicals. This knowledge is
critical to the safe handling and use of the chemicals in their own
facilities, as well as in their customers' facilities. It is also
critical to handling product liability concerns for their materials.
The current HCS requires chemical manufacturers to remain vigilant
regarding new information about their chemicals, and to add significant
new information about hazards or protective measures to their hazard
communication documents within three months of learning about them. See
29 CFR 1910.1200(f)(11), (g)(5). This has always been seen by OSHA as a
more rigorous, but essential, requirement than some other countries'
provisions, which only require these documents to be reviewed every few
years. It should be noted that OSHA has not been enforcing the current
requirement to change labels within three months of getting new
information. This stay on enforcement began some years ago when the
standard was first promulgated, and involved concerns about existing
stockpiles of chemicals and other related information. The stay does
not apply to safety data sheets. OSHA proposed to reinstate the
requirement and lift the stay, making the updating period consistent
with that required for safety data sheets (See the discussion below on
labels).
At the time the HCS was promulgated, the standard's provisions and
approach were quite novel, and there were concerns that chemical
manufacturers and importers would need more guidance regarding what
chemicals to consider hazardous. Thus OSHA included provisions in the
hazard determination paragraph that established certain chemicals as
being hazardous. Chemical manufacturers and importers still had to
complete a hazard evaluation and determination of what hazards were
posed, but for these designated chemicals, there was no decision to be
made as to whether they were hazardous or not. These chemicals were
considered to be a "floor" of chemicals covered by the rule, and
included those for which OSHA has permissible exposure limits in 29 CFR
Part 1910, as well as those for which the American Conference of
Governmental Industrial Hygienists (ACGIH) has recommended Threshold
Limit Values (TLVs). In addition, given that carcinogenicity was the
most controversial and difficult health effect to address, OSHA
indicated that, at a minimum, chemicals found to be carcinogenic in the
National Toxicology Program's biennial Report on Carcinogens (RoC), or
in monographs published by the International Agency for Research on
Cancer, were to be considered to be carcinogens in addition to those
regulated by OSHA as carcinogens.
The current HCS also includes provisions regarding hazard
determinations for mixtures. 29 CFR 1910.1200(d)(5). Where such
mixtures have been tested to determine their hazardous effects, the
data on the mixture as a whole are used. Where testing has not been
done, OSHA promulgated an approach based on the percentage of a
hazardous chemical in a mixture to determine if the mixture is
hazardous. Therefore, if a mixture contains one percent (by weight or
volume) or more of a chemical determined to present a health hazard,
the mixture is assumed to have the same effect. The one exception is
carcinogens--a mixture is considered to be carcinogenic if it contains
0.1% or more of a chemical found to be carcinogenic.
In all cases, a mixture will still be considered to be hazardous if
there is evidence that it poses a health risk when the hazardous
chemical is present in concentrations below the cut-offs. This was
included to ensure that chemicals that can have effects at very low
concentrations, such as sensitizers, will be adequately addressed.
For physical hazards, the evaluator must determine based on
whatever objective evidence is available whether the hazardous effect
is still possible in smaller concentrations. This recognizes that, for
physical effects, such a determination may be made based on factors
such as dilution, and there are readily available means to make an
appropriate assessment.
The approach in the current HCS is considered to be a self-
classification system. In other words, the chemical manufacturer or
importer reviews the available information, and makes the determination
as to whether the product presents a potential hazardous effect. This
is different than some other systems where the regulatory authority
makes the determination, and publishes a list of hazardous chemicals
that must be used by the chemical manufacturer or importer.
The hazard determination is to be completed based on available
information. The current HCS does not require testing of chemicals to
produce information where it is not available.
The hazard determination approach in the current HCS recognizes
that information about chemicals changes, new chemicals are introduced,
others cease to be used--in other words, the world of chemicals in the
workplace changes constantly, and the standard is designed to ensure
that employees receive the most up-to-date information available
regarding the chemicals to which they are currently being exposed.
Employers who simply use chemicals, rather than producing or
importing them, are permitted to rely on the information received from
their suppliers. 29 CFR 1910.1200(d)(1). This downstream flow of
information recognizes that the chemical manufacturers and importers
have access to information about the chemicals they sell that is not
available to those who only use them. It also reduces duplication of
effort by focusing the hazard determination process at the source,
rather than having everyone who uses a chemical trying to complete such
a process.
The current HCS requires chemical manufacturers and importers to
maintain a copy of the procedures they follow to make hazard
determinations. 29 CFR 1910.1200(d)(6). If OSHA finds errors in a label
or SDS, the chemical manufacturer or importer that prepared the
document will be held responsible--not the employer using the chemical.
The hazard determination procedures in the current HCS, including
the definitions and Appendixes A and B, have been in place since the
standard was promulgated in 1983.
Hazard classification under the GHS. The challenge in negotiating
an international approach was to create a system that did not require
frequent changes yet remained current and protective, incorporating the
best parts of the approaches in the existing systems. The GHS embodies
an approach that is very similar to the current HCS in scope and
concept, but builds in additional details and parameters to help to
ensure consistency worldwide. Like the HCS, the GHS approach is based
on a downstream flow of information from suppliers to users; self-
classification; use of available information with no new testing; and a
broad approach to definitions of hazard. The GHS has further refined
the approach to include addressing the degree of severity of the
hazardous effects by assigning categories of hazard within hazard
classes; providing detailed scientific approaches to evaluating the
available data to help ensure that multiple evaluators produce similar
results when classifying hazards; and allowing a broader use of
available data by establishing principles where data can be
extrapolated in situations regarding mixtures. OSHA believes that these
additional provisions in the GHS enhance employee protection in
addition to the benefits of having an internationally harmonized
approach when preparing labels and SDSs.
To accommodate these refinements, and improve protection for
employees exposed to chemicals in the U.S., the final rule modifies the
current HCS as follows. First, paragraph (d) is re-named "hazard
classification" rather than the current "hazard determination." This
re-naming is consistent with the approach and terminology used in the
GHS.
Similarly, final paragraph (d)(1), like the proposal, modifies the
current HCS to indicate that chemical manufacturers and importers are
required to classify the chemicals' health and physical hazards in
accordance with this section. For each chemical, the chemical
manufacturer or importer must determine the hazard classes, and the
category of each class, that apply to the chemical being classified.
Final paragraph (d)(1) allows employers to rely on information
received from suppliers (i.e., chemical manufacturers or importers). In
the final rule, OSHA made two minor changes to the proposed text.
Instead of saying that chemical manufacturers would be required to
classify "their" physical and health hazards, OSHA has replaced
"their" with "the chemicals" for clarification purposes. In
addition, OSHA has added the phrase "where appropriate" to add
clarity that not all hazard classes have more than one category. The
final paragraph (d)(1) now reads as set forth in the regulatory text of
this final rule.
Final paragraph (d)(2), which is identical to the proposal,
similarly modifies the current HCS's terminology regarding
classification. However, the final paragraph also includes
modifications to address the evaluation process and the role of
testing. The paragraph specifically states that evaluation of the
hazards of chemicals requires the evaluator to "identify and consider
the full range of available scientific literature and other evidence
concerning the potential hazards." This is consistent with the current
HCS, but re-emphasizes the responsibility to fully characterize the
hazard of the chemicals. To clarify that available evidence is to be
used, final paragraph (d)(2) specifically states that there is no
requirement to test a chemical to classify its hazards under the
modified provisions--just as there is no such requirement under the
current HCS. Dow Chemical Company (Document ID 0353) suggested
that OSHA revert to the current text of paragraph (d)(2), which simply
referred to Appendix B for the parameters of the hazard determination.
This would not be appropriate since Appendix B no longer exists in its
current form. But OSHA does not believe that what is written in
paragraph (d)(2) is inconsistent with what is currently required in
Appendix B. It is not intended to mean (and does not say) that an
evaluator must identify every "shred" of information as Dow has
indicated in its comment, but rather that the evaluator cannot, for
example, only review acute toxicity data and consider that a complete
evaluation. The extent of the literature search must be what the
reasonably prudent classifier would do to assure themselves that
evidence for the range of hazards covered by the rule has been
identified, and a thorough evaluation has been done of the potential
effects. That is what is required today under the current HCS.
On the other hand, the Styrene Information and Research Center
(SIRC) (Document ID 0361) commented on the same paragraph as
follows:
SIRC supports hazard classification for carcinogenicity and
other endpoints based on a comprehensive assessment of the "full
range of available scientific literature and other evidence
concerning the potential hazards," within a best available science
framework. This approach should provide optimum precision assessing
potential hazards and a sound basis for maintaining a safe and
healthy workplace.
Final paragraph (d)(2) refers to Appendixes A and B for further
information on classification as in the current standard. However, the
Appendixes have been completely changed from the current text. New
Appendix A includes the criteria for classification of health hazards,
and new Appendix B includes the criteria for classification of physical
hazards. These mandatory appendixes have to be used for the hazard
classification process under the revised standard. The Appendixes have
been adopted in the final rule, with some changes as described below.
Reference to these appendixes is also included in final paragraph
(d)(3), which addresses mixtures. Final paragraph (d)(3)(i), like the
proposal, states that chemical manufacturers and importers must follow
the procedures in Appendixes A and B to classify hazards for mixtures
as well as for individual chemicals. Proposed paragraph (d)(3)(ii)
stated that the chemical manufacturer or importer "shall be
responsible for the accuracy of the classification even when relying on
the classifications for individual ingredients received from the
ingredient manufacturers or importers on the safety data sheets." SIRC
expressed reservations about this proposed paragraph (Document ID
0494 Tr. 128-29; See also Document ID 0361). In
commenting on this provision, SIRC said it was uncertain whether this
provision meant that a classifier could rely on the classifications
found in SDSs from the ingredient supplier, or whether the classifier
was required to ensure that the supplier's classification was correct.
It was OSHA's intent in the proposal to clarify that generally
classifiers may rely on the classifications found on the SDSs received
from suppliers. The final rule revises (d)(3)(ii) to state that when
chemical manufacturers and importers are classifying mixtures, they may
rely on the information provided on current safety data sheets of the
individual ingredients, except where the chemical manufacturer or
importer knows, or in the exercise of reasonable diligence should know,
that the safety data sheet misstates or omits required information.
In reconsidering the language proposed, OSHA wanted to ensure that
chemical manufacturers and importers know that, in most cases, they can
continue to rely on their suppliers' SDS information for ingredients
they will be using in formulations. However, where they know
information is incomplete or wrong, they have some responsibility for
ensuring they have the correct information before using it for their
own evaluations.
During implementation of the current HCS, OSHA allowed formulators
of chemicals to develop an SDS by simply providing the SDSs for all the
ingredients rather than compiling a specific SDS for the product. OSHA
does not believe that this practice of providing the SDSs for all the
ingredients is widely pursued, but it will not be permitted under the
final rule. The revisions to the approach to classifying mixtures do
not lend themselves to such a practice. Hazard classification requires
consideration and application of bridging principles based on the
constituents, as well as the application of a formula when there are
multiple ingredients with acute toxicity. These approaches require the
evaluator to determine a classification for the mixture as a whole. In
addition, this practice places more of a burden on the user of the
product to sort out the relevant information for protection of their
employees. The formulator is in a better position to assess the
information and provide what is needed to their customers.
Under the current HCS, paragraph (d)(6) requires chemical
manufacturers, importers, or employers performing hazard determinations
to keep a copy of the procedures they follow in the hazard
determination process. This provision has been deleted in the final
rule because the hazard classification procedures have been specified,
and thus all evaluators are following the same process.
Final paragraph (d) is thus much shorter and less detailed than
paragraph (d) in the existing standard. This is largely due to the
approach in the GHS to include the details regarding classification in
hazard-specific discussions that address both the individual substance
and that substance in mixtures. Given the volume of these criteria, it
appeared to OSHA that presenting the relevant information in mandatory
appendixes was a more efficient way to describe the criteria than
including it all in the primary text of the standard. This is
particularly true for those many employers reading the standard who do
not have to perform hazard classification--the revisions only apply to
chemical manufacturers and importers, unless an employer chooses not to
rely on information received from them.
The GHS criteria. A number of commenters expressed their general
support for the GHS criteria, and agreed that the criteria will result
in thorough, harmonized hazard evaluations (See, e.g., Document ID
0329, 0330, 0335, 0339, 0370, 0375, and 0389). In adopting the
GHS approach, the final rule deletes from the hazard classification
requirements the "floor" of hazardous chemicals described above--
established lists of chemicals that are considered hazardous under the
HCS in all situations. In addition, OSHA deleted the across-the-board
"one study" rule described above, wherein one good scientific study
established that a substance is a hazard. However, the one-study
approach is still included in some of the criteria in the GHS, and thus
in the revised OSHA rule.
With the detailed criteria, and the weight of evidence approach in
the GHS, OSHA indicated in the NPRM that it appeared to no longer be
necessary to have such a floor or the one study rule. Many commenters
agreed with OSHA (See, e.g., Document ID 0313, 0327, 0328,
0336, 0338, 0339, 0344, 0351, 0361, 0363, 0365, 0367, 0370, 0371, 0375,
0376, 0377, 0379, 0381, 0382, 0383, 0393, 0399, 0405, 0408, and 0410).
For example, the Alliance of Hazardous Materials Professionals
(Document ID 0327) indicated:
Elimination of the "floor" definition of hazardous (as
consistent with the GHS) would require producers and users to more
closely examine the properties of the materials they produce or
handle. While this would increase the effort necessary to determine
that some substances are hazardous, it would also force a more
careful examination of the underlying reasons that the substance is
hazardous.
There were few comments that questioned taking the floor out of the
requirements given the detailed nature of the criteria to evaluate
hazards. It was noted that the lack of a floor may result in some
inconsistencies in evaluations (Document ID 0352). There were
also some concerns about removing IARC and NTP as sources to evaluate
chemicals (Document ID 0321). Conversely, others supported
elimination of these resources because inclusion violated the Data
Quality Act (Document ID 0417)--a conclusion that OSHA does
not believe is accurate. Evaluation of carcinogens will be
addressed further below. OSHA has not included a "floor" of hazardous
chemicals in the final standard.
As OSHA indicated in the proposed rule (74 FR 50282, Sept. 30,
2009), the Agency planned to adopt all of the health and physical
hazard classes in the GHS, but not all of the hazard categories. In
keeping with its intent to maintain the scope of coverage of the
existing rule to the extent possible, as well as to be as consistent as
possible with the scope of the European implementation of the GHS, OSHA
did not propose to adopt Acute Toxicity, Category 5; Skin Corrosion/
Irritation, Category 3; and Aspiration Hazard, Category 2.
Many commenters agreed that the categories selected in the proposal
were appropriate (See, e.g., Document ID 0313, 0327, 0329,
0330, 0338, 0344, 0351, 0353, 0365, 0367, 0370, 0376, 0377, 0379, 0381,
0382, 0383, 0393, 0399, 0402, 0408, and 0410), although there were some
who thought all hazard categories should be adopted to be completely
consistent with the GHS (See, e.g., Document ID 0328, 0335,
0336, and 0339). There were other comments that supported streamlining
the document by omitting the guidance portions of the GHS (Document ID
0328, 0399, and 0408); stated that the goal should be
harmonization with trading partners, so if they exclude categories,
OSHA should exclude them too (Document ID 0335 and 0389); or
indicated that OSHA should accept labels and SDSs that include the
excluded hazard categories (Document ID 0328, 0379, and 0405).
OSHA indicated in the NPRM (74 FR 50383, Sept. 30, 2009) that
additional information could be included on labels and SDSs in any
event, and that is the position in the final rule as well. (See (g)(2);
Appendix C.3.)
While the decision logics for the health and physical hazard
criteria were omitted from the regulatory text, OSHA indicated that it
would consider publishing them as guidance. Commenters agreed with this
concept (See, e.g., Document ID 0344, 0351, 0370, 0381, 0410,
and 0453). It was further suggested that the diagrams be made simple so
all workers can understand them (Document ID 0336). The
decision logics are already part of the GHS, and are graphic
representations of the process of determining each type of hazard. As
such, they are tools for preparers of labels and SDSs, rather than for
exposed workers. Another comment was that public comment should be
sought on the decision logics before publishing them (Document ID
0379). Given that they are already part of the agreed text of
the GHS, and are guidance, OSHA will make them readily available on the
Agency's Web page.
There were also comments that OSHA should publish guidance on its
interpretation of criteria application, and indicate whether it agrees
or disagrees with interpretations published by other countries
(Document ID 0382). OSHA is considering many different types
of guidance documents, but has not made final decisions in this regard.
Background on Appendices A and B
The text of Appendixes A and B is the bulk of what was proposed to
be adopted essentially verbatim from the GHS. While some of the
provisions of the GHS have been adopted into the final rule with OSHA-
developed language that is specific to the regulatory system of the
U.S., OSHA has strived in these appendixes to retain the text of the
GHS intact. In order to understand the context of this language, and
OSHA's approach to its inclusion, a brief history of its development is
necessary.
Most people think of the labels and SDSs as the products of the GHS
that are harmonized since they are the system's "output" that are
seen most frequently. But harmonization of these documents cannot occur
unless the underlying criteria are harmonized, and countries adopting
them implement them similarly. The health hazard criteria were
developed in the Organization for Economic Cooperation and Development
(OECD)--an organization of 34 countries that "provides a forum in
which governments can work together to share experiences and seek
solutions to common problems." See www.oecd.org. One of the areas in
which the OECD has long been actively involved is chemicals. As such,
the OECD provides a forum for countries' experts to discuss and resolve
issues of mutual concern. In addition, the OECD works with business,
through the Business and Industry Advisory Committee, and with labor,
through the Trade Union Advisory Committee. Perhaps its most visible
contribution in the area of chemicals is test guidelines to assess the
hazards of chemicals. These test guidelines address many different
health effects; are considered to be scientifically robust, validated
test methods; and are widely used around the world.
It was this expertise and recognition that led to the OECD being
the "focal point" for development of the health hazard criteria. The
OECD also uses a process of consensus to develop their documents,
requiring agreement from all countries to move forward rather than a
simple majority vote. Working on a consensus basis is much more
difficult to accomplish, but is advantageous in other ways since it
helps to ensure that the concerns of all parties are taken into
consideration, and thus are more likely to remain consistent with the
results.
A disadvantage is that the text must satisfy all parties, and thus
it is not always written in the clearest fashion. The text was also
reviewed further when it was submitted to the UN Sub-committee, and
additional editing was done to address concerns. Therefore, it is fair
to say that it was written by expert committees, and reflects the
involvement of many different people and ideas.
The criteria in Appendix B, unlike those in Appendix A, were not
developed "from scratch," but were based on the harmonized criteria
developed to classify the physical hazards of chemicals involved in
transport by the UN Sub-committee of Experts on the Transport of
Dangerous Goods (TDG). The TDG Sub-committee includes many subject
experts in areas such as explosives and flammability. The TDG Sub-
committee and the International Labor Organization (ILO) were jointly
tasked to review the TDG criteria for application to other sectors such
as the workplace. This review not only took advantage of the UN and ILO
expertise, but also created a system that is harmonized with transport
in terms of criteria.
When OSHA developed the proposed rule, it considered editing the
text of the criteria for purposes of improving the language. However,
the trade-off is inconsistency with the GHS, and the potential for
people to believe that OSHA means something different because the text
has been revised. Thus, as noted in the NPRM (74 FR 50392, Sept. 30,
2009), OSHA chose to take the approach of adopting the language as
stated in the GHS. Editing of the criteria focused on what needed to be
changed for purposes of putting it into mandatory regulatory language,
including deleting what was clearly identified as guidance.
Therefore, while we have reviewed every suggestion that was made to
the text of the Appendixes, our general approach was not to make
changes unless they were truly necessary. Editorial changes for
purposes of clarification are more appropriately made through the UN
Sub-committee process, and OSHA participates actively in that activity,
and chairs the primary correspondence group. Those changes that were
suggested that OSHA believes have merit in terms of clarifying
provisions will be worked through this correspondence group so the UN
Sub-committee can make the changes. Then OSHA will adopt them into the
revised standard through rulemaking processes discussed elsewhere in
this preamble. To avoid giving this same response repeatedly, OSHA will
not be individually addressing the many suggestions for clarifications
in this preamble.
In general, there were very few substantive technical comments
provided on the approaches in the criteria, and OSHA assumes that
reflects the fact that the criteria were developed by technical experts
from countries and stakeholder organizations. There were some
suggestions received that certain parts of Appendix A be withdrawn so
OSHA can consult with toxicologists (Document ID 0353).
Numerous toxicologists and other health professionals from the U.S., as
well as many other countries, have been involved in the development and
review of the text in Appendix A, and it has been subject to extensive
scientific and policy discourse. Furthermore, this rulemaking was also
the opportunity for others who have not been involved to provide input.
If OSHA had received significant comments on the technical aspects of
the criteria that indicated a systemic concern about the criteria, it
may have been cause for reconsideration. But most of the comments that
were received were more reflective of differences on policy positions
than truly technical issues. Therefore, there are relatively few
changes to Appendixes A and B as a result of record input. These
changes are discussed below.
As described in the NPRM and this document, in Appendixes A and B
OSHA has maintained its general approach (supported by stakeholders)
of: (a) Limiting changes to the HCS to those that are required to align
with the GHS; and (b) remaining as consistent with the GHS as possible
within the need to use appropriate regulatory language and maintain or
enhance current protections. OSHA has also remained mindful of the
approaches of its trading partners, although it notes that some
proponents of that principle were quite inconsistent themselves when
using this particular argument. Therefore, while this argument was used
to support choosing higher cut-offs for mixtures, for example, some of
these same commenters also suggested not covering hazard classes or
categories that are both covered by the EU and currently addressed by
OSHA (See, e.g., Document ID 0344, 0381, and 0393). These
comments are addressed below.
Appendix A, Health Hazards. Proposed Appendix A began with an
introduction that includes material related to principles of
classification taken from Chapter 1 of the GHS. These address both
weight of the evidence, and the approach to mixtures. In A.0.3.2, the
proposed text referred to both positive and negative results being
"assembled together." Dow (Document ID 0353) expressed
concern about the implications of the word "assembled." In the final
rule, OSHA has revised this language throughout the chapter to say
"shall be considered together." Dow also commented that in the
discussion regarding acceptable data in A.0.2.2 and A.0.2.3, the text
should refer to "valid" methods, rather than "validated." OSHA does
not agree that this change is warranted. To be "valid" data, the
methods used to produce the data must be validated. In order to clarify
the discussion, OSHA has revised the text by adding two sentences from
the GHS to A.0.2.3 as follows:
Any test that determines hazardous properties, which is
conducted according to recognized scientific principles, can be used
for purposes of a hazard determination for health hazards. Test
conditions need to be standardized so that the results are
reproducible with a given substance, and the standardized test
yields 'valid' data for defining the hazard class of concern.
As mentioned below in the discussion on mixtures, OSHA has also
revised Appendix A to use "cut-offs/concentration limits" everywhere
one of these terms was formerly used in order to be consistent, and
make clear the terms are interchangeable.
The remainder of Appendix A is taken from Chapter 3 of the GHS on
Health Hazards. OSHA has included the specific discussions of all of
the health hazards covered by the HCS in proposed Appendix A, extracted
from Chapter 3 of the GHS. OSHA removed the decision logics that are in
the GHS from the criteria, and is considering including them in a
guidance document to be made available at the time the final rule is
published. As discussed above, stakeholders generally supported this
approach. The hazard communication portions of the criteria chapters
have also been removed since all of this information is already
available in Appendix C and would thus be duplicative. In addition,
edits have been made where OSHA is not adopting all of the categories
of a particular hazard class.
The chapters on Skin Corrosion/Irritation (Chapter A.2) and Serious
Eye Damage/Irritation (Chapter A.3) have been modified more extensively
than the other chapters on health hazards in the GHS. In these
chapters, the GHS leads the evaluator to conduct additional testing on
the chemical when information is not available. While the GHS does not
require such testing, the criteria for these effects imply that it
should be conducted to complete an evaluation. The HCS is based solely
on available information, and no testing is ever required. Therefore,
OSHA has modified these chapters to eliminate any references to
additional testing and limit the evaluation to what is known based on
available information. It should be noted that the UNSCEGHS has
initiated work to edit these chapters and make them easier to follow.
OSHA will continue to participate in this activity.
Coverage of Mixtures
The coverage of mixtures in terms of health hazards is addressed in
two places in the revised rule. First, general principles that apply to
multiple effects are addressed in the introductory part of Appendix A
in Chapter A.0, "General Classification Considerations." Second, each
hazard class discussion includes the criteria for classifying a
substance or a mixture. Unlike the current HCS, which defines across-
the-board percentage cut-offs for all health hazard classes, the GHS
employs a tiered approach to classification. Like the HCS,
classification would be based on test data for a mixture as a whole for
most hazard classes where it is available. However, where it is not
available, but there are data on ingredients and similar mixtures, the
GHS allows extrapolation or bridging of data to classify a mixture.
This allows greater use of available data before resorting to a
percentage cut-off or similar approach. Where such data are not
available, the criteria address how to classify mixtures based on cut-
offs specific to that hazard. In the case of acute toxicity, this
includes calculations based on the acute toxicity of each ingredient in
the mixture.
The tiered scheme is somewhat different for certain hazard classes.
As described, usually the evaluation is based first on test data
available on the complete mixture, followed by the applicable bridging
principles and, lastly, cut-offs/concentration limits or additivity.
The criteria for Germ Cell Mutagenicity, Carcinogenicity, and
Reproductive Toxicity take a different approach by considering the cut-
off levels as the primary tier and allowing the classification to be
modified on a case-by-case basis based on available test data for the
mixture as a whole. This approach is related to the sensitivity of
available test methods to detect these types of effects at small
concentrations in the mixture as a whole.
The approach to mixture classification may result in some mixtures
that are currently considered to pose a particular hazard not being so
classified under the GHS. OSHA believes that the protections of the GHS
approach are appropriate, and that these changes will not result in an
inappropriate reduction in protection. For example, if there is a
mixture that is comprised of 1% of an acutely toxic material,
regardless of the severity of that effect, and 99% water, the current
HCS would require that mixture to be considered acutely toxic. Under
the GHS, it is unlikely to be considered as such. Based on the dilution
effect of the water, the acute toxicity is no longer a concern. Thus
the bridging principles under the GHS allow for a more accurate
assessment of the potential harm of the mixture, whereas the strict
cut-off approach under the current HCS may provide hazard information
in cases where the exposure is minimal and the occurrence of an adverse
effect is unlikely. In the example described, the presence of the water
in the mixture as used by the workers reduces the potential for
exposure to the hazardous ingredient to such a small amount that no
effect is expected to result. The GHS approach is not as simple to
apply as the current HCS, but the resulting approximation of the
hazards of the mixture will be more accurate.
The GHS uses both the term "cut-off" (which is what is used in
the current HCS), and "concentration limit" (which is used in the EU
requirements). The terms are used interchangeably and often appear
together (i.e., cut-offs/concentration limits). Several commenters
indicated that OSHA should define these terms (Document ID
0344, 0381, and 0393). There are no definitions in the GHS
since the terms are self-evident when viewed in the context of how they
are used. OSHA does not believe that definitions are needed for these
terms. However, Appendix A has been reviewed to make sure the terms are
both used consistently throughout the Appendix. The GHS was also
reviewed, and it appears the terms are not necessarily used
consistently in that text.
Several commenters indicated that language in A.0.5.1.1(a), in the
bridging principle that addresses dilution, was inappropriately changed
from "may" to "shall" in the NPRM (See, e.g., Document ID
0344, 0381, 0382, and 0393). OSHA changed the language to
track the mandatory nature of the provision when present in a standard
versus a non-mandatory recommendation such as the GHS. Therefore, the
language remains as "shall" in the final rule.
In another part of the bridging principles, the term "commercial
product" is used in the GHS, and was thus used by OSHA in the NPRM
(A.0.5.1.2). Commenters asked that this term be defined (Document ID
0344 and 0381). OSHA reviewed the text, and has changed the
term to "mixture" instead of "commercial product". This is
accurate, and the term is already defined.
There are several hazard classes in the GHS that give competent
authorities such as OSHA a choice of cut-offs/concentration limits to
apply when classifying a mixture containing ingredients that pose these
effects (e.g., reproductive toxicity, sensitization, target organ
effects). The reason the GHS includes a choice of cut-offs to trigger
label disclosure is that countries involved in the negotiations on
mixtures had different views on the issue that could not be resolved.
All countries agreed to use the lower of the two cut-offs for SDSs, so
information will be provided consistently for those documents in all
cases. But for labels, some countries had what were described as
"downstream consequences" that were linked to label disclosures, and
therefore did not want to adopt the lower level and trigger those
consequences (e.g., banning the use of the chemical for consumer
products).
In North America, Canada and the U.S. do not have such consequences
linked to label statements, and their requirements are based on giving
workers the right-to-know about the hazards and identities of the
chemicals in their workplaces. Additionally, Canada has the lower cut-
offs in most cases in their current requirements, and OSHA already has
the 0.1% cut-off for carcinogenicity. Adoption of the lower cut-offs
for both labels and SDSs was supported by both Canada and the U.S. from
the outset.
As has been described, OSHA has used consistent cut-offs for
purposes of hazard determination for mixtures since the HCS was
promulgated in 1983. OSHA described the proposal as follows in the 1983
final rule preamble (48 FR 53290, Nov. 25, 1983):
The rationale of the proposal was that when the hazard of a
mixture is unknown, all hazardous ingredients should be indicated on
the material safety data sheet. The user would then have the most
complete information available to predict the potential hazards of
the mixture. The one percent exclusion was included to absolve the
employer from having to evaluate and list chemicals in small
quantities, which are not likely to result in substantial exposures.
In the 1982 proposal, the one percent cut-off would have applied to
all health and physical hazards. As a result of the comments submitted
to the record, OSHA took a different approach to physical hazards in
the final rule (no percentage cut-off applies to physical hazards), and
also lowered the cut-off for carcinogenicity to 0.1 percent. In
addition, a provision that required inclusion of chemicals below these
cut-offs in certain situations was also part of the 1983 final rule.
In proposing the one percent cut-off, OSHA noted that "there was
no scientifically correct delineation, but that the one percent cut-off
is apparently considered reasonable by a number of parties" (47 FR
12102, Mar. 19, 1982). OSHA's intent was "to absolve the employer from
having to evaluate and list chemicals present in mixtures in small
quantities, which are not likely to result in substantial exposures"
(48 FR 53290, Nov. 25, 1983). These cut-offs were practical
accommodations, had been used in other regulatory settings (See, e.g.,
29 CFR 1910.1003(a)(2), 13 Carcinogens), and in the 1983 final rule
were accompanied by a provision that also covered those situations
where the cut-offs were too high for protection purposes. Science
regarding potential health hazards in the workplace does not provide
evidence that would allow the Agency to draw a bright line to indicate
specific concentrations of a chemical in a mixture are, or are not, a
potential hazard to workers. Therefore, the establishment of such cut-
off levels is a policy decision based on scientific considerations, as
well as concerns regarding practicality and utility, but not on studies
that can be linked to a particular level for each type of health
effect.
That being said, however, the scientific knowledge about these
health effects has increased significantly since the HCS was first
adopted, as has the concern about their occurrence in the work force.
At that time, carcinogenicity was the primary concern in terms of
chronic and/or significant health effects, and this concern was
reflected in the lower cut-off value adopted by OSHA for that effect.
Most of OSHA's substance-specific rulemakings were done for the purpose
of addressing carcinogenicity. Now, however, there is more evidence
that raises significant concerns about other types of effects.
Sensitization is a key example. Respiratory sensitization leads to
asthma, and substantial evidence has
developed over the last few decades showing this effect is of
increasing concern. For example, a study by Frazier et al. (2001,
Document ID 0587) notes that the incidence of occupational
asthma has increased by 50% over the last two decades, and that
population-based surveys have reported that 5% to 21% of asthma cases
are caused or exacerbated by occupational exposure. The authors
extrapolated this to the estimated 12 million adults who have asthma in
the U.S., and concluded that this suggested that between 500,000 and
2.5 million Americans had occupational asthma. This study was published
in 2001, and the numbers are likely to be larger today. The study also
examined SDSs for chemicals containing toluene diisocyanate, a known
respiratory sensitizer, and found only half the SDSs noted asthma as a
potential health effect, and one in four noted neither asthma nor
respiratory sensitization effects. Other studies have also examined the
increasing concerns about occupational asthma (Document ID
0588, 0591, 0592, and 0593).
Further, the most recent science shows that respiratory and skin
sensitization can be caused at very low concentrations. A 2006 paper by
Arts et al. summarizes human and animal studies on skin and respiratory
sensitizers, and finds that sensitization effects often result from
exposures to chemicals at concentrations below 1% in studied
populations (Document ID 0593). Likewise, the World Health
Organization's report, "Skin Sensitization in Chemical Risk
Assessment," also reports positive results for skin sensitization well
below the 1% cut-off used by the current HCS (Document ID
0586). Moreover, once an individual is sensitized, a response
can be triggered at even lower levels than those required initially to
induce sensitization (Document ID 0585 and 0593). OSHA has
often used sensitizers as an example of why SDS preparers need to
consider whether information should be provided below the 1% cut-off.
For example, in OSHA's compliance directive for the HCS (CPL 02-02-
038), the following guidance is given:
If the components of a mixture could be released in
concentrations which would exceed an OSHA PEL, an ACGIH TLV, or
could present a health risk to employees, information on these
components must be included on the MSDS regardless if their final
concentration in the mixture is less than 1% (or 0.1% for
carcinogens). For instance, TDI is a sensitizer at very small
concentrations and despite its low concentration in a mixture, can
be offgassed in quantities which may present a health risk that must
be noted on the MSDS.
But sensitization is not the only effect of concern. Reproductive
toxicity is a serious hazard that includes both fertility and effects
on the offspring. Recent research concerning endocrine disruptors
suggests that these chemicals can have adverse reproductive effects at
very low levels (Document ID 0583, 0584, and 626). Likewise,
occupational disease mortality and morbidity statistics indicate a
number of cases related to target organ effects as well (Document ID
0291, e.g., heart disease and renal effects).
OSHA proposed to use the most protective of the GHS concentration
limits for these hazard classes. For sensitizers and reproductive
toxins, the final rule requires information to be provided on labels
and safety data sheets at concentrations above 0.1%. Other countries
may choose to only provide the information on SDSs when the
concentration is higher. However, as indicated, these particular health
effects are among the most significant to employees, and OSHA believes
the provision of information on labels will help both employers and
employees ensure that appropriate protective measures are followed. (On
the other hand, it should be noted that OSHA was persuaded that the
current 1% cut-off may be too conservative for many acute toxins and
Category 3 Single Target Organ Toxicants, and the final rule is likely
to result in fewer mixtures being covered for these effects than under
the current approach.)
In addition to concerns regarding protection for these health
effects, there is also a concern about the communication difficulties
of having different hazard information on a label versus a safety data
sheet. As indicated, the GHS negotiators agreed that all countries
would use the lower levels in the criteria for providing information on
SDSs. Using a different cut-off for labels would create a situation
where there may be hazards on the SDS that do not appear on a label.
This inconsistency makes training more difficult, and creates confusion
for downstream employers as well when they are deciding about
appropriate protective measures. Under the current rule, the mixture
cut-offs apply to both the label and the SDS. Several commenters
indicated that OSHA should provide guidance indicating specific
threshold cut-offs (Document ID 0344, 0381, and 0399). The
table below indicates what the cut-offs are for different health
hazards. These commenters also suggested OSHA provide guidance on
opting out of the cut-offs if data override the threshold. This is
already addressed in A.0.4.3.2 (if the classifier has information that
the hazard of an ingredient will be evident (i.e., it presents a health
risk) below the specified cut-off/concentration limit, the mixture
containing that ingredient shall be classified accordingly). A.0.4.3.3
also allows the cut-off/concentration limit to be higher in exceptional
cases. The evaluator must have conclusive data demonstrating that the
hazard of an ingredient will not present a health risk. OSHA
anticipates that the criteria of A.0.4.3.3 would rarely permit this
approach to be used.
Table XIII-1
------------------------------------------------------------------------
Hazard class Label cut-offs SDS cut-offs
------------------------------------------------------------------------
Respiratory/Skin sensitization.... >=0.1% >=0.1%
Germ cell mutagenicity (Category >=0.1% >=0.1%
1)...............................
Germ cell mutagenicity (Category >=1.0% >=1.0%
2)...............................
Carcinogenicity................... >=0.1% >=0.1%
Reproductive toxicity............. >=0.1% >=0.1%
Specific target organ toxicity >=1.0% >=1.0%
(single exposure)................
Specific target organ toxicity >=1.0% >=1.0%
(repeated exposure)..............
Specific target organ toxicity >20% >20%
Category 3.......................
------------------------------------------------------------------------
During the hearing, worker representatives were asked to comment on
whether consistency between the information on the label and the SDS
was important for worker protection. They all indicated that it was
important. For example, Mr. Platner, who represented the Building and
Construction Trades Department of the AFL-CIO stated
(Document ID 0494 Tr. 25):
Oh, absolutely. An example of a sensitizer that's very common is
isocyanate components or polyurethane spray foams or coatings.
They're potent sensitizers, and that information very rarely gets to
the label. It's usually appropriately in the MSDS, but it rarely
makes it to the label.
Similarly, Mr. Kojola of the AFL-CIO, commented (Document ID
0494 Tr. 33):
Oh, absolutely. What it does is it provides a consistent message
that workers are getting both in labels and on safety data sheets.
And I think it enhanced the ability to, for example, translate that
information into other languages, so I think that alone is a major
step forward in enhancing worker protection.
Some commenters argued that OSHA should adopt the higher cut-off
levels where given a choice by the GHS (Document ID 0344,
0361, 0367, 0371, 0376, 0381, 0392, and 0393). They questioned whether
there was a scientific justification for the lower levels, and
suggested that the U.S. should harmonize with the EU approach.
As OSHA described above, there are two primary reasons for the
lower levels. First, OSHA believes it is important for effective
communication to have the same hazards on the label and SDS to as great
a degree as possible. Labels are in an employee's work area, and thus
provide the most immediate source of information. While SDSs must be
available, they are longer and more complicated, and workers are less
likely to review them on a regular basis. For downstream employers, it
is also important to maintain consistency and reduce confusion where
possible by having the information on hazards the same on the label and
SDS.
Secondly, as discussed above, increased knowledge of these health
effects in the scientific literature, as well as studies indicating
that they are often not reported when they should be, or the
information is lacking, has led OSHA to the conclusion that
communication at the lower levels is appropriate and necessary for
worker protection. It is particularly critical in the area of
sensitizers since the incidence of occupational asthma is increasing,
and sensitization can occur at lower levels as it progresses. But with
the advent of information on effects like those of endocrine
disruptors, and the increased awareness of the possible effects of low
levels of exposure, it is necessary for all of these effects.
As for the argument regarding consistency with the EU, OSHA has
sought to be consistent where possible. However, the EU has a different
regulatory structure for dealing with these effects downstream, and
what is appropriate for their classification and labeling system is not
necessarily appropriate for ours in the U.S. (See, e.g.,
http://ec.europa.eu/environment/chemicals/dansub/pdfs/30_atp.pdf: "Under
Directive 76/769/EEC on the restrictions of certain dangerous
substances and preparations, the Commission is, in principle, obliged
(within six months of the publication of the classification) to propose
a ban on their placing on the market and use by consumers as substances
or in preparations (above specified concentrations).")
There are relatively few chemicals for which there are data
indicating the types of effects of concern with regard to these lower
cut-offs (e.g., sensitizers), and fewer still that would fall into the
range between the lower and higher cut-offs (e.g., between 0.1% and
0.3% for reproductive toxicity). Furthermore, as suggested in one
comment, disclosing at different levels on labels versus SDSs may
actually create a product liability issue under U.S. law that would
argue against taking such an approach (Document ID 0353).
While product liability is not one of the issues that influenced OSHA's
decision-making, it may be important to these commenters in the future.
The American Chemistry Council asked during the hearing why OSHA
adopted the cut-off levels 25 years ago if the Agency thought they
weren't protective, or whether there is information to indicate that
they have not been protective (Document ID 0494 Tr. 174). In
response to questions from OSHA as to what the scientific basis would
be for communicating a hazard on an SDS and not a label, they responded
(Document ID 0494 Tr. 177): "A scientific basis? Well, most
of these are obligatory regulatory cut-offs for mixtures. There really
is not much scientific basis for any of the mixture cut-offs." In
other words, ACC concedes that there is also no scientific basis for
the higher cut-offs it advocates--rather the EU cut-offs are simply
policy choices made by a different authority with a distinct regulatory
structure. As described previously, OSHA believes there is evidence
that these cut-offs are no longer sufficiently protective in light of
additional information developed since the HCS was adopted in 1983.
Furthermore, having inconsistencies in information on a label versus a
safety data sheet impacts the effectiveness of the communication to
workers and downstream employers. The cut-offs/concentration levels in
the final rule are the same as proposed, and are the lower levels of
those the GHS allows countries to choose from when implementing.
The Styrene Information and Research Center (SIRC) argues that OSHA
may not lower the mixture cut-off thresholds for sensitizers and
reproductive toxicants without establishing that a significant risk
exists at that lower threshold (Document ID 0361, 0467, and
0642). OSHA disagrees.
As discussed in Section V, Pertinent Legal Authority, OSHA has
found that inadequate hazard communication creates a significant risk
and that the final rule will reduce that risk. Contrary to what the
SIRC says, OSHA need not support each requirement in a standard with
its own significant risk finding. Public Citizen Health Research Group
v. Tyson, 796 F.2d 1479, 1502 n. 16 (D.C. Cir. 1986). Indeed, when the
Supreme Court first construed the OSH Act as imposing a significant
risk requirement, it spoke in terms of the Agency making findings about
unsafe workplaces, not individual hazards. Benzene, 448 U.S. at 642
("before promulgating any standard, the Secretary must make a finding
that the workplaces in question are not safe [and] * * * a workplace
can hardly be considered 'unsafe' unless it threatens the workers with
a significant risk of harm"). See also, for example, id. (framing the
"significant risk" requirement as requiring OSHA "to make a
threshold finding that a place of employment is unsafe--in the sense
that significant risks are present and can be eliminated or lessened by
a change in practices."); Texas Indep. Ginners Ass'n v. Marshall, 630
F.2d 398, 400 (5th Cir. 1980) ("The Supreme Court recently ruled that
the Act requires OSHA to provide substantial evidence that a
significant risk of harm arises from a workplace or employment.").
Moreover, courts have held that the OSH Act does not require the
disaggregation of significant risk analyses along other lines. See, for
example, Lockout/Tagout II, 37 F.3d at 670 (upholding OSHA's decision
not to conduct individual significant risk analyses for various
affected industries); American Dental Ass'n v. Martin, 984 F.2d 823,
827 (7th Cir. 1993) (OSHA is not required to evaluate risk "workplace
by workplace"); Associated Builders and Contractors, Inc. v. Brock,
862 F.2d 63, 68 (3d Cir. 1988) ("the significant risk requirement must
of necessity be satisfied by a general finding concerning all
potentially covered industries").
Indeed, a contrary rule would impose an unworkable burden on OSHA.
As the Third Circuit held Associated Builders and Contractors, Inc. v.
Brock, 862 F.2d 63 (3rd Cir. 1988), stating:
The holdings in USWA I and USWA II sustained a general
significant risk finding. Assuming, however, that those opinions
were construed as leaving open the significant risk issue, as
presently presented, the outcome would be no different. This
rulemaking proceeding produced a performance-oriented information
disclosure standard covering thousands of chemical substances used
in numerous industries. For such a standard the significant risk
requirement must of necessity be satisfied by a general finding
concerning all potentially covered industries. A requirement that
the Secretary assess risk to workers and need for disclosure with
respect to each substance in each industry would effectively cripple
OSHA's performance of the duty imposed on it by 29 U.S.C. Sec.
655(b)(5); a duty to protect all employees, to the maximum extent
feasible.
Id. at 68. Thus, OSHA need not make the sort of significant risk
finding suggested by SIRC.
Rather, once OSHA makes a general significant risk finding in
support of a standard, the next question is whether a particular
standard's requirements are reasonably related to the purpose of the
standard as a whole. Asbestos Information Ass'n/N. Am. v. Reich, 117
F.3d 891, 894 (5th Cir. 1997); Forging Indust. Ass'n v. Secretary of
Labor, 773 F.2d 1436, 1447 (4th Cir. 1985); United Steelworkers of Am.,
AFL-CIO-CLC v. Marshall, 647 F. 2d 1189, 1237-38 (D.C. Cir. 1980). The
use of a threshold to govern when the standard applies is reasonably
related to the purposes of hazard communication. It limits
communication to those situations in which a chemical is present in
sufficient quantities that workers might experience substantial
exposures to its hazards. Hazard communication can be undermined just
as much by overcommunication of risks as by undercommunication. An
avalanche of information about less significant hazards on a label or
SDS could obscure important information on substantial hazards faced by
the worker. Thresholds also save manufacturers and importers the burden
of evaluating and listing chemicals present in only small quantities
and not likely to result in substantial exposures (48 FR 53280, 53290
(Nov. 25, 1983). And as noted above, OSHA has provided a justification
for the lower levels challenged by the Styrene Institute and Research
Center: chemicals presenting these hazards may be especially hazardous
at low levels, and the potential effects are of high concern.
In addition, SIRC seems to challenge only the reduction of the
threshold for disclosure on labels, not the identical reduction of the
threshold for disclosing the hazard on SDS for these hazards. Under the
final rule, the same information for sensitizers and reproductive
toxicants must appear on both the label and the SDS, avoiding the
potential for confusion. The reproductive toxicant and sensitizer cut-
offs are reasonably related to the purposes of the Hazard Communication
Standard.
The courts have upheld similar requirements even in the absence of
a significant risk finding, provided the requirements were reasonable.
In National Cottonseed Products Ass'n v. Brock, 825 F.2d 482, 487 (D.C.
Cir. 1987), the court upheld medical monitoring for cottonseed workers
where OSHA found no significant risk. OSHA had eliminated the PEL but
imposed the monitoring as a "backstop" to the "no significant risk"
determination, and the court upheld the monitoring requirement because
the "evidence indicates that there is a real possibility of
significant health risks" where no PEL was imposed. Likewise, in
National Mining Ass'n v. MSHA, 116 F.3d 520, 527-28 (D.C. Cir. 1997),
the court upheld MSHA's decision to require oxygen at a 19.5% level,
even though the evidence only showed that adverse worker effects were
experienced at a lower level of 18%. The proper minimum oxygen level
was "a technical decision entrusted to the expertise of the agency,"
which was "entitled to 'err' on the side of overprotection." Id. at
528. And in Public Citizen, the court upheld a requirement to post
signs to warn employees of the hazards presented by ethylene oxide
exposures without a separate significant risk determination, noting
that signs and labels were specifically contemplated by section 6(b)(7)
of the OSH Act and a "reasonably necessary and appropriate" part of a
standard. 796 F.2d at 1502 n.16.
As explained in the Pertinent Legal Authorities section, the
mixture cut-off levels are part of the HCS's general approach of
providing prophylaxis against the exposure to significant risks,
similar to the medical monitoring requirement in National Cottonseed,
the higher oxygen level requirement in National Mining Ass'n, and the
sign requirement of Public Citizen. The mixture cut-off thresholds are
supported by substantial evidence, as discussed above and, therefore,
authorized by the Act.
A related issue is the cut-off in Category 3 of Specific Target
Organ Toxicity, both in Single Exposure and Repeat Exposure. Under the
GHS, a cut-off/concentration limit of 20% is suggested as guidance. It
is an additive cut-off, meaning that the percentages of the ingredients
that meet the definition for Category 3 would be added together and
compared to the cut-off. Consistent with other revisions to the GHS
language that are appropriate for a mandatory standard versus a non-
mandatory recommendation, OSHA proposed to make the 20% cut-off
mandatory, but requested comment on it. (74 FR 50282, Sept. 30, 2009;
see also A.8.3.4.5 and A.9.3.4.4.) A limit that is not mandatory will
be difficult for chemical manufacturers to know how to comply with, and
it will also be difficult for OSHA to enforce. Furthermore, OSHA views
this provision as relaxing the current requirement, which is a cut-off
of one percent for each of the ingredients in the mixture that are in
and of themselves hazardous. However, consistent with A.0.4.3.2, if the
classifier has information that the hazard will be evident below the
specified concentration limit, the mixture is to be classified
accordingly. Therefore, where the 20% is too high, the classifier will
nevertheless be required to classify it appropriately below that level.
There were a number of commenters who supported making the 20%
level mandatory, suggesting that it was reasonable for the U.S.,
promoted consistency, and that the level could be lower if data warrant
(See, e.g., Document ID 0313, 0324, 0327, 0329, 0330, 0338,
0339, 0353, 0365, 0381, 0410, and 0412). Others did not agree (Document
ID 0323, 0328, 0344, 0376, 0379, 0382, 0393, 0399, and 0405).
Some of these commenters suggested that OSHA should provide data to
support making it mandatory. The GHS is drafted in voluntary terms, but
the HCS is a mandatory standard, meaning that all of its provisions are
mandatory as well. OSHA is unaware of specific data one way or the
other on the question, but notes that this is a significant relaxation
of the applicable cut-off under the current rule. Given the minor
hazard presented by these chemicals, OSHA believes the 20% cut-off is
appropriate to guard against overwarning. Because no alternatives were
presented (other than making the provision voluntary, which is not an
acceptable solution), OSHA has included the mandatory requirement in
the final rule. Again, as noted above, chemical manufacturers or
importers are still required to classify mixtures at lower
concentrations if they have evidence that it presents a hazard, so OSHA
does not believe the final rule is less protective.
Acute toxicity. In Appendix A, Chapter A.1 ("Acute Toxicity"),
OSHA proposed to adopt GHS Categories 1 through 4, but not 5. The
current coverage of the HCS is greater than Category 3 of the GHS, but does not
include all of Category 4. If OSHA were to adopt only three categories,
it would reduce protections with regard to acute toxicity. Adopting
Category 4 expands coverage somewhat. However, chemicals meeting the
definition of Category 4 are already covered under the national
consensus standard on labeling that many chemical manufacturers already
follow (ANSI Z129). In addition, the EU covered them under their
previous classification, packaging, and labeling of dangerous
substances (Directive 67/548/EEC) and preparations (Directive 1999/45/
EC) directives, and their adopted GHS provisions. These countries
comprise the largest trading partner in chemicals for the U.S. Thus,
many manufacturers are already classifying their chemicals as acutely
toxic to comply with European requirements.
Adopting Category 5 would not only expand coverage significantly,
it would lead to inconsistency with Europe and with the current
national consensus standard. OSHA also believes that exposures of this
magnitude are not likely to be encountered in the occupational setting,
and that such coverage would be excessive.
Since OSHA raised this issue for comment in the ANPR, a number of
respondents specifically addressed acute toxicity. The responses
varied, although a number supported the approach proposed to cover
through Category 4 (Document ID 0021, 0046, 0047, 0077, 0104,
0123, 0135, 0145, 0155, 0163, and 0171). For example, Dow (Document ID
0047) stated:
Dow believes that OSHA should adopt all health hazard criteria
and categories, except Acute Toxicity Category 5. While this
category may be useful for characterizing consumer products, its use
with the substances characterized under the HCS would be confusing
and unnecessary. Dow understands that the EU and Australia have both
chosen not to include Acute Toxicity Category 5 in their
implementation of the GHS and that Canada is currently considering
doing the same. Dow believes that the U.S. should be consistent with
these other major trading partners by not including this category
when it adopts the GHS.
Others suggested that OSHA propose to adopt Categories 1 through 3
(Document ID 0034, 0128, and 0141). Some argued that all
categories should be adopted to ensure harmonization (See, e.g.,
Document ID 0018, 0036, 0050, 0078, 0106, and 0116).
OSHA believes that coverage provided by Categories 1 through 4 is
appropriately protective for the workplace, and leads to the greatest
harmonization with workplace authorities in other countries. With
regard to coverage provided by Category 5, OSHA does not preclude
inclusion of information on Category 5 on the label or the SDS. Thus
chemical manufacturers or importers who wish to have one label that
suffices for the workplace and the consumer sector, for example, could
do that and still be in compliance with the HCS. As noted earlier,
commenters on the NPRM supported the categories chosen by OSHA, except
for a few who thought OSHA should adopt all categories in the GHS to
promote complete harmonization. However, OSHA believes that this
concern is addressed by permitting such categories to be addressed on
labels and SDSs with no penalty.
OSHA did not propose to adopt Category 5. The final standard does
not adopt Category 5, nor include it in Table A.1.1, which describes
the criteria for acute toxicity. However, calculations for the acute
toxicity of mixtures that are comprised of one or more ingredients that
fall into Category 5 must include the acute toxicity estimate for the
Category 5 ingredients. Proposed Paragraph A.1.3.6.1(a) indicated that
the calculation of the acute toxicity of mixtures would "[i]nclude
ingredients with a known acute toxicity, which fall into any of the
acute toxicity categories." This is consistent with the GHS
(Subparagraph 3.1.3.6.1(a)).
As discussed in the Proposal, OSHA believes that the exclusion of
Category 5 from the criteria Table A.1.1 may lead to classifiers
overlooking substances falling into this category in the mixture
calculation, which could result in a higher (less protective)
classification. This could also mean a lack of harmonization within the
U.S. if other Federal agencies adopt Category 5, potentially requiring
inclusion of these data in the calculation. To avoid this situation,
OSHA has clarified the text for the mixture calculation to ensure that
the ingredients that would be classified as Category 5, and thus would
not be classified under the HCS, are included in the mixture
calculation. Paragraph A.1.3.6.1(a) has been modified to indicate the
calculation must "[i]nclude ingredients with a known acute toxicity,
which fall into any of the acute toxicity categories, or which have an
oral or dermal LD50 greater than 2000 but less than or equal
to 5000 mg/kg body weight (or the equivalent dose for inhalation);".
OSHA has modified the text of Note (d) to Table A.1.1 to help
clarify the requirements. This was done in response to a comment from
Dow (Document ID 0526), which stated that they were "confused
about the table," and that OSHA should revisit the table and the
definitions to properly harmonize the provisions.
Several commenters noted that there were errors in Table A.1.2 in
the NPRM (Document ID 0376, 0393, and 0405). The errors have
been corrected in the final rule.
One commenter stated that the criteria seem to assume that acute
lethality data are available in all situations, and they are not
(Document ID 0321). As with all other health hazard criteria
in the standard, the HCS does not require data to be generated to
comply with the standard. And the final rule recognizes that many
chemicals have not been tested to ascertain their hazards. For example,
the formula used to calculate the acute toxicity of a mixture makes an
adjustment for ingredients whose acute toxicity is unknown. In
addition, the fact that a mixture contains an ingredient of unknown
toxicity must be indicated on the label and SDS. This is important
because in some mixtures the unknown percentage could be significant,
and therefore the estimation of toxicity for the mixture has less
credibility than in a situation where the majority of the ingredients
have data available.
It was also suggested that the formula used for acute toxicity be
displayed in a way that is more commonly used for such equations
(Document ID 0641). OSHA agrees that it could be displayed in
a different way, but wanted to ensure it appeared the same in the
regulatory text as it appears in the GHS. However, in guidance for
application of the final rule, OSHA will include the formula in the
alternative format as well to assist in understanding it.
The Styrene Information and Research Center (SIRC) challenged the
proposal's requirement to disclose the concentration of ingredients in
a mixture whose acute toxicity was unknown (Document ID 0361).
It argued that "[i]t is unclear how that requirement would pass a
significant risk test" and that "[i]t seems unlikely to make the user
more cautious." However, the record shows the contrary. Both workers
and union representatives testified at the public hearing on this
rulemaking that workers would be more cautious when dealing with
chemicals of unknown toxicity and would look for substitutes where
possible (Document ID 0494). Further, Cathy Cole, President of
the American Industrial Hygiene Association, testified that industrial
hygienists use the fact that a chemical's acute toxicity is unknown
when they perform qualitative risk assessments. She testified (
Document ID 0496 Tr. 425):
[W]e would take that information and use it to weigh it against
all the other information within that mixture. If there's an
unknown, then we would most likely provide a safety factor as we did
our risk assessment * * *. If there's a mixture that has a number of
unknowns, then we would treat that very carefully and we would have
a high risk ranking for it.
The final rule's hazard classification scheme for mixtures
presenting acute toxicity hazards treats unknown toxicity in a similar
way. When testing data on the mixture as a whole are not available, the
acute toxicity of the mixture is determined by assuming that the
nontoxic ingredients dilute the toxicity of the acutely toxic
ingredients. (See A.1.3.6.2.) However, where the acute toxicity of a
particular ingredient is not known, the final rule excludes it from the
toxicity calculation. (A.1.3.6.2.4.) In effect, this means that
ingredients with unknown toxicity are assumed not to dilute the
toxicity of the known acute toxicants. This approach reflects the same
cautious treatment of ingredients having unknown acute toxicity that
the witnesses testified to, as discussed above. In addition, it is
necessary to disclose the concentration of ingredients with unknown
toxicity because downstream users need that information to classify any
products they make with the mixture.
OSHA has also made two minor, clarifying changes to paragraph
A.1.3.6.2.4 that are consistent with changes that were approved by the
UN Sub-committee in December. The word "relevant" has been added in
front of "ingredient," and the word "total" was deleted before
"percentage." Therefore, A.1.3.6.2.4 in the final rule requires that
if the total concentration of the relevant ingredient(s) with unknown
acute toxicity is <=10% then the following formula must be used:
[GRAPHIC] [TIFF OMITTED] TR26MR12.047
However, if the total concentration of the relevant ingredient(s)
with unknown acute toxicity is >10%, the formula presented above is
corrected to adjust for the percentage of the unknown ingredient(s) as
follows:
[GRAPHIC] [TIFF OMITTED] TR26MR12.048
The above discussion shows that SIRC's concerns about the unknown
toxicity requirement are unfounded. Employers use the fact that a
chemical's acute toxicity is unknown in determining how chemicals
should be handled. As such, the disclosure requirement is reasonably
related to the purpose of hazard communication and, therefore, within
OSHA's authority. In addition, by providing the worker with information
about the limits of the known information, the requirement provides the
sort of prophylactic function that has been upheld even in situations
where the Agency has not made a significant risk finding. The unknown
toxicity requirement is consistent with the OSH Act.
Another commenter suggested the trade secret provisions should
apply to the requirement for disclosing the concentration of
ingredients with unknown toxicity (Document ID 0353). The
revised rule (and the GHS) do not suggest that the names of the
components be disclosed--simply the aggregate percentage of the total
composition that has unknown acute toxicity. So if there are three
ingredients in a mixture that have no acute toxicity data available,
and they comprise 20% of the mixture, the label and SDS must indicate
that 20% of the mixture has unknown acute toxicity. The names of the
chemicals do not have to be disclosed, and neither does the number of
chemicals involved. Therefore, there should be no trade secret issue.
Skin corrosion/irritation. OSHA proposed to adopt Categories 1 and
2, but not Category 3, for skin corrosion/irritation. Category 3 covers
more than the criteria for this hazardous effect under the current HCS.
In addition, the irritant effects covered by Category 3 are very minor
and transient, and of limited applicability in the workplace setting.
The Agency received several ANPR comments supporting such an approach
(Document ID 0034, 0077, 0128, 0145, and 0171). This approach
is also consistent with the European Union.
As OSHA noted in the preamble to the NPRM (74 FR 50392-93, Sept.
30, 2009), significant editing was done to the GHS text for this health
hazard. The criteria in the GHS lead the evaluator to conduct
additional testing when information is not available. While the GHS
does not require testing, the criteria imply that it should be done to
complete an evaluation. This implication is not acceptable under the
HCS, which is based solely on available evidence.
As noted in the NPRM discussion, work had already been initiated in
the UN Sub-committee to modify the chapter on skin corrosion/irritation
to address inconsistencies and clarify provisions. That work has
proceeded since the NPRM, and is on the work program for the next two
years as well. OSHA has made modifications to the HCS criteria to
reflect discussions in the Sub-committee, and clarify areas of concern.
In particular, Chapter A.2 of Appendix A, "Skin Corrosion/
Irritation," was reorganized in the final rule so that text and
figures are consistent. Paragraph A.2.1's title was changed to
"Definitions and general considerations." Paragraph A.2.1.2 was added
to introduce a tiered approach to follow when classifying for skin
corrosion/irritation. Paragraph A.2.2, "Classification criteria for
substances using test data," has been modified to reflect that it
covers animal test data. In Paragraph A.2.3, "Irritation," the
factors used to determine the corrosion/irritation potential of a
substance were deleted, and the text was reorganized to follow the
tiered approach to classify substances using other data elements.
Figure A.2.1 was updated to make it consistent with the text, and to
show the tiered evaluation scheme instead of a testing scheme. Comments
had been received that indicated this figure was confusing (Document ID
0344 and 0381). Another commenter noted that the criteria are
provided without indicating how they were derived (Document ID
0321). The criteria were developed by a group of experts in
the OECD and were derived from the existing criteria of the countries
involved. They do not specify a test method because the GHS is test
method neutral, but the OECD testing guidelines are generally agreed to
provide the type of information needed for classification under the
GHS.
There were also several comments that pH criteria are not
appropriate to use in some situations (for example, the pH of the
ingredients in a mixture may not predict the pH of the mixture)
(Document ID 0321, 0335, and 0381). The criteria recognize
that test data for these effects provide better information
to base a classification on, but pH information can be of assistance
when such data are not available.
OSHA believes the edits and changes make the chapter less confusing
and clarify that testing is not required to achieve compliance. The
basic provisions and approach remain the same as the GHS. The Agency is
participating in the continuing work of the UN Sub-committee on this
topic, and will revise the HCS if any additional clarifications are
made in the criteria for these hazards that will help classifiers
follow the provisions.
Serious eye damage/irritation. Proposed Appendix A, Chapter A.3
("Serious Eye Damage/Eye Irritation"), did not include the criteria
for Category 2B of eye irritation, but addressed the label elements for
the category in Appendix C. A number of commenters indicated that OSHA
should include the criteria for Category 2B (Document IDs
0344, 0351, 0367, 0371, 0381, and 0393), clarify coverage of
Category 2B (Document ID 0376 and 0382), or exclude it
(Document ID 0405). The omission of the criteria was an
oversight, and OSHA has added the criteria for Category 2B to the final
rule.
The text for GHS Chapter 3.3, "Serious Eye Damage/Eye
Irritation," posed similar issues to those described above for skin
corrosion/irritation. The criteria in the GHS implied that testing
might be needed to complete classification in the absence of data. This
is required by neither the GHS nor the HCS. OSHA made a number of
modifications to the parallel text in Appendix A, Chapter A.3, of the
HCS proposal to address the perception that testing might be required
when it is not. And the UN Sub-committee is also reviewing this chapter
for purposes of clarifying the requirements.
As with the skin chapter, in the final rule OSHA has reorganized
Chapter A.3 so that the text and figures are consistent, and so that it
is clear that what must be followed is a tiered approach. The title of
A.3.1 was modified to indicate it covers definitions and general
considerations, and paragraph A.3.1.2 was added to introduce the tiered
approach for classification. Paragraph A.3.2 ("Classification criteria
for substances using animal test data") was modified to indicate it
addresses animal data. Table A.3.1 was modified to indicate that
Category 1 corresponds to Serious Eye Damage and not to eye irritants,
and Table A.3.2 adds the criteria for Category 2B. In A.3.3
("Classification criteria for substances using other data elements"),
the classification criteria for substances were reorganized using other
data elements to make it consistent with Figure A.3.1, and to show the
tiered evaluation strategy for classification. Figure A.3.1 was updated
to make it consistent with the text. And Table A.3.3 now has a note to
indicate that a mixture may be classified as Category 2B in cases when
all relevant ingredients are classified as Category 2B. As with skin
corrosion/irritation, OSHA will continue to monitor work in the UN Sub-
committee to clarify these criteria, and will modify the rule to update
the chapter as necessary if changes are made.
One additional issue was raised concerning the coverage of the GHS
criteria for eye irritation in comparison to current criteria used by
CPSC and EPA. The National Toxicology Program Interagency Center for
the Evaluation of Alternative Toxicological Methods (NICEATM) (Document
ID 0384) suggests that the GHS criteria are not as protective
as the current criteria used by CPSC and EPA. OSHA uses the CPSC
criteria in the current HCS, but does not use EPA criteria. NICEATM did
an analysis of a group of chemicals to determine what their
classifications would be under the different criteria, and concluded
that at least 14 of 149 chemicals it reviewed (17%) would not be
classified under the GHS criteria, but would have been under current
HCS criteria.
OSHA asked a consulting toxicologist familiar with the GHS criteria
to review the comment and the analysis, and the results of his review
have been entered into the public record (Document ID 0576,
0577, and 0578). The results of this review show that all of the 14
chemicals are differently classified because they present transitory
effects that resolve in 72 hours or less; the difference in
classification results from the way each method accumulates transitory
positive results across test animals. While there may be some
differences in conclusions made under the differing criteria, the
differences are less pronounced when variance in transient effects is
considered (as it is under the criteria as proposed). This is explained
as follows in the toxicologist's report:
In order to compensate for this difference in approaches, OSHA
has proposed to also adopt the GHS concept of "pronounced
variability". Under this concept, for those chemicals where there
is pronounced variability among animal responses, such information
may be taken into account in determining the classification. As
discussed specifically under OSHA's proposed criteria for
Classification and Categorization of Skin Corrosion/Irritation, but
only mentioned in passing under Serious Eye Damage/Eye Irritation,
this notion would allow for classification in cases where there are
very definite, positive irritant effects related to chemical
exposure in a single animal, but the overall data set does not
support classification. In cases where the response is borderline
but persistent or severe but transient, the Assessor would likely
classify a substance as irritating. It is noted that there are at
least two chemicals among those under examination where "pronounced
variability" would likely cause the Assessor to classify them as
irritants (see data for ethyl thioglycolate and glycidyl
methacrylate; fomesafen, 2,2-dimethyl-3-pentanol, and cellosolve
acetate might also be classified as irritants under this concept).
The final rule retains the pronounced variability language at A.2.2.2.2
and A.3.2.3. The toxicologist also noted that:
Finally, a quick search of secondary and tertiary sources
available on-line indicates that 12 of the 14 chemicals in question
would be classified as hazardous materials under both the current
and proposed classification criteria. Those that would not be
classified are N,N-dimethylguanidine sulfate (sub-EU classification
eye and skin irritation responses; not a sensitizer; no other data
found); and tetraaminopyrimidine sulfate (not an acute or chronic
toxicant; identified as non-irritating by EU Scientific Committee on
Cosmetic Products and Non-Food Products intended for Consumers
(SCCNFP)).
Therefore, although the chemical may not be addressed as an eye
irritant, it would still be considered a health hazard under the GHS--
and the HCS--and thus have information available about its effects on
labels and SDSs.
While OSHA appreciates the concerns raised by NICEATM, the criteria
are being finalized as proposed, other than the modifications made for
clarification purposes. It appears that the pronounced variability
considerations will address some of the concerns raised, and that the
primary remaining differences involve transient effects of relatively
low concern. Both CPSC and EPA were involved in the development of the
criteria in the GHS, and were aware of the differences between their
existing systems and the agreed harmonized criteria. In harmonizing
between the existing systems, the criteria selected were between what
currently exists in the U.S. and in the EU. The classification criteria
in each existing system is not a bright line determined by science, but
rather a scientifically influenced policy determination, and as
discussed elsewhere, an inevitable part of adopting harmonized criteria
is that a few borderline chemicals might be dropped. No other
stakeholders have raised the issue of whether the criteria are
protective enough. OSHA is proceeding with the final rule because it
believes that in this situation,
maintaining harmonization with the GHS is ultimately more important for
worker health. This situation will continue to be monitored as
implementation takes place to ensure that it is appropriate.
Respiratory or skin sensitization. The final rule makes only minor
changes to the proposed text of Appendix A, Chapter A.4, "Respiratory
or Skin Sensitization." The footnotes have been re-numbered since they
were out of sequence in the NPRM. And the term EC3 has been explained
in a footnote to Tables A.4.3 and A.4.4 (estimated concentration of
test chemical required to induce a stimulation index of 3 in the local
lymph node assay).
The GHS criteria for respiratory and skin sensitizers have one
category for each type of sensitization, but also give the option of
dividing that one category into two sub-categories, which involves a
differentiation in the type of evidence available. In the NPRM, OSHA
proposed to adopt the sub-categories for classification. One commenter
strongly supported adopting sub-categories for these sensitizers
(Document ID 0381), while another did not support it because
the EU has not adopted sub-categories (Document ID 0376). OSHA
is adopting the sub-categories as proposed. However, the Agency
recognizes that there are situations where data are not available to
place the chemical into one of the sub-categories. The GHS itself
addresses this in 3.4.2.1.1.1 (respiratory sensitization), and
3.4.2.2.1.1 (skin sensitization). Therefore, under the revised HCS,
simply classifying the chemical as Category 1 will be sufficient in
cases where data are insufficient to assign a subcategory. The American
Chemistry Council (Document ID 0393) suggested that more
guidance is needed to differentiate potential and severe sensitizers
for placement into the sub-categories. OSHA believes that this type of
guidance should be developed through the Sub-committee process, rather
than by countries independently developing guidance for application.
The Agency will consider requesting the Sub-committee to develop such
guidance.
Germ cell mutagenicity. The comments on this health hazard centered
on whether or not it should be included in Appendix A. Procter & Gamble
(Document ID 0381) and the American Chemistry Council
(Document ID 0393) argued that it should not be included. The
Soap and Detergent Association (Document ID 0344) also argued
for exclusion, but said if it is included, only Category 1A should be
covered. Ecolab (Document ID 0351) also argued that only
Category 1A should be covered. These commenters argued that it is
already covered by reproductive toxicity and carcinogenicity, and
adding a separate hazard class would create a training burden.
OSHA disagrees. First, while the current HCS does not define
mutagenicity as a separate health hazard, it is covered by the
reproductive toxin definition. Under the GHS, mutagenicity is not
covered by reproductive toxicity, and OSHA's failure to adopt the
mutagenicity category would render the final less protective than the
current HCS. The hazard class will have to be adopted to maintain
coverage. Secondly, though mutagenicity data are used to predict
carcinogenicity, the mutagenicity hazard is not covered by the
carcinogenicity criteria. Furthermore, little additional burden for
training can be claimed for what is already covered under reproductive
toxicity in the current HCS.
All of these commenters argue that the HCS should be as consistent
with the EU as possible. The EU has already adopted these criteria, so
excluding them would not be consistent with the EU. OSHA is maintaining
the hazard class as part of the HCS, and including both categories. It
is OSHA's understanding that at present there are no chemicals that
meet the criteria for Category 1A, so currently this has no burden
associated with it--although there may be minimal burdens if new data
in the future place chemicals in this category. (See, e.g., Annex VI to
the EU's former directive on classification and labeling, which states:
"To place a substance in category 1, positive evidence from human
mutation epidemiology studies will be needed. Examples of such
substances are not known to date. It is recognized that it is extremely
difficult to obtain reliable information from studies on the incidence
of mutations in human populations, or on possible increases in their
frequencies.") Chemicals in Category 2 are frequently used already in
discussions of potential carcinogenicity, since mutagenicity test
results are used to predict carcinogenicity. Thus, there is little
burden associated with adopting that category either. Therefore, OSHA
has retained Appendix A, Chapter A.5, "Germ Cell Mutagenicity."
OSHA included a new heading in A.5.4 entitled "Examples of
scientifically validated test methods." In the interest of maintaining
current protections, as well as being consistent with implementation in
the EU, germ cell mutagenicity is adopted in the final rule as
proposed.
Carcinogenicity. The primary change to the carcinogenicity hazard
class as proposed in Appendix A, Chapter A.6, "Carcinogenicity," is
the addition of A.6.4, "Classification of carcinogenicity." In the
current HCS, carcinogenicity was determined in part by consulting the
National Toxicology Program's biennial Report on Carcinogens (RoC), or
the International Agency for Research on Cancer's monographs. In
addition, chemicals that are regulated by OSHA based on their
carcinogenicity (i.e., there is a substance-specific standard
addressing the chemical, and the chemical poses a risk of
carcinogenicity), are always covered by the HCS. The IARC and NTP
documents are prepared based on the evaluation of data by experts
convened by these organizations. A number of commenters suggested that
this should still be permitted under the GHS-aligned criteria. For
example, the United Steelworkers argued (Document ID 0403):
The current Hazard Communication standard includes a reference
to several lists of chemicals automatically presumed to be
hazardous, such as the lists of carcinogens published by the
National Toxicology Program (NTP) and the International Agency for
Research on Cancer (IARC). The proposal removes references to such
lists, in favor of a more detailed and complicated classification
system. While that classification system is required by the GHS, the
lists provide useful guidance and should not be removed altogether.
We suggest the following compromise: OSHA should state in the
regulatory text that a classifier may presume that the presence of a
chemical on one or more of those lists is sufficient to classify the
chemical as hazardous with respect to the hazard covered by the
list. (OSHA should also state that the inverse is not true: The
absence from a list does not indicate the lack of a hazard.) This
does not mean that the classifier is required to classify a chemical
as hazardous based solely on the list, only that he or she is free
to do so. OSHA should also indicate in the preamble that the Agency
will use the lists as guidance in enforcement, and that a classifier
who ignores the lists should be prepared to show why his or her
judgment is better than the judgment of, for example, NTP or IARC.
Similarly, Morganite Industries, Inc. and Morgan Technical Ceramics,
stated (Document ID 0321):
For example, IARC, NTP and other qualified organizations assess
carcinogenicity and come to published conclusions. We do not
understand why the proposed Hazard Communication Standard
establishes procedures for chemical suppliers to conduct such
assessments, seemingly asking them to conduct their own evaluations
in the manner
of similar to these expert agencies. That makes no sense to us. Why
not just refer to the conclusions published by these agencies? That
would shorten and simplify the regulation, it would eliminate large
parts of the difficult language and it would eliminate regulatory
requirements that are in fact infeasible for most preparers of MSDS
to comply with.
OSHA agrees with these commenters that allowing evaluators to rely
on IARC and NTP could make classification easier for them, as well as
lead to greater consistency. Therefore, A.6.4.1 has been added to the
criteria in the final rule to indicate that classifiers may treat these
sources as establishing that a chemical is a carcinogen without
applying the criteria themselves. And A.6.4.2 reiterates that OSHA-
regulated carcinogens are covered under the HCS.
In order to facilitate the use of IARC and NTP determinations as
sources for purposes of classification, non-mandatory Appendix F has
been significantly modified. In the NPRM, Appendix F was simply a
verbatim quote of guidance from IARC on determining carcinogenicity. In
the final rule, Appendix F has been updated to reflect the latest
version of that IARC text, but also includes additional guidance on how
to use IARC and NTP to make carcinogenicity classifications. The
inclusion of this guidance should make classification easier for
chemicals addressed by these sources, and should also provide
parameters for the type of weight-of-evidence decisions that are
appropriate under the GHS-aligned criteria.
The following table is included in Part D of Appendix F, and may be
used to perform hazard classifications for carcinogenicity under the
HCS. It relates the approximated GHS hazard categories for
carcinogenicity to the classifications provided by IARC and NTP, as
described in Parts B and C of Appendix F:
Table XIII-2
------------------------------------------------------------------------
Approximate equivalences among carcinogen classification schemes
-------------------------------------------------------------------------
IARC GHS NTP RoC
------------------------------------------------------------------------
Group 1......................... Category 1A....... Known.
Group 2A........................ Category 1B....... Reasonably
Anticipated (See
Note 1).
Group 2B........................ Category 2 .......
------------------------------------------------------------------------
Note 1:
1. Limited evidence of carcinogenicity from studies in humans
(corresponding to IARC 2A/GHS 1B);
2. Sufficient evidence of carcinogenicity from studies in experimental
animals (again, essentially corresponding to IARC 2A/GHS 1B);
3. Less than sufficient evidence of carcinogenicity in humans or
laboratory animals; however:
a. The agent, substance, or mixture belongs to a well-defined,
structurally-related class of substances whose members are listed in a
previous RoC as either "Known" or "Reasonably Anticipated" to be a
human carcinogen, or
b. There is convincing relevant information that the agent acts through
mechanisms indicating it would likely cause cancer in humans.
While the criteria for carcinogenicity (as well as other health
effects) are largely based on weight of evidence evaluations, there are
also provisions in the GHS for countries that want to ensure that all
potential carcinogens are adequately captured by the criteria. Thus
paragraph 3.6.2.6 of the GHS chapter on carcinogenicity states:
* * * For inclusion into Safety Data Sheets, positive results in
any carcinogenicity study performed according to good scientific
principles with statistically significant results may be considered.
OSHA chose to include this requirement in Figure A.6.1 of Appendix A in
the NPRM under Category 2, suspected human carcinogen. Specifically,
the statement read:
Positive results in any carcinogenicity study performed
according to good scientific principles with statistically
significant results qualifies for referencing the chemical as, at
the least a Category 2 carcinogen.
The Styrene Information and Research Council (SIRC) (Document ID
0361) argues that the "one positive study" criterion is
inconsistent with the weight of evidence approach. In fact, it is not
part of the weight of evidence approach, but rather reflects the
Agency's decision to ensure that the current level of protection in
terms of identifying potential carcinogens in the workplace is
maintained in the HCS as permitted by the GHS provisions.
SIRC also indicated that it is not clear what is meant by
"referencing" the chemical as, at the least, a Category 2 carcinogen.
OSHA agrees that the inclusion of this language in Figure A.6.1 is not
as clear as it could be in terms of what is required. In the final
rule, OSHA has separated this requirement from Category 2, and added a
new heading of "Other considerations" to the table. The text for the
"Other considerations" is: "Where the weight of evidence for the
carcinogenicity of a substance does not meet the above criteria, any
positive study conducted in accordance with established scientific
principles, and which reports statistically significant findings
regarding the carcinogenic potential of the substance, must be noted on
the safety data sheet." Categories 1 and 2 will remain based on weight
of evidence, but the data that meet the definition of "other
considerations" must also be provided on the SDS for the chemical.
This will maintain the protections of the current rule and provide
information to downstream users so they can determine the appropriate
protective measures to be taken in these situations.
In paragraph A.6.3.2 of the NPRM, OSHA included the mixture
approach in GHS paragraph 3.6.3.1 regarding use of test data as a whole
to characterize the carcinogenic potential of a mixture:
A mixture may be classified based on the available test data for
the mixture as a whole. In such cases, the test results for the
mixture as a whole must be shown to be conclusive taking into
account dose and other factors such as duration, observations and
analysis (e.g., statistical analysis, test sensitivity) of
carcinogenicity test systems.
SIRC (Document ID 0361) similarly took issue with this
provision:
Again, the use of the word "conclusive" appears to be an
inappropriate attempt to apply the European Precautionary Principle
to this issue. It is inconsistent with the fundamental principle
that hazard communication is to be based on the application of
expert judgment to known information and not require chemical
testing (either explicitly or as an inevitable practical requirement
to avoid unacceptable economic consequences). The word
"conclusive" should be replaced with the word "adequate" or
"persuasive."
The provision in A.6.3.2 recognizes that it is difficult to accurately
characterize the carcinogenicity of a mixture with an ingredient that
is clearly carcinogenic. It requires skilled, expert judgment, and test
results on the mixture as a whole may be misleading. Therefore, the
experts who developed the carcinogenicity criteria believed that given
the critical nature of this effect and the known limitations of
assessing carcinogenic potential in a mixture, it was appropriate to
allow testing of the mixture as a whole to supersede an evaluation
based on the carcinogenic potential of a known ingredient only when the
data allow a sufficient level of confidence about the mixture's
hazards. OSHA agrees with the findings of these experts, and does not
believe that the word "conclusive" needs to be replaced. This
provision remains the same in the final rule. It also does not require
or imply that any testing of chemicals be performed. It is actually
rather unusual to have mixtures tested for any types of effects, so it
is expected that this provision will not be applied frequently. If a
test is performed voluntarily with the purpose of avoiding
characterization of a mixture as a carcinogen, it is very important
that the test provide conclusive evidence before depriving downstream
users of information that ingredients in the mixture present a
carcinogenicity hazard.
In addition to the technical considerations, SIRC (Document ID
0361) (as well as SPI, Document ID 0392), repeatedly
suggests that the precautionary principle, or European approaches, are
the genesis of various provisions. First, OSHA does not agree that the
precautionary principle had any part in the GHS, or the HCS,
provisions. The HCS is an information transmittal standard, not a
standard that requires the implementation of controls or other risk
management approaches. The precautionary principle generally applies to
competent authorities, and allows them to regulate or establish
controls in situations where complete information is not available
about the situation. That certainly does not apply to this provision in
the HCS, which requires definitive data before allowing a chemical
manufacturer or importer to designate a mixture as not being
carcinogenic, although it contains an ingredient that clearly has a
carcinogenic potential. The HCS is a standard that is intended to
provide information to users of chemicals so they can make their own
determinations as to what controls are needed to prevent adverse health
effects or the effects of physical hazards. The better information they
have about the chemicals in their workplaces, the more likely they will
be able to make their own risk assessments, and choose appropriate risk
management measures. The provisions of the HCS--as well as the GHS--are
designed to ensure that such information is available to users.
The criteria proposed are adopted in the final standard, with the
addition of the paragraphs referring to NTP, IARC, and OSHA-regulated
substances, and supplemented by the revised non-mandatory Appendix F.
Reproductive toxicity. This hazard class, described in Appendix A,
Chapter A.7, was proposed to have two hazard categories (Category 1,
which is subdivided into two sub-categories based on human evidence,
and Category 2, which also includes evidence from animal studies). In
addition, it requires consideration of effects on or via lactation.
Several commenters argued that OSHA should not adopt effects on or via
lactation (Document ID 0344, 0351, and 0381). The rationale
provided is that there is no standard assessment method. However, the
criteria already recognize that there is no standard assessment method,
and provide the types of information that can be used to assess whether
a chemical poses this effect. While such information may not be
available for many chemicals, there are certain types of products that
may have such information available, and it is information that needs
to be provided to exposed workers. Therefore, OSHA is maintaining
effects on or via lactation in the final rule. In addition, this
maintains consistency with the EU approach.
The only change OSHA has made in the final rule is to change
"should" to "shall" in A.7.2.5.4, since it is mandatory in the HCS.
Otherwise, the criteria are adopted as proposed in the text of the
final rule.
Specific target organ toxicity single exposure (STOT-SE). This
hazard class, described in Appendix A, Chapter A.8, was proposed to
have three categories. The first two categories deal with differences
in the type of evidence available to assess the effect, while the third
addresses transient target organ effects, such as narcotic effects and
respiratory irritation. Several commenters indicated that Category 3
could be adopted without adopting Category 2 (Document ID
0344, 0351, 0381, and 0393). Procter & Gamble (P&G) (Document
ID 0381) argues that Category 2 should not be adopted:
There are also a significant difficulty and potential unintended
outcome that weigh against applying Category 2. Animal studies may
be done for a variety of purposes, some of which are not relevant to
consumer product uses, and the interpretations of animal data from
these types of studies often yield conclusions not relevant to
consumer products. Using the outcomes from animal studies for
classification into Category 2, especially studies at exposures near
the point of morbidity, requires an unusual level of expertise that
many classifiers would not possess. In addition, classification into
Category 2 relies on interpretation of the phrase "relevant to
human health," which would involve an additional expertise.
Therefore, Category 2 should not be adopted.
There are a number of difficulties with this argument. First, this
section addresses protection of workers exposed to chemicals, and not
the assessment of consumer products and exposures. Many consumer
products are not covered by the HCS, although provisions in the scope
and application cover those products where they are used in the
workplace in a manner different than consumers would use them, or with
a more extensive duration and frequency of use.
In devising the Category 1/Category 2 approach to classifying
specific target organ toxicity after single (and repeated) exposure,
the framers of the STOT-SE (and STOT-Repeated Exposure) criteria sought
to establish a means by which the chemical manufacturers and importers
could communicate to the worker information as to both the nature and
the severity of adverse systemic and target organ effects. The final
rule provides detailed criteria to clarify what would be considered an
"adverse" effect (See A.8.2.1.7.3), and it also provides specific
examples of effects ("changes") that might be seen in animal studies,
yet would not be considered to be "adverse" (A.8.2.1.8).
Using these criteria and examples, classifiers will be able to
consider whether a change was, as required by Category 2, "of
relevance for human health." In specific cases when an evaluated
change was deemed not to be relevant, the classifier is allowed to
discount specific toxicological study findings that are not relevant to
human hazard assessment and not classify. OSHA believes that
classification under Category 2 will be no more difficult than other
hazards under the rule, and that no "special additional experience"
will be needed to classify for Category 2, as suggested by P&G.
Additionally, the GHS-based STOT criteria proposed for adoption by
OSHA sought to introduce the concept of dose response to the
communication of specific target organ toxicity hazards. Such a concept
has long been part of the assessment of acute toxicity hazards, but has
been missing from the communication of many other health hazard
endpoints. Adoption of both Categories 1 and 2, as proposed, allows the
chemical manufacturer/importer of a chemical to convey to the worker
additional information as to the
characterization of the specific target organ hazard by providing some
general measure of whether an effect (change) might be expected at low
(presumably occupationally relevant) exposure, or whether it would be
seen only in cases of unusually high exposure (e.g., catastrophic loss
of safety controls).
P&G also suggests that Category 2 is inconsistent with paragraph
3.8.1.3 of the GHS in that "it does not rely primarily on human data *
* *." However, while GHS 3.8.1.3 (A.8.1.3 in the final rule) does say
that human data will be the "primary source for classification," it
also specifically states that classification in this hazard class may
also be made on reliable evidence "in experimental animals,
toxicologically significant changes * * *." Thus, P&G's contention is
not accurate. In addition, animal data are used or referred to
throughout the criteria in the GHS for health hazards, and the use of
such data to predict effects in exposed humans is a standard
toxicological approach.
In addition to its appropriateness for protection of workers,
Category 2 has been adopted by the EU, and adopting it in the final
rule will thus maintain consistency with the EU as well.
Aspiration hazard. OSHA did not propose to adopt Category 2 for
aspiration hazards covered by the GHS. This category appeared to be
more appropriate for the consumer sector than the workplace. OSHA does
not specifically address aspiration hazards in the current HCS although
the Agency believes the more relevant and serious Category 1 aspiration
hazards are captured under the broad scope of the rule. Several ANPR
commenters agreed that Category 2 should not be covered in the HCS
(Document ID 0034, 0077, 0128, 0145, and 0171), and the EU
does not include it in their requirements. Others suggested that
aspiration should not be covered at all since it is not relevant to the
occupational setting (Document ID 0102, 0104, and 0163).
Several commenters on the NPRM also argued that aspiration hazard
should be completely excluded from the revised HCS (See, e.g., Document
ID 0373, 0393, 0398, 0486, and 0528). In addition, one comment
suggested that the criteria could be interpreted as applying to
drowning, and is overbroad (Document ID 0353).
The primary proponent for complete exclusion of aspiration as a
hazard in the revised GHS was the Hydrocarbon Solvents Panel (the
Panel) of the American Chemistry Council. In their post-hearing
comments, the Panel summarized their position as follows (Document ID
0528):
(1) OSHA should not adopt the Aspiration Toxicity class under
the GHS because, as demonstrated by data submitted to the record,
aspiration as a route of exposure is not common in the industrial
setting, and is not a significant cause of occupationally related
severe or fatal poisonings.
(2) Should OSHA include Aspiration Toxicity as one of the Health
Hazard classes, the Panel urged that OSHA not require the Health
Hazard Symbol be used as part of the pictogram because it does not
accurately symbolize the nature of the hazard represented by the
aspiration route of entry, and could be potentially misleading.
(3) Should OSHA include Aspiration Toxicity and a symbol, the
Exclamation Mark symbol is more appropriate for the Aspiration
Hazard Pictogram. Of the existing symbols in the proposed rule, the
Exclamation Mark symbol is more representative of an actual
aspiration episode. The Exclamation Mark would be a better choice to
connote the hazard endpoints and response necessary in an aspiration
event, due to the immediate need for intervention in an aspiration
episode.
(4) If OSHA is unwilling to adopt the Exclamation Mark symbol
for Aspiration Toxicity, we request that OSHA forward the concern to
the UNSCEGHS for its consideration.
With regard to the Panel's first point, OSHA agrees that this route of
exposure is not frequently found in the occupational setting. But that
is different than saying it does not occur, or should not be a concern.
NIOSH has submitted a number of studies and reports to the record that
document concerns about aspiration (Document ID 0523 and
0524), and address occupational exposures as well (Document ID
0523). For example:
Amoruso et al. [2008] reported that aspiration of mineral
spirits into the lungs may produce serious damage leading to
bronchopneumonia that may be fatal within 24 h[ours] * * *.
Rodriguez et al. [1991] reported a case incident where deaths in
3 crude oil tanker workers were reported as attributed to pulmonary
aspiration as evidenced by histopathology studies. The hypothesized
mechanism of deaths included the contributing factors of asphyxia by
toxic gases leading to loss of consciousness, traumatic injury and
aspiration.
A number of other cases are described in the NIOSH comment. The Panel
itself noted two aspiration fatalities in the period from 2003 to 2007,
one of which was related to a corrosion inhibitor, and the other to
sodium bisulfate (Document ID 0486, 0494 Tr. 212). Moreover,
the Panel's chair testified that her company includes aspiration hazard
warnings on all of its products (Document ID 494 Tr. 214-15).
Therefore, it is clear to OSHA that there are legitimate concerns about
aspiration in terms of both occupational injuries and fatalities, and
that aspiration hazards need to be included in the scope of the HCS.
Thus, OSHA included Chapter A.10, "Aspiration Hazard," in Appendix A
in the proposed rule and has retained it in the final rule.
With regard to the symbol, the application of the more severe
health hazard symbol to a Category 1 hazard category is consistent with
how the symbols are applied to all of the health hazards. Adopting the
exclamation mark in the U.S. for aspiration Category 1 would make the
HCS inconsistent with other countries' rules regarding aspiration
hazard, which would present difficulties for countries exporting to the
U.S., and potentially create inconsistencies in what workers see on
labels and SDSs. This would not be an effective communication approach
to aspiration hazards. Therefore, OSHA does not agree that the
exclamation mark should be permitted for Category 1 aspiration hazards.
In terms of presenting it to the UN Sub-committee as an issue, OSHA
will take that suggestion under advisement. However, industry
stakeholders are free to make this suggestion to the Sub-committee
themselves through submission of a paper.
With regard to the contention that drowning in water could
conceivably be read as being covered by the aspiration hazard criteria,
OSHA assures stakeholders that drowning in water is not covered and
that the HCS will not be interpreted as addressing drowning in water as
an effect covered by the rule.
Aspiration Hazard, Category 1, is included in the final rule as
proposed.
Appendix B, Physical Hazards. Appendix B includes the criteria for
the physical hazards proposed to be covered by the HCS to be consistent
with the GHS. The current HCS covers these hazards, but the
definitions, while similar, are not the same as those included in the
GHS. The GHS based its physical hazard criteria on those incorporated
into the United Nations' Recommendations on the Transport of Dangerous
Goods. In the U.S., the Department of Transportation (DOT) has already
harmonized its definitions with the UN, and thus, with few exceptions,
the GHS. While OSHA's initial physical hazard definitions were
consistent with the DOT definitions at the time the current HCS was
promulgated, DOT's harmonization with the international requirements
resulted in the two agencies having different definitions. Thus the
U.S. has not been domestically harmonized for some years. Adopting the
same definitions in this rulemaking as DOT has in this rulemaking will
have the additional benefit of accomplishing substantial domestic
harmonization.
As with Appendix A and the health hazard criteria, OSHA edited
Chapter 2 of the GHS ("Physical Hazards") to shorten the discussions
and focus only on the criteria in the proposed revisions. Decision
logics and hazard communication information are not included. As with
health hazards, OSHA tried to maintain the current scope of the HCS for
physical hazards in the proposal, as well as being as consistent as
possible with trading partners, particularly the European Union. One
exception may be flammable gases, where it appears that more flammable
gases will be covered by OSHA adopting Category 2 than are currently
covered by the HCS. OSHA is adopting all of the physical hazards in the
GHS.
The one deviation from the approach adopted by the European Union
is in the proposed adoption of Categories 1 through 4 for flammable
liquids. The European system only addresses Categories 1 through 3. The
current HCS covers flammable liquids in Category 4, and exclusion of
this category would result in reduced protection, which OSHA does not
believe is appropriate. Thus Category 4 is included in the revised HCS.
One edit that should be noted occurs in the criteria for
explosives. The GHS criteria currently use the term "article" in a
manner that is inconsistent with that term as used in the workplace in
the U.S. OSHA has changed the term to "item" in these criteria. This
modification was supported by stakeholders (See, e.g., Document ID
0362).
While OSHA believes that harmonizing with DOT provides significant
benefits, there were some concerns regarding this approach that arose
in reviewing the physical hazard criteria. These concerns involved the
test methods referred to in the GHS criteria, which are based on issues
related to the packaging and volume in transportation. Packaging is
obviously a major concern in transport, and is used to address or
mitigate the risk of conveying certain types of chemicals. These
chemicals may or may not be present in the workplace in the same size
or type of packaging and the relevance of these factors in the test
methods are questionable in terms of workplace exposures. OSHA invited
comment on these factors, including comments on the appropriateness of
the criteria (including the test methods and references to packaging or
volume) when applied to the workplace, and any suggestions that
interested parties have to address these issues. Of particular interest
were criteria for self-reactive chemicals, organic peroxides, self-
heating chemicals, and explosives. Commenters indicated that the
criteria could be applied to the workplace (See, e.g., Document ID
0330, 0336, 0383, and 0405). Others specifically noted that
OSHA should maintain consistency with DOT (See, e.g., Document ID
0338, 0344, 0351, 0376, 0379, 0381, and 0392). For example,
the Industry Minerals Association--North America stated (Document ID
0379):
The classification, labeling, handling and storage of chemicals
related to transport concerns should remain aligned with the
principles of HCS. OSHA should seek where possible to reduce
incompatibilities between HCS criteria and US DOT transportation
requirements.
Accordingly, OSHA has decided to carry through these requirements
to the final rule as proposed. OSHA is satisfied that, in this respect,
the criteria proposed are appropriate.
The Society of the Plastics Industry, Inc. (SPI) (Document ID
0392) contends that the requirements will not be possible to
implement for organic peroxides:
The GHS would require that the SDS for organic peroxide include:
(1) Recommended use of the chemical and restrictions on use;
(2) Precautions for safe handling;
(3) Conditions for safe storage, including any
incompatibilities, and
(4) Appropriate engineering controls.
Compliance with these requirements, which include principles
from the EU regulation for the Registration, Evaluation,
Authorisation and Restriction of Chemicals (REACH), presents a
particular concern for organic peroxide producers following
transportation and initial storage in the DOT-regulated transport
container. As written, compliance would present unreasonable
difficulties and appears to be infeasible for suppliers of these
chemicals. Customers are likely to handle and use these materials
under significantly different conditions once they remove the
organic peroxides from the packages in which they were transported.
SPI further recommends that OSHA require "that labels and SDSs include
a generic statement of fact indicating that changes in risk and hazard
can occur when these self-reactive materials are moved from normal
transport and storage conditions into process settings, and that they
may require assessments by specialists." SPI also suggests that OSHA
should be harmonizing with DOT in this area.
SPI indicates that these requirements for information on SDSs
originate with REACH requirements in Europe. In fact, OSHA has always
required such information on SDSs (with the exception of intended use
of the chemical, and restrictions on use), and these requirements
preceded REACH by many years--as did the negotiated text of the GHS. In
Sec. 1910.1200 (g)(2)(viii) and (ix) of the HCS promulgated in 1983,
the preparer of the MSDS is required to provide any generally
applicable precautions for safe handling and use, and any generally
applicable control measures such as engineering controls, which are
known to the chemical manufacturer, importer or employer. OSHA also
notes that the manual supplied and written by SPI: "SAFETY AND
HANDLING OF ORGANIC PEROXIDES: A Guide" (dated August 1999),
recommends that downstream users consult labels and MSDSs for handling
information (Document ID 0392). OSHA does not agree that the
SDS requirements in the NPRM, and the final rule, are infeasible or
even substantially different than what has been required by OSHA since
1983. The Agency does not agree that the suggested statement should be
required by OSHA regarding organic peroxides. Chemical manufacturers
and importers of organic peroxides are free to provide whatever advice
they deem appropriate in the supplementary information part of the
label, or on the SDS, to guide downstream users for appropriate
handling, as long as the advice does not conflict with the required
hazard communication information.
With regard to harmonizing with DOT, the criteria in the final rule
are the criteria that DOT adopted from the UN Transport
recommendations. Therefore, OSHA is harmonizing with DOT through this
rulemaking.
One commenter indicated that there was concern that criteria based
on transport classification may confuse workplace application, and
guidance would be needed (Document ID 0339):
Concerns have been expressed that the criteria developed for
transport concerns, as stated in the GHS, express very specific
constraints, or "worse case scenarios", which can be confusing to
suppliers and users of chemicals who are reading the Safety Data
Sheets (SDSs)/labels, etc., without benefit of the context. PRR
believes this is an area in which OSHA could develop informational
materials to help chemical suppliers and users understand the
rationale behind physical hazard classifications.
OSHA will keep this suggestion in mind as guidance materials are
developed.
Only minor editorial revisions have been made to Appendix B after
reviewing all of the comments received. While a great number of changes
were suggested by one commenter (Document ID 0353), most have
not been adopted, consistent with the discussion above on
the background for Appendixes A and B. This approach is to maintain
consistency with the GHS and DOT, as well as the EU.
The modifications made in the final rule include changing metric
references to units used in the U.S., and modifying references to
documents incorporated by reference to make them consistent with OSHA's
requirements for such references. There are no technical changes to the
criteria. Therefore, Appendix B in the final rule is substantially the
same as proposed.
Classification Database
One interesting comment that was submitted by a number of
respondents to the ANPR involved development of a classification
database (Document ID 0047, 0050, 0053, 0054, 0038, 0155,
0160, and 0165). Opinions as to who would develop and maintain such a
database varied (OSHA, U.S. industry, and an international body were
all mentioned). It appears that the European Union will be making such
a database available for compliance with its requirements, as have
Japan, Taiwan, South Korea, and New Zealand. Concerns have been raised
by stakeholders that classifications in these databases are different
for the same chemical. OSHA invited additional comment on this issue in
the NPRM (74 FR 50284, Sept. 30, 2009), and received a number of
responses.
Many supported the concept of having such a database (Document ID
0328, 0329, 0330, 0335, 0336, 0339, 0341, 0352, 0365, 0366,
0379, 0383, 0389, 0408, 0410, and 0453). There were also various
comments about how a database might be done. Some thought OSHA should
do the classifications and maintain them online, or that the
classifications should be considered "official" (Document ID
0330, 0341, and 0453). Others were concerned about the
Agency's ability to develop and maintain a database (Document ID
0339), or said it should only be done if resources were
provided to maintain it (Document ID 0365). Alternatively,
resources could be provided for classifiers to help improve the quality
of their classifications (Document ID 0365).
Others suggested that NIOSH could be tasked with developing and
maintaining the database (Document ID 0341 and 0408). NIOSH
commented that funding is not currently available, and that OSHA may
wish to partner with the EU database efforts (Document ID
0412). Additionally, NIOSH and another commenter (Document ID
0383) suggested alternatives to developing a database using
existing information such as the Department of Homeland Security's
database; using International Chemical Safety Cards that currently
cover 1,650 substances and are translated into many languages; or
adding GHS classifications to the National Library of Medicine,
including its Hazardous Substances Data Bank. NIOSH is also updating
its Pocket Guide to include GHS classifications.
Another suggestion was to have the UN develop a database so there
is a globally harmonized list, and the Department of Labor could help
support it (Document ID 0328 and 0335). The National Fire
Protection Association (NFPA) (Document ID 0366) suggested
that its database of 2,500 chemicals could be useful in the transition.
Other commenters suggested that suppliers can provide classifications
to a central repository (Document ID 0352, 0408, and 0410),
but one commenter warned that if left to manufacturers, there would be
differences that would have to be resolved downstream (Document ID
0328). Another comment raised a concern that, while a common
database might be useful, it could also interfere with weight-of-
evidence determinations (Document ID 0379). However, such a
database could prove useful for substances, which would provide the
basis for mixture classifications (Document ID 0335).
Other commenters did not support having a classification database
(Document ID 0324, 0344, 0351, 0370, and 0377), or indicated
that if OSHA were to develop a classification list, it should be non-
binding guidance, and include stakeholder input and global
accessibility (Document ID 0344, 0381, 0393, and 0405). Others
were concerned that a common database would create another unharmonized
list of classifications compared to lists in other countries (Document
ID 0344), and that manufacturers should have the
responsibility for classification (Document ID 0324 and 0405).
Also, a company could have valid data that contradicts a classification
assigned in a database, and should be allowed to use its own
information (Document ID 0351). There was also a concern that
such a list might impede progress by not using the best available data
(Document ID 0377). Another commenter argued that the database
would need to be internationally developed and maintained to be useful,
which would result in the elimination of national or regional lists
(Document ID 0376).
OSHA is very interested in whether an international database of
classifications could be developed and maintained. It is not likely to
be feasible for OSHA to develop and maintain a U.S.-based database,
which, as some have noted, would be less useful than an internationally
harmonized approach that preempts countries and regions from developing
their own approaches. The subject has been raised and discussed in the
UN Sub-committee, and a correspondence group has been established to
explore the issue further. OSHA has volunteered to lead that group and
to help form a consensus position in the Sub-committee on options to
address this issue. In the meantime, some of the suggested sources can
provide extensive information to assist businesses with GHS
classifications, particularly small businesses with fewer technical
resources. The International Chemical Safety Cards--which are linked on
both OSHA and NIOSH Web pages--are one such resource. The OECD has also
established a global chemical portal that includes extensive
information on chemicals (www.oecd.org/ehs/eChemPortal).
(e) Written hazard communication program. The GHS does not include
provisions for a written hazard communication program. Thus the
provisions of this paragraph are not directly affected by
implementation of the GHS. The only changes proposed align terminology
(i.e., the proposal uses the term "safety data sheet" rather than
"material safety data sheet").
The written hazard communication program requirements in paragraph
(e) are intended to ensure that hazard communication in a given
workplace is coordinated and comprehensive. An employer's program must
include a list of the hazardous chemicals known to be present in the
workplace (paragraph (e)(1)(i)). This list is basically an inventory of
the chemicals the employer must have safety data sheets for, and must
be available to employees so they, too, can determine what chemicals
should be included under the hazard communication programs in their
workplace. The list can be maintained by work area or for the workplace
as a whole, and must be kept by an "identity" of the chemicals (which
will be the "product identifier" under the final rule). In other
words, the inventory can be common names or product names, rather than
individual chemical ingredients of each product by specific chemical
identity or chemical name.
The employer's hazard communication program must also include how
the standard's requirements for labels, SDSs, and training will be met
(paragraph (e)(1)); how the hazards of non-routine tasks will be
addressed (paragraph (e)(1)(ii)); and how hazard communication will be
handled in a multi-employer workplace situation (paragraph (e)(2)).
OSHA has provided guidance over the years on completing a written
program, and there are many sample programs in circulation. The program
need not be lengthy or complicated, but it should have enough detail to
provide the reader with a blueprint of the workplace-specific program.
Several comments to the ANPR were received from the Small Business
Administration (SBA) and others that suggested there would be
significant burdens associated with revising the written program as a
result of implementing the GHS (See, e.g., Document ID 0022,
0027, 0111, and 0164). Revising the chemical inventory was cited by
these commenters as one aspect that was likely to be burdensome. Since
the chemical inventory is basically a list of the products an employer
has in the workplace that are considered hazardous, the only way this
list would change as a result of implementing the GHS would be if
something that was not hazardous before is now, or vice versa. OSHA
believes that this is not a significant concern for three reasons.
First, it would be unusual for a chemical to only have one hazardous
effect associated with it so that the overall determination of hazard
would be affected by a change in classification in one hazard class.
Second, because HCS currently covers hazardous chemicals, unless the
chemical is new, it is highly probable that it is already covered.
Third, as discussed above in relation to paragraph (b) (Scope and
application), OSHA does not believe that the scope of hazards covered
by the final rule is substantially different than the current HCS.
The most likely differences resulting from re-classification under
the final rule are that a chemical would be placed in a category under
a hazard class that does not currently include categories. It may also
be possible that a chemical may fall into a different category where
there are already defined categories (such as flammability). Neither of
these differences would necessitate a change in the inventory.
With regard to other changes in an employer's program, it does not
appear likely there would be many, if any at all. Written hazard
communication programs usually include provisions such as who in the
organization is responsible for implementing different parts of the
program, or the type of in-plant labeling system used. The final HCS
will not affect those provisions. OSHA does not believe that extensive
revisions would have to be made to written programs, including the
inventory, under the final rule.
OSHA did not propose any substantive modifications to the written
hazard communication program, and it does not anticipate any
significant new burdens associated with revising the program as a
result of other modifications in the final rule.
While the written hazard communication program was mentioned
several times in relation to the costs of compliance, or the burdens on
small businesses, it was generally not discussed in a substantive way
by rulemaking participants. The Building and Construction Trades
Department of the AFL-CIO (Document ID 0359) expressed
concerns about the challenges associated with implementation of the HCS
on multi-employer worksites, a subject that is addressed in the written
hazard communication program requirements. They suggested that the
controlling employer on a site coordinate hazard communication
activities. This is not a subject related to adopting the GHS, and no
changes are being made to the rule to address it. The written program
must address how the exchange of information will be accomplished, and
that will continue under the final rule.
(f) Labels and other forms of warning. The HCS is designed to
provide information through three different media: labels or other
forms of immediate warning; safety data sheets; and training. Labels
are attached to the container of chemicals, and thus provide the
information that employees have the most ready access to in the
workplace. Given that they are attached to containers, they are by
necessity somewhat limited in the amount of information they can
present. The labels provide a snapshot or brief summary of the more
detailed information provided to employees in training programs, or
available to them on safety data sheets. They are not intended to be a
complete or detailed source of information on the chemical.
In the current HCS, the requirements for labels are performance-
oriented. At the time the standard was promulgated, there were many
different types of labels in use. A common label format used by
industry was that provided by the ANSI Z129, Hazardous Industrial
Chemicals--Precautionary Labeling standard. Employers following this
format at the time provided a number of different types of information
on the chemicals involved. However, there were two areas where
employers were inconsistent or did not necessarily provide what was
needed when following the national consensus standard. The first was
provision of an identity on the label that could lead a chemical user
to the specific chemical identities for the hazardous ingredients. It
was common practice to provide a trade name for a product, but not the
names of ingredients, on either the label or the safety data sheet. The
second was provision of specific information on the hazards involved,
such as the target organ affected.
The current HCS label provisions focus on this typically missing
information. On shipped containers, chemical manufacturers or importers
are required to include an identity, and appropriate hazard warnings,
as well as their name and address or that of a responsible party. The
term "identity" is defined in the current HCS definitions (paragraph
(c)) as "any chemical or common name which is indicated on the
material safety data sheet (MSDS) for the chemical. The identity used
shall permit cross-references to be made among the required list of
hazardous chemicals, the label and the MSDS." The hazard warning is to
provide specific information about the health or physical hazards posed
by the chemical. The term is defined as "any words, pictures, symbols,
or combination thereof appearing on a label or other appropriate form
of warning which convey the specific physical and health hazard(s),
including target organ effects, of the chemical(s) in the container(s).
(See the definitions for 'physical hazard' and 'health hazard' to
determine the hazards which must be covered.)"
The current HCS similarly requires identity and appropriate hazard
warnings for in-plant containers. OSHA has taken a flexible approach to
in-plant labeling, allowing a wide variety of systems to be used as
long as all of the required information is readily available to
employees when they are in their work areas. Thus the current standard
allows employers to continue to use systems such as the Hazardous
Materials Information System (HMIS) and the National Fire Protection
Association (NFPA) labeling systems that use numerical rankings of
hazard.
The labeling provisions of the current HCS exemplify the overall
performance orientation of the rule. They establish the basic
information requirements for chemical manufacturers and importers, but
do not specify a format, or any particular label elements to be used.
As a result, labels are often quite different when the same chemical is
addressed by different suppliers, creating the potential for employee
confusion. While many manufacturers follow the ANSI national consensus
standard, others do
not. Large manufacturers have frequently developed their own libraries
or repositories of standard phrases, with decision logics for when to
apply them to convey a hazard or a precaution. Therefore, not only does
this approach lead to labels that are different, it also results in a
large duplication of effort by chemical manufacturers developing their
own systems.
This performance-oriented approach also did not lend itself to
harmonization. Other countries often use more specific approaches,
including assignment of standard phrases to certain hazardous effects,
symbols, and other label elements. It was clear that the performance
orientation of HCS, with its many acceptable varieties of labels, could
not be standardized through agreement on content to achieve
harmonization.
Given that a more specified approach would also lead to consistency
among manufacturers, as well as helping to ensure the same message is
received by all exposed employees, OSHA agreed to negotiate a
harmonized approach that was more specific than the current standard.
This was also agreed to by stakeholder representatives involved in the
negotiations. Thus once a chemical is classified as to its hazard
classes and corresponding categories, the GHS specifies exactly what
information is to appear on a label for that chemical. As described in
Part IV of this preamble, OSHA believes that these specific labeling
requirements will be more protective of employee health and safety than
the current performance-oriented standard.
The NPRM proposed more modifications for paragraph (f) than most of
the other paragraphs of the existing standard. It changed the title of
paragraph (f)(1) to indicate it addresses labels on shipped containers.
OSHA also proposed adding a number of new types of information to the
label: Product identifier, signal word, hazard statement(s),
pictogram(s), precautionary statement(s), and the name, address, and
telephone number of the chemical manufacturer, importer, or other
responsible party. One commenter (Document ID 0520) proposed a
different format for the requirements in paragraph (f). While OSHA
appreciates the suggestion, the format followed by OSHA is dictated to
a large extent by document drafting requirements of the Federal
Register, and remains the same in the final rule. Commenters suggested
that OSHA add the words "where specified" to paragraph (f)(1) because
there are a few hazard categories that do not require all of the
elements listed (for example, there may be no symbol required for the
category (Document ID 0344, 0381, 0381, and 0393)). However,
this concern is addressed in paragraph (f)(2), which states that the
information has to be consistent with Appendix C. Therefore, the change
has not been made. There was also a suggestion that the language in
(f)(1) conflicts with the definition of label (Document ID
0353). OSHA reviewed both the paragraph language and the
definition, and does not agree. Therefore, this change has not been
made.
The final rule requires that labels on shipped containers contain
much more information than required by the current standard. However,
much of this additional information has already been included by
manufacturers, particularly when following the ANSI standard for
precautionary labeling. In addition, the OSHA requirements are intended
to be the minimum information to be provided by manufacturers and
importers. Under the GHS, as well as the current HCS and the final
rule, chemical manufacturers and importers are free to provide
additional information regarding the hazardous chemical and precautions
for safe handling and use. The GHS and the final rule refer to this as
supplemental information. Several commenters requested that this be
permitted (Document ID 0132 and 0145). As has already been
discussed above with regard to the definitions for hazard statements
and precautionary statements, such additional information is permitted
in Appendix C of the rule as long as it is accurate and does not
conflict with the required label elements. Paragraph (f)(1) is adopted
in the final rule as proposed except to provide clarity in light of
OSHA deleting the requirement for labeling for hazards not otherwise
classified. OSHA has modified paragraph (f)(1) to explicitly state that
hazards not otherwise classified do not have to be addressed on
container labels. Paragraph (f)(1) in this final rule now requires that
chemical manufacturers, importers, or distributors ensure that each
container of hazardous chemical leaving the workplace is labeled,
tagged, or marked. Hazards not otherwise classified do not have to be
addressed on the container. The paragraph also includes the information
that the chemical manufacturer or importer must provide on the label,
tag, or mark.
Paragraph (f)(2) of the proposal addressed labeling for
unclassified hazards. As noted in the discussion on definitions, this
has been changed to Hazards Not Otherwise Classified in the final rule.
In addition to the change in the definition, OSHA has removed the
proposed requirement for labeling unclassified hazards. Since there are
no label elements in the rule to address these hazards, the Agency
decided to cover them in a more limited fashion, and removed the
requirement for labeling them from the final rule. Hazards not
otherwise classified will still be addressed on the SDS.
Paragraph (f)(3) in the proposal elaborated the label requirements
by stating that the required information would be taken from new
Appendix C of the standard on Allocation of Label Elements, which
incorporates the GHS labeling requirements. This Appendix specifies the
signal word, hazard statement, pictogram, and precautionary statements
for each hazard class and category. It also includes a few basic rules
about preparing labels that address precedence of hazards and other
topics. Thus once a hazard classification is completed, the chemical
manufacturer or importer can refer to Appendix C to determine what
information must be included on the label. Since paragraph (f)(2) of
the proposal has been deleted from the final standard, paragraph (f)(3)
of the proposal is now paragraph (f)(2) in the final rule. Each of the
subsequent paragraph numbers have changed accordingly. New paragraph
(f)(2) also requires that the label be prominently displayed, and in
English (although other languages may also be included).
New paragraph (f)(3) requires the harmonized information to be
located together on the label, tag, or mark. This paragraph has been
adopted in the final standard as it was proposed.
The rest of paragraph (f) in the current standard remained largely
the same in the proposed modified text, although conforming changes to
terminology were made throughout the paragraph. The current standard's
accommodation for labels associated with solid metal was maintained in
the revised text, although OSHA has added a heading of "Solid
materials" to it. The provision regarding conflicts with the
requirements of DOT has also been maintained. In fact, since transport
rules have been harmonized with the other sectors under the GHS, the
possibility of a conflict in information is less likely when the HCS is
consistent with the international approach. Two ANPR commenters
specifically noted that OSHA should avoid conflict with DOT (Document
ID 0064 and 0066). This is already addressed in paragraph
(f)(5) in the final standard. NPRM commenters further noted that the
exterior package should be for displaying DOT labels, rather than for
OSHA labels (Document ID 0345). In general, this would be
true, although there are some cases where the single container serves
as both the shipping container and the workplace container, such as
drums. In these situations, there are rules in the GHS regarding which
pictograms take precedence and the ways in which to display the
information. These rules are set forth in Appendix C of the final
standard.
The American Trucking Association (ATA) also raised the issue as to
whether a GHS-compliant label might lead to a carrier's violation under
DOT based on the carrier's "constructive knowledge" that a shipment
contains a hazardous material (Document ID 0345). ATA
suggested that OSHA and DOT need to work together to address this
issue. OSHA contacted DOT and was told that this issue is addressed in
49 CFR 172.401, Prohibited Labeling. Specifically, GHS labels are
exempted under 49 CFR 172.401(c)(5).
Under proposed paragraph (f)(7) (paragraph (f)(6) in the final
rule), OSHA addressed workplace labeling. As noted previously, the
current standard provides employers with flexibility regarding the type
of system to be used in their workplaces. Some ANPR comments suggested
that OSHA maintain this flexibility in the proposed standard (See,
e.g., Document ID 0047, 0145, and 0157). OSHA agrees, and the
final rule retains the flexibility by indicating that the employer can
choose to label workplace containers either with the same label that
would be on shipped containers for the chemical under the revised rule,
or with label alternatives that meet the requirements for the standard.
It should be noted that while alternatives are permitted for workplace
containers, the information supplied must be consistent with the
revised HCS. Hazard classifications must be revised as necessary to
conform with the final rule, and the other information provided must be
revised accordingly to ensure the appropriate message is conveyed.
Final paragraph (f)(7) remains the same as proposed.
OSHA did not propose to modify the remaining paragraphs on labels
in the current HCS, including those that deal with alternatives to
affixing labels to stationary containers; labeling of portable
containers where the materials are transferred from a labeled
container, used within a work shift, and under the control of the
employee who performs the transfer; ensuring that all containers in the
workplace have a label; a requirement for workplace labels to be in
English and prominently displayed, while allowing the information to be
in other languages as well; and the requirement for updating label
information when there is new and significant information regarding the
hazards of a chemical.
The only one of these provisions that received significant comment
was the one regarding updating of label information within three months
of receiving new and significant information regarding the hazards of a
chemical. This provision ((f)(11) in the final rule) has been in the
HCS since the 1994 revisions, but an administrative stay was placed on
it shortly after it was promulgated in response to manufacturers'
concerns. That administrative stay was never reconsidered or removed by
OSHA, so the provision was not enforced. OSHA noted in the NPRM (74 FR
50283, Sept. 30, 2009) its intent to lift the stay, and requested
comment and input on whether the time frame is appropriate. It should
also be noted that an administrative stay is a tool available to OSHA
to cease enforcement for reasons the Agency finds appropriate. It is
not, as some appeared to assume, something that is adjudicated by an
outside body, nor does it involve publication or documentation based on
any type of record. It is usually a short-term solution to a problem
that can be resolved through discussions with affected parties.
The current HCS requires that SDSs be updated within three months
of learning of significant new hazard information, and that requirement
has been enforced since the standard first went into effect in 1983. 29
CFR 1910.1200(g)(5). It is important to ensure that labels are
similarly updated in a timely fashion, particularly since they provide
the most immediate information in the workplace.
It appears that some commenters thought this provision was the
effective date for updating the labels with the new GHS-aligned
provisions (Document ID 0400, 0502, and 0513). This is not the
case. Paragraph (j) of the final rule gives a much longer time period
to implement the new GHS label requirements. Paragraph (f)(11), by
contrast, addresses situations when a label must be changed because
there is new and significant information about the hazards of the
chemical. For example, there may be new studies that indicate an
ingredient of the product is a potential carcinogen. This happens
infrequently, so it is not anticipated that this provision would apply
in many cases.
The key concern of commenters is what to do about stockpiles of
chemicals that are already labeled. As noted by one commenter (Document
ID 0370), new technology is available that links labels and
SDSs, making new label generation more efficient. Stockpiles and
distribution are now managed through computer programs that were not
widely available in 1983. These programs can affect the amount of
product kept in stockpiles, as well as the distribution of products in
the supply chain, and thus the ability to deal with this updating
issue. Consequently, a number of participants agreed that three months
was an acceptable time frame (Document ID 0330, 0335, 0336,
0339, 0349, 0351, 0370, 0383, 0408, and 0410). Other commenters
suggested that it was reasonable to allow sales to continue of products
that are already labeled (Document ID 0313, 0323, 0327, 0328,
0329, 0344, 0351, 0361, 0375, 0377, 0381, 0399, and 0410). For example,
Ecolab (Document ID 0351) stated:
Ecolab agrees that three months for labels to be updated with
significant changes to the hazards is acceptable. However, it would
also be reasonable to allow the sell-through of product that is
already produced and labeled. By three months, we agree new
production of that product should occur with the significant new
information, as long as existing date-coded inventory can be sold
without modification. * * *
Others thought the administrative stay should be continued
(Document ID 0353 and 0405). Of those who suggested
alternative time frames, a number thought twelve months would be
appropriate (Document ID 0328, 0352, 0372, 0376, 0382, 0399,
0402, and 0405). Others indicated three months was not enough (Document
ID 0379); updating at some time interval is needed (Document
ID 0365); six months would be the minimum (Document ID
0324, 0344, and 0361); or a range of six or seven to twelve
months would be appropriate (Document ID 0411).
The North American Insulation Manufacturers Association (NAIMA)
detailed some of the factors that influence the ability of a
manufacturer to update a label: (1) Identification of the products
whose labels need to be changed; (2) drafting new label language, which
might require redesign of the packaging; (3) the ability to obtain new
label or packing stock for printing; (4) the availability of printers
to print the new material within the required time; (5) and
transportation time for stock to the printer, from the printer to the
manufacturer, and from the manufacturer through the supply chain
(Document ID 411). NAIMA argues that many of these factors may
be beyond the control of the manufacturer.
OSHA will not maintain the stay. It is necessary that labels be
updated to ensure that users have the appropriate information in a timely manner.
OSHA is also not convinced that any difficulties in updating labels
justify a full year's delay in providing significant new information.
However, OSHA is persuaded that, in some cases at least, it may be
difficult to update labels within three months. Thus, final paragraph
(f)(11) allows six months to begin labeling shipped containers with the
new information. As noted above, there are few situations where this
provision will come into play. It is not related to every modification
of the label, just those that are significant with regard to hazard
information. Six months should be long enough to revise labels, and
allow for the depletion of already labeled product. While some
commenters discussed the need for global compliance associated with
different labels (Document ID 0376), OSHA is only requiring
domestic compliance within this time frame. Therefore, the provision is
adopted in the final rule with a six-month time period for updating
product labels when there is new and significant information about the
hazards.
One commenter suggested that OSHA add a new requirement that
importers, distributors, and employers inform the chemical manufacturer
in writing, within three months, when they become aware of significant
information about the hazards of a chemical (unless they have already
received this information from the chemical manufacturer) (Document ID
0520). The HCS has always been designed on the premise that
the chemical manufacturer is in the best position to know what
information is available about the chemicals produced. This information
is then to be disseminated downstream to distributors and users of the
chemical. This suggestion would create a very extensive new burden on
parties in the distribution chain who are not responsible for the
chemical or the information regarding it as required under the GHS. It
is not consistent with the approach in the rule, and is not the most
effective and efficient way to identify and distribute information.
Therefore, OSHA rejects this suggestion. However, downstream users are
free to inform manufacturers of new hazards of which they learn, and
OSHA encourages the sharing of such information.
A few commenters on the ANPR also argued that a small package
exemption, or some type of prioritization of information on small
packages, should be permitted (Document ID 0043, 0046, and
0080). The current HCS does not have such an exemption or limitation,
but the Agency has allowed practical accommodations in enforcement
policies for those situations where an issue has occurred. (See, e.g.,
CPL 02-02-038" Inspection Procedures for the Hazard Communication
Standard: "CSHOs must consider alternate labeling provisions (for
example, tags or markings) for containers which are of unusual shape or
proportion and do not easily accommodate a legible label.")
In Revision 3 of the GHS, some provisions regarding small package
labels have been included (1.4.10.5.4.4, Labelling of small
packagings). The competent authority is given the discretion to
implement changes that allow label preparers to reduce the required
information to accommodate a small package size. OSHA did not propose
to adopt such a provision, and has retained its current approach
regarding small packages in the final rule. Very small packages are
less frequent in the workplace than in consumer settings, and it is
difficult to argue that employees should get less information just
because of the size of the package. The practical accommodation
approach OSHA has been utilizing addresses those situations where there
is a valid issue, and ensures that workers receive all of the required
information.
Following the NPRM, further comments were received on the issue of
labeling small packages. Some suggested that OSHA should provide clear
guidance for small containers, including perhaps a suggested priority
for the label information (Document ID 0313, 0327, and 0339).
Others thought the manufacturer should be permitted to pick the most
important hazard and precautionary statements to include on small
packages (Document ID 0405), or that OSHA should use the GHS
guidance on the issue (Document ID 0342). Particular problems
were noted, such as labeling small containers for reference standards
(Document ID 0342). Phylmar Regulatory Roundtable testified
during the hearing, and suggested that OSHA should either establish a
priority for information on a small package label, or clarify what is
meant by practical accommodations (Document ID 0497 Tr. 113).
The guidance in the GHS (1.4.10.5.4.4) basically allows countries
to introduce a consideration of risk by determining that small
quantities of the chemical are not a concern, or that information may
be omitted because of the small volume. This approach is not consistent
with the HCS, or with the concept of right-to-know. It is also
unacceptable to OSHA to allow manufacturers to decide which information
is the most important. Essentially, all of the suggested solutions
result in less information being available to exposed employees than
other employees would receive when exposed to the same chemical
packaged in a larger container.
The concept of practical accommodations is difficult to define,
since it entails a judgment by OSHA staff when confronted with the
details of a specific situation. The point, however, is to find a way
to provide the required information in every situation, and not to
start with the premise that the solution is to omit such information.
Ensuring that workers receive the required information may be
accomplished in ways other than simply attaching it directly to each
small container. OSHA will examine the situation to make sure that the
information is associated with the proper containers, and that it is
complete. OSHA is not adopting any regulatory requirements for small
packages, but will consider whether any additional guidance is needed
as the standard is implemented.
While the GHS specifies the information to be placed on a label, it
does not provide a specific format for placement, which is similar to
current HCS requirements. At least one commenter noted that the GHS
does not specify a location or size of core information on a shipment
(Document ID 0066). OSHA believes that the performance-
oriented approach of paragraphs (f)(3) and (f)(10) is preferable. The
Agency will allow accommodations to be made as long as the information
is located together, and is prominently displayed as required.
A number of commenters endorsed the overall approach or specific
parts of the label requirements. Comments included adopting the GHS
labels (Document ID 0324 and 0339), supporting the flexibility
of the in-plant labeling (Document ID 0392), and the use of
signal words (Document ID 0321). Others wanted to ensure that
hazards are conveyed accurately to all levels of education in the work
force (Document ID 0331); supported allowing other languages
on labels (Document ID 0381); suggested OSHA should allow
flexibility of format and placement of required label elements
(Document ID 0405); and suggested that OSHA should follow
Revision 3 of the GHS for label requirements (Document ID
0382). OSHA believes that the final standard incorporates all
of these concepts.
Appendix C details how the specified label elements apply to each
hazard class and hazard category. OSHA has made some modifications to
the introductory text to Appendix C regarding the combination of hazard and
precautionary statements, and these modifications were discussed under
paragraph (c), Definitions. Comments received regarding red border
frames for pictograms, and making the precautionary statements
mandatory, are also discussed above in the explanation of paragraph
(c), Definitions. Also, as discussed in the explanation of that
paragraph, OSHA has added definitions to the final standard for simple
asphyxiant and pyrophoric gas. The Agency has also added a new section
to Appendix C to provide the label elements for these hazards (C.4.30,
Label Elements for OSHA Defined Hazards).
In C.2.1, "Precedence of hazard information," addressing
precedence of symbols, OSHA indicated that where the skull and
crossbones is on a label, the exclamation point should not be included
for acute toxicity. In the GHS, the statement simply says the
exclamation point should not be included where the skull and crossbones
is on the label. This is followed in the GHS by two other statements
about not using the exclamation point for specific hazards when there
is already a symbol for the more severe category of the same hazard.
OSHA received a comment that the phrase "where it is used for acute
toxicity" should be deleted since it is not in the GHS (Document ID
0393). OSHA believes that this phrase is appropriate for
clarity and parallel construction with the other provisions of the
paragraph. The skull and crossbones symbol only addresses acute
toxicity, and does not convey other types of effects.
One commenter indicated that paragraph C.2.3.3 should not be
mandatory (Document ID 0335). The paragraph indicates that
when there is a DOT pictogram for a hazard on a label, an additional
GHS pictogram for the same hazard must not appear. The reason it is
mandatory is that having two different pictograms addressing the same
hazard may lead to confusion for people handling the chemical.
OSHA also indicated that it was proposing to exclude ammunition and
ammunition components under Division 1.4S from having the exploding
bomb symbol and precautionary statements normally used for explosives
(74 FR 50283, Sept. 30, 2009). This proposed exclusion was based on
discussions during OSHA's rulemaking to update the explosives standard,
and the issue of ammunition being sold in retail establishments. The
Agency asked for input on whether the exclusion of the symbol was
sufficiently protective, and whether any adjustments needed to be made.
Several people thought the symbol should be included on ammunition and
components since they are explosive (Document ID 0313, 0327,
and 0328). However, others thought it was appropriate to treat
ammunition and components differently, and that the exploding bomb does
not represent the hazards of ammunition (Document ID 0330,
0336, 0338, 0370, and 0376). OSHA agrees with these commenters that the
exploding bomb does not represent the hazards of ammunition, implying
that there is a mass explosion hazard when handling these items,
although that is not the case. Therefore, the Agency is maintaining the
proposed provisions in the final standard, and will not be requiring a
symbol or precautionary statements for ammunition and ammunition
components.
A question was raised by the National Propane Gas Association
(Document ID 0400) regarding signal words for propane if both
simple asphyxiant and flammability hazards are covered since they have
different signal words (warning and danger, respectively). Appendix C
explains the precedence rules for signal words. Only one is ever
required on a label. If one of the hazards warrants a "danger" signal
word, then that will be the only one required on the label.
A few comments were also received about the interface of the new
OSHA label requirements with the requirements of other agencies. For
example, it was noted that it would be difficult to use one label to
comply with both OSHA and CPSC (Document ID 0405), and that
EPA and CPSC should accept GHS labels until they adopt the system
themselves (Document ID 0328). OSHA does not have authority to
determine the policies of other agencies with regard to accepting the
new GHS-aligned labels. Another commenter noted that fireworks are
regulated by other agencies, and therefore additional requirements are
burdensome (Document ID 0355). The new OSHA requirements will
be essentially harmonized with DOT's requirements, which will
facilitate compliance with both agencies. Lastly, it was noted that
OSHA should coordinate label implementation with Canada's Workplace
Hazardous Material Information System (WHMIS) (Document ID
0461). As was noted earlier, OSHA does have bilateral
discussions with Canada on implementation issues--however, Canada has
not yet adopted the GHS or initiated implementation by regulation.
(g) Safety data sheets. The proposed revisions to this paragraph
were confined primarily to paragraph (g)(2), other than conforming
terminology regarding classification and SDSs. Paragraph (g)(2) of the
current HCS indicates what information must be included on an SDS. It
does not specify a format for presentation, or an order of information.
Chemical manufacturers and importers have been free to use whatever
format they choose, as long as the information is provided.
While this performance orientation was supported by chemical
manufacturers when the standard was originally promulgated, it was
largely based on the positions of those who were already providing SDSs
and did not want to change their format. As the scope of the standard
was expanded to cover other industries, it became clear that SDS users
preferred a uniform order of information or a format. In particular,
stakeholders such as emergency responders were concerned that
information not being located in the same place on every SDS could
create an increased risk in situations where the information was needed
quickly.
Several years after the HCS was adopted, the chemical manufacturers
themselves responded to these concerns by developing a national
voluntary industry consensus standard that included a 16-section SDS
(ANSI Z400, Hazardous Industrial Chemicals--Material Safety Data
Sheets--Preparation). This consensus standard establishes the titles of
each section and the order of presentation. It addresses concerns
raised by also putting information of most use to those exposed in the
beginning of the SDS, with the more technical data required by health
and safety professionals in later sections. ANSI Z400 also responded to
comments indicating that the SDS should be essentially "one stop
shopping" in terms of information on a chemical, and should include
other information such as how it is regulated by other Federal
agencies, including transport requirements and environmental
information by having sections for each of those categories of
information.
In 1990, OSHA published a Request for Information (RFI) that
addressed the issues of comprehensibility of labels and SDSs (55 FR
20580, May 17, 1990). Nearly 600 comments were received, and the
majority of respondents sought an order of information or format for
SDSs. Since the international harmonization process had begun at that
point, OSHA thought it would be useful to wait until a globally
harmonized SDS was available before changing the
requirements. However, through interpretation, OSHA has made clear for
many years that the ANSI format is acceptable, as long as the SDS
includes the required information (See CPL 02-02-038, "Inspection
Procedures for the Hazard Communication Standard" (Mar. 20, 1998), the
compliance directive for the HCS). As explained in Section IV of this
preamble, OSHA believes that the implementation of a standardized SDS
format will enhance hazard communication and be more protective of
employee health than the current performance-oriented standard.
The 16-section format continued to be recognized in different
countries and organizations over the years, including an International
Labour Organization (ILO) recommendation on chemical safety, the
European SDS requirements, and an International Standards Organization
standard on SDSs. When the GHS was developed, it was decided that this
16-section format was already a de facto international approach, so it
was adapted to be part of the GHS. One small change was made to reverse
sections 2 and 3 so that hazard information comes before the chemical
names of ingredients. This change has subsequently been adopted by ANSI
and other groups to be consistent with the GHS.
Since the 16-section SDS was initiated in the U.S. by industry,
many companies have been using it. This adoption by industry will
reduce the impact of the harmonized GHS requirements. Others who
continued to use different formats will need to change their SDSs to
conform. There is already software available to assist in developing
SDSs in the 16-section format, and it is expected that more tools will
be available as the dates for SDS compliance approach.
OSHA proposed to modify paragraph (g)(2) to establish the section
numbers and title headings of the sections of the SDS to be consistent
with the GHS. Furthermore, a new Appendix D was proposed to be added to
the standard to address safety data sheets, and it indicates what
information must be included in each section.
As OSHA indicated in the ANPR and the NPRM, sections 12 through 15
of the SDS require information on subjects that are outside the
Agency's jurisdiction (See the list of sections below). OSHA will not
be making these sections mandatory for inclusion, nor will any
enforcement activity be directed to these sections. However, inclusion
of the sections in an SDS is not precluded, and they have been included
in the text of the revised standard so people will be aware that a
fully GHS-compliant SDS will have to address those areas in addition to
the ones mandated by OSHA.
The revised SDS would require the following sections:
Section 1. Identification.
Section 2. Hazard(s) identification.
Section 3. Composition/Information on ingredients.
Section 4. First-aid measures.
Section 5. Fire-fighting measures.
Section 6. Accidental release measures.
Section 7. Handling and storage.
Section 8. Exposure controls/personal protection.
Section 9. Physical and chemical properties.
Section 10. Stability and reactivity.
Section 11. Toxicological information.
Section 16. Other information, including date of preparation of
the last revision.
A note in the revised text addresses the other sections that are
not mandatory for OSHA:
Section 12. Ecological information.
Section 13. Disposal considerations.
Section 14. Transport information.
Section 15. Regulatory information.
The remainder of the paragraph on SDSs remains the same as the
current HCS. The final rule, like the proposal, retains the current HCS
design, ensuring the downstream flow of information from the chemical
manufacturer or importer to the distributor and ultimately the
employer. Other provisions (completion of all sections of the SDS;
provisions for complex mixtures; the requirement for information to be
accurate and reflect the scientific evidence; the need to update the
SDS when new and significant information is available; maintenance of
SDSs so they are accessible to employees; accommodations for situations
where employees travel between workplaces during a work shift; and
access for OSHA and NIOSH) remain in this final standard as they are in
the current standard, although they have been re-numbered.
As was the case with labels, relatively few comments were submitted
in response to the ANPR or the NPRM on the specific provisions for
SDSs. The final provisions are generally consistent with the current
HCS, with the exception of the standardized approach described above
that OSHA proposed and adopted in the final rule.
The only text changes that were made to the provisions that follow
(g)(2) in the standard were to revise the terminology to be consistent
with the new approach. However, there were some editorial suggestions
for other changes (Document ID 0353). Consistent with OSHA's
stated intent to not change anything that does not require change to
align with the GHS, these suggestions have not been implemented in the
final rule.
A number of rulemaking participants stated that they support the
standardization of SDSs, and some noted that standardization would
facilitate training (Document ID 0307, 0321, 0322, 0349, 0456,
and 0463). It was suggested that OSHA update (g)(8) to (g)(10) to
indicate that electronic distribution is acceptable (Document ID
0376 and 0395). It is already stated in (g)(8) that electronic
access is acceptable for employees (although OSHA has removed
"microfiche" from this provision since that technology is outdated
and rarely used and in any event is captured under the broader term
"other alternatives," which is retained in the final rule).
Electronic distribution is not precluded, although the employer on the
receiving end of the information must be able to access it in that
form. The general issue of electronic distribution and access is
addressed in the compliance directive for the standard (CPL 02-02.038),
and is based on recommendations made by the National Advisory Committee
on Occupational Safety and Health (NACOSH). As explained in the
directive, electronic distribution is permitted, but the
appropriateness of its implementation will be judged as follows:
MSDSs must be readily accessible and there must be no barriers
to employee access during the work shift. The Agency interprets the
term "readily accessible" to mean immediate access to MSDSs. The
employer has flexibility to determine how this will be accomplished.
The use of electronic means such as computers with printers,
microfiche machines, the Internet, CD-ROMS, fax machines, etc., is
acceptable. Employers using electronic means to supply MSDSs to
their employees must ensure that reliable devices are readily
accessible in the workplace at all times; that workers are trained
in the use of these devices, including specific software; that there
is an adequate back-up system for rapid access to MSDSs in the event
of an emergency, including power outages, equipment, and on-line
access delays; and that the system is part of the overall hazard
communication program of the workplace. Additionally, employees must
be able to access hard copies of the MSDSs, and in the event of
medical emergencies, employers must be able to immediately provide
copies of MSDSs to medical personnel. Mere transmission of the
requested information orally via telephone is not acceptable.
Employers may use off-site MSDS management services to meet the
requirements of the HCS only if MSDSs are readily available to
employees, either as hard copies in the workplace or through
electronic means and as long as the provisions outlined
in the previous paragraph are ensured. Despite the use of an MSDS
management service, the employer maintains primary responsibility
for the hazard communication program, including receipt and use of
the information to develop and implement a site-specific hazard
communication program under paragraph (e) of the HCS.
When immediate access to paper or hard copy MSDSs does not
exist, CSHOs should evaluate the performance of the employer's
system by requesting a specific MSDS. Ultimately, the evaluation of
an adequate system will rely on the professional judgment of the
CSHO. Factors that may be appropriate to consider when determining
if MSDSs are readily accessible include:
(1) Are the sheets or alternative methods maintained at a
location and under conditions where employees can access them during
each work shift, when they are in their work areas?
(2) If an electronic system is used for MSDS access (computer,
fax, etc.) do employees know how to operate and obtain information
from the system? (CSHOs should request an employee to retrieve MSDSs
using the electronic system.)
(3) Was there an emergency/accident where immediate access was
critical?
(4) How quickly did the employer respond to the employee's
request?
Employees must have immediate access to MSDSs and be able to get
information when they need it in order for an employer to be in
compliance.
On multi-employer job sites, employers who produce, use or store
hazardous chemicals in such a way that other employers' employees
are exposed or potentially exposed, must communicate to other
employers how the means of access to MSDSs will be accomplished.
Various suggestions were made for improvements to SDSs. For
example, it was suggested that the SDS be limited to five pages
(Document ID 0415); that a one-page, eighth-grade reading
level summary of its contents should be provided (Document ID
0306); and that SDSs be written in plain and simple language
(Document ID 0347). OSHA agrees that SDS preparers should try
to ensure the SDSs are written clearly, and preparers should consider
the audience in determining how the information may be best
communicated. As originally designed by ANSI, the sections in the
beginning of the SDS are intended to be written in plain language, with
fewer technical terms where possible. This information should be of
immediate use in emergency situations, and addresses information that
exposed workers are most likely to need (summary of hazards for
example). But many of the remaining sections of the SDS require
technical information, and they are intended to be of use primarily to
professionals designing protective measures or providing services such
as medical surveillance to exposed employees. These sections need to
retain their technical terminology in order to be useful to the
professionals for these purposes. It is difficult to regulate those
aspects of preparing documents that are intended to convey technical
information, and no specific requirements of this type have been
included in the final standard.
There was also a comment that the Superfund Amendments and
Reauthorization Act (SARA) refers to material safety data sheets (See
42 U.S.C. 11022), and that changing the name to safety data sheets
would violate the Paperwork Reduction Act (PRA)(Document ID
0350). Changing the references to the data sheet does not
violate PRA or SARA. As is clear from the foregoing discussion, MSDSs
under the current standard and SDSs under the final rule both serve the
same function and communicate the same types of information. OSHA
believes that an SDS under the final rule should be treated as an MSDS
under SARA, but if the regulated community needs additional clarity, it
can ask EPA to issue an interpretation to ensure there are no
compliance issues. Similarly, because the change of the regulatory term
from material safety data sheet to safety data sheet does not, by
itself, create a paperwork burden, there are no PRA implications.
One commenter suggested that OSHA add to the SDS the date the
chemical was produced, where chemical testing occurred to determine SDS
data, and the manufacturer's Web site (Document ID 0346). OSHA
rejects this suggestion, noting that the final rule does not require
adding information to the SDS that would make it significantly
different from the GHS harmonized information requirements.
Furthermore, it would not be practical to require either the date the
chemical was produced (which would result in a costly requirement to
revise SDSs for every day the chemical was produced), or where chemical
testing occurred (which may not be known, given that such information
is obtained from many different sources, and studies do not frequently
indicate where the testing occurred). However, suppliers are free to
provide this information on their Web sites, and often do.
In the NPRM, OSHA noted that mixture safety data sheets could no
longer be prepared by attaching multiple SDSs for the ingredients, but
rather would have to be an SDS for the mixture as a whole (74 FR 50392,
Sept. 30, 2009). One commenter (Document ID 0334) thought the
multiple SDSs practice should continue to be allowed, particularly to
minimize burdens for small businesses. OSHA believes that this approach
is not in compliance with the GHS-aligned requirements. It also does
not provide the best information for those downstream, including small
business users.
New mandatory Appendix D, "Safety Data Sheets," provides
additional requirements for the information to be included under each
section heading. The sub-headings used to indicate the additional
information were lettered (e.g., (a) product identifier used on the
label, (b) Other means of identification, and so forth). Questions were
raised as to whether the letters identifying each subheading were
considered mandatory (Document ID 0382, 0376, and 0393).
Apparently, the EU requires the subheadings to be numbered. OSHA does
not consider the letters to be mandatory, but the information each
subheading identifies is required to be included. A similar comment
indicated that the format of Section 9, Physical and chemical
properties should be clarified (Document ID 0339). No
particular format is required. Appendix D simply requires that
information responsive to that heading and its subheadings must be
included. If applicable information is not available, the SDS must
state so.
Another commenter indicated concern that Appendix D does not refer
to ANSI Z400.1 or Annex 4 of the GHS (Document ID 0336). OSHA
does not believe that reference to either of these documents is
necessary since Appendix D is self-contained. As Appendix D is
mandatory, those documents would have to be incorporated by reference
to be referred to, and that is not necessary for purposes of compliance
with the standard. However, both ANSI Z400.1 and Annex 4 would be
useful references for SDS preparers since they provide additional
guidance for completing an SDS.
In the final rule, a small modification has been made to the
introduction to Appendix D to indicate that a subheading "within a
section" needs to be marked when no relevant information is available.
Also, OSHA has added column identifiers of "heading" and "sub-
heading" to clarify what is being referred to by that terminology.
Additional comments were received on specific sections of the SDS.
For example, in section 1, "Identification," the American Chemistry
Council wanted clarification of subheading (c), "Recommended use of
the chemical and restrictions on use" (Document ID 0393). As
explained in Annex 4 of the GHS, A4.3.1.3, the SDS preparer should
"provide the recommended or intended use of the substance or mixture,
including a brief description of what it actually does, e.g., flame
retardant, anti-oxidant, etc. Restrictions on use should, as far as
possible, be stated including non-statutory recommendations by the
supplier." Section 1 is adopted in the final rule as proposed.
On Section 2 of the SDS, "Hazard identification," the Soap and
Detergent Association argued that the requirement for precautionary
statements in subheading (b) should not be included because they are
not mandatory in the GHS (Document ID 0344). However, the GHS
requires that precautionary statements appear on a label
(1.4.10.5.2(c)), and Annex 4 (A.4.3.2.2) indicates that the GHS label
elements, including precautionary statements, should be included in
Section 2 of the SDS. As has already been discussed, OSHA is adopting
the GHS precautionary statements, so they are mandatory for purposes of
complying with this standard.
Other commenters questioned what was meant by "unknown toxicity"
in Section 2, subheading (d) (Document ID 0367 and 0371). This
term refers to the criteria for determining the acute toxicity of a
mixture where there are ingredients that have no available acute
toxicity data. In this case, the percentage of ingredients that have no
data to consider in the calculations must be indicated in Section 2. In
the final rule, OSHA has slightly modified sub-heading (d) to clarify
this reference.
In addition to this clarification, two other changes have been made
in Section 2. First, references to paragraphs (d) and (f) said
"paragraph (d)[(f)] of this section," which is the normal regulatory
reference since the entire standard is called a "section" of the Code
of Federal Regulations. However, since parts of the SDS under the
"Headings" column are also referred to as sections, it was confusing.
Section 2 now refers to the section number of the standard, 1910.1200.
This change is tracked in other parts of Appendix D as well. Second,
subheading (c) has been revised to refer to hazards not otherwise
classified, rather than unclassified hazards, consistent with
modifications to the regulatory text.
In Section 3, "Composition/information on ingredients,"
commenters indicated that OSHA had left out a phrase that appears in
the GHS with regard to identification of ingredients in a mixture
(Document ID 0344 and 0393). This was an oversight, and OSHA
has added the language "and are present above their concentration
limits/cut-off levels" into Section 3. To ensure consistency with the
classification criteria, OSHA has also clarified that ingredients that
present a health risk below the cut-off/concentration limits would also
need to be disclosed in section 3 of the SDS. It was also suggested
that where the SDS discloses only the range of concentrations, the
narrowest range possible should be permitted (Document ID
0395). Neither the GHS provisions for information on SDSs, nor
the guidance for completing them, address specific limits for
concentration limits. Under the current rule, concentrations of
chemicals in a mixture are not required to be disclosed at all. OSHA
agrees with the commenter that when SDS preparers use ranges rather
than a specific percentage composition, the range must be limited in
terms of the percentage concentration variation, and the variation in
concentration must have no effect on the hazard of the mixture.
In order to help ensure that use of concentration ranges is
understood, OSHA has added the term "concentration" in parentheses
after the "exact percentage" terminology used in paragraph (i)(1)
regarding trade secret protection. Similarly, the term "exact
percentage" has been added in parentheses after "concentration" in
Section 3 requirements for the SDS. These terms refer to situations
where the mixture has a set formula, and the amount of a substance in
the mixture is consistent from batch-to-batch. OSHA recognizes that
there are some very small variances in this situation that have no
impact on the hazard of the overall mixture. "Exact percentage" is
the terminology used in the GHS guidance for preparation of SDSs, but
these small variations or tolerances are expected and acceptable when
reporting the anticipated percentage based on the formula.
Concentration ranges, rather than concentrations, may be used in
other situations. For example, the final standard includes the
longstanding provision that addresses the use of a single SDS for
complex mixtures in paragraph (g)(4). Under this provision, where
complex mixtures have similar hazards and contents (the ingredients are
essentially the same, but the specific composition varies from mixture
to mixture), one SDS may be used for all of these similar mixtures.
Petroleum streams would be an example of a type of complex mixture to
which this provision applies. In this situation, concentration ranges
may be used for the ingredients that vary from stream to stream.
A chemical manufacturer or importer may also have a line of
products that are very similar, but can be varied slightly in
composition to meet the needs of customers. For example, toner colors
may be changed by varying the amount of pigment. The variances are
small, and the hazard remains the same. In these situations,
concentration ranges may be used for multiple, similar products.
Trade secret status may be claimed for exact percentage composition
but not for concentration ranges. Where a trade secret claim is made
for exact percentage, the chemical manufacturer or importer may choose
to provide a concentration range to assist downstream users in
providing appropriate protections and, at the same time, potentially
eliminating requests from users for disclosure of the trade secret in
accordance with Sec. 1910.1200. However, Section 3 must indicate that
a trade secret claim is being made and information has been withheld.
Section 8 addresses exposure controls and personal protection. Some
commenters noted that the information provided should have more detail
than what was proposed in Appendix D, such as requiring information on
specific PPE materials that provide protection (Document ID
0359 and 0456). OSHA agrees that SDS preparers should provide
the most specific information available for the material so that the
appropriate protective measures can be implemented. Annex 4 of the GHS,
guidance for preparing the SDS, addresses the specific type of
information on personal protective equipment that should be provided in
Section 8 of the SDS in paragraph A4.3.8.3. OSHA will be making
additional guidance available when the rule is implemented.
Section 8 also addresses inclusion of occupational exposure limits
(OELs) on the SDS. Comments were received on inclusion of exposure
limits on SDSs in response to the ANPR, and a number of different
opinions were expressed, particularly regarding TLVs being required.
Many ANPR commenters argued that TLVs should be included on the SDSs,
as is currently required under the HCS (See, e.g., Document ID
0042, 0179, 0021, 0038, 0124, and 0149). Others suggested they
should not be required (See, e.g., Document ID 0036, 0058,
0064, 0129, 0151, and 0163). A number of commenters suggested other
types of occupational exposure limits that should be included on SDSs,
such as levels from other countries, those recommended by NIOSH, and
those recommended by the American Industrial Hygiene Association (See,
e.g., Document ID 0018, 0024, 0109, 0147, and 0171).
In the NPRM, OSHA proposed to maintain the requirement to include
its mandatory permissible exposure limits
(PELs) on the SDSs, and to specify, as in the existing HCS, that
manufacturers should include "any other exposure limit used or
recommended by the chemical manufacturer, importer, or employer
preparing the safety data sheet." This would allow inclusion of any of
the different types of occupational exposure limits commenters
recommended for inclusion where the SDS preparer deems it appropriate.
It also helps to minimize differences between the U.S. and other
countries by not providing (except for PELs) a list of U.S.-specific
occupational exposure limits that must be included, yet provides
protection for employees by allowing inclusion of various
recommendations that will help employers design appropriate protective
measures. OSHA requested comment on this approach, and received many
opinions from rulemaking participants.
First, many people agreed that the PEL should be on the SDS
(although some acknowledged that they are out-of-date) (See, e.g.,
Document ID 0328, 0330, 0332, 0336, 0338, 0339, 0340, 0341,
0344, 0349, 0351, 0352, 0354, 0357, 0359, 0375, 0379, 0382, 0399, 0412,
and 0414). For example, the American Foundry Society (Document ID
0375) supported including the PEL, but thought other limits
should only be included at the discretion of the SDS preparer:
Our industry generally supports the requirement to include OSHA
PELs, but not require the other recommended limits on SDSs. In
particular, the American Conference of Industrial Hygienists
(ACGIH)--TLVs, while able to provide useful information often lack
credibility. As the result of a sometimes flawed development
process, the TLVs can be misleading and their use can reduce clarity
of communication. For certain materials, some manufacturers may
choose to include TLVs on an SDS, or include other non-mandatory
exposure values, including their own recommendations, but this
should not be mandatory. The relevance of such other non-mandatory
guidelines should be determined by the manufacturer who can best
explain the meaning, context and limitations of such values.
Others specifically supported the approach proposed (See, e.g.,
Document ID 0351, 0366, 0370, 0376, 0381, 0383, 0393, 0408,
and 0411). Clariant Corporation (Document ID 0383) indicated
they would support the proposed text, as well as a non-mandatory
appendix listing other exposure limits:
Clariant supports the recommendation to "include other
occupational exposure limits used or recommended". Clariant would
also support a non-mandatory appendix to the HCS to include
reference to the TLVs and other occupational exposure limits such as
the AIHA WEELs. Many companies already include other occupational
exposure limits on their SDS. In most cases, those other limits are
more up-to-date than the OSHA PELs.
The American Industrial Hygiene Association (AIHA) also suggested
inclusion of a non-mandatory appendix listing other exposure limits
such as the TLVs and WEELs (Document ID 0365).
Many commenters supported mandatory disclosure of applicable TLVs
on the SDS in Section 8 (See, e.g., Document ID 0313, 0315,
0317, 0319, 0323, 0327, 0328, 0330, 0332, 0336, 0340, 0347, 0349, 0353,
0354, 0357, 0359, 0401, 0403, 0410, 0412, 0413, 0414, 0463, and 0464).
Others argued that inclusion of the TLVs would be inappropriate because
such inclusion does not meet the Information (or Data) Quality Act, the
development process is flawed, or they are non-governmental (See, e.g.,
Document ID 0325, 0375, 0379, 0408, and 0409).
For example, the Center for Regulatory Effectiveness argued that
OSHA's decision to require the disclosure of ACGIH TLVs on SDSs is
inconsistent with the requirements of the Information Quality Act,
Public Law 106-554, Sec. 1(a)(3), Title V, Sec. 515, 114 Stat. 2763
(2000). That act required OMB and DOL to issue guidelines "ensuring
and maximizing the quality, objectivity, utility, and integrity of
information * * * disseminated by the agency." 44 U.S.C. 3516, note,
at (b)(2)(A). Both OMB and DOL have issued such guidelines, and in
addition OMB issued the "Peer Review Bulletin," citing the authority
of the Information Quality Act. OMB, Guidelines for Ensuring and
Maximizing the Quality, Objectivity, Utility, and Integrity of
Information Disseminated by Federal Agencies, 67 FR 8452 (Feb. 22,
2002) (hereafter "OMB Guidelines"); DOL, Guidelines for Ensuring and
Maximizing the Quality, Objectivity, Utility, and Integrity of
Information Disseminated by the Department of Labor (Oct. 1, 2002),
found at http://www.dol.gov/cio/programs/infoguidelines/informationqualitytext.htm
(hereafter "DOL Guidelines"); OMB, Final
Information Quality Bulletin for Peer Review, 70 FR 2664 (Jan. 14,
2005) (hereafter "Peer Review Bulletin"). Each of these guidelines
specifies certain steps an agency should take when engaged in the
"dissemination" of "information." OSHA does not believe that it is
disseminating "information," as defined by these documents, in
requiring disclosure of TLVs on SDSs.
All three documents except from the definition of information
"opinions, where the agency's presentation makes it clear that what is
being offered is someone's opinion rather than fact or the agency's
views." (OMB Guidelines V.5; DOL Guidelines at 5, 13-14; Peer Review
Bulletin I.5.) OSHA understands this to mean that the guidelines do not
apply unless the public could reasonably understand the information
being disseminated as the official view of the agency. This
understanding is supported by a number of statements by OMB and DOL. In
the preamble to the Peer Review Bulletin, for example, OMB states that
"[a]n information product is not covered by the Bulletin unless it
represents an official view of one or more departments or agencies of
the federal government." 70 FR at 2667/2. Likewise, DOL's guidelines
do not apply to information "clearly represented as opinion and not an
official agency or Departmental representation." DOL Guidelines at 3.
Hyperlinks on an agency's Web site to information on non-governmental
Web sites are not an agency dissemination of information, nor is a
private researcher's publication and communication of the results of a
government-funded study, where an appropriate disclaimer appears. OMB
Guidelines V.5; 67 FR 8454/1; DOL Guidelines at 5, 13-14.
Users of hazardous chemicals could not reasonably think that ACGIH
TLVs listed on an SDS are OSHA's dissemination of information as to the
correct or feasible level of exposure to the chemical. As explained on
the ACGIH Web site, TLVs are the ACGIH's statements of "scientific
opinion" (Document ID 0529). The SDS is prepared by the
manufacturer and represents the manufacturer's understanding of the
hazards of the chemical, the appropriate conditions of use, and the
necessary protective measures to be employed. It is hard to see, in
that context, how a user of the SDS could understand that the TLVs
listed on the SDS represent information disseminated by OSHA. The TLV
will be identified as such on the SDS. Indeed, in the many cases where
there is an applicable OSHA PEL, the PEL will also be listed in
addition to the TLV.
Further, if TLVs are "information" for purposes of the IQA, then
so too is everything in the SDS. If that were true, it would render the
approach of the HCS unworkable because it would require OSHA to review
and approve every manufacturer's label and SDS. OSHA does not believe
Congress intended such a result in enacting the IQA.
The Center for Regulatory Effectiveness and the AFL-CIO's Building
and Construction Trades Department suggested that OSHA could
require SDS preparers to add a statement to the SDS saying that the TLV
does not represent OSHA's view of a safe level (Document ID
0325 and 0644). OSHA has decided against such an approach.
First, as explained above, OSHA does not believe that a reasonable SDS
user would understand the TLV to be OSHA's official representation.
Second, such a disclaimer could cause confusion, creating the incorrect
impression that the remainder of the information on the SDS does
represent OSHA's official representation about the hazards of the
chemical in question.
There are other reasons the IQA guidelines do not apply here. The
OMB and DOL guidelines only apply to information "first disseminated
after October 1, 2002" (OMB Guidelines III.4; DOL Guidelines at 2),
and OSHA has required TLVs to be disclosed on MSDSs since 1983.
Moreover, the guidelines are "not intended to impose any binding
requirements on DOL or the public or * * * to provide any right to
judicial review" (DOL Guidelines at 2). Rather, "information quality
[is] an important management objective." (Id.) Courts have accordingly
rejected private attempts to force agency compliance with the data
quality guidelines. See, e.g., Salt Institute v. Leavitt, 440 F.3d 156,
159 (4th Cir. 2006) (IQA "does not create a legal right to access to
information or to correctness"); Single Stick, Inc. v. Johanns, 601 F.
Supp. 2d 307, 316 (D.D.C. 2009) (same), aff'd in relevant part on other
grounds sub nom Prime Time Int'l Co. v. Vilsack, 599 F.3d 678, 686
(D.C. Cir. 2010). Likewise, the Peer Review Bulletin is "intended to
improve the internal management of the executive branch, and is not
intended to, and does not, create any right or benefit, substantive or
procedural" enforceable against the federal government (Peer Review
Bulletin XII). OSHA finds that the DOL and OMB Guidelines and the Peer
Review Bulletin do not require the Agency to take the additional step
of analysis before requiring the disclosure of TLVs on safety data
sheets.
At least one commenter suggested that requiring disclosure of the
TLV would violate the Administrative Procedure Act's notice and comment
requirements, to the extent that the SDSs were required to disclose
TLVs that the ACGIH might adopt after the final rule is published
(Document ID 0361). That contention was rejected in National
Ass'n of Manufacturers v. OSHA, 485 F.3d 1201, 1204 (D.C. Cir. 2007),
where the court held that the hazard communication standard does not
prescribe particular chemicals for which hazard communications are
required, but rather a system by which manufacturers and the ACGIH
evaluate and communicate chemical hazards. This system is not changed
when the ACGIH modifies a TLV, and therefore no new notice and comment
is required. Id. Nor is OSHA impermissibly delegating its authority to
the ACGIH by requiring that TLVs be listed, as argued by the National
Association of Home Builders (Document ID 0372). The Third
Circuit rejected that argument in a challenge to the current standard,
which also required that manufacturers and importers perform hazard
determinations for all chemicals for which the ACGIH had published
TLVs. Associated Builders and Contractors v. Brock, 862 F.2d 63, 68 (3d
Cir. 1988). The final rule's requirement to list nonbinding TLVs is an
a fortiori case.
Finally, a number of commenters expressed concerns about the
procedures ACGIH uses in adopting TLVs (Document ID 0083,
0084, 0361, 0371, 0372, and 0529). Typical of these is the comment from
the Independent Lubricant Manufacturers Association:
TLVs are developed by way of ACGIH committees that operate in
secret with anonymous authors. Though the opportunity to provide
written comments exists, there is no "appeal" process to
challenge, question or even engage in a professional discourse with
the people responsible for developing and finalizing the TLVs. ILMA
believes that because the TLV development process is closed, TLVs
have compromised scientific value and limited utility in addressing
occupational health and safety matters. Indeed, this non-consensus
process can generate defective decisions that have the potential to
compromise the health and safety of the very workers the TLVs are
designed to help. In addition to issues of transparency and
fairness, TLVs are developed without any regard to the economic and
technical feasibility of its recommendations or the availability of
acceptable methods to determine compliance.
(Document ID 0371 (emphasis in original)). Other commenters
also objected to the fact that the ACGIH provides no public hearing,
that the extent of review ACGIH committees devote to TLV
recommendations before adopting them is unclear, and that TLVs are not
"consensus standards" within the meaning of the OSH Act (Document ID
0372 and 0529).
As explained on its Web site, ACGIH TLVs "represent conditions
under which ACGIH believes that nearly all workers may be repeatedly
exposed without adverse health effects. They are not fine lines between
safe and dangerous exposures" (Document ID 0529). TLVs are to
be used by industrial hygienists in determining safe exposures in
workplace, according to the ACGIH, but "are only one of multiple
factors to be considered in evaluating specific workplace situations
and conditions." (Id.)
The record evidence shows that the ACGIH uses a reliable and open
method to develop TLVs with ample opportunity for public input. ACGIH
TLVs are set by the Threshold Limit Value Chemical Substances Committee
(Document ID 0536). Members of this committee are chosen for
their expertise in industrial hygiene, occupational medicine,
epidemiology, toxicology, or related fields such as statistics or
chemistry, and members are selected to maintain a balance between these
specialties. (Id.) Membership preference is given, among other things,
to those with 10 or more years experience and advanced degrees within
their field. (Id.) A majority of committee members must be "Regular"
ACGIH members, that is, those occupational hygiene, occupational
health, environmental health, or safety professionals whose primary
employment is with a government agency or an educational institution.
(Id.; See also http://www.acgih.org/Members/memdescrip.htm.)
The ACGIH has a conflict of interest policy, requiring that members
disclose, both orally and in writing, "potential, real, or perceived
conflict[s] of interest" with respect to a substance under
consideration (Document ID 0536). The Committee chair is
required to conduct a conflict of interest presentation annually, and
Sub-committee chairs will typically inquire at the beginning of
meetings as to whether members' conflict status has changed. (Id.)
Where conflicts arise, the steps to be taken--such as recusal,
abstention, or disclosure--are decided based on the nature of the
conflict involved. (Id.)
Once the relevant ACGIH sub-committee decides to consider a new
TLV, it is included on an "Under Study" list that the ACGIH publishes
each February 1. (Id.) Each July 31, that list is updated to indicate
the substances for which the ACGIH anticipates issuing a "Notice of
Intended Change" in the coming year. (Id.) An author is assigned to
prepare a draft "documentation" supporting a proposed new TLV; the
author or ACGIH staff must conduct a full literature search on the
substance; and only published, peer-reviewed data may be relied upon in
the documentation. (Id.) The ACGIH has detailed guidelines governing
the content of documentations and the method of conducting literature
searches. (Id.) Once the draft documentation is approved by a sub-
committee (by consensus) and the full TLV committee, ACGIH issues a
public Notice of Intended Change and makes the draft documentation
available to the public for at least a year to submit comments. (Id.)
The author and the sub-committee review the public comments
received, and the draft documentation is amended if necessary. (Id.)
Once the sub-committee reaches consensus, the draft documentation is
forwarded to the full committee with a proposal to (1) retain the
current TLV and publish the draft documentation for comment for an
additional year; (2) change the TLV but publish the draft documentation
for comment for an additional year; (3) adopt the proposed TLV and
draft documentation; or (4) withdraw the proposal. (Id.) The proposal
is then voted on by the full committee, and then the committee's
recommendation is sent to the ACGIH board of directors for
"ratification." (Id.) Generally ACGIH does not hold meetings with
interested parties during this process, but its rules allow for public
discussion of the evidence on a chemical's hazard at ACGIH-sponsored
symposia, and allows for meetings where new evidence has been developed
and is "essential to the Committee's deliberations." (Id.)
NIOSH, the Kentucky Labor Cabinet, the American Industrial Hygiene
Association, the American Society of Safety Engineers, the Alliance of
Hazardous Materials Professionals, and several occupational safety and
health consulting firms support the TLV requirement, stating that ACGIH
TLVs are useful in developing health and safety programs and are widely
used in industry (Document ID 0313, 0323, 0327, 0336, 0354,
0365, 0410, 0412, 0496, and 0521). A number of manufacturers and
manufacturer associations also support the TLV requirement (Document ID
0328, 0330, 0332, 0353, 0413, and 495). The International
Chemical Safety Cards, prepared under the auspices of the UN, list TLVs
(Document ID 0497). TLVs are currently required to be
disclosed under the HCS, and witnesses testified that failure to
include TLVs on SDSs in the final rule would render the standard less
protective of worker health because TLVs are more up to date and cover
more substances than OSHA's PELs (Document ID 494 Tr. 28-29,
94; Document ID 496 Tr. 368, 382).
Based on this record, OSHA finds that commenters' objections to
TLVs are without merit. TLVs are set through an open process with ample
opportunity for public input through the comment and symposium process;
the fact that the ACGIH does not hold public hearings on proposed TLVs
does not undermine the fairness of the process. While OSHA agrees that
TLVs do not address feasibility concerns, it finds that TLVs are useful
information for employers and employees to use in evaluating the
hazards presented by chemicals used in their workplaces. OSHA finds
that the record does not support the contention that TLVs have
"compromised scientific value" because of the process used by the
ACGIH. Each TLV is supported by a documentation explaining the evidence
and assumptions on which it relies; these documentations are subjected
to public comment and approved at several levels within the
organization. It is certainly possible that a manufacturer or importer
might disagree with the scientific judgments embodied in a TLV, but the
final rule allows them to set forth their own recommendations about an
appropriate exposure level on the SDS. Based on the ACGIH's procedures
and the evidence of TLV use by industry, occupational safety and health
professionals, and NIOSH, OSHA reaffirms its position that, in general,
TLVs provide useful information that should be disclosed to employers
and employees using hazardous chemicals.
Some commenters supported requiring other limits to be on the SDS
in addition to the TLVs, such as the NIOSH Recommended Exposure Limits
(RELs); the AIHA Workplace Environmental Exposure Limits (WEELs); and
the German maximum allowable concentrations (MAKs) (See, e.g., Document
ID 0323, 0330, 0336, 0340, 0349, 0354, 0357, 0359, 0401, 0410,
0412, and 0414). NIOSH recommended broad inclusion of available
occupational exposure limits (Document ID 0412):
Providing occupational exposure limits (OELs) helps workers and
employers understand the relationship between exposure concentration
and adverse health effects. NIOSH supports the requirement of
including PELs on the SDSs and further suggests that OSHA consider
adding additional exposure limits, whenever available, such as NIOSH
recommended exposure limits (RELs), American Conference of
Governmental Industrial Hygienists (ACGIH) Threshold Limit Values
(TLVs), American Industrial Hygiene Association (AIHA) Workplace
Environmental Exposure Limits (WEELs), and German maximum allowable
concentrations (MAKs) * * *.
There were a number of other comments on the issue of exposure
limits in Section 8 of the SDSs, such as asking for an explanation of
"any other exposure limit used or recommended" by the SDS preparer
(Document ID 0329, 0351, 0382, 0381, and 0393), including
whether this means exposure limits from other countries. There was also
a suggestion to delete "used or" from the requirement (Document ID
0339). This language is in the current HCS, and is intended to
include any exposure limits developed by the producer to protect their
own employees, as well as other exposure limits commonly available such
as the TLV or REL. It may also include exposure limits from other
countries, but there is no intent to require that every known exposure
limit in the world be provided. OSHA does not agree that it is
appropriate to delete "used or" since companies often have exposure
limits to protect their own employees, and this information can help
their customers to determine what is needed to protect downstream
employees as well. Others thought inclusion of exposure limits in
addition to the PELs would confuse small businesses (Document ID
0372), or be detrimental to harmonization (Document ID
0464).
The AFL-CIO summarized their view of the record on this issue, as
well as that of other worker representatives, in their post-hearing
brief (Document ID 0645):
We believe that OSHA needs to issue a final rule that restores
the requirement to list the TLV on the SDS and strong record
evidence supports our position. There is broad support for this
position, covering a wide range of organizations including NIOSH
(Ex. 0412.1) unions (AFL-CIO, Ex. 340.1; Building and Construction
Trades Department, Ex. 0359.1; and the Steelworkers, Ex. 0403.2);
safety and health professional associations (American Society of
Safety Engineers, Ex. 0336.1); employers and their representatives
(Dow Chemical Company, Ex. 03353.1); Patton Boggs, Ex. 0413.1); and
individual experts (Adam Finkel, Ex. 0401.1; Harry Ettinger, Ex.
0319.1).
In Section 8 of the SDS in the final rule, OSHA has included the
language used in the current rule to describe what exposure limits are
to be addressed: "OSHA permissible exposure limit (PEL), American
Conference of Governmental Industrial Hygienists (ACGIH) Threshold
Limit Value (TLV), and any other exposure limit used or recommended by
the chemical manufacturer, importer, or employer preparing the safety
data sheet, where available."
As noted in the NPRM, OSHA took the reference to TLVs out of
Section 8 of the SDS in the interest of limiting country-specific
deviations from the GHS. However, based on many comments in the record,
OSHA has concluded that the TLVs provide useful information for those
designing protection programs for employees exposed to the chemicals involved,
and are already widely used and applied for that purpose in American
workplaces, as well as around the world. Referencing TLVs on the SDSs
does not make them mandatory or establish them as control guidelines.
It simply provides additional information that can help employers
determine the proper levels of protections in their workplaces.
With regard to the recommendations for other exposure limits to be
included on the SDS, OSHA agrees that referring to those exposure
limits could also be useful, and would encourage SDS preparers to
include them where available. However, the Agency is still concerned
about including additional country-specific deviations, especially for
limits that are less available than the TLVs. Providing too many
different exposure limits may also be confusing to employers.
Publication of a non-mandatory appendix would require OSHA to
continually update it, as these different lists are prepared by various
organizations. Since the Code of Federal Regulations is only updated
annually, the Appendix would always be out-of-date. We do not believe
this would be helpful in the long term, and that resources would be
better put to other purposes than updating a non-mandatory appendix.
In the NPRM, OSHA did not propose to continue to require specific
mention of IARC, NTP, and OSHA as sources of determinations regarding
carcinogenicity. The requirement to consider these sources definitive
in terms of a carcinogen determination was not included in the NPRM
since it was not part of the GHS approach. However, as was discussed
above, OSHA has modified Appendix F to allow classifiers to use these
sources when assessing carcinogenicity, rather than applying the
criteria to the data themselves. In order to facilitate this, OSHA has
provided a table in Appendix F that aligns the GHS criteria with those
of IARC and NTP. In addition, OSHA has decided to retain the
requirement to include this information on the SDS in Section 11. This
information will be of use to classifiers, as well as to employers and
employees, when ascertaining potential hazards and determining
appropriate control measures. This was supported by some commenters
(See, e.g., Document ID 0321, 0335, and 0403), while others
argued that the determinations of such organizations should not be
included because of issues with their process of making determinations
(See, e.g., Document ID 0379, 0417, and 0529). OSHA believes
that this information from organizations that are recognized as expert
in the field of carcinogenicity will continue to be helpful to both
classifiers and users of chemicals, and does not agree with the
commenters who argue about the process followed to make such
determinations. The arguments were similar to those discussed above
regarding inclusion of TLVs on SDSs, and OSHA's response to such
arguments apply here as well. OSHA finds that both IARC and NTP use
reliable procedures and criteria in making their determinations.
OSHA indicated in the NPRM that Sections 12 through 15 of the SDS
were not going to be mandatory since they involved information that is
outside OSHA's jurisdiction. With regard to Section 12 on environmental
effects, some commenters expressed concern about the lack of
harmonization with trading partners on environmental issues, or
suggested that OSHA should work with EPA on this issue (See, e.g.,
Document ID 0351 and 0377). OSHA and EPA have discussed this
issue, and EPA's Office of Chemical Safety and Pollution Prevention
will be updating applicable Toxic Substances Control Act (TSCA)
regulations consistent with modifications made in this Federal Register
Notice. Dates will be published in the Unified Regulatory Agenda
(www.reginfo.gov). As noted previously, OSHA encourages SDS preparers
to complete Section 12, as well as Sections 13 through 15, so as to
have an SDS that is compatible with other international requirements,
as well as ensuring customers have complete information.
Similarly, comment was received suggesting that Section 14 on
transport information should be required, and producers should indicate
whether the product is, or is not, covered by DOT's Hazardous Material
Regulations (Document ID 0345). While OSHA does not have
authority to require this to be included in Section 14, we certainly
agree that it would be useful information for users of the chemical,
and encourage producers to complete Section 14.
In the final rule, non-mandatory Section 15 of the SDS is intended
to provide other regulatory information. OSHA raised as an issue for
comment whether this section should be made mandatory by requiring
regulatory information on OSHA's substance-specific standards be
included in it. Employers can, of course, voluntarily list information
about other OSHA standards (Document ID 0376), but voluntarily
provided information is not subject to enforcement. Many of the
respondents commented that Section 15 should not be made mandatory
(See, e.g., Document ID 0324, 0335, 0344, 0352, 0353, 0355,
0370, 0372, 0376, 0377, 0379, 0381, 0385, 0393, 0399, 0402, 0405, and
0408). Some questioned whether information about substance-specific
standards would be useful to users of the SDS (See, e.g., Document ID
0329, 0335, 0372, and 0405). Others thought that OSHA should
require the substance-specific standards to be indicated, and that
Section 15 should thus be mandatory (See, e.g., Document ID
0328, 0330, 0336, 0338, 0339, 0340, 0347, 0349, 0351, 0354,
0357, 0365, 0383, 0389, 0403, 0410, 0414, and 0453).
While OSHA agrees that there is merit in including the substance-
specific standards in Section 15 to inform chemical users of their
existence and applicability, it is difficult to make completion of
Section 15 mandatory since there is likely to be considerable other
information in the section that would not be enforceable by OSHA.
Having a section that includes both mandatory and non-mandatory
information is potentially confusing to the regulated community.
Additionally, the PELs will already be indicated in Section 8, and will
thus inform the user when there is a substance-specific standard of
concern. Therefore, while OSHA encourages additional information in
Section 15, it remains non-mandatory in the final rule.
One suggestion received for Section 16 indicated that the preparer
should identify the exact changes made to the SDS when revising it so
the user can determine if re-training is needed (Document ID
0469). Presumably, the user would review the changes to decide
whether re-training is needed. However, the success of such an approach
would depend on how often the chemical is purchased, and a new SDS is
received. If the chemical has not been purchased for a while, and a new
SDS only indicates what changes have been made since the last update,
the user could have missed versions of the SDS in the interim, and thus
would not know all of the changes that had been made since the last SDS
was received. In addition, adding such a requirement would make the
OSHA provisions internationally inconsistent.
(h) Employee information and training. The GHS does not include
harmonized training requirements, but does recognize the important role
that training plays in hazard communication. For example, 1.1.3.1.3 of
the GHS states:
In the workplace, it is expected that all of the GHS elements
will be adopted, including labels that have the harmonized core
information under the GHS, and safety data
sheets. It is also anticipated that this will be supplemented by
employee training to help ensure effective communication.
OSHA agrees that training is key to ensuring effective hazard
communication. Under the current HCS, training is used to explain the
label and SDS systems used in a workplace, and to address the hazards
of chemicals and protective measures. While the written information
provided is clearly important, training is an opportunity to explain
the data and helps to ensure that the messages are being received
accurately so they can be acted on appropriately. (See Section IV of
this preamble.)
The training provisions in the HCS do not need to be modified to be
consistent with the GHS since it does not include such requirements.
However, OSHA proposed small revisions to track terminology used in
other paragraphs, as well as to clarify the requirement to train on the
details of the hazard communication program in (h)(3)(iv). While
training on the program has always been required in the HCS, OSHA
believed that modifying the text slightly would convey the need to
address both the labels that will arrive on shipped containers, as well
as any workplace-specific system that the employer uses. In addition,
the training on SDSs must include the order of information. The final
rule requires that training include the details of the hazard
communication program developed by the employer, including an
explanation of the labels received on shipped containers and the
workplace labeling system used by their employer; the safety data
sheets, including the order of information and how employees can obtain
and use the appropriate hazard information.
OSHA proposed that employers train or re-train employees regarding
the new labels and safety data sheets within two years after the rule
is promulgated. The Agency believes that the training needs to be
completed by the time employees begin to see labels and safety data
sheets with the new information on them, rather than waiting until
after the transition has been completed.
Some commenters to the ANPR noted that training would be required
to ensure employees understand, in particular, the symbols and
pictograms that will be used on labels. Some argued that the burden
would be substantial given that all training would have to be revised,
and the time and resources required would be significant (See, e.g.,
Document ID 0153 and 0178). However, many agreed that having a
standardized approach to labels and SDSs will make training easier in
the future than training under the current rule where chemical
manufacturers and importers can use whatever formats they choose (See,
e.g., Document ID 0030, 0042, 0072, and 0077).
Marshfield Clinic (Document ID 0028) noted that
communication of information about chemicals and other hazardous
substances:
* * * is one of the more difficult to get across to workers. It
is very appreciated that OSHA is revisiting this. Standardization
will greatly assist in giving workers a better understanding of the
hazards they may encounter when working with chemicals and other
hazardous substances.
Similarly, Alcoa (Document ID 0042) suggested: "A
standardized format will simplify hazard communication training and the
use of pictograms will alleviate some of the problems presented by poor
language skills."
There were a few commenters who argued that the standardized
approach either would not simplify training, or they did not know if it
would (See, e.g., Document ID 0065 and 0078). Another noted
that the current approach is fine for companies that are domestic only
(Document ID 0026).
The majority of the comments made on the training provisions
suggested additions to the existing requirements to further specify
what is expected, and to improve the training. These comments were
submitted primarily by worker representatives, or by the National
Institute of Environmental Health Sciences (NIEHS) (See, e.g., Document
ID 0340, 0347, 0349, 0357, and 0403). For example, the
Communication Workers of America (CWA) (Document ID 0349)
suggested:
* * * Given the significance of education and training, OSHA
should develop a mandatory appendix to the Proposed Rule that sets
forth the elements (including an evaluative component) of an
acceptable education and training program.
As noted above, OSHA agrees with these commenters that effective
training is a key part of hazard communication. While the GHS does not
include such requirements, the developers also recognized the
importance of including training in national programs, and encouraged
countries to do that. In addition, the United Nations Institute for
Training and Research (UNITAR), which is the international focal point
for capacity building on the GHS, is developing training courses to be
made available to developing countries, in particular to assist them in
adopting the GHS.
As described, OSHA proposed a slight modification to ensure that
employers are aware that they need to train specifically on the new
label elements and SDS format. This modification is in the final rule,
and the training on these aspects is to be completed prior to other
provisions going into full effect. OSHA does not agree that other
changes should be made to the training provisions of the HCS at this
time. As also indicated in this document, the changes to the HCS being
promulgated are focused on what is necessary to comply with the GHS.
Since the GHS does not have any training requirements, the modification
proposed and adopted by OSHA is what is necessary to ensure appropriate
compliance with the revised standard, and does not introduce any new
approaches or requirements.
OSHA is planning to provide additional guidance to help ensure
appropriate training is conducted when complying with the revised HCS.
A draft Model Training Program was posted for comment on OSHA's Web
page some years ago. It includes many of the concepts addressed in the
comments received, but was never finalized. While it was designed to
provide an array of tools from which employers could choose what they
needed based on their workplaces (lesson plans and slides), there were
comments received at the time that it was too long for small employers.
OSHA believes that the model program includes important information
about conducting appropriate training (which was also the view of other
commenters on the program). It is being revised and updated to be
consistent with the revised rule, and will be made available on OSHA's
Web page. A shorter guidance document for small employers is also being
developed.
In addition to these training-specific tools, OSHA has other tools
under development that could be used in training (e.g., a quick card
with the new symbols). These too will help to address some of the
issues that have been raised.
Based on the above reasons, the final rule adopts the training
provisions in the proposal. OSHA will address other comments provided
through guidance and compliance assistance materials, rather than
through further revisions to the rule.
OSHA has made minor changes to the training provisions to reflect
the new definition of hazardous chemical in the final rule. In (h)(1),
OSHA is replacing the phrase "new physical or health hazard" with the
broader term "chemical hazard." Final paragraph (h)(1) requires that
employers provide employees with effective information and training on
hazardous chemicals in their work area at the time of their initial
assignment, and whenever a new chemical hazard the employees have not
previously been trained about is introduced into their work area.
Information and training may be designed to cover categories of hazards
(e.g., flammability, carcinogenicity) or specific chemicals. Chemical-
specific information must always be available through labels and safety
data sheets.
Similarly in paragraph (h)(3)(ii), OSHA is replacing the phrase
"The physical and health hazards" with all of the hazards identified
as well as the hazards not otherwise classified. Final paragraph
(h)(3)(ii) requires that the training include the physical, health,
simple asphyxiation, combustible dust, and pyrophoric gas hazards, as
well as hazards not otherwise classified, of the chemicals in the work
area. This change was necessary because the final rule covers simple
asphyxiants, pyrophoric gas, combustible dust, and hazards not
otherwise classified, in addition to what falls under the new
definitions for physical and health hazards. The modification to
paragraph (h)(3)(ii) requires employers to train employees on all of
the chemical hazards in the workplace, rather than only physical and
health hazards as defined in the final rule.
(i) Trade secrets. The current HCS includes provisions that define
what can be considered trade secret information under the rule, as well
as delineate the conditions under which this information must be
disclosed to ensure the safety and health of exposed employees. These
provisions were a significant focus of the original rulemaking on the
HCS, and reflect the common law of the United States on this topic. In
the years since the rule has been in effect, however, this issue has
not been as important. Overall, since these provisions were
promulgated, it appears that fewer claims of trade secrecy have been
made, and fewer requests for trade secret disclosure have been
received, than were anticipated during the original rulemaking process.
The negotiations for development of the GHS recognized at the
outset that trade secrets--generally referred to internationally as
confidential business information--would be an issue of concern.
Guiding principles included the following (See 1.1.1.6(j) of the GHS):
In relation to chemical hazard communication, the safety and
health of workers, consumers and the public in general, as well as
the protection of the environment, should be ensured while
protecting confidential business information, as prescribed by the
competent authorities.
As the issue was considered further, it was recognized that laws
regarding confidential business information were very much country-
specific, and had a broader context than rules for classification and
labeling. Such laws could not be modified or harmonized through the
process of harmonizing classification and labeling. Thus it was
determined that the GHS would recognize the importance of trade
secrets, and provide principles for countries to follow when adopting
the GHS. These principles are consistent with the approach already
incorporated into the HCS.
The type of information that can be considered confidential or
trade secret is limited to the names of chemicals and their
concentrations in mixtures. Under the current HCS, OSHA did not require
that concentrations in mixtures be disclosed, and thus limited trade
secret claims to specific chemical identities. This was the primary
difference between the current rule and the proposed revisions to the
HCS. To be consistent with GHS, OSHA proposed to add percentage
composition information to the SDS. This introduces the possibility
that trade secret claims will be made for this type of information, as
well as specific chemical identities. Thus the proposal revised the
text of the current rule to add consideration of percentage composition
everywhere specific chemical identity is addressed in the provisions.
The GHS further suggests that SDSs indicate when information has
been withheld as confidential; that the information be disclosed to the
competent authority upon request and under condition of
confidentiality; that the information must be disclosed in a medical
emergency, with mechanisms to protect it while ensuring timely
disclosure; that the information be disclosed in non-emergency
situations, also under conditions of protecting confidentiality; and
that the competent authority have procedures to deal with challenges to
this process. All of these principles have already been included in the
trade secret provisions of the HCS, and are maintained in the final
rule as previously promulgated. The proposed revisions simply conformed
terminology, and added text regarding percentage composition being
subject to the same provisions as specific chemical identity.
Very few comments on trade secrets or confidential business
information were received in response to the ANPR. It was suggested
that protection of confidential business information should be an
implementation principle for the GHS modifications to HCS (Document ID
0072 and 0179), and that the current trade secret position
should be retained (Document ID 0049). There was also a
comment that indicated that full disclosure of all ingredients should
be required on the SDS unless the employer provides a justification to
the Agency showing that a particular ingredient is a trade secret, and
demonstrating that the economic damage of disclosure exceeds the damage
associated with the potential health effects to exposed employees
(Document ID 0044). In addition, the National Paints and
Coatings Association (NPCA) argued that the approaches to protection of
confidential business information need to be harmonized (Document ID
0050). As NPCA stated, different approaches may lead to
development of different SDSs for various authorities.
As noted above, laws regarding confidential business information
are generally not specific to classification and labeling requirements,
but rather reflect an overall approach of a country. It was not
possible to change such laws through the harmonization of
classification and labeling, and thus the limit of the agreement was to
establish the principles already described. Those principles are
consistent with law in the United States, and do not require any
modifications to the current HCS approach to be consistent with the
GHS.
There were a few comments on the trade secret provisions proposed.
Some expressed their support for maintaining the current approach, with
the small revisions to conform to the GHS (Document ID 0353,
0367, and 0371). Several indicated that the trade secret provisions
should be extended to labels because the name of unclassified hazards
was proposed to be included on labels, and when there is an ingredient
of unknown toxicity, this must be indicated as well. For example, the
American Petroleum Institute (Document ID 0376) indicated:
Under certain conditions both the SDS and label can require text
such as: x percent of the mixture consists of ingredient(s) of
unknown toxicity. This statement may apply to an ingredient of a
mixture whose percentage of composition is a trade secret. In such a
case the trade secret provisions only apply when this statement is
on the SDS. The current trade secret provisions do not apply to
labels. Since the percentage composition of an ingredient can be
required on labels as well as SDSs, the trade secret provisions
should also apply to labels.
(Footnote omitted; See also Document ID 0344, 0381, 0382,
and 0393.)
With regard to the inclusion of the name of unclassified hazards on
a label, this requirement has been deleted from the final rule.
Therefore, listing unclassified hazards on the label no longer raises a
trade secret concern. It should be noted that there was never a
requirement proposed for the "specific chemical identity" to be on
the label for unclassified hazards, so even if the provision had been
included in the final rule, it still would not have been analogous to
the specific chemical identity required on an SDS.
With regard to the statement regarding unknown toxicity, OSHA does
not find that this statement merits a change to allow the trade secret
provisions to apply to labels. It is noted in paragraph A.1.3.6.2.3
that, where there is one or more ingredient of unknown toxicity in a
mixture of other ingredients known to be acutely toxic, the calculation
for predicting the acute toxicity cannot be completely accurate.
Therefore, as suggested in the GHS, OSHA has indicated that a statement
must be on the label and SDS indicating that a percentage of the
mixture has unknown acute toxicity. There is no requirement to relate
that general statement to specific ingredients, and specific chemical
identities are not required on the label. Therefore, no trade secret
information is required to be disclosed, and protection of the
information under the trade secret provisions is not necessary.
There were also comments that OSHA should allow for flexibility in
terms for indicating information is being withheld as a trade secret,
such as "confidential," "confidential business information," or
"proprietary" (Document ID 0376 and 0393). OSHA has never
indicated specific terminology for claiming that information is subject
to the trade secret provisions of the HCS, and would accept language
such as "confidential," "confidential business information," or
"proprietary" when indicating on an SDS that information is being
withheld. This has never been an issue in OSHA enforcement of the HCS.
As implementation moves forward in different countries and regions,
conformance to the GHS principles should lead to increased
harmonization of approaches. This is an area that should be monitored
to determine if further action can be defined and implemented. OSHA
does not believe it would be prudent to implement changes in the
approach to trade secret protection and disclosure before that time.
Therefore, the final maintains the proposed language for the trade
secret provisions.
(j) Effective dates. OSHA proposed to require training on the new
labels and SDSs two years after publication, and all other provisions
within three years. During the three-year transition period, employers
would be required to be in compliance with either the existing HCS or
the modified GHS, or both. OSHA recognized that hazard communication
programs will go through a period of time where labels and safety data
sheets under both standards will be present in the workplace. It was
indicated that this would be considered acceptable, and employers would
not be required to maintain two sets of labels or safety data sheets
for compliance purposes. However, given the longstanding requirements
for a hazard communication program, there must be no time during the
transition period when hazard communication is not in effect in the
workplace, and information is not available under either the existing
requirements or the new final standard for exposed employees.
It should be noted that due to requirements of the Federal
Register, a revision date of October 1, 2009, was entered into the
proposed language to indicate the version to be used as the existing
HCS standard. This confused some commenters (See, e.g., Document ID
0376). There were no actual revisions introduced as of that
date, and it is irrelevant to this final rule.
Many comments were received in response to the ANPR on the issue of
phasing in the requirements of the GHS, as well as on current practices
and time frames required for various activities. There was a wide
variety of opinions, as well as a number of factors that commenters
suggested should be considered in establishing effective dates.
OSHA specifically requested input on the possibility of phasing in
requirements based on the size of the business. While a few commenters
supported this approach (See, e.g., Document ID 0022, 0144,
0146, and 0151), many more indicated that this would not be appropriate
(See, e.g., Document ID 0018, 0033, 0107, 0116, 0123, 0147,
0154, and 0171). One reason given was that the supply chain may involve
large businesses purchasing from small businesses, and thus the large
businesses would need information from the small businesses in order to
comply themselves (Document ID 0080 and 0123).
There were also those who thought the phasing should be coordinated
with other trading partners, particularly the European Union (Document
ID 0024, 0072, 0080, 0081, 0163, 0171, and 0179). The European
phasing is taking place over a long period of time because of the REACH
requirements for chemicals that are going into effect and not
necessarily because of the amount of time needed just for compliance
with GHS. Another suggestion that had support from commenters was to
phase in substances first, and then cover mixtures, or to have a three-
step phase-in that includes intermediates before mixtures (See, e.g.,
Document ID 0021, 0024, 0034, 0036, 0122, 0141, and 0154).
There were also suggestions for a specific number of years, or a
range of years, for phase-in. Some of these suggested less than 3 years
(See, e.g., Document ID 0019, 0028, and 0064). A number
suggested 3 to 5 years, or in some cases, 6 years (See, e.g., Document
ID 0015, 0032, 0038, 0111, 0125, and 0163). And there were
some commenters who suggested anywhere from 7 to 13 years for full
compliance (See, e.g., Document ID 0018, 0050, 0077, 0078,
0116, 0129, 0141, and 0164).
OSHA decided on the three-year proposal based on a consideration of
the widely diverse viewpoints expressed, as well as information
provided by commenters about stockpiles and other issues. It is clear
that activities have already begun by a number of vendors of software
programs for hazard classification and labeling to convert to the GHS,
and make programs available for companies to use to comply with
requirements around the world as countries adopt the GHS.
Stakeholders provided many comments, as well as testimony, on the
proposed effective dates. As with the record submitted in response to
the ANPR described above, the opinions ranged over a wide variety of
effective date options.
As noted, OSHA proposed that employers provide training regarding
the new labels and safety data sheets two years after publication of
the final rule. The intent of this training is to ensure that when
employees begin to see such labels and SDSs in their workplaces, they
understand how to use them and access the information effectively.
Given the number of chemicals imported into American workplaces, as
well as the number of employers who are already beginning to change
over to the new formats, OSHA believes it is important to have this
introductory training done before all of the labels and SDSs will be
changed. It is not possible to pick a time frame that would ensure that
such training is done before employees see any of these documents, but
two years is a reasonable period of time and helps to ensure that
employees will be trained before the new formats become the standard
practice.
This training is not required to address the specific hazards of
the chemicals, or the protective measures. Employees will have already
been trained on hazards and protective
measures under the existing hazard communication requirements, but they
will not have had training on the new label elements (e.g., pictograms
and signal words) and SDS format, nor have learned how this information
is to be used in their workplaces. Completion of such training in two
years will help to ensure they can use the new documents effectively
when they begin to arrive in their workplaces.
Some commenters thought two years would not be enough time, or who
appeared to misunderstand what training was to be done by this date
(See, e.g., Document ID 0330, 0344, 0351, 0361, 0390, 0397,
and 0399). For example, the American Society of Safety Engineers and
Industrial Health and Safety consultants indicated that the training
should be completed within one year of the final rule (Document ID
0336 and 0410). But the majority of those who commented agreed
that two years was an appropriate time period in which to complete the
training on the new label and SDS formats (See, e.g., Document ID
0324, 0329, 0335, 0338, 0346, 0370, and 0405).
The three-year time frame for compliance with all other
requirements generated significant comment. Many commenters supported
this time frame as being appropriate and feasible (See, e.g., Document
ID 0313, 0322, 0324, 0327, 0329, 0335, 0339, 0376, 0390, 0395,
and 0405). Others indicated that three years would not be adequate
(See, e.g., Document ID 0342, 0371, 0399, and 0402). There
were also comments that suggested additional time should be provided to
distributors to ensure they have the information from suppliers to
provide it downstream. For example, the National Association of
Chemical Distributors (Document ID 0341) stated:
OSHA should consider an additional 18-month phase in period for
chemical distributors after the 3-year implementation date expires.
This would allow for a more effective GHS while reducing any
potential negative economic impact on small chemical distributors.
NACD members have expressed concern that a three-year transition
time for the entire value chain (suppliers, distributors, customers)
presents the possibility of a bottleneck in the supply of chemicals
* * *
Many commenters indicated that the time frame should be longer and
tiered, with either substances first, and then mixtures, or a three-
tiered system with substances, intermediate mixtures, and complex
mixtures. The latter approach has been used by the EU. (See, e.g.,
Document ID 0328, 0341, 0352, 0363, 0367, 0392, 0393, and
0400.) For example, comments on behalf of the Soap and Detergent
Association and the Consumer Specialty Products Association indicated
(Document ID 0344):
Therefore, SDA and CPSA support either a sequenced approach of
substance suppliers first and formulators last, or a longer overall
timeframe in order to minimize the impact of undertaking this
significant effort to reclassify substances and mixtures, develop
revised labeling, while allowing time to deplete inventories of
labels and products with a current label. Any consideration of
business size for a phase-in approach would be unacceptable as
businesses large and small use each other's products in their end-
use products; each one may rely on the upstream supplier for
information in hazard classification.
While the Agency wants to provide sufficient time for compliance,
there is also a concern about the effect on employees of dealing with
multiple systems during a transition period. While some time period
when the currently required labels and SDSs, and the new GHS labels and
SDSs, will co-exist in workplaces is inevitable, hazard communication
during this transition period will be confusing and less effective. It
is therefore important to minimize the effects of the transition on the
effectiveness of hazard communication by ensuring that it is completed
in a timely fashion, while allowing adequate time for an orderly
changeover.
Requiring the phasing in of substances first, and then mixtures,
clearly has some persuasive logic as an approach. However, the supply
chain is not always orderly and logical. It cannot be assumed, for
example, that no mixtures can be completed until all substances are
done. Mixtures that are comprised of substances that are widely
available, and their hazards are well known, do not need an extensive
time period to complete. Some mixtures are comprised of other mixtures
rather than substances, and producers of such mixtures will need
information on the component mixtures before they can comply. If
manufacturers of mixtures wait until the end of an extensive time
period to complete their work, their customers might not meet the
compliance dates. These types of issues are generally addressed by the
market, and the needs of a manufacturer's customers, and cannot be
individually addressed in a phasing-in period.
OSHA is also mindful of the fact that the initial HCS had a two-
year phase-in period for completion and distribution of all labels and
SDSs, and an additional six months for all other provisions of the rule
to be completed. There was no tiered approach to substances and
mixtures. In that situation, the requirements for labels and safety
data sheets were completely new, and yet timely compliance was achieved
by most employers. Where there were situations that needed special
consideration (such as an employer not receiving the required
information from suppliers), the Agency made adjustments through
enforcement policies. It should also be noted that this took place
nearly thirty years ago, and pre-dated many of the resources available
today that can facilitate compliance--such as access to extensive
information online.
As was the case in the comments to the ANPR, a number of NPRM
participants referenced the timeline for compliance with European CLP
requirements (See, e.g., Document ID 0328, 0361, 0367, 0377,
and 0392). When discussing this issue in the NPRM, OSHA noted that the
dates selected for CLP compliance were influenced significantly by
compliance dates for REACH, rather than providing an indication of how
long compliance should take in the absence of such competing
responsibilities (74 FR 50403, Sept. 30, 2009).
That being said, however, nearly two years have elapsed since the
NPRM was published, and the EU requirements for notifications regarding
classification of substances are now in effect. In January 2011, the
European Chemicals Agency (ECHA) indicated that it had received over
three million such classifications (See discussion earlier in the
Summary and Explanation). These substance classifications are being
made available in a public database. The availability of this
information clearly facilitates compliance with this revised HCS. While
chemical manufacturers and importers must review the information if
they are using classifications performed by someone else, many of the
classifications were being submitted by U.S. companies, and thus they
are already substantially in compliance with the new U.S. requirements
as well.
Taking into consideration all of the information received from the
public during the comment periods and in hearing testimony, as well as
the results of the economic analysis which examines the effects of
different compliance dates on the overall costs of compliance, the
following effective dates have been included in the final rule. Rather
than specifying a time frame related to the publication date of the
final rule, OSHA is establishing dates certain for these activities to
be completed. The following table summarizes the requirements in the
final rule:
Table XIII-3--Effective Dates and Requirements
----------------------------------------------------------------------------------------------------------------
Effective completion date Requirement(s) Who
----------------------------------------------------------------------------------------------------------------
December 1, 2013..................... Train employees on the new label elements Employers.
and SDS format.
June 1, 2015......................... Compliance with all modified provisions of Chemical manufacturers,
this final rule, except: importers, distributors and
employers.
December 1, 2015..................... The Distributor shall not ship containers
labeled by the chemical manufacturer or
importer unless it is a GHS label.
June 1, 2016......................... Update alternative workplace labeling and Employers.
hazard communication program as
necessary, and provide additional
employee training for newly identified
physical or health hazards.
Transition Period 5/25/12 to the May comply with either 29 CFR 1910.1200 Chemical manufacturers,
effective completion dates noted (this final standard), or the current importers, distributors, and
above. standard, or both. employers.
----------------------------------------------------------------------------------------------------------------
First, final paragraph (j)(1) requires training regarding the new
label and SDS formats to be completed by all covered employers by
December 1, 2013. OSHA has concluded that it is necessary and
appropriate to complete this training prior to all of the new labels
and SDSs being completed and received in workplaces so that employees
know how to access and use the information appropriately. Most of those
who commented on this issue agreed with that position, and with the
timing proposed. Those who didn't may have misunderstood exactly what
training is being required, but we have clarified that in this
document.
Secondly, OSHA has not found the arguments regarding phasing in
based on whether the product is a substance or a mixture to be
convincing. There are many variations in the supply chain that impact
the logic of this approach. In addition, given the current situation
where substance classifications for the GHS have already had to be
completed for both the EU countries, as well as other countries such as
Japan, many suppliers involved in international trade have already had
to complete substance evaluations. For those who have not, there is
extensive information available as a result of these classifications
having been done for the purpose of compliance with other authorities'
requirements. Thus little time should be necessary to complete this
part of the work.
Final paragraph (j)(2) requires compliance with all of the
provisions for preparation of new labels and safety data sheets by June
1, 2015. This compliance date is consistent with the EU requirements
for classification of mixtures. It also provides almost a year more
time for compliance than was proposed. Thus it addresses a number of
the suggestions received, but is still a reasonable time frame in terms
of employee protections. There are two exceptions to this date. First,
final paragraph (j)(2)(i) gives distributors an additional six months
to distribute containers received from chemical manufacturers and
importers with the new labels and SDSs in order to accommodate those
they receive very close to the compliance date. Accordingly, by
December 1, 2015, all their distributed containers must be
appropriately labeled, and have the new SDS. Second, final paragraph
(j)(2)(ii) gives employers until June 1, 2016, to make sure that their
workplace labels and training programs reflect any new information
received as a result of the final rule.
As was proposed, final paragraph (j)(3) states that employers will
be considered to be in compliance with the HCS during the transition
period as long as they are complying with either the existing HCS as of
October 1, 2011, or this revised HCS.
Employers are encouraged to work with their suppliers to ensure
they get the information they need by the dates they need it. While the
final rule gives distributors and employers extra time to ensure they
have the information before they have to be in compliance with all
requirements, coordination will still be key to ensure everything is
done on time. For example, mixture formulators need to make sure their
suppliers are aware of their need to receive substance classifications
as soon as possible. Employers would be best served to start evaluating
their workplaces long before the year after suppliers must be in
compliance to assess what they will need to do to bring their programs
in line with the new requirements. As with the original rule, OSHA will
handle individual problems through enforcement policies that recognize
difficult issues or situations that impede compliance. Nevertheless,
given the long time frame involved, and recognition of different
players in the supply chain of the needs of others, OSHA expects that
these situations will be minimal.
Summary and Explanation of Requirements in OSHA Standards Affected by
the GHS Modifications to HCS
General Explanation
In this final standard, OSHA has modified its current standards in
General Industry (29 CFR Part 1910), Construction (29 CFR Part 1926),
and Maritime (Shipyards, Marine Terminals, and Longshoring (29 CFR
Parts 1915, 1917, and 1918, respectively)) that contain hazard
classification and communication provisions so that they will be
internally consistent and aligned with the GHS modifications to the
HCS. OSHA proposed to do so on the basis of the strong support in the
record of comments on the ANPR. The majority of commenters who
addressed the impact of the GHS on other OSHA standards recommended the
Agency review all its standards and update them for consistency with
GHS (71 FR 53617, Sept. 12, 2006) (Document ID 0031, 0038,
0046, 0050, 0054, 0072, 0077, 0107, 0116, 0145, 0147, 0154, 0155, 0163,
0165, 0171, and 0179). OSHA did so, and this rule contains the updates
to the requirements in OSHA standards affected by the GHS modifications
to HCS. Commenters also urged OSHA to complete these revisions in one
rulemaking (Document ID 0079, 0123, 0137, 0154, and 0157). The
comments on the proposed standard and testimony at the hearing also
strongly supported modifying these standards for consistency with the
GHS (Document ID 0313, 0327, 0328, 0329, 0336, 0338, 0352,
0359, 0365, 0370, 0372, 0405, 0408, 0410, 0412, and 494 Tr. 91, 162).
Of the commenters who specifically addressed adopting GHS provisions on
physical hazards, many urged the
Agency to conform the OSHA standards to the GHS in order to minimize
discrepancies and ensure consistency (Document ID 0012, 0018,
0050, 0072, 0104, 0105, 0139, 0140, and 0144). One commenter, 3M, noted
that adoption of the GHS physical hazard criteria (without changing
OSHA standards) would "create unacceptable inconsistencies between
OSHA standards" (Document ID 0128).
Several other commenters to the ANPR pointed out some of the
difficulties with adoption of the GHS physical hazards criteria in OSHA
standards (Document ID 0031, 0034, 0038, 0077, 0145, and
0166). BASF was concerned that modifying OSHA standards to conform to
the GHS will cause them to deviate from the national consensus
standards they were based on (Document ID 077). In addition,
some ANPR commenters recommended that OSHA limit changes only to
standards that directly refer to the HCS (Document ID 0047,
0064, 0077, 0104, and 0115). OSHA acknowledged these concerns when
developing the NPRM.
OSHA's NPRM reflected the advantages of harmonizing OSHA's
standards, but also took into account the places where harmonization
might be too difficult at this time because it would substantially
change the scope of coverage of a current standard or make OSHA's
standards incompatible with other widely accepted standards (74 FR
50280, Sept. 30, 2009). OSHA proposed modifying requirements in
primarily the substance-specific health standards and in physical
hazards definitions and terminology for the purposes of internal
consistency and compatibility with the GHS-modified Hazard
Communication Standard (HCS).
Building and Trades Construction Department of AFL-CIO (BTCD) and
Northrup Grumman Shipbuilding, in response to the NPRM, requested that
OSHA again review the standards, and the Agency has done so (Document
ID 0359 and 0395). OSHA reviewed all its standards, the
comments, and the entire record and has decided to maintain the
modifications to the substance-specific standards as proposed, except
for some minor changes that are explained below.
Substance-Specific Health Standards
In the NPRM, OSHA updated the substance-specific health standards
in General Industry, Construction, and Maritime, whether they
specifically referenced HCS or contained their own hazard communication
requirements. OSHA proposed to modify these standards as follows:
[ssquf] Revise the provisions covering workplace signs to require
warning statements that are consistent with the GHS modifications to
HCS;
[ssquf] Revise all standards to reference the modified HCS for
labels, safety data sheets, and training, and identify the hazards that
need to be addressed;
[ssquf] Maintain the requirement to avoid creating dust currently
in some substance-specific health standards for which GHS modifications
contain no equivalent statements at this time;
[ssquf] Maintain or specify language for contaminated clothing and
debris;
[ssquf] Update definitions in Sec. 1910.1450, Occupational
Exposure to Hazardous Chemicals in Laboratories, to maintain
compatibility with the modified HCS; and
[ssquf] Change the name Material Safety Data Sheets to Safety Data
Sheets and require information on them to be compliant with GHS in
content, format, and order.
Workplace Warning Language on Signs and Labels
OSHA proposed to update the language for workplace signs and labels
to incorporate the GHS hazard statement and the applicable
precautionary statement(s), where required. Most OSHA substance-
specific heath standards require hazard warning signs, usually for
regulated areas, and the language required on the signs varies greatly
(e.g., Asbestos, 4-Nitrobiphenyl, 13 Carcinogens, Vinyl Chloride,
Inorganic Arsenic, Cadmium, Benzene, Coke Oven Emissions, Cotton Dust,
DBCP, Acrylonitrile, Formaldehyde, Methylenedianiline, 1,3-Butadiene,
Methylene Chloride, and Lead). With the GHS revision, these standards
retain the requirements for specific warning language for specific
signs; however, OSHA proposed to modify the language to be compatible
with GHS and consistent throughout the OSHA standards. Labels for
products, mixtures, and raw materials are included in the GHS-modified
HCS and are required to be compliant with it. Labels required by the
current standards for contaminated clothing, PPE, and waste and debris,
which are not addressed in the GHS, are retained, but their language
has been changed to be as reflective of GHS terminology as possible.
The vast majority of persons and entities who commented on the
issue in response to the NPRM supported OSHA's harmonization of the
signage and labeling currently required in its substance-specific
standards with the modifications to HCS (Document ID 0313,
0315, 0327, 0328, 0329, 0330, 0336, 0338, 0344, 0365, 0370, 0372, 0376,
0381, 0382, 0383, 0405, 0408, and 0410). NIOSH pointed out that the
consistent language on signs, labels, and SDSs would avoid confusion
and allow for easy translation into other languages (Document ID
0414). AIHA, in supporting the modification of language for
signs and labels, noted that the action was consistent with GHS and the
goal of harmonization. They envisioned clearer warnings, improved
comprehension, and better self-protection by workers (Document ID
0365). Companies such as Ecolab, Product Safety Solutions,
DuPont Company, Phylmar Group, Stericycle, Procter & Gamble, Clariant
Corporation, 3M, Industrial Health and Safety Consultants, and Wacker
Chemical specifically addressed the issue of affected standards and
stressed that aligning the standards with GHS would bring needed
consistency and aid employee understanding (Document ID 0313,
0329, 0335, 0338, 0339, 0351, 0381, 0383, 0405, and 0410). Lawrence R.
Klein of DuPont (Document ID 0329) commented that:
* * * hazard communication regardless of whether * * * general
chemicals or substance specific chemicals regulated under other OSHA
standards, will prove to be beneficial for industry. Through
adequate training * * * and consistent, easily comprehensible hazard
and precautionary statements, via workplace signs or chemical
labels, the safety and protection of employees will be enhanced.
Ameren added that the language proposed for the substance-specific
standards accurately conveyed the hazards (Document ID 0330).
Associations that addressed this issue also provided strong support.
ORC, ASSE, NAHB, API, Alliance of Hazardous Materials Professionals,
National Paint and Coatings Association, Soap and Detergent
Association, ACC, and AISI agreed with OSHA that modifying the
standards will provide consistency and aid in employee understanding
(Document ID 0327, 0328, 0336, 0344, 0370, 0376, 0393, and
0408). Many commenters followed up with testimony at the informal
public hearings. NIOSH testified that there would be better
identification of what was a hazard and the nature of the hazard
(Document ID 0494 Tr. 50). BCTD AFL-CIO testified that the
specific format and vocabulary for labels would facilitate hazard
communication across a range of English literacy, as one in four
construction workers speaks a language other than English, and two in
three entering workers speak Spanish. They said that the signs,
symbols, and phrases will make it easier for employees to work safely
with hazardous products
(Document ID 0497 Tr. 7, 16, 33-34, 62, 66). ORC Worldwide
testified their companies have significant global operations and so
support concurrent harmonization of hazardous communication components
(Document ID 0497 Tr. 88, 91, 99). SIRC generally supported
the principles of the GHS update (Document ID 0494 Tr. 118).
ASSE agreed that it is important for consistency to have the same
language on the label, SDS, and regulated area sign (Document ID
0496 Tr. p. 362). In speaking about all labeling requirements,
USSW (Document ID 0499 Tr. 136-37) testified:
It's imperative that the information on the labels is consistent
from product to product. Incorporating the GHS labeling system with
pictograms and single-word hazard statements will assist workers to
quickly recognize hazards.
AIHA summed up the support from commenters and testifiers by declaring
that the GHS modifications will improve quality and consistency of
hazard communication information (Document ID 0496 Tr. 415).
Several commenters to the NPRM, while supporting the modifications,
raised potential problems with warnings for substance-specific health
standards' labels and regulated area signs. Northrop Grumman agreed
with the wording of the regulated area signs and that it would enhance
employee information, although there was concern that this was a change
in OSHA's policy of allowing supplemental language on labels and signs
that would enhance the information (Document ID 0395). OSHA
has not changed its policy on regulated area signs with this rulemaking
and will continue to allow supplemental language on labels and signs.
ASSE suggested that, under the proposal, the term "Cancer Agent"
would be retained in the thirteen identified carcinogens standard,
though the ASSE did not believe the problems caused by this
inconsistency would be significant (Document ID 0336). OSHA
notes that all cancer warnings, including "Cancer Agent" and "Cancer
Suspect Agent," have been changed to "May Cause Cancer," so there is
no inconsistency. NAHB addressed the issue of the cancer warning in a
comment to the ANPR, positing that the different signal words
("Danger" versus "Warning") and different hazard statements ("May
Cause Cancer" versus "Suspected of Causing Cancer") may create
confusion (Document ID 0065). Like other commenters, NIEHS
supported consistency, but thought "May Cause Cancer" may not be
strong enough, and recommended "Causes Cancer" be retained. The
International Chemical Workers Union Council agreed that "May Cause
Cancer" was not strong enough; they preferred "Causes Cancer"
because it was a more definite statement about the health hazard. They
were concerned that some workers might not see the warning as a clear
indication of the material causing cancer and act accordingly (Document
ID 0456). Dr. Michelle Sullivan also supported consistency
among SDSs, labels, and in-plant warning signs, but cautioned that
training would be needed especially on "May Cause Cancer" (Document
ID 0382). OSHA agrees that training will be needed and that
appropriately trained workers who see the phrase "May Cause Cancer"
will be well warned and benefit from the use of a consistent hazard
statement for all carcinogens.
The current substance-specific health standards that are regulated
as carcinogens have varying hazard statements on signs and labels, as,
for example, from "Cancer Hazard" for inorganic arsenic (29 CFR
1910.1018) to "Cancer-Suspect Agent" for vinyl chloride (29 CFR
1910.1017) to "May Cause Cancer" for methylenedianiline (MDA) (29 CFR
1910.1050). As stated in the preamble to the proposed standard, these
warnings appeared to suggest gradations of cancer hazards, but they
were not intended that way. The standards were promulgated over many
years, and the differences in the warning language reflect the language
widely used for each cancer warning at the time of promulgation, not
the degree of hazard (74 FR 50405, Sept. 30, 2009). This inconsistency
has long been a problem, especially in workplaces where two or more
OSHA-regulated carcinogens are used. The final rule's revision to the
substance-specific health standards will solve the problem of different
warning statements by standardizing the carcinogen warning language to
"May Cause Cancer" for each standard. This will lead to clearer and
more timely recognition of the hazard and, with training, better
understanding of the potential for developing cancer.
OSHA understands the points made by commenters who argued for
another warning for cancer that might appear stronger, but any other
warning would not be consistent with GHS and thus workers would not
benefit from the global consistency of a single hazard statement for
carcinogenicity. Moreover, OSHA believes that, with training, workers
will understand the seriousness of the warning and benefit from seeing
only one warning on carcinogens in the workplace. OSHA has concluded
that the signal words and hazard statements, including "May Cause
Cancer," in its substance-specific health standards will provide
better hazard information to employers, and has carried through the
changes proposed in the NPRM to the final rule.
See Table XIII-4 for a comparison of the signs' final language to
that currently required.
BILLING CODE 4510-26-P
[GRAPHIC] [TIFF OMITTED] TR26MR12.049
[GRAPHIC] [TIFF OMITTED] TR26MR12.050
[GRAPHIC] [TIFF OMITTED] TR26MR12.051
[GRAPHIC] [TIFF OMITTED] TR26MR12.052
[GRAPHIC] [TIFF OMITTED] TR26MR12.053
BILLING CODE 4510-26-C
OSHA's proposal to change the signage requirements in the
substance-specific standards was nearly universally supported by
commenters. Product Safety Solutions, AHMP, National Paint and Coatings
Association, Ameren, Wacker Chemical Corp, ASSE, Stericycle, Phylmar
Regulatory Roundtable, Soap and Detergent Association/Consumer
Specialty Products Association, Ecolab, Inc., AIHA, ORC Worldwide,
National Association of Homebuilders, API, Procter & Gamble Company,
Dr. Michelle Sullivan, Clariant Corporation, American Chemical Council,
3M, AISI (American Coke and Coal Chemicals Institute), Industrial
Health and Safety Consultants, and NIOSH, in their comments to the
NPRM, specifically supported the harmonization of signage required in
the substance-specific standards (Document ID 0313, 0327,
0328, 0330, 0335, 0336, 0338, 0339, 0344, 0351, 0365, 0370, 0372, 0376,
0381, 0382, 0383, 0393, 0405, 0408, 0410, and 0412). USSE, agreeing
with the commenters above, testified that having the same wording on
regulated-areas signs would be helpful to workers as they move around
and it is better for them to see the same information they have been
trained on (Document ID 0499 Tr. 165).
Commenters raised several signage issues. Dow Chemical advocated
the elimination of signs in substance-specific health standards,
arguing that there was no need for signs since the chemical will be
labeled and workers can also refer to an SDS (Document ID
0353). OSHA disagrees. The substance-specific standards' sign
requirements cover regulated areas of facilities that are by definition
high-exposure or potentially high-exposure areas. They are among the
most dangerous areas in a facility, which is why OSHA requires signs.
Moreover, contrary to what Dow assumes, product labels may not always
be available in these circumstances. Thus, OSHA disagrees with Dow
Chemical and believes the signs convey crucial information about the
chemical hazard in a regulated area and that the signs benefit not only
the well-trained worker but also other workers who might be near, or
inadvertently enter, the regulated area.
The Battery Council International (Document ID 0390) had
suggestions for language on regulated area signs for the lead standard,
29 CFR 1910.1025. First, they requested that OSHA change the language
from "Causes Damage to the Central Nervous System" to "May Cause
Damage to the Central Nervous System," since nerve damage may or may
not occur depending on whether or not the facility has taken proper
precautions. However, as discussed above, OSHA has updated the signs to
be consistent with GHS labeling to ensure that the worker is receiving
the same message and this would provide better identification of the
hazard. Therefore, OSHA has retained the proposed language for lead
regulated area signs in the final.
The Battery Council International also requested that OSHA retain
the original language Sec. 1910.1025(m)(2) so that it would be clear
that other signage may also be used in places where required (Document
ID 0390). For example, it reported that California has such a
signage requirement under Proposition 65. OSHA agrees that, in some
very specific cases, other warnings may be necessary for lead. Thus,
the current requirement that, "The employer may use signs required by
other statutes, regulations or ordinances in addition to, or in
combination with, signs required by this paragraph," has been retained
in the final rule for the lead standard at Sec. 1910.1025(m)(2)(iv).
OSHA concludes that the proposed changes, which are as close as
possible to the GHS terminology, are essential in order to make the
warnings on signs consistent with each other, as well as labels, to the
extent possible. These consistent warning signs will provide the best
hazard communication in the relevant workplace regulated areas. The
proposed changes to the signage requirements of the substance-specific
standards have been carried through to the final rule.
Hazard Communication, Classification and Labels
OSHA's current substance-specific standards are inconsistent in
that some have their own communication of hazards requirements, while
other standards reference the HCS, and still other standards have no
requirements for labels and safety data sheets in their sections.
Although these latter standards are missing requirements, they still
are covered by HCS. Similarly for labels, while most substance-specific
standards require labels on containers of raw materials, mixtures, and
products, some specify specific language while others reference the
HCS. As proposed, and as carried forward in this final rule, OSHA has
standardized the language for hazard communication and has removed the
requirements for specific language labels from the "Communications of
hazards" paragraphs of the substance-specific standards. The new
paragraph in each substance-specific standard uses the following model
format:
Hazard Communication--General.
(i) Chemical manufacturers, importers, distributors and
employers shall comply with all requirements of the Hazard
Communication Standard (HCS) (29 CFR 1910.1200) for [chemical name].
(ii) In classifying the hazards of [chemical name] at least the
following hazards are to be addressed: [hazard information].
(iii) Employers shall include [chemical name] in the hazard
communication program established to comply with the HCS. Employers
shall ensure that each employee has access to labels on containers
of [chemical name] and to safety data sheets, and is trained in
accordance with the requirements of HCS and paragraph [Training
paragraph] of this section.
By adding this paragraph in each substance-specific health
standard, OSHA achieves consistency across standards and with GHS
principles. Some commenters indicated that the chemicals covered by the
substance-specific standards should not be classified any differently
than any other chemical in regard to the health hazards included on a
label or SDS (See, e.g., Document ID 0365). That was OSHA's
intent. OSHA has clarified the regulatory language to minimize
confusion. The final rule, like the proposal, requires compliance with
the HCS in each substance-specific standard.
OSHA believes that requiring standards to reference HCS will ensure
consistency with the GHS revisions and across the standards and
consistency when the specific chemical is part of a mixture. Removal of
the current specific warning language was essential for adoption of the
GHS language. Retention of these provisions in the standards would
result in the untenable situation of two potentially conflicting
requirements, only one of which (the reference to HCS) would be in
accord with the GHS-modified HCS. Moreover, as OSHA noted in the
preamble to the proposed standard, the hazard statements specified for
the chemical in the standard might not be correct when the chemical is
part of a mixture. As for the current standards that simply referenced
HCS, employers could choose any language and format that conveyed the
necessary information. This approach is no longer allowed because, as
OSHA has found in adopting the GHS approach, consistency in labeling is
key to effective communication of hazards. The vast majority of
commenters agreed. For example, AHMP noted that eliminating language
inconsistent with established hazard statements will facilitate hazard
communication and should not result in lower protection (Document ID
0327). Others, including NIOSH, DuPont,
Ameren, ASSE, Ecolab, Inc., AIHA, ORC Worldwide, NAHB, API, Procter &
Gamble, Dr. Michelle Sullivan, ACC, 3M, AISI, Industrial Health and
Safety Consultants, and American Coke and Coal Chemicals Institute,
agreed (Document ID 0329, 0330, 0336, 0351, 0365, 0370, 0372,
0376, 0381, 0382, 0393, 0405, 0408, 0410, and 0412). Commenters noted
that for the benefits of consistency to accrue, harmonization is
essential (Document ID 0313, 0315, 0327, 0328, 0329, 0330,
0335, 0336, 0338, 0344, 0365, 0370, 0372, 0376, 0381, 0382, 0383, 0393,
0405, 0408, and 0410). NIEHS Worker Education and Training Program
agreed, testifying that consistency of labels and safety data sheets is
important to help employees recognize hazards and be able to deal with
them effectively (Document ID 0497 Tr. 104). Phylmar
Regulatory said that standardized label elements will be more effective
in communicating hazard information (Document ID 0497 Tr. 108-
109). AIHA testified that standardized labels will make hazard
identification easier and the pictograms will be useful in workplaces
where English language reading is limited (Document ID 0496
Tr. 415). USSW affirmed that one hazard communication system would be
best (Document ID 0499 Tr. 178). OSHA believes all these
commenters provide important and compelling reasons for the labels
required by the substance-specific standards to be consistent with the
GHS modifications to HCS.
For classification purposes, OSHA proposed to provide guidance on
the potential health outcomes that must be addressed when classifying a
substance by setting forth the health end-points (outcomes) for each
substance-specific health standard. The Agency did not attempt to
formally classify each substance; rather, OSHA provided a proposed list
of health effects to assist the classifier in determining what must be
considered for inclusion on the new labels. The GHS classification
process for a specific substance dictates the actual hazard warnings
and precautionary statements that are required on the new GHS-compliant
labels and SDSs. In determining the hazards to include for each
substance-specific health standard, the Agency's primary sources on
health effects were the information gained in its own rulemakings and
subsequent experience, the NIOSH Pocket Guide to Chemical Hazards
(2005), and the International Chemical Safety Cards (ICSCs). The ICSCs
are an undertaking of the International Programme on Chemical Safety
(IPCS) (a joint activity of three cooperating International
Organizations, namely, the United Nations Environment Programme (UNEP),
the International Labour Office (ILO), and the World Health
Organization (WHO)) and are peer reviewed by a group of internationally
recognized experts (Document ID 0412.2). As a secondary
source, OSHA also considered the European Union's (EU) "Proposal for a
Regulation of the European Parliament and of the Council on
Classification, Labeling and Packaging of Substances and Mixtures, and
amending Directive 67/548/EEC and Regulation (EC) No 1907/2006." From
these sources, OSHA developed hazard endpoints to be considered for
hazard classification in the substance-specific health standards based
on either of two criteria: (1) The health hazard was the basis for the
original rulemaking; or (2) the health hazard was asserted by OSHA,
NIOSH, or IPCS, and confirmed by a second source. For example,
acrylonitrile (AN) (Sec. 1910.1045) was regulated by OSHA based on its
carcinogenicity. Skin sensitization was acknowledged by OSHA, IPCS, and
EU; skin irritation by OSHA, NIOSH, and EU; respiratory tract
irritation by IPCS and EU; eye irritation by OSHA, NIOSH, and IPCS;
liver effects and central nervous system effects by IPCS and NIOSH;
acute toxicity by OSHA, IPCS, and EU; and flammability by IPCS, NIOSH,
and EU. Because all these effects met the criteria for inclusion, skin
sensitization, skin irritation, respiratory irritation, eye irritation,
liver effects, central nervous system effects, acute toxicity, and
flammability were listed as potential hazards in the acrylonitrile
standard.
OSHA's approach, including its choice of sources for health
effects, was generally supported by many commenters to the proposal
(Document ID 0329, 0339, 0351, 0370, and 0376). However, some,
including NIOSH, AIHA, ASSE, Ameren, Stericycle, Wacker Chemical
Corporation, and 3M Corporation, wanted OSHA to add other sources
(Document ID 0233, 0330, 0338, 0365, 0405, and 0412). NIOSH
suggested OSHA look at OECD SIDS, ESIS, NOAA, NLM, NLM-TOXSEEK, NLM-
TOXNET, IPCS, CCOHS, and GESTIS (Document ID 0412). AIHA
commented that substance-specific health standards should be classified
the same as other chemicals and that other references such as ATSDR
Toxicological Profiles, IRIS Toxicological Reviews, WHC Monographs,
CICADS, OECD SIDS, and Patty's Toxicology should be used (Document ID
0365). Wacker Chemical Corporation recommended IARC be
included and that one recognized body's determination of a hazard
should be sufficient (Document ID 0335). ASSE urged inclusion
of ACGIH documentation of TLVs and RELs and precautions developed by
manufacturers from testing and epidemiological studies. ASSE submitted
a long list of sources including NSC's Fundamentals of Industrial
Hygiene, The Industrial Environment--Its Evaluation and Control,
Patty's Industrial Hygiene and Toxicology, Casarett & Doull's
Toxicology, The Dose Makes the Poison, Quick Selection Guide to
Chemical Protective Clothing, U.S. DHHS Seventh Annual Report on
Carcinogens, AIHA Engineering Field Reference Manual, and 17 others
(Document ID 0336). AIHA urged OSHA to have the hazards for
the substance-specific standards considered, but not be mandatory. It
recommended additional references such as ATSDR Toxicological Profiles,
IRIS Toxicological Reviews, EHC Monographs, CICADS, OECD SIDS, and
Patty's Toxicology (Document ID 0365). Ameren would have OSHA
add ACHIS and AIHA sources (Document ID 0330). Stericycle
advocated adding Industrial Chemical Safety Cards, European Commission,
and ACGIH as secondary sources (Document ID 0338). Still
others, such as ASSE, API, AHMP, Product Safety Solutions, National
Paint and Coatings Association, and Industrial Minerals Association--
North America, deemed OSHA's choice of sources inadequate (Document ID
0313, 0327, 0328, 0338, 0376, and 0379). USSW (Document ID
0403) found lists such as IARC's and NTP's useful, but wanted
OSHA to state in the regulatory text that a chemical on one or more of
these lists was sufficient to classify it as hazardous (although the
absence of a chemical on a list does not mean it is not hazardous). It
also wanted OSHA to use lists in enforcement.
Commenters also raised other issues in this regard. API believed
OSHA should just reference the GHS criteria, while ASSE wanted OSHA to
use other authoritative references (Document ID 0336 and
0376). Both AHMP and Product Safety Solutions were concerned the NIOSH
Pocket Guide and International Chemical Safety Cards had not been
subject to rulemaking and could be overly conservative, even though
they felt these sources could be used as information, but not as
precedent if significant contradictory information is presented
(Document ID 0313 and 0327). National Paint and Coatings
Association commented that the substance-specific standards' health
hazards should remain as published and only new information should be
subject to the two-reference rule (Document ID 0328). Still
other commenters, including DuPont Company, Soap and Detergent
Association and Consumer Specialty Products Association, Procter &
Gamble, and Dr. Michelle Sullivan, expressed concern about whether the
sources OSHA was using were to be current or updated, as newer editions
become available (Document ID 0329, 0344, 0381, and 0382).
OSHA believes these comments reflect a misunderstanding of what
OSHA proposed for its substance-specific health standards and how the
sources were used to yield health effects to be considered in
classifying all health hazards but not to perform a formal
classification. (See 74 FR at 50411, Sept. 30, 2009, for the preamble
explanation). The substance-specific health standards are unique in
that they were all the subject of rulemaking, enabling the Agency to
collect extensive information on sources and on health effects. That
collection of information, coupled with the Agency's own expertise,
enabled the Agency to confidently select sources for these regulated
chemicals that would provide adequate information to classifiers. OSHA
disagrees with commenters who suggested its chosen sources were
inadequate. Some commenters recommended other sources. OSHA believes
that these other sources can be useful in classifying hazards, and can
certainly be used by classifiers in evaluating the hazards related to
chemicals regulated by the substance-specific standards. At issue here,
though, is the method OSHA has determined to use for selecting a list
of hazard endpoints that, at a minimum, must be considered to provide
accurate warnings on labels for its substance-specific standards. OSHA
has concluded that the method it used in the proposal is scientifically
sound and appropriate.
In complying with the HCS, as discussed above, classifiers must
take into account available scientific information about the hazards of
the chemical being classified, which could include information found in
the other sources noted by the commenters. The manufacturer,
distributor, or importer must still classify and categorize each
regulated chemical (in the substance-specific health standards) in
compliance with the GHS-modified HCS and its appendices. The lists of
endpoints for each substance-specific standard are the minimum that
must be considered. The manufacturer or importer has leeway to use
additional primary studies and sources to evaluate the substance-
specific chemical and is free to add health effects' endpoints as
appropriate according to the studies or sources. As discussed
previously in this section, the HCS generally uses a weight-of-evidence
approach in classifying health hazards. Therefore, a superior source or
significant and compelling contradictory information to a particular
source usually must be weighed with the total body of evidence.
IMA-NA suggested that OSHA's methodology for determining the list
of health effects to be considered by classifiers does not meet the
requirements of the Information Quality Act (Document ID
0233). OSHA disagrees. That statute, and the guidelines
published under it (discussed in more detail above), require that
agencies take steps to ensure the "quality, objectivity, utility, and
integrity" of information they disseminate. Similar to its response to
the concern regarding TLVs, discussed above, OSHA does not believe that
it is disseminating information for purposes of the IQA when it merely
requires that manufacturers and importers consider specific health
effects listed for each substance-specific standard in classifying the
chemical under the HCS. However, even if it were disseminating
information in the final rule, OSHA believes that it has complied with
the applicable requirements of the IQA. OSHA has fully described the
methods by which it determined the listed health effects for each
substance, relied only on respected health compilations prepared by
governmental agencies or subject to peer review, and subjected its
analysis to notice and comment in this rulemaking. This adequately
assures the quality, objectivity, utility, and integrity of any
dissemination of information involved in these provisions of the final
rule.
OSHA received no comments on the particular hazards proposed for
each substance-specific health standard, and retained them in the final
rule. The endpoints for each substance-specific standard are listed in
Table XIII-5, "Health Effects Determined for the Substance-Specific
Standards."
Table XIII-5--Health Effects Determined for the Substance-Specific
Standards
------------------------------------------------------------------------
Standard No. Substance Health effects
------------------------------------------------------------------------
1910.1001, 1915.1001, Asbestos............ Cancer and lung
1926.1101. effects.
1910.1003................... 4-Nitrobiphenyl..... Cancer.
1910.1003................... Alpha-Naphthylamine. Cancer; skin
irritation; and
acute toxicity
effects.
1910.1003................... Methyl chloromethyl Cancer; skin, eye,
ether. and respiratory
effects; acute
toxicity effects;
and flammability.
1910.1003................... 3,3'- Cancer and skin
Dichlorobenzidine sensitization.
(and its salts).
1910.1003................... Bis-Chloromethyl Cancer; skin, eye,
ether. and respiratory
tract effects;
acute toxicity
effects; and
flammability.
1910.1003................... Beta-Naphthylamine.. Cancer and acute
toxicity effects.
1910.1003................... Benzidine........... Cancer and acute
toxicity effects.
1910.1003................... 4-Aminodiphenyl..... Cancer.
1910.1003................... Ethyleneimine....... Cancer;
mutagenicity; skin
and eye effects;
liver effects;
kidney effects;
acute toxicity
effects; and
flammability.
1910.1003................... Beta-Propiolactone.. Cancer; skin
irritation; eye
effects; and acute
toxicity effects.
1910.1003................... 2- Cancer.
Acetylaminofluorene.
1910.1003................... 4-Dimethylaminoazo- Cancer; skin
benzene. effects; and
respiratory tract
irritation.
1910.1003................... N- Cancer; liver
Nitrosodimethylamin effects; and acute
e. toxicity effects.
1910.1017................... Vinyl chloride...... Cancer; central
nervous system
effects; liver
effects; blood
effects; and
flammability.
1910.1018................... Inorganic arsenic... Cancer; liver
effects; skin
effects;
respiratory
irritation; nervous
system effects; and
acute toxicity
effects.
1910.1025, 1926.62.......... Lead................ Reproductive/
developmental
toxicity; central
nervous system
effects; kidney
effects; blood
effects; and acute
toxicity effects.
1910.1026, 1915.1026, Chromium VI......... Cancer; skin
1926.1126. sensitization; and
eye irritation.
1910.1027, 1926.1127........ Cadmium............. Cancer; lung
effects; kidney
effects; and acute
toxicity effects.
1910.1028................... Benzene............. Cancer; central
nervous system
effects; blood
effects;
aspiration; skin,
eye, and
respiratory tract
irritation; and
flammability.
1910.1029................... Coke oven emissions. Cancer.
1910.1043................... Cotton Dust......... Lung effects.
1910.1044................... 1,2-dibromo-3- Cancer; reproductive
chloropropane effects; liver
(DBCP). effects; kidney
effects; central
nervous system
effects; skin, eye,
and respiratory
tract irritation;
and acute toxicity
effects.
1910.1045................... Acrylonitrile (AN).. Cancer; central
nervous system
effects; liver
effects; skin
sensitization;
skin, respiratory,
and eye irritation;
acute toxicity
effects; and
flammability.
1910.1047................... Ethylene oxide (EtO) Cancer; reproductive
effects;
mutagenicity;
central nervous
system; skin
sensitization;
skin, eye, and
respiratory tract
irritation; acute
toxicity effects;
and flammability.
1910.1048................... Formaldehyde........ Cancer; skin and
respiratory
sensitization; eye,
skin, and
respiratory track
irritation; acute
toxicity effects;
and flammability.
1910.1050, 1926.62.......... Methylenedianiline Cancer; liver
(MDA). effects; and skin
sensitization.
1910.1051................... 1,3 Butadiene (BD).. Cancer; eye and
respiratory tract
irritation; center
nervous system
effects; and
flammability.
1910.1052................... Methylene chloride.. Cancer; cardiac
effects; central
nervous system
effects; liver
effects; and skin
and eye irritation.
------------------------------------------------------------------------
The NPRM retained specific language for labels in the substance-
specific health standards for containers of contaminated clothing or
waste and debris to ensure that protection gained from communicating
these hazards to the downstream recipients of the materials would not
be lessened. The proposal, however, updated the language to be
consistent with the GHS. The labeling requirements in these standards
are part of broad protections, resulting from PELs and ancillary
provisions such as exposure monitoring, personal protective equipment,
and medical surveillance. These requirements for labeling containers of
contaminated clothing, PPE, and waste and debris have been an integral
part of the standards since their promulgation. To simply conform the
labeling requirements for these kinds of containers to the GHS-modified
HCS rule would not offer the extra protection currently provided in
these standards; because of the variation in the quantity of chemicals
in the containers of contaminated clothing, PPE, and waste and debris,
the chemical concentration may be lower than the specified cut-off
values/concentration limits. In such a case, if OSHA only relied on the
GHS-modified HCS labeling requirement, labeling for these containers
may not be triggered and protections would be lessened.
Commenters agreed that specific language for labels on containers
of contaminated clothing and waste and debris should be maintained. For
example, Ameren and 3M Corporation commented that maintaining specific
language for labels on contaminated clothing and waste/debris
containers for the substance-specific health standards will provide
adequate warnings to all (Document ID 0330 and 0405). AIHA, in
supporting the specific labels, noted that the workplace-contaminated
materials are not hazardous chemicals in commerce; thus, these special
labels are not inconsistent with GHS. Further, AIHA said that because
recipients of these containers are accustomed to specific warnings, a
change, such as elimination of the specific warning language because it
might not be required by GHS, might be perceived as a change in hazard
(Document ID 0365). The Battery Council International urged
OSHA not to eliminate the label language requiring the disposal of
lead-contaminated waste water in accordance with applicable local,
state, or federal regulations in Sec. 1910.1025(m)(2) for contaminated
clothing. OSHA agrees that this information is important and is not
inconsistent with GHS labeling. Therefore, OSHA has retained this
language for the labels for contaminated clothing and equipment in the
final rule. AISI and Industrial Health and Safety Consultants urged
OSHA to require that the language on containers of contaminated
clothing and waste/debris be in accord with the GHS guidelines. Such
harmonization would maintain consistency with other labeling and
minimize confusion of downstream handlers (Document ID 0408).
In addition, Industrial Health and Safety Consultants felt that
containers of contaminated clothing and waste/debris should be
classified according to the HCS and the specific language on the label
should be eliminated (Document ID 0410). As discussed below,
OSHA does not agree. Industrial Health and Safety Consultants also
suggested that OSHA require HCS classification and labeling of
contaminated waste clothing and waste for all chemicals (Document ID
0410). OSHA did not propose such extensive new requirements
for containers of chemically contaminated clothing and waste and
debris. These requirements were not part of HCS and would be a
significant addition to the final rule.
OSHA agrees with the commenters who advocate retaining the warnings
and harmonizing these labels for contaminated clothing and waste and
debris containers, and did so to the extent possible. (See 74 FR 50434-
50439, Sept. 30, 2009). However, classifying containers of chemically
contaminated clothing and waste and debris consistent with GHS would be
an impossible task, as substances found on contaminated clothing and
waste and debris often occur in unknown, varying, and frequently small
quantities. In order to ensure and maintain protection for employees in
workplaces that receive these containers, labeling of the hazards with
specific language is essential. The warnings, like all other warnings,
are most effective when they are consistent with each other and, to the
extent possible, with the GHS language. This consistency was achieved
with the proposed language. Therefore, the proposed language for the
substance-specific standards remains unchanged and is finalized in this
rulemaking.
OSHA is adding two warnings to the Cadmium standard, which were
left out of one paragraph of the proposal, through an error. In the
NPRM, OSHA proposed that the warning labels for waste, scrap, or debris
be required to include "Danger"; "Contains Cadmium"; and "May
Cause Cancer" in paragraph 1910.1027(m)(3)(ii). The warnings "Causes
Damage to Lungs and Kidneys" and "Avoid Creating Dust" were
inadvertently left out of this paragraph. (The NPRM properly included
these two warnings in paragraph 1910.1027(i)(2)(iv) for bags and
containers of contaminated protective clothing and equipment.) OSHA is
correcting this error by adding these warnings in this final standard,
making the Cadmium standard consistent with the other substance-
specific standards and, to the extent possible, with GHS.
In addition, for labels of bags or containers of contaminated
clothing and equipment, OSHA has determined precautionary statements
that address creating dust in the current substance-specific health
standards must be retained even though there is no GHS equivalent. At
this time, a work group formed under the UN Sub-Committee of Experts
for the GHS (UN Sub-committee) is working to finalize issues related to
hazard and precautionary statements. OSHA has recommended to the UN
Sub-committee to adopt the phrase "avoid creating dust" as a
precautionary statement, if this statement is adopted as a
precautionary statement, then this statement will be consistent with
the GHS. However, if the UN Sub-committee does not adopt such a
statement, OSHA intends to continue to require the dust statements in
those paragraphs for labels of bags and containers of contaminated
clothing and equipment since OSHA has concluded that removing these
statements would be a lessening of protection. An example of
requirements for those statements can be found in OSHA's Cadmium
standard, Sec. 1910.1027(i), (k), and (m). OSHA also inadvertently
removed the term "Avoid Creating Dust" from the Asbestos labeling
requirements in Sec. 1910.1001(j) and Sec. 1926.1101(l) of the
proposal. As discussed above, OSHA believes that this is a unique
statement and should be retained. OSHA is correcting this error by
reinstating this phrase in the asbestos labeling requirements in Sec.
1910.1001(j) and Sec. 1926.1101(l).
Occupational Exposure To Hazardous Chemicals in Laboratories:
Definitions
OSHA proposed to modify most of paragraph (b), Definitions, in
Sec. 1910.1450, Occupational Exposure to Hazardous Chemicals in
Laboratories (the laboratory standard), in order to maintain
compatibility with HCS. In particular, OSHA removed the definitions of
Combustible liquid, Compressed gas, Explosive, Flammable, Flashpoint,
Organic peroxide, Oxidizer, Unstable (reactive), and Water-reactive
from paragraph (b). In addition, in the NPRM, OSHA revised the
definitions of Hazardous chemical, Physical hazard, and Reproductive
toxins in paragraph (b) and added definitions for Health hazard and
Mutagen in paragraph (b). By these modifications to Sec. 1910.1450,
the proposal sought to ensure that the definitions to the GHS-modified
HCS also apply to the laboratory standard (Sec. 1910.1450). The
modification is consistent with the goal of this rulemaking and the
original intent of the laboratory standard. OSHA explained in the
preamble to the laboratory standard the importance of having the HCS
and the laboratory standard both use the same definitions for hazardous
chemicals:
The term "hazardous chemical" used in this final rule relies
on the definition of "health hazard" found in the OSHA Hazard
Communication Standard. As discussed in the scope and application
section above, commenters urged OSHA to maintain consistency in
terms between the Hazard Communication Standard and this final
standard since laboratories are subject to both regulations.
(55 FR 3315, Jan. 31, 1990).
Ameren agreed with OSHA that "combustible liquid" should be
removed from paragraph (b) (Document ID 0330). However, the
company recommended that OSHA replace the term with specific flashpoint
criteria. OSHA disagrees that a definition for combustible liquid with
specific flashpoint criteria differing from GHS-modified HCS should be
contained in the laboratory standard. OSHA's intention is to harmonize
the laboratory standard with the GHS-modified HCS. The final HCS rule
contains definitions of flammable liquids with flashpoint criteria in
Appendix B, and these flashpoint criteria include what are currently
the combustible liquid classes. The laboratory standard does not
contain specific requirements for physical hazards, including flammable
or combustible liquids. Rather, this program standard contains
requirements for such things as a chemical hygiene plan, employee
exposure determination, training, medical consultation and
examinations, and recordkeeping. Thus, OSHA does not see a need for
including separate flashpoint criteria for flammable or combustible
liquids and believes that reference to the flammable liquid categories
in HCS is appropriate for Sec. 1910.1450.
OSHA proposed to maintain the current definition of "select
carcinogens" in the laboratory standard since the original purpose of
the standard was to deviate from the HCS definition and narrow the
scope of the standard. As noted in the preamble to the final rule for
the laboratory standard, the scope was set for "select carcinogens"
based on the small, often minute, quantities of substances handled.
OSHA stated its reasons for this deviation in that preamble, and those
reasons remain persuasive:
This final rule, however, modifies the carcinogen definition and
the obligatory action so that special provisions must be explicitly
considered by the employer, but need only be implemented when the
employer deems them appropriate on the basis of the specific
conditions existing in his/her laboratory. Moreover, the term,
"carcinogen" has been replaced by "select carcinogen" which
covers a narrower range of substances * * *
(55 FR 3315, Jan. 31, 1990).
OSHA has thus incorporated in the final rule its proposed changes
to the definitions in the laboratory standard.
Appendices
OSHA reviewed the appendices to each of its substance-specific
health standards and made the following minor changes necessary to
align the appendices with their GHS-harmonized standards.
The language in Appendix B, "Employee Standard Summary," chapter
XI, "Signs," in both the general industry and the construction
standards for lead (Sec. 1910.1025 and Sec. 1926.62, respectively)
has been made consistent with the language in their regulatory texts.
In Asbestos Sec. 1910.1001, Appendix F, "Work Practices and
Engineering Controls for Automotive Brake and Clutch Inspection,
Disassembly, Repair and Assembly (Mandatory)," a reference to
paragraph (j)(4) of the standard has been redesignated as paragraph
(j)(5) to be consistent with the changes in the regulatory text for
Sec. 1910.1001. No changes were made to the construction Asbestos
standard Sec. 1926.1101, as none were needed.
Safety Data Sheets
OSHA has changed the term "material safety data sheets" when it
appears to "safety data sheets" in both the substance-specific health
standards and their appendices. As discussed above, this change
reflects the GHS terminology.
Compliance Dates for Substance-Specific Health Standards
OSHA proposed to require implementation of all but one of the
revisions to the HCS in three years following completion or
promulgation of the final rule. Training was proposed to be required in
two years. OSHA noted that during the transition period, an employer
could be in compliance with either the current HCS or the revised HCS
(the final rule), but there could not be a lapse in compliance. For the
final standard, OSHA has decided to align implementation of GHS with
the final implementation of GHS in the EU for labeling and SDSs. A full
explanation of the information and comments and the Agency's reasoning
is set out above in this section.
The proposed changes to the substance-specific health standards
required compliance with the HCS, thus incorporating the proposed
compliance dates for the revised HCS. One commenter suggested that the
proposed sign and label updates be done in accordance with the
facility's normal replacement schedule (Document ID 0376).
OSHA finds that this is too indefinite a period, because it essentially
leaves the compliance date in the hands of each employer. OSHA has
concluded that the administration of HCS programs by employers and the
communication and comprehension of the hazards by employees will be
most effective if the requirement for completion of changes for the
substance-specific health standards is the same as for all other
chemicals. In a sense, this is just another example of the consistency
that was approved by so many of the commenters and hearing witnesses.
Thus, the final rule keeps the compliance dates for the new
substance-specific health standard requirements in line with those for
the revisions to the HCS. Employers must be using new labels for
contaminated clothing and waste and debris by June 1, 2015, the date by
which manufacturers and importers must comply with the labeling and SDS
requirements of the revised HCS. Employers must post the new signs by
June 1, 2016, the same date by which employers must also update their
hazard communication plans for any new hazard information they receive
as a result of the final rule. In the meantime, as with the revised
HCS, employers must comply with either the old or new labeling and
signage requirements. Provisions to this effect are inserted for each
substance-specific standard in this final rule.
Safety Standards
OSHA proposed modifying safety standards that either directly
reference the HCS or provide information pertinent to the SDSs, in
particular regarding the storage and handling of chemicals. As noted
above, many commenters supported standardizing physical hazard criteria
across all applicable OSHA standards (Document ID 0034, 0104,
0105, 0155, 0170, 0171, 0313, 0324, 0327, 0328, 0329, 0336, 0338, 0359,
0365, 0376, 0382, 0395, 0405, 0408, 0410, and 0494 Tr. 91, 162). For
example, the Compressed Gas Association (CGA) (Document ID
0324) stated:
CGA agrees with the harmonization to GHS to align the
definitions of the physical hazards to the requirements of the GHS
categories in safety standards for general industry, construction,
and maritime standards, which either directly reference the Hazard
Communication Standard * * * or provide information pertinent to the
SDS.
However, some other commenters, and even some who supported
applying physical hazard criteria across all standards, raised concerns
about storage and handling requirements; degree of impact; potential
effects on the scope of the Process Safety Management of Highly
Hazardous Chemicals (PSM) standard; and potential conflicts with widely
accepted consensus standards (Document ID 0038, 0077, 0104,
0163, 0329, 0335, 0336, 0339, 0366, 0370, 0381, 0383, 0393, 0399, 0414,
0500, 0514, 0530, 0643, 0494 Tr. 91, 162, and 0497 Tr. 81-84).
OSHA agrees with the commenters who supported standardizing
physical hazard criteria and is doing so except in some standards, such
as OSHA's electrical standards, where conflicts with referenced
consensus standards make harmonization inappropriate at this time. OSHA
proposed to:
Incorporate the current HCS definitions of flammable
liquid and gas into PSM and health hazard into Hazardous Waste
Operations and Emergency Response (HAZWOPER) standards;
Modify the Welding standard (Sec. 1910.252) requirements
on labeling welding consumables to be consistent with GHS modifications
to HCS;
Amend paragraphs on flammable and combustible liquids to
conform categories, terminology, flashpoints (FP), and boiling points
to the GHS modifications to HCS;
Incorporate the modified-HCS definition of flammable
aerosols into the Flammable and Combustible Liquids standard, Sec.
1910.106. (In Sec. 1910.106, OSHA is also correcting a rounding error
in the conversion from 12 feet to meters. The change is from 3.648
meters to 3.658 meters); and
Update the acceptable methods for determining flashpoints;
but
Leave unchanged electrical standards in Subpart S for
general industry and Subpart K for construction, and explosive
standards for general industry (Sec. 1910.109) and for construction
(Sec. 1926.914).
Commenters overwhelmingly supported ensuring consistency in OSHA
standards, while maintaining scope of coverage. (Document ID
0049, 0050, 0077, 0105, 0123, 0145, 0163, 0170, 0313, 0324, 0327, 0328,
0351, 0359, 0365, 0376, and 0494 Tr. 91, 162). Organization Resource
Counselors (ORC) (Document ID 0494 Tr. 91) testified:
ORC supports concurrent harmonization of hazard definitions in
most OSHA standards. ORC agrees with OSHA's proposal to harmonize
hazardous communication components across most other OSHA standards
in this rulemaking. ORC believes this is the most efficient way to
address this necessary step in ensuring consistent hazard
information and eliminating conflicting requirements.
Many comments to the ANPR and the NPRM supported OSHA exempting
certain standards such as electrical and explosive standards from
harmonization at this time (Document ID 0047, 0075, 0076,
0104, 0113, 0145, 0163, 0328, 0330, 0336, 0370, 0393, and 0408). For
example, the standards in Subpart S contain requirements such as
internal design criteria that, if changed, would impact their scope.
OSHA's reasons for excluding these standards are explained below. In
testimony at the hearing, the ACC (Document ID 0494 Tr. 162)
agreed, stating:
We agree with this approach and therefore would expect that
there would be no impact on electrical area classification, facility
[s]iting, mechanical integrity, electrical classification, storage
quantities, unloading and storage location, ventilation
requirements, spill protection, grounding and bonding, tank and
vessel design, interlocks and safety devices and process hazard
analysis.
As discussed in detail below, in the final rule PSM retains its
current scope; HAZWOPER's definition of "health hazard" is modified;
the definitions in the Flammable and Combustible Liquids standards are
aligned with the GHS modifications to HCS; Welding, Cutting and Brazing
labeling requirements were also modified to be consistent with HCS; and
a few technical amendments have been made to other safety standards
that currently use the term "combustible" in order to keep their
scope the same. Also, no changes were made to standards that OSHA
proposed to exclude from this rulemaking.
PSM
PSM standards for general industry and construction reference the
HCS for their scopes, which are currently set forth in Sec.
1910.119(a)(1)(ii) and Sec. 1926.64(a)(1)(ii) as covering a process
which involves a flammable liquid or gas (as defined in Sec.
1910.1200(c) [Sec. 1926.59(c)] on site in one location, in a quantity
of 10,000 pounds (4535.9 kg) or more, followed by the listed exceptions
in the paragraph.
If OSHA did not modify this provision in this rulemaking, the scope
of PSM would expand since the HCS's definition of flammable liquid
changes from liquids with a flashpoint below 100 [deg]F (37.8 [deg]C)
to the new GHS definition of liquids with a flashpoint at or below
199.4 [deg]F (93 [deg]C) (though, as discussed above, the scope of the
HCS is unaffected). Keeping the reference to the HCS definition would
mean that many more processes would have been covered by the PSM
standards than when those standards were promulgated. OSHA does not
intend to expand the scope of the PSM standards. Therefore, to maintain
the scope of those standards, OSHA proposed to modify the language in
the scope paragraphs Sec. 1910.119(a)(1)(ii) and Sec.
1926.64(a)(1)(ii) to read:
A process which involves a Category 1 flammable gas (as defined
in Sec. 1910.1200(c)) or flammable liquid with a flashpoint below
100 [deg]F (37.8 [deg]C) on site in one location, in a quantity of
10,000 pounds (4535.9 kg) or more * * *
In other words, for PSM, "flammable gas" includes Category 1
flammable gases and liquids only if they have flashpoints below 100
[deg]F (37.8 [deg]C) to be consistent with the criteria specified in
the current HCS.
Commenters who considered the issue differed on what should be done
(Document ID 0324, and 0402). For example, ACC, in responding
the NPRM, supported OSHA's approach (Document ID 0393). ACC
noted that OSHA's proposed regulatory language for Sec. 1910.119, the
general industry PSM, appropriately reflected the new cut-off without
changing the scope of the regulation (Document ID 0393).
However, CGA requested that OSHA update paragraph (a)(1)(ii) of Sec.
1910.119 to use GHS Category 1 flammable liquids as a cutoff for PSM
coverage, stating, "This would maintain consistency throughout the
OSHA standards and harmonization with the GHS" (Document ID
0324). The Society of Chemical Manufacturers and Affiliates
(SOCMA) (Document ID 0402) was concerned that the change in
the flashpoint trigger for flammable liquids from "the current 100
[deg]F to the new 140 [deg]F * * * would significantly expand the
number of products subject to OSHA 1910.106 (flammable liquids), and
OSHA 1910.119 (Process Safety Standards)."
While OSHA agrees with CGA that using GHS Category 1 flammable
liquids would maintain consistency throughout the OSHA standards, to do
so would change the scope of the PSM standard by making it applicable
only to flammable liquids with flashpoints below 73 [deg]F (23 [deg]C).
This would significantly narrow the scope of PSM and lessen worker
protection by eliminating from coverage flammable liquids with
flashpoints from 73 [deg]F to below 100 [deg]F. However, to set the
coverage of PSM to 140 [deg]F (flammable liquid categories 1, 2 and 3
which require the hazard warning "flammable" to appear on labels), as
SOCMA noted, would expand the coverage beyond the scope of the original
standard.
OSHA has concluded that setting the flashpoint below 100 [deg]F
(37.8 [deg]C), the previous HCS level, properly maintains the scope of
the PSM standards as they were promulgated. As explained in the
proposal, OSHA's approach to the other affected standards is to
"modify provisions of the standards that reference the HCS definitions
to maintain coverage or consistency with the modified HCS" (74 FR
50404, Sept. 30, 2009). It is beyond the scope of this rulemaking to
consider whether, as a substantive matter, the scope of the PSM
standards should be changed. Thus, OSHA is neither increasing nor
decreasing the scope of the PSM standard; consequently, the same
products in the same quantities will be covered. The final rule adopts
the proposed changes to the PSM standards noted above.
HAZWOPER
In the NPRM, OSHA updated the definition of health hazard in its
HAZWOPER standards, Sec. 1910.120(a)(3) for general industry and Sec.
1926.65(a)(3) for construction, so that the terminology is aligned with
the GHS health hazards in Sec. 1910.1200, Appendix A. The final rule
retains the proposed definition.
In proposing this change, OSHA was concerned that some of the
terminology in HAZWOPER, such as neurotoxin and nephrotoxin, which were
partly defined by reference to the HCS, would no longer be consistent
with the GHS-modified HCS. For consistency, the proposal removed such
terms from HAZWOPER and are now subsumed within the HCS specific target
organ toxicity category, thus maintaining the same hazard communication
requirements in both HAZWOPER and HCS. By updating the definition of
"health hazard" in the HAZWOPER standards to clearly reference HCS,
employers will have the proper reference to HCS and, in there, the
proper guidance on how to classify the health hazards. OSHA received no
contrary comment, and the final rule adopts the definition of health
hazard as proposed.
The ACC requested that OSHA clarify how the HAZWOPER standards
would be affected by OSHA's adoption of the GHS flammable and
combustible liquid classifications in Sec. 1910.106, Sec. 1926.152,
and Sec. 1926.155 (Document ID 0393 and 0530). ACC seems to
be asking why OSHA did not reference the new definitions (GHS
categories) of flammable liquids in HAZWOPER. OSHA believes the
HAZWOPER standards would not be directly affected by the GHS-harmonized
categories of flammable liquids, and therefore ACC's concern is
misplaced. The HAZWOPER standards are program standards, and they do
not contain any specific references to flammable or combustible
liquids. It is true that the HAZWOPER standards state that all
requirements of Parts 1910 and 1926 of CFR title 29 apply to hazardous
waste and emergency response (Sec. 1910.120(a)(2) and Sec.
1926.65(a)(2)). Thus, where HAZWOPER-covered employees are responding
to an emergency situation where flammable liquids have been stored or
need to be temporarily stored during clean-up, the flammable liquid
standards might apply. OSHA believes that even in those situations, GHS
harmonization of flammable liquids will
have little or no effect on the HAZWOPER standards, because the
substantive requirements of these standards have not significantly
changed.
Welding, Cutting and Brazing--General Requirements
OSHA is harmonizing the requirements in the Welding, Cutting and
Brazing standard, Sec. 1910.252, by adding a Hazard Communication
paragraph and bringing in line with the GHS and OSHA's substance-
specific health standards the terminology in the labeling requirements
for filler metals and fusible granular materials, filler metals
containing cadmium, and fluxes containing fluorine compounds.
The final rule retains the proposed text of the Hazard
Communication paragraph at Sec. 1910.252(c)(1)(iv). Similar to the
substance-specific standards, the welding standard's hazard
communication paragraph requires employers to include welding
contaminants in a program established to comply with the HCS (Sec.
1910.1200). Also, similar to the substance-specific standards, OSHA has
added a date paragraph requiring employers to be using new labels by
June 1, 2015, the date by which manufacturers and importers must comply
with the labeling and SDS requirements of the revised HCS.
In addition to adding the general Hazard Communication paragraph,
OSHA reorganized some of the paragraphs in Sec. 1910.252 so as to
place the general reference to HCS in the correct position in the
standard, Sec. 1910.252(c)(1)(iv). To accomplish this, OSHA moved the
"Additional considerations for hazard communication in welding,
cutting, and brazing," including filler and fusible granular
materials, materials containing cadmium, and materials containing
fluorine compounds, from paragraphs (c)(1)(iv)(A) through (C) to new
paragraphs (c)(1)(v)(A) through (D).
The proposal inserted a cross reference to Sec. 1910.1200 in the
welding standards hazard determination section. In addition, as with
the substance-specific standards, the proposal deleted specific label
language requirements for welding materials containing cadmium and
fluorine and instead listed specific health endpoints to be considered
in the classification.
OSHA received one comment on the proposed changes from the Gases
and Welding Distributors Association, Inc. (GAWDA). While GAWDA
generally supported OSHA's rulemaking effort, GAWDA requested that OSHA
change "suppliers" of welding materials to "manufacturers" in Sec.
1910.252(c)(1)(v)(A) of the proposal (Document ID 0388).
GAWDA stated the term "supplier" is undefined and might include
different entities in the supply chain; furthermore, elsewhere OSHA
places the responsibility of hazard determination on manufacturers,
importers, and distributors. However, OSHA would like to point out that
the term "supplier" is used in the current standard, which requires
suppliers to determine the hazards in Sec. 1910.252(1)(c)(iv): "The
suppliers of welding materials shall determine the hazard, if any,
associated with the use of their materials in welding, cutting, etc."
OSHA assumes that "suppliers" will continue to use the same method
that they are currently using to determine the hazards of their
materials. To change this term could result in a substantive change in
the scope of this standard and would be beyond the scope of this
rulemaking. Therefore OSHA will retain the word "suppliers" as
proposed.
In addition, as discussed in the preamble to the proposal, See 74
FR 50417 (Sept. 30, 2009), current Sec. 1910.252(c)(iv) does not
merely require suppliers to determine the hazards of their products,
but also to ensure that labels properly convey those hazards. A
requirement that a supplier only determine the hazard of its products
is of little value if they do not also convey information about those
hazards on to the persons who use it. The final rule provides
additional clarity that suppliers of welding products covered by the
standard label as well as determine the hazard.
The changes to this standard were predicated on achieving
consistency with the GHS modifications to HCS and other OSHA substance-
specific standards, and OSHA has concluded that the modifications as
proposed and as explained in the previous paragraphs will effectuate
harmonizing the standard's terminology with HCS. In addition, this
action also contributes to internal consistency by making the Welding,
Cutting, and Brazing standard similar to the substance-specific health
standards.
Flammable and Combustible Liquids
OSHA proposed to align the definitions of flammable and combustible
liquids in both the general industry and construction standards to
conform to the GHS modifications to the HCS. In particular, the
proposal changed the definitions of flammable liquid categories and
deleted the term and definition of combustible liquids (See Table XIII-
6 for comparison of the GHS-modified HCS definitions and the current
flammable and combustible definitions that were contained in 29 CFR
1910.106 and 29 CFR 1926.155). OSHA has concluded that the proposed
changes to the Sec. 1910.106 and Sec. 1926.155 definitions are
reasonably necessary and appropriate and carried them forward into the
final rule. In addition, to essentially maintain the scope of the
standards, OSHA proposed, and is maintaining in the final rule, the
addition of the flashpoint cut-off value where the GHS flammable liquid
categories overlapped with the current HCS classes. The Alliance of
Hazardous Materials Professionals and David Levine of Product Safety
Solutions agreed, stating: "The elimination of the term 'combustible'
and substitution of actual flash point data provide a more meaningful
definition in the affected standards" (Document ID 0313 and
0327).
OSHA proposed to drop the current rules' classifications of
flammable and combustible liquids in favor of the GHS flammable liquid
classifications. This meant that all liquids under the proposal would
fall into GHS flammable liquid Categories 1 through 4, and that the
term "Combustible Liquids" in Sec. Sec. 1910.106, 1910.107,
1910.123, 1910.125, 1926.152, and 1926.155 was proposed to be deleted
since the GHS does not have a hazard class titled "Combustible
liquids." However, the GHS does require the hazard statement
"combustible liquid" on the label for Category 4 Flammable liquids
(flashpoint greater than 60 [deg]C (140 [deg]F) but not greater than 93
[deg]C (199.4[deg]F)).
In addition, the current general industry Spray Finishing standard,
Sec. 1910.107, relies on the current Sec. 1910.106 definition of
Class IIIB liquids (liquids with a flashpoint over 93 [deg]C).
Therefore the proposal amends Sec. 1910.107 to replace its use of the
term "combustible liquids," which has no corresponding GHS category,
with the phrase "Liquids with a Flashpoint Greater than 93 [deg]C
(199.4 [deg]F)." With the new terminology, the protection provided by
the original standards remains the same.
OSHA believed that most of the proposed changes in the definitions
were not significant. The move to GHS categories entails nominal
changes to the flashpoint values for flammable and combustible liquids
from 22.8 [deg]C (73 [deg]F) (current Class IA/B cut-off) to 23 [deg]C
(73.4 [deg]F) (GHS Category 1/2 cut-off) and from 93.3 [deg]C (200
[deg]F) (current Class IIIB cut-off) to 93 [deg]C (199.4 [deg]F) (GHS
Category 4). OSHA believes these changes in flash point represent
simple rounding to the closest significant value
and that they would have no significant effect on the scope of its
standards or on employee safety. ACC agreed with OSHA, stating that
"the elimination of the term 'combustible liquid' in Sec. 1910.107
does not significantly change the requirements of the standards and
should not adversely affect industry's ability to comply with the
standard" (Document ID 0393). OSHA has concluded these new
whole numbers are minute changes and that the rounded numbers coincide
with GHS, are easier to understand and remember, and therefore will
improve communication of hazards.
However, OSHA requested comment in the proposal on one change that
was potentially significant. Under the proposal, the boiling points
used to define the threshold for the current Flammable Class IA in
Sec. 1910.106 shifted from the cut-off value of 37.8 [deg]C (100
[deg]F) to a cut-off value of 35 [deg]C (95 [deg]F) for GHS Category 1.
Likewise, the boiling points in the proposed definition of Flammable
Class IB (Sec. 1910.106) shift from equal to or greater than (>=) 37.8
[deg]C (100 [deg]F) to greater than (>=) 35 [deg]C (95 [deg]F) in GHS
Category 2 (See Table XIII-6). The Agency believed the changes would be
necessary to make OSHA standards internally consistent and consistent
with the GHS modifications to HCS. However, as discussed in the NPRM,
OSHA was concerned that changing the boiling point cut-off for the
highly flammable liquids classified as Flammable IA could, under the
GHS modifications to HCS, lead to a subset of these chemicals being
classified as GHS Category 2 Flammable Liquids. Since some of the
storage and handling requirements are based on the hazard category, the
proposal would allow a facility to use larger tanks to store liquids
with boiling points between 37.8 [deg]C (100 [deg]F) and 35 [deg]C (95
[deg]F). OSHA was concerned that this practice could decrease safety.
OSHA reviewed the properties related to the flammability of
approximately 900 chemical substances (754 liquids) listed in the CRC
Handbook of Chemistry and Physics [85th edition]. Approximately 1
percent of this list of flammable liquids would result in a
reclassification from the current Flammable and Combustible Liquids
Standard Class IA to GHS Category 2. While this is a small percentage
of the total flammable liquids, it represents approximately 15 percent
of the Flammable and Combustible Liquids Standard Class IA liquids on
this list. OSHA was concerned that this was an instance where the
benefits of harmonization could have been in conflict with the measure
of safety currently provided and therefore requested comments on this
issue.
Most agreed with OSHA that resulting reclassifications of liquids
with borderline flashpoints from the old Class IA to the GHS Category 2
was not significant (Document ID 0313, 0324, 0327, 0328, 0338,
0352, 0365, 0366, 0370, 0376, 0382, 0383, 0393, 0405, 0408, 0410, and
0494 Tr. 56). National Association of Chemical Distributers (NACD)
stated that "Several NACD members handle flammable liquids under
Category 1 and 2. However, the proposed changes would result in few
operational changes" (Document ID 0341). Several commenters
pointed out that aligning the definitions for flammable liquids is
consistent with the single worldwide definition for these hazards
(Document ID 0313 and 0327). ORC (Document ID 0370)
stated:
ORC agrees that the methods OSHA proposes to classify flammable
liquids Category 1and 2 and flammable aerosols are similar enough to
the current definitions that substances that are currently regulated
by OSHA would continue to be regulated and that few, if any, changes
would result in a shift in regulatory coverage.
The National Fire Protection Association (NFPA) (Document ID
0366 and 0497 Tr. 56) stated:
NFPA agrees with OSHA's assessment regarding the slight adjustment
resulting from the change in criteria for flash point and boiling
point for flammable liquid categories when applying the GHS
criteria. NFPA believes the overall impact of the changed flash
point and boiling point will be negligible.
The American Petroleum Institute (API) urged OSHA to be consistent
across all standards (Document ID 0376). Further, the ACC
commented that in reference to the boiling point cut-off for Category 1
and 2 flammable liquids, they believe the language (in the NPRM) is
sufficient to reflect the cut-off without changing the scope of the
regulation (Document ID 0393).
However, some commenters expressed concern that the shift in
flammability criteria would require facilities to modify their storage
facilities to maintain compliance with Sec. 1910.106, and consequently
storage receptacles would have to be smaller, leading to less storage
and greater costs (ISSA, Document ID 0399). That concern is
misplaced because the change from OSHA's old flammable and combustible
classes to GHS categories involves a lowering of the boiling point cut-
offs by 2.8 [deg]C (5.04 [deg]F), so that employers will still be able
to use current handling and storage practices affected by the change.
Likewise, current storage and handling practices for chemicals whose
boiling points fall between 37.8 [deg]C and 35 [deg]C would still be
allowed under the proposal. SOCMA commented that changing the
definition would expand the number of products subject to Sec.
1910.106 (Document ID 0402). That is also not correct. Due to
the rounding of GHS flashpoints, cut-offs are slightly less stringent
(See Table XIII-6) and no new chemicals would be regulated.
Table XIII-6--Flammable Liquid Definitions
----------------------------------------------------------------------------------------------------------------
GHS Flammable and combustible liquids standard (29
--------------------------------------------------------------- CFR 1910.106)
-------------------------------------------------
Flashpoint Boiling point Flashpoint Boiling point
Category [deg]C [deg]C Class [deg]C [deg]C
([deg]F) ([deg]F) ([deg]F) ([deg]F)
----------------------------------------------------------------------------------------------------------------
Flammable 1.................. <23 (73.4)..... <=35 (95) Flammable Class <22.8 (73)..... <37.8 (100)
IA.
Flammable 2.................. <23 (73.4)..... >35 (95) Flammable Class <22.8 (73)..... >=37.8 (100)
IB.
Flammable 3.................. >=23 (73.4) and .............. Flammable Class >=22.8 (73) and ..............
<=60 (140). IC Combustible <37.8 (100). ..............
Class II. >=37.8 (100)
and <60 (140).
Flammable 4.................. >60 (140) and .............. Combustible >=60 (140) and ..............
<=93 (199.4). Class IIIA. <93.3 (200).
None......................... ............... .............. Combustible >=93.3 (200)... ..............
Class IIIB.
----------------------------------------------------------------------------------------------------------------
The American Society of Safety Engineers (ASSE) agreed with OSHA's
assessment of the storage issue. ASSE noted that the differences in
boiling points from the original Sec. 1910.106 to the GHS Categories
could increase the number of gallons allowed to be stored in rooms and
cabinets as well as the size of containers for certain liquids.
However, in its opinion, the "slightly" increased boiling point would
be of "little significance" (Document ID 0336). Therefore,
based on the analysis discussed above and the comments received, OSHA
has concluded that the shift in boiling point and the minor changes in
temperatures and the re-categorizing of flammable liquids are
insignificant and will have a negligible impact on the protection
provided by the standards that use these terms.
Most commenters supported OSHA's proposal to incorporate the GHS
definitions for flammable liquids into its safety standards (Document
ID 0313, 0327, 0328, 0338, 0365, 0376, 0405, 0408, and 0410).
Some stressed the "consistency" benefits from harmonization (Document
ID 0338, 0405, and 0408). ASSE (Document ID 0336)
said:
In response to OSHA's proposal to eliminate the term "combustible
liquid" in 29 CFR 1910.106, 1910.107, 1910.123, 1910.124, 1910.125,
and 1926.155 for liquids with a flashpoint above 100 degrees F.,
ASSE believes this list of standards is appropriate. * * * However,
ASSE urges OSHA to remove the term "combustible liquid" for all
liquids and use the GHS criteria for all flammable liquids.
The National Paint and Coatings Association (NPCA), in supporting the
removal of the term "combustible liquid," noted that it was
consistent with DOT (Document ID 0328).
Although there was considerable support for the changes OSHA made
in the proposal to the flammable and combustible liquid categories,
OSHA also received comments suggesting that the deletion of the
"combustible" designation and the combining of NFPA Class 1C
flammable and Class II combustible liquids into new Category Flammable
3, would lead to confusion among engineers, employers, and employees,
which could result in potential accidents (Document ID 0344,
0366, 0381, 0399, 0402, 0498, 0500, 0514, and 0643). In addition, some
commenters questioned whether the OSHA standards that address flammable
liquids that are not covered by GHS (Combustible Class IIIB) are best
handled by replacing the term "combustible" with a quantitative
definition so as to maintain their coverage (Document ID 0336,
0366, and 0497 Tr. 56-58 and 68).
Some organizations, though they supported the proposed changes in
general, had some specific concerns, particularly with how the OSHA GHS
harmonization works with other national standards, including consensus
standards. Clariant Corporation opined that eliminating the term
"combustible liquid" will likely cause some confusion since it is
still used by NFPA and DOT but urged OSHA to adopt the GHS criteria to
maintain global consistency (Document ID 0383). However, OSHA
points out that, as mentioned above by NPCA, the GHS criteria are
consistent with DOT. The American Federation of State, County and
Municipal Employees (AFSCME) favored OSHA's GHS harmonization, but
sought clarification or additional guidance on how secondary labeling
systems such as NFPA's 704 Diamond or the Hazardous Materials
Information System (HMIS) would be used once GHS was in effect
(Document ID 0414).
NFPA testified that the GHS categories would conflict with NFPA's
established hazard ratings in NFPA 704, which has been in effect since
the 1950s. NFPA recommended that the term "combustible liquid" not be
deleted (Document ID 0497 Tr. 59-64). In addition, NFPA
expressed concern that there may be additional confusion since the
rating system in NFPA 704 expresses the most hazardous as a "4" while
the GHS classification criteria expresses the most hazardous as
Category "1". The International Fire Marshals Association (IFMA),
echoing the sentiments of the NFPA, agreed that users have been relying
on the NFPA 704 Hazard Rating and the Hazardous Material Information
System (HMIS) systems for a long time and would be confused by the
change (Document ID 0497 Tr. 80-84).
These commenters were concerned that the proposed realignment of
the flammable liquid categories would result in confusion among
employees, emergency responders, authorities having jurisdiction, and
others who have been used to the distinction between flammable and
combustible liquids (Document ID 0344, 0366, 0381, 0399, 0402,
0498, 0500, 0514, 0643, and 0497 Tr. 56-58). NFPA (Document ID
0366) stated:
NFPA is also concerned with the elimination of the "combustible
liquids" classification that will occur with the adoption of GHS as
we believe there will be considerable confusion among the workers
who have been instructed to take specific precautions for various
liquids based on whether they were identified as "flammable or
combustible." Further, we believe that the elimination of the
"combustible liquid" classification may cause confusion among
emergency responders and authorities having jurisdiction, who have
until now understood that "flammable liquids" can be expected to
be ignitable at ambient temperatures, while "combustible liquids"
typically require some degree of heating to reach their flash point
temperatures. This lack of definition may also be an issue, albeit
to a lesser extent, among designers who have been trained to apply
certain fire protection measures to "flammable liquids", but not
to "combustible liquids." The immediate recognition that has
existed in the workplace for decades may be removed by the proposed
rule; NFPA cautions OSHA that confusion among workers has the
potential to be more significant than OSHA has acknowledged.
See also Document ID 0497 Tr. 56-58.
As an initial matter, OSHA notes liquids with a flashpoint greater
than or equal to 60 [deg]C (140 [deg]F) and less than 93.3 [deg]C (200
[deg]F), which are currently classified as "combustible," will be
labeled as "combustible liquids" under the final rule. Thus this
minimizes the potential for the confusion that NFPA suggests for these
chemicals.
In any event, OSHA believes that there is currently confusion and
inconsistency in this area. For example, OSHA standards have several
cutoff values for flammable and combustible liquids. In OSHA's general
industry standard at Sec. 1910.106, 100 [deg]F is the cut-off between
flammable liquids and combustible liquids, but in construction, Sec.
1926.155, 140 [deg]F is the cut-off between flammable and combustible.
Even the NFPA's standards are confusing. In NFPA 30, the hazard levels
are structured from Ia/b/c to III b, with Ia being the highest, while
in NFPA 704 the hazard levels range from 1 to 4, where the highest
hazard category is 4 and the lowest is 1. NFPA classification and
rating systems have been in existence since the 1950s and while the
NFPA rating system is widely used, it is still not universally used or
understood. Testimony from Mr. Frederick of the United Steelworkers
indicated that NFPA is a good quick reference although (he believed) it
does not cover all hazards, but it is used to alert workers that they
must look elsewhere for additional information (Document ID
0499 Tr. 155-169).
In addition, OSHA reviewed randomly chosen SDSs for liquids
classified under the current standard to determine how NFPA ratings
correlated to hazard warnings. As shown in Table XIII-7, the hazard
warnings were inconsistent, while the MSDSs were all technically
correct for physical properties. For example, the hazard warning for
flammable liquids with a
NFPA rating of 3 ranges from "Flammable Liquid" to "Extremely
Flammable" to "Severe." Notably, cyclohexanone, currently classified
as a combustible liquid under Sec. 1910.106, bears the hazard
statement "Flammable."
Table XIII-7--MSDS Communications of Flammable Liquid Hazard Warnings
----------------------------------------------------------------------------------------------------------------
Docket Chemical name Flashpoint NFPA rating listed Hazard warning
----------------------------------------------------------------------------------------------------------------
0565........................... Toluene............ 40.7 [deg]F....... 3................. Flammable Liquid
0566........................... Turpentine......... 95 [deg]F......... 3................. Flammable Liquid
0570........................... Aliphatic 120 [deg]F........ None listed....... Flammable Liquid
Hydrocarbons.
0571........................... Reagent N Hexane... -22 [deg]F........ 3................. Extremely
Flammable
0567........................... Paint Thinner...... 104 [deg]F........ 2................. Combustible
0557........................... Reagent Alcohol.... 55 [deg]F......... 3................. Severe (flammable)
0599........................... Cyclohexane........ 0 [deg]F.......... 3................. Extremely
Flammable
0560........................... Cyclohexanone...... 111 [deg]F........ 2................. Flammable
----------------------------------------------------------------------------------------------------------------
OSHA believes that this rulemaking will promote greater
harmonization of hazard warnings in the future. Now, when a chemical
falls in a particular flammable liquid hazard category, the HCS
requirements will dictate the appropriate hazard warning. At least one
comment alleges this has already happened in the United States. Dr.
Michele Sullivan pointed out that the U.S. Department of Transportation
(DOT) is already aligned with the GHS physical hazard criteria (the GHS
criteria for physical hazard was based on the DOT physical hazard
criteria); thus is already aligned with GHS flammable liquid criteria.
Therefore, OSHA is aligning with DOT with this rulemaking (49 CFR
173.120 and Document ID 0382).
Neither the proposal nor final rule prohibits the use of NFPA or
HMIS rating systems. They do not prohibit the use of NFPA definitions
for employers taking preventive measures in designing facilities or
implementing fire protection systems such as automatic sprinklers to
ensure a safer situation. OSHA's requirements, even with the
substitution of the term "flammable" for "combustible," do not
prohibit safer workplace designs or installations. Furthermore, OSHA
expects that engineers and other professionals will use the actual
flashpoints and other properties of the liquids themselves in design
and installation of controls rather than a designation of a liquid as
"flammable" or "combustible." IFMA agreed with this premise
(Document ID 0497 Tr. 84-85). In any event, even if the
engineer, facility designer, or employer is somehow misled by Sec.
1910.106's use of the term "flammable," which has traditionally
connoted a higher level of hazard, the result should be an error on the
side of safety, rather than of less protection.
During the public hearings, ORC Worldwide commented on OSHA's
review of the standards affected by this rulemaking, stating support
for the "concurrent harmonization of hazard definitions in most OSHA
standards." However, ORC also "agrees with member concern that
changes to definitions in Sec. 1910.106, Flammable and Combustible
Liquids, while not increasing the scope of the standard, may cause
confusion to workers who are familiar with NFPA nomenclature for these
materials" (Document ID 0494 Tr. 91-92). OSHA asked ORC to
elaborate on this concern and provide support for their testimony. In
response, ORC (Document ID 0643) provided two hypothetical
situations it believes show that confusion over the realignment of
flammable and combustible liquid categories could be significant:
Consider an engineer who is designing a new warehouse. (New)
Category 3 liquids are to be stored therein, and these are liquids
which were previously called "combustible." Engineer does not
design an electrical classification for the area. He does not
realize that the new category may also include some liquids which
are flammable. Because of this design outage, an electrical issue
causes a fire and the warehouse burns down.
Consider a dry cleaning business that is using a (new) Category 3
solvent and does not include automatic sprinklers because the team
is familiar with this solvent as being "combustible" under the
previous NFPA definitions. A different, more effective solvent is
proposed, also (new) Category 3, and is accepted as being
"similar"--the manufacturer reassures them that the new solvent is
in the same flammability category as the previous one. But this one
is indeed flammable and would require automatic sprinkler protection
under NFPA rules. A fire starts with the new solvent, and because no
automatic sprinklers exist onsite, the dry cleaner burns down.
OSHA thanks ORC Worldwide for their testimony and for providing
examples of where revisions to standards affected by this rulemaking
might cause confusion. With regard to the situations presented by ORC,
OSHA understands that the engineer designing the sprinkler system would
be required to follow local and state building codes, along with NFPA
codes or other building codes, such as NFPA 1 (Fire Code), NFPA 13
(Standard for the Installation of Sprinkler Systems), NFPA 30
(Flammable and Combustible Liquids Code), NFPA 32 (Standard for Dry
Cleaning Plants), NFPA 5000 (Building Construction and Safety Code),
and the International Building Code (published by the International
Code Council) as well as any OSHA standards that would apply.
The design of a system is not predicated on one physical property,
and a prudent engineer or sprinkler designer should be aware that there
are special requirements for the storage of combustible and flammable
liquids. The codes and standards mentioned above all refer to NFPA 30
for requirements related to the storage and use of flammable and
combustible liquids. There are restrictions on maximum container size,
maximum storage height, and maximum total quantity stored based on
flashpoint.
With regard to the change in solvent used at a dry cleaning
facility, the argument remains the same as for the design engineer
mentioned above. The flashpoint determines the classification of the
chemical. The automatic sprinkler system design would be based on the
flashpoint and not the class of chemical being used. OSHA concludes
that commenters' concerns about confusion are not well founded and has
decided to retain the GHS definition for flammable liquids as proposed
in the final rule.
Two commenters, Procter & Gamble and ISSA, believed OSHA was
adopting the 140 [deg]F flashpoint cut-off as the definition of a
flammable liquid and that this would conflict with the current
flashpoint cut-off of 100 [deg]F in Sec. 1910.106 (Document ID
0381 and 0399). Procter & Gamble, arguing that the GHS was
designed for hazard communications and not intended to regulate design
criteria and that aligning the GHS criteria for flammable liquids in
OSHA's safety standards would have unintended consequences
(Document ID 0381), offered OSHA two options:
Option 1: Leave the current OSHA definition of flammable liquids
unchanged. This is easy, clear, and no-cost to U.S. industry.
Option 2: In principle, GHS is a labeling and hazard communication
system, and was not intended to regulate the design and operation of
facilities. OSHA 1910.106, by comparison, is a risk management
regulation used in such design and operation. If OSHA adopts the GHS
Building Block of 140 [deg]F, leave the parallel definition of 100
[deg]F intact in 1910.106. This dual system will create some
confusion, but will minimize the negative effects listed above.
As an initial matter, Procter & Gamble misunderstood how OSHA
incorporated the GHS flammable liquid definitions into the safety
standards. This change was made only to align terminology. In fact,
OSHA agrees that the GHS was not intended to regulate design criteria.
Therefore, OSHA proposed to leave the standard's design criteria intact
by using the actual measurable flashpoint as the defining criterion.
The proposal, adopted by the final rule, is similar to Procter &
Gamble's Option 2 and accomplishes both harmonization with GHS and
retention of OSHA's long-established and effective risk management
practice.
Finally, there were concerns that realigning the flammability
criteria could affect contracts. Phylmar Regulatory Roundtable (PRR),
which did not oppose OSHA's alignment of definitions of flammable and
combustible liquids with the GHS categories, was concerned the
reclassification of chemicals may cause conflicts in contracts with
customers. PRR stated that the contracts require specifications in
products manufactured, engineering controls, personal protective
equipment, and specified instructions. PRR claimed that in such a
situation the manufacturer by contract is permitted no deviation from
the contract or process standards (Document ID 0514). However,
as stated above, OSHA has not changed the scope or the requirements of
its standards. Therefore, OSHA has concluded there is unlikely to be
any interference with contracts. Moreover, where distinctions must be
made in the OSHA requirements between the former Class 1C flammable
liquids and Class II combustible liquids, the OSHA requirements have
specified such distinctions with specific flashpoints. The contents and
scopes of the regulatory paragraphs are not affected by GHS
reclassifications or terminology changes, nor are OSHA's ventilation,
respiratory protection, and personal protective equipment standards. In
addition, OSHA did not change standards, like its electrical standards,
that address internal design criteria.
OSHA has decided to remain consistent with GHS and not create
additional flammable liquid categories. However, Sec.
1910.106(18)(ii)(b) defines Combustible Class IIIB liquids as liquids
with flashpoints at or above 200 [deg]F (93.3 [deg]C). While Class IIIB
liquids are not included in the scope of Sec. 1910.106, there is no
such exemption in the Spray Finishing standard, Sec. 1910.107 (OSHA
letter of interpretation, Aug. 15, 2006). In order to preserve coverage
in standards such as Spray Finishing, these liquids are now called
"Liquids with a Flashpoint of >93 [deg]C (199.4 [deg]F)." Similar to
Sec. 1910.106, the use of the flashpoint cut-off is the best way to
stay as close to the GHS and maintain scope and consistency within the
standards. The Soap and Detergent Association (SDA) and the Consumer
Specialty Products Association (CSPA) in a joint comment stated that
OSHA should "correct" Sec. 1910.107(e) and (e)(4) and Sec.
1910.124(c)(2) to read "Liquids with a Flashpoint at or below 199.4
[deg]F" to be consistent with the GHS criteria (Document ID
0344). However, if OSHA were to adhere strictly to GHS in this
instance and drop the higher flashpoint category, protection from this
hazard would be lost and safety compromised.
Several commenters addressed this issue. ASSE stated that their
"members do not see the need for the fifth category of 'Flammable
Liquids Over a Flash Point of 93.3 [deg]C.' Specific flash point
criteria should be used" (Document ID 0336). NFPA expressed
general concern about the elimination of the Class IIIB liquids by the
adoption of the GHS categorization system, though they acknowledged
that OSHA had proposed to extend liquids as "flammable liquid with
flash point greater than 93 [deg]C" (Document ID 0366 and
0497 Tr. 56-58). The point was further clarified upon questioning at
the hearing where NFPA agreed that by extending the liquids to
flashpoints greater than 199.4 [deg]F, OSHA was providing the coverage
for Sec. 1910.107 that had always been there. In addition, NFPA
recommended it be further clarified that these liquids with the higher
flashpoints belong to Sec. 1910.107 and are not part of GHS Category 4
(Document ID 0497 Tr. 68).
In addition, Intercontinental Chemical Corporation recommended that
OSHA create six new categories matching the six classes in the original
Sec. 1910.106 (Document ID 0500). The Agency believes that
this approach would be inconsistent with GHS, since the GHS
classifications and categories, including flammable liquid Categories
1-4, were established by international committees and are in place.
OSHA's intent in this rulemaking is to harmonize the HCS with the
existing GHS classifications and categories, not to make new
categories.
In summary, OSHA views this rulemaking as a step towards
eliminating current inconsistencies. OSHA believes the potential
confusion with other agency policies, standards, consensus standards,
and traditional practices suggested by the commenters are not likely to
occur for several reasons. First, the changes in the final rule will
bring internal consistency to the OSHA standards covered. OSHA
standards currently have several cut-off values for flammable and
combustible liquids. In OSHA's general industry standard (Sec.
1910.106), 100 [deg]F is the cut-off between the flammable and
combustible liquids, but in construction, Sec. 1926.155, 140 [deg]F is
the cut-off between flammable and combustible. Harmonizing these
standards, which have been out of sync for many years, will bring
needed consistency to the safety standards. In addition, as noted
above, substantive requirements have not changed, and therefore designs
are not affected.
Second, the changes to the standards do not require changes in work
practices. Rather, what have changed are a few regulatory terms used in
the standards. Commenters who thought that such changes in definitions
and terminology would result in significant and costly modifications to
facility design and operation are incorrect, as the old requirements in
the standards remain and no facility design and operation changes are
required (Document ID 0344, 0381, and 0399). The requirements
for what were known formerly as combustible liquids remain the same
even though they are now categorized as flammable liquids.
Third, there is growing awareness of the GHS "flammable liquids"
definition. Other agencies, such as DOT, are already aligned with the
GHS definition for flammable liquids (49 CFR 173.120), and OSHA
believes that its ANPR and NPRM have raised awareness of the
definition.
Change occurs in every area of employment, and employers and
workers get trained and adjust to the change; OSHA believes these minor
changes will be accepted and adopted. OSHA's flammable and combustible
liquid storage requirements have always been based on the flashpoint
and boiling point of the liquid; OSHA does not
believe that facility designs rely on whether the liquid is labeled as
flammable or combustible. (See Document ID 0497 Tr. 84-85)
Thus, OSHA has concluded that the allegations of impacts on facility
design and operations are perceptual rather than actual. This is
especially true in light of the fact that certain OSHA standards were
exempted from the terminology changes if these changes were to affect
internal design criteria of any area of the workplace. OSHA has
therefore concluded that the proposed changes to the Sec. 1910.106
definitions are reasonably necessary and appropriate and has carried
them forward into the final rule.
OSHA will be doing outreach to affected parties and working with
professional and trade associations to help users become familiar with
and competent in applying these modifications. ORC testified that the
changes may cause confusion to workers familiar with NFPA nomenclature,
and agreed with OSHA that, with training, any confusion resulting from
the change from NFPA definitions and terminology to GHS definitions and
terminology would be overcome. ORC (Document ID 0494 Tr. 100-
101) further stated that potential confusion would not be a reason to
delay moving forward with finalizing the standard:
There's a significant problem with lack of harmonization of chemical
control approaches in the United States, and we would like to see,
as we said in our testimony, some sort of formalization because we
think it's the only thing that's going to work here, formalization
of regular contacts between the NFPA and OSHA.
Mike Wright, representing the United Steelworkers (Document ID
0494 Tr. 76-77), put it succinctly, stating:
The whole point of harmonization is to reconcile different
standards, which may be conflicting. That means something has to
change. * * * Ultimately, in the short term will there be some
confusion? Yes. Can we minimize that through good training, through
good information? Yes, and we ought to, but ultimately I think we
have a globally harmonized system that's been adopted on a worldwide
level and then we have various national organizations--very
important ones like the NFPA--which may deviate in the way they
communicate hazards from that globally harmonized system.
With respect to my friends at the NFPA, who I think do wonderful
work, I think their job is to harmonize their system to the Globally
Harmonized System. We hope that happens as soon as possible, and I'm
confident that it will.
You know, ultimately we need to go to one * * * system worldwide. We
have that system now. It will take some time and a little bit of
confusion to conform every other kind of national voluntary system
to that, but that work has to be done.
OSHA agrees and believes users of the new GHS flammable liquid
categories will implement its new terminology in their work.
Minor Safety Standard Changes
The note in the PSM construction standard, Sec.
1926.64(d)(1)(vii), has been changed. In the current standard,
paragraph (d)(1)(vii), the note states, "Material Safety Data Sheets
meeting the requirements of 29 CFR 1926.59(g) may be used to comply
with this requirement to the extent they contain the information
required by this subparagraph." The note has been changed to "Safety
Data Sheets (SDSs) meeting the requirements of 29 CFR 1910.1200(g) . *
* *"
To correct a technical error and to complete alignment across
standards, Sec. 1910.106(j), Scope, has been made consistent with
Sec. 1910.106(a)(19) and Sec. 1910.1200, Appendix B. Proposed Sec.
1910.106(j) stated that it "applie[d] to the handling, storage, and
use of flammable liquids with a flashpoint below 199.4 [deg]F (93
[deg]C) unless otherwise noted." (Emphasis added). Final Sec.
1910.106(j) is now consistent with Sec. 1910.106(a)(19) and Sec.
1910.1200 in that it applies to "* * * flammable liquids with a
flashpoint at or below 199.4 [deg]F (93 [deg]C) * * *" (Emphasis
added).
In Sec. 1926.155, OSHA proposed to harmonize the definitions of
flammable and combustible liquids to be consistent with the GHS
categories of flammable liquids (i.e., the updating of the definition
of flammable liquids and the removal of the definition for combustible
liquids), and this change is carried through to the final rule. The
final rule also removes "or combustible" in the other standards in
Subpart F, to maintain consistency with the "Definitions" in Sec.
1926.155. In Sec. 1926.150(c)(vi), which currently states, "A fire
extinguisher, rated not less than 10B, shall be provided within 50 feet
of wherever more than 5 gallons of flammable or combustible liquids or
5 pounds of flammable gas are being used on the jobsite," the term
"or combustible" has been removed. Likewise, the Agency is correcting
Sec. 1926.151(b)(3) by removing "or combustible." In Sec.
1926.151(a)(4), Portable battery powered lighting, which states that
"the storage, handling, or use of flammable gases or liquids, shall be
* * * approved for the hazardous locations," the term "flammable
liquids" has been changed to "Category 1, 2, or 3 flammable
liquids." This change maintains the scope set by the flashpoint ranges
for the Subpart (as defined by the original Sec. 1926.155 paragraphs
(c) and (h)).
The Soap and Detergent Association and Consumer Specialty Products
Association, in a joint comment (Document ID 0344), suggested
that OSHA change the term "pilot light" to "indicating light." As
discussed previously, this type of change is outside of the scope of
this rulemaking since it does not pertain to hazard communication or
GHS harmonization. Therefore, OSHA is not adopting that suggestion at
this time.
Methods To Determine Flashpoints
OSHA proposed to update the methods that may be used to determine
flashpoints in the NPRM. These methods include updated ASTM methods,
ISO methods, and British, French, and German national standards for the
testing. The methods are listed in Appendix B.6 of Sec. 1910.1200 and
are also referenced in Revision 3 of the GHS (2009), Chapter 2.6.
In the definitions of Sec. 1910.106, the current standard allowed
only ASTM D-56-70 and ASTM D-93-71 as testing methods to determine
flashpoints. In Sec. 1926.155, which applies to Subpart F of the
construction standards (Fire Protection and Prevention), OSHA currently
allows only ASTM D-56-69 and ASTM D-93-69 for such determinations. The
current HCS allows only ASTM D 56-79, ASTM D 93-79, and ASTM D 3278-78.
The methods allowed in Sec. 1910.155 were adopted in the late 1960s,
and the methods for Sec. 1910.106 and Sec. 1926.1200 were adopted in
the 1970s.
The NPRM updated the methods in Sec. 1910.1200 to conform to the
GHS. However, flashpoint methods in Sec. 1910.1200 had always differed
from methods in Sec. 1910.106 and Sec. 1926.155. Instead of revamping
the older test methods in OSHA's other standards, the proposal allowed
a broader test selection. OSHA kept the tests currently permitted in
Sec. 1910.106 and Sec. 1926.155 because they were in the original
OSHA standards, but allowed methods in the GHS-modified HCS be used as
well. The final rule adopts these changes.
Thus, the final rule amends Sec. 1910.106 and Sec. 1926.155 to
allow ASTM D-56-70 and ASTM D-93-71 for Sec. 1910.106; ASTM D-56-69
and ASTM D-93-69 for Sec. 1910.155; and the equivalent testing methods
permitted in the HCS, Sec. 1910.1200, Appendix B.6, Physical Hazard
Criteria. For example, as amended by the final rule, Sec.
1910.106(a)(14)(i) states that for a liquid which has a viscosity of
less than 45 SUS at 100 [deg]F (37.8 [deg]C), does not
contain suspended solids, and does not have a tendency to form a
surface film while under test, the procedure specified in the Standard
Method of Test for Flashpoint by Tag Closed Tester (ASTM D-56-70),
which is incorporated by reference as specified in Sec. 1910.6, or an
equivalent test method as defined in Appendix B to Sec. 1910.1200--
Physical Hazard Criteria, must be used.
By equivalent test method, OSHA means employers can select any of
the test methods in Appendix B.6 or in Chapter 2.6 of Revision 3 of the
GHS (2009).
The only comments on this issue recommended additional methods for
determining flashpoints (Document ID 0344 and 0381). The Soap
and Detergent Association/Consumer Specialty Products Association
(Document ID 0344) and the Procter & Gamble Company (Document
ID 0381) recommended OSHA include ASTM D6450 on the list of
approved methods for determining the flashpoints of liquids in the
"incorporation by reference" list in Sec. 1910.106. OSHA is not
prepared to adopt this method at this time. The determination of
flashpoint test methods for GHS falls under a Sub-committee of the
United Nations Economic and Social Council's Committee of Experts on
the Transport of Dangerous Goods (UNCEDTG). Commenters who wish the GHS
to incorporate ASTM D6450 should direct their requests to that body,
and if the method is incorporated into the GHS, OSHA will consider the
matter at that time.
Flammable Aerosols
OSHA currently defines the term "flammable aerosol" in Sec.
1910.106 and in Sec. 1910.1200 by reference to a definition developed
by the Consumer Product Safety Commission under the Federal Hazardous
Substances Act. See 16 CFR 1500.45; See also 15 U.S.C. 1261(l). The
current HCS defines flammable aerosol as an aerosol that, when tested
by the method described in 16 CFR 1500.45, yields a flame projection
exceeding 18 inches at full valve opening, or a flashback (a flame
extending back to the valve) at any degree of valve opening.
The current Sec. 1910.106 definitions for "aerosol" and
"flammable aerosol" are provided in (Sec. 1910.106(a)(1)) and (Sec.
1910.106(a)(13)) and are different from those in the revised Hazard
Communication Standard. In the current Sec. 1910.106, an aerosol is
defined as a material which is dispensed from its container as a mist,
spray, or foam by a propellant under pressure. However, in the current
Sec. 1910.106, a flammable aerosol is defined as an aerosol which is
required to be labeled "Flammable" under the Federal Hazardous
Substances Labeling Act (15 U.S.C. 1261). For the purposes of Sec.
1910.106(d), such aerosols are considered Class IA liquids.
OSHA proposed to remove the definitions of "aerosol" and
"flammable aerosol" from Sec. 1910.106 and instead insert its GHS-
consistent definitions along with references to Appendix B.3 of the
GHS-modified HCS. In response to OSHA's proposed action, National Paint
and Coatings Association and Alliance of Hazardous Materials
Professionals both said that, while they were not prepared to offer
specific impact information on operations, "to align OSHA definitions
for * * * Flammable Aerosols is fully consistent with the concept of a
'single world-wide' definition for these hazards." (Document ID
0313 and 0327).
OSHA agrees with these comments and has included the revised
definition of "flammable aerosols" in the final rule. The revised
definition in the Flammable liquids standard, Sec. 1910.106,
duplicates the flammable aerosols definition contained in Appendix B to
Sec. 1910.1200--Physical Hazard Criteria. For the purposes of Sec.
1910.106(d), such aerosols are considered Category 1 flammable liquids.
The GHS-modified definition and classification criteria for
flammable aerosols can be found in Appendix B.3 of HCS.
OSHA's decision to change the definition of aerosols to be
consistent with the GHS-modified HCS is based not only upon harmonizing
its own standards with those followed by other countries who have or
are considering adopting GHS, but also to harmonize with DOT's
definition for flammable aerosols, which is also consistent with the
GHS. See 49 CFR 173.115(k).
Dr. Michelle Sullivan (Document ID 0382), alluding to
flammable aerosols, pointed out that flammable categories will differ
among regulatory authorities. She stated:
[T]he GHS flammable aerosol criteria are linked to the criteria for
flammable liquid, flammable solid and flammable gas, the flammable
aerosol criteria depend on the hazard categories/building blocks of
these other hazards * * * some regulatory authorities will adopt
categories 1-4 while others will adopt categories 1-3 * * * [and
thus] * * * the flammable aerosol criteria will differ for these
regulatory authorities.
Regarding Dr. Sullivan's comment, OSHA acknowledges that other
regulatory bodies, when adopting GHS, may choose different building
blocks. However, the basis for classification will still be based on
the same criteria and will lead to harmonization of similarly covered
materials. This does not affect OSHA's decision to strive for both
domestic and international harmonization.
Finally, OSHA believes that the GHS classification criteria are
similar enough to the current Sec. 1910.106 and Sec. 1910.1200
criteria that all aerosols currently regulated by OSHA would continue
to be so, and that few, if any, new aerosols would be subject to OSHA
regulation. Indeed, OSHA raised this issue in the NPRM and received no
comments to the contrary.
Standards Not Included in This Rulemaking
OSHA did not propose to change standards that incorporate by
reference other consensus standards, such as NFPA codes, or are based
on consensus standards when those consensus standards are used for
internal design criteria only and do not reference the HCS for
applicable scope or incorporation into the SDS. These standards include
Subpart S--Electrical, in Part 1910 (General Industry), and Subpart K--
Electrical, in Part 1926 (Construction). Many commenters on the ANPR
were particularly concerned that a change in OSHA's definitions would
create an incompatibility with local building codes (Document ID
0047, 0075, 0076, 0104, 0113, 0145 and 0163). They alleged
that, in many cases, this would require extensive rewiring to meet the
Subpart S requirements on hazardous locations and would lead to
conflicts with local electrical codes.
Many commenters on the NPRM supported OSHA's exemption of these
standards (Document ID 0328, 0330, 0336, 0370, 0393, and
0408). Ameren expressed concern that if OSHA harmonized the electrical
and blasting agents standards (Part 1910 Subpart S, Sec. 1910.109, and
Part 1926 Subpart K, Sec. 1926.914) with the GHS, such changes would
require training of affected employees on the changes (Document ID
0330). ASSE agreed with OSHA's decision not to propose updates
to the electrical standards (general industry 1910 Subpart S and
construction 1926 Subpart K) or explosives and blasting agents (general
industry Sec. 1910.109 and construction Sec. 1926.914), since these
subparts are "self-contained" in that they do not rely on other OSHA
standards for regulatory scope or definitions but reference external
organizations such as the National Fire Protection Association (NFPA)
(Document ID 0336). The American Iron and Steel Institute
agreed (Document ID 0408). ORC strongly supported OSHA's approach of not
updating these standards but waiting until the referenced external
organizations adopted the GHS elements (Document ID 0370).
Wacker Chemical Company, PRR, and ACC urged OSHA to update
electrical and explosive and blasting agents standards if the consensus
organizations could come to agreement, and they expressed their
concerns regarding potential conflicts with local codes and regulations
(Document ID 0335, 0339, and 0393). Wacker Chemical
Corporation encouraged OSHA to work closely with organizations (NFPA
and others) that develop fire and electrical codes to ensure there is
consistent application of these codes to area classification, building
construction, equipment electrical ratings, etc. (Document ID
0335). Wacker Chemical suggested that OSHA could make progress
with the consensus organizations (Document ID 0335). PRR
recommended harmonization updates of electrical and explosive standards
if the updates would enhance safety and the ease of doing business in
the global market (Document ID 0339). The ACC agreed with
OSHA's decision not to change standards that incorporate consensus
standards by reference (i.e., design criteria) (Document ID
0393). ACC requested OSHA clarify in its final rule that
harmonization would not affect the International Building Code and the
International Fire Code such that users will not be unduly required to
upgrade buildings to conform to requirements for hazardous occupancies.
By its decision regarding standards not included in this rulemaking,
OSHA is making it clear that upgrading buildings is not within the
scope of this rulemaking.
OSHA agrees with those comments that expressed the desire to
harmonize but also expressed concern over the potential effects of
internal codes. OSHA concluded that exempting those standards where
conflicts with internal codes could occur at this time was appropriate.
OSHA agrees with ACC that impacting electrical area classification,
facility siting, and wiring configuration is not appropriate.
Therefore, because of these potential conflicts with internal design
criteria, OSHA is not harmonizing the electrical and other standards
that depend on internal design criteria and local building codes.
Explosives and Blasting Agents
OSHA did not propose to harmonize the Explosive and Blasting Agents
standards, Sec. 1910.109 (general industry) and Sec. 1926, Subpart U
(construction). At the time of the proposal, a separate rulemaking to
revise them was in progress. That rulemaking has since been terminated
(75 FR 5545, Feb. 3, 2010). However, the HCS has always covered
hazardous chemicals regulated by OSHA's Explosive and Blasting Agents
standards. Although the rulemaking on explosives and blasting agents
has ceased, the general requirements in the GHS-modified HCS and
specific requirements in its appendices still apply to explosives and
blasting agents that can be considered hazardous chemicals.
Manufacturers and importers must evaluate chemicals to classify their
health and physical hazards in accordance with paragraph (d) of the
HCS, must affix labels in accordance with paragraph (f) in HCS, and
must provide SDSs in accordance with paragraph (g) of the HCS. Appendix
B.1 of the GHS-modified HCS contains specific classification criteria
for explosives. Furthermore, labels are required by the Department of
Transportation (DOT) for the transportation of packages or containment
devices that contain hazardous materials meeting one or more of DOT's
hazard class definitions. See 49 CFR Part 172 Subpart E. In addition,
OSHA's general industry standard Sec. 1910.1201, "Retention of DOT
markings, placards, and labels," requires that DOT labels, placards,
or markings be retained under certain conditions. Thus, explosives and
blasting agents are already covered by the GHS-modified HCS and Sec.
1910.1201.
The few commenters who addressed the issue supported OSHA's
decision not to include the Explosive and Blasting Agents standards
(Sec. 1910.109 and Sec. 1926.914) in the proposal (Document ID
0328, 0330, 0336, 0362, and 0370).
As to the continuing coverage of HCS, a representative from
Institute Makers of Explosives stated that the commercial explosives
industry understands the importance of GHS, has been prepared for
several years to implement GHS, and would not experience any impacts to
explosives operations that were not already anticipated (Document ID
0362).
Galaxy Fireworks noted that Sec. 1910.109(k)(1) excludes the sale
and "use (public display)" of pyrotechnics (fireworks) from the
explosives standard (Document ID 0355). Galaxy Fireworks'
concern was the potential for the proposal to create a regulation that
overlaps with the existing requirements of the Department of
Transportation and the Consumer Product Safety Commission. Galaxy urged
OSHA to work with these other agencies in amending the HCS to develop
regulations that would apply uniformly to the fireworks industry and
with other organizations to further harmonization (Document ID
0335). OSHA agrees and believes its global harmonization
efforts embodied in this rulemaking go a long way toward the overall
goal of consistency.
Maritime
OSHA received one comment, from Northrop Grumman Shipbuilding,
which stated that OSHA had omitted modification of the shipyard Part
1915 safety standards for GHS harmonization (Document ID
0395). More specifically, Northrop Grumman believed that the maritime
standards that contain requirements for flammable and combustible
liquids required review and updating to be GHS harmonized, just as the
flammable and combustible liquids the General Industry Part 1910 and
Construction Part 1926 standards were proposed to be reviewed and
updated.
OSHA did not propose to update the maritime standards, other than
the substance-specific standards mentioned above, in this rulemaking.
Unlike the standards in general industry and construction, the maritime
standards (Shipyard Employment, Part 1915; Marine Terminals, Part 1917;
and Longshoring, Part 1918) have always addressed flammables and
combustibles in their own unique way, reflecting the special conditions
of maritime work. These parts do not use flashpoint criteria to
distinguish between flammable and combustible liquids. The terminology
in the maritime standards that addresses flammable and combustible
materials, including liquids, differs from the general industry and
construction standards. For example, Sec. 1915.12(b)(1) (Flammable
atmospheres) and Sec. 1915.54 (Welding, cutting and heating of hollow
metal containers not covered by Sec. 1915.12) require competent-person
testing and contain detailed instructions on the specific maritime work
covered.
There are a few paragraphs in the maritime standards where
flammable and combustible liquids requirements reference flashpoint
criteria but in these cases, flashpoints are not used for the purpose
of distinguishing flammable from combustible liquids. Examples include
Subpart P, Fire Protection, Sec. 1915.501 through Sec. 1915.509,
where flammable liquid is defined as liquids with flashpoints below 100
[deg]F (37.8 [deg]C). Combustible liquids are neither defined nor
mentioned in this Subpart, although combustible materials are mentioned
and not defined. Other maritime standards such as Sec. 1915.14 (Hot
work) and Sec. 1915.35 (Painting) specify flashpoints for certain
requirements, but these are not distinctions of flashpoints defining
flammable or combustible liquids. The final rule does not modify these
criteria.
OSHA has issued a maritime compliance tool, "Tool Bag Directive
for the Part 1915 Shipyard Employment Standards," that includes
specific interpretations of the maritime standards. The Tool Bag
Directive references specific general industry standards in order to
provide further guidance related to some of the more general maritime
requirements. A specific case is how general industry standard Sec.
1910.106 is used. The Tool Bag Directive informs users that if specific
Part 1915 shipyard requirements give flashpoint criteria, those
requirements take precedence. However, where definitions of flammable
and combustible liquids are not specified in the Part 1915 shipyard
standards, the definitions of Sec. 1910.106 are to apply. The final
rule's changes do not significantly modify the substantive requirements
of Sec. 1910.106, and the Tool Bag Directive's interpretive policy
will continue after the final rule becomes effective, using the new
definitions in Sec. 1910.106.
In a similar manner, OSHA has a compliance tool for Parts 1917
"Marine Terminals" and 1918 "Longshoring" called the Tool Shed
Directive. This Directive notes that the requirements of Sec.
1910.1200 apply to operations covered by Parts 1917 and 1918. See also
1917.1(a)(2)(vi); 1918.1(b)(4). Therefore, all the requirements in the
GHS-modified HCS (Sec. 1910.1200), and its appendices will apply to
the maritime industry. In addition, part 1910 applies to marine
terminal operations that fall within the exception found at Sec.
1917.1(a)(1)(i): "facilities used solely for the bulk storage,
handling, and transfer of flammable, non-flammable, and combustible
liquids and gases." The final rule's changes to Sec. 1910.106 will
therefore apply to facilities handling flammable and combustible
liquids that fall within this exclusion, but again, as explained above,
the substantive requirements of Sec. 1910.106 have not changed
significantly.
Construction
The Building and Construction Trades Department (BCTD) requested
that OSHA clarify inconsistencies in the construction standards,
particularly by updating the Part 1926 standards to conform to the
proposed requirements for and definitions of "flammable" and the
related deletion of the term "combustible" liquids (Document ID
0359). BCTD gave examples of Sec. Sec. 1926.152, 1926.155,
1926.66 and Subpart K of Part 1926 and requested that OSHA conduct a
thorough review of the Part 1926 construction standards. Though it had
done so once in preparing the NPRM, OSHA again conducted a thorough
review of Part 1926. OSHA had already proposed to modify Sec. 1926.152
(Flammable and combustible liquids) and Sec. 1926.155 (Definitions) as
well as Sec. 1926.64 (Process Safety Management), Sec. 1926.65
(HAZWOPER), and the substance-specific health standards in construction
in the NPRM. As explained above, OSHA has made further revisions in the
construction regulations regarding process safety management (Sec.
1926.64(d)(1)(vii)) and fire protection and prevention (Sec.
1926.150(c)(vi), Sec. 1926.151(a)(4)), and Sec. 1926.151(b)(3)) in
this final rule.
Like Subpart S in general industry, Sec. 1926.66 (Criteria for
design and construction of spray booths) belongs to the category of
construction standards that incorporate other consensus standards by
reference, such as NFPA codes, or are based on consensus standards when
those consensus standards are used for internal design criteria only
and do not reference HCS for applicable scope or incorporation into the
SDS. Clearly, there is no reason to change the terminology in Sec.
1926.66. As noted above, Part 1926, Subpart K (Electrical), belongs in
this category. Other similar standards are Sec. 1926.351 (Arc Welding
and Cutting), and Part 1926, Subpart V (Power Transmission and
Distribution). OSHA is not modifying these standards for the same
reasons listed above for general industry.
Similar to the discussion regarding the Maritime standards, OSHA
did not propose modifications of standards that do not contain
definitions that are applicable to standards in the Subpart or
explicitly reference standards that contain the definitions. The
standards may contain phrases with the terms "flammable liquid" or
"combustible liquid," but the definitions of the terms are absent.
Standards belonging to this category of undefined terms include Sec.
1926.66(c)(9)(i) (Criteria for design and construction of spray
booths), Sec. 1926.252(e) (Disposal of waste materials), Sec.
1926.307(p)(2)(ii) (Mechanical power-transmission apparatus), Sec.
1926.352(c) and (h) (Fire prevention), Sec. 1926.803(l)(13)
(Compressed air), and Sec. 1926.1101, Appendix B (Sampling and
Analysis for Asbestos). In addition, some of these standards'
requirements use the term "flammable liquid" without the term
"combustible liquid," and some of the requirements use the term
"combustible liquid" without the term "flammable liquid." As with
the maritime standards, since OSHA has not changed the actual
requirements of Sec. 1910.106 or Sec. 1926.155, OSHA does not
anticipate that the final rule will affect the requirements of other
OSHA standards that use some of the same terminology.
In addition, OSHA did not modify standards that refer to flammable
and combustible materials, storage piles, etc. that are not liquids.
Examples are Sec. 1926.550(a)(15)(vii)(C) (Cranes and derricks), which
refers to combustible and flammable materials; Sec. 1926.956(b)(3)
(Underground lines), which refers to combustible gases; and Sec.
1926.352(c) (Fire prevention), which refers to flammable compounds. In
addition, Sec. 1926.154(e)(1) (Temporary heating devices) mentions
"flammable liquids," but the term was not the focus of the standard.
The requirement mentions flammable liquid-fired heaters, but the focus
is on safety controls for the particular piece of equipment. Safety
training and education, Sec. 1926.21(b)(5), is another example that
contains some of the terminology, but its focus is on safety training.
Flammable liquids are treated in a general sense, i.e., grouped with
gases or toxic materials.
Miscellaneous
A commenter from the International Chemical Workers Union Council
recommended OSHA include a conversion formula for Centigrade and
Fahrenheit or, at a minimum, provide the equivalent degrees when
addressing flammable and combustible liquids, since in general
employers and employees in the U.S. are more familiar with degrees
Fahrenheit (Document ID 456). OSHA proposed to provide
temperature equivalents, and in the final standard equivalents are
included where there are requirements for flammable and combustible
liquids. The formulas for conversion are:
(\9/5\) [deg]C + 32 = [deg]F or (\5/9\)([deg]F-32) = [deg]C
Since the formulas for conversion are standard formulas found in
textbooks, and since equivalents have been provided wherever possible
for flammable and combustible liquids, OSHA has determined that it is
not necessary to state the formulas for conversion in the actual
regulations.
XIV. Authority and Signature
This document was prepared under the direction of David Michaels,
Assistant Secretary of Labor for Occupational Safety and Health, U.S.
labor, 200 Constitution Avenue NW., Washington, DC 20210.
It is issued under the authority of sections 4, 6, and 8 of the
Occupational Safety and Health Act of 1970 (29 U.S.C. 653, 655, 657); 5
U.S.C. 553; Section 304, Clean Air Act Amendments of 1990 (Pub. L. 101-
549, reprinted at 29 U.S.C.A. 655 Note); Section 41, Longshore and
Harbor Workers' Compensation Act (33 U.S.C. 941); Section 107, Contract
Work Hours and Safety Standards Act (40 U.S.C. 3704); Section 1031,
Housing and Community Development Act of 1992 (42 U.S.C. 4853); Section
126, Superfund Amendments and Reauthorization Act of 1986, as amended
(reprinted at 29 U.S.C.A. 655 Note); Secretary of Labor's Order No. 1-
2012 (77 FR 3912); and 29 CFR Part 1911.
List of Subjects
29 CFR Part 1910
Asbestos, Blood, Chemicals, Diving, Fire prevention, Gases, Hazard
communication, Hazardous substances, Health records, Incorporation by
reference, Labeling, Labels, Laboratories, Occupational safety and
health, Reporting and recordkeeping requirements, Safety data sheets,
Signs and symbols, and Training.
29 CFR Part 1915
Hazard communication, Hazardous substances, Labels, Longshore and
harbor workers, Occupational safety and health, Reporting and
recordkeeping requirements, Safety data sheets, Signs and symbols,
Training, and Vessels.
29 CFR Part 1926
Chemicals, Construction industry, Diving, Fire prevention, Gases,
Hazard communication, Hazardous substances, Health records, Labels,
Lead, Occupational safety and health, Reporting and recordkeeping
requirements, Safety data sheets, Signs and symbols, and Training.
Signed at Washington, DC, on February 23, 2012.
David Michaels,
Assistant Secretary of Labor for Occupational Safety and Health.
Final Amendments
For the reasons discussed in the preamble, the Occupational Safety
and Health Administration amends 29 CFR parts 1910, 1915 and 1926 as
set forth below:
PART 1910--OCCUPATIONAL SAFETY AND HEALTH STANDARDS
Subpart A--[Amended]
0
1. Revise the authority citation for subpart A of part 1910 to read as
follows:
Authority: Sections 4, 6, and 8 of the Occupational Safety and
Health Act of 1970 (29 U.S.C. 653, 655, 657); Secretary of Labor's
Order No. 12-71 (36 FR 8754), 8-76 (41 FR 25059), 9-83 (48 FR
35736), 1-90 (55 FR 9033), 6-96 (62 FR 111), 3-2000 (65 FR 50017),
5-2002 (67 FR 65008), 5-2007 (72 FR 31159), 4-2010 (75 FR 55355) or
1-2012 (77 FR 3912), as applicable.
Section 1910.6 also issued under 5 U.S.C. 553. Sections 1910.6,
1910.7, and 1910.8 also issued under 29 CFR Part 1911. Section
1910.7(f) also issued under 31 U.S.C. 9701, 29 U.S.C. 9a, 5 U.S.C.
553; Pub. L. 106-113 (113 Stat. 1501A-222); Pub. L. 111-8 and 111-
317 and OMB Circular A-25 (dated July 8, 1993) (58 FR 38142, July
15, 1993).
0
2. Amend Sec. 1910.6 by revising paragraphs (a)(4) and (h), the
introductory text of paragraph (q), and by adding new paragraphs
(q)(37), (y), and (z) to read as follows:
Sec. 1910.6 Incorporation by reference
(a) * * *
(4) Copies of standards listed in this section and issued by
private standards organizations are available for purchase from the
issuing organizations at the addresses or through the other contact
information listed below for these private standards organizations. In
addition, these standards are available for inspection at any Regional
Office of the Occupational Safety and Health Administration (OSHA), or
at the OSHA Docket Office, U.S. Department of Labor, 200 Constitution
Avenue NW., Room N-2625, Washington, DC 20210; telephone: 202-693-2350
(TTY number: 877-889-5627). They are also available for inspection at
the National Archives and Records Administration (NARA). For
information on the availability of these standards at NARA, telephone:
202-741-6030, or go to http://www.archives.gov/federal_register/code_of_federal_regulations/ibr_locations.html.
* * * * *
(h) Copies of the standards listed below in this paragraph (h) are
available for purchase from ASTM International, 100 Barr Harbor Drive,
P.O. Box C700, West Conshohocken, PA 19428-2959; Telephone: 610-832-
9585; Fax: 610-832-9555; Email: seviceastm.org; Web site: http://www.astm.org.
Copies of historical standards or standards that ASTM
does not have may be purchased from Information Handling Services,
Global Engineering Documents, 15 Inverness Way East, Englewood, CO
80112; Telephone: 1-800-854-7179; Email: global@ihs.com; Web sites:
http://global.ihs.com or http://www.store.ihs.com.
(1) ASTM A 47-68, Malleable Iron Castings, IBR approved for Sec.
1910.111.
(2) ASTM A 53-69, Welded and Seamless Steel Pipe, IBR approved for
Sec. Sec. 1910.110 and 1910.111.
(3) ASTM A 126-66, Gray Iron Casting for Valves, Flanges and Pipe
Fitting, IBR approved for Sec. 1910.111.
(4) ASTM A 391-65 (ANSI G61.1-1968), Alloy Steel Chain, IBR
approved for Sec. 1910.184.
(5) ASTM A 395-68, Ductile Iron for Use at Elevated Temperatures,
IBR approved for Sec. 1910.111.
(6) ASTM B 88-66A, Seamless Copper Water Tube, IBR approved for
Sec. 1910.252.
(7) ASTM B 88-69, Seamless Copper Water Tube, IBR approved for
Sec. 1910.110.
(8) ASTM B 117-64, Salt Spray (Fog) Test, IBR approved for Sec.
1910.268.
(9) ASTM B 210-68, Aluminum-Alloy Drawn Seamless Tubes, IBR
approved for Sec. 1910.110.
(10) ASTM B 241-69, Standard Specifications for Aluminum-Alloy
Seamless Pipe and Seamless Extruded Tube, IBR approved for Sec.
1910.110.
(11) ASTM D 5-65, Test for Penetration by Bituminous Materials, IBR
approved for Sec. 1910.106.
(12) ASTM D 56-70, Test for Flash Point by Tag Closed Tester, IBR
approved for Sec. 1910.106.
(13) ASTM D 56-05, Standard Test Method for Flash Point by Tag
Closed Cup Tester, Approved May 1, 2005, IBR approved for Appendix B to
Sec. 1910.1200.
(14) ASTM D 86-62, Test for Distillation of Petroleum Products, IBR
approved for Sec. Sec. 1910.106 and 1910.119.
(15) ASTM D 86-07a, Standard Test Method for Distillation of
Petroleum Products at Atmospheric Pressure, Approved April 1, 2007, IBR
approved for Appendix B to Sec. 1910.1200.
(16) ASTM D 88-56, Test for Saybolt Viscosity, IBR approved for
Sec. 1910.106.
(17) ASTM D 93-71, Test for Flash Point by Pensky Martens, IBR
approved for Sec. 1910.106.
(18) ASTM D 93-08, Standard Test Methods for Flash Point by Pensky-
Martens Closed Cup Tester, Approved Oct. 15, 2008, IBR approved for
Appendix B to Sec. 1910.1200.
(19) ASTM D 240-02 (Reapproved 2007), Standard Test Method for Heat
of Combustion of Liquid Hydrocarbon Fuels by Bomb Calorimeter, Approved
May 1, 2007, IBR approved for Appendix B to Sec. 1910.1200.
(20) ASTM D 323-68, Standard Test Method of Test for Vapor Pressure
of Petroleum Products (Reid Method), IBR approved for Sec. 1910.106.
(21) ASTM D 445-65, Test for Viscosity of Transparent and Opaque
Liquids, IBR approved for Sec. 1910.106.
(22) ASTM D 1078-05, Standard Test Method for Distillation Range of
Volatile Organic Liquids, Approved May 15, 2005, IBR approved for
Appendix B to Sec. 1910.1200.
(23) ASTM D 1692-68, Test for Flammability of Plastic Sheeting and
Cellular Plastics, IBR approved for Sec. 1910.103.
(24) ASTM D 2161-66, Conversion Tables for SUS, IBR approved for
Sec. 1910.106.
(25) ASTM D 3278-96 (Reapproved 2004) E1, Standard Test Methods for
Flash Point of Liquids by Small Scale Closed-Cup Apparatus, Approved
November 1, 2004, IBR approved for Appendix B to Sec. 1910.1200.
(26) ASTM D 3828-07a, Standard Test Methods for Flash Point by
Small Scale Closed Cup Tester, Approved July 15, 2007, IBR approved for
Appendix B to Sec. 1910.1200.
(27) ASTM F-2412-2005, Standard Test Methods for Foot Protection,
IBR approved for Sec. 1910.136.
(28) ASTM F-2413-2005, Standard Specification for Performance
Requirements for Protective Footwear, IBR approved for Sec. 1910.136.
* * * * *
(q) The following material is available for purchase from the
National Fire Protection Association (NFPA), 1 Batterymarch Park,
Quincy, MA 02269; Telephone: 800-344-3555 or 617-770-3000; Fax: 1-800-
593-6372 or 1-508-895-8301; Email: custserv@nfpa.org; Web site: http://www.nfpa.org.
* * * * *
(37) NFPA 30B, Code for the Manufacture and Storage of Aerosol
Products, 2007 Edition, Approved August 17, 2006, IBR approved for
Appendix B to Sec. 1910.1200.
* * * * *
(y)(1) The following materials are available for purchase from the
International Standards Organization (ISO) through ANSI, 25 West 43rd
Street, Fourth Floor, New York, NY 10036-7417; Telephone: 212-642-4980;
Fax: 212-302-1286; Email: info@ansi.org; Web site: http://www.ansi.org.
(2) Documents not available in the ANSI store may be purchased
from:
(i) Document Center Inc., 111 Industrial Road, Suite 9, Belmont,
94002; Telephone: 650-591-7600; Fax: 650-591-7617; Email:
center.com">info@document-center.com; Web site: www.document-center.com.
(ii) DECO--Document Engineering Co., Inc., 15210 Stagg Street, Van
Nuys, CA 91405; Telephone: 800-645-7732 or 818-782-1010; Fax: 818-782-
2374; Email: doceng@doceng.com; Web site: www.doceng.com
(iii) Global Engineering Documents, 15 Inverness Way East,
Englewood, CO 80112; Telephone: 1-800-854-7179 or 303-397-7956; Fax:
303-397-2740; Email: global@ihs.com; Web sites: http://global.ihs.com
or http://www.store.ihs.com;
(iv) ILI Infodisk, Inc., 610 Winters Avenue, Paramus, NJ 07652;
Telephone: 201-986-1131; Fax: 201-986-7886; Email: sales@ili-info.com;
Web site: www.ili-info.com.
(v) Techstreet, a business of Thomson Reuters, 3916 Ranchero Drive,
Ann Arbor, MI 48108; Telephone: 800-699-9277 or 734-780-8000; Fax: 734-
780-2046; Email: techstreet.service@thomsonreuters.com; Web site:
www.Techstreet.com.
(3) ISO 10156:1996 (E), Gases and Gas Mixtures--Determination of
Fire Potential and Oxidizing Ability for the Selection of Cylinder
Valve Outlets, Second Edition, Feb. 15, 1996, IBR approved for Appendix
B to Sec. 1910.1200.
(4) ISO 10156-2:2005 (E), Gas cylinders--Gases and Gas Mixtures--
Part 2: Determination of Oxidizing Ability of Toxic and Corrosive Gases
and Gas Mixtures, First Edition, Aug. 1, 2005, IBR approved for
Appendix B to Sec. 1910.1200.
(5) ISO 13943:2000 (E/F), Fire Safety--Vocabulary, First Edition,
April, 15, 2000, IBR approved for Appendix B to Sec. 1910.1200.
(z)(1) The following document is available for purchase from United
Nations Publications, Customer Service, c/o National Book Network,
15200 NBN Way, PO Box 190, Blue Ridge Summit, PA 17214; telephone: 1-
888-254-4286; fax: 1-800-338-4550; email: unpublications@nbnbooks.com.
Other distributors of United Nations Publications include:
(i) Bernan, 15200 NBN Way, Blue Ridge Summit, PA 17214; telephone:
1-800-865-3457; fax: 1-800-865-3450; email: customercare@bernan; Web
site: http://www.bernan.com; and
(ii) Renouf Publishing Co. Ltd., 812 Proctor Avenue, Ogdensburg, NY
13669-2205; telephone: 1-888-551-7470; Fax: 1-888-551-7471; email:
orders@renoufbooks.com; Web site: http://www.renoufbooks.com.
(2) UN ST/SG/AC.10/Rev.4, The UN Recommendations on the Transport
of Dangerous Goods, Manual of Tests and Criteria, Fourth Revised
Edition, 2003, IBR approved for Appendix B to Sec. 1910.1200.
Subpart H--[Amended]
0
3. The authority citation for subpart H is revised to read as follows:
Authority: Sections 4, 6, and 8 of the Occupational Safety and
Health Act of 1970 (29 U.S.C. 653, 655, 657); Secretary of Labor's
Order No. 12-71 (36 FR 8754), 8-76 (41 FR 25059), 9-83 (48 FR
35736), 1-90 (55 FR 9033), 6-96 (62 FR 111), 3-2000 (65 FR 50017),
or 5-2007 (72 FR 31159), 4-2010 (75 FR 55355) or 1-2012 (77 FR
3912), as applicable; and 29 CFR part 1911.
Sections 1910.103, 1910.106 through 1910.111, and 1910.119,
1910.120, and 1910.122 through 1910.126 also issued under 29 CFR
part 1911.
Section 1910.119 also issued under Section 304, Clean Air Act
Amendments of 1990 (Pub. L. 101-549), reprinted at 29 U.S.C.A. 655
Note.
Section 1910.120 also issued under Section 126, Superfund
Amendments and Reauthorization Act of 1986 as amended (29 U.S.C.A.
655 Note), and 5 U.S.C. 553.
0
4. Amend Sec. 1910.106 as follows:
0
A. Revise the section heading;
0
B. Revise paragraphs (a)(13), (a)(14)(i) through (a)(14)(iii), and
(a)(19);
0
C. Remove the last sentence of paragraph (a)(17);
0
D. Remove and reserve paragraph (a)(18);
0
E. Remove the words "or combustible" wherever they appear in Sec.
1910.106.
0
F. Remove the words "and combustible" in paragraphs (d)(5)(vi)
introductory text, (e)(2) introductory text, (j)(1) and (j)(3);
0
G. Revise paragraphs (b)(2)(iv)(f) and (g), (b)(2)(vi)(b),
(b)(2)(viii)(e), (b)(3)(i), (b)(3)(iv)(a), (b)(3)(iv)(c), (b)(3)(v)(d),
and (b)(4)(iv)(e);
0
H. Revise paragraphs (d)(1)(ii)(b), (d)(2)(iii) introductory text and
(d)(2)(iii)(a)(2), Table H-12, paragraphs (d)(3)(i), (d)(4)(iii),
(d)(4)(iv), Tables H-14 through H-17, and paragraph (d)(7)(i)(b);
0
I. Revise paragraphs (e)(2)(ii)(b)(1), (e)(2)(ii)(b)(2),
(e)(2)(ii)(b)(3), (e)(2)(iv)(a), (e)(2)(iv)(c), (e)(3)(v)(a),
(e)(3)(v)(b), (e)(4)(i), (e)(6)(ii), and (e)(7)(i)(c);
0
J. Revise paragraphs (f)(1)(i), (f)(1)(ii), (f)(2)(ii), (f)(2)(iii)(a),
(f)(2)(iii)(b), (f)(2)(iii)(c), (f)(3)(i), (f)(3)(ii),
(f)(3)(iv)(a)(1), (f)(3)(iv)(a)(2), (f)(3)(iv)(d)(2), (f)(3)(v),
(f)(3)(vi), (f)(4)(viii)(e), (f)(5)(i), (f)(6), and (f)(8);
0
K. Revise paragraphs (g)(1)(i)(c), (g)(1)(i)(e) introductory text,
(g)(1)(i)(f), (g)(1)(iii)(a), (g)(1)(iii)(b), (g)(1)(iii)(c),
(g)(1)(v), (g)(3)(iv)(a), (g)(3)(iv)(b),
(g)(3)(iv)(c), (g)(3)(v)(a), (g)(3)(vi)(a), Table H-19, and paragraphs
(g)(4)(iii)(d), (g)(5)(i), (g)(6)(iv), and (g)(7); and
0
L. Revise paragraphs (h)(3)(i)(a), (h)(3)(iii)(b), (h)(3)(iv), (h)(5),
(h)(7)(i)(b), (h)(7)(iii)(c), and (j).
The revisions read as follows:
Sec. 1910.106 Flammable liquids.
* * * * *
(a) * * *
(13) Flammable aerosol shall mean a flammable aerosol as defined by
Appendix B to Sec. 1910.1200--Physical Hazard Criteria. For the
purposes of paragraph (d) of this section, such aerosols are considered
Category 1 flammable liquids.
(14) * * *
(i) For a liquid which has a viscosity of less than 45 SUS at 100
[deg]F (37.8 [deg]C), does not contain suspended solids, and does not
have a tendency to form a surface film while under test, the procedure
specified in the Standard Method of Test for Flashpoint by Tag Closed
Tester (ASTM D-56-70), which is incorporated by reference as specified
in Sec. 1910.6, or an equivalent test method as defined in Appendix B
to Sec. 1910.1200--Physical Hazard Criteria, shall be used.
(ii) For a liquid which has a viscosity of 45 SUS or more at 100
[deg]F (37.8 [deg]C), or contains suspended solids, or has a tendency
to form a surface film while under test, the Standard Method of Test
for Flashpoint by Pensky-Martens Closed Tester (ASTM D-93-71) or an
equivalent method as defined by Appendix B to Sec. 1910.1200--Physical
Hazard Criteria, shall be used except that the methods specified in
Note 1 to section 1.1 of ASTM D-93-71 may be used for the respective
materials specified in the Note. The preceding ASTM standard is
incorporated by reference as specified in Sec. 1910.6.
(iii) For a liquid that is a mixture of compounds that have
different volatilities and flashpoints, its flashpoint shall be
determined by using the procedure specified in paragraph (a)(14)(i) or
(ii) of this section on the liquid in the form it is shipped.
* * * * *
(18) [Reserved]
(19) Flammable liquid means any liquid having a flashpoint at or
below 199.4 [deg]F (93 [deg]C). Flammable liquids are divided into four
categories as follows:
(i) Category 1 shall include liquids having flashpoints below 73.4
[deg]F (23 [deg]C) and having a boiling point at or below 95 [deg]F (35
[deg]C).
(ii) Category 2 shall include liquids having flashpoints below 73.4
[deg]F (23 [deg]C) and having a boiling point above 95 [deg]F (35
[deg]C).
(iii) Category 3 shall include liquids having flashpoints at or
above 73.4 [deg]F (23 [deg]C) and at or below 140 [deg]F (60 [deg]C).
When a Category 3 liquid with a flashpoint at or above 100 [deg]F (37.8
[deg]C) is heated for use to within 30 [deg]F (16.7 [deg]C) of its
flashpoint, it shall be handled in accordance with the requirements for
a Category 3 liquid with a flashpoint below 100 [deg]F (37.8 [deg]C).
(iv) Category 4 shall include liquids having flashpoints above 140
[deg]F (60 [deg]C) and at or below 199.4 [deg]F (93 [deg]C). When a
Category 4 flammable liquid is heated for use to within 30 [deg]F (16.7
[deg]C) of its flashpoint, it shall be handled in accordance with the
requirements for a Category 3 liquid with a flashpoint at or above 100
[deg]F (37.8 [deg]C).
(v) When liquid with a flashpoint greater than 199.4 [deg]F (93
[deg]C) is heated for use to within 30 [deg]F (16.7 [deg]C) of its
flashpoint, it shall be handled in accordance with the requirements for
a Category 4 flammable liquid.
* * * * *
(b) * * *
(2) * * *
(iv) * * *
(f)(1) Tanks and pressure vessels storing Category 1 flammable
liquids shall be equipped with venting devices which shall be normally
closed except when venting to pressure or vacuum conditions. Tanks and
pressure vessels storing Category 2 flammable liquids and Category 3
flammable liquids with a flashpoint below 100 [deg]F (37.8 [deg]C)
shall be equipped with venting devices which shall be normally closed
except when venting under pressure or vacuum conditions, or with
approved flame arresters.
(2) Exemption: Tanks of 3,000 bbls (barrels). capacity or less
containing crude petroleum in crude-producing areas and outside
aboveground atmospheric tanks under 1,000 gallons capacity containing
other than Category 1 flammable liquids may have open vents. (See
paragraph (b)(2)(vi)(b) of this section.)
(g) Flame arresters or venting devices required in paragraph
(b)(2)(iv)(f) of this section may be omitted for Category 2 flammable
liquids and Category 3 flammable liquids with a flashpoint below 100
[deg]F (37.8 [deg]C) where conditions are such that their use may, in
case of obstruction, result in tank damage.
* * * * *
(vi) * * *
(b) Where vent pipe outlets for tanks storing Category 1 or 2
flammable liquids, or Category 3 flammable liquids with a flashpoint
below 100 [deg]F (37.8 [deg]C), are adjacent to buildings or public
ways, they shall be located so that the vapors are released at a safe
point outside of buildings and not less than 12 feet above the adjacent
ground level. In order to aid their dispersion, vapors shall be
discharged upward or horizontally away from closely adjacent walls.
Vent outlets shall be located so that flammable vapors will not be
trapped by eaves or other obstructions and shall be at least five feet
from building openings.
* * * * *
(viii) * * *
(e) For Category 2 flammable liquids and Category 3 flammable
liquids with a flashpoint below 100 [deg]F (37.8 [deg]C), other than
crude oils, gasolines, and asphalts, the fill pipe shall be so designed
and installed as to minimize the possibility of generating static
electricity. A fill pipe entering the top of a tank shall terminate
within 6 inches of the bottom of the tank and shall be installed to
avoid excessive vibration.
* * * * *
(3) * * *
(i) Location. Excavation for underground storage tanks shall be
made with due care to avoid undermining of foundations of existing
structures. Underground tanks or tanks under buildings shall be so
located with respect to existing building foundations and supports that
the loads carried by the latter cannot be transmitted to the tank. The
distance from any part of a tank storing Category 1 or 2 flammable
liquids, or Category 3 flammable liquids with a flashpoint below 100
[deg]F (37.8 [deg]C), to the nearest wall of any basement or pit shall
be not less than 1 foot, and to any property line that may be built
upon, not less than 3 feet. The distance from any part of a tank
storing Category 3 flammable liquids with a flashpoint at or above 100
[deg]F (37.8 [deg]C) or Category 4 flammable liquids to the nearest
wall of any basement, pit or property line shall be not less than 1
foot.
* * * * *
(iv) * * *
(a) Location and arrangement of vents for Category 1 or 2 flammable
liquids, or Category 3 flammable liquids with a flashpoint below 100
[deg]F (37.8 [deg]C). Vent pipes from tanks storing Category 1 or 2
flammable liquids, or Category 3 flammable liquids with a flashpoint
below 100 [deg]F (37.8 [deg]C), shall be so located that the discharge
point is outside of buildings, higher than the fill pipe opening, and
not less than 12 feet above the adjacent ground level. Vent pipes shall
discharge only upward in order to disperse vapors. Vent pipes 2 inches
or less in nominal inside diameter shall not be obstructed by devices
that will cause excessiveback pressure. Vent pipe outlets shall be so
located that flammable vapors will not enter building openings, or be
trapped under eaves or other obstructions. If the vent pipe is less
than 10 feet in length, or greater than 2 inches in nominal inside
diameter, the outlet shall be provided with a vacuum and pressure relief
device or there shall be an approved flame arrester located in the vent
line at the outlet or within the approved distance from the outlet.
* * * * *
(c) Location and arrangement of vents for Category 3 flammable
liquids with a flashpoint at or above 100 [deg]F (37.8 [deg]C) or
Category 4 flammable liquids. Vent pipes from tanks storing Category 3
flammable liquids with a flashpoint at or above 100 [deg]F (37.8
[deg]C) or Category 4 flammable liquids shall terminate outside of the
building and higher than the fill pipe opening. Vent outlets shall be
above normal snow level. They may be fitted with return bends, coarse
screens or other devices to minimize ingress of foreign material.
* * * * *
(v) * * *
(d) For Category 2 flammable liquids and Category 3 flammable
liquids with a flashpoint below 100 [deg]F (37.8 [deg]C), other than
crude oils, gasolines, and asphalts, the fill pipe shall be so designed
and installed as to minimize the possibility of generating static
electricity by terminating within 6 inches of the bottom of the tank.
* * * * *
(4) * * *
(iv) * * *
(e) For Category 2 flammable liquids and Category 3 flammable
liquids with a flashpoint below 100 [deg]F (37.8 [deg]C), other than
crude oils, gasoline, and asphalts, the fill pipe shall be so designed
and installed as to minimize the possibility of generating static
electricity by terminating within 6 inches of the bottom of the tank.
* * * * *
(d) * * *
(1) * * *
(ii) * * *
(b) Category 1, 2, or 3 flammable liquids in the fuel tanks of a
motor vehicle, aircraft, boat, or portable or stationary engine;
* * * * *
(2) * * *
(iii) Size. Flammable liquid containers shall be in accordance with
Table H-12, except that glass or plastic containers of no more than 1-
gallon capacity may be used for a Category 1 or 2 flammable liquid if:
(a) * * *
(2) The user's process either would require more than 1 pint of a
Category 1 flammable liquid or more than 1 quart of a Category 2
flammable liquid of a single assay lot to be used at one time, or would
require the maintenance of an analytical standard liquid of a quality
which is not met by the specified standards of liquids available, and
the quantity of the analytical standard liquid required to be used in
any one control process exceeds one-sixteenth the capacity of the
container allowed under Table H-12 for the category of liquid; or
* * * * *
Table H-12--Maximum Allowable Size of Containers and Portable Tanks for Flammable Liquids
----------------------------------------------------------------------------------------------------------------
Container type Category 1 Category 2 Category 3 Category 4
----------------------------------------------------------------------------------------------------------------
Glass or approved plastic....... 1 pt.............. 1 qt.............. 1 gal............. 1 gal.
Metal (other than DOT drums).... 1 gal............. 5 gal............. 5 gal............. 5 gal.
Safety cans..................... 2 gal............. 5 gal............. 5 gal............. 5 gal.
Metal drums (DOT specifications) 60 gal............ 60 gal............ 60 gal............ 60 gal.
Approved portable tanks......... 660 gal........... 660 gal........... 660 gal........... 660 gal.
----------------------------------------------------------------------------------------------------------------
Note: Container exemptions: (a) Medicines, beverages, foodstuffs, cosmetics, and other common consumer items,
when packaged according to commonly accepted practices, shall be exempt from the requirements of
1910.106(d)(2)(i) and (ii).
(3) * * *
(i) Maximum capacity. Not more than 60 gallons of Category 1, 2, or
3 flammable liquids, nor more than 120 gallons of Category 4 flammable
liquids may be stored in a storage cabinet.
* * * * *
(4) * * *
(iii) Wiring. Electrical wiring and equipment located in inside
storage rooms used for Category 1 or 2 flammable liquids, or Category 3
flammable liquids with a flashpoint below 100 [deg]F (37.8 [deg]C),
shall be approved under subpart S of this part for Class I, Division 2
Hazardous Locations; for Category 3 flammable liquids with a flashpoint
at or above 100 [deg]F (37.8 [deg]C) and Category 4 flammable liquids,
shall be approved for general use.
(iv) Ventilation. Every inside storage room shall be provided with
either a gravity or a mechanical exhaust ventilation system. Such
system shall be designed to provide for a complete change of air within
the room at least six times per hour. If a mechanical exhaust system is
used, it shall be controlled by a switch located outside of the door.
The ventilating equipment and any lighting fixtures shall be operated
by the same switch. A pilot light shall be installed adjacent to the
switch if Category 1 or 2 flammable liquids, or Category 3 flammable
liquids with a flashpoint below 100 [deg]F (37.8 [deg]C), are dispensed
within the room. Where gravity ventilation is provided, the fresh air
intake, as well as the exhaust outlet from the room, shall be on the
exterior of the building in which the room is located.
* * * * *
BILLING CODE 4510-26-P
[GRAPHIC] [TIFF OMITTED] TR26MR12.054
[GRAPHIC] [TIFF OMITTED] TR26MR12.055
[GRAPHIC] [TIFF OMITTED] TR26MR12.056
[GRAPHIC] [TIFF OMITTED] TR26MR12.057
BILLING CODE 4510-26-C
(7) * * *
(i) * * *
(b) At least one portable fire extinguisher having a rating of not
less than 12-B units must be located not less than 10 feet, nor more
than 25 feet, from any Category 1, 2, or 3 flammable liquid storage
area located outside of a storage room but inside a building.
* * * * *
(e) * * *
(2) * * *
(ii) * * *
(b) * * *
(1) 25 gallons of Category 1 flammable liquids in containers
(2) 120 gallons of Category 2, 3, or 4 flammable liquids in
containers
(3) 660 gallons of Category 2, 3, or 4 flammable liquids in a
single portable tank.
* * * * *
(iv) * * *
(a) Category 1 or 2 flammable liquids, or Category 3 flammable
liquids with a flashpoint below 100 [deg]F (37.8 [deg]C), shall be kept
in covered containers when not actually in use.
* * * * *
(c) Category 1 or 2 flammable liquids, or Category 3 flammable
liquids with a flashpoint below 100 [deg]F (37.8 [deg]C), may be used
only where there are no open flames or other sources of ignition within
the possible path of vapor travel.
* * * * *
(3) * * *
(v) * * *
(a) Areas as defined in paragraph (e)(3)(i) of this section using
Category 1 or 2 flammable liquids, or Category 3 flammable liquids with
a flashpoint below 100 [deg]F (37.8 [deg]C), shall be ventilated at a
rate of not less than 1 cubic foot per minute per square foot of solid
floor area. This shall be accomplished by natural or mechanical
ventilation with discharge or exhaust to a safe location outside of the
building. Provision shall be made for introduction of makeup air in
such a manner as not to short circuit the ventilation. Ventilation
shall be arranged to include all floor areas or pits where flammable
vapors may collect.
(b) Equipment used in a building and the ventilation of the
building shall be designed so as to limit flammable vapor-air mixtures
under normal operating conditions to the interior of equipment, and to
not more than 5 feet from equipment which exposes Category 1 or 2
flammable liquids, or Category 3 flammable liquids with a flashpoint
below 100 [deg]F (37.8 [deg]C), to the air. Examples of such equipment
are dispensing stations, open centrifuges, plate and frame filters,
open vacuum filters, and surfaces of open equipment.
* * * * *
(4) * * *
(i) Tank vehicle and tank car loading or unloading facilities shall
be separated from aboveground tanks, warehouses, other plant buildings
or nearest line of adjoining property which may be built upon by a
distance of 25 feet for Category 1 or 2 flammable liquids, or Category
3 flammable liquids with a flashpoint below 100 [deg]F (37.8 [deg]C),
and 15 feet for Category 3 flammable liquids with a flashpoint at or
above 100 [deg]F (37.8 [deg]C) and Category 4 flammable liquids,
measured from the nearest position of any fill stem. Buildings for
pumps or shelters for personnel may be a part of the facility.
Operations of the facility shall comply with the appropriate portions
of paragraph (f)(3) of this section.
* * * * *
(6) * * *
(ii) Grounding. Category 1 or 2 flammable liquids, or Category 3
flammable liquids with a flashpoint below 100 [deg]F (37.8 [deg]C),
shall not be dispensed into containers unless the nozzle and container
are electrically interconnected. Where the metallic floorplate on which
the container stands while filling is electrically connected to the
fill stem or where the fill stem is bonded to the container during
filling operations by means of a bond wire, the provisions of this
section shall be deemed to have been complied with.
(7) * * *
(i) * * *
(c) Locations where flammable vapor-air mixtures may exist under
abnormal conditions and for a distance beyond Division 1 locations
shall be classified Division 2 according to the requirements of subpart
S of this part. These locations include an area within 20 feet
horizontally, 3 feet vertically beyond a Division 1 area, and up to 3
feet above floor or grade level within 25 feet, if indoors, or 10 feet
if outdoors, from any pump, bleeder, withdrawal fitting, meter, or
similar device handling Category 1 or 2 flammable liquids, or Category
3 flammable liquids with a flashpoint below 100 [deg]F (37.8 [deg]C).
Pits provided with adequate mechanical ventilation within a Division 1
or 2 area shall be classified Division 2. If only Category 3 flammable
liquids with a flashpoint at or above 100 [deg]F (37.8 [deg]C) or
Category 4 flammable liquids are handled, then ordinary electrical
equipment is satisfactory though care shall be used in locating
electrical apparatus to prevent hot metal from falling into open
equipment.
* * * * *
(f) * * *
(1) * * *
(i) Category 1 or 2 flammable liquids, or Category 3 flammable
liquids with a flashpoint below 100 [deg]F (37.8 [deg]C). Category 1 or
2 flammable liquids, or Category 3 flammable liquids with a flashpoint
below 100 [deg]F (37.8 [deg]C), shall be stored in closed containers,
or in storage tanks above ground outside of buildings, or underground
in accordance with paragraph (b) of this section.
(ii) Category 3 flammable liquids with a flashpoint at or above 100
[deg]F (37.8 [deg]C) and Category 4 flammable liquids. Category 3
flammable liquids with a flashpoint at or above 100 [deg]F (37.8
[deg]C) and Category 4 flammable liquids shall be stored in containers,
or in tanks within buildings or above ground outside of buildings, or
underground in accordance with paragraph (b) of this section.
* * * * *
(2) * * *
(ii) Heating. Rooms in which Category 1 or 2 flammable liquids, or
Category 3 flammable liquids with a flashpoint below 100 [deg]F (37.8
[deg]C), are stored or handled shall be heated only by means not
constituting a source of ignition, such as steam or hot water. Rooms
containing heating appliances involving sources of ignition shall be
located and arranged to prevent entry of flammable vapors.
(iii) * * *
(a) Ventilation shall be provided for all rooms, buildings, or
enclosures in which Category 1 or 2 flammable liquids, or Category 3
flammable liquids with a flashpoint below 100 [deg]F (37.8 [deg]C), are
pumped or dispensed. Design of ventilation systems shall take into
account the relatively high specific gravity of the vapors. Ventilation
may be provided by adequate openings in outside walls at floor level
unobstructed except by louvers or coarse screens. Where natural
ventilation is inadequate, mechanical ventilation shall be provided.
(b) Category 1 or 2 flammable liquids, or Category 3 flammable
liquids with a flashpoint below 100 [deg]F (37.8 [deg]C), shall not be
stored or handled within a building having a basement or pit into which
flammable vapors may travel, unless such area is provided with
ventilation designed to prevent the accumulation of flammable vapors
therein.
(c) Containers of Category 1 or 2 flammable liquids, or Category 3
flammable liquids with a flashpoint below 100 [deg]F (37.8 [deg]C),
shall not be drawn from or filled within buildings unless provision is
made to prevent the accumulation of flammable vapors in hazardous
concentrations. Where mechanical ventilation is required, it shall be
kept in operation while flammable liquids with a flashpoint below 100
[deg]F (37.8 [deg]C) are being handled.
(3) * * *
(i) Separation. Tank vehicle and tank car loading or unloading
facilities shall be separated from aboveground tanks, warehouses, other
plant buildings or nearest line of adjoining property that may be built
upon by a distance of 25 feet for Category 1 or 2 flammable liquids, or
Category 3 flammable liquids with a flashpoint below 100 [deg]F (37.8
[deg]C), and 15 feet for Category 3 flammable liquids with a flashpoint
at or above 100 [deg]F (37.8 [deg]C) and Category 4 flammable liquids
measured from the nearest position of any fill spout. Buildings for
pumps or shelters for personnel may be a part of the facility.
(ii) Category restriction. Equipment such as piping, pumps, and
meters used for the transfer of Category 1 or 2 flammable liquids, or
Category 3 flammable liquids with a flashpoint below 100 [deg]F (37.8
[deg]C), between storage tanks and the fill stem of the loading rack
shall not be used for the transfer of Category 3 flammable liquids with
a flashpoint at or above 100 [deg]F (37.8 [deg]C) or Category 4
flammable liquids.
* * * * *
(iv) * * *
(a) * * *
(1) Where Category 1 or 2 flammable liquids, or Category 3
flammable liquids with a flashpoint below 100 [deg]F (37.8 [deg]C), are
loaded, or
(2) Where Category 3 flammable liquids with a flashpoint at or
above 100 [deg]F (37.8 [deg]C) or Category 4 flammable liquids are
loaded into vehicles which may contain vapors from previous cargoes of
Category 1 or 2 flammable liquids, or Category 3 flammable liquids with
a flashpoint below 100 [deg]F (37.8 [deg]C).
* * * * *
(d) * * *
(2) Where no Category 1 or 2 flammable liquids, or Category 3
flammable liquids with a flashpoint below 100 [deg]F (37.8 [deg]C), are
handled at the loading facility and the tank vehicles loaded are used
exclusively for Category 3 flammable liquids with a flashpoint at or
above 100 [deg]F (37.8 [deg]C) and Category 4 flammable liquids; and
* * * * *
(v) Stray currents. Tank car loading facilities where Category 1 or
2 flammable liquids, or Category 3 flammable liquids with a flashpoint
below 100 [deg]F (37.8 [deg]C), are loaded through open domes shall be
protected against stray currents by bonding the pipe to at least one
rail and to the rack structure if of metal. Multiple lines entering the
rack area shall be electrically bonded together. In addition, in areas
where excessive stray currents are known to exist, all pipe entering
the rack area shall be provided with insulating sections to
electrically isolate the rack piping from the pipelines. No bonding
between the tank car and the rack or piping is required during either
loading or unloading of Category 3 flammable liquids with a
flashpoint at or above 100 [deg]F (37.8 [deg]C) or Category 4 flammable
liquids.
(vi) Container filling facilities. Category 1 or 2 flammable
liquids, or Category 3 flammable liquids with a flashpoint below 100
[deg]F (37.8 [deg]C), shall not be dispensed into containers unless the
nozzle and container are electrically interconnected. Where the
metallic floorplate on which the container stands while filling is
electrically connected to the fill stem or where the fill stem is
bonded to the container during filling operations by means of a bond
wire, the provisions of this section shall be deemed to have been
complied with.
(4) * * *
(viii) * * *
(e) In addition to the requirements of paragraph (f)(4)(viii)(d) of
this section, each line conveying Category 1 or 2 flammable liquids, or
Category 3 flammable liquids with a flashpoint below 100 [deg]F (37.8
[deg]C), leading to a wharf shall be provided with a readily accessible
block valve located on shore near the approach to the wharf and outside
of any diked area. Where more than one line is involved, the valves
shall be grouped in one location.
* * * * *
(5) * * *
(i) Application. This paragraph (f)(5)(i) shall apply to areas
where Category 1 or 2 flammable liquids, or Category 3 flammable
liquids with a flashpoint below 100 [deg]F (37.8 [deg]C), are stored or
handled. For areas where only Category 3 flammable liquids with a
flashpoint at or above 100 [deg]F (37.8 [deg]C) or Category 4 flammable
liquids are stored or handled, the electrical equipment may be
installed in accordance with the provisions of Subpart S of this part,
for ordinary locations.
* * * * *
(6) Sources of ignition. Category 1 or 2 flammable liquids, or
Category 3 flammable liquids with a flashpoint below 100 [deg]F (37.8
[deg]C), shall not be handled, drawn, or dispensed where flammable
vapors may reach a source of ignition. Smoking shall be prohibited
except in designated localities. "No Smoking" signs shall be
conspicuously posted where hazard from flammable liquid vapors is
normally present.
* * * * *
(8) Fire control. Suitable fire-control devices, such as small hose
or portable fire extinguishers, shall be available to locations where
fires are likely to occur. Additional fire-control equipment may be
required where a tank of more than 50,000 gallons individual capacity
contains Category 1 or 2 flammable liquids, or Category 3 flammable
liquids with a flashpoint below 100 [deg]F (37.8 [deg]C), and where an
unusual exposure hazard exists from surrounding property. Such
additional fire-control equipment shall be sufficient to extinguish a
fire in the largest tank. The design and amount of such equipment shall
be in accordance with approved engineering standards.
* * * * *
(g) * * *
(1) * * *
(i) * * *
(c) Apparatus dispensing Category 1 or 2 flammable liquids, or
Category 3 flammable liquids with a flashpoint below 100 [deg]F (37.8
[deg]C), into the fuel tanks of motor vehicles of the public shall not
be located at a bulk plant unless separated by a fence or similar
barrier from the area in which bulk operations are conducted.
* * * * *
(e) The provisions of paragraph (g)(1)(i)(a) of this section shall
not prohibit the dispensing of flammable liquids with a flashpoint
below 100 [deg]F (37.8 [deg]C) in the open from a tank vehicle to a
motor vehicle. Such dispensing shall be permitted provided:
* * * * *
(f) Category 1 or 2 flammable liquids, or Category 3 flammable
liquids with a flashpoint below 100 [deg]F (37.8 [deg]C), shall not be
stored or handled within a building having a basement or pit into which
flammable vapors may travel, unless such area is provided with
ventilation designed to prevent the accumulation of flammable vapors
therein.
* * * * *
(iii) * * *
(a) Except where stored in tanks as provided in paragraph
(g)(1)(ii) of this section, no Category 1 or 2 flammable liquids, or
Category 3 flammable liquids with a flashpoint below 100 [deg]F (37.8
[deg]C), shall be stored within any service station building except in
closed containers of aggregate capacity not exceeding 60 gallons. One
container not exceeding 60 gallons capacity equipped with an approved
pump is permitted.
(b) Category 1 or 2 flammable liquids, or Category 3 flammable
liquids with a flashpoint below 100 [deg]F (37.8 [deg]C), may be
transferred from one container to another in lubrication or service
rooms of a service station building provided the electrical
installation complies with Table H-19 and provided that any heating
equipment complies with paragraph (g)(6) of this section.
(c) Category 3 flammable liquids with a flashpoint at or above 100
[deg]F (37.8 [deg]C) and Category 4 flammable liquids may be stored and
dispensed inside service station buildings from tanks of not more than
120 gallons capacity each.
* * * * *
(v) Dispensing into portable containers. No delivery of any
Category 1 or 2 flammable liquids, or Category 3 flammable liquids with
a flashpoint below 100 [deg]F (37.8 [deg]C), shall be made into
portable containers unless the container is constructed of metal, has a
tight closure with screwed or spring cover, and is fitted with a spout
or so designed so the contents can be poured without spilling.
* * * * *
(3) * * *
(iv) * * *
(a) Category 1 or 2 flammable liquids, or Category 3 flammable
liquids with a flashpoint below 100 [deg]F (37.8 [deg]C), shall be
transferred from tanks by means of fixed pumps so designed and equipped
as to allow control of the flow and to prevent leakage or accidental
discharge.
(b)(1) Only listed devices may be used for dispensing Category 1 or
2 flammable liquids, or Category 3 flammable liquids with a flashpoint
below 100 [deg]F (37.8 [deg]C). No such device may be used if it shows
evidence of having been dismantled.
(2) Every dispensing device for Category 1 or 2 flammable liquids,
or Category 3 flammable liquids with a flashpoint below 100 [deg]F
(37.8 [deg]C), installed after December 31, 1978, shall contain
evidence of listing so placed that any attempt to dismantle the device
will result in damage to such evidence, visible without disassembly or
dismounting of the nozzle.
(c) Category 1 or 2 flammable liquids, or Category 3 flammable
liquids with a flashpoint below 100 [deg]F (37.8 [deg]C), shall not be
dispensed by pressure from drums, barrels, and similar containers.
Approved pumps taking suction through the top of the container or
approved self-closing faucets shall be used.
* * * * *
(v) * * *
(a) This paragraph (g)(3)(v) shall apply to systems for dispensing
Category 1 or 2 flammable liquids, or Category 3 flammable liquids with
a flashpoint below 100 [deg]F (37.8 [deg]C), where such liquids are
transferred from storage to individual or multiple dispensing units by
pumps located elsewhere than at the dispensing units.
* * * * *
(vi) * * *
(a) A listed manual or automatic-closing type hose nozzle valve
shall be provided on dispensers used for the dispensing of Category 1
or 2 flammable
liquids, or Category 3 flammable liquids with a flashpoint below 100
[deg]F (37.8 [deg]C).
* * * * *
(4) * * *
(iii) * * *
BILLING CODE 4510-26-P
[GRAPHIC] [TIFF OMITTED] TR26MR12.058
[GRAPHIC] [TIFF OMITTED] TR26MR12.059
BILLING CODE 4510-26-C
(d) Piping handling Category 1 or 2 flammable liquids, or Category
3 flammable liquids with a flashpoint below 100 [deg]F (37.8 [deg]C),
shall be grounded to control stray currents.
(5) * * *
(i) Application. This paragraph (g)(5) shall apply to areas where
Category 1 or 2 flammable liquids, or Category 3 flammable liquids with
a flashpoint below 100 [deg]F (37.8 [deg]C), are stored or handled. For
areas where Category 3 flammable liquids with a flashpoint at or above
100 [deg]F (37.8 [deg]C) or Category 4 flammable liquids are stored or
handled the electrical equipment may be installed in accordance with
the provisions of subpart S of this part, for ordinary locations.
* * * * *
(6) * * *
(iv) Work areas. Heating equipment using gas or oil fuel may be
installed in the lubrication, sales, or service room where there is no
dispensing or transferring of Category 1 or 2 flammable liquids or 3
flammable liquids with a flashpoint below 100 [deg]F (37.8 [deg]C),
provided the bottom of the combustion chamber is at least 18 inches
above the floor and the heating equipment is protected from physical
damage by vehicles. Heating equipment using gas or oil fuel listed for
use in garages may be installed in the lubrication or service room
where Category 1 or 2 flammable liquids, or Category 3 flammable
liquids with a flashpoint below 100 [deg]F (37.8 [deg]C), are dispensed
provided the equipment is installed at least 8 feet above the floor.
* * * * *
(7) Drainage and waste disposal. Provision shall be made in the
area where Category 1 or 2 flammable liquids, or Category 3 flammable
liquids with a flashpoint below 100 [deg]F (37.8 [deg]C), are dispensed
to prevent spilled liquids from flowing into the interior of service
station buildings. Such provision may be by grading driveways, raising
door sills, or other equally effective means. Crankcase drainings and
flammable liquids shall not be dumped into sewers but shall be stored
in tanks or drums outside of any building until removed from the premises.
* * * * *
(h) * * *
(3) * * *
(i) * * *
(a) Processing buildings shall be of fire-resistance or
noncombustible construction, except heavy timber construction with
load-bearing walls may be permitted for plants utilizing only stable
Category 3 flammable liquids with a flashpoint at or above 100 [deg]F
(37.8 [deg]C) or Category 4 flammable liquids. Except as provided in
paragraph (h)(2)(ii) of this section or in the case of explosion
resistant walls used in conjunction with explosion relieving
facilities, see paragraph (h)(3)(iv) of this section, load-bearing
walls are prohibited. Buildings shall be without basements or covered
pits.
* * * * *
(iii) * * *
(b) Equipment used in a building and the ventilation of the
building shall be designed so as to limit flammable vapor-air mixtures
under normal operating conditions to the interior of equipment, and to
not more than 5 feet from equipment which exposes Category 1 or 2
flammable liquids, or Category 3 flammable liquids with a flashpoint
below 100 [deg]F (37.8 [deg]C), to the air. Examples of such equipment
are dispensing stations, open centrifuges, plate and frame filters,
open vacuum filters, and surfaces of open equipment.
(iv) Explosion relief. Areas where Category 1 or unstable liquids
are processed shall have explosion venting through one or more of the
following methods:
* * * * *
(5) Tank vehicle and tank car loading and unloading. Tank vehicle
and tank car loading or unloading facilities shall be separated from
aboveground tanks, warehouses, other plant buildings, or nearest line
of adjoining property which may be built upon by a distance of 25 feet
for Category 1 or 2 flammable liquids, or Category 3 flammable liquids
with a flashpoint below 100 [deg]F (37.8 [deg]C), and 15 feet for
Category 3 flammable liquids with a flashpoint at or above 100 [deg]F
(37.8 [deg]C) and Category 4 flammable liquids measured from the
nearest position of any fill stem. Buildings for pumps or shelters for
personnel may be a part of the facility. Operations of the facility
shall comply with the appropriate portions of paragraph (f)(3) of this
section.
* * * * *
(7) * * *
(i) * * *
(b) Category 1 or 2 flammable liquids, or Category 3 flammable
liquids with a flashpoint below 100 [deg]F (37.8 [deg]C), shall not be
dispensed into containers unless the nozzle and container are
electrically interconnected. Where the metallic floorplate on which the
container stands while filling is electrically connected to the fill
stem or where the fill stem is bonded to the container during filling
operations by means of a bond wire, the provisions of this section
shall be deemed to have been complied with.
* * * * *
(iii) * * *
(c) Locations where flammable vapor-air mixtures may exist under
abnormal conditions and for a distance beyond Division 1 locations
shall be classified Division 2 according to the requirements of subpart
S of this part. These locations include an area within 20 feet
horizontally, 3 feet vertically beyond a Division 1 area, and up to 3
feet above floor or grade level within 25 feet, if indoors, or 10 feet
if outdoors, from any pump, bleeder, withdrawal fitting, meter, or
similar device handling Category 1 or 2 flammable liquids, or Category
3 flammable liquids with a flashpoint below 100 [deg]F (37.8 [deg]C).
Pits provided with adequate mechanical ventilation within a Division 1
or 2 area shall be classified Division 2. If Category 3 flammable
liquids with a flashpoint at or above 100 [deg]F (37.8 [deg]C) or
Category 4 flammable liquids only are handled, then ordinary electrical
equipment is satisfactory though care shall be used in locating
electrical apparatus to prevent hot metal from falling into open
equipment.
* * * * *
(j) Scope. This section applies to the handling, storage, and use
of flammable liquids with a flashpoint at or below 199.4 [deg]F (93
[deg]C) unless otherwise noted. This section does not apply to:
* * * * *
0
5. Amend Sec. 1910.107 as follows:
0
A. Amend paragraphs (c)(9)(i), (e)(1), (e)(2), (e)(3), (e)(6)(iv),
(e)(8), and (e)(9) by removing the terms "flammable or combustible
liquids" wherever it appears and adding in its place the phrase
"flammable liquids or liquids with a flashpoint greater than 199.4
[deg]F (93 [deg]C)"; and
0
B. Revise the heading of paragraph (e), and (e)(4) to read as follows:
Sec. 1910.107 Spray finishing using flammable and combustible
materials.
* * * * *
(e) Flammable liquids and liquids with a flashpoint greater than
199.4 [deg]F (93 [deg]C)
* * * * *
(4) Transferring liquids. Except as provided in paragraph (e)(5) of
this section the withdrawal of flammable liquids and liquids with a
flashpoint greater than 199.4 [deg]F (93 [deg]C) from containers having
a capacity of greater than 60 gallons shall be by approved pumps. The
withdrawal of flammable liquids or liquids with a flashpoint greater
than 199.4 [deg]F (93 [deg]C) from containers and the filling of
containers, including portable mixing tanks, shall be done only in a
suitable mixing room or in a spraying area when the ventilating system
is in operation. Adequate precautions shall be taken to protect against
liquid spillage and sources of ignition.
* * * * *
0
6. Amend Sec. 1910.119 to revise paragraphs (a)(1)(ii) introductory
text, (a)(1)(ii)(B) and the definition of "Trade secret" in paragraph
(b) to read as follows:
Sec. 1910.119 Process safety management of highly hazardous
chemicals.
* * * * *
(a) * * *
(1) * * *
(ii) A process which involves a Category 1 flammable gas (as
defined in 1910.1200(c)) or a flammable liquid with a flashpoint below
100 [deg]F (37.8 [deg]C) on site in one location, in a quantity of
10,000 pounds (4535.9 kg) or more except for:
* * * * *
(B) Flammable liquids with a flashpoint below 100 [deg]F (37.8
[deg]C) stored in atmospheric tanks or transferred which are kept below
their normal boiling point without benefit of chilling or
refrigeration.
* * * * *
(b) * * *
Trade secret means any confidential formula, pattern, process,
device, information or compilation of information that is used in an
employer's business, and that gives the employer an opportunity to
obtain an advantage over competitors who do not know or use it. See
Appendix E to Sec. 1910.1200--Definition of a Trade Secret (which sets
out the criteria to be used in evaluating trade secrets).
* * * * *
0
7. In Sec. 1910.120, revise the definition of the term Health hazard
in paragraph (a)(3) to read as follows:
Sec. 1910.120 Hazardous waste operations and emergency response.
(a) * * *
(3) * * *
Health hazard means a chemical or a pathogen where acute or chronic
health effects may occur in exposed employees. It also includes stress due to
temperature extremes. The term health hazard includes chemicals that
are classified in accordance with the Hazard Communication Standard, 29
CFR 1910.1200, as posing one of the following hazardous effects: Acute
toxicity (any route of exposure); skin corrosion or irritation; serious
eye damage or eye irritation; respiratory or skin sensitization; germ
cell mutagenicity; carcinogenicity; reproductive toxicity; specific
target organ toxicity (single or repeated exposure); aspiration
toxicity or simple asphyxiant. (See Appendix A to Sec. 1910.1200--
Health Hazard Criteria (Mandatory) for the criteria for determining
whether a chemical is classified as a health hazard.)
* * * * *
0
8. Amend paragraph (d) of Sec. 1910.123 by removing the definition of
"Combustible liquid" and revising the definitions of the terms
"Flammable liquid" and "Flashpoint" to read as follows:
Sec. 1910.123 Dipping and coating operations: Coverage and
definitions.
* * * * *
(d) * * *
Flammable liquid means any liquid having a flashpoint at or below
199.4[emsp14][deg]F (93 [deg]C).
Flashpoint means the minimum temperature at which a liquid gives
off a vapor in sufficient concentration to ignite if tested in
accordance with the test methods in Appendix B to Sec. 1910.1200--
Physical Hazard Criteria.
* * * * *
0
9. In Sec. 1910.124, revise paragraph (c)(2) introductory text to read
as follows:
Sec. 1910.124 General requirements for dipping and coating
operations.
* * * * *
(c) * * *
(2) You must ensure that any exhaust air re-circulated from a
dipping or coating operation using flammable liquids or liquids with
flashpoints greater than 199.4 [deg]F (93 [deg]C) is:
* * * * *
0
10. Amend Sec. 1910.125 by revising the section heading and the
introductory text (including the table) to read as follows:
Sec. 1910.125 Additional requirements for dipping and coating
operations that use flammable liquids or liquids with flashpoints
greater than 199.4 [deg]F (93 [deg]C).
If you use flammable liquids, you must comply with the requirements
of this section as well as the requirements of Sec. Sec. 1910.123,
1910.124, and 1910.126, as applicable.
------------------------------------------------------------------------
You must also comply with this section
if: And:
------------------------------------------------------------------------
The flashpoint of the liquid The liquid is heated
is 199.4 [deg]F (93 [deg]C) or above. as part of the operation; or
A heated object is
placed in the liquid.
------------------------------------------------------------------------
0
11. Amend the introductory text of paragraph (c) of Sec. 1910.126 by
removing the words "or combustible".
Subpart Q--[Amended]
0
12. The authority citation for subpart Q continues to read as follows:
Authority: Sections 4, 6, and 8 of the Occupational Safety and
Health Act of 1970 (29 U.S.C. 653, 655, and 657); Secretary of
Labor's Orders Nos. 12-71 (36 FR 8754), 8-76 (41 FR 25059), 9-83 (48
FR 35736), 1-90 (55 FR 9033), 6-96 (62 FR 111), 3-2000 (65 FR
50017), 5-2002 (67 FR 65008), 5-2007 (72 FR 31159), 4-2010 (75 FR
55355), or 1-2012 (77 FR 3912), as applicable; and 29 CFR part 1911.
0
13. Amend Sec. 1910.252 as follows;
0
A. Revise paragraph (c)(1)(iv);
0
B. Add new paragraphs (c)(1)(v) and (c)(1)(vi).
Sec. 1910.252 General requirements.
* * * * *
(c) * * *
(1) * * *
(iv) Hazard communication. The employer shall include the
potentially hazardous materials employed in fluxes, coatings,
coverings, and filler metals, all of which are potentially used in
welding and cutting, or are released to the atmosphere during welding
and cutting, in the program established to comply with the Hazard
Communication Standard (HCS) (Sec. 1910.1200). The employer shall
ensure that each employee has access to labels on containers of such
materials and safety data sheets, and is trained in accordance with the
provisions of Sec. 1910.1200. Potentially hazardous materials shall
include but not be limited to the materials itemized in paragraphs
(c)(5) through (c)(12) of this section.
(v) Additional considerations for hazard communication in welding,
cutting, and brazing. (A) The suppliers shall determine and shall label
in accordance with Sec. 1910.1200 any hazards associated with the use
of their materials in welding, cutting, and brazing.
(B) In addition to any requirements imposed by Sec. 1910.1200, all
filler metals and fusible granular materials shall carry the following
notice, as a minimum, on tags, boxes, or other containers:
Do not use in areas without adequate ventilation. See ANSI Z49.1-
1967 Safety in Welding, Cutting, and Allied Processes published by the
American Welding Society.
(C) Where brazing (welding) filler metals contain cadmium in
significant amounts, the labels shall indicate the hazards associated
with cadmium including cancer, lung and kidney effects, and acute
toxicity effects.
(D) Where brazing and gas welding fluxes contain fluorine
compounds, the labels shall indicate the hazards associated with
fluorine compounds including eye and respiratory tract effects.
(vi) Prior to June 1, 2015, employers may include the following
information on labels in lieu of the labeling requirements in paragraph
(c)(1)(v) of this section:
(A) All filler metals and fusible granular materials shall carry
the following notice, as a minimum, on tags, boxes, or other
containers:
CAUTION
Welding may produce fumes and gases hazardous to health. Avoid
breathing these fumes and gases. Use adequate ventilation. See ANSI
Z49.1-1967 Safety in Welding and Cutting published by the American
Welding Society.
(B) Brazing (welding) filler metals containing cadmium in
significant amounts shall carry the following notice on tags, boxes, or
other containers:
WARNING
CONTAINS CADMIUM--POISONOUS FUMES MAY BE FORMED ON HEATING
Do not breathe fumes. Use only with adequate ventilation such as
fume collectors, exhaust ventilators, or air-supplied respirators. See
ANSI Z49.1-1967. If chest pain, cough, or fever develops after use call
physician immediately.
(C) Brazing and gas welding fluxes containing fluorine compounds
shall have a cautionary wording to indicate that they contain fluorine
compounds. One such cautionary wording recommended by the American
Welding Society for brazing and gas welding fluxes reads as follows:
CAUTION
CONTAINS FLUORIDES
This flux when heated gives off fumes that may irritate eyes, nose
and throat.
1. Avoid fumes--use only in well-ventilated spaces.
2. Avoid contact of flux with eyes or skin.
3. Do not take internally.
* * * * *
Subpart Z--[Amended]
0
14. Revise the authority citation for subpart Z to read as follows:
Authority: Sections 4, 6, 8 of the Occupational Safety and
Health Act of 1970 (29 U.S.C. 653, 655, 657); Secretary of Labor's
Order No. 12-71 (36 FR 8754), 8-76 (41 FR 25059), 9-83 (48 FR
35736), 1-90 (55 FR 9033), 6-96 (62 FR 111), 3-2000 (65 FR 50017),
5-2002 (67 FR 65008), 5-2007 (72 FR 31159), 4-2010 (75 FR 55355), or
1-2012 (77 FR 3912), as applicable; and 29 CFR part 1911.
All of subpart Z issued under section 6(b) of the Occupational
Safety and Health Act of 1970, except those substances that have
exposure limits listed in Tables Z-1, Z-2, and Z-3 of 29 CFR
1910.1000. The latter were issued under section 6(a) (29 U.S.C.
655(a)).
Section 1910.1000, Tables Z-1, Z-2 and Z-3 also issued under 5
U.S.C. 553, but not under 29 CFR part 1911 except for the arsenic
(organic compounds), benzene, cotton dust, and chromium (VI)
listings.
Section 1910.1001 also issued under section 107 of the Contract
Work Hours and Safety Standards Act (40 U.S.C. 3704) and 5 U.S.C.
553.
Section 1910.1002 also issued under 5 U.S.C. 553, but not under
29 U.S.C. 655 or 29 CFR part 1911.
Sections 1910.1018, 1910.1029, and 1910.1200 also issued under
29 U.S.C. 653.
Section 1910.1030 also issued under Pub. L. 106-430, 114 Stat.
1901.
Section 1910.1201 also issued under 49 U.S.C. 1801-1819 and 5
U.S.C. 533.
0
15. Amend Sec. 1910.1001 as follows:
0
A. Remove paragraph (j)(5);
0
B. Redesignate paragraphs (j)(1) through (j)(4) as paragraphs (j)(2)
through (j)(5);
0
C. Revise paragraphs (h)(2)(iv), (h)(3)(vi), the newly redesignated
paragraphs (j)(4), (j)(5), and the introductory text of paragraph
(j)(6);
0
D. Add new paragraph (j)(1);
0
E. Amend Appendix F, to Sec. 1910.1001, Paragraph [A] (6) by removing
"(j)(4)" and adding in its place "(j)(5)".
The revisions and additions read as follows:
Sec. 1910.1001 Asbestos.
* * * * *
(h) * * *
(2) * * *
(iv) The employer shall ensure that containers of contaminated
protective devices or work clothing, which are to be taken out of
change rooms or the workplace for cleaning, maintenance or disposal,
bear labels in accordance with paragraph (j) of this section.
(3) * * *
(vi) The employer shall ensure that contaminated clothing is
transported in sealed impermeable bags, or other closed, impermeable
containers, and labeled in accordance with paragraph (j) of this
section.
* * * * *
(j) * * *
(1) Hazard communication--general. (i) Chemical manufacturers,
importers, distributors and employers shall comply with all
requirements of the Hazard Communication Standard (HCS) (Sec.
1910.1200) for asbestos.
(ii) In classifying the hazards of asbestos at least the following
hazards are to be addressed: Cancer and lung effects.
(iii) Employers shall include asbestos in the hazard communication
program established to comply with the HCS (Sec. 1910.1200). Employers
shall ensure that each employee has access to labels on containers of
asbestos and to safety data sheets, and is trained in accordance with
the requirements of HCS and paragraph (j)(7) of this section.
* * * * *
(4) Warning signs--(i) Posting. Warning signs shall be provided and
displayed at each regulated area. In addition, warning signs shall be
posted at all approaches to regulated areas so that an employee may
read the signs and take necessary protective steps before entering the
area.
(ii) Sign specifications:
(A) The warning signs required by paragraph (j)(4)(i) of this
section shall bear the following legend:
DANGER
ASBESTOS
MAY CAUSE CANCER
CAUSES DAMAGE TO LUNGS
AUTHORIZED PERSONNEL ONLY
(B) In addition, where the use of respirators and protective
clothing is required in the regulated area under this section, the
warning signs shall include the following:
WEAR RESPIRATORY PROTECTION AND PROTECTIVE CLOTHING IN THIS AREA
(C) Prior to June 1, 2016, employers may use the following legend
in lieu of that specified in paragraph (j)(4)(ii)(A) of this section:
DANGER
ASBESTOS
CANCER AND LUNG DISEASE
HAZARD
AUTHORIZED PERSONNEL ONLY
(D) Prior to June 1, 2016, employers may use the following legend
in lieu of that specified in paragraph (j)(4)(ii)(B) of this section:
RESPIRATORS AND PROTECTIVE CLOTHING ARE REQUIRED IN THIS AREA
(iii) The employer shall ensure that employees working in and
contiguous to regulated areas comprehend the warning signs required to
be posted by paragraph (j)(4)(i) of this section. Means to ensure
employee comprehension may include the use of foreign languages,
pictographs and graphics.
(iv) At the entrance to mechanical rooms/areas in which employees
reasonably can be expected to enter and which contain ACM and/or PACM,
the building owner shall post signs which identify the material which
is present, its location, and appropriate work practices which, if
followed, will ensure that ACM and/or PACM will not be disturbed. The
employer shall ensure, to the extent feasible, that employees who come
in contact with these signs can comprehend them. Means to ensure
employee comprehension may include the use of foreign languages,
pictographs, graphics, and awareness training.
(5) Warning labels--(i) Labeling. Labels shall be affixed to all
raw materials, mixtures, scrap, waste, debris, and other products
containing asbestos fibers, or to their containers. When a building
owner or employer identifies previously installed ACM and/or PACM,
labels or signs shall be affixed or posted so that employees will be
notified of what materials contain ACM and/or PACM. The employer shall
attach such labels in areas where they will clearly be noticed by
employees who are likely to be exposed, such as at the entrance to
mechanical room/areas. Signs required by paragraph (j) of this section
may be posted in lieu of labels so long as they contain the information
required for labeling.
(ii) Label specifications. In addition to the requirements of
paragraph (j)(1), the employer shall ensure that labels of bags or
containers of protective clothing and equipment, scrap, waste, and
debris containing asbestos fibers include the following information:
DANGER
CONTAINS ASBESTOS FIBERS
MAY CAUSE CANCER
CAUSES DAMAGE TO LUNGS
DO NOT BREATHE DUST
AVOID CREATING DUST
(iii) Prior to June 1, 2015, employers may include the following
information on raw materials, mixtures or labels of bags or containers
of protective clothing and equipment, scrap, waste, and debris
containing asbestos fibers in lieu of the labeling requirements in
paragraphs (j)(1)(i) and (j)(5)(ii) of this section:
DANGER
CONTAINS ASBESTOS FIBERS
AVOID CREATING DUST
CANCER AND LUNG DISEASE HAZARD
(6) The provisions for labels and for safety data sheets required
by paragraph (j) of this section do not apply where:
* * * * *
0
16. Amend Sec. 1910.1003 as follows:
0
A. In the last sentence in paragraph (c)(4)(v) remove the words
"paragraphs (e)(2), (3), and (4)" and add the words "paragraph (e)"
in their place;
0
B. Revise the heading of paragraph (e);
0
C. Revise paragraphs (e)(1) and (e)(2).
0
D. Remove paragraph (e)(3); and
0
E. Redesignate paragraphs (e)(4) and (e)(5) as (e)(3) and (e)(4).
The revisions read as follows:
Sec. 1910.1003 13 Carcinogens (4-nitrobiphenyl, etc.).
* * * * *
(e) Communication of hazards--(1) Hazard communication. (i)
Chemical manufacturers, importers, distributors and employers shall
comply with all requirements of the Hazard Communication Standard (HCS)
(Sec. 1910.1200) for each carcinogen listed in paragraph (e)(1)(iv) of
this section.
(ii) In classifying the hazards of carcinogens listed in paragraph
(e)(1)(iv) of this section, at least the hazards listed in paragraph
(e)(1)(iv) are to be addressed.
(iii) Employers shall include the carcinogens listed in paragraph
(e)(1)(iv) of this section in the hazard communication program
established to comply with the HCS (Sec. 1910.1200). Employers shall
ensure that each employee has access to labels on containers of the
carcinogens listed in paragraph (e)(1)(iv) and to safety data sheets,
and is trained in accordance with the requirements of HCS and paragraph
(e)(4) of this section.
(iv) List of Carcinogens:
(A) 4-Nitrobiphenyl: Cancer.
(B) alpha-Naphthylamine: Cancer; skin irritation; and acute
toxicity effects.
(C) Methyl chloromethyl ether: Cancer; skin, eye and respiratory
effects; acute toxicity effects; and flammability.
(D) 3,3'-Dichlorobenzidine (and its salts): Cancer and skin
sensitization.
(E) bis-Chloromethyl ether: Cancer; skin, eye, and respiratory
tract effects; acute toxicity effects; and flammability.
(F) beta-Naphthylamine: Cancer and acute toxicity effects.
(G) Benzidine: Cancer and acute toxicity effects.
(H) 4-Aminodiphenyl: Cancer.
(I) Ethyleneimine: Cancer; mutagenicity; skin and eye effects;
liver effects; kidney effects; acute toxicity effects; and
flammability.
(J) beta-Propiolactone: Cancer; skin irritation; eye effects; and
acute toxicity effects.
(K) 2-Acetylaminofluorene: Cancer.
(L) 4-Dimethylaminoazo-benzene: Cancer; skin effects; and
respiratory tract irritation.
(M) N-Nitrosodimethylamine: Cancer; liver effects; and acute
toxicity effects.
(2) Signs. (i) The employer shall post entrances to regulated areas
with signs bearing the legend:
DANGER
(CHEMICAL IDENTIFICATION)
MAY CAUSE CANCER
AUTHORIZED PERSONNEL ONLY
(ii) The employer shall post signs at entrances to regulated areas
containing operations covered in paragraph (c)(5) of this section. The
signs shall bear the legend:
DANGER
(CHEMICAL IDENTIFICATION)
MAY CAUSE CANCER
WEAR AIR-SUPPLIED HOODS, IMPERVIOUS SUITS, AND PROTECTIVE EQUIPMENT
IN THIS AREA
AUTHORIZED PERSONNEL ONLY
(iii) Prior to June 1, 2016, employers may use the following legend
in lieu of that specified in paragraph (e)(2)(i) of this section:
CANCER-SUSPECT AGENT
AUTHORIZED PERSONNEL ONLY
(iv) Prior to June 1, 2016, employers may use the following legend
in lieu of that specified in paragraph (e)(2)(ii) of this section:
CANCER-SUSPECT AGENT EXPOSED IN THIS AREA
IMPERVIOUS SUIT INCLUDING GLOVES, BOOTS, AND AIR-SUPPLIED HOOD
REQUIRED AT ALL TIMES
AUTHORIZED PERSONNEL ONLY
(v) Appropriate signs and instructions shall be posted at the
entrance to, and exit from, regulated areas, informing employees of the
procedures that must be followed in entering and leaving a regulated
area.
* * * * *
0
17. Revise Sec. 1910.1017 paragraph (l) to read as follows:
Sec. 1910.1017 Vinyl chloride.
* * * * *
(l) Communication of hazards--(1) Hazard communication--general.
(i) Chemical manufacturers, importers, distributors and employers shall
comply with all requirements of the Hazard Communication Standard (HCS)
(Sec. 1910.1200) for vinyl chloride and polyvinyl chloride.
(ii) In classifying the hazards of vinyl chloride at least the
following hazards are to be addressed: Cancer; central nervous system
effects; liver effects; blood effects; and flammability.
(iii) Employers shall include vinyl chloride in the hazard
communication program established to comply with the HCS (Sec.
1910.1200). Employers shall ensure that each employee has access to
labels on containers of vinyl chloride and to safety data sheets, and
is trained in accordance with the requirements of HCS and paragraph (j)
of this section.
(2) Signs. (i) The employer shall post entrances to regulated areas
with legible signs bearing the legend:
DANGER
VINYL CHLORIDE
MAY CAUSE CANCER
AUTHORIZED PERSONNEL ONLY
(ii) The employer shall post signs at areas containing hazardous
operations or where emergencies currently exist. The signs shall be
legible and bear the legend:
DANGER
VINYL CHLORIDE
MAY CAUSE CANCER
WEAR RESPIRATORY PROTECTION AND PROTECTIVE CLOTHING IN THIS AREA
AUTHORIZED PERSONNEL ONLY
(iii) Prior to June 1, 2016, employers may use the following legend
in lieu of that specified in paragraph (l)(2)(i) of this section:
CANCER-SUSPECT AGENT AREA
AUTHORIZED PERSONNEL ONLY
(iv) Prior to June 1, 2016, employers may use the following legend
in lieu of that specified in paragraph (l)(2)(ii) of this section:
CANCER-SUSPECT AGENT IN THIS AREA
PROTECTIVE EQUIPMENT REQUIRED
AUTHORIZED PERSONNEL ONLY
(3) Labels. (i) In addition to the other requirements in this
paragraph (l), the employer shall ensure that labels for containers of
polyvinyl chloride resin waste from reactors or other waste
contaminated with vinyl chloride are legible and include the following
information:
CONTAMINATED WITH VINYL CHLORIDE
MAY CAUSE CANCER
(ii) Prior to June 1, 2015, employers may include the following
information on labels of containers of polyvinyl chloride resin waste
from reactors or other waste contaminated with vinyl chloride in lieu
of the labeling requirements in paragraphs (l)(3)(i)of this section:
CONTAMINATED WITH VINYL CHLORIDE
CANCER-SUSPECT AGENT
(4) Prior to June 1, 2015, employers may include the following
information for containers of polyvinyl chloride in lieu of the
labeling requirements in paragraphs (l)(1)(i) of this section:
POLYVINYL CHLORIDE (OR TRADE NAME)
Contains
VINYL CHLORIDE
VINYL CHLORIDE IS A CANCER-SUSPECT AGENT
(5)(i) Prior to June 1, 2015, employers may include either the
following information in either paragraph (l)(5)(i) or (l)(5)(ii) of
this section on containers of vinyl chloride in lieu of the labeling
requirements in paragraph (l)(1)(i) of this section:
VINYL CHLORIDE
EXTREMELY FLAMMABLE GAS UNDER PRESSURE
CANCER-SUSPECT AGENT
(ii) In accordance with 49 CFR Parts 170-189, with the additional
legend applied near the label or placard:
CANCER-SUSPECT AGENT
(6) No statement shall appear on or near any required sign, label,
or instruction which contradicts or detracts from the effect of any
required warning, information, or instruction.
* * * * *
0
18. Revise Sec. 1910.1018 paragraphs (j)(2)(vii) and (p) to read as
follows:
Sec. 1910.1018 Inorganic arsenic.
* * * * *
(j) * * *
(2) * * *
(vii) Labels on contaminated protective clothing and equipment.
(A) The employer shall ensure that the containers of contaminated
protective clothing and equipment in the workplace or which are to be
removed from the workplace are labeled and that the labels include the
following information:
DANGER: CONTAMINATED WITH INORGANIC ARSENIC. MAY CAUSE CANCER. DO
NOT REMOVE DUST BY BLOWING OR SHAKING. DISPOSE OF INORGANIC ARSENIC
CONTAMINATED WASH WATER IN ACCORDANCE WITH APPLICABLE LOCAL, STATE
OR FEDERAL REGULATIONS.
(B) Prior to June 1, 2015, employers may include the following
information on containers of protective clothing and equipment in lieu
of the labeling requirements in paragraphs (j)(2)(vii) of this section:
CAUTION: Clothing contaminated with inorganic arsenic; do not remove
dust by blowing or shaking. Dispose of inorganic arsenic contaminated
wash water in accordance with applicable local, State or Federal
regulations.
* * * * *
(p) Communication of hazards--(1) Hazard communication--General.
(i) Chemical manufacturers, importers, distributors and employers shall
comply with all requirements of the Hazard Communication Standard (HCS)
(Sec. 1910.1200) for inorganic arsenic.
(ii) In classifying the hazards of inorganic arsenic at least the
following hazards are to be addressed: Cancer; liver effects; skin
effects; respiratory irritation; nervous system effects; and acute
toxicity effects.
(iii) Employers shall include inorganic arsenic in the hazard
communication program established to comply with the HCS (Sec.
1910.1200). Employers shall ensure that each employee has access to
labels on containers of inorganic arsenic and to safety data sheets,
and is trained in accordance with the requirements of HCS and paragraph
(o) of this section.
(iv) The employer shall ensure that no statement appears on or near
any sign or label required by this paragraph (p) which contradicts or
detracts from the meaning of the required sign or label.
(2) Signs. (i) The employer shall post signs demarcating regulated
areas bearing the legend:
DANGER
INORGANIC ARSENIC
MAY CAUSE CANCER
DO NOT EAT, DRINK OR SMOKE
WEAR RESPIRATORY PROTECTION IN THIS AREA
AUTHORIZED PERSONNEL ONLY
(ii) Prior to June 1, 2016, employers may use the following legend
in lieu of that specified in paragraph (p)(2)(i) of this section:
DANGER
INORGANIC ARSENIC
CANCER HAZARD
AUTHORIZED PERSONNEL ONLY
NO SMOKING OR EATING
RESPIRATOR REQUIRED
(iii) The employer shall ensure that signs required by this
paragraph (p) are illuminated and cleaned as necessary so that the
legend is readily visible.
(3)(i) Prior to June 1, 2015, in lieu of the labeling requirements
in paragraphs (p)(1)(i) of this section, employers may apply
precautionary labels to all shipping and storage containers of
inorganic arsenic, and to all products containing inorganic arsenic,
bearing the following legend:
DANGER
CONTAINS INORGANIC ARSENIC
CANCER HAZARD
HARMFUL IF INHALED OR SWALLOWED
USE ONLY WITH ADEQUATE VENTILATION OR RESPIRATORY PROTECTION
(ii) Labels are not required when the inorganic arsenic in the
product is bound in such a manner so as to make unlikely the
possibility of airborne exposure to inorganic arsenic. (Possible
examples of products not requiring labels are semiconductors, light
emitting diodes and glass.)
* * * * *
0
19. Amend Sec. 1910.1025 as follows:
0
A. Revise paragraph (g)(2)(vii) and paragraph (m);
0
B. Revise Appendix B to Sec. 1910.1025, paragraph xi.
The revisions read as follows:
Sec. 1910.1025 Lead.
* * * * *
(g) * * *
(2) * * *
(vii) Labeling of contaminated protective clothing and equipment.
(A) The employer shall ensure that labels of bags or containers of
contaminated protective clothing and equipment include the following
information:
DANGER: CLOTHING AND EQUIPMENT CONTAMINATED WITH LEAD. MAY DAMAGE
FERTILITY OR THE UNBORN CHILD. CAUSES DAMAGE TO THE CENTRAL NERVOUS
SYSTEM. DO NOT EAT, DRINK OR SMOKE WHEN HANDLING. DO NOT REMOVE DUST
BY BLOWING OR SHAKING. DISPOSE OF LEAD CONTAMINATED WASH WATER IN
ACCORDANCE WITH APPLICABLE LOCAL, STATE, OR FEDERAL REGULATIONS.
(B) Prior to June 1, 2015, employers may include the following
information on bags or containers of contaminated protective clothing
and equipment in lieu of the labeling requirements in paragraphs
(g)(2)(vii)(A) of this section:
CAUTION: CLOTHING CONTAMINATED WITH LEAD. DO NOT REMOVE DUST BY
BLOWING OR SHAKING. DISPOSE OF LEAD CONTAMINATED WASH WATER IN
ACCORDANCE WITH APPLICABLE LOCAL, STATE, OR FEDERAL REGULATIONS.
* * * * *
(m) Communication of hazards--(1) Hazard communication--general.
(i) Chemical manufacturers, importers, distributors and employers shall
comply with all requirements of the Hazard Communication Standard (HCS)
(Sec. 1910.1200) for lead.
(ii) In classifying the hazards of lead at least the following
hazards are to be addressed: Reproductive/developmental
toxicity; central nervous system effects; kidney effects; blood
effects; and acute toxicity effects.
(iii) Employers shall include lead in the hazard communication
program established to comply with the HCS (Sec. 1910.1200). Employers
shall ensure that each employee has access to labels on containers of
lead and to safety data sheets, and is trained in accordance with the
requirements of HCS and paragraph (l) of this section.
(2) Signs. (i) The employer shall post the following warning signs
in each work area where the PEL is exceeded:
DANGER
LEAD
MAY DAMAGE FERTILITY OR THE UNBORN CHILD
CAUSES DAMAGE TO THE CENTRAL NERVOUS SYSTEM
DO NOT EAT, DRINK OR SMOKE IN THIS AREA
(ii) The employer shall ensure that no statement appears on or near
any sign required by this paragraph (m)(2) which contradicts or
detracts from the meaning of the required sign.
(iii) The employer shall ensure that signs required by this
paragraph (m)(2) are illuminated and cleaned as necessary so that the
legend is readily visible.
(iv) The employer may use signs required by other statutes,
regulations, or ordinances in addition to, or in combination with,
signs required by this paragraph (m)(2).
(v) Prior to June 1, 2016, employers may use the following legend
in lieu of that specified in paragraph (m)(2)(ii) of this section:
WARNING
LEAD WORK AREA
POISON
NO SMOKING OR EATING
* * * * *
Appendix B to Sec. 1910.1025--Employee Standard Summary
* * * * *
xi. SIGNS--PARAGRAPH (m)
The standard requires that the following warning sign be posted in
the work areas when the exposure to lead exceeds the PEL:
DANGER
LEAD
MAY DAMAGE FERTILITY OR THE UNBORN CHILD
CAUSES DAMAGE TO THE CENTRAL NERVOUS SYSTEM
DO NOT EAT, DRINK OR SMOKE IN THIS AREA
However, prior to June 1, 2016, employers may use the following
legend in lieu of that specified above:
WARNING
LEAD WORK AREA
POISON
NO SMOKING OR EATING
* * * * *
0
20. Revise Sec. 1910.1026, paragraphs (h)(2)(iv) and (l)(1) to read as
follows:
Sec. 1910.1026 Chromium (VI).
* * * * *
(h) * * *
(2) * * *
(iv) The employer shall ensure that bags or containers of
contaminated protective clothing or equipment that are removed from
change rooms for laundering, cleaning, maintenance, or disposal are
labeled in accordance with the requirements of the Hazard Communication
Standard, Sec. 1910.1200.
* * * * *
(l) * * *
(1) Hazard communication--general (i) Chemical manufacturers,
importers, distributors and employers shall comply with all
requirements of the Hazard Communication Standard (HCS) (Sec.
1910.1200) for chromium (VI).
(ii) In classifying the hazards of chromium (VI) at least the
following hazards are to be addressed: Cancer, eye irritation, and skin
sensitization.
(iii) Employers shall include chromium (VI) in the hazard
communication program established to comply with the HCS (Sec.
1910.1200). Employers shall ensure that each employee has access to
labels on containers of chromium (VI) and to safety data sheets, and is
trained in accordance with the requirements of HCS and paragraph (l)(2)
of this section.
* * * * *
0
21. Revise Sec. 1910.1027 paragraphs (k)(7), (m)(1), (m)(2), and
(m)(3) to read as follows:
Sec. 1910.1027 Cadmium.
* * * * *
(k) * * *
(7) Waste, scrap, debris, bags, containers, personal protective
equipment, and clothing contaminated with cadmium and consigned for
disposal shall be collected and disposed of in sealed impermeable bags
or other closed, impermeable containers. These bags and containers
shall be labeled in accordance with paragraph (m) of this section.
* * * * *
(m) * * *
(1) Hazard communication.--general. (i) Chemical manufacturers,
importers, distributors and employers shall comply with all
requirements of the Hazard Communication Standard (HCS) (Sec.
1910.1200) for cadmium.
(ii) In classifying the hazards of cadmium at least the following
hazards are to be addressed: Cancer; lung effects; kidney effects; and
acute toxicity effects.
(iii) Employers shall include cadmium in the hazard communication
program established to comply with the HCS (Sec. 1910.1200). Employers
shall ensure that each employee has access to labels on containers of
cadmium and to safety data sheets, and is trained in accordance with
the requirements of HCS and paragraph (m)(4) of this section.
(2) Warning signs. (i) Warning signs shall be provided and
displayed in regulated areas. In addition, warning signs shall be
posted at all approaches to regulated areas so that an employee may
read the signs and take necessary protective steps before entering the
area.
(ii) Warning signs required by paragraph (m)(2)(i) of this section
shall bear the following legend:
DANGER
CADMIUM
MAY CAUSE CANCER
CAUSES DAMAGE TO LUNGS AND KIDNEYS
WEAR RESPIRATORY PROTECTION IN THIS AREA
AUTHORIZED PERSONNEL ONLY
(iii) The employer shall ensure that signs required by this
paragraph (m)(2) are illuminated, cleaned, and maintained as necessary
so that the legend is readily visible.
(iv) Prior to June 1, 2016, employers may use the following legend
in lieu of that specified in paragraph (m)(2)(ii) of this section:
DANGER
CADMIUM
CANCER HAZARD
CAN CAUSE LUNG AND KIDNEY DISEASE
AUTHORIZED PERSONNEL ONLY
RESPIRATORS REQUIRED IN THIS AREA
(3) Warning labels. (i) Shipping and storage containers containing
cadmium or cadmium compounds shall bear appropriate warning labels, as
specified in paragraph (m)(1) of this section.
(ii) The warning labels for containers of contaminated protective
clothing, equipment, waste, scrap, or debris shall include at least the
following information:
DANGER
CONTAINS CADMIUM
MAY CAUSE CANCER
CAUSES DAMAGE TO LUNGS AND KIDNEYS
AVOID CREATING DUST
(iii) Prior to June 1, 2015, employers may include the following
information on shipping and storage containers containing cadmium,
cadmium compounds, or cadmium contaminated clothing, equipment, waste,
scrap, or debris in lieu of the labeling requirements specified in
paragraphs (m)(1)(i) and (m)(3)(ii) of this section:
DANGER
CONTAINS CADMIUM
CANCER HAZARD
AVOID CREATING DUST
CAN CAUSE LUNG AND KIDNEY DISEASE
(iv) Where feasible, installed cadmium products shall have a
visible label or other indication that cadmium is present.
* * * * *
0
22. Revise Sec. 1910.1028, paragraph (j) heading, and paragraphs
(j)(1) and (j)(2) to read as follows:
Sec. 1910.1028 Benzene.
* * * * *
(j) Communication of hazards--(1) Hazard communication--general.
(i) Chemical manufacturers, importers, distributors and employers shall
comply with all requirements of the Hazard Communication Standard (HCS)
(Sec. 1910.1200) for benzene.
(ii) In classifying the hazards of benzene at least the following
hazards are to be addressed: Cancer; central nervous system effects;
blood effects; aspiration; skin, eye, and respiratory tract irritation;
and flammability.
(iii) Employers shall include benzene in the hazard communication
program established to comply with the HCS (Sec. 1910.1200). Employers
shall ensure that each employee has access to labels on containers of
benzene and to safety data sheets, and is trained in accordance with
the requirements of HCS and paragraph (j)(3) of this section.
(2) Warning signs and labels. (i)The employer shall post signs at
entrances to regulated areas. The signs shall bear the following
legend:
DANGER
BENZENE
MAY CAUSE CANCER
HIGHLY FLAMMABLE LIQUID AND VAPOR
DO NOT SMOKE
WEAR RESPIRATORY PROTECTION IN THIS AREA
AUTHORIZED PERSONNEL ONLY
(ii) Prior to June 1, 2016, employers may use the following legend
in lieu of that specified in paragraph (j)(2)(i) of this section:
DANGER
BENZENE
CANCER HAZARD
FLAMMABLE--NO SMOKING
AUTHORIZED PERSONNEL ONLY
RESPIRATOR REQUIRED
(iii) The employer shall ensure that labels or other appropriate
forms of warning are provided for containers of benzene within the
workplace. There is no requirement to label pipes. The labels shall
comply with the requirements of paragraph (j)(1) of this section and
Sec. 1910.1200(f).
(iv) Prior to June 1, 2015, employers shall include the following
legend or similar language on the labels or other appropriate forms of
warning:
DANGER
CONTAINS BENZENE
CANCER HAZARD
* * * * *
0
23. Revise Sec. 1910.1029 paragraph (l) heading, and paragraphs (l)(1)
through (l)(3) to read as follows:
Sec. 1910.1029 Coke oven emissions.
* * * * *
(l) Communication of hazards--(1) Hazard communication--general.
The employer shall include coke oven emissions in the program
established to comply with the Hazard Communication Standard (HCS)
(Sec. 1910.1200). The employer shall ensure that each employee has
access to labels on containers of chemicals and substances associated
with coke oven processes and to safety data sheets, and is trained in
accordance with the provisions of HCS and paragraph (k) of this
section. The employer shall ensure that at least the following hazard
is addressed: Cancer.
(2) Signs. (i) The employer shall post signs in the regulated area
bearing the legend:
DANGER
COKE OVEN EMISSIONS
MAY CAUSE CANCER
DO NOT EAT, DRINK OR SMOKE
WEAR RESPIRATORY PROTECTION IN THIS AREA
AUTHORIZED PERSONNEL ONLY
(ii) In addition, the employer shall post signs in the areas where
the permissible exposure limit is exceeded bearing the legend:
WEAR RESPIRATORY PROTECTION IN THIS AREA
(iii) The employer shall ensure that no statement appears on or
near any sign required by this paragraph (l) which contradicts or
detracts from the effects of the required sign.
(iv) The employer shall ensure that signs required by this
paragraph (l)(2) are illuminated and cleaned as necessary so that the
legend is readily visible.
(v) Prior to June 1, 2016, employers may use the following legend
in lieu of that specified in paragraph (l)(2)(i) of this section:
DANGER
CANCER HAZARD
AUTHORIZED PERSONNEL ONLY
NO SMOKING OR EATING
(vi) Prior to June 1, 2016, employers may use the following legend
in lieu of that specified in paragraph (l)(2)(ii) of this section:
DANGER
RESPIRATOR REQUIRED
(3) Labels. (i) The employer shall ensure that labels of containers
of contaminated protective clothing and equipment include the following
information:
CONTAMINATED WITH COKE EMISSIONS
MAY CAUSE CANCER
DO NOT REMOVE DUST BY BLOWING OR SHAKING
(ii) Prior to June 1, 2015, employers may include the following
information on contaminated protective clothing and equipment in lieu
of the labeling requirements in paragraph (l)(3)(i) of this section:
CAUTION
CLOTHING CONTAMINATED WITH COKE EMISSIONS
DO NOT REMOVE DUST BY BLOWING OR SHAKING
* * * * *
0
24. Revise Sec. 1910.1043 paragraph (j) to read as follows:
Sec. 1910.1043 Cotton dust.
* * * * *
(j) Signs. (1) The employer shall post the following warning sign
in each work area where the permissible exposure limit for cotton dust
is exceeded:
DANGER
COTTON DUST
CAUSES DAMAGE TO LUNGS
(BYSSINOSIS)
WEAR RESPIRATORY PROTECTION IN THIS AREA
(2) Prior to June 1, 2016, employers may use the following legend
in lieu of that specified in paragraph (j)(1) of this section:
WARNING
COTTON DUST WORK AREA
MAY CAUSE ACUTE OR DELAYED
LUNG INJURY
(BYSSINOSIS)
RESPIRATORS
REQUIRED IN THIS AREA
* * * * *
0
25. Revise Sec. 1910.1044 paragraphs (j)(2)(v), (k)(1)(iii)(b), and
paragraph (o) to read as follows:
Sec. 1910.1044 1,2-dibromo-3-chloropropane.
* * * * *
(j) * * *
(2) * * *
(v) Containers of DBCP-contaminated protective devices or work
clothing which are to be taken out of change rooms or the workplace for
cleaning, maintenance or disposal shall bear labels with the following
information: CONTAMINATED WITH 1,2-Dibromo-3-chloropropane (DBCP),
MAY CAUSE CANCER.
* * * * *
(k) * * *
(1) * * *
(iii) * * *
(b) Portable vacuum units used to collect DBCP may not be used for
other cleaning purposes and shall be labeled as prescribed by paragraph
(j)(2)(v) of this section.
* * * * *
(o) Communication of hazards--(1) Hazard communication--general.
(i) Chemical manufacturers, importers, distributors and employers shall
comply with all requirements of the Hazard Communication Standard (HCS)
(Sec. 1910.1200) for DBCP.
(ii) In classifying the hazards of DBCP at least the following
hazards are to be addressed: Cancer; reproductive effects; liver
effects; kidney effects; central nervous system effects; skin, eye and
respiratory tract irritation; and acute toxicity effects.
(iii) Employers shall include DBCP in the hazard communication
program established to comply with the HCS (Sec. 1910.1200). Employers
shall ensure that each employee has access to labels on containers of
DBCP and to safety data sheets, and is trained in accordance with the
requirements of HCS and paragraph (n) of this section.
(iv) The employer shall ensure that no statement appears on or near
any sign or label required by this paragraph (o) which contradicts or
detracts from the meaning of the required sign or label.
(2) Signs. (i) The employer shall post signs to clearly indicate
all regulated areas. These signs shall bear the legend:
DANGER
1,2-Dibromo-3-chloropropane
MAY CAUSE CANCER
WEAR RESPIRATORY PROTECTION IN THIS AREA
AUTHORIZED PERSONNEL ONLY
(ii) Prior to June 1, 2016, employers may use the following legend
in lieu of that specified in paragraph (o)(2) of this section:
DANGER
1,2-Dibromo-3-chloropropane
(Insert appropriate trade or common names)
CANCER HAZARD
AUTHORIZED PERSONNEL ONLY
RESPIRATOR REQUIRED
(3) Labels. (i) Where DBCP or products containing DBCP are sold,
distributed or otherwise leave the employer's workplace bearing
appropriate labels required by EPA under the regulations in 40 CFR Part
162, the labels required by this paragraph (o)(3) need not be affixed.
(ii) The employer shall ensure that the precautionary labels
required by this paragraph (o)(3) are readily visible and legible.
(iii) Prior to June 1, 2015, employers may include the following
information on containers of DBCP or products containing DBCP, DBCP-
contaminated protective devices or work clothing or DBCP-contaminated
portable vacuums in lieu of the labeling requirements in paragraphs
(j)(2)(v), (k)(l)(iii)(b) and (o)(1)(i) of this section:
DANGER
1,2-Dibromo-3-chloropropane
CANCER HAZARD
* * * * *
0
26. Revise Sec. 1910.1045 paragraph (p) to read as follows:
Sec. 1910.1045 Acrylonitrile.
* * * * *
(p) Communication of hazards--(1) Hazard communication--general.
(i) Chemical manufacturers, importers, distributors and employers shall
comply with all requirements of the Hazard Communication Standard (HCS)
(Sec. 1910.1200) for AN and AN-based materials not exempted under
paragraph (a)(2) of this section.
(ii) In classifying the hazards of AN and AN-based materials at
least the following hazards are to be addressed: Cancer; central
nervous system effects; liver effects; skin sensitization; skin,
respiratory, and eye irritation; acute toxicity effects; and
flammability.
(iii) Employers shall include AN and AN-based materials in the
hazard communication program established to comply with the HCS (Sec.
1910.1200). Employers shall ensure that each employee has access to
labels on containers of AN and AN-based materials and to safety data
sheets, and is trained in accordance with the requirements of HCS and
paragraph (o) of this section.
(iv) The employer shall ensure that no statement appears on or near
any sign or label required by this paragraph (p) that contradicts or
detracts from the required sign or label.
(2) Signs. (i) The employer shall post signs to clearly indicate
all workplaces where AN concentrations exceed the permissible exposure
limits. The signs shall bear the following legend:
DANGER
ACRYLONITRILE (AN)
MAY CAUSE CANCER
RESPIRATORY PROTECTION MAY BE REQURED IN THIS AREA
AUTHORIZED PERSONNEL ONLY
(ii) The employer shall ensure that signs required by this
paragraph (p)(2) are illuminated and cleaned as necessary so that the
legend is readily visible.
(iii) Prior to June 1, 2016, employers may use the following legend
in lieu of that specified in paragraph (p)(2)(i) of this section:
DANGER
ACRYLONITRILE (AN)
CANCER HAZARD
AUTHORIZED PERSONNEL ONLY
RESPIRATORS MAY BE REQUIRED
(3) Labels. (i) The employer shall ensure that precautionary labels
are in compliance with paragraph (p)(1)(i) of this section and are
affixed to all containers of liquid AN and AN-based materials not
exempted under paragraph (a)(2) of this section. The employer shall
ensure that the labels remain affixed when the materials are sold,
distributed, or otherwise leave the employer's workplace.
(ii) Prior to June 1, 2015, employers may include the following
information on precautionary labels required by this paragraph (p)(3)
in lieu of the labeling requirements in paragraph (p)(1) of this
section:
DANGER
CONTAINS ACRYLONITRILE (AN)
CANCER HAZARD
(iii) The employer shall ensure that the precautionary labels
required by this paragraph (p)(3) are readily visible and legible.
* * * * *
0
27. Revise Sec. 1910.1047 paragraph (j) heading, and paragraphs (j)(1)
and (j)(2) to read as follows:
Sec. 1910.1047 Ethylene oxide.
* * * * *
(j) Communication of hazards--(1) Hazard communication--general.
(i) Chemical manufacturers, importers, distributors and employers shall
comply with all requirements of the Hazard Communication Standard (HCS)
(Sec. 1910.1200) for EtO.
(ii) In classifying the hazards of EtO at least the following
hazards are to be addressed: Cancer; reproductive effects;
mutagenicity; central nervous system; skin sensitization; skin, eye and
respiratory tract irritation; acute toxicity effects; and flammability.
(iii) Employers shall include EtO in the hazard communication
program established to comply with the HCS (Sec. 1910.1200). Employers
shall ensure that each employee has access to labels on containers of
EtO and to safety data sheets, and is trained in accordance with the
requirements of HCS and paragraph (j)(3) of this section.
(2) Signs and labels--(i) Signs. (A) The employer shall post and
maintain legible signs demarcating regulated areas and entrances or
access ways to regulated areas that bear the following legend:
DANGER
ETHYLENE OXIDE
MAY CAUSE CANCER
MAY DAMAGE FERTILITY OR THE UNBORN CHILD
RESPIRATORY PROTECTION AND PROTECTIVE CLOTHING MAY BE REQUIRED IN
THIS AREA
AUTHORIZED PERSONNEL ONLY
(B) Prior to June 1, 2016, employers may use the following legend
in lieu of that specified in paragraph (j)(2)(i)(A) of this section:
DANGER
ETHYLENE OXIDE
CANCER HAZARD AND REPRODUCTIVE HAZARD
AUTHORIZED PERSONNEL ONLY
RESPIRATORS AND PROTECTIVE CLOTHING MAY BE REQUIRED TO BE WORN IN
THIS AREA
(ii) Labels. (A) The employer shall ensure that labels are affixed
to all containers of EtO whose contents are capable of causing employee
exposure at or above the action level or whose contents may reasonably
be foreseen to cause employee exposure above the excursion limit, and
that the labels remain affixed when the containers of EtO leave the
workplace. For the purposes of this paragraph (j)(2)(ii), reaction
vessels, storage tanks, and pipes or piping systems are not considered
to be containers.
(B) Prior to June 1, 2015, employers may include the following
information on containers of EtO in lieu of the labeling requirements
in paragraph (j)(1)(i) of this section:
(1) DANGER
CONTAINS ETHYLENE OXIDE
CANCER HAZARD AND REPRODUCTIVE HAZARD;
(2) A warning statement against breathing airborne concentrations of
EtO.
(C) The labeling requirements under this section do not apply where
EtO is used as a pesticide, as such term is defined in the Federal
Insecticide, Fungicide, and Rodenticide Act (7 U.S.C. 136 et seq.),
when it is labeled pursuant to that Act and regulations issued under
that Act by the Environmental Protection Agency.
* * * * *
0
28. Revise Sec. 1910.1048 paragraphs (e)(1), (h)(2)(ii), (j)(4) and
(m) to read as follows:
Sec. 1910.1048 Formaldehyde.
* * * * *
(e) * * *
(1) Signs. (i) The employer shall establish regulated areas where
the concentration of airborne formaldehyde exceeds either the TWA or
the STEL and post all entrances and access ways with signs bearing the
following legend:
DANGER
FORMALDEHYDE
MAY CAUSE CANCER
CAUSES SKIN, EYE, AND RESPIRATORY IRRITATION
AUTHORIZED PERSONNEL ONLY
(ii) Prior to June 1, 2016, employers may use the following legend
in lieu of that specified in paragraph (e)(1)(i) of this section:
DANGER
FORMALDEHYDE
IRRITANT AND POTENTIAL CANCER HAZARD
AUTHORIZED PERSONNEL ONLY
* * * * *
(h) * * *
(2) * * *
(ii) When formaldehyde-contaminated clothing and equipment is
ventilated, the employer shall establish storage areas so that employee
exposure is minimized.
(A) Signs. Storage areas for contaminated clothing and equipment
shall have signs bearing the following legend:
DANGER
FORMALDEHYDE-CONTAMINATED [CLOTHING] EQUIPMENT
MAY CAUSE CANCER
CAUSES SKIN, EYE AND RESPIRATORY IRRITATION
DO NOT BREATHE VAPOR
DO NOT GET ON SKIN
(B) Labels. The employer shall ensure containers for contaminated
clothing and equipment are labeled consistent with the Hazard
Communication Standard, Sec. 1910.1200, and shall, as a minimum,
include the following:
DANGER
FORMALDEHYDE-CONTAMINATED [CLOTHING] EQUIPMENT
MAY CAUSE CANCER
CAUSES SKIN, EYE, AND RESPIRATORY IRRITATION
DO NOT BREATHE VAPOR
DO NOT GET ON SKIN
(C) Prior to June 1, 2016, employers may use the following legend
in lieu of that specified in paragraph (h)(2)(ii)(A) of this section:
DANGER
FORMALDEHYDE-CONTAMINATED [CLOTHING] EQUIPMENT
AVOID INHALATION AND SKIN CONTACT
(D) Prior to June 1, 2015, employers may include the following
information on containers of protective clothing and equipment in lieu
of the labeling requirements in paragraphs (h)(2)(ii)(B) of this
section:
DANGER
FORMALDEHYDE-CONTAMINATED [CLOTHING] EQUIPMENT
AVOID INHALATION AND SKIN CONTACT
* * * * *
(j) * * *
(4) Formaldehyde-contaminated waste and debris resulting from leaks
or spills shall be placed for disposal in sealed containers bearing a
label warning of formaldehyde's presence and of the hazards associated
with formaldehyde. The employer shall ensure that the labels are in
accordance with paragraph (m) of this section.
* * * * *
(m) Communication of hazards. (1) Hazard communication--General.
(i) Chemical manufacturers, importers, distributors and employers shall
comply with all requirements of the Hazard Communication Standard (HCS)
(Sec. 1910.1200) for formaldehyde.
(ii) In classifying the hazards of formaldehyde at least the
following hazards are to be addressed: Cancer; skin and respiratory
sensitization; eye, skin and respiratory tract irritation; acute
toxicity effects; and flammability.
(iii) Employers shall include formaldehyde in the hazard
communication program established to comply with the HCS (Sec.
1910.1200). Employers shall ensure that each employee has access to
labels on containers of formaldehyde and to safety data sheets, and is
trained in accordance with the requirements of HCS and paragraph (n) of
this section.
(iv) Paragraphs (m)(1)(i), (m)(1)(ii), and (m)(1)(iii) of this
section apply to chemicals associated with formaldehyde gas, all
mixtures or solutions composed of greater than 0.1 percent
formaldehyde, and materials capable of releasing formaldehyde into the
air at concentrations reaching or exceeding 0.1 ppm.
(v) In making the determinations of anticipated levels of
formaldehyde release, the employer may rely on objective data
indicating the extent of potential formaldehyde release under
reasonably foreseeable conditions of use.
(2)(i) In addition to the requirements in paragraphs (m)(1) through
(m)(1)(iv) of this section, for materials listed in paragraph
(m)(1)(iv) capable of releasing formaldehyde at levels above 0.5 ppm,
labels shall appropriately address all hazards as defined in paragraph
(d) of Sec. 1910.1200 and Appendices A and B to Sec. 1910.1200,
including cancer and respiratory sensitization, and shall
contain the hazard statement "May Cause Cancer."
(ii) As a minimum, for all materials listed in paragraph (m)(1)(i)
and (iv) of this section capable of releasing formaldehyde at levels of
0.1 ppm to 0.5 ppm, labels shall identify that the product contains
formaldehyde; list the name and address of the responsible party; and
state that physical and health hazard information is readily available
from the employer and from safety data sheets.
(iii) Prior to June 1, 2015, employers may include the phrase
"Potential Cancer Hazard" in lieu of "May Cause Cancer" as
specified in paragraph (m)(2)(i) of this section.
* * * * *
0
29. Amend Sec. 1910.1050 as follows:
0
A. Revise the heading of paragraph (k);
0
B. Revise paragraphs (k)(1) and (k)(2);
0
C. Redesignate paragraphs (k)(3) and (k)(4) as (k)(4) and (k)(5);
0
D. Add new paragraph (k)(3).
The revisions and additions read as follows:
Sec. 1910.1050 Methylenedianiline.
* * * * *
(k) Communication of hazards--(1) Hazard communication--general.
(i) Chemical manufacturers, importers, distributors and employers
shall comply with all requirements of the Hazard Communication Standard
(HCS) (Sec. 1910.1200) for MDA.
(ii) In classifying the hazards of MDA at least the following
hazards are to be addressed: Cancer; liver effects; and skin
sensitization.
(iii) Employers shall include MDA in the hazard communication
program established to comply with the HCS (Sec. 1910.1200). Employers
shall ensure that each employee has access to labels on containers of
MDA and to safety data sheets, and is trained in accordance with the
requirements of HCS and paragraph (k)(4) of this section.
(2) Signs and labels--(i) Signs. (A) The employer shall post and
maintain legible signs demarcating regulated areas and entrances or
access ways to regulated areas that bear the following legend:
DANGER
MDA
MAY CAUSE CANCER
CAUSES DAMAGE TO THE LIVER
RESPIRATORY PROTECTION AND PROTECTIVE CLOTHING MAY BE REQUIRED IN
THIS AREA
AUTHORIZED PERSONNEL ONLY
(B) Prior to June 1, 2016, employers may use the following legend
in lieu of that specified in paragraph (k)(2)(i)(A) of this section:
DANGER
MDA
MAY CAUSE CANCER
LIVER TOXIN
AUTHORIZED PERSONNEL ONLY
RESPIRATORS AND PROTECTIVE CLOTHING MAY BE REQUIRED TO BE WORN IN
THIS AREA
(ii) Labels. Prior to June 1, 2015, employers may include the
following information workplace labels in lieu of the labeling
requirements in paragraph (k)(1) of this section:
(A) For pure MDA:
DANGER
CONTAINS MDA
MAY CAUSE CANCER
LIVER TOXIN
(B) For mixtures containing MDA:
DANGER
CONTAINS MDA
CONTAINS MATERIALS WHICH MAY CAUSE CANCER
LIVER TOXIN
(3) Safety data sheets (SDS). In meeting the obligation to provide
safety data sheets, employers shall make appropriate use of the
information found in Appendices A and B to Sec. 1910.1050.
* * * * *
0
30. Revise Sec. 1910.1051 paragraph (l)(1) to read as follows:
Sec. 1910.1051 1,3-Butadiene.
* * * * *
(l) * * *
(1) Hazard communication--general. (i) Chemical manufacturers,
importers, distributors and employers shall comply with all
requirements of the Hazard Communication Standard (HCS) (Sec.
1910.1200) for BD.
(ii) In classifying the hazards of BD at least the following
hazards are to be addressed: Cancer; eye and respiratory tract
irritation; center nervous system effects; and flammability.
(iii) Employers shall include BD in the hazard communication
program established to comply with the HCS (Sec. 1910.1200). Employers
shall ensure that each employee has access to labels on containers of
BD and to safety data sheets, and is trained in accordance with the
requirements of HCS and paragraph (l)(2) of this section.
* * * * *
0
31. Amend Sec. 1910.1052 as follows:
0
A. Revise paragraph (k);
0
B. Remove the phrase "material safety data sheets (MSDS)" and add in
its place the phrase "safety data sheets (SDS)" where it appears in
Appendix A, Paragraph X.E.
The revisions read as follows:
Sec. 1910.1052 Methylene chloride.
* * * * *
(k) Hazard communication.--(1) Hazard communication--general. (i)
Chemical manufacturers, importers, distributors and employers shall
comply with all requirements of the Hazard Communication Standard (HCS)
(Sec. 1910.1200) for MC.
(ii) In classifying the hazards of MC at least the following
hazards are to be addressed: Cancer, cardiac effects (including
elevation of carboxyhemoglobin), central nervous system effects, liver
effects, and skin and eye irritation.
(iii) Employers shall include MC in the hazard communication
program established to comply with the HCS (Sec. 1910.1200). Employers
shall ensure that each employee has access to labels on containers of
MC and to safety data sheets, and is trained in accordance with the
requirements of HCS and paragraph (l) of this section.
(2) [Reserved]
* * * * *
0
32. Amend Sec. 1910.1200 as follows:
0
A. Remove the word "material" before the word "safety" in the
phrase "material safety data sheet" or "material safety data
sheets" wherever they appear in paragraphs (b)(3)(ii), (b)(4)(ii),
(e)(1) introductory text, (e)(2)(i), (g)(4), (g)(6)(i) through (iv),
(g)(7)(i) through (vii), (g)(9), (g)(11), (h)(1), (h)(2)(iii), and
(i)(1)(ii);
0
B. Remove the word "Material" before the word "safety" in the
phrase "Material safety data sheets" wherever they appear in
paragraphs (g)(10) and (g)(11). In paragraphs (g)(10) and (g)(11) in
the first sentence, capitalize the first letter of the word "safety".
0
C. Remove the following definitions in paragraph (c) Combustible
liquid, Compressed gas, Explosive, Flammable, Flashpoint, Hazard
warning, Identity, Material safety data sheet (MSDS), Organic peroxide,
Oxidizer, Pyrophoric, Unstable (reactive), and Water-reactive;
0
D. Revise the following definitions in paragraph (c) Chemical, Chemical
name, Health hazard, Label, Mixture, Physical hazard, and Trade secret;
0
E. Redesignate the definition of the term Hazardous chemical in
alphabetical order in paragraph (c) and revise the definition;
0
F. Add the following definitions in alphabetical order in paragraph (c)
Classification, Hazard category, Hazard class, Hazard not otherwise
classified, Hazard statement, Label elements, Pictogram, Precautionary
statement, Product identifier, Pyrophoric gas, Safety Data Sheet (SDS),
Signal word, Simple asphyxiant, and Substance;
0
G. Remove the following phrases: "in" before the phrase "in their
work area(s)"
in paragraph (g)(10); "specific chemical identity" in paragraph
(i)(10)(ii); and "or percentage of mixture" in paragraph (i)(13);
0
H. Revise paragraphs (a), (b)(1), (b)(3)(iv), (b)(5)(iv), (b)(6)(ii),
paragraph (d) (heading), paragraphs (d)(1) through (d)(3), (e)(1)(i),
(f), paragraph (g) (heading), paragraphs (g)(1), (g)(2), (g)(3),
(g)(5), (g)(8), (g)(11), (h)(1), (h)(3)(ii), (h)(3)(iv), (i)(1)
introductory text, (i)(1)(iii) and (iv), (i)(2), (i)(3) introductory
text, (i)(3)(iii), (i)(7) introductory text, (i)(7)(iii), (i)(7)(v),
(i)(9)(i), (i)(10), (i)(11), and (j).
0
I. Remove Appendices A, B, and E to Sec. 1910.1200.
0
J. Redesignate Appendix D to Sec. 1910.1200 as Appendix E to Sec.
1910.1200.
0
K. Add new Appendices A, B, C, D and F to Sec. 1910.1200.
The revisions and additions read as follows:
Sec. 1910.1200 Hazard communication.
(a) Purpose. (1) The purpose of this section is to ensure that the
hazards of all chemicals produced or imported are classified, and that
information concerning the classified hazards is transmitted to
employers and employees. The requirements of this section are intended
to be consistent with the provisions of the United Nations Globally
Harmonized System of Classification and Labelling of Chemicals (GHS),
Revision 3. The transmittal of information is to be accomplished by
means of comprehensive hazard communication programs, which are to
include container labeling and other forms of warning, safety data
sheets and employee training.
(2) This occupational safety and health standard is intended to
address comprehensively the issue of classifying the potential hazards
of chemicals, and communicating information concerning hazards and
appropriate protective measures to employees, and to preempt any
legislative or regulatory enactments of a state, or political
subdivision of a state, pertaining to this subject. Classifying the
potential hazards of chemicals and communicating information concerning
hazards and appropriate protective measures to employees, may include,
for example, but is not limited to, provisions for: developing and
maintaining a written hazard communication program for the workplace,
including lists of hazardous chemicals present; labeling of containers
of chemicals in the workplace, as well as of containers of chemicals
being shipped to other workplaces; preparation and distribution of
safety data sheets to employees and downstream employers; and
development and implementation of employee training programs regarding
hazards of chemicals and protective measures. Under section 18 of the
Act, no state or political subdivision of a state may adopt or enforce
any requirement relating to the issue addressed by this Federal
standard, except pursuant to a Federally-approved state plan.
(b) * * *
(1) This section requires chemical manufacturers or importers to
classify the hazards of chemicals which they produce or import, and all
employers to provide information to their employees about the hazardous
chemicals to which they are exposed, by means of a hazard communication
program, labels and other forms of warning, safety data sheets, and
information and training. In addition, this section requires
distributors to transmit the required information to employers.
(Employers who do not produce or import chemicals need only focus on
those parts of this rule that deal with establishing a workplace
program and communicating information to their workers.)
* * * * *
(3) * * *
(iv) Laboratory employers that ship hazardous chemicals are
considered to be either a chemical manufacturer or a distributor under
this rule, and thus must ensure that any containers of hazardous
chemicals leaving the laboratory are labeled in accordance with
paragraph (f) of this section, and that a safety data sheet is provided
to distributors and other employers in accordance with paragraphs
(g)(6) and (g)(7) of this section.
* * * * *
(5) * * *
(iv) Any distilled spirits (beverage alcohols), wine, or malt
beverage intended for nonindustrial use, as such terms are defined in
the Federal Alcohol Administration Act (27 U.S.C. 201 et seq.) and
regulations issued under that Act, when subject to the labeling
requirements of that Act and labeling regulations issued under that Act
by the Bureau of Alcohol, Tobacco, Firearms and Explosives;
* * * * *
(6) * * *
(ii) Any hazardous substance as such term is defined by the
Comprehensive Environmental Response, Compensation and Liability Act
(CERCLA) (42 U.S.C. 9601 et seq.) when the hazardous substance is the
focus of remedial or removal action being conducted under CERCLA in
accordance with Environmental Protection Agency regulations.
* * * * *
(c) * * *
Chemical means any substance, or mixture of substances.
* * * * *
Chemical name means the scientific designation of a chemical in
accordance with the nomenclature system developed by the International
Union of Pure and Applied Chemistry (IUPAC) or the Chemical Abstracts
Service (CAS) rules of nomenclature, or a name that will clearly
identify the chemical for the purpose of conducting a hazard
classification.
Classification means to identify the relevant data regarding the
hazards of a chemical; review those data to ascertain the hazards
associated with the chemical; and decide whether the chemical will be
classified as hazardous according to the definition of hazardous
chemical in this section. In addition, classification for health and
physical hazards includes the determination of the degree of hazard,
where appropriate, by comparing the data with the criteria for health
and physical hazards.
* * * * *
Hazard category means the division of criteria within each hazard
class, e.g., oral acute toxicity and flammable liquids include four
hazard categories. These categories compare hazard severity within a
hazard class and should not be taken as a comparison of hazard
categories more generally.
Hazard class means the nature of the physical or health hazards,
e.g., flammable solid, carcinogen, oral acute toxicity.
Hazard not otherwise classified (HNOC) means an adverse physical or
health effect identified through evaluation of scientific evidence
during the classification process that does not meet the specified
criteria for the physical and health hazard classes addressed in this
section. This does not extend coverage to adverse physical and health
effects for which there is a hazard class addressed in this section,
but the effect either falls below the cut-off value/concentration limit
of the hazard class or is under a GHS hazard category that has not been
adopted by OSHA (e.g., acute toxicity Category 5).
Hazard statement means a statement assigned to a hazard class and
category that describes the nature of the hazard(s) of a chemical,
including, where appropriate, the degree of hazard.
Hazardous chemical means any chemical which is classified as a
physical hazard or a health hazard, a simple asphyxiant,
combustible dust, pyrophoric gas, or hazard not otherwise classified.
Health hazard means a chemical which is classified as posing one of
the following hazardous effects: acute toxicity (any route of
exposure); skin corrosion or irritation; serious eye damage or eye
irritation; respiratory or skin sensitization; germ cell mutagenicity;
carcinogenicity; reproductive toxicity; specific target organ toxicity
(single or repeated exposure); or aspiration hazard. The criteria for
determining whether a chemical is classified as a health hazard are
detailed in Appendix A to Sec. 1910.1200--Health Hazard Criteria.
* * * * *
Label means an appropriate group of written, printed or graphic
information elements concerning a hazardous chemical that is affixed
to, printed on, or attached to the immediate container of a hazardous
chemical, or to the outside packaging.
Label elements means the specified pictogram, hazard statement,
signal word and precautionary statement for each hazard class and
category.
Mixture means a combination or a solution composed of two or more
substances in which they do not react.
Physical hazard means a chemical that is classified as posing one
of the following hazardous effects: explosive; flammable (gases,
aerosols, liquids, or solids); oxidizer (liquid, solid or gas); self-
reactive; pyrophoric (liquid or solid); self-heating; organic peroxide;
corrosive to metal; gas under pressure; or in contact with water emits
flammable gas. See Appendix B to Sec. 1910.1200--Physical Hazard
Criteria.
Pictogram means a composition that may include a symbol plus other
graphic elements, such as a border, background pattern, or color, that
is intended to convey specific information about the hazards of a
chemical. Eight pictograms are designated under this standard for
application to a hazard category.
Precautionary statement means a phrase that describes recommended
measures that should be taken to minimize or prevent adverse effects
resulting from exposure to a hazardous chemical, or improper storage or
handling.
Product identifier means the name or number used for a hazardous
chemical on a label or in the SDS. It provides a unique means by which
the user can identify the chemical. The product identifier used shall
permit cross-references to be made among the list of hazardous
chemicals required in the written hazard communication program, the
label and the SDS.
* * * * *
Pyrophoric gas means a chemical in a gaseous state that will ignite
spontaneously in air at a temperature of 130 degrees F (54.4 degrees C)
or below.
* * * * *
Safety data sheet (SDS) means written or printed material
concerning a hazardous chemical that is prepared in accordance with
paragraph (g) of this section.
Signal word means a word used to indicate the relative level of
severity of hazard and alert the reader to a potential hazard on the
label. The signal words used in this section are "danger" and
"warning." "Danger" is used for the more severe hazards, while
"warning" is used for the less severe.
Simple asphyxiant means a substance or mixture that displaces
oxygen in the ambient atmosphere, and can thus cause oxygen deprivation
in those who are exposed, leading to unconsciousness and death.
* * * * *
Substance means chemical elements and their compounds in the
natural state or obtained by any production process, including any
additive necessary to preserve the stability of the product and any
impurities deriving from the process used, but excluding any solvent
which may be separated without affecting the stability of the substance
or changing its composition.
Trade secret means any confidential formula, pattern, process,
device, information or compilation of information that is used in an
employer's business, and that gives the employer an opportunity to
obtain an advantage over competitors who do not know or use it.
Appendix E to Sec. 1910.1200--Definition of Trade Secret, sets out the
criteria to be used in evaluating trade secrets.
* * * * *
(d) Hazard classification. (1) Chemical manufacturers and importers
shall evaluate chemicals produced in their workplaces or imported by
them to classify the chemicals in accordance with this section. For
each chemical, the chemical manufacturer or importer shall determine
the hazard classes, and, where appropriate, the category of each class
that apply to the chemical being classified. Employers are not required
to classify chemicals unless they choose not to rely on the
classification performed by the chemical manufacturer or importer for
the chemical to satisfy this requirement.
(2) Chemical manufacturers, importers or employers classifying
chemicals shall identify and consider the full range of available
scientific literature and other evidence concerning the potential
hazards. There is no requirement to test the chemical to determine how
to classify its hazards. Appendix A to Sec. 1910.1200 shall be
consulted for classification of health hazards, and Appendix B to Sec.
1910.1200 shall be consulted for the classification of physical
hazards.
(3) Mixtures. (i) Chemical manufacturers, importers, or employers
evaluating chemicals shall follow the procedures described in
Appendices A and B to Sec. 1910.1200 to classify the hazards of the
chemicals, including determinations regarding when mixtures of the
classified chemicals are covered by this section.
(ii) When classifying mixtures they produce or import, chemical
manufacturers and importers of mixtures may rely on the information
provided on the current safety data sheets of the individual
ingredients, except where the chemical manufacturer or importer knows,
or in the exercise of reasonable diligence should know, that the safety
data sheet misstates or omits information required by this section.
* * * * *
(e) * * *
(1) * * *
(i) A list of the hazardous chemicals known to be present using a
product identifier that is referenced on the appropriate safety data
sheet (the list may be compiled for the workplace as a whole or for
individual work areas); and,
* * * * *
(f) Labels and other forms of warning--(1) Labels on shipped
containers. The chemical manufacturer, importer, or distributor shall
ensure that each container of hazardous chemicals leaving the workplace
is labeled, tagged, or marked. Hazards not otherwise classified do not
have to be addressed on the container. Where the chemical manufacturer
or importer is required to label, tag or mark the following information
shall be provided:
(i) Product identifier;
(ii) Signal word;
(iii) Hazard statement(s);
(iv) Pictogram(s);
(v) Precautionary statement(s); and,
(vi) Name, address, and telephone number of the chemical
manufacturer, importer, or other responsible party.
(2) The chemical manufacturer, importer, or distributor shall
ensure that the information provided under paragraphs (f)(1)(i) through
(v) of this section is in accordance with Appendix
C to Sec. 1910.1200, for each hazard class and associated hazard
category for the hazardous chemical, prominently displayed, and in
English (other languages may also be included if appropriate).
(3) The chemical manufacturer, importer, or distributor shall
ensure that the information provided under paragraphs (f)(1)(ii)
through (iv) of this section is located together on the label, tag, or
mark.
(4) Solid materials. (i) For solid metal (such as a steel beam or a
metal casting), solid wood, or plastic items that are not exempted as
articles due to their downstream use, or shipments of whole grain, the
required label may be transmitted to the customer at the time of the
initial shipment, and need not be included with subsequent shipments to
the same employer unless the information on the label changes;
(ii) The label may be transmitted with the initial shipment itself,
or with the safety data sheet that is to be provided prior to or at the
time of the first shipment; and,
(iii) This exception to requiring labels on every container of
hazardous chemicals is only for the solid material itself, and does not
apply to hazardous chemicals used in conjunction with, or known to be
present with, the material and to which employees handling the items in
transit may be exposed (for example, cutting fluids or pesticides in
grains).
(5) Chemical manufacturers, importers, or distributors shall ensure
that each container of hazardous chemicals leaving the workplace is
labeled, tagged, or marked in accordance with this section in a manner
which does not conflict with the requirements of the Hazardous
Materials Transportation Act (49 U.S.C. 1801 et seq.) and regulations
issued under that Act by the Department of Transportation.
(6) Workplace labeling. Except as provided in paragraphs (f)(7) and
(f)(8) of this section, the employer shall ensure that each container
of hazardous chemicals in the workplace is labeled, tagged or marked
with either:
(i) The information specified under paragraphs (f)(1)(i) through
(v) of this section for labels on shipped containers; or,
(ii) Product identifier and words, pictures, symbols, or
combination thereof, which provide at least general information
regarding the hazards of the chemicals, and which, in conjunction with
the other information immediately available to employees under the
hazard communication program, will provide employees with the specific
information regarding the physical and health hazards of the hazardous
chemical.
(7) The employer may use signs, placards, process sheets, batch
tickets, operating procedures, or other such written materials in lieu
of affixing labels to individual stationary process containers, as long
as the alternative method identifies the containers to which it is
applicable and conveys the information required by paragraph (f)(6) of
this section to be on a label. The employer shall ensure the written
materials are readily accessible to the employees in their work area
throughout each work shift.
(8) The employer is not required to label portable containers into
which hazardous chemicals are transferred from labeled containers, and
which are intended only for the immediate use of the employee who
performs the transfer. For purposes of this section, drugs which are
dispensed by a pharmacy to a health care provider for direct
administration to a patient are exempted from labeling.
(9) The employer shall not remove or deface existing labels on
incoming containers of hazardous chemicals, unless the container is
immediately marked with the required information.
(10) The employer shall ensure that workplace labels or other forms
of warning are legible, in English, and prominently displayed on the
container, or readily available in the work area throughout each work
shift. Employers having employees who speak other languages may add the
information in their language to the material presented, as long as the
information is presented in English as well.
(11) Chemical manufacturers, importers, distributors, or employers
who become newly aware of any significant information regarding the
hazards of a chemical shall revise the labels for the chemical within
six months of becoming aware of the new information, and shall ensure
that labels on containers of hazardous chemicals shipped after that
time contain the new information. If the chemical is not currently
produced or imported, the chemical manufacturer, importer, distributor,
or employer shall add the information to the label before the chemical
is shipped or introduced into the workplace again.
(g) Safety data sheets. (1) Chemical manufacturers and importers
shall obtain or develop a safety data sheet for each hazardous chemical
they produce or import. Employers shall have a safety data sheet in the
workplace for each hazardous chemical which they use.
(2) The chemical manufacturer or importer preparing the safety data
sheet shall ensure that it is in English (although the employer may
maintain copies in other languages as well), and includes at least the
following section numbers and headings, and associated information
under each heading, in the order listed (See Appendix D to Sec.
1910.1200--Safety Data Sheets, for the specific content of each section
of the safety data sheet):
(i) Section 1, Identification;
(ii) Section 2, Hazard(s) identification;
(iii) Section 3, Composition/information on ingredients;
(iv) Section 4, First-aid measures;
(v) Section 5, Fire-fighting measures;
(vi) Section 6, Accidental release measures;
(vii) Section 7, Handling and storage;
(viii) Section 8, Exposure controls/personal protection;
(ix) Section 9, Physical and chemical properties;
(x) Section 10, Stability and reactivity;
(xi) Section 11, Toxicological information;
(xii) Section 12, Ecological information;
(xiii) Section 13, Disposal considerations;
(xiv) Section 14, Transport information;
(xv) Section 15, Regulatory information; and
(xvi) Section 16, Other information, including date of preparation
or last revision.
Note 1 to paragraph (g)(2): To be consistent with the GHS, an SDS
must also include the headings in paragraphs (g)(2)(xii) through
(g)(2)(xv) in order.
Note 2 to paragraph (g)(2): OSHA will not be enforcing information
requirements in sections 12 through 15, as these areas are not under
its jurisdiction.
(3) If no relevant information is found for any sub-heading within
a section on the safety data sheet, the chemical manufacturer, importer
or employer preparing the safety data sheet shall mark it to indicate
that no applicable information was found.
* * * * *
(5) The chemical manufacturer, importer or employer preparing the
safety data sheet shall ensure that the information provided accurately
reflects the scientific evidence used in making the hazard
classification. If the chemical manufacturer, importer or employer
preparing the safety data sheet becomes newly aware of any significant
information regarding the hazards of a chemical, or ways to protect
against the hazards, this new information shall be added to the safety
data sheet within three months. If the chemical is not
currently being produced or imported, the chemical manufacturer or
importer shall add the information to the safety data sheet before the
chemical is introduced into the workplace again.
* * * * *
(8) The employer shall maintain in the workplace copies of the
required safety data sheets for each hazardous chemical, and shall
ensure that they are readily accessible during each work shift to
employees when they are in their work area(s). (Electronic access and
other alternatives to maintaining paper copies of the safety data
sheets are permitted as long as no barriers to immediate employee
access in each workplace are created by such options.)
* * * * *
(11) Safety data sheets shall also be made readily available, upon
request, to designated representatives, the Assistant Secretary, and
the Director, in accordance with the requirements of Sec.
1910.1020(e).
(h) * * *
(1) Employers shall provide employees with effective information
and training on hazardous chemicals in their work area at the time of
their initial assignment, and whenever a new chemical hazard the
employees have not previously been trained about is introduced into
their work area. Information and training may be designed to cover
categories of hazards (e.g., flammability, carcinogenicity) or specific
chemicals. Chemical-specific information must always be available
through labels and safety data sheets.
* * * * *
(3) * * *
(ii) The physical, health, simple asphyxiation, combustible dust,
and pyrophoric gas hazards, as well as hazards not otherwise
classified, of the chemicals in the work area;
* * * * *
(iv) The details of the hazard communication program developed by
the employer, including an explanation of the labels received on
shipped containers and the workplace labeling system used by their
employer; the safety data sheet, including the order of information and
how employees can obtain and use the appropriate hazard information.
(i) * * *
(1) The chemical manufacturer, importer, or employer may withhold
the specific chemical identity, including the chemical name, other
specific identification of a hazardous chemical, or the exact
percentage (concentration) of the substance in a mixture, from the
safety data sheet, provided that:
* * * * *
(iii) The safety data sheet indicates that the specific chemical
identity and/or percentage of composition is being withheld as a trade
secret; and,
(iv) The specific chemical identity and percentage is made
available to health professionals, employees, and designated
representatives in accordance with the applicable provisions of this
paragraph (i).
(2) Where a treating physician or nurse determines that a medical
emergency exists and the specific chemical identity and/or specific
percentage of composition of a hazardous chemical is necessary for
emergency or first-aid treatment, the chemical manufacturer, importer,
or employer shall immediately disclose the specific chemical identity
or percentage composition of a trade secret chemical to that treating
physician or nurse, regardless of the existence of a written statement
of need or a confidentiality agreement. The chemical manufacturer,
importer, or employer may require a written statement of need and
confidentiality agreement, in accordance with the provisions of
paragraphs (i)(3) and (4) of this section, as soon as circumstances
permit.
(3) In non-emergency situations, a chemical manufacturer, importer,
or employer shall, upon request, disclose a specific chemical identity
or percentage composition, otherwise permitted to be withheld under
paragraph (i)(1) of this section, to a health professional (i.e.
physician, industrial hygienist, toxicologist, epidemiologist, or
occupational health nurse) providing medical or other occupational
health services to exposed employee(s), and to employees or designated
representatives, if:
* * * * *
(iii) The request explains in detail why the disclosure of the
specific chemical identity or percentage composition is essential and
that, in lieu thereof, the disclosure of the following information to
the health professional, employee, or designated representative, would
not satisfy the purposes described in paragraph (i)(3)(ii) of this
section:
* * * * *
(7) If the chemical manufacturer, importer, or employer denies a
written request for disclosure of a specific chemical identity or
percentage composition, the denial must:
* * * * *
(iii) Include evidence to support the claim that the specific
chemical identity or percent of composition is a trade secret;
* * * * *
(v) Explain in detail how alternative information may satisfy the
specific medical or occupational health need without revealing the
trade secret.
* * * * *
(9) * * *
(i) The chemical manufacturer, importer, or employer has supported
the claim that the specific chemical identity or percentage composition
is a trade secret;
* * * * *
(10) * * *
(i) If OSHA determines that the specific chemical identity or
percentage composition requested under paragraph (i)(3) of this section
is not a "bona fide" trade secret, or that it is a trade secret, but
the requesting health professional, employee, or designated
representative has a legitimate medical or occupational health need for
the information, has executed a written confidentiality agreement, and
has shown adequate means to protect the confidentiality of the
information, the chemical manufacturer, importer, or employer will be
subject to citation by OSHA.
(ii) If a chemical manufacturer, importer, or employer demonstrates
to OSHA that the execution of a confidentiality agreement would not
provide sufficient protection against the potential harm from the
unauthorized disclosure of a trade secret, the Assistant Secretary may
issue such orders or impose such additional limitations or conditions
upon the disclosure of the requested chemical information as may be
appropriate to assure that the occupational health services are
provided without an undue risk of harm to the chemical manufacturer,
importer, or employer.
(11) If a citation for a failure to release trade secret
information is contested by the chemical manufacturer, importer, or
employer, the matter will be adjudicated before the Occupational Safety
and Health Review Commission in accordance with the Act's enforcement
scheme and the applicable Commission rules of procedure. In accordance
with the Commission rules, when a chemical manufacturer, importer, or
employer continues to withhold the information during the contest, the
Administrative Law Judge may review the citation and supporting
documentation "in camera" or issue appropriate orders to protect the
confidentiality of such matters.
* * * * *
(j) Effective dates. (1) Employers shall train employees regarding
the new label elements and safety data sheets format by December 1,
2013.
(2) Chemical manufacturers, importers, distributors, and employers
shall be in compliance with all modified provisions of this section no
later than June 1, 2015, except:
(i) After December 1, 2015, the distributor shall not ship
containers labeled by the chemical manufacturer or importer unless the
label has been modified to comply with paragraph (f)(1) of this
section.
(ii) All employers shall, as necessary, update any alternative
workplace labeling used under paragraph (f)(6) of this section, update
the hazard communication program required by paragraph (h)(1), and
provide any additional employee training in accordance with paragraph
(h)(3) for newly identified physical or health hazards no later than
June 1, 2016.
(3) Chemical manufacturers, importers, distributors, and employers
may comply with either Sec. 1910.1200 revised as of October 1, 2011,
or the current version of this standard, or both during the transition
period.
Appendix A to Sec. 1910.1200--Health Hazard Criteria (Mandatory)
A.0 GENERAL CLASSIFICATION CONSIDERATIONS
A.0.1 Classification
A.0.1.1 The term "hazard classification" is used to indicate
that only the intrinsic hazardous properties of chemicals are
considered. Hazard classification incorporates three steps:
(a) Identification of relevant data regarding the hazards of a
chemical;
(b) Subsequent review of those data to ascertain the hazards
associated with the chemical;
(c) Determination of whether the chemical will be classified as
hazardous and the degree of hazard.
A.0.1.2 For many hazard classes, the criteria are semi-
quantitative or qualitative and expert judgment is required to
interpret the data for classification purposes.
A.0.2 Available Data, Test Methods and Test Data Quality
A.0.2.1 There is no requirement for testing chemicals.
A.0.2.2 The criteria for determining health hazards are test
method neutral, i.e., they do not specify particular test methods,
as long as the methods are scientifically validated.
A.0.2.3 The term "scientifically validated" refers to the
process by which the reliability and the relevance of a procedure
are established for a particular purpose. Any test that determines
hazardous properties, which is conducted according to recognized
scientific principles, can be used for purposes of a hazard
determination for health hazards. Test conditions need to be
standardized so that the results are reproducible with a given
substance, and the standardized test yields "valid" data for
defining the hazard class of concern.
A.0.2.4 Existing test data are acceptable for classifying
chemicals, although expert judgment also may be needed for
classification purposes.
A.0.2.5 The effect of a chemical on biological systems is
influenced, by the physico-chemical properties of the substance and/
or ingredients of the mixture and the way in which ingredient
substances are biologically available. A chemical need not be
classified when it can be shown by conclusive experimental data from
scientifically validated test methods that the chemical is not
biologically available.
A.0.2.6 For classification purposes, epidemiological data and
experience on the effects of chemicals on humans (e.g., occupational
data, data from accident databases) shall be taken into account in
the evaluation of human health hazards of a chemical.
A.0.3 Classification Based on Weight of Evidence
A.0.3.1 For some hazard classes, classification results directly
when the data satisfy the criteria. For others, classification of a
chemical shall be determined on the basis of the total weight of
evidence using expert judgment. This means that all available
information bearing on the classification of hazard shall be
considered together, including the results of valid in vitro tests,
relevant animal data, and human experience such as epidemiological
and clinical studies and well-documented case reports and
observations.
A.0.3.2 The quality and consistency of the data shall be
considered. Information on chemicals related to the material being
classified shall be considered as appropriate, as well as site of
action and mechanism or mode of action study results. Both positive
and negative results shall be considered together in a single
weight-of-evidence determination.
A.0.3.3 Positive effects which are consistent with the criteria
for classification, whether seen in humans or animals, shall
normally justify classification. Where evidence is available from
both humans and animals and there is a conflict between the
findings, the quality and reliability of the evidence from both
sources shall be evaluated in order to resolve the question of
classification. Reliable, good quality human data shall generally
have precedence over other data. However, even well-designed and
conducted epidemiological studies may lack a sufficient number of
subjects to detect relatively rare but still significant effects, or
to assess potentially confounding factors. Therefore, positive
results from well-conducted animal studies are not necessarily
negated by the lack of positive human experience but require an
assessment of the robustness, quality and statistical power of both
the human and animal data.
A.0.3.4 Route of exposure, mechanistic information, and
metabolism studies are pertinent to determining the relevance of an
effect in humans. When such information raises doubt about relevance
in humans, a lower classification may be warranted. When there is
scientific evidence demonstrating that the mechanism or mode of
action is not relevant to humans, the chemical should not be
classified.
A.0.3.5 Both positive and negative results are considered
together in the weight of evidence determination. However, a single
positive study performed according to good scientific principles and
with statistically and biologically significant positive results may
justify classification.
A.0.4 Considerations for the Classification of Mixtures
A.0.4.1 For most hazard classes, the recommended process of
classification of mixtures is based on the following sequence:
(a) Where test data are available for the complete mixture, the
classification of the mixture will always be based on those data;
(b) Where test data are not available for the mixture itself,
the bridging principles designated in each health hazard chapter of
this appendix shall be considered for classification of the mixture;
(c) If test data are not available for the mixture itself, and
the available information is not sufficient to allow application of
the above-mentioned bridging principles, then the method(s)
described in each chapter for estimating the hazards based on the
information known will be applied to classify the mixture (e.g.,
application of cut-off values/concentration limits).
A.0.4.2 An exception to the above order or precedence is made
for Carcinogenicity, Germ Cell Mutagenicity, and Reproductive
Toxicity. For these three hazard classes, mixtures shall be
classified based upon information on the ingredient substances,
unless on a case-by-case basis, justification can be provided for
classifying based upon the mixture as a whole. See chapters A.5,
A.6, and A.7 for further information on case-by-case bases.
A.0.4.3 Use of cut-off values/concentration limits.
A.0.4.3.1 When classifying an untested mixture based on the
hazards of its ingredients, cut-off values/concentration limits for
the classified ingredients of the mixture are used for several
hazard classes. While the adopted cut-off values/concentration
limits adequately identify the hazard for most mixtures, there may
be some that contain hazardous ingredients at lower concentrations
than the specified cut-off values/concentration limits that still
pose an identifiable hazard. There may also be cases where the cut-
off value/concentration limit is considerably lower than the
established non-hazardous level for an ingredient.
A.0.4.3.2 If the classifier has information that the hazard of
an ingredient will be evident (i.e., it presents a health risk)
below the specified cut-off value/concentration limit, the mixture
containing that ingredient shall be classified accordingly.
A.0.4.3.3 In exceptional cases, conclusive data may demonstrate
that the hazard of an ingredient will not be evident (i.e., it does
not present a health risk) when present at a level above the
specified cut-off value/concentration limit(s). In these cases the
mixture may be classified according to those data. The data must
exclude the possibility that the ingredient will behave in the
mixture in a manner that would increase the hazard over that of the
pure substance. Furthermore, the mixture must not contain
ingredients that would affect that determination.
A.0.4.4 Synergistic or antagonistic effects.
When performing an assessment in accordance with these
requirements, the evaluator must take into account all available
information about the potential occurrence of synergistic effects
among the ingredients of the mixture. Lowering classification of a
mixture to a less hazardous category on the basis of antagonistic
effects may be done only if the determination is supported by
sufficient data.
A.0.5 Bridging Principles for the Classification of Mixtures Where Test
Data Are Not Available for the Complete Mixture
A.0.5.1 Where the mixture itself has not been tested to
determine its toxicity, but there are sufficient data on both the
individual ingredients and similar tested mixtures to adequately
characterize the hazards of the mixture, these data shall be used in
accordance with the following bridging principles, subject to any
specific provisions for mixtures for each hazard class. These
principles ensure that the classification process uses the available
data to the greatest extent possible in characterizing the hazards
of the mixture.
A.0.5.1.1 Dilution.
For mixtures classified in accordance with A.1 through A.10 of
this Appendix, if a tested mixture is diluted with a diluent that
has an equivalent or lower toxicity classification than the least
toxic original ingredient, and which is not expected to affect the
toxicity of other ingredients, then:
(a) The new diluted mixture shall be classified as equivalent to
the original tested mixture; or
(b) For classification of acute toxicity in accordance with A.1
of this Appendix, paragraph A.1.3.6 (the additivity formula) shall
be applied.
A.0.5.1.2 Batching.
For mixtures classified in accordance with A.1 through A.10 of
this Appendix, the toxicity of a tested production batch of a
mixture can be assumed to be substantially equivalent to that of
another untested production batch of the same mixture, when produced
by or under the control of the same chemical manufacturer, unless
there is reason to believe there is significant variation such that
the toxicity of the untested batch has changed. If the latter
occurs, a new classification is necessary.
A.0.5.1.3 Concentration of mixtures.
For mixtures classified in accordance with A.1, A.2, A.3, A.8,
A.9, or A.10 of this Appendix, if a tested mixture is classified in
Category 1, and the concentration of the ingredients of the tested
mixture that are in Category 1 is increased, the resulting untested
mixture shall be classified in Category 1.
A.0.5.1.4 Interpolation within one toxicity category.
For mixtures classified in accordance with A.1, A.2, A.3, A.8,
A.9, or A.10 of this Appendix, for three mixtures (A, B and C) with
identical ingredients, where mixtures A and B have been tested and
are in the same toxicity category, and where untested mixture C has
the same toxicologically active ingredients as mixtures A and B but
has concentrations of toxicologically active ingredients
intermediate to the concentrations in mixtures A and B, then mixture
C is assumed to be in the same toxicity category as A and B.
A.0.5.1.5 Substantially similar mixtures.
For mixtures classified in accordance with A.1 through A.10 of
this Appendix, given the following set of conditions:
(a) Where there are two mixtures:
(i) A + B;
(ii) C + B;
(b) The concentration of ingredient B is essentially the same in
both mixtures;
(c) The concentration of ingredient A in mixture (i) equals that
of ingredient C in mixture (ii);
(d) And data on toxicity for A and C are available and
substantially equivalent; i.e., they are in the same hazard category
and are not expected to affect the toxicity of B; then
If mixture (i) or (ii) is already classified based on test data,
the other mixture can be assigned the same hazard category.
A.0.5.1.6 Aerosols.
For mixtures classified in accordance with A.1, A.2, A.3, A.4,
A.8, or A.9 of this Appendix, an aerosol form of a mixture shall be
classified in the same hazard category as the tested, non-
aerosolized form of the mixture, provided the added propellant does
not affect the toxicity of the mixture when spraying.
A.1 ACUTE TOXICITY
A.1.1 Definition
Acute toxicity refers to those adverse effects occurring
following oral or dermal administration of a single dose of a
substance, or multiple doses given within 24 hours, or an inhalation
exposure of 4 hours.
A.1.2 Classification Criteria for Substances
A.1.2.1 Substances can be allocated to one of four toxicity
categories based on acute toxicity by the oral, dermal or inhalation
route according to the numeric cut-off criteria as shown in Table
A.1.1. Acute toxicity values are expressed as (approximate) LD50
(oral, dermal) or LC50 (inhalation) values or as acute toxicity
estimates (ATE). See the footnotes following Table A.1.1 for further
explanation on the application of these values.
Table A.1.1--Acute Toxicity Hazard Categories and Acute Toxicity Estimate (ATE) Values Defining the Respective
Categories
----------------------------------------------------------------------------------------------------------------
Exposure route Category 1 Category 2 Category 3 Category 4
----------------------------------------------------------------------------------------------------------------
Oral (mg/kg bodyweight)
see: Note (a), Note (b)...... <=5 >5 and <=50........ >50 and <=300...... >300 and <=2000.
Dermal (mg/kg bodyweight)
see: Note (a), Note (b)...... <=5 >50 and <=200...... >200 and <=1000.... >1000 and <=2000.
Inhalation--Gases (ppmV)
see: Note (a), Note (b), Note <=100 >100 and <=500..... >500 and <=2500.... >2500 and <=20000.
(c).
Inhalation--Vapors (mg/l)
see: Note (a), Note (b), Note <=0.5 >0.5 and <=2.0..... >2.0 and <=10.0.... >10.0 and <=20.0.
(c), Note (d).
Inhalation--Dusts and Mists (mg/
l)
see: Note (a), Note (b), Note <=0.05 >0.05 and <=0.5.... >0.5 and <=1.0..... >1.0 and <=5.0.
(c).
----------------------------------------------------------------------------------------------------------------
Note: Gas concentrations are expressed in parts per million per volume (ppmV).
Notes to Table A.1.1:
(a) The acute toxicity estimate (ATE) for the classification of a substance is derived using the LD50/LC50
Stewardwhere available;
(b) The acute toxicity estimate (ATE) for the classification of a substance or ingredient in a mixture is
derived using:
(i) the LD50/LC50 where available. Otherwise,
(ii) the appropriate conversion value from Table 1.2 that relates to the results of a range test, or
(iii) the appropriate conversion value from Table 1.2 that relates to a classification category;
(c) Inhalation cut-off values in the table are based on 4 hour testing exposures. Conversion of existing
inhalation toxicity data which has been generated according to 1 hour exposure is achieved by dividing by a
factor of 2 for gases and vapors and 4 for dusts and mists;
(d) For some substances the test atmosphere will be a vapor which consists of a combination of liquid and
gaseous phases. For other substances the test atmosphere may consist of a vapor which is nearly all the
gaseous phase. In these latter cases, classification is based on ppmV as follows: Category 1 (100 ppmV),
Category 2 (500 ppmV), Category 3 (2500 ppmV), Category 4 (20000 ppmV).
The terms "dust", "mist" and "vapor" are defined as follows:
(i) Dust: solid particles of a substance or mixture suspended in a gas (usually air);
(ii) Mist: liquid droplets of a substance or mixture suspended in a gas (usually air);
(iii) Vapor: the gaseous form of a substance or mixture released from its liquid or solid state.
A.1.2.3 The preferred test species for evaluation of acute
toxicity by the oral and inhalation routes is the rat, while the rat
or rabbit are preferred for evaluation of acute dermal toxicity.
Test data already generated for the classification of chemicals
under existing systems should be accepted when reclassifying these
chemicals under the harmonized system. When experimental data for
acute toxicity are available in several animal species, scientific
judgment should be used in selecting the most appropriate
LD50 value from among scientifically validated tests.
A.1.3 Classification Criteria for Mixtures
A.1.3.1 The approach to classification of mixtures for acute
toxicity is tiered, and is dependent upon the amount of information
available for the mixture itself and for its ingredients. The flow
chart of Figure A.1.1 indicates the process that must be followed:
[GRAPHIC] [TIFF OMITTED] TR26MR12.060
A.1.3.2 Classification of mixtures for acute toxicity may be
carried out for each route of exposure, but is only required for one
route of exposure as long as this route is followed (estimated or
tested) for all ingredients and there is no relevant evidence to
suggest acute toxicity by multiple routes. When there is relevant
evidence of acute toxicity by multiple routes of exposure,
classification is to be conducted for all appropriate routes of
exposure. All available information shall be considered. The
pictogram and signal word used shall reflect the most severe hazard
category; and all relevant hazard statements shall be used.
A.1.3.3 For purposes of classifying the hazards of mixtures in
the tiered approach:
(a) The "relevant ingredients" of a mixture are those which
are present in concentrations >=1% (weight/weight for solids,
liquids, dusts, mists and vapors and volume/volume for gases). If
there is reason to suspect that an ingredient present at a
concentration <1% will affect classification of the mixture for
acute toxicity, that ingredient shall also be considered relevant.
Consideration of ingredients present at a concentration <1% is
particularly important when classifying untested mixtures which
contain ingredients that are classified in Category 1 and Category
2;
(b) Where a classified mixture is used as an ingredient of
another mixture, the actual or derived acute toxicity estimate (ATE)
for that mixture is used when calculating the classification of the
new mixture using the formulas in A.1.3.6.1 and A.1.3.6.2.4.
(c) If the converted acute toxicity point estimates for all
ingredients of a mixture are within the same category, then the
mixture should be classified in that category.
(d) When only range data (or acute toxicity hazard category
information) are available for ingredients in a mixture, they may be
converted to point estimates in accordance with Table A.1.2 when
calculating the classification of the new mixture using the formulas
in A.1.3.6.1 and A.1.3.6.2.4.
A.1.3.4 Classification of Mixtures Where Acute Toxicity Test Data Are
Available for the Complete Mixture
Where the mixture itself has been tested to determine its acute
toxicity, it is classified according to the same criteria as those
used for substances, presented in Table A.1.1. If test data for the
mixture are not available, the procedures presented below must be
followed.
A.1.3.5 Classification of Mixtures Where Acute Toxicity Test Data Are
Not Available for the Complete Mixture: Bridging Principles
A.1.3.5.1 Where the mixture itself has not been tested to
determine its acute toxicity, but there are sufficient data on both
the individual ingredients and similar tested mixtures to adequately
characterize the hazards of the mixture, these data will be used in
accordance with the following bridging principles as found in
paragraph A.0.5 of this Appendix: Dilution, Batching, Concentration
of mixtures, Interpolation within one toxicity category,
Substantially similar mixtures, and Aerosols.
A.1.3.6 Classification of Mixtures Based on Ingredients of the Mixture
(Additivity Formula)
A.1.3.6.1 Data available for all ingredients.
The acute toxicity estimate (ATE) of ingredients is considered
as follows:
(a) Include ingredients with a known acute toxicity, which fall
into any of the acute toxicity categories, or have an oral or dermal
LD50 greater than 2000 but less than or equal to 5000 mg/
kg body weight (or the equivalent dose for inhalation);
(b) Ignore ingredients that are presumed not acutely toxic
(e.g., water, sugar);
(c) Ignore ingredients if the data available are from a limit
dose test (at the upper threshold for Category 4 for the appropriate
route of exposure as provided in Table A.1.1) and do not show acute
toxicity.
Ingredients that fall within the scope of this paragraph are
considered to be ingredients with a known acute toxicity estimate
(ATE). See note (b) to Table A.1.1 and paragraph A.1.3.3 for
appropriate application of available data to the equation below, and
paragraph A.1.3.6.2.4.
The ATE of the mixture is determined by calculation from the ATE
values for all relevant ingredients according to the following
formula below for oral, dermal or inhalation toxicity:
[GRAPHIC] [TIFF OMITTED] TR26MR12.061
Where:
Ci = concentration of ingredient i
n ingredients and i is running from 1 to n
ATEi = acute toxicity estimate of ingredient i.
A.1.3.6.2 Data are not available for one or more ingredients of
the mixture.
A.1.3.6.2.1 Where an ATE is not available for an individual
ingredient of the mixture, but available information provides a
derived conversion value, the formula in A.1.3.6.1 may be applied.
This information may include evaluation of:
(a) Extrapolation between oral, dermal and inhalation acute
toxicity estimates. Such an evaluation requires appropriate
pharmacodynamic and pharmacokinetic data;
(b) Evidence from human exposure that indicates toxic effects
but does not provide lethal dose data;
(c) Evidence from any other toxicity tests/assays available on
the substance that indicates toxic acute effects but does not
necessarily provide lethal dose data; or
(d) Data from closely analogous substances using structure/
activity relationships.
A.1.3.6.2.2 This approach requires substantial supplemental
technical information, and a highly trained and experienced expert,
to reliably estimate acute toxicity. If sufficient information is
not available to reliably estimate acute toxicity, proceed to the
provisions of A.1.3.6.2.3.
A.1.3.6.2.3 In the event that an ingredient with unknown acute
toxicity is used in a mixture at a concentration >=1%, and the
mixture has not been classified based on testing of the mixture as a
whole, the mixture cannot be attributed a definitive acute toxicity
estimate. In this situation the mixture is classified based on the
known ingredients only. (Note: A statement that x percent of the
mixture consists of ingredient(s) of unknown toxicity is required on
the label and safety data sheet in such cases; see Appendix C to
this section, Allocation of Label Elements and Appendix D to this
section, Safety Data Sheets.)
Where an ingredient with unknown acute toxicity is used in a
mixture at a concentration >=1%, and the mixture is not classified
based on testing of the mixture as a whole, a statement that X% of
the mixture consists of ingredient(s) of unknown acute toxicity is
required on the label and safety data sheet in such cases; see
Appendix C to this section, Allocation of Label Elements and
Appendix D to this section, Safety Data Sheets.)
A.1.3.6.2.4 If the total concentration of the relevant
ingredient(s) with unknown acute toxicity is <=10% then the formula
presented in A.1.3.6.1 must be used. If the total concentration of
the relevant ingredient(s) with unknown acute toxicity is >10%, the
formula presented in A.1.3.6.1 is corrected to adjust for the
percentage of the unknown ingredient(s) as follows:
[GRAPHIC] [TIFF OMITTED] TR26MR12.062
Table A.1.2--Conversion From Experimentally Obtained Acute Toxicity
Range Values (or Acute Toxicity Hazard Categories) to Acute Toxicity
Point Estimates for Use in the Formulas for the Classification of
Mixtures
------------------------------------------------------------------------
Classification category
or experimentally Converted
Exposure routes obtained acute acute toxicity
toxicity range estimate point estimate
------------------------------------------------------------------------
Oral (mg/kg bodyweight )....... 0 =1 of 3 animals
Category 1: corrosive Corrosive sub- -------------------------------------------------
categories Exposure Observation
----------------------------------------------------------------------------------------------------------------
1A..................... <=3 min................ <=1 h.
1B..................... >3 min <=1 h........... <=14 days.
1C..................... >1 h <=4 h............. <=14 days.
----------------------------------------------------------------------------------------------------------------
A.2.2.2 Irritation
A.2.2.2.1 A single irritant category (Category 2) is presented
in the Table A.2.2. The major criterion for the irritant category is
that at least 2 tested animals have a mean score of >=2.3 <=4.0.
Table A.2.2--Skin Irritation Category
------------------------------------------------------------------------
Criteria
------------------------------------------------------------------------
Irritant (Category 2)........ (1) Mean value of >=2.3 <=4.0 for
erythema/eschar or for edema in at least
2 of 3 tested animals from gradings at
24, 48 and 72 hours after patch removal
or, if reactions are delayed, from
grades on 3 consecutive days after the
onset of skin reactions; or
(2) Inflammation that persists to the end
of the observation period normally 14
days in at least 2 animals, particularly
taking into account alopecia (limited
area), hyperkeratosis, hyperplasia, and
scaling; or
(3) In some cases where there is
pronounced variability of response among
animals, with very definite positive
effects related to chemical exposure in
a single animal but less than the
criteria above.
------------------------------------------------------------------------
A.2.2.2.2 Animal irritant responses within a test can be quite
variable, as they are with corrosion. A separate irritant criterion
accommodates cases when there is a significant irritant response but
less than the mean score criterion for a positive test. For example,
a substance might be designated as an irritant if at least 1 of 3
tested animals shows a very elevated mean score throughout the
study, including lesions persisting at the end of an observation
period of normally 14 days. Other responses could also fulfil this
criterion. However, it should be ascertained that the responses are
the result of chemical exposure. Addition of this criterion
increases the sensitivity of the classification system.
A.2.2.2.3 Reversibility of skin lesions is another consideration
in evaluating irritant responses. When inflammation persists to the
end of the observation period in 2 or more test animals, taking into
consideration alopecia (limited area), hyperkeratosis, hyperplasia
and scaling, then a chemical should be considered to be an irritant.
A.2.3 Classification Criteria for Substances Using Other Data Elements
A.2.3.1 Existing human and animal data including information
from single or repeated exposure should be the first line of
analysis, as they give information directly relevant to effects on
the skin. If a substance is highly toxic by the dermal route, a skin
corrosion/irritation study may not be practicable since the amount
of test substance to be applied would considerably exceed the toxic
dose and, consequently, would result in the death of the animals.
When observations are made of skin corrosion/irritation in acute
toxicity studies and are observed up through the limit dose, these
data may be used for classification provided that the dilutions used
and species tested are equivalent. In vitro alternatives that have
been scientifically validated shall be used to make classification
decisions. Solid substances (powders) may become corrosive or
irritant when moistened or in contact with moist skin or mucous
membranes. Likewise, pH extremes like <=2 and >=11.5 may indicate
skin effects, especially when associated with significant buffering
capacity. Generally, such substances are expected to produce
significant effects on the skin. In the absence of any other
information, a substance is considered corrosive (Skin Category 1)
if it has a pH <=2 or a pH >=11.5. However, if consideration of
alkali/acid reserve suggests the substance or mixture may not be
corrosive despite the low or high pH value, then further evaluation
may be necessary. In some cases enough information may be available
from structurally related compounds to make classification
decisions.
A.2.3.2 A tiered approach to the evaluation of initial
information shall be used (Figure A.2.1) recognizing that all
elements may not be relevant in certain cases.
A.2.3.3 The tiered approach explains how to organize information
on a substance and to make a weight-of-evidence decision about
hazard assessment and hazard classification.
A.2.3.4 All the above information that is available on a
substance shall be evaluated. Although information might be gained
from the evaluation of single parameters within a
tier, there is merit in considering the totality of existing
information and making an overall weight of evidence determination.
This is especially true when there is information available on some
but not all parameters. Emphasis shall be placed upon existing human
experience and data, followed by animal experience and testing data,
followed by other sources of information, but case-by-case
determinations are necessary.
BILLING CODE 4510-26-P
[GRAPHIC] [TIFF OMITTED] TR26MR12.063
[GRAPHIC] [TIFF OMITTED] TR26MR12.064
BILLING CODE 4510-26-C
A.2.4 Classification Criteria for Mixtures
A.2.4.1 Classification of Mixtures When Data Are Available for the
Complete Mixture
A.2.4.1.1 The mixture shall be classified using the criteria for
substances (See A.2.3).
A.2.4.2 Classification of Mixtures When Data Are Not Available for the
Complete Mixture: Bridging Principles
A.2.4.2.1 Where the mixture itself has not been tested to
determine its skin corrosion/irritation, but there are sufficient
data on both the individual ingredients and similar tested mixtures
to adequately characterize the hazards of the mixture, these data
will be used in accordance with the following bridging principles,
as found in paragraph A.0.5 of this Appendix: Dilution, Batching,
Concentration of mixtures, Interpolation within one toxicity
category, Substantially similar mixtures, and Aerosols.
A.2.4.3 Classification of Mixtures When Data Are Available for All
Ingredients or Only for Some Ingredients of the Mixture
A.2.4.3.1 For purposes of classifying the skin corrosion/
irritation hazards of mixtures in the tiered approach:
The "relevant ingredients" of a mixture are those which are
present in concentrations >1% (weight/weight for solids, liquids,
dusts, mists and vapors and volume/volume for gases.) If the
classifier has reason to suspect that an ingredient present at a
concentration <1% will affect classification of the mixture for skin
corrosion/irritation, that ingredient shall also be considered
relevant.
A.2.4.3.2 In general, the approach to classification of mixtures
as irritant or corrosive to skin when data are available on the
ingredients, but not on the mixture as a whole, is based on the
theory of additivity, such that each corrosive or irritant
ingredient contributes to the overall irritant or corrosive
properties of the mixture in proportion to its potency and
concentration. A weighting factor of 10 is used for corrosive
ingredients when they are present at a concentration below the
concentration limit for classification with Category 1, but are at a
concentration that will contribute to the classification of the
mixture as an irritant. The mixture is classified as corrosive or
irritant when the sum of the concentrations of such ingredients
exceeds a cut-off value/concentration limit.
A.2.4.3.3 Table A.2.3 below provides the cut-off value/
concentration limits to be used to determine if the mixture is
considered to be an irritant or a corrosive to the skin.
A.2.4.3.4 Particular care shall be taken when classifying
certain types of chemicals such as acids and bases, inorganic salts,
aldehydes, phenols, and surfactants. The approach explained in
A.2.4.3.1 and A.2.4.3.2 might not work given that many of such
substances are corrosive or irritant at concentrations <1%. For
mixtures containing strong acids or bases the pH should be used as
classification criteria since pH will be a better indicator of
corrosion than the concentration limits of Table A.2.3. A mixture
containing corrosive or irritant ingredients that cannot be
classified based on the additivity approach shown in Table A.2.3,
due to chemical characteristics that make this approach unworkable,
should be classified as Skin Category 1 if it contains >=1% of a
corrosive ingredient and as Skin Category 2 when it contains >=3% of
an irritant ingredient. Classification of mixtures with ingredients
for which the approach in Table A.2.3 does not apply is summarized
in Table A.2.4 below.
A.2.4.3.5 On occasion, reliable data may show that the skin
corrosion/irritation of an ingredient will not be evident when
present at a level above the generic concentration cut-off values
mentioned in Tables A.2.3 and A.2.4. In these cases the mixture
could be classified according to those data (See Use of cut-off
values/concentration limits, paragraph A.0.4.3 of this Appendix).
A.2.4.3.6 If there are data showing that (an) ingredient(s) may
be corrosive or irritant at a concentration of <1% (corrosive) or
<3% (irritant), the mixture shall be classified accordingly (See Use
of cut-off values/concentration limits, paragraph A.0.4.3 of this
Appendix).
Table A.2.3--Concentration of Ingredients of a Mixture Classified as
Skin Category 1 or 2 That Would Trigger Classification of the Mixture as
Hazardous to Skin
[Category 1 or 2]
------------------------------------------------------------------------
Concentration triggering classification
of a mixture as:
Sum of ingredients classified ------------------------------------------
as: Skin corrosive Skin irritant
------------------------------------------
Category 1 Category 2
------------------------------------------------------------------------
Skin Category 1.............. >=5% >=1% but <5%.
Skin Category 2.............. .................. >=10%.
(10 x Skin Category 1) + Skin .................. >=10%.
Category 2.
------------------------------------------------------------------------
Table A.2.4--Concentration of Ingredients of a Mixture for Which the
Additivity Approach Does Not Apply, That Would Trigger Classification of
the Mixture as Hazardous to Skin
------------------------------------------------------------------------
Mixture classified
Ingredient: Concentration: as: Skin
------------------------------------------------------------------------
Acid with pH <=2............. >=1% Category 1.
Base with pH >=11.5.......... >=1% Category 1.
Other corrosive (Category 1) >=1% Category 1.
ingredients for which
additivity does not apply.
Other irritant (Category 2) >=3% Category 2.
ingredients for which
additivity does not apply,
including acids and bases.
------------------------------------------------------------------------
A.3 SERIOUS EYE DAMAGE/EYE IRRITATION
A.3.1 Definitions and General Considerations
A.3.1.1 Serious eye damage is the production of tissue damage in
the eye, or serious physical decay of vision, following application
of a test substance to the anterior surface of the eye, which is not
fully reversible within 21 days of application.
Eye irritation is the production of changes in the eye following
the application of test substance to the anterior surface of the
eye, which are fully reversible within 21 days of application.
A.3.1.2 Serious eye damage/eye irritation shall be classified
using a tiered approach as detailed in Figure A.3.1. Emphasis shall
be placed upon existing human data (See A.0.2.6), followed by animal
data, followed by other sources of information. Classification
results directly when the data satisfy the criteria in this section.
In case the criteria cannot be directly applied, classification of a
substance or a mixture is made on the basis of the total weight of
evidence (See A.0.3.1). This means that all available information
bearing on the determination of serious eye damage/eye irritation is
considered together, including the results of appropriate
scientifically validated in vitro tests, relevant animal data, and
human data such as epidemiological and clinical studies and well-
documented case reports and observations.
A.3.2 Classification Criteria for Substances Using Animal Test Data
A.3.2.1 Irreversible effects on the eye/serious damage to eyes
(Category 1).
A single hazard category is provided in Table A.3.1, for
substances that have the potential to seriously damage the eyes.
Category 1, irreversible effects on the eye, includes the criteria
listed below. These observations include animals with grade 4 cornea
lesions and other severe reactions (e.g. destruction of cornea)
observed at any time during the test, as well as persistent corneal
opacity, discoloration of the cornea by a dye substance, adhesion,
pannus, and interference with the function of the iris or other
effects that impair sight. In this context, persistent lesions are
considered those which are not fully reversible within an
observation period of normally 21 days. Category 1 also contains
substances fulfilling the criteria of corneal opacity >=3 and/or
iritis >1.5 detected in a Draize eye test with rabbits, because
severe lesions like these usually do not reverse within a 21-day
observation period.
Table A.3.1--Irreversible Eye Effects
------------------------------------------------------------------------
-------------------------------------------------------------------------
A substance is classified as Serious Eye Damage Category 1 (irreversible
effects on the eye) when it produces:
(a) at least in one tested animal, effects on the cornea, iris or
conjunctiva that are not expected to reverse or have not fully
reversed within an observation period of normally 21 days; and/or
(b) at least in 2 of 3 tested animals, a positive response of:
(i) corneal opacity >=3; and/or
(ii) iritis >1.5;
calculated as the mean scores following grading at 24, 48 and 72
hours after instillation of the substance.
------------------------------------------------------------------------
A.3.2.2 Reversible effects on the eye (Category 2).
A single category is provided in Table A.3.2 for substances that
have the potential to induce reversible eye irritation.
Table A.3.2--Reversible Eye Effects
------------------------------------------------------------------------
-------------------------------------------------------------------------
A substance is classified as Eye irritant Category 2A (irritating to
eyes) when it produces in at least in 2 of 3 tested animals a positive
response of:
(i) corneal opacity >=1; and/or
(ii) iritis >=1; and/or
(iii) conjunctival redness >=2; and/or
(iv) conjunctival edema (chemosis) >=2
calculated as the mean scores following grading at 24, 48 and 72
hours after instillation of the substance, and which fully reverses
within an observation period of normally 21 days.
An eye irritant is considered mildly irritating to eyes (Category 2B)
when the effects listed above are fully reversible within 7 days of
observation.
------------------------------------------------------------------------
A.3.2.3 For those chemicals where there is pronounced
variability among animal responses, this information may be taken
into account in determining the classification.
A.3.3 Classification Criteria for Substances Using Other Data Elements
A.3.3.1 Existing human and animal data should be the first line
of analysis, as they give information directly relevant to effects
on the eye. Possible skin corrosion shall be evaluated prior to
consideration of serious eye damage/eye irritation in order to avoid
testing for local effects on eyes with skin corrosive substances. In
vitro alternatives that have been scientifically validated and
accepted shall be used to make classification decisions. Likewise,
pH extremes like <=2 and >=11.5, may indicate serious eye damage,
especially when associated with significant buffering capacity.
Generally, such substances are expected to produce significant
effects on the eyes. In the absence of any other information, a
mixture/substance is considered to cause serious eye damage (Eye
Category 1) if it has a pH <=2 or >=11.5. However, if consideration
of acid/alkaline reserve suggests the substance may not have the
potential to cause serious eye damage despite the low or high pH
value, then further evaluation may be necessary. In some cases
enough information may be available from structurally related
compounds to make classification decisions.
A.3.3.2 A tiered approach to the evaluation of initial
information shall be used where applicable, recognizing that all
elements may not be relevant in certain cases (Figure A.3.1).
A.3.3.3 The tiered approach explains how to organize existing
information on a substance and to make a weight-of-evidence
decision, where appropriate, about hazard assessment and hazard
classification.
A.3.3.4 All the above information that is available on a
substance shall be evaluated. Although information might be gained
from the evaluation of single parameters within a tier,
consideration should be given to the totality of existing
information and making an overall weight-of-evidence determination.
This is especially true when there is conflict in information
available on some parameters.
BILLING CODE 4510-26-P
[GRAPHIC] [TIFF OMITTED] TR26MR12.065
[GRAPHIC] [TIFF OMITTED] TR26MR12.066
BILLING CODE 4510-26-C
A.3.4 Classification Criteria for Mixtures
A.3.4.1 Classification of Mixtures When Data Are Available for the
Complete Mixture
A.3.4.1.1 The mixture will be classified using the criteria for
substances.
A.3.4.1.2 Unlike other hazard classes, there are alternative
tests available for skin corrosivity of certain types of chemicals
that can give an accurate result for classification purposes, as
well as being simple and relatively inexpensive to perform. When
considering testing of the mixture, chemical manufacturers are
encouraged to use a tiered weight of evidence strategy as included
in the criteria for classification of substances for skin corrosion
and serious eye damage and eye irritation to help ensure an accurate
classification, as well as avoid unnecessary animal testing. In the
absence of any other information, a mixture is considered to cause
serious eye damage (Eye Category 1) if it has a pH <=2 or >=11.5.
However, if consideration of acid/alkaline reserve suggests the
substance or mixture may not have the potential to cause serious eye
damage despite the low or high pH value, then further evaluation may
be necessary.
A.3.4.2 Classification of Mixtures When Data Are Not Available for the
Complete Mixture: Bridging Principles
A.3.4.2.1 Where the mixture itself has not been tested to
determine its skin corrosivity or potential to cause serious eye
damage or eye irritation, but there are sufficient data on both the
individual ingredients and similar tested mixtures to adequately
characterize the hazards of the mixture, these data will be used in
accordance with the following bridging principles, as found in
paragraph A.0.5 of this Appendix: Dilution, Batching, Concentration
of mixtures, Interpolation within one toxicity category,
Substantially similar mixtures, and Aerosols.
A.3.4.3 Classification of Mixtures When Data Are Available for All
Ingredients or Only for Some Ingredients of the Mixture
A.3.4.3.1 For purposes of classifying the eye corrosion/
irritation hazards of mixtures in the tiered approach:
The "relevant ingredients" of a mixture are those which are
present in concentrations >1% (weight/weight for solids, liquids,
dusts, mists and vapors and volume/volume for gases). If the
classifier has reason to suspect that an ingredient present at a
concentration <1% will affect classification of the mixture for eye
corrosion/irritation, that ingredient shall also be considered
relevant.
A.3.4.3.2 In general, the approach to classification of mixtures
as seriously damaging to the eye or eye irritant when data are
available on the ingredients, but not on the mixture as a whole, is
based on the theory of additivity, such that each corrosive or
irritant ingredient contributes to the overall irritant or corrosive
properties of the mixture in proportion to its potency and
concentration. A weighting factor of 10 is used for corrosive
ingredients when they are present at a concentration below the
concentration limit for classification with Category 1, but are at a
concentration that will contribute to the classification of the
mixture as an irritant. The mixture is classified as seriously
damaging to the eye or eye irritant when the sum of the
concentrations of such ingredients exceeds a threshold cut-off
value/concentration limit.
A.3.4.3.3 Table A.3.3 provides the cut-off value/concentration
limits to be used to determine if the mixture should be classified
as seriously damaging to the eye or an eye irritant.
A.3.4.3.4 Particular care must be taken when classifying certain
types of chemicals such as acids and bases, inorganic salts,
aldehydes, phenols, and surfactants. The approach explained in
A.3.4.3.1 and A.3.4.3.2 might not work given that many of such
substances are corrosive or irritant at concentrations <1%. For
mixtures containing strong acids or bases, the pH should be used as
classification criteria (See A.3.4.1) since pH will be a better
indicator of serious eye damage than the concentration limits of
Table A.3.3. A mixture containing corrosive or irritant ingredients
that cannot be classified based on the additivity approach applied
in Table A.3.3 due to chemical characteristics that make this
approach unworkable, should be classified as Eye Category 1 if it
contains >=1% of a corrosive ingredient and as Eye Category 2 when
it contains >=3% of an irritant ingredient. Classification of
mixtures with ingredients for which the approach in Table A.3.3 does
not apply is summarized in Table A.3.4.
A.3.4.3.5 On occasion, reliable data may show that the
reversible/irreversible eye effects of an ingredient will not be
evident when present at a level above the generic cut-off values/
concentration limits mentioned in Tables A.3.3 and A.3.4. In these
cases the mixture could be classified according to those data (See
also A.0.4.3 Use of cut-off values/concentration limits"). On
occasion, when it is expected that the skin corrosion/irritation or
the reversible/irreversible eye effects of an ingredient will not be
evident when present at a level above the generic concentration/cut-
off levels mentioned in Tables A.3.3 and A.3.4, testing of the
mixture may be considered. In those cases, the tiered weight of
evidence strategy should be applied as referred to in section A.3.3,
Figure A.3.1 and explained in detail in this chapter.
A.3.4.3.6 If there are data showing that (an) ingredient(s) may
be corrosive or irritant at a concentration of <1% (corrosive) or
<3% (irritant), the mixture should be classified accordingly (See
also paragraph A.0.4.3, Use of cut-off values/concentration limits).
Table A.3.3--Concentration of Ingredients of a Mixture Classified as Skin Category 1 and/or Eye Category 1 or 2
That Would Trigger Classification of the Mixtures as Hazardous to the Eye
----------------------------------------------------------------------------------------------------------------
Concentration triggering classification of a mixture as:
------------------------------------------------------------------------
Sum of ingredients classified as: Irreversible eye effects Reversible eye effects
------------------------------------------------------------------------
Category 1 Category 2
----------------------------------------------------------------------------------------------------------------
Eye or Skin Category 1................. >=3% >=1% but <3%.
Eye Category 2......................... ........................... >=10%.
(10 x Eye Category 1) + Eye Category 2. ........................... >=10%.
Skin Category 1 + Eye Category 1....... >=3% >=1% but <3%.
10 x (Skin Category 1 + Eye Category 1) ........................... >=10%.
+ Eye Category 2.
----------------------------------------------------------------------------------------------------------------
Note: A mixture may be classified as Eye Category 2B in cases when all relevant ingredients are classified as
Eye Category 2B.
Table A.3.4--Concentration of Ingredients of a Mixture for Which the
Additivity Approach Does Not Apply, That Would Trigger Classification of
the Mixture as Hazardous to the Eye
------------------------------------------------------------------------
Mixture classified
Ingredient Concentration as: Eye
------------------------------------------------------------------------
Acid with pH <=2.............. >=1% Category 1.
Base with pH >=11.5........... >=1% Category 1.
Other corrosive (Category 1) >=1% Category 1.
ingredients for which
additivity does not apply.
Other irritant (Category 2) >=3% Category 2.
ingredients for which
additivity does not apply,
including acids and bases.
------------------------------------------------------------------------
A.4 RESPIRATORY OR SKIN SENSITIZATION
A.4.1 Definitions and General Considerations
A.4.1.1 Respiratory sensitizer means a chemical that will lead
to hypersensitivity of the airways following inhalation of the
chemical.
Skin sensitizer means a chemical that will lead to an allergic
response following skin contact.
A.4.1.2 For the purpose of this chapter, sensitization includes
two phases: the first phase is induction of specialized
immunological memory in an individual by exposure to an allergen.
The second phase is elicitation, i.e., production of a cell-mediated
or antibody-mediated allergic response by exposure of a sensitized
individual to an allergen.
A.4.1.3 For respiratory sensitization, the pattern of induction
followed by elicitation phases is shared in common with skin
sensitization. For skin sensitization, an induction phase is
required in which the immune system learns to react; clinical
symptoms can then arise when subsequent exposure is sufficient to
elicit a visible skin reaction (elicitation phase). As a
consequence, predictive tests usually follow this pattern in which
there is an induction phase, the response to which is measured by a
standardized elicitation phase, typically involving a patch test.
The local lymph node assay is the exception, directly measuring the
induction response. Evidence of skin sensitization in humans
normally is assessed by a diagnostic patch test.
A.4.1.4 Usually, for both skin and respiratory sensitization,
lower levels are necessary for elicitation than are required for
induction.
A.4.1.5 The hazard class "respiratory or skin sensitization"
is differentiated into:
(a) Respiratory sensitization; and
(b) Skin sensitization.
A.4.2 Classification Criteria for Substances
A.4.2.1 Respiratory Sensitizers
A.4.2.1.1 Hazard Categories.
A.4.2.1.1.1 Effects seen in either humans or animals will
normally justify classification in a weight of evidence approach for
respiratory sensitizers. Substances may be allocated to one of the
two sub-categories 1A or 1B using a weight of evidence approach in
accordance with the criteria given in Table A.4.1 and on the basis
of reliable and good quality evidence from human cases or
epidemiological studies and/or observations from appropriate studies
in experimental animals.
A.4.2.1.1.2 Where data are not sufficient for sub-
categorization, respiratory sensitizers shall be classified in
Category 1.
Table A.4.1--Hazard Category and Sub-Categories for Respiratory
Sensitizers
------------------------------------------------------------------------
Category 1 Respiratory sensitizer
------------------------------------------------------------------------
A substance is classified as a
respiratory sensitizer.
(a) if there is evidence in humans
that the substance can lead to
specific respiratory
hypersensitivity and/or
(b) if there are positive results
from an appropriate animal test.\1\
Sub-category 1A................... Substances showing a high frequency
of occurrence in humans; or a
probability of occurrence of a high
sensitization rate in humans based
on animal or other tests.\1\
Severity of reaction may also be
considered.
Sub-category 1B................... Substances showing a low to moderate
frequency of occurrence in humans;
or a probability of occurrence of a
low to moderate sensitization rate
in humans based on animal or other
tests.\1\ Severity of reaction may
also be considered.
------------------------------------------------------------------------
---------------------------------------------------------------------------
\1\ At this writing, recognized and validated animal models for
the testing of respiratory hypersensitivity are not available. Under
certain circumstances, data from animal studies may provide valuable
information in a weight of evidence assessment.
---------------------------------------------------------------------------
A.4.2.1.2 Human evidence.
A.4.2.1.2.1 Evidence that a substance can lead to specific
respiratory hypersensitivity will normally be based on human
experience. In this context, hypersensitivity is normally seen as
asthma, but other hypersensitivity reactions such as rhinitis/
conjunctivitis and alveolitis are also considered. The condition
will have the clinical character of an allergic reaction. However,
immunological mechanisms do not have to be demonstrated.
A.4.2.1.2.2 When considering the human evidence, it is necessary
that in addition to
the evidence from the cases, the following be taken into account:
(a) The size of the population exposed;
(b) The extent of exposure.
A.4.2.1.2.3 The evidence referred to above could be:
(a) Clinical history and data from appropriate lung function
tests related to exposure to the substance, confirmed by other
supportive evidence which may include:
(i) In vivo immunological test (e.g., skin prick test);
(ii) In vitro immunological test (e.g., serological analysis);
(iii) Studies that may indicate other specific hypersensitivity
reactions where immunological mechanisms of action have not been
proven, e.g., repeated low-level irritation, pharmacologically
mediated effects;
(iv) A chemical structure related to substances known to cause
respiratory hypersensitivity;
(b) Data from positive bronchial challenge tests with the
substance conducted according to accepted guidelines for the
determination of a specific hypersensitivity reaction.
A.4.2.1.2.4 Clinical history should include both medical and
occupational history to determine a relationship between exposure to
a specific substance and development of respiratory
hypersensitivity. Relevant information includes aggravating factors
both in the home and workplace, the onset and progress of the
disease, family history and medical history of the patient in
question. The medical history should also include a note of other
allergic or airway disorders from childhood and smoking history.
A.4.2.1.2.5 The results of positive bronchial challenge tests
are considered to provide sufficient evidence for classification on
their own. It is, however, recognized that in practice many of the
examinations listed above will already have been carried out.
A.4.2.1.3 Animal studies.
A.4.2.1.3.1 Data from appropriate animal studies \2\ which may
be indicative of the potential of a substance to cause sensitization
by inhalation in humans \3\ may include:
---------------------------------------------------------------------------
\2\ At this writing, recognized and validated animal models for
the testing of respiratory hypersensitivity are not available. Under
certain circumstances, data from animal studies may provide valuable
information in a weight of evidence assessment.
\3\ The mechanisms by which substances induce symptoms of asthma
are not yet fully known. For preventive measures, these substances
are considered respiratory sensitizers. However, if on the basis of
the evidence, it can be demonstrated that these substances induce
symptoms of asthma by irritation only in people with bronchial
hyperactivity, they should not be considered as respiratory
sensitizers.
---------------------------------------------------------------------------
(a) Measurements of Immunoglobulin E (IgE) and other specific
immunological parameters, for example in mice
(b) Specific pulmonary responses in guinea pigs.
A.4.2.2 Skin Sensitizers
A.4.2.2.1 Hazard categories.
A.4.2.2.1.1 Effects seen in either humans or animals will
normally justify classification in a weight of evidence approach for
skin sensitizers. Substances may be allocated to one of the two sub-
categories 1A or 1B using a weight of evidence approach in
accordance with the criteria given in Table A.4.2 and on the basis
of reliable and good quality evidence from human cases or
epidemiological studies and/or observations from appropriate studies
in experimental animals according to the guidance values provided in
A.4.2.2.2.1 and A.4.2.2.3.2 for sub-category 1A and in A.4.2.2.2.2
and A.4.2.2.3.3 for sub-category 1B.
A.4.2.2.1.2 Where data are not sufficient for sub-
categorization, skin sensitizers shall be classified in Category 1.
Table A.4.2--Hazard Category and Sub-Categories for Skin Sensitizers
------------------------------------------------------------------------
Category 1 Skin sensitizer
------------------------------------------------------------------------
A substance is classified as a skin
sensitizer.
(a) if there is evidence in humans
that the substance can lead to
sensitization by skin contact in a
substantial number of persons, or
(b) if there are positive results
from an appropriate animal test.
Sub-category 1A................... Substances showing a high frequency
of occurrence in humans and/or a
high potency in animals can be
presumed to have the potential to
produce significant sensitization
in humans. Severity of reaction may
also be considered.
Sub-category 1B................... Substances showing a low to moderate
frequency of occurrence in humans
and/or a low to moderate potency in
animals can be presumed to have the
potential to produce sensitization
in humans. Severity of reaction may
also be considered.
------------------------------------------------------------------------
A.4.2.2.2 Human evidence.
A.4.2.2.2.1 Human evidence for sub-category 1A may include:
(a) Positive responses at <=500 [mu]g/cm\2\ (Human Repeat Insult
Patch Test (HRIPT), Human Maximization Test (HMT)--induction
threshold);
(b) Diagnostic patch test data where there is a relatively high
and substantial incidence of reactions in a defined population in
relation to relatively low exposure;
(c) Other epidemiological evidence where there is a relatively
high and substantial incidence of allergic contact dermatitis in
relation to relatively low exposure.
A.4.2.2.2.2 Human evidence for sub-category 1B may include:
(a) Positive responses at >500 [mu]g/cm\2\ (HRIPT, HMT--
induction threshold);
(b) Diagnostic patch test data where there is a relatively low
but substantial incidence of reactions in a defined population in
relation to relatively high exposure;
(c) Other epidemiological evidence where there is a relatively
low but substantial incidence of allergic contact dermatitis in
relation to relatively high exposure.
A.4.2.2.3 Animal studies
A.4.2.2.3.1 For Category 1, when an adjuvant type test method
for skin sensitization is used, a response of at least 30% of the
animals is considered as positive. For a non-adjuvant Guinea pig
test method a response of at least 15% of the animals is considered
positive. For Category 1, a stimulation index of three or more is
considered a positive response in the local lymph node assay.\4\
---------------------------------------------------------------------------
\4\ Test methods for skin sensitization are described in OECD
Guideline 406 (the Guinea Pig Maximization test and the Buehler
guinea pig test) and Guideline 429 (Local Lymph Node Assay). Other
methods may be used provided that they are scientifically validated.
The Mouse Ear Swelling Test (MEST), appears to be a reliable
screening test to detect moderate to strong sensitizers, and can be
used, in accordance with professional judgment, as a first stage in
the assessment of skin sensitization potential.
---------------------------------------------------------------------------
A.4.2.2.3.2 Animal test results for sub-category 1A can include
data with values indicated in Table A.4.3 below:
Table A.4.3--Animal Test Results for Sub-Category 1A
------------------------------------------------------------------------
Assay Criteria
------------------------------------------------------------------------
Local lymph node assay....... EC3 value <=2%.
Guinea pig maximization test. >=30% responding at <=0.1% intradermal
induction dose or
>=60% responding at >0.1% to <=1%
intradermal induction dose.
Buehler assay................ >=15% responding at <=0.2% topical
induction dose or
>=60% responding at >0.2% to <=20%
topical induction dose.
------------------------------------------------------------------------
Note: EC3 refers to the estimated concentration of test chemical
required to induce a stimulation index of 3 in the local lymph node
assay.
A.4.2.2.3.3 Animal test results for sub-category 1B can include
data with values indicated in Table A.4.4 below:
Table A.4.4--Animal Test Results for Sub-Category 1B
------------------------------------------------------------------------
Assay Criteria
------------------------------------------------------------------------
Local lymph node assay....... EC3 value >2%.
Guinea pig maximization test. >=30% to <60% responding at >0.1% to <=1%
intradermal induction dose or
>=30% responding at >1% intradermal
induction dose.
Buehler assay................ >=15% to <60% responding at >0.2% to
<=20% topical induction dose or
>=15% responding at >20% topical
induction dose.
------------------------------------------------------------------------
Note: EC3 refers to the estimated concentration of test chemical
required to induce a stimulation index of 3 in the local lymph node
assay.
A.4.2.2.4 Specific considerations.
A.4.2.2.4.1 For classification of a substance, evidence shall
include one or more of the following using a weight of evidence
approach:
(a) Positive data from patch testing, normally obtained in more
than one dermatology clinic;
(b) Epidemiological studies showing allergic contact dermatitis
caused by the substance. Situations in which a high proportion of
those exposed exhibit characteristic symptoms are to be looked at
with special concern, even if the number of cases is small;
(c) Positive data from appropriate animal studies;
(d) Positive data from experimental studies in man (See
paragraph A.0.2.6 of this Appendix);
(e) Well documented episodes of allergic contact dermatitis,
normally obtained in more than one dermatology clinic;
(f) Severity of reaction.
A.4.2.2.4.2 Evidence from animal studies is usually much more
reliable than evidence from human exposure. However, in cases where
evidence is available from both sources, and there is conflict
between the results, the quality and reliability of the evidence
from both sources must be assessed in order to resolve the question
of classification on a case-by-case basis. Normally, human data are
not generated in controlled experiments with volunteers for the
purpose of hazard classification but rather as part of risk
assessment to confirm lack of effects seen in animal tests.
Consequently, positive human data on skin sensitization are usually
derived from case-control or other, less defined studies. Evaluation
of human data must, therefore, be carried out with caution as the
frequency of cases reflect, in addition to the inherent properties
of the substances, factors such as the exposure situation,
bioavailability, individual predisposition and preventive measures
taken. Negative human data should not normally be used to negate
positive results from animal studies. For both animal and human
data, consideration should be given to the impact of vehicle.
A.4.2.2.4.3 If none of the above-mentioned conditions are met,
the substance need not be classified as a skin sensitizer. However,
a combination of two or more indicators of skin sensitization, as
listed below, may alter the decision. This shall be considered on a
case-by-case basis.
(a) Isolated episodes of allergic contact dermatitis;
(b) Epidemiological studies of limited power, e.g., where
chance, bias or confounders have not been ruled out fully with
reasonable confidence;
(c) Data from animal tests, performed according to existing
guidelines, which do not meet the criteria for a positive result
described in A.4.2.2.3, but which are sufficiently close to the
limit to be considered significant;
(d) Positive data from non-standard methods;
(e) Positive results from close structural analogues.
A.4.2.2.4.4 Immunological contact urticaria.
A.4.2.2.4.4.1 Substances meeting the criteria for classification
as respiratory sensitizers may, in addition, cause immunological
contact urticaria. Consideration shall be given to classifying these
substances as skin sensitizers.
A.4.2.2.4.4.2 Substances which cause immunological contact
urticaria without meeting the criteria for respiratory sensitizers
shall be considered for classification as skin sensitizers.
A.4.2.2.4.4.3 There is no recognized animal model available to
identify substances which cause immunological contact urticaria.
Therefore, classification will normally be based on human evidence,
similar to that for skin sensitization.
A.4.3 Classification Criteria for Mixtures
A.4.3.1 Classification of Mixtures When Data Are Available for the
Complete Mixture
When reliable and good quality evidence, as described in the
criteria for substances, from human experience or appropriate
studies in experimental animals, is available for the mixture, then
the mixture shall be classified by weight of evidence evaluation of
these data. Care must be exercised in evaluating data on mixtures
that the dose used does not render the results inconclusive.
A.4.3.2 Classification of Mixtures When Data Are Not Available for the
Complete Mixture: Bridging Principles
A.4.3.2.1 Where the mixture itself has not been tested to
determine its sensitizing properties, but there are sufficient data
on both the individual ingredients and similar tested mixtures to
adequately characterize the hazards of the mixture, these data will
be used in accordance with the following agreed bridging principles
as found in paragraph A.0.5 of this Appendix: Dilution, Batching,
Concentration of mixtures, Interpolation, Substantially similar
mixtures, and Aerosols.
A.4.3.3 Classification of Mixtures When Data Are Available for All
Ingredients or Only for Some Ingredients of the Mixture
The mixture shall be classified as a respiratory or skin
sensitizer when at least one ingredient has been classified as a
respiratory or skin sensitizer and is present at or above the
appropriate cut-off value/concentration limit for the specific
endpoint as shown in Table A.4.5.
Table A.4.5--Cut-Off Values/Concentration Limits of Ingredients of a Mixture Classified as Either Respiratory
Sensitizers or Skin Sensitizers That Would Trigger Classification of the Mixture
----------------------------------------------------------------------------------------------------------------
Cut-off values/concentration limits triggering classification of a
mixture as:
--------------------------------------------------------------------------
Ingredient classified as: Respiratory Sensitizer Category 1 Skin Sensitizer
-------------------------------------------------- Category 1
------------------------
Solid/liquid Gas All physical states
----------------------------------------------------------------------------------------------------------------
Respiratory Sensitizer, Category 1... >=0.1% >=0.1% .......................
Respiratory Sensitizer, Sub-category >=0.1% >=0.1% .......................
1A..................................
Respiratory Sensitizer, Sub-category >=1.0% >=0.2% .......................
1B..................................
Skin Sensitizer, Category 1.......... ....................... ....................... >=0.1%
Skin Sensitizer, Sub-category 1A..... ....................... ....................... >=0.1%
Skin Sensitizer, Sub-category 1B..... ....................... ....................... >=1.0%
----------------------------------------------------------------------------------------------------------------
A.5 GERM CELL MUTAGENICITY
A.5.1 Definitions and General Considerations
A.5.1.1 A mutation is defined as a permanent change in the
amount or structure of the genetic material in a cell. The term
mutation applies both to heritable genetic changes that may be
manifested at the phenotypic level and to the underlying DNA
modifications when known (including, for example, specific base pair
changes and chromosomal translocations). The term mutagenic and
mutagen will be used for agents giving rise to an increased
occurrence of mutations in populations of cells and/or organisms.
A.5.1.2 The more general terms genotoxic and genotoxicity apply
to agents or processes which alter the structure, information
content, or segregation of DNA, including those which cause DNA
damage by interfering with normal replication processes, or which in
a non-physiological manner (temporarily) alter its replication.
Genotoxicity test results are usually taken as indicators for
mutagenic effects.
A.5.1.3 This hazard class is primarily concerned with chemicals
that may cause mutations in the germ cells of humans that can be
transmitted to the progeny. However, mutagenicity/genotoxicity tests
in vitro and in mammalian somatic cells in vivo are also considered
in classifying substances and mixtures within this hazard class.
A.5.2 Classification Criteria for Substances
A.5.2.1 The classification system provides for two different
categories of germ cell mutagens to accommodate the weight of
evidence available. The two-category system is described in the
Figure A.5.1.
Figure A.5.1--Hazard Categories for Germ Cell Mutagens
------------------------------------------------------------------------
-------------------------------------------------------------------------
CATEGORY 1: Substances known to induce heritable mutations or to be
regarded as if they induce heritable mutations in the germ cells of
humans.
Category 1A: Substances known to induce heritable mutations in germ
cells of humans.
Positive evidence from human epidemiological studies.
Category 1B: Substances which should be regarded as if they induce
heritable mutations in the germ cells of humans.
(a) Positive result(s) from in vivo heritable germ cell mutagenicity
tests in mammals; or
(b) Positive result(s) from in vivo somatic cell mutagenicity tests
in mammals, in combination with some evidence that the substance
has potential to cause mutations to germ cells. This supporting
evidence may, for example, be derived from mutagenicity/
genotoxicity tests in germ cells in vivo, or by demonstrating the
ability of the substance or its metabolite(s) to interact with the
genetic material of germ cells; or
(c) Positive results from tests showing mutagenic effects in the
germ cells of humans, without demonstration of transmission to
progeny; for example, an increase in the frequency of aneuploidy in
sperm cells of exposed people.
CATEGORY 2: Substances which cause concern for humans owing to the
possibility that they may induce heritable mutations in the germ cells
of humans.
Positive evidence obtained from experiments in mammals and/or in
some cases from in vitro experiments, obtained from:
(a) Somatic cell mutagenicity tests in vivo, in mammals; or
(b) Other in vivo somatic cell genotoxicity tests which are
supported by positive results from in vitro mutagenicity
assays.
Note: Substances which are positive in in vitro mammalian
mutagenicity assays, and which also show chemical structure
activity relationship to known germ cell mutagens, should
be considered for classification as Category 2 mutagens.
------------------------------------------------------------------------
A.5.2.2 Specific considerations for classification of substances
as germ cell mutagens:
A.5.2.2.1 To arrive at a classification, test results are
considered from experiments determining mutagenic and/or genotoxic
effects in germ and/or somatic cells of exposed animals. Mutagenic
and/or genotoxic effects determined in in vitro tests shall also be
considered.
A.5.2.2.2 The system is hazard based, classifying chemicals on
the basis of their intrinsic ability to induce mutations in germ
cells. The scheme is, therefore, not meant for the (quantitative)
risk assessment of chemical substances.
A.5.2.2.3 Classification for heritable effects in human germ
cells is made on the basis of scientifically validated tests.
Evaluation of the test results shall be done using expert judgment
and all the available evidence shall be weighed for classification.
A.5.2.2.4 The classification of substances shall be based on the
total weight of evidence available, using expert judgment. In those
instances where a single well-conducted test is used for
classification, it shall provide clear and unambiguously positive
results. The relevance of the route of exposure used in the study of
the substance compared to the route of human exposure should also be
taken into account.
A.5.3 Classification Criteria for Mixtures \5\
---------------------------------------------------------------------------
\5\ It should be noted that the classification criteria for
health hazards usually include a tiered scheme in which test data
available on the complete mixture are considered as the first tier
in the evaluation, followed by the applicable bridging principles,
and lastly, cut-off values/concentration limits or additivity.
However, this approach is not used for Germ Cell Mutagenicity. These
criteria for Germ Cell Mutagenicity consider the cut-off values/
concentration limits as the primary tier and allow the
classification to be modified only on a case-by-case evaluation
based on available test data for the mixture as a whole.
---------------------------------------------------------------------------
A.5.3.1 Classification of Mixtures When Data Are Available for All
Ingredients or Only for Some Ingredients of the Mixture
A.5.3.1.1 Classification of mixtures shall be based on the
available test data for the
individual ingredients of the mixture using cut-off values/
concentration limits for the ingredients classified as germ cell
mutagens.
A.5.3.1.2 The mixture will be classified as a mutagen when at
least one ingredient has been classified as a Category 1A, Category
1B or Category 2 mutagen and is present at or above the appropriate
cut-off value/concentration limit as shown in Table A.5.1 below for
Category 1 and 2 respectively.
Table A.5.1--Cut-Off Values/Concentration Limits of Ingredients of a Mixture Classified as Germ Cell Mutagens
That Would Trigger Classification of the Mixture
----------------------------------------------------------------------------------------------------------------
Cut-off/concentration limits triggering
classification of a mixture as:
Ingredient classified as: -------------------------------------------------
Category 1 mutagen Category 2 mutagen
----------------------------------------------------------------------------------------------------------------
Category 1A/B mutagen......................................... >=0.1% .......................
Category 2 mutagen............................................ ....................... >=1.0%
----------------------------------------------------------------------------------------------------------------
Note: The cut-off values/concentration limits in the table above apply to solids and liquids (w/w units) as well
as gases (v/v units).
A.5.3.2 Classification of Mixtures When Data Are Available for the
Mixture Itself
The classification may be modified on a case-by-case basis based
on the available test data for the mixture as a whole. In such
cases, the test results for the mixture as a whole must be shown to
be conclusive taking into account dose and other factors such as
duration, observations and analysis (e.g. statistical analysis, test
sensitivity) of germ cell mutagenicity test systems.
A.5.3.3 Classification of Mixtures When Data Are Not Available for the
Complete Mixture: Bridging Principles
A.5.3.3.1 Where the mixture itself has not been tested to
determine its germ cell mutagenicity hazard, but there are
sufficient data on both the individual ingredients and similar
tested mixtures to adequately characterize the hazards of the
mixture, these data will be used in accordance with the following
bridging principles as found in paragraph A.0.5 of this Appendix:
Dilution, Batching, and Substantially similar mixtures.
A.5.4 Examples of Scientifically Validated Test Methods
A.5.4.1 Examples of in vivo heritable germ cell mutagenicity tests
are:
(a) Rodent dominant lethal mutation test (OECD 478)
(b) Mouse heritable translocation assay (OECD 485)
(c) Mouse specific locus test
A.5.4.2 Examples of in vivo somatic cell mutagenicity tests are:
(a) Mammalian bone marrow chromosome aberration test (OECD 475)
(b) Mouse spot test (OECD 484)
(c) Mammalian erythrocyte micronucleus test (OECD 474)
A.5.4.3 Examples of mutagenicity/genotoxicity tests in germ cells
are:
(a) Mutagenicity tests:
(i) Mammalian spermatogonial chromosome aberration test (OECD
483)
(ii) Spermatid micronucleus assay
(b) Genotoxicity tests:
(i) Sister chromatid exchange analysis in spermatogonia
(ii) Unscheduled DNA synthesis test (UDS) in testicular cells
A.5.4.4 Examples of genotoxicity tests in somatic cells are:
(a) Liver Unscheduled DNA Synthesis (UDS) in vivo (OECD 486)
(b) Mammalian bone marrow Sister Chromatid Exchanges (SCE)
A.5.4.5 Examples of in vitro mutagenicity tests are:
(a) In vitro mammalian chromosome aberration test (OECD 473)
(b) In vitro mammalian cell gene mutation test (OECD 476)
(c) Bacterial reverse mutation tests (OECD 471)
A.5.4.6 As new, scientifically validated tests arise, these may also
be used in the total weight of evidence to be considered.
A.6 CARCINOGENICITY
A.6.1 Definitions
Carcinogen means a substance or a mixture of substances which
induce cancer or increase its incidence. Substances and mixtures
which have induced benign and malignant tumors in well-performed
experimental studies on animals are considered also to be presumed
or suspected human carcinogens unless there is strong evidence that
the mechanism of tumor formation is not relevant for humans.
Classification of a substance or mixture as posing a
carcinogenic hazard is based on its inherent properties and does not
provide information on the level of the human cancer risk which the
use of the substance or mixture may represent.
A.6.2 Classification Criteria for Substances 6
---------------------------------------------------------------------------
\6\ See Non-mandatory Appendix F Part A for further guidance
regarding hazard classification for carcinogenicity. This appendix
is consistent with the GHS adn is provided as guidance excerpted
from the International Agency for Research on Cancer (IARC)
"Monographs on the Evaluation of Carcinogenic Risks to Humans"
(2006).
---------------------------------------------------------------------------
A.6.2.1 For the purpose of classification for carcinogenicity,
substances are allocated to one of two categories based on strength
of evidence and additional weight of evidence considerations. In
certain instances, route-specific classification may be warranted.
Figure A.6.1--Hazard Categories for Carcinogens
------------------------------------------------------------------------
-------------------------------------------------------------------------
CATEGORY 1: Known or presumed human carcinogens.
The classification of a substance as a Category 1 carcinogen is done
on the basis of epidemiological and/or animal data. This
classification is further distinguished on the basis of whether the
evidence for classification is largely from human data (Category
1A) or from animal data (Category 1B):
Category 1A: Known to have carcinogenic potential for humans.
Classification in this category is largely based on human evidence.
Category 1B: Presumed to have carcinogenic potential for humans.
Classification in this category is largely based on animal evidence.
The classification of a substance in Category 1A and 1B is based on
strength of evidence together with weight of evidence
considerations (See paragraph A.6.2.5). Such evidence may be
derived from:
--human studies that establish a causal relationship between
human exposure to a substance and the development of cancer
(known human carcinogen); or
--animal experiments for which there is sufficient evidence to
demonstrate animal carcinogenicity (presumed human carcinogen).
In addition, on a case by case basis, scientific judgment may
warrant a decision of presumed human carcinogenicity derived from
studies showing limited evidence of carcinogenicity in humans
together with limited evidence of carcinogenicity in experimental
animals.
CATEGORY 2: Suspected human carcinogens.
The classification of a substance in Category 2 is done on the basis
of evidence obtained from human and/or animal studies, but which is
not sufficiently convincing to place the substance in Category 1A
or B. This classification is based on strength of evidence together
with weight of evidence considerations (See paragraph A.6.2.5).
Such evidence may be from either limited evidence of
carcinogenicity in human studies or from limited evidence of
carcinogenicity in animal studies.
Other considerations: Where the weight of evidence for the
carcinogenicity of a substance does not meet the above criteria, any
positive study conducted in accordance with established scientific
principles, and which reports statistically significant findings
regarding the carcinogenic potential of the substance, must be noted on
the safety data sheet.
------------------------------------------------------------------------
A.6.2.2 Classification as a carcinogen is made on the basis of
evidence from reliable and acceptable methods, and is intended to be
used for substances which have an intrinsic property to produce such
toxic effects. The evaluations are to be based on all existing data,
peer-reviewed published studies and additional data accepted by
regulatory agencies.
A.6.2.3 Carcinogen classification is a one-step, criterion-based
process that involves two interrelated determinations: evaluations
of strength of evidence and consideration of all other relevant
information to place substances with human cancer potential into
hazard categories.
A.6.2.4 Strength of evidence involves the enumeration of tumors
in human and animal studies and determination of their level of
statistical significance. Sufficient human evidence demonstrates
causality between human exposure and the development of cancer,
whereas sufficient evidence in animals shows a causal relationship
between the agent and an increased incidence of tumors. Limited
evidence in humans is demonstrated by a positive association between
exposure and cancer, but a causal relationship cannot be stated.
Limited evidence in animals is provided when data suggest a
carcinogenic effect, but are less than sufficient. (Guidance on
consideration of important factors in the classification of
carcinogenicity and a more detailed description of the terms
"limited" and "sufficient" have been developed by the
International Agency for Research on Cancer (IARC) and are provided
in non-mandatory Appendix F).
A.6.2.5 Weight of evidence: Beyond the determination of the
strength of evidence for carcinogenicity, a number of other factors
should be considered that influence the overall likelihood that an
agent may pose a carcinogenic hazard in humans. The full list of
factors that influence this determination is very lengthy, but some
of the important ones are considered here.
A.6.2.5.1 These factors can be viewed as either increasing or
decreasing the level of concern for human carcinogenicity. The
relative emphasis accorded to each factor depends upon the amount
and coherence of evidence bearing on each. Generally there is a
requirement for more complete information to decrease than to
increase the level of concern. Additional considerations should be
used in evaluating the tumor findings and the other factors in a
case-by-case manner.
A.6.2.5.2 Some important factors which may be taken into
consideration, when assessing the overall level of concern are:
(a) Tumor type and background incidence;
(b) Multisite responses;
(c) Progression of lesions to malignancy;
(d) Reduced tumor latency;
Additional factors which may increase or decrease the level of
concern include:
(e) Whether responses are in single or both sexes;
(f) Whether responses are in a single species or several
species;
(g) Structural similarity or not to a substance(s) for which
there is good evidence of carcinogenicity;
(h) Routes of exposure;
(i) Comparison of absorption, distribution, metabolism and
excretion between test animals and humans;
(j) The possibility of a confounding effect of excessive
toxicity at test doses; and,
(k) Mode of action and its relevance for humans, such as
mutagenicity, cytotoxicity with growth stimulation, mitogenesis,
immunosuppression.
Mutagenicity: It is recognized that genetic events are central
in the overall process of cancer development. Therefore evidence of
mutagenic activity in vivo may indicate that a substance has a
potential for carcinogenic effects.
A.6.2.5.3 A substance that has not been tested for
carcinogenicity may in certain instances be classified in Category
1A, Category 1B, or Category 2 based on tumor data from a structural
analogue together with substantial support from consideration of
other important factors such as formation of common significant
metabolites, e.g., for benzidine congener dyes.
A.6.2.5.4 The classification should also take into consideration
whether or not the substance is absorbed by a given route(s); or
whether there are only local tumors at the site of administration
for the tested route(s), and adequate testing by other major
route(s) show lack of carcinogenicity.
A.6.2.5.5 It is important that whatever is known of the physico-
chemical, toxicokinetic and toxicodynamic properties of the
substances, as well as any available relevant information on
chemical analogues, i.e., structure activity relationship, is taken
into consideration when undertaking classification.
A.6.3 Classification Criteria for Mixtures 7
---------------------------------------------------------------------------
\7\ It should be noted that the classification criteria for
health hazards usually include a tiered scheme in which test data
available on the complete mixture are considered as the first tier
in the evaluation, followed by the applicable bridging principles,
and lastly, cut-off values/concentration limit or additivity.
However, this approach is not used for Carcinogenicity. These
criteria for Carcinogenicity consider the cut-off values/
concentration limits as the primary tier and allow the
classification to be modified only on a case-by-case evaluation
based on available test data for the mixture as a whole.
---------------------------------------------------------------------------
A.6.3.1 The mixture shall be classified as a carcinogen when at
least one ingredient has been classified as a Category 1 or Category
2 carcinogen and is present at or above the appropriate cut-off
value/concentration limit as shown in Table A.6.1.
Table A.6.1--Cut-Off Values/Concentration Limits of Ingredients of a Mixture Classified as Carcinogen That Would
Trigger Classification of the Mixture
----------------------------------------------------------------------------------------------------------------
Category 1
Ingredient classified as: carcinogen Category 2 carcinogen
----------------------------------------------------------------------------------------------------------------
Category 1 carcinogen......................... >=0.1% ............................................
Category 2 carcinogen......................... .................. >=0.1% (note 1).
----------------------------------------------------------------------------------------------------------------
Note: If a Category 2 carcinogen ingredient is present in the mixture at a concentration between 0.1% and 1%,
information is required on the SDS for a product. However, a label warning is optional. If a Category 2
carcinogen ingredient is present in the mixture at a concentration of =1%, both an SDS and a label
is required and the information must be included on each.
A.6.3.2 Classification of Mixtures When Data Are Available for the
Complete Mixture
A mixture may be classified based on the available test data for
the mixture as a whole. In such cases, the test results for the
mixture as a whole must be shown to be conclusive taking into
account dose and other factors such as duration, observations and
analysis (e.g., statistical analysis, test sensitivity) of
carcinogenicity test systems.
A.6.3.3 Classification of Mixtures When Data Are Not Available for the
Complete Mixture: Bridging Principles
Where the mixture itself has not been tested to determine its
carcinogenic hazard, but there are sufficient data on both the
individual ingredients and similar tested mixtures to adequately
characterize the hazards of the mixture, these data will be used in
accordance with the following bridging principles as found in
paragraph A.0.5 of this Appendix: Dilution; Batching; and
Substantially similar mixtures.
A.6.4 Classification of Carcinogenicity 8
---------------------------------------------------------------------------
\8\ See Non-mandatory Appendix F for further guidance regarding
hazard classification for carcinogenicity and how to relate
carcinogenicity classification information from IARC and NTP to GHS.
---------------------------------------------------------------------------
A.6.4.1 Chemical manufacturers, importers and employers
evaluating chemicals may treat the following sources as establishing
that a substance is a carcinogen or potential carcinogen for hazard
communication purposes in lieu of applying the criteria described
herein:
A.6.4.1.1 National Toxicology Program (NTP), "Report on
Carcinogens" (latest edition);
A.6.4.1.2 International Agency for Research on Cancer (IARC)
"Monographs on the Evaluation of Carcinogenic Risks to Humans"
(latest editions)
A.6.4.2 Where OSHA has included cancer as a health hazard to be
considered by classifiers for a chemical covered by 29 CFR part
1910, Subpart Z, Toxic and Hazardous Substances, chemical
manufacturers, importers, and employers shall classify the chemical
as a carcinogen.
A.7 REPRODUCTIVE TOXICITY
A.7.1 Definitions and General Considerations
A.7.1.1 Reproductive toxicity includes adverse effects on sexual
function and fertility in adult males and females, as well as
adverse effects on development of the offspring. Some reproductive
toxic effects cannot be clearly assigned to either impairment of
sexual function and fertility or to developmental toxicity.
Nonetheless, chemicals with these effects shall be classified as
reproductive toxicants.
For classification purposes, the known induction of genetically
based inheritable effects in the offspring is addressed in Germ cell
mutagenicity (See A.5).
A.7.1.2 Adverse effects on sexual function and fertility means
any effect of chemicals that interferes with reproductive ability or
sexual capacity. This includes, but is not limited to, alterations
to the female and male reproductive system, adverse effects on onset
of puberty, gamete production and transport, reproductive cycle
normality, sexual behaviour, fertility, parturition, pregnancy
outcomes, premature reproductive senescence, or modifications in
other functions that are dependent on the integrity of the
reproductive systems.
A.7.1.3 Adverse effects on development of the offspring means
any effect of chemicals which interferes with normal development of
the conceptus either before or after birth, which is induced during
pregnancy or results from parental exposure. These effects can be
manifested at any point in the life span of the organism. The major
manifestations of developmental toxicity include death of the
developing organism, structural abnormality, altered growth and
functional deficiency.
A.7.1.4 Adverse effects on or via lactation are also included in
reproductive toxicity, but for classification purposes, such effects
are treated separately (See A.7.2.1).
A.7.2 Classification Criteria for Substances
A.7.2.1 For the purpose of classification for reproductive
toxicity, substances shall be classified in one of two categories in
accordance with Figure A.7.1(a). Effects on sexual function and
fertility, and on development, shall be considered. In addition,
effects on or via lactation shall be classified in a separate hazard
category in accordance with Figure A.7.1(b).
Figure A.7.1(a)--Hazard Categories for Reproductive Toxicants
------------------------------------------------------------------------
-------------------------------------------------------------------------
CATEGORY 1: Known or presumed human reproductive toxicant.
Substance shall be classified in Category 1 for reproductive
toxicity when they are known to have produced an adverse effect on
sexual function and fertility or on development in humans or when
there is evidence from animal studies, possibly supplemented with
other information, to provide a strong presumption that the
substance has the capacity to interfere with reproduction in
humans. The classification of a substance is further distinguished
on the basis of whether the evidence for classification is
primarily from human data (Category 1A) or from animal data
(Category 1B).
Category 1A: Known human reproductive toxicant.
The classification of a substance in this category is largely based
on evidence from humans.
Category 1B: Presumed human reproductive toxicant.
The classification of a substance in this category is largely based
on evidence from experimental animals. Data from animal studies
shall provide sufficient evidence of an adverse effect on sexual
function and fertility or on development in the absence of other
toxic effects, or if occurring together with other toxic effects
the adverse effect on reproduction is considered not to be a
secondary non-specific consequence of other toxic effects. However,
when there is mechanistic information that raises doubt about the
relevance of the effect for humans, classification in Category 2
may be more appropriate.
CATEGORY 2: Suspected human reproductive toxicant.
Substances shall be classified in Category 2 for reproductive
toxicity when there is some evidence from humans or experimental
animals, possibly supplemented with other information, of an
adverse effect on sexual function and fertility, or on development,
in the absence of other toxic effects, or if occurring together
with other toxic effects the adverse effect on reproduction is
considered not to be a secondary non-specific consequence of the
other toxic effects, and where the evidence is not sufficiently
convincing to place the substance in Category 1. For instance,
deficiencies in the study may make the quality of evidence less
convincing, and in view of this, Category 2 would be the more
appropriate classification.
------------------------------------------------------------------------
Figure A.7.1(b)--Hazard Category for Effects on or Via Lactation
------------------------------------------------------------------------
-------------------------------------------------------------------------
EFFECTS ON OR VIA LACTATION
Effects on or via lactation shall be classified in a separate single
category. Chemicals that are absorbed by women and have been shown to
interfere with lactation or that may be present (including metabolites)
in breast milk in amounts sufficient to cause concern for the health of
a breastfed child, shall be classified to indicate this property
hazardous to breastfed babies. This classification shall be assigned on
the basis of:
(a) absorption, metabolism, distribution and excretion studies that
indicate the likelihood the substance would be present in
potentially toxic levels in breast milk; and/or
(b) results of one or two generation studies in animals which
provide clear evidence of adverse effect in the offspring due to
transfer in the milk or adverse effect on the quality of the milk;
and/or
(c) human evidence indicating a hazard to babies during the
lactation period.
------------------------------------------------------------------------
A.7.2.2 Basis of Classification
A.7.2.2.1 Classification is made on the basis of the criteria,
outlined above, an assessment of the total weight of evidence, and
the use of expert judgment. Classification as a reproductive
toxicant is intended to be used for substances which have an
intrinsic, specific property to produce an adverse effect on
reproduction and substances should not be so classified if such an
effect is produced solely as a non-specific secondary consequence of
other toxic effects.
A.7.2.2.2 In the evaluation of toxic effects on the developing
offspring, it is important to consider the possible influence of
maternal toxicity.
A.7.2.2.3 For human evidence to provide the primary basis for a
Category 1A classification there must be reliable evidence of an
adverse effect on reproduction in humans. Evidence used for
classification shall be from well conducted epidemiological studies,
if available, which include the use of appropriate controls,
balanced assessment, and due consideration of bias or confounding
factors. Less rigorous data from studies in humans may be sufficient
for a Category 1A classification if supplemented with adequate data
from studies in experimental animals, but classification in Category
1B may also be considered.
A.7.2.3 Weight of Evidence
A.7.2.3.1 Classification as a reproductive toxicant is made on
the basis of an assessment of the total weight of evidence using
expert judgment. This means that all available information that
bears on the determination of reproductive toxicity is considered
together. Included is information such as epidemiological studies
and case reports in humans and specific reproduction studies along
with sub-chronic, chronic and special study results in animals that
provide relevant information regarding toxicity to reproductive and
related endocrine organs. Evaluation of substances chemically
related to the material under study may also be included,
particularly when information on the material is scarce. The weight
given to the available evidence will be influenced by factors such
as the quality of the studies, consistency of results, nature and
severity of effects, level of statistical significance for
intergroup differences, number of endpoints affected, relevance of
route of administration to humans and freedom from bias. Both
positive and negative results are considered together in a weight of
evidence determination. However, a single, positive study performed
according to good scientific principles and with statistically or
biologically significant positive results may justify classification
(See also A.7.2.2.3).
A.7.2.3.2 Toxicokinetic studies in animals and humans, site of
action and mechanism or mode of action study results may provide
relevant information, which could reduce or increase concerns about
the hazard to human health. If it is conclusively demonstrated that
the clearly identified mechanism or mode of action has no relevance
for humans or when the toxicokinetic differences are so marked that
it is certain that the hazardous property will not be expressed in
humans then a chemical which produces an adverse effect on
reproduction in experimental animals should not be classified.
A.7.2.3.3 In some reproductive toxicity studies in experimental
animals the only effects recorded may be considered of low or
minimal toxicological significance and classification may not
necessarily be the outcome. These effects include, for example,
small changes in semen parameters or in the incidence of spontaneous
defects in the fetus, small changes in the proportions of common
fetal variants such as are observed in skeletal examinations, or in
fetal weights, or small differences in postnatal developmental
assessments.
A.7.2.3.4 Data from animal studies shall provide sufficient
evidence of specific reproductive toxicity in the absence of other
systemic toxic effects. However, if developmental toxicity occurs
together with other toxic effects in the dam (mother), the potential
influence of the generalized adverse effects should be assessed to
the extent possible. The preferred approach is to consider adverse
effects in the embryo/fetus first, and then evaluate maternal
toxicity, along with any other factors which are likely to have
influenced these effects, as part of the weight of evidence. In
general, developmental effects that are observed at maternally toxic
doses should not be automatically discounted. Discounting
developmental effects that are observed at maternally toxic doses
can only be done on a case-by-case basis when a causal relationship
is established or refuted.
A.7.2.3.5 If appropriate information is available it is
important to try to determine whether developmental toxicity is due
to a specific maternally mediated mechanism or to a non-specific
secondary mechanism, like maternal stress and the disruption of
homeostasis. Generally, the presence of maternal toxicity should not
be used to negate findings of embryo/fetal effects, unless it can be
clearly demonstrated that the effects are secondary non-specific
effects. This is especially the case when the effects in the
offspring are significant, e.g., irreversible effects such as
structural malformations. In some situations it is reasonable to
assume that reproductive toxicity is due to a secondary consequence
of maternal toxicity and discount the effects, for example if the
chemical is so toxic that dams fail to thrive and there is severe
inanition; they are incapable of nursing pups; or they are prostrate
or dying.
A.7.2.4 Maternal Toxicity
A.7.2.4.1 Development of the offspring throughout gestation and
during the early postnatal stages can be influenced by toxic effects
in the mother either through non-specific mechanisms related to
stress and the disruption of maternal homeostasis, or by specific
maternally-mediated mechanisms. So, in the interpretation of the
developmental outcome to decide classification for developmental
effects it is important to consider the possible influence of
maternal toxicity. This is a complex issue because of uncertainties
surrounding the relationship between maternal toxicity and
developmental outcome. Expert judgment and a weight of evidence
approach, using all available studies, shall be used to determine
the degree of influence to be attributed to maternal toxicity when
interpreting the criteria for classification for developmental
effects. The adverse effects in the embryo/fetus shall be first
considered, and then maternal toxicity, along with any other factors
which are likely to have influenced these effects, as weight of
evidence, to help reach a conclusion about classification.
A.7.2.4.2 Based on pragmatic observation, it is believed that
maternal toxicity may, depending on severity, influence development
via non-specific secondary mechanisms, producing effects such as
depressed fetal weight, retarded ossification, and possibly
resorptions and certain malformations in some strains of certain
species. However, the limited numbers of studies which have
investigated the relationship between developmental effects and
general maternal toxicity have failed to demonstrate a consistent,
reproducible relationship across species. Developmental effects
which occur even in the presence of maternal toxicity are considered
to be evidence of developmental toxicity, unless it can be
unequivocally demonstrated on a case by case basis that the
developmental effects are secondary to maternal toxicity. Moreover,
classification shall be considered where there is a significant
toxic effect in the offspring, e.g., irreversible effects such as
structural malformations, embryo/fetal lethality, or significant
post-natal functional deficiencies.
A.7.2.4.3 Classification shall not automatically be discounted
for chemicals that produce developmental toxicity only in
association with maternal toxicity, even if a specific maternally-
mediated mechanism has been demonstrated. In such a case,
classification in Category 2 may be considered more appropriate than
Category 1. However, when a chemical is so toxic that maternal death
or severe inanition results, or the dams (mothers) are prostrate and
incapable of nursing the pups, it is reasonable to assume that
developmental toxicity is produced solely as a secondary consequence
of maternal toxicity and discount the developmental effects.
Classification is not necessarily the outcome in the case of minor
developmental changes, e.g., a small reduction in fetal/pup body
weight or retardation of ossification when seen in association with
maternal toxicity.
A.7.2.4.4 Some of the endpoints used to assess maternal toxicity
are provided below. Data on these endpoints, if available, shall be
evaluated in light of their statistical or biological significance
and dose-response relationship.
(a) Maternal mortality: An increased incidence of mortality
among the treated dams over the controls shall be considered
evidence of maternal toxicity if the increase occurs in a dose-
related manner and can be attributed to the systemic toxicity of the
test material. Maternal mortality greater than 10% is considered
excessive and the data for that dose level shall not normally be
considered to need further evaluation.
(b) Mating index (Number of animals with seminal plugs or sperm/
Number of mated x 100)
(c) Fertility index (Number of animals with implants/Number of
matings x 100)
(d) Gestation length (If allowed to deliver)
(e) Body weight and body weight change: Consideration of the
maternal body weight change and/or adjusted (corrected) maternal
body weight shall be included in the evaluation of maternal toxicity
whenever such data are available. The calculation of an adjusted
(corrected) mean maternal body weight change, which is the
difference between the initial and terminal body weight minus the
gravid uterine weight (or alternatively, the sum of the weights of
the fetuses), may indicate whether the effect is maternal or
intrauterine. In rabbits, the body weight gain may not be a useful
indicator of maternal toxicity because of normal fluctuations in
body weight during pregnancy.
(f) Food and water consumption (if relevant): The observation of
a significant decrease in the average food or water consumption in
treated dams (mothers) compared to the control group may be useful
in evaluating maternal toxicity, particularly when the test material
is administered in the diet or drinking water. Changes in food or
water consumption must be evaluated in conjunction with maternal
body weights when determining if the effects noted are reflective of
maternal toxicity or more simply, unpalatability of the test
material in feed or water.
(g) Clinical evaluations (including clinical signs, markers, and
hematology and clinical chemistry studies): The observation of
increased incidence of significant clinical signs of toxicity in
treated dams (mothers) relative to the control group is useful in
evaluating maternal toxicity. If this is to be used as the basis for
the assessment of maternal toxicity, the types, incidence, degree
and duration of clinical signs shall be reported in the study.
Clinical signs of maternal intoxication include, but are not limited
to: coma, prostration, hyperactivity, loss of righting reflex,
ataxia, or labored breathing.
(h) Post-mortem data: Increased incidence and/or severity of
post-mortem findings may be indicative of maternal toxicity. This
can include gross or microscopic pathological findings or organ
weight data, including absolute organ weight, organ-to-body weight
ratio, or organ-to-brain weight ratio. When supported by findings of
adverse histopathological effects in the affected organ(s), the
observation of a significant change in the average weight of
suspected target organ(s) of treated dams (mothers), compared to
those in the control group, may be considered evidence of maternal
toxicity.
A.7.2.5 Animal and Experimental Data
A.7.2.5.1 A number of scientifically validated test methods are
available, including methods for developmental toxicity testing
(e.g., OECD Test Guideline 414, ICH Guideline S5A, 1993), methods
for peri- and post-natal toxicity testing (e.g., ICH S5B, 1995), and
methods for one or two-generation toxicity testing (e.g., OECD Test
Guidelines 415, 416)
A.7.2.5.2 Results obtained from screening tests (e.g., OECD
Guidelines 421--Reproduction/Developmental Toxicity Screening Test,
and 422--Combined Repeated Dose Toxicity Study with Reproduction/
Development Toxicity Screening Test) can also be used to justify
classification, although the quality of this evidence is less
reliable than that obtained through full studies.
A.7.2.5.3 Adverse effects or changes, seen in short- or long-
term repeated dose toxicity studies, which are judged likely to
impair reproductive function and which occur in the absence of
significant generalized toxicity, may be used as a basis for
classification, e.g., histopathological changes in the gonads.
A.7.2.5.4 Evidence from in vitro assays, or non-mammalian tests,
and from analogous substances using structure-activity relationship
(SAR), can contribute to the procedure for classification. In all
cases of this nature, expert judgment must be used to assess the
adequacy of the data. Inadequate data shall not be used as a primary
support for classification.
A.7.2.5.5 It is preferable that animal studies are conducted
using appropriate routes of administration which relate to the
potential route of human exposure. However, in practice,
reproductive toxicity studies are commonly conducted using the oral
route, and such studies will normally be suitable for evaluating the
hazardous properties of the substance with respect to reproductive
toxicity. However, if it can be conclusively demonstrated that the
clearly identified mechanism or mode of action has no relevance for
humans or when the toxicokinetic differences are so marked that it
is certain that the hazardous property will not be expressed in
humans then a substance which produces an adverse effect on
reproduction in experimental animals should not be classified.
A.7.2.5.6 Studies involving routes of administration such as
intravenous or intraperitoneal injection, which may result in
exposure of the reproductive organs to unrealistically high levels
of the test substance, or elicit local damage to the reproductive
organs, e.g., by irritation, must be interpreted with extreme
caution and on their own are not normally the basis for
classification.
A.7.2.5.7 There is general agreement about the concept of a
limit dose, above which the production of an adverse effect may be
considered to be outside the criteria which lead to classification.
Some test guidelines specify a limit dose, other test guidelines
qualify the limit dose with a statement that higher doses may be
necessary if anticipated human exposure is sufficiently high that an
adequate margin of exposure would not be achieved. Also, due to
species differences in toxicokinetics, establishing a specific limit
dose may not be adequate for situations where humans are more
sensitive than the animal model.
A.7.2.5.8 In principle, adverse effects on reproduction seen
only at very high dose levels in animal studies (for example doses
that induce prostration, severe inappetence, excessive mortality) do
not normally lead to classification, unless other information is
available, for example, toxicokinetics information indicating that
humans may be more susceptible than animals, to suggest that
classification is appropriate.
A.7.2.5.9 However, specification of the actual "limit dose"
will depend upon the test method that has been employed to provide
the test results.
A.7.3 Classification Criteria for Mixtures 9
---------------------------------------------------------------------------
\9\ It should be noted that the classification criteria for
health hazards usually include a tiered scheme in which test data
available on the complete mixture are considered as the first tier
in the evaluation, followed by the applicable bridging principles,
and lastly, cut-off values/concentration limits or additivity.
However, this approach is not used for Reproductive Toxicity. These
criteria for Reproductive Toxicity consider the cut-off values/
concentration limits as the primary tier and allow the
classification to be modified only on a case-by-case evaluation
based on available test data for the mixture as a whole.
---------------------------------------------------------------------------
A.7.3.1 Classification of Mixtures When Data Are Available for All
Ingredients or Only for Some Ingredients of the Mixture
A.7.3.1.1 The mixture shall be classified as a reproductive
toxicant when at least one ingredient has been classified as a
Category 1 or Category 2 reproductive toxicant and is present at or
above the appropriate cut-off value/concentration limit specified in
Table A.7.1 for Category 1 and 2, respectively.
A.7.3.1.2 The mixture shall be classified for effects on or via
lactation when at least one ingredient has been classified for
effects on or via lactation and is present at or above the
appropriate cut-off value/concentration limit specified in Table
A.7.1 for the additional category for effects on or via lactation.
Table A.7.1--Cut-Off Values/Concentration Limits of Ingredients of a Mixture Classified as Reproductive
Toxicants or for Effects on or via Lactation That Trigger Classification of the Mixture
----------------------------------------------------------------------------------------------------------------
Cut-off values/concentration limits triggering classification of a mixture
as:
-----------------------------------------------------------------------------
Ingredients classified as: Additional category for
Category 1 reproductive Category 2 reproductive effects on or via
toxicant toxicant lactation
----------------------------------------------------------------------------------------------------------------
Category 1 reproductive toxicant.. >=0.1% ........................ ........................
Category 2 reproductive toxicant.. ........................ >=0.1% ........................
Additional category for effects on ........................ ........................ >=0.1%
or via lactation.................
----------------------------------------------------------------------------------------------------------------
A.7.3.2 Classification of Mixtures When Data Are Available for the
Complete Mixture
Available test data for the mixture as a whole may be used for
classification on a case-by-case basis. In such cases, the test
results for the mixture as a whole must be shown to be conclusive
taking into account dose and other factors such as duration,
observations and analysis (e.g., statistical analysis, test
sensitivity) of reproduction test systems.
A.7.3.3 Classification of Mixtures When Data Are Not Available for the
Complete Mixture: Bridging Principles
A.7.3.3.1 Where the mixture itself has not been tested to
determine its reproductive toxicity, but there are sufficient data
on both the individual ingredients and similar tested mixtures to
adequately characterize the hazards of the mixture, these data shall
be used in accordance with the following bridging principles as
found in paragraph A.0.5 of this Appendix: Dilution, Batching, and
Substantially similar mixtures.
A.8 SPECIFIC TARGET ORGAN TOXICITY SINGLE EXPOSURE
A.8.1 Definitions and General Considerations
A.8.1.1 Specific target organ toxicity--single exposure, (STOT-
SE) means specific, non-lethal target organ toxicity arising from a
single exposure to a chemical. All significant health effects that
can impair function, both reversible and irreversible, immediate
and/or delayed and not specifically addressed in A.1 to A.7 and A.10
of this Appendix are included. Specific target organ toxicity
following repeated exposure is classified in accordance with
SPECIFIC TARGET ORGAN TOXICITY--REPEATED EXPOSURE (A.9 of this
Appendix) and is therefore not included here.
A.8.1.2 Classification identifies the chemical as being a
specific target organ toxicant and, as such, it presents a potential
for adverse health effects in people who are exposed to it.
A.8.1.3 The adverse health effects produced by a single exposure
include consistent and identifiable toxic effects in humans; or, in
experimental animals, toxicologically significant changes which have
affected the function or morphology of a tissue/organ, or have
produced serious changes to the biochemistry or hematology of the
organism, and these changes are relevant for human health. Human
data is the primary source of evidence for this hazard class.
A.8.1.4 Assessment shall take into consideration not only
significant changes in a single organ or biological system but also
generalized changes of a less severe nature involving several
organs.
A.8.1.5 Specific target organ toxicity can occur by any route
that is relevant for humans, i.e., principally oral, dermal or
inhalation.
A.8.1.6 The classification criteria for specific organ systemic
toxicity single exposure are organized as criteria for substances
Categories 1 and 2 (See A.8.2.1), criteria for substances Category 3
(See A.8.2.2) and criteria for mixtures (See A.8.3). See also Figure
A.8.1.
A.8.2 Classification Criteria for Substances
A.8.2.1 Substances of Category 1 and Category 2
A.8.2.1.1 Substances shall be classified for immediate or
delayed effects separately, by the use of expert judgment on the
basis of the weight of all evidence available, including the use of
recommended guidance values (See A.8.2.1.9). Substances shall then
be classified in Category 1 or 2, depending upon the nature and
severity of the effect(s) observed, in accordance with Figure A.8.1.
Figure A.8.1--Hazard Categories for Specific Target Organ Toxicity
Following Single Exposure
------------------------------------------------------------------------
-------------------------------------------------------------------------
CATEGORY 1: Substances that have produced significant toxicity in
humans, or that, on the basis of evidence from studies in experimental
animals can be presumed to have the potential to produce significant
toxicity in humans following single exposure
Substances are classified in Category 1 for STOT-SE on the basis of:
(a) reliable and good quality evidence from human cases or
epidemiological studies; or
(b) observations from appropriate studies in experimental animals in
which significant and/or severe toxic effects of relevance to human
health were produced at generally low exposure concentrations. Guidance
dose/concentration values are provided below (See A.8.2.1.9) to be used
as part of weight-of-evidence evaluation.
CATEGORY 2: Substances that, on the basis of evidence from studies in
experimental animals, can be presumed to have the potential to be
harmful to human health following single exposure
Substances are classified in Category 2 for STOT-SE on the basis of
observations from appropriate studies in experimental animals in which
significant toxic effects, of relevance to human health, were produced
at generally moderate exposure concentrations. Guidance dose/
concentration values are provided below (See A.8.2.1.9) in order to
help in classification.
In exceptional cases, human evidence can also be used to place a
substance in Category 2 (See A.8.2.1.6).
CATEGORY 3: Transient target organ effects
There are target organ effects for which a substance does not meet the
criteria to be classified in Categories 1 or 2 indicated above. These
are effects which adversely alter human function for a short duration
after exposure and from which humans may recover in a reasonable period
without leaving significant alteration of structure or function. This
category only includes narcotic effects and respiratory tract
irritation. Substances are classified specifically for these effects as
discussed in A.8.2.2.
Note: The primary target organ/system shall be identified where
possible, and where this is not possible, the substance shall be
identified as a general toxicant. The data shall be evaluated and,
where possible, shall not include secondary effects (e.g., a
hepatotoxicant can produce secondary effects in the nervous or gastro-
intestinal systems).
------------------------------------------------------------------------
A.8.2.1.2 The relevant route(s) of exposure by which the
classified substance produces damage shall be identified.
A.8.2.1.3 Classification is determined by expert judgment, on
the basis of the weight of all evidence available including the
guidance presented below.
A.8.2.1.4 Weight of evidence of all available data, including
human incidents, epidemiology, and studies conducted in experimental
animals is used to substantiate specific target organ toxic effects
that merit classification.
A.8.2.1.5 The information required to evaluate specific target
organ toxicity comes either from single exposure in humans (e.g.,
exposure at home, in the workplace or environmentally), or from
studies conducted in experimental animals. The standard animal
studies in rats or mice that provide this information are acute
toxicity studies which can include clinical observations and
detailed macroscopic and microscopic examination to enable the toxic
effects on target tissues/organs to be identified. Results of acute
toxicity studies conducted in other species may also provide
relevant information.
A.8.2.1.6 In exceptional cases, based on expert judgment, it may
be appropriate to place certain substances with human evidence of
target organ toxicity in Category 2: (a) when the weight of human
evidence is not sufficiently convincing to warrant Category 1
classification, and/or (b) based on the nature and severity of
effects. Dose/concentration levels in humans shall not be considered
in the classification and any available evidence from animal studies
shall be consistent with the Category 2 classification. In other
words, if there are also animal data available on the substance that
warrant Category 1 classification, the chemical shall be classified
as Category 1.
A.8.2.1.7 Effects considered to support classification for
Category 1 and 2
A.8.2.1.7.1 Classification is supported by evidence associating
single exposure to the substance with a consistent and identifiable
toxic effect.
A.8.2.1.7.2 Evidence from human experience/incidents is usually
restricted to reports of adverse health consequences, often with
uncertainty about exposure conditions, and may not provide the
scientific detail that can be obtained from well-conducted studies
in experimental animals.
A.8.2.1.7.3 Evidence from appropriate studies in experimental
animals can furnish much more detail, in the form of clinical
observations, and macroscopic and microscopic pathological
examination and this can often reveal hazards that may not be life-
threatening but could indicate functional impairment. Consequently
all available evidence, and evidence relevance to human health, must
be taken into consideration in the classification process. Relevant
toxic effects in humans and/or animals include, but are not limited
to:
(a) Morbidity resulting from single exposure;
(b) Significant functional changes, more than transient in
nature, in the respiratory system, central or peripheral nervous
systems, other organs or other organ systems, including signs of
central nervous system depression and effects on special senses
(e.g., sight, hearing and sense of smell);
(c) Any consistent and significant adverse change in clinical
biochemistry, hematology, or urinalysis parameters;
(d) Significant organ damage that may be noted at necropsy and/
or subsequently seen or confirmed at microscopic examination;
(e) Multi-focal or diffuse necrosis, fibrosis or granuloma
formation in vital organs with regenerative capacity;
(f) Morphological changes that are potentially reversible but
provide clear evidence of marked organ dysfunction; and,
(g) Evidence of appreciable cell death (including cell
degeneration and reduced cell number) in vital organs incapable of
regeneration.
A.8.2.1.8 Effects considered not to support classification for
Category 1 and 2
Effects may be seen in humans and/or animals that do not justify
classification. Such effects include, but are not limited to:
(a) Clinical observations or small changes in bodyweight gain,
food consumption or water intake that may have some toxicological
importance but that do not, by themselves, indicate "significant"
toxicity;
(b) Small changes in clinical biochemistry, hematology or
urinalysis parameters and/or transient effects, when such changes or
effects are of doubtful or of minimal toxicological importance;
(c) Changes in organ weights with no evidence of organ
dysfunction;
(d) Adaptive responses that are not considered toxicologically
relevant; and,
(e) Substance-induced species-specific mechanisms of toxicity,
i.e., demonstrated with reasonable certainty to be not relevant for
human health, shall not justify classification.
A.8.2.1.9 Guidance values to assist with classification based on
the results obtained from studies conducted in experimental animals
for Category 1 and 2
A.8.2.1.9.1 In order to help reach a decision about whether a
substance shall be classified or not, and to what degree it shall be
classified (Category 1 vs. Category 2), dose/concentration
"guidance values" are provided for consideration of the dose/
concentration which has been shown to produce significant health
effects. The principal argument for proposing such guidance values
is that all chemicals are potentially toxic and there has to be a
reasonable dose/concentration above which a degree of toxic effect
is acknowledged.
A.8.2.1.9.2 Thus, in animal studies, when significant toxic
effects are observed that indicate classification, consideration of
the dose/concentration at which these effects were seen, in relation
to the suggested guidance values, provides useful information to
help assess the need to classify (since the toxic effects are a
consequence of the hazardous property(ies) and also the dose/
concentration).
A.8.2.1.9.3 The guidance value (C) ranges for single-dose
exposure which has produced a significant non-lethal toxic effect
are those applicable to acute toxicity testing, as indicated in
Table A.8.1.
Table A.8.1--Guidance Value Ranges for Single-Dose Exposures
----------------------------------------------------------------------------------------------------------------
Guidance value ranges for:
Route of exposure Units -----------------------------------------------------------
Category 1 Category 2 Category 3
----------------------------------------------------------------------------------------------------------------
Oral (rat)...................... mg/kg body weight. C <=300........... 2000 >=C >300..... Guidance values do
not apply.
Dermal (rat or rabbit).......... mg/kg body weight. C <=1,000......... 2000 >=C >1,000...
Inhalation (rat) gas............ ppmV/4h........... C <=2,500......... 20,000 >=C >2,500.
Inhalation (rat) vapor.......... mg/1/4h........... C <=10............ 20 >=C >10........
Inhalation (rat) dust/mist/fume. mg/l/4h........... C <=1.0........... 5.0 >=C >1.0......
----------------------------------------------------------------------------------------------------------------
A.8.2.1.9.4 The guidance values and ranges mentioned in Table
A.8.1 are intended only for guidance purposes, i.e., to be used as
part of the weight of evidence approach, and to assist with
decisions about classification. They are not intended as strict
demarcation values. Guidance values are not provided for Category 3
since this classification is primarily based on human data; animal
data may be included in the weight of evidence evaluation.
A.8.2.1.9.5 Thus, it is feasible that a specific profile of
toxicity occurs at a dose/concentration below the guidance value,
e.g., <2000 mg/kg body weight by the oral route, however the nature
of the effect may result in the decision not to classify.
Conversely, a specific profile of toxicity may be seen in animal
studies occurring at above a guidance value, e.g., >=2000 mg/kg body
weight by the oral route, and in addition there is supplementary
information from other sources, e.g., other single dose studies, or
human case experience, which supports a conclusion that, in view of
the weight of evidence, classification is the prudent action to
take.
A.8.2.1.10 Other considerations
A.8.2.1.10.1 When a substance is characterized only by use of
animal data the classification process includes reference to dose/
concentration guidance values as one of
the elements that contribute to the weight of evidence approach.
A.8.2.1.10.2 When well-substantiated human data are available
showing a specific target organ toxic effect that can be reliably
attributed to single exposure to a substance, the substance shall be
classified. Positive human data, regardless of probable dose,
predominates over animal data. Thus, if a substance is unclassified
because specific target organ toxicity observed was considered not
relevant or significant to humans, if subsequent human incident data
become available showing a specific target organ toxic effect, the
substance shall be classified.
A.8.2.1.10.3 A substance that has not been tested for specific
target organ toxicity shall, where appropriate, be classified on the
basis of data from a scientifically validated structure activity
relationship and expert judgment-based extrapolation from a
structural analogue that has previously been classified together
with substantial support from consideration of other important
factors such as formation of common significant metabolites.
A.8.2.2 Substances of Category 3
A.8.2.2.1 Criteria for respiratory tract irritation
The criteria for classifying substances as Category 3 for
respiratory tract irritation are:
(a) Respiratory irritant effects (characterized by localized
redness, edema, pruritis and/or pain) that impair function with
symptoms such as cough, pain, choking, and breathing difficulties
are included. It is recognized that this evaluation is based
primarily on human data;
(b) Subjective human observations supported by objective
measurements of clear respiratory tract irritation (RTI) (e.g.,
electrophysiological responses, biomarkers of inflammation in nasal
or bronchoalveolar lavage fluids);
(c) The symptoms observed in humans shall also be typical of
those that would be produced in the exposed population rather than
being an isolated idiosyncratic reaction or response triggered only
in individuals with hypersensitive airways. Ambiguous reports simply
of "irritation" should be excluded as this term is commonly used
to describe a wide range of sensations including those such as
smell, unpleasant taste, a tickling sensation, and dryness, which
are outside the scope of classification for respiratory tract
irritation;
(d) There are currently no scientifically validated animal tests
that deal specifically with RTI; however, useful information may be
obtained from the single and repeated inhalation toxicity tests. For
example, animal studies may provide useful information in terms of
clinical signs of toxicity (dyspnoea, rhinitis etc) and
histopathology (e.g., hyperemia, edema, minimal inflammation,
thickened mucous layer) which are reversible and may be reflective
of the characteristic clinical symptoms described above. Such animal
studies can be used as part of weight of evidence evaluation; and,
(e) This special classification will occur only when more severe
organ effects including the respiratory system are not observed as
those effects would require a higher classification.
A.8.2.2.2 Criteria for narcotic effects
The criteria for classifying substances in Category 3 for
narcotic effects are:
(a) Central nervous system depression including narcotic effects
in humans such as drowsiness, narcosis, reduced alertness, loss of
reflexes, lack of coordination, and vertigo are included. These
effects can also be manifested as severe headache or nausea, and can
lead to reduced judgment, dizziness, irritability, fatigue, impaired
memory function, deficits in perception and coordination, reaction
time, or sleepiness; and,
(b) Narcotic effects observed in animal studies may include
lethargy, lack of coordination righting reflex, narcosis, and
ataxia. If these effects are not transient in nature, then they
shall be considered for classification as Category 1 or 2.
A.8.3 Classification Criteria for Mixtures
A.8.3.1 Mixtures are classified using the same criteria as for
substances, or alternatively as described below. As with substances,
mixtures may be classified for specific target organ toxicity
following single exposure, repeated exposure, or both.
A.8.3.2 Classification of Mixtures When Data Are Available for the
Complete Mixture
When reliable and good quality evidence from human experience or
appropriate studies in experimental animals, as described in the
criteria for substances, is available for the mixture, then the
mixture shall be classified by weight of evidence evaluation of this
data. Care shall be exercised in evaluating data on mixtures, that
the dose, duration, observation or analysis, do not render the
results inconclusive.
A.8.3.3 Classification of Mixtures When Data Are Not Available for the
Complete Mixture: Bridging Principles
A.8.3.3.1 Where the mixture itself has not been tested to
determine its specific target organ toxicity, but there are
sufficient data on both the individual ingredients and similar
tested mixtures to adequately characterize the hazards of the
mixture, these data shall be used in accordance with the following
bridging principles as found in paragraph A.0.5 of this Appendix:
Dilution, Batching, Concentration of mixtures, Interpolation within
one toxicity category, Substantially similar mixtures, or Aerosols.
A.8.3.4 Classification of Mixtures When Data Are Available for All
Ingredients or Only for Some Ingredients of the Mixture
A.8.3.4.1 Where there is no reliable evidence or test data for
the specific mixture itself, and the bridging principles cannot be
used to enable classification, then classification of the mixture is
based on the classification of the ingredient substances. In this
case, the mixture shall be classified as a specific target organ
toxicant (specific organ specified), following single exposure,
repeated exposure, or both when at least one ingredient has been
classified as a Category 1 or Category 2 specific target organ
toxicant and is present at or above the appropriate cut-off value/
concentration limit specified in Table A.8.2 for Categories 1 and 2,
respectively.
Table A.8.2--Cut-Off Values/Concentration Limits of Ingredients of a
Mixture Classified as a Specific Target Organ Toxicant That Would
Trigger Classification of the Mixture as Category 1 or 2
------------------------------------------------------------------------
Cut-off values/concentration
limits triggering
classification of a mixture
Ingredient classified as: as:
-------------------------------
Category 1 Category 2
------------------------------------------------------------------------
Category 1 Target organ toxicant........ >=1.0% ..............
Category 2 Target organ toxicant........ .............. >=1.0%
------------------------------------------------------------------------
A.8.3.4.2 These cut-off values and consequent classifications
shall be applied equally and appropriately to both single- and
repeated-dose target organ toxicants.
A.8.3.4.3 Mixtures shall be classified for either or both single
and repeated dose toxicity independently.
A.8.3.4.4 Care shall be exercised when toxicants affecting more
than one organ system are combined that the potentiation or
synergistic interactions are considered, because certain substances
can cause target organ toxicity at <1% concentration when other
ingredients in the mixture are known to potentiate its toxic effect.
A.8.3.4.5 Care shall be exercised when extrapolating the
toxicity of a mixture that contains Category 3 ingredient(s). A cut-
off value/concentration limit of 20%, considered as an additive of
all Category 3 ingredients for each hazard endpoint, is appropriate;
however, this cut-off value/concentration limit may be higher or
lower depending on the Category 3 ingredient(s) involved and the
fact that some effects such as respiratory tract irritation may not
occur below a certain concentration while other effects such as
narcotic effects may occur below this 20% value. Expert judgment
shall be exercised. Respiratory tract irritation and narcotic
effects are to be evaluated separately in accordance with the
criteria given in A.8.2.2. When conducting classifications for these
hazards, the contribution of each ingredient should be considered
additive, unless there is evidence that the effects are not
additive.
A.9 SPECIFIC TARGET ORGAN TOXICITY REPEATED OR PROLONGED EXPOSURE
A.9.1 Definitions and general considerations
A.9.1.1 Specific target organ toxicity--repeated exposure (STOT-
RE) means specific target organ toxicity arising from repeated
exposure to a substance or mixture. All significant health effects
that can impair function, both reversible and irreversible,
immediate and/or delayed and not specifically addressed in A.1 to
A.7 and A.10 of this Appendix are included. Specific target organ
toxicity following a single-event exposure is classified in
accordance with SPECIFIC TARGET ORGAN TOXICITY--SINGLE EXPOSURE (A.8
of this Appendix) and is therefore not included here.
A.9.1.2 Classification identifies the substance or mixture as
being a specific target organ toxicant and, as such, it may present
a potential for adverse health effects in people who are exposed to
it.
A.9.1.3 These adverse health effects produced by repeated
exposure include consistent and identifiable toxic effects in
humans, or, in experimental animals, toxicologically significant
changes which have affected the function or morphology of a tissue/
organ, or have produced serious changes to the biochemistry or
hematology of the organism and these changes are relevant for human
health. Human data will be the primary source of evidence for this
hazard class.
A.9.1.4 Assessment shall take into consideration not only
significant changes in a single organ or biological system but also
generalized changes of a less severe nature involving several
organs.
A.9.1.5 Specific target organ toxicity can occur by any route
that is relevant for humans, e.g., principally oral, dermal or
inhalation.
A.9.2 Classification Criteria for Substances
A.9.2.1 Substances shall be classified as STOT-RE by expert
judgment on the basis of the weight of all evidence available,
including the use of recommended guidance values which take into
account the duration of exposure and the dose/concentration which
produced the effect(s), (See A.9.2.9). Substances shall be placed in
one of two categories, depending upon the nature and severity of the
effect(s) observed, in accordance with Figure A.9.1.
Figure A.9.1--Hazard Categories for Specific Target Organ Toxicity
Following Repeated Exposure
------------------------------------------------------------------------
-------------------------------------------------------------------------
CATEGORY 1: Substances that have produced significant toxicity in
humans, or that, on the basis of evidence from studies in experimental
animals can be presumed to have the potential to produce significant
toxicity in humans following repeated or prolonged exposure
Substances are classified in Category 1 for specific target organ
toxicity (repeated exposure) on the basis of:
(a) reliable and good quality evidence from human cases or
epidemiological studies; or,
(b) observations from appropriate studies in experimental animals in
which significant and/or severe toxic effects, of relevance to
human health, were produced at generally low exposure
concentrations. Guidance dose/concentration values are provided
below (See A.9.2.9) to be used as part of weight-of-evidence
evaluation.
CATEGORY 2: Substances that, on the basis of evidence from studies in
experimental animals can be presumed to have the potential to be
harmful to human health following repeated or prolonged exposure
Substances are classified in Category 2 for specific target organ
toxicity (repeated exposure) on the basis of observations from
appropriate studies in experimental animals in which significant
toxic effects, of relevance to human health, were produced at
generally moderate exposure concentrations. Guidance dose/
concentration values are provided below (See A.9.2.9) in order to
help in classification.
In exceptional cases human evidence can also be used to place a
substance in Category 2 (See A.9.2.6).
Note: The primary target organ/system shall be identified where
possible, or the substance shall be identified as a general toxicant.
The data shall be carefully evaluated and, where possible, shall not
include secondary effects (e.g., a hepatotoxicant can produce secondary
effects in the nervous or gastro-intestinal systems).
------------------------------------------------------------------------
A.9.2.2 The relevant route of exposure by which the classified
substance produces damage shall be identified.
A.9.2.3 Classification is determined by expert judgment, on the
basis of the weight of all evidence available including the guidance
presented below.
A.9.2.4 Weight of evidence of all data, including human
incidents, epidemiology, and studies conducted in experimental
animals, is used to substantiate specific target organ toxic effects
that merit classification.
A.9.2.5 The information required to evaluate specific target
organ toxicity comes either from repeated exposure in humans, e.g.,
exposure at home, in the workplace or environmentally, or from
studies conducted in experimental animals. The standard animal
studies in rats or mice that provide this information are 28 day, 90
day or lifetime studies (up to 2 years) that include hematological,
clinico-chemical and detailed macroscopic and microscopic
examination to enable the toxic effects on target tissues/organs to
be identified. Data from repeat dose studies performed in other
species may also be used. Other long-term exposure studies, e.g.,
for carcinogenicity, neurotoxicity or reproductive toxicity, may
also provide evidence of specific target organ toxicity that could
be used in the assessment of classification.
A.9.2.6 In exceptional cases, based on expert judgment, it may
be appropriate to place certain substances with human evidence of
specific target organ toxicity in Category 2: (a) when the weight of
human evidence is not sufficiently convincing to warrant Category 1
classification, and/or (b) based on the nature and severity of
effects. Dose/concentration levels in humans shall not be considered
in the classification and any available evidence from animal studies
shall be consistent with the Category 2 classification. In other
words, if there are also animal data available on the substance that
warrant Category 1 classification, the substance shall be classified
as Category 1.
A.9.2.7 Effects Considered To Support Classification
A.9.2.7.1 Classification is supported by reliable evidence
associating repeated exposure to the substance with a consistent and
identifiable toxic effect.
A.9.2.7.2 Evidence from human experience/incidents is usually
restricted to reports of adverse health consequences, often with
uncertainty about exposure conditions, and may not provide the
scientific detail that can be obtained from well-conducted studies
in experimental animals.
A.9.2.7.3 Evidence from appropriate studies in experimental
animals can furnish much more detail, in the form of clinical
observations, hematology, clinical chemistry, macroscopic and
microscopic pathological examination and this can often reveal
hazards that may not be life-threatening but could indicate
functional impairment. Consequently all available evidence, and
relevance to human health, must be taken into consideration in the
classification process. Relevant toxic effects in humans and/or
animals include, but are not limited to:
(a) Morbidity or death resulting from repeated or long-term
exposure. Morbidity or death may result from repeated exposure, even
to relatively low doses/concentrations, due to bioaccumulation of
the substance or its metabolites, or due to the overwhelming of the
de-toxification process by repeated exposure;
(b) Significant functional changes in the central or peripheral
nervous systems or other organ systems, including signs of central
nervous system depression and effects on special senses (e.g.,
sight, hearing and sense of smell);
(c) Any consistent and significant adverse change in clinical
biochemistry, hematology, or urinalysis parameters;
(d) Significant organ damage that may be noted at necropsy and/
or subsequently seen or confirmed at microscopic examination;
(e) Multi-focal or diffuse necrosis, fibrosis or granuloma
formation in vital organs with regenerative capacity;
(f) Morphological changes that are potentially reversible but
provide clear evidence of marked organ dysfunction (e.g., severe
fatty change in the liver); and,
(g) Evidence of appreciable cell death (including cell
degeneration and reduced cell number) in vital organs incapable of
regeneration.
A.9.2.8 Effects Considered Not To Support Classification
Effects may be seen in humans and/or animals that do not justify
classification. Such effects include, but are not limited to:
(a) Clinical observations or small changes in bodyweight gain,
food consumption or water intake that may have some toxicological
importance but that do not, by themselves, indicate "significant"
toxicity;
(b) Small changes in clinical biochemistry, hematology or
urinalysis parameters and/or transient effects, when such changes or
effects are of doubtful or of minimal toxicological importance;
(c) Changes in organ weights with no evidence of organ
dysfunction;
(d) Adaptive responses that are not considered toxicologically
relevant;
(e) Substance-induced species-specific mechanisms of toxicity,
i.e., demonstrated with reasonable certainty to be not relevant for
human health, shall not justify classification.
A.9.2.9 Guidance Values To Assist With Classification Based on the
Results Obtained From Studies Conducted in Experimental Animals
A.9.2.9.1 In studies conducted in experimental animals, reliance
on observation of effects alone, without reference to the duration
of experimental exposure and dose/concentration, omits a fundamental
concept of toxicology, i.e., all substances are potentially toxic,
and what determines the toxicity is a function of the dose/
concentration and the duration of exposure. In most studies
conducted in experimental animals the test guidelines use an upper
limit dose value.
A.9.2.9.2 In order to help reach a decision about whether a
substance shall be classified or not, and to what degree it shall be
classified (Category 1 vs. Category 2), dose/concentration
"guidance values" are provided in Table A.9.1 for consideration of
the dose/concentration which has been shown to produce significant
health effects. The principal argument for proposing such guidance
values is that all chemicals are potentially toxic and there has to
be a reasonable dose/concentration above which a degree of toxic
effect is acknowledged. Also, repeated-dose studies conducted in
experimental animals are designed to produce toxicity at the highest
dose used in order to optimize the test objective and so most
studies will reveal some toxic effect at least at this highest dose.
What is therefore to be decided is not only what effects have been
produced, but also at what dose/concentration they were produced and
how relevant is that for humans.
A.9.2.9.3 Thus, in animal studies, when significant toxic
effects are observed that indicate classification, consideration of
the duration of experimental exposure and the dose/concentration at
which these effects were seen, in relation to the suggested guidance
values, provides useful information to help assess the need to
classify (since the toxic effects are a consequence of the hazardous
property(ies) and also the duration of exposure and the dose/
concentration).
A.9.2.9.4 The decision to classify at all can be influenced by
reference to the dose/concentration guidance values at or below
which a significant toxic effect has been observed.
A.9.2.9.5 The guidance values refer to effects seen in a
standard 90-day toxicity study conducted in rats. They can be used
as a basis to extrapolate equivalent guidance values for toxicity
studies of greater or lesser duration, using dose/exposure time
extrapolation similar to Haber's rule for inhalation, which states
essentially that the effective dose is directly proportional to the
exposure concentration and the duration of exposure. The assessment
should be done on a case-by-case basis; for example, for a 28-day
study the guidance values below would be increased by a factor of
three.
A.9.2.9.6 Thus for Category 1 classification, significant toxic
effects observed in a 90-day repeated-dose study conducted in
experimental animals and seen to occur at or below the (suggested)
guidance values (C) as indicated in Table A.9.1 would justify
classification:
Table A.9.1--Guidance Values To Assist in Category 1 Classification
[Applicable to a 90-day study]
------------------------------------------------------------------------
Guidance values
Route of exposure Units (dose/concentration)
------------------------------------------------------------------------
Oral (rat).................. mg/kg body weight/ C <=10.
day.
Dermal (rat or rabbit)...... mg/kg body weight/ C <=20.
day.
Inhalation (rat) gas........ ppmV/6h/day......... C <=50.
Inhalation (rat) vapor...... mg/liter/6h/day..... C <=0.2.
Inhalation (rat) dust/mist/ mg/liter/6h/day..... C <=0.02.
fume.
------------------------------------------------------------------------
A.9.2.9.7 For Category 2 classification, significant toxic
effects observed in a 90-day repeated-dose study conducted in
experimental animals and seen to occur within the (suggested)
guidance value ranges as indicated in Table A.9.2 would justify
classification:
Table A.9.2--Guidance Values To Assist in Category 2 Classification
[Applicable to a 90-day study]
------------------------------------------------------------------------
Guidance values
Route of exposure Units (dose/concentration)
------------------------------------------------------------------------
Oral (rat).................. mg/kg body weight/ 10 =100 mg/kg body weight/day by the oral route, and in addition there
is supplementary information from other sources, e.g., other long-
term administration studies, or human case experience, which
supports a conclusion that, in view of the weight of evidence,
classification is prudent.
A.9.2.10 Other Considerations
A.9.2.10.1 When a substance is characterized only by use of
animal data the classification process includes reference to dose/
concentration guidance values as one of the elements that contribute
to the weight of evidence approach.
A.9.2.10.2 When well-substantiated human data are available
showing a specific target organ toxic effect that can be reliably
attributed to repeated or prolonged exposure to a substance, the
substance shall be classified. Positive human data, regardless of
probable dose, predominates over animal data. Thus, if a substance
is unclassified because no specific target organ toxicity was seen
at or below the dose/concentration guidance value for animal
testing, if subsequent human incident data become available showing
a specific target organ toxic effect, the substance shall be
classified.
A.9.2.10.3 A substance that has not been tested for specific
target organ toxicity may in certain instances, where appropriate,
be classified on the basis of data from a scientifically validated
structure activity relationship and expert judgment-based
extrapolation from a structural analogue that has previously been
classified together with substantial support from consideration of
other important factors such as formation of common significant
metabolites.
A.9.3 Classification Criteria for Mixtures
A.9.3.1 Mixtures are classified using the same criteria as for
substances, or alternatively as described below. As with substances,
mixtures may be classified for specific target organ toxicity
following single exposure, repeated exposure, or both.
A.9.3.2 Classification of Mixtures When Data Are Available for the
Complete Mixture
When reliable and good quality evidence from human experience or
appropriate studies in experimental animals, as described in the
criteria for substances, is available for the mixture, then the
mixture shall be classified by weight of evidence evaluation of
these data. Care shall be exercised in evaluating data on mixtures,
that the dose, duration, observation or analysis, do not render the
results inconclusive.
A.9.3.3 Classification of Mixtures When Data Are Not Available for the
Complete Mixture: Bridging Principles
A.9.3.3.1 Where the mixture itself has not been tested to
determine its specific target organ toxicity, but there are
sufficient data on both the individual ingredients and similar
tested mixtures to adequately characterize the hazards of the
mixture, these data shall be used in accordance with the following
bridging principles as found in paragraph A.0.5 of this Appendix:
Dilution; Batching; Concentration of mixtures; Interpolation within
one toxicity category; Substantially similar mixtures; and Aerosols.
A.9.3.4 Classification of Mixtures When Data Are Available for All
Ingredients or Only for Some Ingredients of the Mixture
A.9.3.4.1 Where there is no reliable evidence or test data for
the specific mixture itself, and the bridging principles cannot be
used to enable classification, then classification of the mixture is
based on the classification of the ingredient substances. In this
case, the mixture shall be classified as a specific target organ
toxicant (specific organ specified), following single exposure,
repeated exposure, or both when at least one ingredient has been
classified as a Category 1 or Category 2 specific target organ
toxicant and is present at or above the appropriate cut-off value/
concentration limit specified in Table A.9.3 for Category 1 and 2
respectively.
Table A.9.3--Cut-Off Value/Concentration Limits of Ingredients of a
Mixture Classified as a Specific Target Organ Toxicant That Would
Trigger Classification of the Mixture as Category 1 or 2
------------------------------------------------------------------------
Cut-off values/concentration
limits triggering
classification of a mixture
Ingredient classified as: as:
-------------------------------
Category 1 Category 2
------------------------------------------------------------------------
Category 1 Target organ toxicant........ >=1.0% ..............
Category 2 Target organ toxicant........ .............. >=1.0%
------------------------------------------------------------------------
A.9.3.4.2 These cut-off values and consequent classifications
shall be applied equally and appropriately to both single- and
repeated-dose target organ toxicants.
A.9.3.4.3 Mixtures shall be classified for either or both
single- and repeated-dose toxicity independently.
A.9.3.4.4 Care shall be exercised when toxicants affecting more
than one organ system are combined that the potentiation or
synergistic interactions are considered, because certain substances
can cause specific target organ toxicity at <1% concentration when
other ingredients in the mixture are known to potentiate its toxic
effect.
A.10 ASPIRATION HAZARD
A.10.1 Definitions and General and Specific Considerations
A.10.1.1 Aspiration means the entry of a liquid or solid
chemical directly through the oral or nasal cavity, or indirectly
from vomiting, into the trachea and lower respiratory system.
A.10.1.2 Aspiration toxicity includes severe acute effects such
as chemical pneumonia, varying degrees of pulmonary injury or death
following aspiration.
A.10.1.3 Aspiration is initiated at the moment of inspiration,
in the time required to take one breath, as the causative material
lodges at the crossroad of the upper respiratory and digestive
tracts in the laryngopharyngeal region.
A.10.1.4 Aspiration of a substance or mixture can occur as it is
vomited following ingestion. This may have consequences for
labeling, particularly where, due to acute toxicity, a
recommendation may be considered to induce vomiting after ingestion.
However, if the substance/mixture also presents an aspiration
toxicity hazard, the recommendation to induce vomiting may need to
be modified.
A.10.1.5 Specific Considerations
A.10.1.5.1 The classification criteria refer to kinematic
viscosity. The following provides the conversion between dynamic and
kinematic viscosity:
[GRAPHIC] [TIFF OMITTED] TR26MR12.130
A.10.1.5.2 Although the definition of aspiration in A.10.1.1
includes the entry of solids into the respiratory system,
classification according to (b) in table A.10.1 for Category 1 is
intended to apply to liquid substances and mixtures only.
A.10.1.5.3 Classification of aerosol/mist products.
Aerosol and mist products are usually dispensed in containers
such as self-pressurized containers, trigger and pump sprayers.
Classification for these products shall be considered if their use
may form a pool of product in the mouth, which then may be
aspirated. If the mist or aerosol from a pressurized container is
fine, a pool may not be formed. On the other hand, if a pressurized
container dispenses product in a stream, a pool may be formed that
may then be aspirated. Usually, the mist produced by trigger and
pump sprayers is coarse and therefore, a pool may be formed that
then may be aspirated. When the pump mechanism may be removed and
contents are available to be swallowed then the classification of
the products should be considered.
A.10.2 Classification Criteria for Substances
Table A.10.1--Criteria for Aspiration Toxicity
------------------------------------------------------------------------
Category Criteria
------------------------------------------------------------------------
Category 1: Chemicals known to cause A substance shall be classified
human aspiration toxicity hazards or in Category 1:
to be regarded as if they cause human (a) If reliable and good
aspiration toxicity hazard. quality human evidence
indicates that it causes
aspiration toxicity (See
note); or
(b) If it is a hydrocarbon and
has a kinematic viscosity
<=20.5 mm\2\/s, measured at 40
[deg]C.
------------------------------------------------------------------------
Note: Examples of substances included in Category 1 are certain
hydrocarbons, turpentine and pine oil.
A.10.3 Classification Criteria for Mixtures
A.10.3.1 Classification When Data Are Available for the Complete
Mixture
A mixture shall be classified in Category 1 based on reliable
and good quality human evidence.
A.10.3.2 Classification of Mixtures When Data Are Not Available for the
Complete Mixture: Bridging Principles
A.10.3.2.1 Where the mixture itself has not been tested to
determine its aspiration toxicity, but there are sufficient data on
both the individual ingredients and similar tested mixtures to
adequately characterize the hazard of the mixture, these data shall
be used in accordance with the following bridging principles as
found in paragraph A.0.5 of this Appendix: Dilution; Batching;
Concentration of mixtures; Interpolation within one toxicity
category; and Substantially similar mixtures. For application of the
dilution bridging principle, the concentration of aspiration
toxicants shall not be less than 10%.
A.10.3.3 Classification of Mixtures When Data Are Available for All
Ingredients or Only for Some Ingredients of the Mixture
A.10.3.3.1 A mixture which contains >=10% of an ingredient or
ingredients classified in Category 1, and has a kinematic viscosity
<=20.5 mm\2\/s, measured at 40 [deg]C, shall be classified in
Category 1.
A.10.3.3.2 In the case of a mixture which separates into two or
more distinct layers, one of which contains >=10% of an ingredient
or ingredients classified in Category 1 and has a kinematic
viscosity <=20.5 mm\2\/s, measured at 40 [deg]C, then the entire
mixture shall be classified in Category 1.
APPENDIX B TO Sec. 1910.1200--PHYSICAL CRITERIA (MANDATORY)
B.1 EXPLOSIVES
B.1.1 Definitions and General Considerations
B.1.1.1 An explosive chemical is a solid or liquid chemical
which is in itself capable by chemical reaction of producing gas at
such a temperature and pressure and at such a speed as to cause
damage to the surroundings. Pyrotechnic chemicals are included even
when they do not evolve gases.
A pyrotechnic chemical is a chemical designed to produce an
effect by heat, light, sound, gas or smoke or a combination of these
as the result of non-detonative self-sustaining exothermic chemical
reactions.
An explosive item is an item containing one or more explosive
chemicals.
A pyrotechnic item is an item containing one or more pyrotechnic
chemicals.
An unstable explosive is an explosive which is thermally
unstable and/or too sensitive for normal handling, transport, or
use.
An intentional explosive is a chemical or item which is
manufactured with a view to produce a practical explosive or
pyrotechnic effect.
B.1.1.2 The class of explosives comprises:
(a) Explosive chemicals;
(b) Explosive items, except devices containing explosive
chemicals in such quantity or of such a character that their
inadvertent or accidental ignition or initiation shall not cause any
effect external to the device either by projection, fire, smoke,
heat or loud noise; and
(c) Chemicals and items not included under (a) and (b) above
which are manufactured with the view to producing a practical
explosive or pyrotechnic effect.
B.1.2 Classification Criteria
Chemicals and items of this class shall be classified as
unstable explosives or shall be assigned to one of the following six
divisions depending on the type of hazard they present:
(a) Division 1.1--Chemicals and items which have a mass
explosion hazard (a mass explosion is one which affects almost the
entire quantity present virtually instantaneously);
(b) Division 1.2--Chemicals and items which have a projection
hazard but not a mass explosion hazard;
(c) Division 1.3--Chemicals and items which have a fire hazard
and either a minor blast hazard or a minor projection hazard or
both, but not a mass explosion hazard:
(i) Combustion of which gives rise to considerable radiant heat;
or
(ii) Which burn one after another, producing minor blast or
projection effects or both;
(d) Division 1.4--Chemicals and items which present no
significant hazard: chemicals and items which present only a small
hazard in the event of ignition or initiation. The effects are
largely confined to the package and no projection of fragments of
appreciable size or range is to be expected. An external fire shall
not cause virtually instantaneous explosion of almost the entire
contents of the package;
(e) Division 1.5--Very insensitive chemicals which have a mass
explosion hazard: chemicals which have a mass explosion hazard but
are so insensitive that there is very little probability of
initiation or of transition from burning to detonation under normal
conditions;
(f) Division 1.6--Extremely insensitive items which do not have
a mass explosion hazard: items which contain only extremely
insensitive detonating chemicals and which demonstrate a negligible
probability of accidental initiation or propagation.
B.1.3 Additional Classification Considerations
B.1.3.1 Explosives shall be classified as unstable explosives or
shall be assigned to one of the six divisions identified in B.1.2 in
accordance with the three step procedure in Part I of the UN ST/SG/
AC.10 (incorporated by reference; See Sec. 1910.6). The first step
is to ascertain whether the substance or mixture has explosive
effects (Test Series 1). The second step is the acceptance procedure
(Test Series 2 to 4) and the third step is the assignment to a
hazard division (Test Series 5 to 7). The assessment whether a
candidate for "ammonium nitrate emulsion or suspension or gel,
intermediate for blasting explosives (ANE)" is insensitive enough
for inclusion as an oxidizing liquid (See B.13) or an oxidizing
solid (See B.14) is determined by Test Series 8 tests.
Note: Classification of solid chemicals shall be based on tests
performed on the chemical as presented. If, for example, for the
purposes of supply or transport, the same
chemical is to be presented in a physical form different from that
which was tested and which is considered likely to materially alter
its performance in a classification test, classification must be
based on testing of the chemical in the new form.
B.1.3.2 Explosive properties are associated with the presence of
certain chemical groups in a molecule which can react to produce
very rapid increases in temperature or pressure. The screening
procedure in B.1.3.1 is aimed at identifying the presence of such
reactive groups and the potential for rapid energy release. If the
screening procedure identifies the chemical as a potential
explosive, the acceptance procedure (See section 10.3 of the UN ST/
SG/AC.10 (incorporated by reference; See Sec. 1910.6)) is necessary
for classification.
Note: Neither a Series 1 type (a) propagation of detonation test
nor a Series 2 type (a) test of sensitivity to detonative shock is
necessary if the exothermic decomposition energy of organic
materials is less than 800 J/g.
B.1.3.3 If a mixture contains any known explosives, the
acceptance procedure is necessary for classification.
B.1.3.4 A chemical is not classified as explosive if:
(a) There are no chemical groups associated with explosive
properties present in the molecule. Examples of groups which may
indicate explosive properties are given in Table A6.1 in Appendix 6
of the UN ST/SG/AC.10 (incorporated by reference; See Sec. 1910.6);
or
(b) The substance contains chemical groups associated with
explosive properties which include oxygen and the calculated oxygen
balance is less than -200.
The oxygen balance is calculated for the chemical reaction:
CxHyOz + [x + (y/4) - (z/2)]
O2 [rarr] x. CO2 + (y/2) H2O
using the formula:
oxygen balance = -1600 [2x +(y/2) -z]/molecular weight;
or
(c) The organic substance or a homogenous mixture of organic
substances contains chemical groups associated with explosive
properties but the exothermic decomposition energy is less than 500
J/g and the onset of exothermic decomposition is below 500 [deg]C
(932 [deg]F). The exothermic decomposition energy may be determined
using a suitable calorimetric technique; or
(d) For mixtures of inorganic oxidizing substances with organic
material(s), the concentration of the inorganic oxidizing substance
is:
(i) Less than 15%, by mass, if the oxidizing substance is
assigned to Category 1 or 2;
(ii) Less than 30%, by mass, if the oxidizing substance is
assigned to Category 3.
B.2 FLAMMABLE GASES
B.2.1 Definition
Flammable gas means a gas having a flammable range with air at
20 [deg]C (68 [deg]F) and a standard pressure of 101.3 kPa (14.7
psi).
B.2.2 Classification Criteria
A flammable gas shall be classified in one of the two categories
for this class in accordance with Table B.2.1:
Table B.2.1--Criteria for Flammable Gases
----------------------------------------------------------------------------------------------------------------
Category Criteria
----------------------------------------------------------------------------------------------------------------
1........................................... Gases, which at 20 [deg]C (68 [deg]F) and a standard pressure of
101.3 kPa (14.7 psi):
(a) are ignitable when in a mixture of 13% or less by volume in
air; or
(b) have a flammable range with air of at least 12 percentage
points regardless of the lower flammable limit.
2........................................... Gases, other than those of Category 1, which, at 20 [deg]C (68
[deg]F) and a standard pressure of 101.3 kPa (14.7 psi), have a
flammable range while mixed in air.
----------------------------------------------------------------------------------------------------------------
Note: Aerosols should not be classified as flammable gases. See
B.3.
B.2.3 Additional Classification Considerations
Flammability shall be determined by tests or by calculation in
accordance with ISO 10156 (incorporated by reference; See Sec.
1910.6). Where insufficient data are available to use this method,
equivalent validated methods may be used.
B.3 FLAMMABLE AEROSOLS
B.3.1 Definition
Aerosol means any non-refillable receptacle containing a gas
compressed, liquefied or dissolved under pressure, and fitted with a
release device allowing the contents to be ejected as particles in
suspension in a gas, or as a foam, paste, powder, liquid or gas.
B.3.2 Classification Criteria
B.3.2.1 Aerosols shall be considered for classification as
flammable if they contain any component which is classified as
flammable in accordance with this Appendix, i.e.:
Flammable liquids (See B.6);
Flammable gases (See B.2);
Flammable solids (See B.7).
Note 1: Flammable components do not include pyrophoric, self-
heating or water-reactive chemicals.
Note 2: Flammable aerosols do not fall additionally within the
scope of flammable gases, flammable liquids, or flammable solids.
B.3.2.2 A flammable aerosol shall be classified in one of the
two categories for this class in accordance with Table B.3.1.
Table B.3.1--Criteria for Flammable Aerosols
----------------------------------------------------------------------------------------------------------------
Category Criteria
----------------------------------------------------------------------------------------------------------------
1........................................... Contains >=85% flammable components and the chemical heat of
combustion is >=30 kJ/g; or
(a) For spray aerosols, in the ignition distance test, ignition
occurs at a distance >=75 cm (29.5 in), or
(b) For foam aerosols, in the aerosol foam flammability test.
(i) The flame height is >=20 cm (7.87 in) and the flame duration
>=2 s; or
(ii) The flame height is >=4 cm (1.57 in) and the flame duration
>=7 s.
2........................................... Contains >1% flammable components, or the heat of combustion is
>=20 kJ/g; and
(a) for spray aerosols, in the ignition distance test, ignition
occurs at a distance >=15 cm (5.9 in), or in the enclosed space
ignition test, the
(i) Time equivalent is <=300 s/m\3\; or
(ii) Deflagration density is <=300 g/m\3\.
(b) For foam aerosols, in the aerosol foam flammability test, the
flame height is >=4 cm and the flame duration is >=2 s and it
does not meet the criteria for Category 1.
----------------------------------------------------------------------------------------------------------------
Note: Aerosols not submitted to the flammability classification
procedures in this Appendix shall be classified as extremely
flammable (Category 1).
B.3.3 Additional Classification Considerations
B.3.3.1 To classify a flammable aerosol, data on its flammable
components, on its chemical heat of combustion and, if applicable,
the results of the aerosol foam flammability test (for foam
aerosols) and of the ignition distance test and enclosed space test
(for spray aerosols) are necessary.
B.3.3.2 The chemical heat of combustion ([Delta]Hc), in
kilojoules per gram (kJ/g), is the product of the theoretical heat
of combustion ([Delta]Hcomb), and a combustion efficiency, usually
less than 1.0 (a typical combustion efficiency is 0.95 or 95%).
For a composite aerosol formulation, the chemical heat of
combustion is the summation of the weighted heats of combustion for
the individual components, as follows:
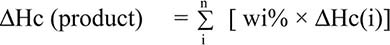 Where:
[Delta]Hc = chemical heat of combustion (kJ/g);
wi% = mass fraction of component i in the product;
[Delta]Hc(i) = specific heat of combustion (kJ/g) of component i in
the product;
The chemical heats of combustion shall be found in literature,
calculated or determined by tests (See ASTM D240-02, ISO 13943,
Sections 86.1 to 86.3, and NFPA 30B (incorporated by reference; See
Sec. 1910.6)).
B.3.3.3 The Ignition Distance Test, Enclosed Space Ignition Test
and Aerosol Foam Flammability Test shall be performed in accordance
with sub-sections 31.4, 31.5 and 31.6 of the of the UN ST/SG/AC.10
(incorporated by reference; See Sec. 1910.6).
B.4 OXIDIZING GASES
B.4.1 Definition
Oxidizing gas means any gas which may, generally by providing
oxygen, cause or contribute to the combustion of other material more
than air does.
Note: "Gases which cause or contribute to the combustion of
other material more than air does" means pure gases or gas mixtures
with an oxidizing power greater than 23.5% (as determined by a
method specified in ISO 10156 or 10156-2 (incorporated by reference,
See Sec. 1910.6) or an equivalent testing method.)
B.4.2 Classification Criteria
An oxidizing gas shall be classified in a single category for
this class in accordance with Table B.4.1:
Table B.4.1--Criteria for Oxidizing Gases
----------------------------------------------------------------------------------------------------------------
Category Criteria
----------------------------------------------------------------------------------------------------------------
1........................................... Any gas which may, generally by providing oxygen, cause or
contribute to the combustion of other material more than air
does.
----------------------------------------------------------------------------------------------------------------
B.4.3 Additional Classification Considerations
Classification shall be in accordance with tests or calculation
methods as described in ISO 10156 (incorporated by reference; See
Sec. 1910.6) and ISO 10156-2 (incorporated by reference; See Sec.
1910.6).
B.5 GASES UNDER PRESSURE
B.5.1 Definition
Gases under pressure are gases which are contained in a
receptacle at a pressure of 200 kPa (29 psi) (gauge) or more, or
which are liquefied or liquefied and refrigerated.
They comprise compressed gases, liquefied gases, dissolved gases
and refrigerated liquefied gases.
B.5.2 Classification Criteria
Gases under pressure shall be classified in one of four groups
in accordance with Table B.5.1:
Table B.5.1--Criteria for Gases Under Pressure
----------------------------------------------------------------------------------------------------------------
Group Criteria
----------------------------------------------------------------------------------------------------------------
Compressed gas.............................. A gas which when under pressure is entirely gaseous at -50 [deg]C
(-8 [deg]F), including all gases with a critical temperature\1\
<=-50 [deg]C (-58 [deg]F).
Liquefied gas............................... A gas which when under pressure is partially liquid at
temperatures above -50 [deg]C (-58 [deg]F). A distinction is made
between:
(a) High pressure liquefied gas: A gas with a critical
temperature \1\ between -50 [deg]C (-58 [deg]F) and +65 [deg]C
(149 [deg]F); and
(b) Low pressure liquefied gas: A gas with a critical
temperature \1\ above +65 [deg]C (149 [deg]F).
Refrigerated liquefied gas.................. A gas which is made partially liquid because of its low
temperature.
Dissolved gas............................... A gas which when under pressure is dissolved in a liquid phase
solvent.
----------------------------------------------------------------------------------------------------------------
\1\ The critical temperature is the temperature above which a pure gas cannot be liquefied, regardless of the
degree of compression.
B.6 FLAMMABLE LIQUIDS
B.6.1 Definition
Flammable liquid means a liquid having a flash point of not more
than 93 [deg]C (199.4 [deg]F).
Flash point means the minimum temperature at which a liquid
gives off vapor in sufficient concentration to form an ignitable
mixture with air near the surface of the liquid, as determined by a
method identified in Section B.6.3.
B.6.2 Classification Criteria
A flammable liquid shall be classified in one of four categories
in accordance with Table B.6.1:
Table B.6.1--Criteria for Flammable Liquids
--------------------------------------------------------------------------------------------------------------------------------------------------------
Category Criteria
--------------------------------------------------------------------------------------------------------------------------------------------------------
1........................................... Flash point <23 [deg]C (73.4 [deg]F) and initial boiling point <=35 [deg]C (95 [deg]F).
2........................................... Flash point <23 [deg]C (73.4 [deg]F) and initial boiling point >35 [deg]C (95 [deg]F).
3........................................... Flash point >=23 [deg]C (73.4 [deg]F) and <=60 [deg]C (140 [deg]F).
4........................................... Flash point >60 [deg]C (140 [deg]F) and <=93 [deg]C (199.4 [deg]F).
--------------------------------------------------------------------------------------------------------------------------------------------------------
B.6.3 Additional Classification Considerations
The flash point shall be determined in accordance with ASTM D56-
05, ASTM D3278, ASTM D3828, ASTM D93-08 (incorporated by reference;
See Sec. 1910.6), or any other method specified in GHS Revision 3,
Chapter 2.6.
The initial boiling point shall be determined in accordance with
ASTM D86-07a or ASTM D1078 (incorporated by reference; See Sec.
1910.6).
B.7 FLAMMABLE SOLIDS
B.7.1 Definitions
Flammable solid means a solid which is a readily combustible
solid, or which may cause or contribute to fire through friction.
Readily combustible solids are powdered, granular, or pasty
chemicals which are dangerous if they can be easily ignited by brief
contact with an ignition source, such as a burning match, and if the
flame spreads rapidly.
B.7.2 Classification Criteria
B.7.2.1 Powdered, granular or pasty chemicals shall be
classified as flammable solids when the time of burning of one or
more of the test runs, performed in accordance with the test method
described in the UN ST/SG/AC.10 (incorporated by reference; See
Sec. 1910.6), Part III, sub-section 33.2.1, is less than 45 s or
the rate of burning is more than 2.2 mm/s (0.0866 in/s).
B.7.2.2 Powders of metals or metal alloys shall be classified as
flammable solids when they can be ignited and the reaction spreads
over the whole length of the sample in 10 min or less.
B.7.2.3 Solids which may cause fire through friction shall be
classified in this class by analogy with existing entries (e.g.,
matches) until definitive criteria are established.
B.7.2.4 A flammable solid shall be classified in one of the two
categories for this class using Method N.1 as described in Part III,
sub-section 33.2.1 of the UN ST/SG/AC.10 (incorporated by reference;
See Sec. 1910.6), in accordance with Table B.7.1:
Table B.7.1--Criteria for Flammable Solids
------------------------------------------------------------------------
Category Criteria
------------------------------------------------------------------------
1...................................... Burning rate test:
Chemicals other than metal
powders:
(a) Wetted zone does not
stop fire; and
(b) Burning time <45 s or
burning rate >2.2 mm/s.
Metal powders: Burning time
<=5 min.
2...................................... Burning rate test:
Chemicals other than metal
powders:
(a) Wetted zone stops the
fire for at least 4 min;
and
(b) Burning time <45 s or
burning rate >2.2 mm/s.
Metal powders: Burning time
>5 min and <=10 min.
------------------------------------------------------------------------
Note: Classification of solid chemicals shall be based on tests
performed on the chemical as presented. If, for example, for the
purposes of supply or transport, the same chemical is to be
presented in a physical form different from that which was tested
and which is considered likely to materially alter its performance
in a classification test, classification must be based on testing of
the chemical in the new form.
B.8 SELF-REACTIVE CHEMICALS
B.8.1 Definitions
Self-reactive chemicals are thermally unstable liquid or solid
chemicals liable to undergo a strongly exothermic decomposition even
without participation of oxygen (air). This definition excludes
chemicals classified under this section as explosives, organic
peroxides, oxidizing liquids or oxidizing solids.
A self-reactive chemical is regarded as possessing explosive
properties when in laboratory testing the formulation is liable to
detonate, to deflagrate rapidly or to show a violent effect when
heated under confinement.
B.8.2 Classification Criteria
B.8.2.1 A self-reactive chemical shall be considered for
classification in this class unless:
(a) It is classified as an explosive according to B.1 of this
appendix;
(b) It is classified as an oxidizing liquid or an oxidizing
solid according to B.13 or B.14 of this appendix, except that a
mixture of oxidizing substances which contains 5% or more of
combustible organic substances shall be classified as a self-
reactive chemical according to the procedure defined in B.8.2.2;
(c) It is classified as an organic peroxide according to B.15 of
this appendix;
(d) Its heat of decomposition is less than 300 J/g; or
(e) Its self-accelerating decomposition temperature (SADT) is
greater than 75 [deg]C (167 [deg]F) for a 50 kg (110 lb) package.
B.8.2.2 Mixtures of oxidizing substances, meeting the criteria
for classification as oxidizing liquids or oxidizing solids, which
contain 5% or more of combustible organic substances and which do
not meet the criteria mentioned in B.8.2.1 (a), (c), (d) or (e),
shall be subjected to the self-reactive chemicals classification
procedure in B.8.2.3. Such a mixture showing the properties of a
self-reactive chemical type B to F shall be classified as a self-
reactive chemical.
B.8.2.3 Self-reactive chemicals shall be classified in one of
the seven categories of "types A to G" for this class, according
to the following principles:
(a) Any self-reactive chemical which can detonate or deflagrate
rapidly, as packaged, will be defined as self-reactive chemical TYPE
A;
(b) Any self-reactive chemical possessing explosive properties
and which, as packaged, neither detonates nor deflagrates rapidly,
but is liable to undergo a thermal explosion in that package will be
defined as self-reactive chemical TYPE B;
(c) Any self-reactive chemical possessing explosive properties
when the chemical as packaged cannot detonate or deflagrate rapidly
or undergo a thermal explosion will be defined as self-reactive
chemical TYPE C;
(d) Any self-reactive chemical which in laboratory testing meets
the criteria in (d)(i), (ii), or (iii) will be defined as self-
reactive chemical TYPE D:
(i) Detonates partially, does not deflagrate rapidly and shows
no violent effect when heated under confinement; or
(ii) Does not detonate at all, deflagrates slowly and shows no
violent effect when heated under confinement; or
(iii) Does not detonate or deflagrate at all and shows a medium
effect when heated under confinement;
(e) Any self-reactive chemical which, in laboratory testing,
neither detonates nor deflagrates at all and shows low or no effect
when heated under confinement will be defined as self-reactive
chemical TYPE E;
(f) Any self-reactive chemical which, in laboratory testing,
neither detonates in the cavitated state nor deflagrates at all and
shows only a low or no effect when heated under confinement as well
as low or no explosive power will be defined as self-reactive
chemical TYPE F;
(g) Any self-reactive chemical which, in laboratory testing,
neither detonates in the cavitated state nor deflagrates at all and
shows no effect when heated under confinement nor any explosive
power, provided that it is thermally stable (self-accelerating
decomposition temperature is 60 [deg]C (140 [deg]F) to 75 [deg]C
(167 [deg]F) for a 50 kg (110 lb) package), and, for liquid
mixtures, a diluent having a boiling point greater than or equal to
150 [deg]C (302 [deg]F) is used for desensitization will be defined
as self-reactive chemical TYPE G. If the mixture is not thermally
stable or a diluent having a boiling point less than 150 [deg]C (302
[deg]F) is used for desensitization, the mixture shall be defined as
self-reactive chemical TYPE F.
B.8.3 Additional Classification Considerations
B.8.3.1 For purposes of classification, the properties of self-
reactive chemicals shall be determined in accordance with test
series A to H as described in Part II of the UN ST/SG/AC.10
(incorporated by reference; See Sec. 1910.6).
B.8.3.2 Self-accelerating decomposition temperature (SADT) shall
be determined in accordance with the UN ST/SG/AC.10, Part II,
section 28 (incorporated by reference; See Sec. 1910.6).
B.8.3.3 The classification procedures for self-reactive
substances and mixtures need not be applied if:
(a) There are no chemical groups present in the molecule
associated with explosive or self-reactive properties; examples of
such groups are given in Tables A6.1 and A6.2 in the Appendix 6 of
the UN ST/SG/AC.10 (incorporated by reference; See Sec. 1910.6); or
(b) For a single organic substance or a homogeneous mixture of
organic substances, the estimated SADT is greater than 75 [deg]C
(167 [deg]F) or the exothermic decomposition energy is less than 300
J/g. The onset temperature and decomposition energy may be estimated
using a suitable calorimetric technique (See 20.3.3.3 in Part II of
the UN ST/SG/AC.10 (incorporated by reference; See Sec. 1910.6)).
B.9 PYROPHORIC LIQUIDS
B.9.1 Definition
Pyrophoric liquid means a liquid which, even in small
quantities, is liable to ignite within five minutes after coming
into contact with air.
B.9.2 Classification Criteria
A pyrophoric liquid shall be classified in a single category for
this class using test N.3 in Part III, sub-section 33.3.1.5 of the
UN ST/SG/AC.10 (incorporated by reference; See Sec. 1910.6), in
accordance with Table B.9.1:
Table B.9.1--Criteria for Pyrophoric Liquids
----------------------------------------------------------------------------------------------------------------
Category Criteria
----------------------------------------------------------------------------------------------------------------
1........................................... The liquid ignites within 5 min when added to an inert carrier and
exposed to air, or it ignites or chars a filter paper on contact
with air within 5 min.
----------------------------------------------------------------------------------------------------------------
B.9.3 Additional Classification Considerations
The classification procedure for pyrophoric liquids need not be
applied when experience in production or handling shows that the
chemical does not ignite spontaneously on coming into contact with
air at normal temperatures (i.e., the substance is known to be
stable at room temperature for prolonged periods of time (days)).
B.10 PYROPHORIC SOLIDS
B.10.1 Definition
Pyrophoric solid means a solid which, even in small quantities,
is liable to ignite within five minutes after coming into contact
with air.
B.10.2 Classification Criteria
A pyrophoric solid shall be classified in a single category for
this class using test N.2 in Part III, sub-section 33.3.1.4 of the
UN ST/SG/AC.10 (incorporated by reference; See Sec. 1910.6), in
accordance with Table B.10.1:
Table B.10.1--Criteria for Pyrophoric Solids
--------------------------------------------------------------------------------------------------------------------------------------------------------
Category Criteria
--------------------------------------------------------------------------------------------------------------------------------------------------------
1............................................. The solid ignites within 5 min of coming into contact with air.
--------------------------------------------------------------------------------------------------------------------------------------------------------
Note: Classification of solid chemicals shall be based on tests
performed on the chemical as presented. If, for example, for the
purposes of supply or transport, the same chemical is to be
presented in a physical form different from that which was tested
and which is considered likely to materially alter its performance
in a classification test, classification must be based on testing of
the chemical in the new form.
B.10.3 Additional Classification Considerations
The classification procedure for pyrophoric solids need not be
applied when experience in production or handling shows that the
chemical does not ignite spontaneously on coming into contact with
air at normal temperatures (i.e., the chemical is known to be stable
at room temperature for prolonged periods of time (days)).
B.11 SELF-HEATING CHEMICALS
B.11.1 Definition
A self-heating chemical is a solid or liquid chemical, other
than a pyrophoric liquid or solid, which, by reaction with air and
without energy supply, is liable to self-heat; this chemical differs
from a pyrophoric liquid or solid in that it will ignite only when
in large amounts (kilograms) and after long periods of time (hours
or days).
Note: Self-heating of a substance or mixture is a process where
the gradual reaction of that substance or mixture with oxygen (in
air) generates heat. If the rate of heat production exceeds the rate
of heat loss, then the temperature of the substance or mixture will
rise which, after an induction time, may lead to self-ignition and
combustion.
B.11.2 Classification Criteria
B.11.2.1 A self-heating chemical shall be classified in one of
the two categories for this class if, in tests performed in
accordance with test method N.4 in Part III, sub-section 33.3.1.6 of
the UN ST/SG/AC.10 (incorporated by reference; See Sec. 1910.6),
the result meets the criteria shown in Table B.11.1.
Table B.11.1--Criteria for Self-Heating Chemicals
----------------------------------------------------------------------------------------------------------------
Category Criteria
----------------------------------------------------------------------------------------------------------------
1........................................... A positive result is obtained in a test using a 25 mm sample cube
at 140 [deg]C (284 [deg]F).
2........................................... A negative result is obtained in a test using a 25 mm cube sample
at 140 [deg]C (284 [deg]F), a positive result is obtained in a
test using a 100 mm sample cube at 140 [deg]C (284 [deg]F), and:
(a) The unit volume of the chemical is more than 3 m\3\; or
(b) A positive result is obtained in a test using a 100 mm cube
sample at 120 [deg]C (248 [deg]F) and the unit volume of the
chemical is more than 450 liters; or
(c) A positive result is obtained in a test using a 100 mm cube
sample at 100 [deg]C (212 [deg]F).
----------------------------------------------------------------------------------------------------------------
B.11.2.2 Chemicals with a temperature of spontaneous combustion
higher than 50 [deg]C (122 [deg]F) for a volume of 27 m\3\ shall not
be classified as self-heating chemicals.
B.11.2.3 Chemicals with a spontaneous ignition temperature
higher than 50 [deg]C (122 [deg]F) for a volume of 450 liters shall
not be classified in Category 1 of this class.
B.11.3 Additional Classification Considerations
B.11.3.1 The classification procedure for self-heating chemicals
need not be applied if the results of a screening test can be
adequately correlated with the classification test and an
appropriate safety margin is applied.
B.11.3.2 Examples of screening tests are:
(a) The Grewer Oven test (VDI guideline 2263, part 1, 1990, Test
methods for the Determination of the Safety Characteristics of
Dusts) with an onset temperature 80[deg]K above the reference
temperature for a volume of 1 l;
(b) The Bulk Powder Screening Test (Gibson, N. Harper, D. J.
Rogers, R. Evaluation of the fire and explosion risks in drying
powders, Plant Operations Progress, 4 (3), 181-189, 1985) with an
onset temperature 60[deg]K above the reference temperature for a
volume of 1 l.
B.12 CHEMICALS WHICH, IN CONTACT WITH WATER, EMIT FLAMMABLE GASES
B.12.1 Definition
Chemicals which, in contact with water, emit flammable gases are
solid or liquid chemicals which, by interaction with water, are
liable to become spontaneously flammable or to give off flammable
gases in dangerous quantities.
B.12.2 Classification Criteria
B.12.2.1 A chemical which, in contact with water, emits
flammable gases shall be classified in one of the three categories
for this class, using test N.5 in Part III, sub-section 33.4.1.4 of
the UN ST/SG/AC.10 (incorporated by reference; See Sec. 1910.6), in
accordance with Table B.12.1:
Table B.12.1--Criteria for Chemicals Which, in Contact With Water, Emit Flammable Gases
----------------------------------------------------------------------------------------------------------------
Category Criteria
----------------------------------------------------------------------------------------------------------------
1........................................... Any chemical which reacts vigorously with water at ambient
temperatures and demonstrates generally a tendency for the gas
produced to ignite spontaneously, or which reacts readily with
water at ambient temperatures such that the rate of evolution of
flammable gas is equal to or greater than 10 liters per kilogram
of chemical over any one minute.
2........................................... Any chemical which reacts readily with water at ambient
temperatures such that the maximum rate of evolution of flammable
gas is equal to or greater than 20 liters per kilogram of
chemical per hour, and which does not meet the criteria for
Category 1.
3........................................... Any chemical which reacts slowly with water at ambient
temperatures such that the maximum rate of evolution of flammable
gas is equal to or greater than 1 liter per kilogram of chemical
per hour, and which does not meet the criteria for Categories 1
and 2.
----------------------------------------------------------------------------------------------------------------
Note: Classification of solid chemicals shall be based on tests
performed on the chemical as presented. If, for example, for the
purposes of supply or transport, the same chemical is to be
presented in a physical form different from that which was tested
and which is considered likely to materially alter its performance
in a classification test, classification must be based on testing of
the chemical in the new form.
B.12.2.2 A chemical is classified as a chemical which, in
contact with water emits flammable gases if spontaneous ignition
takes place in any step of the test procedure.
B.12.3 Additional Classification Considerations
The classification procedure for this class need not be applied
if:
(a) The chemical structure of the chemical does not contain
metals or metalloids;
(b) Experience in production or handling shows that the chemical
does not react with water, (e.g., the chemical is manufactured with
water or washed with water); or
(c) The chemical is known to be soluble in water to form a
stable mixture.
B.13 OXIDIZING LIQUIDS
B.13.1 Definition
Oxidizing liquid means a liquid which, while in itself not
necessarily combustible, may, generally by yielding oxygen, cause,
or contribute to, the combustion of other material.
B.13.2 Classification Criteria
An oxidizing liquid shall be classified in one of the three
categories for this class using test O.2 in Part III, sub-section
34.4.2 of the UN ST/SG/AC.10 (incorporated by reference; See Sec.
1910.6), in accordance with Table B.13.1:
Table B.13.1--Criteria for Oxidizing Liquids
----------------------------------------------------------------------------------------------------------------
Category Criteria
----------------------------------------------------------------------------------------------------------------
1........................................... Any chemical which, in the 1:1 mixture, by mass, of chemical and
cellulose tested, spontaneously ignites; or the mean pressure
rise time of a 1:1 mixture, by mass, of chemical and cellulose is
less than that of a 1:1 mixture, by mass, of 50% perchloric acid
and cellulose;
2........................................... Any chemical which, in the 1:1 mixture, by mass, of chemical and
cellulose tested, exhibits a mean pressure rise time less than or
equal to the mean pressure rise time of a 1:1 mixture, by mass,
of 40% aqueous sodium chlorate solution and cellulose; and the
criteria for Category 1 are not met;
3........................................... Any chemical which, in the 1:1 mixture, by mass, of chemical and
cellulose tested, exhibits a mean pressure rise time less than or
equal to the mean pressure rise time of a 1:1 mixture, by mass,
of 65% aqueous nitric acid and cellulose; and the criteria for
Categories 1 and 2 are not met.
----------------------------------------------------------------------------------------------------------------
B.13.3 Additional Classification Considerations
B.13.3.1 For organic chemicals, the classification procedure for
this class shall not be applied if:
(a) The chemical does not contain oxygen, fluorine or chlorine;
or
(b) The chemical contains oxygen, fluorine or chlorine and these
elements are chemically bonded only to carbon or hydrogen.
B.13.3.2 For inorganic chemicals, the classification procedure
for this class shall not be applied if the chemical does not contain
oxygen or halogen atoms.
B.13.3.3 In the event of divergence between test results and
known experience in the handling and use of chemicals which shows
them to be oxidizing, judgments based on known experience shall take
precedence over test results.
B.13.3.4 In cases where chemicals generate a pressure rise (too
high or too low), caused by chemical reactions not characterizing
the oxidizing properties of the chemical, the test described in Part
III, sub-section 34.4.2 of the UN ST/SG/AC.10 (incorporated by
reference; See Sec. 1910.6) shall be repeated with an inert
substance (e.g., diatomite (kieselguhr)) in place of the cellulose
in order to clarify the nature of the reaction.
B.14 OXIDIZING SOLIDS
B.14.1 Definition
Oxidizing solid means a solid which, while in itself is not
necessarily combustible, may, generally by yielding oxygen, cause,
or contribute to, the combustion of other material.
B.14.2 Classification Criteria
An oxidizing solid shall be classified in one of the three
categories for this class using test O.1 in Part III, sub-section
34.4.1 of the UN ST/SG/AC.10 (incorporated by reference; See Sec.
1910.6), in accordance with Table B.14.1:
Table B.14.1--Criteria for Oxidizing Solids
----------------------------------------------------------------------------------------------------------------
Category Criteria
----------------------------------------------------------------------------------------------------------------
1........................................... Any chemical which, in the 4:1 or 1:1 sample-to-cellulose ratio
(by mass) tested, exhibits a mean burning time less than the mean
burning time of a 3:2 mixture, by mass, of potassium bromate and
cellulose.
2........................................... Any chemical which, in the 4:1 or 1:1 sample-to-cellulose ratio
(by mass) tested, exhibits a mean burning time equal to or less
than the mean burning time of a 2:3 mixture (by mass) of
potassium bromate and cellulose and the criteria for Category 1
are not met.
3........................................... Any chemical which, in the 4:1 or 1:1 sample-to-cellulose ratio
(by mass) tested, exhibits a mean burning time equal to or less
than the mean burning time of a 3:7 mixture (by mass) of
potassium bromate and cellulose and the criteria for Categories 1
and 2 are not met.
----------------------------------------------------------------------------------------------------------------
Note 1: Some oxidizing solids may present explosion hazards
under certain conditions (e.g., when stored in large quantities).
For example, some types of ammonium nitrate may give rise to an
explosion hazard under extreme conditions and the "Resistance to
detonation test" (IMO: Code of Safe Practice for Solid Bulk
Cargoes, 2005, Annex 3, Test 5) may be used to assess this hazard.
When information indicates that an oxidizing solid may present an
explosion hazard, it shall be indicated on the Safety Data Sheet.
Note 2: Classification of solid chemicals shall be based on
tests performed on the chemical as presented. If, for example, for
the purposes of supply or transport, the same chemical is to be
presented in a physical form different from that which was tested
and which is considered likely to materially alter its performance
in a classification test, classification must be based on testing of
the chemical in the new form.
B.14.3 Additional Classification Considerations
B.14.3.1 For organic chemicals, the classification procedure for
this class shall not be applied if:
(a) The chemical does not contain oxygen, fluorine or chlorine;
or
(b) The chemical contains oxygen, fluorine or chlorine and these
elements are chemically bonded only to carbon or hydrogen.
B.14.3.2 For inorganic chemicals, the classification procedure
for this class shall not be applied if the chemical does not contain
oxygen or halogen atoms.
B.14.3.3 In the event of divergence between test results and
known experience in the handling and use of chemicals which shows
them to be oxidizing, judgements based on known experience shall
take precedence over test results.
B.15 ORGANIC PEROXIDES
B.15.1 Definition
B.15.1.1 Organic peroxide means a liquid or solid organic
chemical which contains the bivalent -0-0- structure and as such is
considered a derivative of hydrogen peroxide, where one or both of
the hydrogen atoms have been replaced by organic radicals. The term
organic peroxide includes organic peroxide mixtures containing at
least one organic peroxide. Organic peroxides are thermally unstable
chemicals, which may undergo exothermic self-accelerating
decomposition. In addition, they may have one or more of the
following properties:
(a) Be liable to explosive decomposition;
(b) Burn rapidly;
(c) Be sensitive to impact or friction;
(d) React dangerously with other substances.
B.15.1.2 An organic peroxide is regarded as possessing explosive
properties when in laboratory testing the formulation is liable to
detonate, to deflagrate rapidly or to show a violent effect when
heated under confinement.
B.15.2 Classification Criteria
B.15.2.1 Any organic peroxide shall be considered for
classification in this class, unless it contains:
(a) Not more than 1.0% available oxygen from the organic
peroxides when containing not more than 1.0% hydrogen peroxide; or
(b) Not more than 0.5% available oxygen from the organic
peroxides when containing more than 1.0% but not more than 7.0%
hydrogen peroxide.
Note: The available oxygen content (%) of an organic peroxide
mixture is given by the formula:
Where:
[Delta]Hc = chemical heat of combustion (kJ/g);
wi% = mass fraction of component i in the product;
[Delta]Hc(i) = specific heat of combustion (kJ/g) of component i in
the product;
The chemical heats of combustion shall be found in literature,
calculated or determined by tests (See ASTM D240-02, ISO 13943,
Sections 86.1 to 86.3, and NFPA 30B (incorporated by reference; See
Sec. 1910.6)).
B.3.3.3 The Ignition Distance Test, Enclosed Space Ignition Test
and Aerosol Foam Flammability Test shall be performed in accordance
with sub-sections 31.4, 31.5 and 31.6 of the of the UN ST/SG/AC.10
(incorporated by reference; See Sec. 1910.6).
B.4 OXIDIZING GASES
B.4.1 Definition
Oxidizing gas means any gas which may, generally by providing
oxygen, cause or contribute to the combustion of other material more
than air does.
Note: "Gases which cause or contribute to the combustion of
other material more than air does" means pure gases or gas mixtures
with an oxidizing power greater than 23.5% (as determined by a
method specified in ISO 10156 or 10156-2 (incorporated by reference,
See Sec. 1910.6) or an equivalent testing method.)
B.4.2 Classification Criteria
An oxidizing gas shall be classified in a single category for
this class in accordance with Table B.4.1:
Table B.4.1--Criteria for Oxidizing Gases
----------------------------------------------------------------------------------------------------------------
Category Criteria
----------------------------------------------------------------------------------------------------------------
1........................................... Any gas which may, generally by providing oxygen, cause or
contribute to the combustion of other material more than air
does.
----------------------------------------------------------------------------------------------------------------
B.4.3 Additional Classification Considerations
Classification shall be in accordance with tests or calculation
methods as described in ISO 10156 (incorporated by reference; See
Sec. 1910.6) and ISO 10156-2 (incorporated by reference; See Sec.
1910.6).
B.5 GASES UNDER PRESSURE
B.5.1 Definition
Gases under pressure are gases which are contained in a
receptacle at a pressure of 200 kPa (29 psi) (gauge) or more, or
which are liquefied or liquefied and refrigerated.
They comprise compressed gases, liquefied gases, dissolved gases
and refrigerated liquefied gases.
B.5.2 Classification Criteria
Gases under pressure shall be classified in one of four groups
in accordance with Table B.5.1:
Table B.5.1--Criteria for Gases Under Pressure
----------------------------------------------------------------------------------------------------------------
Group Criteria
----------------------------------------------------------------------------------------------------------------
Compressed gas.............................. A gas which when under pressure is entirely gaseous at -50 [deg]C
(-8 [deg]F), including all gases with a critical temperature\1\
<=-50 [deg]C (-58 [deg]F).
Liquefied gas............................... A gas which when under pressure is partially liquid at
temperatures above -50 [deg]C (-58 [deg]F). A distinction is made
between:
(a) High pressure liquefied gas: A gas with a critical
temperature \1\ between -50 [deg]C (-58 [deg]F) and +65 [deg]C
(149 [deg]F); and
(b) Low pressure liquefied gas: A gas with a critical
temperature \1\ above +65 [deg]C (149 [deg]F).
Refrigerated liquefied gas.................. A gas which is made partially liquid because of its low
temperature.
Dissolved gas............................... A gas which when under pressure is dissolved in a liquid phase
solvent.
----------------------------------------------------------------------------------------------------------------
\1\ The critical temperature is the temperature above which a pure gas cannot be liquefied, regardless of the
degree of compression.
B.6 FLAMMABLE LIQUIDS
B.6.1 Definition
Flammable liquid means a liquid having a flash point of not more
than 93 [deg]C (199.4 [deg]F).
Flash point means the minimum temperature at which a liquid
gives off vapor in sufficient concentration to form an ignitable
mixture with air near the surface of the liquid, as determined by a
method identified in Section B.6.3.
B.6.2 Classification Criteria
A flammable liquid shall be classified in one of four categories
in accordance with Table B.6.1:
Table B.6.1--Criteria for Flammable Liquids
--------------------------------------------------------------------------------------------------------------------------------------------------------
Category Criteria
--------------------------------------------------------------------------------------------------------------------------------------------------------
1........................................... Flash point <23 [deg]C (73.4 [deg]F) and initial boiling point <=35 [deg]C (95 [deg]F).
2........................................... Flash point <23 [deg]C (73.4 [deg]F) and initial boiling point >35 [deg]C (95 [deg]F).
3........................................... Flash point >=23 [deg]C (73.4 [deg]F) and <=60 [deg]C (140 [deg]F).
4........................................... Flash point >60 [deg]C (140 [deg]F) and <=93 [deg]C (199.4 [deg]F).
--------------------------------------------------------------------------------------------------------------------------------------------------------
B.6.3 Additional Classification Considerations
The flash point shall be determined in accordance with ASTM D56-
05, ASTM D3278, ASTM D3828, ASTM D93-08 (incorporated by reference;
See Sec. 1910.6), or any other method specified in GHS Revision 3,
Chapter 2.6.
The initial boiling point shall be determined in accordance with
ASTM D86-07a or ASTM D1078 (incorporated by reference; See Sec.
1910.6).
B.7 FLAMMABLE SOLIDS
B.7.1 Definitions
Flammable solid means a solid which is a readily combustible
solid, or which may cause or contribute to fire through friction.
Readily combustible solids are powdered, granular, or pasty
chemicals which are dangerous if they can be easily ignited by brief
contact with an ignition source, such as a burning match, and if the
flame spreads rapidly.
B.7.2 Classification Criteria
B.7.2.1 Powdered, granular or pasty chemicals shall be
classified as flammable solids when the time of burning of one or
more of the test runs, performed in accordance with the test method
described in the UN ST/SG/AC.10 (incorporated by reference; See
Sec. 1910.6), Part III, sub-section 33.2.1, is less than 45 s or
the rate of burning is more than 2.2 mm/s (0.0866 in/s).
B.7.2.2 Powders of metals or metal alloys shall be classified as
flammable solids when they can be ignited and the reaction spreads
over the whole length of the sample in 10 min or less.
B.7.2.3 Solids which may cause fire through friction shall be
classified in this class by analogy with existing entries (e.g.,
matches) until definitive criteria are established.
B.7.2.4 A flammable solid shall be classified in one of the two
categories for this class using Method N.1 as described in Part III,
sub-section 33.2.1 of the UN ST/SG/AC.10 (incorporated by reference;
See Sec. 1910.6), in accordance with Table B.7.1:
Table B.7.1--Criteria for Flammable Solids
------------------------------------------------------------------------
Category Criteria
------------------------------------------------------------------------
1...................................... Burning rate test:
Chemicals other than metal
powders:
(a) Wetted zone does not
stop fire; and
(b) Burning time <45 s or
burning rate >2.2 mm/s.
Metal powders: Burning time
<=5 min.
2...................................... Burning rate test:
Chemicals other than metal
powders:
(a) Wetted zone stops the
fire for at least 4 min;
and
(b) Burning time <45 s or
burning rate >2.2 mm/s.
Metal powders: Burning time
>5 min and <=10 min.
------------------------------------------------------------------------
Note: Classification of solid chemicals shall be based on tests
performed on the chemical as presented. If, for example, for the
purposes of supply or transport, the same chemical is to be
presented in a physical form different from that which was tested
and which is considered likely to materially alter its performance
in a classification test, classification must be based on testing of
the chemical in the new form.
B.8 SELF-REACTIVE CHEMICALS
B.8.1 Definitions
Self-reactive chemicals are thermally unstable liquid or solid
chemicals liable to undergo a strongly exothermic decomposition even
without participation of oxygen (air). This definition excludes
chemicals classified under this section as explosives, organic
peroxides, oxidizing liquids or oxidizing solids.
A self-reactive chemical is regarded as possessing explosive
properties when in laboratory testing the formulation is liable to
detonate, to deflagrate rapidly or to show a violent effect when
heated under confinement.
B.8.2 Classification Criteria
B.8.2.1 A self-reactive chemical shall be considered for
classification in this class unless:
(a) It is classified as an explosive according to B.1 of this
appendix;
(b) It is classified as an oxidizing liquid or an oxidizing
solid according to B.13 or B.14 of this appendix, except that a
mixture of oxidizing substances which contains 5% or more of
combustible organic substances shall be classified as a self-
reactive chemical according to the procedure defined in B.8.2.2;
(c) It is classified as an organic peroxide according to B.15 of
this appendix;
(d) Its heat of decomposition is less than 300 J/g; or
(e) Its self-accelerating decomposition temperature (SADT) is
greater than 75 [deg]C (167 [deg]F) for a 50 kg (110 lb) package.
B.8.2.2 Mixtures of oxidizing substances, meeting the criteria
for classification as oxidizing liquids or oxidizing solids, which
contain 5% or more of combustible organic substances and which do
not meet the criteria mentioned in B.8.2.1 (a), (c), (d) or (e),
shall be subjected to the self-reactive chemicals classification
procedure in B.8.2.3. Such a mixture showing the properties of a
self-reactive chemical type B to F shall be classified as a self-
reactive chemical.
B.8.2.3 Self-reactive chemicals shall be classified in one of
the seven categories of "types A to G" for this class, according
to the following principles:
(a) Any self-reactive chemical which can detonate or deflagrate
rapidly, as packaged, will be defined as self-reactive chemical TYPE
A;
(b) Any self-reactive chemical possessing explosive properties
and which, as packaged, neither detonates nor deflagrates rapidly,
but is liable to undergo a thermal explosion in that package will be
defined as self-reactive chemical TYPE B;
(c) Any self-reactive chemical possessing explosive properties
when the chemical as packaged cannot detonate or deflagrate rapidly
or undergo a thermal explosion will be defined as self-reactive
chemical TYPE C;
(d) Any self-reactive chemical which in laboratory testing meets
the criteria in (d)(i), (ii), or (iii) will be defined as self-
reactive chemical TYPE D:
(i) Detonates partially, does not deflagrate rapidly and shows
no violent effect when heated under confinement; or
(ii) Does not detonate at all, deflagrates slowly and shows no
violent effect when heated under confinement; or
(iii) Does not detonate or deflagrate at all and shows a medium
effect when heated under confinement;
(e) Any self-reactive chemical which, in laboratory testing,
neither detonates nor deflagrates at all and shows low or no effect
when heated under confinement will be defined as self-reactive
chemical TYPE E;
(f) Any self-reactive chemical which, in laboratory testing,
neither detonates in the cavitated state nor deflagrates at all and
shows only a low or no effect when heated under confinement as well
as low or no explosive power will be defined as self-reactive
chemical TYPE F;
(g) Any self-reactive chemical which, in laboratory testing,
neither detonates in the cavitated state nor deflagrates at all and
shows no effect when heated under confinement nor any explosive
power, provided that it is thermally stable (self-accelerating
decomposition temperature is 60 [deg]C (140 [deg]F) to 75 [deg]C
(167 [deg]F) for a 50 kg (110 lb) package), and, for liquid
mixtures, a diluent having a boiling point greater than or equal to
150 [deg]C (302 [deg]F) is used for desensitization will be defined
as self-reactive chemical TYPE G. If the mixture is not thermally
stable or a diluent having a boiling point less than 150 [deg]C (302
[deg]F) is used for desensitization, the mixture shall be defined as
self-reactive chemical TYPE F.
B.8.3 Additional Classification Considerations
B.8.3.1 For purposes of classification, the properties of self-
reactive chemicals shall be determined in accordance with test
series A to H as described in Part II of the UN ST/SG/AC.10
(incorporated by reference; See Sec. 1910.6).
B.8.3.2 Self-accelerating decomposition temperature (SADT) shall
be determined in accordance with the UN ST/SG/AC.10, Part II,
section 28 (incorporated by reference; See Sec. 1910.6).
B.8.3.3 The classification procedures for self-reactive
substances and mixtures need not be applied if:
(a) There are no chemical groups present in the molecule
associated with explosive or self-reactive properties; examples of
such groups are given in Tables A6.1 and A6.2 in the Appendix 6 of
the UN ST/SG/AC.10 (incorporated by reference; See Sec. 1910.6); or
(b) For a single organic substance or a homogeneous mixture of
organic substances, the estimated SADT is greater than 75 [deg]C
(167 [deg]F) or the exothermic decomposition energy is less than 300
J/g. The onset temperature and decomposition energy may be estimated
using a suitable calorimetric technique (See 20.3.3.3 in Part II of
the UN ST/SG/AC.10 (incorporated by reference; See Sec. 1910.6)).
B.9 PYROPHORIC LIQUIDS
B.9.1 Definition
Pyrophoric liquid means a liquid which, even in small
quantities, is liable to ignite within five minutes after coming
into contact with air.
B.9.2 Classification Criteria
A pyrophoric liquid shall be classified in a single category for
this class using test N.3 in Part III, sub-section 33.3.1.5 of the
UN ST/SG/AC.10 (incorporated by reference; See Sec. 1910.6), in
accordance with Table B.9.1:
Table B.9.1--Criteria for Pyrophoric Liquids
----------------------------------------------------------------------------------------------------------------
Category Criteria
----------------------------------------------------------------------------------------------------------------
1........................................... The liquid ignites within 5 min when added to an inert carrier and
exposed to air, or it ignites or chars a filter paper on contact
with air within 5 min.
----------------------------------------------------------------------------------------------------------------
B.9.3 Additional Classification Considerations
The classification procedure for pyrophoric liquids need not be
applied when experience in production or handling shows that the
chemical does not ignite spontaneously on coming into contact with
air at normal temperatures (i.e., the substance is known to be
stable at room temperature for prolonged periods of time (days)).
B.10 PYROPHORIC SOLIDS
B.10.1 Definition
Pyrophoric solid means a solid which, even in small quantities,
is liable to ignite within five minutes after coming into contact
with air.
B.10.2 Classification Criteria
A pyrophoric solid shall be classified in a single category for
this class using test N.2 in Part III, sub-section 33.3.1.4 of the
UN ST/SG/AC.10 (incorporated by reference; See Sec. 1910.6), in
accordance with Table B.10.1:
Table B.10.1--Criteria for Pyrophoric Solids
--------------------------------------------------------------------------------------------------------------------------------------------------------
Category Criteria
--------------------------------------------------------------------------------------------------------------------------------------------------------
1............................................. The solid ignites within 5 min of coming into contact with air.
--------------------------------------------------------------------------------------------------------------------------------------------------------
Note: Classification of solid chemicals shall be based on tests
performed on the chemical as presented. If, for example, for the
purposes of supply or transport, the same chemical is to be
presented in a physical form different from that which was tested
and which is considered likely to materially alter its performance
in a classification test, classification must be based on testing of
the chemical in the new form.
B.10.3 Additional Classification Considerations
The classification procedure for pyrophoric solids need not be
applied when experience in production or handling shows that the
chemical does not ignite spontaneously on coming into contact with
air at normal temperatures (i.e., the chemical is known to be stable
at room temperature for prolonged periods of time (days)).
B.11 SELF-HEATING CHEMICALS
B.11.1 Definition
A self-heating chemical is a solid or liquid chemical, other
than a pyrophoric liquid or solid, which, by reaction with air and
without energy supply, is liable to self-heat; this chemical differs
from a pyrophoric liquid or solid in that it will ignite only when
in large amounts (kilograms) and after long periods of time (hours
or days).
Note: Self-heating of a substance or mixture is a process where
the gradual reaction of that substance or mixture with oxygen (in
air) generates heat. If the rate of heat production exceeds the rate
of heat loss, then the temperature of the substance or mixture will
rise which, after an induction time, may lead to self-ignition and
combustion.
B.11.2 Classification Criteria
B.11.2.1 A self-heating chemical shall be classified in one of
the two categories for this class if, in tests performed in
accordance with test method N.4 in Part III, sub-section 33.3.1.6 of
the UN ST/SG/AC.10 (incorporated by reference; See Sec. 1910.6),
the result meets the criteria shown in Table B.11.1.
Table B.11.1--Criteria for Self-Heating Chemicals
----------------------------------------------------------------------------------------------------------------
Category Criteria
----------------------------------------------------------------------------------------------------------------
1........................................... A positive result is obtained in a test using a 25 mm sample cube
at 140 [deg]C (284 [deg]F).
2........................................... A negative result is obtained in a test using a 25 mm cube sample
at 140 [deg]C (284 [deg]F), a positive result is obtained in a
test using a 100 mm sample cube at 140 [deg]C (284 [deg]F), and:
(a) The unit volume of the chemical is more than 3 m\3\; or
(b) A positive result is obtained in a test using a 100 mm cube
sample at 120 [deg]C (248 [deg]F) and the unit volume of the
chemical is more than 450 liters; or
(c) A positive result is obtained in a test using a 100 mm cube
sample at 100 [deg]C (212 [deg]F).
----------------------------------------------------------------------------------------------------------------
B.11.2.2 Chemicals with a temperature of spontaneous combustion
higher than 50 [deg]C (122 [deg]F) for a volume of 27 m\3\ shall not
be classified as self-heating chemicals.
B.11.2.3 Chemicals with a spontaneous ignition temperature
higher than 50 [deg]C (122 [deg]F) for a volume of 450 liters shall
not be classified in Category 1 of this class.
B.11.3 Additional Classification Considerations
B.11.3.1 The classification procedure for self-heating chemicals
need not be applied if the results of a screening test can be
adequately correlated with the classification test and an
appropriate safety margin is applied.
B.11.3.2 Examples of screening tests are:
(a) The Grewer Oven test (VDI guideline 2263, part 1, 1990, Test
methods for the Determination of the Safety Characteristics of
Dusts) with an onset temperature 80[deg]K above the reference
temperature for a volume of 1 l;
(b) The Bulk Powder Screening Test (Gibson, N. Harper, D. J.
Rogers, R. Evaluation of the fire and explosion risks in drying
powders, Plant Operations Progress, 4 (3), 181-189, 1985) with an
onset temperature 60[deg]K above the reference temperature for a
volume of 1 l.
B.12 CHEMICALS WHICH, IN CONTACT WITH WATER, EMIT FLAMMABLE GASES
B.12.1 Definition
Chemicals which, in contact with water, emit flammable gases are
solid or liquid chemicals which, by interaction with water, are
liable to become spontaneously flammable or to give off flammable
gases in dangerous quantities.
B.12.2 Classification Criteria
B.12.2.1 A chemical which, in contact with water, emits
flammable gases shall be classified in one of the three categories
for this class, using test N.5 in Part III, sub-section 33.4.1.4 of
the UN ST/SG/AC.10 (incorporated by reference; See Sec. 1910.6), in
accordance with Table B.12.1:
Table B.12.1--Criteria for Chemicals Which, in Contact With Water, Emit Flammable Gases
----------------------------------------------------------------------------------------------------------------
Category Criteria
----------------------------------------------------------------------------------------------------------------
1........................................... Any chemical which reacts vigorously with water at ambient
temperatures and demonstrates generally a tendency for the gas
produced to ignite spontaneously, or which reacts readily with
water at ambient temperatures such that the rate of evolution of
flammable gas is equal to or greater than 10 liters per kilogram
of chemical over any one minute.
2........................................... Any chemical which reacts readily with water at ambient
temperatures such that the maximum rate of evolution of flammable
gas is equal to or greater than 20 liters per kilogram of
chemical per hour, and which does not meet the criteria for
Category 1.
3........................................... Any chemical which reacts slowly with water at ambient
temperatures such that the maximum rate of evolution of flammable
gas is equal to or greater than 1 liter per kilogram of chemical
per hour, and which does not meet the criteria for Categories 1
and 2.
----------------------------------------------------------------------------------------------------------------
Note: Classification of solid chemicals shall be based on tests
performed on the chemical as presented. If, for example, for the
purposes of supply or transport, the same chemical is to be
presented in a physical form different from that which was tested
and which is considered likely to materially alter its performance
in a classification test, classification must be based on testing of
the chemical in the new form.
B.12.2.2 A chemical is classified as a chemical which, in
contact with water emits flammable gases if spontaneous ignition
takes place in any step of the test procedure.
B.12.3 Additional Classification Considerations
The classification procedure for this class need not be applied
if:
(a) The chemical structure of the chemical does not contain
metals or metalloids;
(b) Experience in production or handling shows that the chemical
does not react with water, (e.g., the chemical is manufactured with
water or washed with water); or
(c) The chemical is known to be soluble in water to form a
stable mixture.
B.13 OXIDIZING LIQUIDS
B.13.1 Definition
Oxidizing liquid means a liquid which, while in itself not
necessarily combustible, may, generally by yielding oxygen, cause,
or contribute to, the combustion of other material.
B.13.2 Classification Criteria
An oxidizing liquid shall be classified in one of the three
categories for this class using test O.2 in Part III, sub-section
34.4.2 of the UN ST/SG/AC.10 (incorporated by reference; See Sec.
1910.6), in accordance with Table B.13.1:
Table B.13.1--Criteria for Oxidizing Liquids
----------------------------------------------------------------------------------------------------------------
Category Criteria
----------------------------------------------------------------------------------------------------------------
1........................................... Any chemical which, in the 1:1 mixture, by mass, of chemical and
cellulose tested, spontaneously ignites; or the mean pressure
rise time of a 1:1 mixture, by mass, of chemical and cellulose is
less than that of a 1:1 mixture, by mass, of 50% perchloric acid
and cellulose;
2........................................... Any chemical which, in the 1:1 mixture, by mass, of chemical and
cellulose tested, exhibits a mean pressure rise time less than or
equal to the mean pressure rise time of a 1:1 mixture, by mass,
of 40% aqueous sodium chlorate solution and cellulose; and the
criteria for Category 1 are not met;
3........................................... Any chemical which, in the 1:1 mixture, by mass, of chemical and
cellulose tested, exhibits a mean pressure rise time less than or
equal to the mean pressure rise time of a 1:1 mixture, by mass,
of 65% aqueous nitric acid and cellulose; and the criteria for
Categories 1 and 2 are not met.
----------------------------------------------------------------------------------------------------------------
B.13.3 Additional Classification Considerations
B.13.3.1 For organic chemicals, the classification procedure for
this class shall not be applied if:
(a) The chemical does not contain oxygen, fluorine or chlorine;
or
(b) The chemical contains oxygen, fluorine or chlorine and these
elements are chemically bonded only to carbon or hydrogen.
B.13.3.2 For inorganic chemicals, the classification procedure
for this class shall not be applied if the chemical does not contain
oxygen or halogen atoms.
B.13.3.3 In the event of divergence between test results and
known experience in the handling and use of chemicals which shows
them to be oxidizing, judgments based on known experience shall take
precedence over test results.
B.13.3.4 In cases where chemicals generate a pressure rise (too
high or too low), caused by chemical reactions not characterizing
the oxidizing properties of the chemical, the test described in Part
III, sub-section 34.4.2 of the UN ST/SG/AC.10 (incorporated by
reference; See Sec. 1910.6) shall be repeated with an inert
substance (e.g., diatomite (kieselguhr)) in place of the cellulose
in order to clarify the nature of the reaction.
B.14 OXIDIZING SOLIDS
B.14.1 Definition
Oxidizing solid means a solid which, while in itself is not
necessarily combustible, may, generally by yielding oxygen, cause,
or contribute to, the combustion of other material.
B.14.2 Classification Criteria
An oxidizing solid shall be classified in one of the three
categories for this class using test O.1 in Part III, sub-section
34.4.1 of the UN ST/SG/AC.10 (incorporated by reference; See Sec.
1910.6), in accordance with Table B.14.1:
Table B.14.1--Criteria for Oxidizing Solids
----------------------------------------------------------------------------------------------------------------
Category Criteria
----------------------------------------------------------------------------------------------------------------
1........................................... Any chemical which, in the 4:1 or 1:1 sample-to-cellulose ratio
(by mass) tested, exhibits a mean burning time less than the mean
burning time of a 3:2 mixture, by mass, of potassium bromate and
cellulose.
2........................................... Any chemical which, in the 4:1 or 1:1 sample-to-cellulose ratio
(by mass) tested, exhibits a mean burning time equal to or less
than the mean burning time of a 2:3 mixture (by mass) of
potassium bromate and cellulose and the criteria for Category 1
are not met.
3........................................... Any chemical which, in the 4:1 or 1:1 sample-to-cellulose ratio
(by mass) tested, exhibits a mean burning time equal to or less
than the mean burning time of a 3:7 mixture (by mass) of
potassium bromate and cellulose and the criteria for Categories 1
and 2 are not met.
----------------------------------------------------------------------------------------------------------------
Note 1: Some oxidizing solids may present explosion hazards
under certain conditions (e.g., when stored in large quantities).
For example, some types of ammonium nitrate may give rise to an
explosion hazard under extreme conditions and the "Resistance to
detonation test" (IMO: Code of Safe Practice for Solid Bulk
Cargoes, 2005, Annex 3, Test 5) may be used to assess this hazard.
When information indicates that an oxidizing solid may present an
explosion hazard, it shall be indicated on the Safety Data Sheet.
Note 2: Classification of solid chemicals shall be based on
tests performed on the chemical as presented. If, for example, for
the purposes of supply or transport, the same chemical is to be
presented in a physical form different from that which was tested
and which is considered likely to materially alter its performance
in a classification test, classification must be based on testing of
the chemical in the new form.
B.14.3 Additional Classification Considerations
B.14.3.1 For organic chemicals, the classification procedure for
this class shall not be applied if:
(a) The chemical does not contain oxygen, fluorine or chlorine;
or
(b) The chemical contains oxygen, fluorine or chlorine and these
elements are chemically bonded only to carbon or hydrogen.
B.14.3.2 For inorganic chemicals, the classification procedure
for this class shall not be applied if the chemical does not contain
oxygen or halogen atoms.
B.14.3.3 In the event of divergence between test results and
known experience in the handling and use of chemicals which shows
them to be oxidizing, judgements based on known experience shall
take precedence over test results.
B.15 ORGANIC PEROXIDES
B.15.1 Definition
B.15.1.1 Organic peroxide means a liquid or solid organic
chemical which contains the bivalent -0-0- structure and as such is
considered a derivative of hydrogen peroxide, where one or both of
the hydrogen atoms have been replaced by organic radicals. The term
organic peroxide includes organic peroxide mixtures containing at
least one organic peroxide. Organic peroxides are thermally unstable
chemicals, which may undergo exothermic self-accelerating
decomposition. In addition, they may have one or more of the
following properties:
(a) Be liable to explosive decomposition;
(b) Burn rapidly;
(c) Be sensitive to impact or friction;
(d) React dangerously with other substances.
B.15.1.2 An organic peroxide is regarded as possessing explosive
properties when in laboratory testing the formulation is liable to
detonate, to deflagrate rapidly or to show a violent effect when
heated under confinement.
B.15.2 Classification Criteria
B.15.2.1 Any organic peroxide shall be considered for
classification in this class, unless it contains:
(a) Not more than 1.0% available oxygen from the organic
peroxides when containing not more than 1.0% hydrogen peroxide; or
(b) Not more than 0.5% available oxygen from the organic
peroxides when containing more than 1.0% but not more than 7.0%
hydrogen peroxide.
Note: The available oxygen content (%) of an organic peroxide
mixture is given by the formula:
 Where:
ni = number of peroxygen groups per molecule of organic peroxide i;
ci = concentration (mass %) of organic peroxide i;
mi = molecular mass of organic peroxide i.
B.15.2.2 Organic peroxides shall be classified in one of the
seven categories of "Types A to G" for this class, according to
the following principles:
(a) Any organic peroxide which, as packaged, can detonate or
deflagrate rapidly shall be defined as organic peroxide TYPE A;
(b) Any organic peroxide possessing explosive properties and
which, as packaged, neither detonates nor deflagrates rapidly, but
is liable to undergo a thermal explosion in that package shall be
defined as organic peroxide TYPE B;
(c) Any organic peroxide possessing explosive properties when
the chemical as packaged cannot detonate or deflagrate rapidly or
undergo a thermal explosion shall be defined as organic peroxide
TYPE C;
(d) Any organic peroxide which in laboratory testing meets the
criteria in (d)(i), (ii), or (iii) shall be defined as organic
peroxide TYPE D:
(i) Detonates partially, does not deflagrate rapidly and shows
no violent effect when heated under confinement; or
(ii) Does not detonate at all, deflagrates slowly and shows no
violent effect when heated under confinement; or
(iii) Does not detonate or deflagrate at all and shows a medium
effect when heated under confinement;
(e) Any organic peroxide which, in laboratory testing, neither
detonates nor deflagrates at all and shows low or no effect when
heated under confinement shall be defined as organic peroxide TYPE
E;
(f) Any organic peroxide which, in laboratory testing, neither
detonates in the cavitated state nor deflagrates at all and shows
only a low or no effect when heated under confinement as well as low
or no explosive power shall be defined as organic peroxide TYPE F;
(g) Any organic peroxide which, in laboratory testing, neither
detonates in the cavitated state nor deflagrates at all and shows no
effect when heated under confinement nor any explosive power,
provided that it is thermally stable (self-accelerating
decomposition temperature is 60 [deg]C (140 [deg]F) or higher for a
50 kg (110 lb) package), and, for liquid mixtures, a diluent having
a boiling point of not less than 150 [deg]C (302 [deg]F) is used for
desensitization, shall be defined as organic peroxide TYPE G. If the
organic peroxide is not thermally stable or a diluent having a
boiling point less than 150 [deg]C (302 [deg]F) is used for
desensitization, it shall be defined as organic peroxide TYPE F.
B.15.3 Additional Classification Considerations
B.15.3.1 For purposes of classification, the properties of
organic peroxides shall be determined in accordance with test series
A to H as described in Part II of the UN ST/SG/AC.10 (incorporated
by reference; See Sec. 1910.6).
B.15.3.2 Self-accelerating decomposition temperature (SADT)
shall be determined in accordance with the UN ST/SG/AC.10
(incorporated by reference; See Sec. 1910.6), Part II, section 28.
B.15.3.3 Mixtures of organic peroxides may be classified as the
same type of organic peroxide as that of the most dangerous
ingredient. However, as two stable ingredients can form a thermally
less stable mixture, the SADT of the mixture shall be determined.
B.16 CORROSIVE TO METALS
B.16.1 Definition
A chemical which is corrosive to metals means a chemical which
by chemical action will materially damage, or even destroy, metals.
B.16.2 Classification Criteria
A chemical which is corrosive to metals shall be classified in a
single category for this class, using the test in Part III, sub-
section 37.4 of the UN ST/SG/AC.10 (incorporated by reference; See
Sec. 1910.6), in accordance with Table B.16.1:
Table B.16.1--Criteria for Chemicals Corrosive to Metal
----------------------------------------------------------------------------------------------------------------
Category Criteria
----------------------------------------------------------------------------------------------------------------
1........................................... Corrosion rate on either steel or aluminium surfaces exceeding
6.25 mm per year at a test temperature of 55 [deg]C (131 [deg]F)
when tested on both materials.
----------------------------------------------------------------------------------------------------------------
Note: Where an initial test on either steel or aluminium
indicates the chemical being tested is corrosive, the follow-up test
on the other metal is not necessary.
B.16.3 Additional Classification Considerations
The specimen to be used for the test shall be made of the
following materials:
(a) For the purposes of testing steel, steel types S235JR+CR
(1.0037 resp.St 37-2), S275J2G3+CR (1.0144 resp.St 44-3), ISO 3574,
Unified Numbering System (UNS) G 10200, or SAE 1020;
(b) For the purposes of testing aluminium: Non-clad types 7075-
T6 or AZ5GU-T6.
APPENDIX C TO Sec. 1910.1200--ALLOCATION OF LABEL ELEMENTS (MANDATORY)
C.1 The label for each hazardous chemical shall include the
product identifier used on the safety data sheet.
C.1.1 The labels on shipped containers shall also include the
name, address, and telephone number of the chemical manufacturer,
importer, or responsible party.
C.2 The label for each hazardous chemical that is classified
shall include the signal word, hazard statement(s), pictogram(s),
and precautionary statement(s) specified in C.4 for each hazard
class and associated hazard category, except as provided for in
C.2.1 through C.2.4.
C.2.1 Precedence of Hazard Information
C.2.1.1 If the signal word "Danger" is included, the signal
word "Warning" shall not appear;
C.2.1.2 If the skull and crossbones pictogram is included, the
exclamation mark pictogram shall not appear where it is used for
acute toxicity;
C.2.1.3 If the corrosive pictogram is included, the exclamation
mark pictogram shall not appear where it is used for skin or eye
irritation;
C.2.1.4 If the health hazard pictogram is included for
respiratory sensitization, the exclamation mark pictogram shall not
appear where it is used for skin sensitization or for skin or eye
irritation.
C.2.2 Hazard Statement Text
C.2.2.1 The text of all applicable hazard statements shall
appear on the label, except as otherwise specified. The information
in italics shall be included as part of the hazard statement as
provided. For example: "causes damage to organs (state all organs
affected) through prolonged or repeated exposure (state route of
exposure if no other routes of exposure cause the hazard)". Hazard
statements may be combined where appropriate to reduce the
information on the label and improve readability, as long as all of
the hazards are conveyed as required.
C.2.2.2 If the chemical manufacturer, importer, or responsible
party can demonstrate that all or part of the hazard statement is
inappropriate to a specific substance or mixture, the corresponding
statement may be omitted from the label.
C.2.3 Pictograms
C.2.3.1 Pictograms shall be in the shape of a square set at a
point and shall include a black hazard symbol on a white background
with a red frame sufficiently wide to be clearly visible. A square
red frame set at a point without a hazard symbol is not a pictogram
and is not permitted on the label.
BILLING CODE 4510-26-P
[GRAPHIC] [TIFF OMITTED] TR26MR12.069
[GRAPHIC] [TIFF OMITTED] TR26MR12.070
BILLING CODE 4510-26-C
C.2.4 Precautionary Statement Text
C.2.4.1 There are four types of precautionary statements
presented, "prevention," "response," "storage," and
"disposal." The core part of the precautionary statement is
presented in bold print. This is the text, except as otherwise
specified, that shall appear on the label. Where additional
information is required, it is indicated in plain text.
C.2.4.2 When a backslash or diagonal mark (/) appears in the
precautionary statement text, it indicates that a choice has to be
made between the separated phrases. In such cases, the chemical
manufacturer, importer, or responsible party can choose the most
appropriate phrase(s). For example, "Wear protective gloves/
protective clothing/eye protection/face protection" could read
"wear eye protection".
C.2.4.3 When three full stops (* * *) appear in the
precautionary statement text, they indicate that all applicable
conditions are not listed. For example, in "Use explosion-proof
electrical/ventilating/lighting/* * */equipment", the use of "* *
*" indicates that other equipment may need to be specified. In such
cases, the chemical manufacturer, importer, or responsible party can
choose the other conditions to be specified.
C.2.4.4 When text in italics is used in a precautionary
statement, this indicates specific conditions applying to the use or
allocation of the precautionary statement. For example, "Use
explosion-proof electrical/ventilating/lighting/* * */equipment" is
only required for flammable solids "if dust clouds can occur".
Text in italics is intended to be an explanatory, conditional note
and is not intended to appear on the label.
C.2.4.5 Where square brackets ([ ]) appear around text in a
precautionary statement, this indicates that the text in square
brackets is not appropriate in every case and should be used only in
certain circumstances. In these cases, conditions for use explaining
when the text should be used are provided. For example, one
precautionary statement states: "[In case of inadequate
ventilation] wear respiratory protection." This statement is given
with the condition for use "- text in square brackets may be used
if additional information is provided with the chemical at the point
of use that explains what type of ventilation would be adequate for
safe use". This means that, if additional information is provided
with the chemical explaining what type of ventilation would be
adequate for safe use, the text in square brackets should be used
and the statement would read: "In case of inadequate ventilation
wear respiratory protection." However, if the chemical is supplied
without such ventilation information, the text in square brackets
should not be used, and the precautionary statement should read:
"Wear respiratory protection."
C.2.4.6 Precautionary statements may be combined or consolidated
to save label space and improve readability. For example, "Keep
away from heat, sparks and open flame," "Store in a well-
ventilated place" and "Keep cool" can be combined to read "Keep
away from heat, sparks and open flame and store in a cool, well-
ventilated place."
C.2.4.7 In most cases, the precautionary statements are
independent (e.g., the phrases for explosive hazards do not modify
those related to certain health hazards, and products that are
classified for both hazard classes shall bear appropriate
precautionary statements for both). Where a chemical is classified
for a number of hazards, and the precautionary statements are
similar, the most stringent shall be included on the label (this
will be applicable mainly to preventive measures). An order of
precedence may be imposed by the chemical manufacturer, importer or
responsible party in situations where phrases concern "Response."
Rapid action may be crucial. For example, if a chemical is
carcinogenic and acutely toxic, rapid action may be crucial, and
first aid measures for acute toxicity will take precedence over
those for long-term effects. In addition, medical attention to
delayed health effects may be required in cases of incidental
exposure, even if not associated with immediate symptoms of
intoxication.
C.2.4.8 If the chemical manufacturer, importer, or responsible
party can demonstrate that a precautionary statement is
inappropriate to a specific substance or mixture, the precautionary
statement may be omitted from the label.
C.3 Supplementary Hazard Information
C.3.1 To ensure that non-standardized information does not lead
to unnecessarily wide variation or undermine the required
information, supplementary information on the label is limited to
when it provides further detail and does not contradict or cast
doubt on the validity of the standardized hazard information.
C.3.2 Where the chemical manufacturer, importer, or distributor
chooses to add supplementary information on the label, the placement
of supplemental information shall not impede identification of
information required by this section.
C.3.3 Where an ingredient with unknown acute toxicity is used in
a mixture at a concentration >=1%, and the mixture is not classified
based on testing of the mixture as a whole, a statement that X% of
the mixture consists of ingredient(s) of unknown acute toxicity is
required on the label.
BILLING CODE 4510-26-P
[GRAPHIC] [TIFF OMITTED] TR26MR12.071
[GRAPHIC] [TIFF OMITTED] TR26MR12.072
[GRAPHIC] [TIFF OMITTED] TR26MR12.073
[GRAPHIC] [TIFF OMITTED] TR26MR12.074
[GRAPHIC] [TIFF OMITTED] TR26MR12.075
[GRAPHIC] [TIFF OMITTED] TR26MR12.076
[GRAPHIC] [TIFF OMITTED] TR26MR12.077
[GRAPHIC] [TIFF OMITTED] TR26MR12.078
[GRAPHIC] [TIFF OMITTED] TR26MR12.079
[GRAPHIC] [TIFF OMITTED] TR26MR12.080
[GRAPHIC] [TIFF OMITTED] TR26MR12.081
[GRAPHIC] [TIFF OMITTED] TR26MR12.082
[GRAPHIC] [TIFF OMITTED] TR26MR12.083
[GRAPHIC] [TIFF OMITTED] TR26MR12.084
[GRAPHIC] [TIFF OMITTED] TR26MR12.085
[GRAPHIC] [TIFF OMITTED] TR26MR12.086
[GRAPHIC] [TIFF OMITTED] TR26MR12.087
[GRAPHIC] [TIFF OMITTED] TR26MR12.088
[GRAPHIC] [TIFF OMITTED] TR26MR12.089
[GRAPHIC] [TIFF OMITTED] TR26MR12.090
[GRAPHIC] [TIFF OMITTED] TR26MR12.091
[GRAPHIC] [TIFF OMITTED] TR26MR12.092
[GRAPHIC] [TIFF OMITTED] TR26MR12.093
[GRAPHIC] [TIFF OMITTED] TR26MR12.094
[GRAPHIC] [TIFF OMITTED] TR26MR12.095
[GRAPHIC] [TIFF OMITTED] TR26MR12.096
[GRAPHIC] [TIFF OMITTED] TR26MR12.097
[GRAPHIC] [TIFF OMITTED] TR26MR12.098
[GRAPHIC] [TIFF OMITTED] TR26MR12.099
[GRAPHIC] [TIFF OMITTED] TR26MR12.100
[GRAPHIC] [TIFF OMITTED] TR26MR12.101
[GRAPHIC] [TIFF OMITTED] TR26MR12.102
[GRAPHIC] [TIFF OMITTED] TR26MR12.103
[GRAPHIC] [TIFF OMITTED] TR26MR12.104
[GRAPHIC] [TIFF OMITTED] TR26MR12.105
[GRAPHIC] [TIFF OMITTED] TR26MR12.106
[GRAPHIC] [TIFF OMITTED] TR26MR12.107
[GRAPHIC] [TIFF OMITTED] TR26MR12.108
[GRAPHIC] [TIFF OMITTED] TR26MR12.109
[GRAPHIC] [TIFF OMITTED] TR26MR12.110
[GRAPHIC] [TIFF OMITTED] TR26MR12.111
[GRAPHIC] [TIFF OMITTED] TR26MR12.112
[GRAPHIC] [TIFF OMITTED] TR26MR12.113
[GRAPHIC] [TIFF OMITTED] TR26MR12.114
[GRAPHIC] [TIFF OMITTED] TR26MR12.115
[GRAPHIC] [TIFF OMITTED] TR26MR12.116
[GRAPHIC] [TIFF OMITTED] TR26MR12.117
[GRAPHIC] [TIFF OMITTED] TR26MR12.118
[GRAPHIC] [TIFF OMITTED] TR26MR12.119
[GRAPHIC] [TIFF OMITTED] TR26MR12.120
[GRAPHIC] [TIFF OMITTED] TR26MR12.121
[GRAPHIC] [TIFF OMITTED] TR26MR12.122
[GRAPHIC] [TIFF OMITTED] TR26MR12.123
[GRAPHIC] [TIFF OMITTED] TR26MR12.124
[GRAPHIC] [TIFF OMITTED] TR26MR12.125
[GRAPHIC] [TIFF OMITTED] TR26MR12.126
[GRAPHIC] [TIFF OMITTED] TR26MR12.127
BILLING CODE 4510-26-C
Appendix D to Sec. 1910.1200--Safety Data Sheets (Mandatory)
A safety data sheet (SDS) shall include the information
specified in Table D.1 under the section number and heading
indicated for sections 1-11 and 16. If no relevant information is
found for any given subheading within a section, the SDS shall
clearly indicate that no applicable information is available.
Sections 12-15 may be included in the SDS, but are not mandatory.
Table D.1--Minimum Information for an SDS
------------------------------------------------------------------------
Heading Subheading
------------------------------------------------------------------------
1. Identification............ (a) Product identifier used on the label;
(b) Other means of identification;
(c) Recommended use of the chemical and
restrictions on use;
(d) Name, address, and telephone number
of the chemical manufacturer, importer,
or other responsible party;
(e) Emergency phone number.
2. Hazard(s) identification.. (a) Classification of the chemical in
accordance with paragraph (d) of Sec.
1910.1200;
(b) Signal word, hazard statement(s),
symbol(s) and precautionary statement(s)
in accordance with paragraph (f) of Sec.
1910.1200. (Hazard symbols may be
provided as graphical reproductions in
black and white or the name of the
symbol, e.g., flame, skull and
crossbones);
(c) Describe any hazards not otherwise
classified that have been identified
during the classification process;
(d) Where an ingredient with unknown
acute toxicity is used in a mixture at a
concentration >=1% and the mixture is
not classified based on testing of the
mixture as a whole, a statement that X%
of the mixture consists of ingredient(s)
of unknown acute toxicity is required.
3. Composition/information on Except as provided for in paragraph (i)
ingredients. of Sec. 1910.1200 on trade secrets:
For Substances
(a) Chemical name;
(b) Common name and synonyms;
(c) CAS number and other unique
identifiers;
(d) Impurities and stabilizing additives
which are themselves classified and
which contribute to the classification
of the substance.
For Mixtures
In addition to the information required
for substances:
(a) The chemical name and concentration
(exact percentage) or concentration
ranges of all ingredients which are
classified as health hazards in
accordance with paragraph (d) of Sec.
1910.1200 and
(1) Are present above their cut-off/
concentration limits; or
(2) Present a health risk below the cut-
off/concentration limits.
(b) The concentration (exact percentage)
shall be specified unless a trade secret
claim is made in accordance with
paragraph (i) of Sec. 1910.1200, when
there is batch-to-batch variability in
the production of a mixture, or for a
group of substantially similar mixtures
(See A.0.5.1.2) with similar chemical
composition. In these cases,
concentration ranges may be used.
For All Chemicals Where a Trade Secret is
Claimed
Where a trade secret is claimed in
accordance with paragraph (i) of Sec.
1910.1200, a statement that the specific
chemical identity and/or exact
percentage (concentration) of
composition has been withheld as a trade
secret is required.
4. First-aid measures........ (a) Description of necessary measures,
subdivided according to the different
routes of exposure, i.e., inhalation,
skin and eye contact, and ingestion;
(b) Most important symptoms/effects,
acute and delayed.
(c) Indication of immediate medical
attention and special treatment needed,
if necessary.
5. Fire-fighting measures.... (a) Suitable (and unsuitable)
extinguishing media.
(b) Specific hazards arising from the
chemical (e.g., nature of any hazardous
combustion products).
(c) Special protective equipment and
precautions for fire-fighters.
6. Accidental release (a) Personal precautions, protective
measures. equipment, and emergency procedures.
(b) Methods and materials for containment
and cleaning up.
7. Handling and storage...... (a) Precautions for safe handling.
(b) Conditions for safe storage,
including any incompatibilities.
8. Exposure controls/personal (a) OSHA permissible exposure limit
protection. (PEL), American Conference of
Governmental Industrial Hygienists
(ACGIH) Threshold Limit Value (TLV), and
any other exposure limit used or
recommended by the chemical
manufacturer, importer, or employer
preparing the safety data sheet, where
available.
(b) Appropriate engineering controls.
(c) Individual protection measures, such
as personal protective equipment.
9. Physical and chemical (a) Appearance (physical state, color,
properties. etc.);
(b) Odor;
(c) Odor threshold;
(d) pH;
(e) Melting point/freezing point;
(f) Initial boiling point and boiling
range;
(g) Flash point;
(h) Evaporation rate;
(i) Flammability (solid, gas);
(j) Upper/lower flammability or explosive
limits;
(k) Vapor pressure;
(l) Vapor density;
(m) Relative density;
(n) Solubility(ies);
(o) Partition coefficient: n-octanol/
water;
(p) Auto-ignition temperature;
(q) Decomposition temperature;
(r) Viscosity.
10. Stability and reactivity. (a) Reactivity;
(b) Chemical stability;
(c) Possibility of hazardous reactions;
(d) Conditions to avoid (e.g., static
discharge, shock, or vibration);
(e) Incompatible materials;
(f) Hazardous decomposition products.
11. Toxicological information Description of the various toxicological
(health) effects and the available data
used to identify those effects,
including:
(a) Information on the likely routes of
exposure (inhalation, ingestion, skin
and eye contact);
(b) Symptoms related to the physical,
chemical and toxicological
characteristics;
(c) Delayed and immediate effects and
also chronic effects from short- and
long-term exposure;
(d) Numerical measures of toxicity (such
as acute toxicity estimates).
(e) Whether the hazardous chemical is
listed in the National Toxicology
Program (NTP) Report on Carcinogens
(latest edition) or has been found to be
a potential carcinogen in the
International Agency for Research on
Cancer (IARC) Monographs (latest
edition), or by OSHA.
12. Ecological information (a) Ecotoxicity (aquatic and terrestrial,
(Non-mandatory). where available);
(b) Persistence and degradability;
(c) Bioaccumulative potential;
(d) Mobility in soil;
(e) Other adverse effects (such as
hazardous to the ozone layer).
13. Disposal considerations Description of waste residues and
(Non-mandatory). information on their safe handling and
methods of disposal, including the
disposal of any contaminated packaging.
14. Transport information (a) UN number;
(Non-mandatory).
(b) UN proper shipping name;
(c) Transport hazard class(es);
(d) Packing group, if applicable;
(e) Environmental hazards (e.g., Marine
pollutant (Yes/No));
(f) Transport in bulk (according to Annex
II of MARPOL 73/78 and the IBC Code);
(g) Special precautions which a user
needs to be aware of, or needs to comply
with, in connection with transport or
conveyance either within or outside
their premises.
15. Regulatory information Safety, health and environmental
(Non-mandatory). regulations specific for the product in
question.
16. Other information, The date of preparation of the SDS or the
including date of last change to it.
preparation or last revision.
------------------------------------------------------------------------
Appendix F to Sec. 1910.1200--Guidance for Hazard Classifications Re:
Carcinogenicity (Non-Mandatory)
The mandatory criteria for classification of a chemical for
carcinogenicity under HCS (Sec. 1910.1200) are found in Appendix
A.6 to this section. This non-mandatory Appendix provides additional
guidance on hazard classification for carcinogenicity. Part A of
Appendix F includes background guidance provided by GHS based on the
Preamble of the International Agency for Research on Cancer (IARC)
"Monographs on the Evaluation of Carcinogenic Risks to Humans"
(2006). Part B provides IARC classification information. Part C
provides background guidance from the National Toxicology Program
(NTP) "Report on Carcinogens" (RoC), and Part D is a table that
compares GHS carcinogen hazard categories to carcinogen
classifications under IARC and NTP, allowing classifiers to be able
to use information from IARC and NTP RoC carcinogen classifications
to complete their classifications under the GHS, and thus the HCS.
Part A: Background Guidance 1
---------------------------------------------------------------------------
\1\ The text of Appendix F, Part A, on the IARC Monographs, is
paraphrased from the 2006 Preamble to the "Monographs on the
Evaluation of Carcinogenic Risks to Humans"; the Classifier is
referred to the full IARC Preamble for the complete text. The text
is not part of the agreed GHS text on the harmonized system
developed by the OECD Task Force-HCL.
---------------------------------------------------------------------------
As noted in Footnote 6 of Appendix A.6. to this section, the GHS
includes as guidance for classifiers information taken from the
Preamble of the International Agency for Research on Cancer (IARC)
"Monographs on the Evaluation of Carcinogenic Risks to Humans"
(2006), providing guidance on the evaluation of the strength and
evidence of carcinogenic risks to humans. This guidance also
discusses some additional considerations in classification and an
approach to analysis, rather than hard-and-fast rules. Part A is
consistent with Appendix A.6, and should help in evaluating
information to determine carcinogenicity.
Carcinogenicity in humans:
The evidence relevant to carcinogenicity from studies in humans
is classified into one of the following categories:
(a) Sufficient evidence of carcinogenicity: A causal
relationship has been established between exposure to the agent and
human cancer. That is, a positive relationship has been observed
between the exposure and cancer in studies in which chance, bias and
confounding could be ruled out with reasonable confidence.
(b) Limited evidence of carcinogenicity: A positive association
has been observed between exposure to the agent and cancer for which
a causal interpretation is considered by the Working Group to be
credible, but chance, bias or confounding could not be ruled out
with reasonable confidence.
In some instances, the above categories may be used to classify
the degree of evidence related to carcinogenicity in specific organs
or tissues.
Carcinogenicity in experimental animals:
The evidence relevant to carcinogenicity in experimental animals
is classified into one of the following categories:
(a) Sufficient evidence of carcinogenicity: A causal
relationship has been established between the agent and an increased
incidence of malignant neoplasms or of an appropriate combination of
benign and malignant neoplasms in two or more species of animals or
two or more independent studies in one species carried out at
different times or in different laboratories or under different
protocols. An increased incidence of tumors in both sexes of a
single species in a well-conducted study, ideally conducted under
Good Laboratory Practices, can also provide sufficient evidence.
Exceptionally, a single study in one species and sex might be
considered to provide sufficient evidence of carcinogenicity when
malignant neoplasms occur to an unusual degree with regard to
incidence, site, type of tumor or age at onset, or when there are
strong findings of tumors at multiple sites.
(a) Limited evidence of carcinogenicity: The data suggest a
carcinogenic effect but are limited for making a definitive
evaluation because, e.g. the evidence of carcinogenicity is
restricted to a single experiment; there are unresolved questions
regarding the adequacy of the design, conduct or interpretation of
the studies; the agent increases the incidence only of benign
neoplasms or lesions of uncertain neoplastic potential; or the
evidence of carcinogenicity is restricted to studies that
demonstrate only promoting activity in a narrow range of tissues or
organs.
Guidance on How To Consider Important Factors in Classification of
Carcinogenicity (See Reference Section)
The weight of evidence analysis called for in GHS and the HCS
(Sec. 1910.1200) is an integrative approach that considers
important factors in determining carcinogenic potential along with
the strength of evidence analysis. The IPCS "Conceptual Framework
for Evaluating a Mode of Action for Chemical Carcinogenesis"
(2001), International Life Sciences Institute (ILSI) "Framework for
Human Relevance Analysis of Information on Carcinogenic Modes of
Action" (Meek, et al., 2003; Cohen et al., 2003, 2004), and
Preamble to the IARC Monographs (2006; Section B.6. (Scientific
Review and Evaluation; Evaluation and Rationale)) provide a basis
for systematic assessments that may be performed in a consistent
fashion. The IPCS also convened a panel in 2004 to further develop
and clarify the human relevance framework. However, the above
documents are not intended to dictate answers, nor provide lists of
criteria to be checked off.
Mode of Action
Various documents on carcinogen assessment all note that mode of
action in and of itself, or consideration of comparative metabolism,
should be evaluated on a case-by-case basis and are part of an
analytic evaluative approach. One must look closely at any mode of
action in animal experiments, taking into consideration comparative
toxicokinetics/toxicodynamics between the animal test species and
humans to determine the relevance of the results to humans. This may
lead to the possibility of discounting very specific effects of
certain types of substances. Life stage-dependent effects on
cellular differentiation may also lead to qualitative differences
between animals and humans. Only if a mode of action of tumor
development is conclusively determined not to be operative in humans
may the carcinogenic evidence for that tumor be discounted. However,
a weight of evidence evaluation for a substance calls for any other
tumorigenic activity to be evaluated, as well.
Responses in Multiple Animal Experiments
Positive responses in several species add to the weight of
evidence that a substance is a carcinogen. Taking into account all
of the factors listed in A.6.2.5.2 and more, such chemicals with
positive outcomes in two or more species would be provisionally
considered to be classified in GHS Category 1B until human relevance
of animal results are assessed in their entirety. It should be
noted, however, that positive results for one species in at least
two independent studies, or a single positive study showing
unusually strong evidence of malignancy may also lead to Category
1B.
Responses Are in One Sex or Both Sexes
Any case of gender-specific tumors should be evaluated in light
of the total tumorigenic response to the substance observed at other
sites (multi-site responses or incidence above background) in
determining the carcinogenic potential of the substance.
If tumors are seen only in one sex of an animal species, the
mode of action should be carefully evaluated to see if the response
is consistent with the postulated mode of action. Effects seen only
in one sex in a test species may be less convincing than effects
seen in both sexes, unless there is a clear patho-physiological
difference consistent with the mode of action to explain the single
sex response.
Confounding Effects of Excessive Toxicity or Localized Effects
Tumors occurring only at excessive doses associated with severe
toxicity generally have doubtful potential for carcinogenicity in
humans. In addition, tumors occurring only at sites of contact and/
or only at excessive doses need to be carefully evaluated for human
relevance for carcinogenic hazard. For example, forestomach tumors,
following administration by gavage of an irritating or corrosive,
non-mutagenic chemical, may be of questionable relevance. However,
such determinations must be evaluated carefully in justifying the
carcinogenic potential for humans; any occurrence of other tumors at
distant sites must also be considered.
Tumor Type, Reduced Tumor Latency
Unusual tumor types or tumors occurring with reduced latency may
add to the weight of evidence for the carcinogenic potential of a
substance, even if the tumors are not statistically significant.
Toxicokinetic behavior is normally assumed to be similar in
animals and humans, at least from a qualitative perspective. On the
other hand, certain tumor types in animals may be associated with
toxicokinetics or toxicodynamics that are unique to the animal
species tested and may not be predictive of carcinogenicity in
humans. Very few such examples have been agreed internationally.
However, one example is the lack of human relevance of kidney tumors
in male rats associated with compounds causing [alpha]2u-globulin
nephropathy (IARC, Scientific Publication N[deg] 147 \2\). Even when
a particular tumor type may be discounted, expert judgment must be
used in assessing the total tumor profile in any animal experiment.
---------------------------------------------------------------------------
\2\ While most international agencies do not consider kidney
tumors coincident with [alpha]2u-globulin nephropathy to be a
predictor of risk in humans, this view is not universally held.
(See: Doi et al., 2007).
---------------------------------------------------------------------------
Part B: International Agency for Research on Cancer (IARC) 3
---------------------------------------------------------------------------
\3\ Preamble of the International Agency for Research on Cancer
(IARC) "Monographs on the Evaluation of Carcinogenic Risks to
Humans" (2006).
---------------------------------------------------------------------------
IARC Carcinogen Classification Categories:
Group 1: The agent is carcinogenic to humans
This category is used when there is sufficient evidence of
carcinogenicity in humans. Exceptionally, an agent may be placed in
this category when evidence of carcinogenicity in humans is less
than sufficient but there is sufficient evidence of carcinogenicity
in experimental animals and strong evidence in exposed humans that
the agent acts through a relevant mechanism of carcinogenicity.
Group 2:
This category includes agents for which, at one extreme, the
degree of evidence of carcinogenicity in humans is almost
sufficient, as well as those for which, at the other extreme, there
are no human data but for which there is evidence of carcinogenicity
in experimental animals. Agents are assigned to either Group 2A
(probably carcinogenic to humans) or Group 2B (possibly carcinogenic
to humans) on the basis of epidemiological and experimental evidence
of carcinogenicity and mechanistic and other relevant data. The
terms probably carcinogenic and possibly carcinogenic have no
quantitative significance and are used simply as descriptors of
different levels of evidence of human carcinogenicity, with probably
carcinogenic signifying a higher level of evidence than possibly
carcinogenic.
Group 2A: The agent is probably carcinogenic to human.
This category is used when there is limited evidence of
carcinogenicity in humans and sufficient evidence of carcinogenicity
in experimental animals. In some cases, an agent may be classified
in this category when there is inadequate evidence of
carcinogenicity in humans and sufficient evidence of carcinogenicity
in experimental animals and strong evidence that the carcinogenesis
is mediated by a mechanism that also operates in humans.
Exceptionally, an agent may be classified in this category solely on
the basis of limited evidence of carcinogenicity in humans. An agent
may be assigned to this category if it clearly belongs, based on
mechanistic considerations, to a class of agents for which one or
more members have been classified in Group 1 or Group 2A.
Group 2B: The agent is possibly carcinogenic to humans.
This category is used for agents for which there is limited
evidence of carcinogenicity in humans and less than sufficient
evidence of carcinogenicity in experimental animals. It may also be
used when there is inadequate evidence of carcinogenicity in humans
but there is sufficient evidence of carcinogenicity in experimental
animals. In some instances, an agent for which there is inadequate
evidence of carcinogenicity in humans and less than sufficient
evidence of carcinogenicity in experimental animals together with
supporting evidence from mechanistic and other relevant data may be
placed in this group. An agent may be classified in this category
solely on the basis of strong evidence from mechanistic and other
relevant data.
Part C: National Toxicology Program (NTP), "Report on Carcinogens",
Background Guidance
NTP Listing Criteria \4\:
---------------------------------------------------------------------------
\4\ See: http://ntp.niehs.nih.gov/go/15209.
---------------------------------------------------------------------------
The criteria for listing an agent, substance, mixture, or
exposure circumstance in the Report on Carcinogens (RoC) are as
follows:
Known To Be A Human Carcinogen: There is sufficient evidence of
carcinogenicity from studies in humans \5\ that indicates a causal
relationship between exposure to the agent, substance, or mixture,
and human cancer.
---------------------------------------------------------------------------
\5\ This evidence can include traditional cancer epidemiology
studies, data from clinical studies, and/or data derived from the
study of tissues or cells from humans exposed to the substance in
question that can be useful for evaluating whether a relevant cancer
mechanism is operating in people.
---------------------------------------------------------------------------
Reasonably Anticipated To Be A Human Carcinogen: There is
limited evidence of carcinogenicity from studies in humans that
indicates that a causal interpretation is credible, but that
alternative explanations, such as chance, bias, or confounding
factors, could not adequately be excluded,
or
there is sufficient evidence of carcinogenicity from studies in
experimental animals that indicates there is an increased incidence
of malignant and/or a combination of malignant and benign tumors in
multiple species or at multiple tissue sites, or by multiple routes
of exposure, or to an unusual degree with regard to incidence, site,
or type of tumor, or age at onset,
or
there is less than sufficient evidence of carcinogenicity in humans
or laboratory animals; however, the agent, substance, or mixture
belongs to a well-defined, structurally-related class of substances
whose members are listed in a previous Report on Carcinogens as
either known to be a human carcinogen or reasonably anticipated to
be a human carcinogen, or there is convincing relevant information
that the agent acts through mechanisms indicating it would likely
cause cancer in humans.
Conclusions regarding carcinogenicity in humans or experimental
animals are based on scientific judgment, with consideration given
to all relevant information. Relevant information includes, but is
not limited to, dose response, route of exposure, chemical
structure, metabolism, pharmacokinetics, sensitive sub-populations,
genetic effects, or other data relating to mechanism of action or
factors that may be unique to a given substance. For example, there
may be substances for which there is evidence of carcinogenicity in
laboratory animals, but there are compelling data indicating that
the agent acts through mechanisms that do not operate in humans and
would therefore not reasonably be anticipated to cause cancer in
humans.
Part D: Table Relating Approximate Equivalences Among IARC, NTP RoC,
and GHS Carcinogenicity Classifications
The following table may be used to perform hazard
classifications for carcinogenicity under the HCS (Sec. 1910.1200).
It relates the approximated GHS hazard categories for
carcinogenicity to the classifications provided by IARC and NTP, as
described in Parts B and C of this Appendix.
Approximate Equivalences Among Carcinogen Classification Schemes
----------------------------------------------------------------------------------------------------------------
IARC GHS NTP RoC
----------------------------------------------------------------------------------------------------------------
Group 1................................. Category 1A................ Known.
Group 2A................................ Category 1B................ Reasonably Anticipated.
Group 2B................................ Category 2................. (See Note 1).
----------------------------------------------------------------------------------------------------------------
Note 1:
1. Limited evidence of carcinogenicity from studies in humans (corresponding to IARC 2A/GHS 1B);
2. Sufficient evidence of carcinogenicity from studies in experimental animals (again, essentially corresponding
to IARC 2A/GHS 1B);
3. Less than sufficient evidence of carcinogenicity in humans or laboratory animals; however:
c. The agent, substance, or mixture belongs to a well-defined, structurally-related class of substances whose
members are listed in a previous RoC as either "Known" or "Reasonably Anticipated" to be a human
carcinogen, or
d. There is convincing relevant information that the agent acts through mechanisms indicating it would likely
cause cancer in humans.
*References
Cohen, S.M., J. Klaunig, M.E. Meek, R.N. Hill, T. Pastoor, L.
Lehman-McKeeman, J. Bucher, D.G. Longfellow, J. Seed, V. Dellarco,
P. Fenner-Crisp, and D. Patton. 2004. Evaluating the human relevance
of chemically induced animal tumors. Toxicol. Sci. 78(2):181-186.
Cohen, S.M., M.E. Meek, J.E. Klaunig, D.E. Patton, P.A. Fenner-
Crisp. 2003. The human relevance of information on carcinogenic
modes of action: Overview. Crit. Rev. Toxicol. 33(6):581-9.
Meek, M.E., J.R. Bucher, S.M. Cohen, V. Dellarco, R.N. Hill, L.
Lehman-McKeeman, D.G. Longfellow, T. Pastoor, J. Seed, D.E. Patton.
2003. A framework for human relevance analysis of information on
carcinogenic modes of action. Crit. Rev. Toxicol. 33(6):591-653.
Sonich-Mullin, C., R. Fielder, J. Wiltse, K. Baetcke, J. Dempsey, P.
Fenner-Crisp, D. Grant, M. Hartley, A. Knapp, D. Kroese, I.
Mangelsdorf, E. Meek, J.M. Rice, and M. Younes. 2001. The conceptual
framework for evaluating a mode of action for chemical
carcinogenesis. Reg. Toxicol. Pharm. 34:146-152.
International Programme on Chemical Safety Harmonization Group.
2004. Report of the First Meeting of the Cancer Working Group. World
Health Organization. Report IPCS/HSC-CWG-1/04. Geneva.
International Agency for Research on Cancer. IARC Monographs on the
Evaluation of Carcinogenic Risks to Human. Preambles to Volumes.
World Health Organization. Lyon, France.
Cohen, S.M., P.A. Fenner-Crisp, and D.E. Patton. 2003. Special
Issue: Cancer Modes of Action and Human Relevance. Critical Reviews
in Toxicology, R.O. McClellan, ed., Volume 33/Issue 6. CRC Press.
Capen, C.C., E. Dybing, and J.D. Wilbourn. 1999. Species differences
in thyroid, kidney and urinary bladder carcinogenesis. International
Agency for Research on Cancer, Scientific Publication N[deg] 147.
Doi, A.M., G. Hill, J. Seely, J.R. Hailey, G. Kissling, and J.R.
Buchera. 2007. [alpha]2u-Globulin nephropathy and renal tumors in
National Toxicology Program studies. Toxicol. Pathol. 35:533-540.
* * * * *
0
33. Amend Sec. 1910.1450 as follows:
0
A. Remove the definitions of Combustible liquid, Compressed gas,
Explosive, Flammable, Flashpoint, Organic peroxide, Oxidizer, Unstable
(reactive), and Water-reactive from paragraph (b);
0
B. Revise the definitions of Hazardous chemical, Physical hazard, and
Reproductive toxins in paragraph (b);
0
C. Add definitions of Health hazard and Mutagen in alphabetical order
in paragraph (b);
0
D. In paragraphs (f)(3)(v), (h)(1) introductory text, (h)(1)(ii) and
(h)(2)(iii), remove the phrases "Material Safety Data Sheets" and
"material safety data sheets" and add in their place "safety data
sheets";
0
E. In Appendix A to Sec. 1910.1450, in the Table of Contents (item
"G") remove "Material Safety Data Sheets" and add in its place
"Safety Data Sheets";
0
F. In Appendix A to Sec. 1910.1450, revise the heading "G. Material
Safety Data Sheets" and revise the text following the heading.
The revisions read as follows:
Sec. 1910.1450 Occupational exposure to hazardous chemicals in
laboratories.
* * * * *
(b) * * *
Hazardous chemical means any chemical which is classified as health
hazard or simple asphyxiant in accordance with the Hazard Communication
Standard (Sec. 1910.1200).
Health hazard means a chemical that is classified as posing one of
the following hazardous effects: Acute toxicity (any route of
exposure); skin corrosion or irritation; serious eye damage or eye
irritation; respiratory or skin sensitization; germ cell mutagenicity;
carcinogenity; reproductive toxicity; specific target organ toxicity
(single or repeated exposure); aspiration hazard. The criteria for
determining whether a chemical is classified as a health hazard are
detailed in Appendix A of the Hazard Communication Standard (Sec.
1910.1200) and Sec. 1910.1200(c) (definition of "simple
asphyxiant").
* * * * *
Mutagen means chemicals that cause permanent changes in the amount
or structure of the genetic material in a cell. Chemicals classified as
mutagens in accordance with the Hazard Communication Standard (Sec.
1910.1200) shall be considered mutagens for purposes of this section.
* * * * *
Physical hazard means a chemical that is classified as posing one
of the following hazardous effects: Explosive; flammable (gases,
aerosols, liquids, or solids); oxidizer (liquid, solid, or gas); self
reactive; pyrophoric (gas, liquid or solid); self-heating; organic
peroxide; corrosive to metal; gas under pressure; in contact with water
emits flammable gas; or combustible dust. The criteria for determining
whether a chemical is classified as a physical hazard are in Appendix B
of the Hazard Communication Standard (Sec. 1910.1200) and Sec.
1910.1200(c) (definitions of "combustible dust" and "pyrophoric
gas").
* * * * *
Reproductive toxins mean chemicals that affect the reproductive
capabilities including adverse effects on sexual function and fertility
in adult males and females, as well as adverse effects on the
development of the offspring. Chemicals classified as reproductive
toxins in accordance with the Hazard Communication Standard (Sec.
1910.1200) shall be considered reproductive toxins for purposes of this
section.
* * * * *
Appendix A to Sec. 1910.1450--National Research Council
Recommendations Concerning Chemical Hygiene in Laboratories (Non-
Mandatory)
* * * * *
G. Safety Data Sheets
Safety data sheets are presented in "Prudent Practices" for the
chemicals listed below. (Asterisks denote that comprehensive safety
data sheets are provided).
* * * * *
PART 1915--OCCUPATIONAL SAFETY AND HEALTH STANDARDS FOR SHIPYARD
EMPLOYMENT
0
34. Revise the authority citation for part 1915 to read as follows:
Authority: Section 41, Longshore and Harbor Workers'
Compensation Act (33 U.S.C. 941); Sections. 4, 6, and 8 of the
Occupational Safety and Health Act of 1970 (29 U.S.C. 653, 655,
657); Secretary of Labor's Order No. 12-71 (36 FR 8754), 8-76 (41 FR
25059), 9-83 (48 FR 35736), 1-90 (55 FR 9033), 6-96 (62 FR 111), 3-
2000 (65 FR 50017), 5-2002 (67 FR 65008), 5-2007 (72 FR 31160), 4-
2010 (75 FR 55355), or 1-2012 (77 FR 3912), as applicable; 29 CFR
Part 1911.
Section 1915.100 also issued under 49 U.S.C. 1801-1819 and 5
U.S.C. 553.
Sections 1915.120 and 1915.152 of 29 CFR also issued under 29
CFR part 1911.
Subpart Z--[Amended]
0
35. Revise Sec. 1915.1001 paragraphs (i)(3), (k)(7), and (k)(8) to
read as follows:
Sec. 1915.1001 Asbestos.
* * * * *
(i) * * *
(3) The employer shall ensure that contaminated clothing is
transported in sealed impermeable bags, or other closed, impermeable
containers, and labeled in accordance with paragraph (k) of this
section.
* * * * *
(k) * * *
(7) Hazard communication. (i) Labels shall be affixed to all
products containing asbestos and to all containers containing such
products, including waste containers. Where feasible, installed
asbestos products shall contain a visible label.
(ii) General. The employer shall include asbestos in the program
established to comply with the Hazard Communication Standard (HCS)
(Sec. 1910.1200). The employer shall ensure that each employee has
access to labels on containers of asbestos and safety data sheets, and
is trained in accordance with the provisions of the HCS and paragraph
(k)(9) of this section. The employer shall ensure that at least the
following hazards are addressed: Cancer and lung effects.
(iii) Labels. (A) The employer shall ensure that labels of bags or
containers of protective clothing and equipment, scrap, waste, and
debris containing asbestos fibers bear the following information:
DANGER
CONTAINS ASBESTOS FIBERS
MAY CAUSE CANCER
CAUSES DAMAGE TO LUNGS
DO NOT BREATHE DUST
AVOID CREATING DUST
(B)(1) Prior to June 1, 2015, employers may include the following
information on raw materials, mixtures or labels of bags or containers
of protective clothing and equipment, scrap, waste, and debris
containing asbestos fibers in lieu of the labeling requirements in
paragraphs (k)(7)(ii) and (k)(7)(iii)(A) of this section:
DANGER
CONTAINS ASBESTOS FIBERS
AVOID CREATING DUST
CANCER AND LUNG DISEASE HAZARD
(2) Labels shall also contain a warning statement against breathing
asbestos fibers.
(iv) The provisions for labels required in paragraph (k)(7) of this
section do not apply where:
(A) Asbestos fibers have been modified by a bonding agent, coating,
binder, or other material, provided that the manufacturer can
demonstrate that, during any reasonably foreseeable use, handling,
storage, disposal, processing, or transportation, no airborne
concentrations of asbestos fibers in excess of the permissible exposure
limit and/or excursion limit will be released, or
(B) Asbestos is present in a product in concentrations less than
1.0 percent.
(8) Signs. (i) Warning signs that demarcate the regulated area
shall be provided and displayed at each location where a regulated area
is required to be established by paragraph (e) of this section. Signs
shall be posted at such a distance from such a location that an
employee may read the signs and take necessary protective steps before
entering the area marked by the signs.
(ii) The warning signs required by this paragraph (k)(8) shall bear
the following legend:
DANGER
ASBESTOS
MAY CAUSE CANCER
CAUSES DAMAGE TO LUNGS
AUTHORIZED PERSONNEL ONLY
(iii) In addition, where the use of respirators and protective
clothing is required in the regulated area under this section, the
warning signs shall include the following:
WEAR RESPIRATORY PROTECTION
AND PROTECTIVE CLOTHING IN THIS AREA
(iv) The employer shall ensure that employees working in and
contiguous to regulated areas comprehend the warning signs required to
be posted by paragraph (k)(8) of this section. Means to ensure employee
comprehension may include the use of foreign languages, pictographs,
and graphics.
(v) When a building/vessel owner or employer identifies previously
installed PACM and/or ACM, labels or signs shall be affixed or posted
so that employees will be notified of what materials contain PACM and/
or ACM. The employer shall attach such labels in areas where they will
clearly be noticed by employees who are likely to be exposed, such as
at the entrance to mechanical room/areas. Signs required by paragraph
(k)(6) of this section may be posted in lieu of labels, so long as they
contain information required for labeling. The employer shall ensure,
to the extent feasible, that employees who come in contact with these
signs or labels can comprehend them. Means to ensure employee
comprehension may include the use of foreign languages, pictographs,
graphics, and awareness training.
(vi) Prior to June 1, 2016, employers may use the following legend
in lieu of that specified in paragraph (k)(8)(ii) of this section:
DANGER
ASBESTOS
CANCER AND LUNG DISEASE HAZARD
AUTHORIZED PERSONNEL ONLY
(vii) Prior to June 1, 2016, employers may use the following legend
in lieu of that specified in paragraph (k)(8)(iii) of this section:
RESPIRATORS AND PROTECTIVE CLOTHING ARE REQUIRED IN THIS AREA
* * * * *
0
36. Revise Sec. 1915.1026 paragraphs (g)(2)(iv) and (j)(1), to read as
follows;
Sec. 1915.1026 Chromium (VI).
* * * * *
(g) * * *
(2) * * *
(iv) The employer shall ensure that bags or containers of
contaminated protective clothing or equipment that are removed from
change rooms for laundering, cleaning, maintenance, or disposal are
labeled in accordance with the requirements of the Hazard Communication
Standard, Sec. 1910.1200.
* * * * *
(j) * * *
(1) Hazard communication. The employer shall include chromium (VI)
in the program established to comply with the Hazard Communication
Standard (HCS) (Sec. 1910.1200). The employer shall ensure that each
employee has access to labels on containers of chromium (VI) and safety
data sheets, and is trained in accordance with the provisions of HCS
and paragraph (j)(2) of this section. The employer shall ensure that at
least the following hazards are addressed: Cancer; skin sensitization;
and eye irritation.
* * * * *
PART 1926--SAFETY AND HEALTH REGULATIONS FOR CONSTRUCTION
Subpart D--[Amended]
0
37. The authority citation for subpart D is revised to read as follows:
Authority: Section 107 of the Contract Work Hours and Safety
Standards Act (40 U.S.C. 3704); Sections 4, 6, and 8 of the
Occupational Safety and Health Act of 1970 (29 U.S.C. 653, 655, and
657); and Secretary of Labor's Order No. 12-71 (36 FR 8754), 8-76
(41 FR 25059), 9-83 (48 FR 35736), 1-90 (55 FR 9033), 6-96 (62 FR
111), 3-2000 (65 FR 50017), 5-2002 (67 FR 65008), 5-2007 (72 FR
31159), 4-2010 (75 FR 55355), or 1-2012 (77 FR 3912) as applicable;
and 29 CFR part 1911.
Sections 1926.58, 1926.59, 1926.60, and 1926.65 also issued
under 5 U.S.C. 553 and 29 CFR part 1911.
Section 1926.61 also issued under 49 U.S.C. 1801-1819 and 6
U.S.C. 553.
Section 1926.62 also issued under section 1031 of the Housing
and Community Development Act of 1992 (42 U.S.C. 4853).
Section 1926.65 also issued under section 126 of the Superfund
Amendments and Reauthorization Act of 1986, as amended (reprinted at
29 U.S.C.A. 655 Note), and 5 U.S.C. 553.
0
38. Revise Sec. 1926.60 paragraphs (l)(1) and (l)(2) to read as
follows:
Sec. 1926.60 Methylenedianiline.
* * * * *
(l) * * *
(1) Hazard communication. The employer shall include
Methylenedianiline (MDA) in the program established to comply with the
Hazard Communication Standard (HCS) (Sec. 1910.1200). The employer
shall ensure that each employee has access to labels on containers of
MDA and safety data sheets, and is trained in accordance with the
provisions of HCS and paragraph (l)(3) of this section. The employer
shall ensure that at least the following hazards are addressed: Cancer;
liver effects; and skin sensitization.
(2) Signs and labels-- (i) Signs. (A) The employer shall post and
maintain legible signs demarcating regulated areas and entrances or
access-ways to regulated areas that bear the following legend:
DANGER
MDA
MAY CAUSE CANCER
CAUSES DAMAGE TO THE LIVER
RESPIRATORY PROTECTION AND PROTECTIVE CLOTHING MAY BE REQUIRED IN
THIS AREA
AUTHORIZED PERSONNEL ONLY
(B) Prior to June 1, 2016, employers may use the following legend
in lieu of that specified in paragraph (l)(2)(i)(A) of this section:
DANGER
MDA
MAY CAUSE CANCER
LIVER TOXIN
AUTHORIZED PERSONNEL ONLY
RESPIRATORS AND PROTECTIVE CLOTHING MAY BE REQUIRED TO BE WORN IN
THIS AREA
(ii) Labels. (A) The employer shall ensure that labels or other
appropriate forms of warning are provided for containers of MDA within
the workplace. The labels shall comply with the requirements of Sec.
1910.1200(f) and shall include at least the following information for
pure MDA and mixtures containing MDA:
DANGER
CONTAINS MDA
MAY CAUSE CANCER
CAUSES DAMAGE TO THE LIVER
(B) Prior to June 1, 2015, employers may include the following
information workplace labels in lieu of the labeling requirements in
paragraph (l)(2)(ii)(A) of this section:
(1) For Pure MDA:
DANGER
CONTAINS MDA
MAY CAUSE CANCER
LIVER TOXIN
(2) For mixtures containing MDA:
DANGER
CONTAINS MDA
CONTAINS MATERIALS WHICH MAY CAUSE CANCER
LIVER TOXIN
* * * * *
0
39. Amend Sec. 1926.62 by revising paragraph (g)(2)(vii), the heading
of paragraph (l), paragraph (l)(1)(i), and paragraph (m), and Appendix
B to Sec. 1926.62 section XI, to read as follows:
Sec. 1926.62 Lead.
* * * * *
(g) * * *
(2) * * *
(vii)(A) The employer shall ensure that the containers of
contaminated protective clothing and equipment required by paragraph
(g)(2)(v) of this section are labeled as follows:
DANGER: CLOTHING AND EQUIPMENT CONTAMINATED WITH LEAD. MAY DAMAGE
FERTILITY OR THE UNBORN CHILD. CAUSES DAMAGE TO THE CENTRAL NERVOUS
SYSTEM. DO NOT EAT, DRINK OR SMOKE WHEN HANDLING. DO NOT REMOVE DUST
BY BLOWING OR SHAKING. DISPOSE OF LEAD CONTAMINATED WASH WATER IN
ACCORDANCE WITH APPLICABLE LOCAL, STATE, OR FEDERAL REGULATIONS.
(B) Prior to June 1, 2015, employers may include the following
information on bags or containers of contaminated protective clothing
and equipment required by paragraph (g)(2)(v) in lieu of the labeling
requirements in paragraph (g)(2)(vii)(A) of this section:
Caution: Clothing contaminated with lead. Do not remove dust by
blowing or shaking. Dispose of lead contaminated wash water in
accordance with applicable local, state, or federal regulations.
* * * * *
(l) Communication of hazards.
(1) * * *
(i) Hazard communication. The employer shall include lead in the
program established to comply with the Hazard Communication Standard
(HCS) (Sec. 1910.1200). The employer shall ensure that each employee
has access to labels on containers of lead and safety data sheets, and
is trained in accordance with the provisions of HCS and paragraph (l)
of this section. The employer shall ensure that at least the following
hazards are addressed:
(A) Reproductive/developmental toxicity;
(B) Central nervous system effects;
(C) Kidney effects;
(D) Blood effects; and
(E) Acute toxicity effects.
* * * * *
(m) Signs.
(1) General.
(i) The employer shall post the following warning signs in each
work area where an employee's exposure to lead is above the PEL.
DANGER
LEAD WORK AREA
MAY DAMAGE FERTILITY OR THE UNBORN CHILD
CAUSES DAMAGE TO THE CENTRAL NERVOUS SYSTEM
DO NOT EAT, DRINK OR SMOKE IN THIS AREA
(ii) The employer shall ensure that no statement appears on or near
any sign required by this paragraph (m) that contradicts or detracts
from the meaning of the required sign.
(iii) The employer shall ensure that signs required by this
paragraph (m) are illuminated and cleaned as necessary so that the
legend is readily visible.
(iv) The employer may use signs required by other statutes,
regulations or ordinances in addition to, or in combination with, signs
required by this paragraph (m).
(v) Prior to June 1, 2016, employers may use the following legend
in lieu of that specified in paragraph (m)(1)(i) of this section:
WARNING
LEAD WORK AREA
POISON
NO SMOKING OR EATING
* * * * *
Appendix B to Sec. 1926.62--Employee Standard Summary
* * * * *
XI. Signs--Paragraph (M)
The standard requires that the following warning sign be posted
in work areas when the exposure to lead is above the PEL:
DANGER
LEAD WORK AREA
MAY DAMAGE FERTILITY OR THE UNBORN CHILD
CAUSES DAMAGE TO THE CENTRAL NERVOUS SYSTEM
DO NOT EAT, DRINK OR SMOKE IN THIS AREA
Prior to June 1, 2016, employers may use the following legend in
lieu of that specified above:
WARNING
LEAD WORK AREA
POISON
NO SMOKING OR EATING
* * * * *
0
40. Revise Sec. 1926.64 paragraphs (a)(1)(ii) introductory text,
(a)(1)(ii)(B), and (d)(1)(vii), and the note following paragraph
(d)(1)(vii), to read as follows:
Sec. 1926.64 Process safety management of highly hazardous chemicals.
* * * * *
(a) * * *
(1) * * *
(ii) A process which involves a Category 1 flammable gas (as
defined in Sec. 1910.1200(c)) or flammable liquid with a flashpoint
below 100 [deg]F (37.8 [deg]C) on site in one location, in a quantity
of 10,000 pounds (4535.9 kg) or more except for:
* * * * *
(B) Flammable liquids with a flashpoint below 100 [deg]F (37.8
[deg]C) stored in atmospheric tanks or transferred that are kept below
their normal boiling point without benefit of chilling or
refrigeration.
* * * * *
(d) * * *
(1) * * *
(vii) Hazardous effects of inadvertent mixing of different
materials that could foreseeably occur.
Note to paragraph (d)(1): Safety data sheets meeting the
requirements of Sec. 1910.1200(g) may be used to comply with this
requirement to the extent they contain the information required by
this paragraph (d)(1).
* * * * *
0
41. Amend Sec. 1926.65 paragraph (a)(3) by revising the definition of
"Health hazard" to read as follows:
Sec. 1926.65 Hazardous waste operations and emergency response.
(a) * * *
(3) * * *
Health hazard means a chemical or a pathogen where acute or chronic
health effects may occur in exposed employees. It also includes stress
due to temperature extremes. The term health hazard includes chemicals
that are classified in accordance with the Hazard Communication
Standard, Sec. 1910.1200, as posing one of the following hazardous
effects: acute toxicity (any route of exposure); skin corrosion or
irritation; serious eye damage or eye irritation; respiratory or skin
sensitization; germ cell mutagenicity; carcinogenicity; reproductive
toxicity; specific target organ toxicity (single or repeated exposure);
aspiration toxicity or simple asphyxiant. (See Appendix A to Sec.
1910.1200--Health Hazard Criteria (Mandatory) for the criteria for
determining whether a chemical is classified as a health hazard.)
* * * * *
Subpart F--[Amended]
0
42. Revise the authority citation for subpart F to read as follows:
Authority: Section 107 of the Contract Work Hours and Safety
Standards Act (40 U.S.C. 3704); Sections 4, 6, and 8 of the
Occupational Safety and Health Act of 1970 (29 U.S.C. 653, 655,
657); Secretary of Labor's Order No. 12-71 (36 FR 8754), 8-76 (41 FR
25059), 9-83 (48 FR 35736),1-90 (55 FR 9033), 6-96 (62 FR 111), 3-
2000 (62 FR 50017), 5-2002 (67 FR 650008), 5-2007 (72 FR 31159), 4-
2010 (75 FR 55355), or 1-2012
(77 FR 3912), as applicable; and 29 CFR part 1911.
* * * * *
0
43. Amend Sec. 1926.152 as follows:
0
A. Revise the section heading;
0
B. Remove the words "and combustible" from the first sentence in
paragraph (a)(1), the heading of paragraph (b), and paragraphs (b)(2)
introductory text, (b)(4)(viii), (h) introductory text, and (h)(1);
0
C. Remove the words "or combustible" wherever it appears in
paragraphs (a)(2), (b)(1), (b)(4)(iii), (b)(5), and (c)(3);
0
D. Remove the words "or combustible" in paragraphs (d) (the heading),
(d)(1), (d)(4), (e)(1), (e)(3), (f)(2), (g)(1), and (g)(8);
0
E. Remove the words "or combustible" wherever it appears in
paragraphs (i)(1)(i)(D) and (F), (i)(1)(iii)(D), (i)(2)(ii)(A), (D),
and (F), (i)(2)(vii)(B)(2), (i)(4)(iv)(C), (i)(5)(vi)(A), (D), (G), (V)
introductory text, and (i)(5)(vi)(V)(1); (j)(1)(i), (j)(2)(ii), (j)(5),
and (k)(4);
0
F. Amend the fifth sentence of paragraph (b)(4)(vi) by adding the words
"Category 1, 2, or 3" before the words "flammable liquids";
0
G. Amend paragraphs (e)(2), (e)(5), (g)(7)(i), and (g)(7)(ii), by
adding the words "Category 1, 2, or 3" before the words "flammable
liquids" ;
0
H. Amend paragraphs (f)(1) and (f)(3) by removing the words "Flammable
liquids" and adding in their place the words "Category 1, 2, or 3
flammable liquids";
0
I. Revise paragraphs (b)(2)(iii), (b)(3), (h) introductory text,
(i)(2)(iv)(F) and (G), (i)(2)(vi)(B), (i)(2)(viii)(E), (i)(3)(i),
(i)(3)(iv)(A) and (C), (i)(3)(v)(D), and (i)(4)(iv)(E);
0
J. Revise Table F-19 and paragraph (k)(3)(iv).
The revisions read as follows:
Sec. 1926.152 Flammable liquids.
* * * * *
(b) * * *
(2) * * *
(iii) Cabinets shall be labeled in conspicuous lettering,
"Flammable-Keep Away from Open Flames."
(3) Not more than 60 gallons of Category 1, 2 and/or 3 flammable
liquids or 120 gallons of Category 4 flammable liquids shall be stored
in any one storage cabinet. Not more than three such cabinets may be
located in a single storage area. Quantities in excess of this shall be
stored in an inside storage room.
* * * * *
(h) Scope. This section applies to the handling, storage, and use
of flammable liquids with a flashpoint at or below 199.4 [deg]F (93
[deg]C). This section does not apply to:
* * * * *
(i) * * *
(2) * * *
(iv) * * *
(F) Tanks and pressure vessels storing Category 1 flammable liquids
shall be equipped with venting devices that shall be normally closed
except when venting to pressure or vacuum conditions. Tanks and
pressure vessels storing Category 2 flammable liquids, or Category 3
flammable liquids with a flashpoint below 100 [deg]F (37.8 [deg]C),
shall be equipped with venting devices that shall be normally closed
except when venting under pressure or vacuum conditions, or with
approved flame arresters.
Exemption: Tanks of 3,000 bbls (barrels) (84 m(3)) capacity or less
containing crude petroleum in crude-producing areas; and, outside
aboveground atmospheric tanks under 1,000 gallons (3,785 L) capacity
containing other than Category 1 flammable liquids may have open vents.
(See paragraph (i)(2)(vi)(B) of this section.)
(G) Flame arresters or venting devices required in paragraph
(i)(2)(iv)(F) of this section may be omitted for Category 2 flammable
liquids or Category 3 flammable liquids with a flashpoint below 100
[deg]F (37.8 [deg]C) where conditions are such that their use may, in
case of obstruction, result in tank damage.
* * * * *
(vi) * * *
(B) Where vent pipe outlets for tanks storing Category 1 or 2
flammable liquids, or Category 3 flammable liquids with a flashpoint
below 100 [deg]F (37.8 [deg]C), are adjacent to buildings or public
ways, they shall be located so that the vapors are released at a safe
point outside of buildings and not less than 12 feet (3.658 m) above
the adjacent ground level. In order to aid their dispersion, vapors
shall be discharged upward or horizontally away from closely adjacent
walls. Vent outlets shall be located so that flammable vapors will not
be trapped by eaves or other obstructions and shall be at least 5 feet
(1.52 m) from building openings.
(viii) * * *
(E) For Category 2 flammable liquids or Category 3 flammable
liquids with a flashpoint below 100 [deg]F (37.8 [deg]C), other than
crude oils, gasolines, and asphalts, the fill pipe shall be so designed
and installed as to minimize the possibility of generating static
electricity. A fill pipe entering the top of a tank shall terminate
within 6 inches (15.24 cm) of the bottom of the tank and shall be
installed to avoid excessive vibration.
* * * * *
(3) * * *
(i) Location. Evacuation for underground storage tanks shall be
made with due care to avoid undermining of foundations of existing
structures. Underground tanks or tanks under buildings shall be so
located with respect to existing building foundations and supports that
the loads carried by the latter cannot be transmitted to the tank. The
distance from any part of a tank storing Category 1 or 2 flammable
liquids, or Category 3 flammable liquids with a flashpoint below 100
[deg]F (37.8 [deg]C), to the nearest wall of any basement or pit shall
be not less than 1 foot (0.304 m), and to any property line that may be
built upon, not less than 3 feet (0.912 m). The distance from any part
of a tank storing Category 3 flammable liquids with a flashpoint at or
above 100 [deg]F (37.8 [deg]C) or Category 4 flammable liquids to the
nearest wall of any basement, pit or property line shall be not less
than 1 foot (0.304 m).
* * * * *
(iv) * * *
(A) Location and arrangement of vents for Category 1 or 2 flammable
liquids, or Category 3 flammable liquids with a flashpoint below 100
[deg]F (37.8 [deg]C). Vent pipes from tanks storing Category 1 or 2
flammable liquids, or Category 3 flammable liquids with a flashpoint
below 100 [deg]F (37.8 [deg]C), shall be so located that the discharge
point is outside of buildings, higher than the fill pipe opening, and
not less than 12 feet (3.658 m) above the adjacent ground level. Vent
pipes shall discharge only upward in order to disperse vapors. Vent
pipes 2 inches (5.08 cm) or less in nominal inside diameter shall not
be obstructed by devices that will cause excessive back pressure. Vent
pipe outlets shall be so located that flammable vapors will not enter
building openings, or be trapped under eaves or other obstructions. If
the vent pipe is less than 10 feet (3.04 m) in length, or greater than
2 inches (5.08 cm) in nominal inside diameter, the outlet shall be
provided with a vacuum and pressure relief device or there shall be an
approved flame arrester located in the vent line at the outlet or
within the approved distance from the outlet.
* * * * *
(C) Location and arrangement of vents for Category 3 flammable
liquids with a flashpoint at or above 100 [deg]F (37.8 [deg]C) or
Category 4 flammable liquids. Vent pipes from tanks storing Category 3
flammable liquids with a flashpoint at or above 100 [deg]F (37.8
[deg]C) or Category 4
flammable liquids shall terminate outside of the building and higher
than the fill pipe opening. Vent outlets shall be above normal snow
level. They may be fitted with return bends, coarse screens or other
devices to minimize ingress of foreign material.
* * * * *
(v) * * *
(D) For Category 2 flammable liquids, or Category 3 flammable
liquids with a flashpoint below 100 [deg]F (37.8 [deg]C), other than
crude oils, gasolines, and asphalts, the fill pipe shall be so designed
and installed as to minimize the possibility of generating static
electricity by terminating within 6 inches (15.24 cm) of the bottom of
the tank.
* * * * *
(4) * * *
(iv) * * *
(E) For Category 2 flammable liquids, or Category 3 flammable
liquids with a flashpoint below 100 [deg]F (37.8 [deg]C), other than
crude oils, gasolines, and asphalts, the fill pipe shall be so designed
and installed as to minimize the possibility of generating static
electricity by terminating within 6 inches (15.24 cm) of the bottom of
the tank.
* * * * *
(k) * * *
(3) * * *
* * * * *
BILLING CODE 4510-26-P
[GRAPHIC] [TIFF OMITTED] TR26MR12.128
[GRAPHIC] [TIFF OMITTED] TR26MR12.129
BILLING CODE 4510-26-C
(iv) Piping handling Category 1 or 2 flammable liquids, or Category
3 flammable liquids with a flashpoint below 100 [deg]F (37.8 [deg]C),
shall be grounded to control stray currents.
* * * * *
0
44. Amend Sec. 1926.155 as follows:
0
A. Remove and reserve paragraph (c);
0
B. Revise paragraphs (h) and (i)(1) and (2).
The revisions read as follows:
Sec. 1926.155 Definitions applicable to this subpart.
* * * * *
(h) Flammable liquid means any liquid having a vapor pressure not
exceeding 40 pounds per square inch (absolute) at 100 [deg]F (37.8
[deg]C) and having a flashpoint at or below 199.4 [deg]F (93 [deg]C).
Flammable liquids are divided into four categories as follows:
(1) Category 1 shall include liquids having flashpoints below 73.4
[deg]F (23 [deg]C) and having a boiling point at or below 95 [deg]F (35
[deg]C).
(2) Category 2 shall include liquids having flashpoints below 73.4
[deg]F (23 [deg]C) and having a boiling point above 95 [deg]F (35
[deg]C).
(3) Category 3 shall include liquids having flashpoints at or above
73.4 [deg]F (23 [deg]C) and at or below 140 [deg]F (60 [deg]C).
(4) Category 4 shall include liquids having flashpoints above 140
[deg]F (60 [deg]C) and at or below 199.4 [deg]F (93 [deg]C).
(i) * * *
(1) The flashpoint of liquids having a viscosity less than 45
Saybolt Universal Second(s) at 100 [deg]F (37.8 [deg]C) and a
flashpoint below 175 [deg]F (79.4 [deg]C) shall be determined in
accordance with the Standard Method of Test for Flash Point by the Tag
Closed Tester, ASTM D-56-69 (incorporated by reference; See Sec.
1926.6), or an equivalent method as defined by Sec. 1910.1200 appendix
B.
(2) The flashpoints of liquids having a viscosity of 45 Saybolt
Universal Second(s) or more at 175 [deg]F (79.4 [deg]C) or higher shall
be determined in accordance with the Standard Method of Test for Flash
Point by the Pensky Martens Closed Tester, ASTM D-93-69 (incorporated
by reference; See Sec. 1926.6), or an equivalent method as defined by
Sec. 1910.1200 appendix B.
* * * * *
Subpart Z--[Amended]
0
45. Revise the authority citation for subpart Z to read as follows:
Authority: Section107 of the Contract Work Hours and Safety
Standards Act (40 U.S.C. 3704); Sections 4, 6, and 8 of the
Occupational Safety and Health Act of 1970 (29 U.S.C. 653, 655,
657); and Secretary of Labor's Order No. 12-71 (36 FR 8754), 8-76
(41 FR 25059), 9-83 (48 FR 35736), 1-90 (55 FR 9033), 6-96 (62 FR
111), 3-2000 (65 FR 50017), 5-2002 (67 FR 65008), 5-2007 (72 FR
31159), 4-2010 (75 FR 55355), or 1-2012 (77 FR 3912) as applicable;
and 29 CFR part 1911.
Section 1926.1102 not issued under 29 U.S.C. 655 or 29 CFR part
1911; also issued under 5 U.S.C. 553.
0
46. Amend Sec. 1926.1101 as follows:
0
A. Redesignate paragraph (k)(1) as (k)(1)(i) and add a new heading to
paragraph (k)(1);
0
B. Add new paragraphs (k)(1)(ii), (k)(7)(ii)(C), (k)(7)(ii)(D), and
(k)(8)(iv);
0
C. Amend paragraphs (k)(2)(i) and (k)(3)(i) by removing the references
to "(k)(1)" and adding in their place "(k)(1)(i)";
0
D. Revise paragraphs (k)(7)(ii)(A) and (B), and (k)(8)(ii) and (iii);
The revisions read as follows:
Sec. 1926.1101 Asbestos.
* * * * *
(k) * * *
(1) Hazard communication.
* * * * *
(ii) The employer shall include asbestos in the program established
to comply with the Hazard Communication Standard (HCS) (Sec.
1910.1200). The employer shall ensure that each employee has access to
labels on containers of asbestos and safety data sheets, and is trained
in accordance with the provisions of HCS and paragraphs (k)(9) and (10)
of this section. The employer shall provide information on at least the
following hazards: Cancer and lung effects.
* * * * *
(7) * * *
(ii) * * *
(A) The warning signs required by paragraph (k)(7) of this section
shall bear the following information.
DANGER
ASBESTOS
MAY CAUSE CANCER
CAUSES DAMAGE TO LUNGS
AUTHORIZED PERSONNEL ONLY
(B) In addition, where the use of respirators and protective
clothing is required in the regulated area under this section, the
warning signs shall include the following:
WEAR RESPIRATORY PROTECTION AND PROTECTIVE CLOTHING IN THIS AREA
(C) Prior to June 1, 2016, employers may use the following legend
in lieu of that specified in paragraph (k)(7)(ii)(A) of this section:
DANGER
ASBESTOS
CANCER AND LUNG DISEASE HAZARD
AUTHORIZED PERSONNEL ONLY
(D) Prior to June 1, 2016, employers may use the following legend
in lieu of that specified in paragraph (k)(7)(ii)(B) of this section:
RESPIRATORS AND PROTECTIVE CLOTHING ARE REQUIRED IN THIS AREA
* * * * *
(8) * * *
(ii) The employer shall ensure that such labels comply with
paragraphs (k) of this section.
(iii) The employer shall ensure that labels of bags or containers
of protective clothing and equipment, scrap, waste, and debris
containing asbestos fibers bear the following information:
DANGER
CONTAINS ASBESTOS FIBERS
MAY CAUSE CANCER
CAUSES DAMAGE TO LUNGS
DO NOT BREATHE DUST
AVOID CREATING DUST
(iv) (A) Prior to June 1, 2015, employers may include the following
information on raw materials, mixtures or labels of bags or containers
of protective clothing and equipment, scrap, waste, and debris
containing asbestos fibers in lieu of the labeling requirements in
paragraphs (k)(8)(ii) and (k)(8)(iii) of this section:
DANGER
CONTAINS ASBESTOS FIBERS
AVOID CREATING DUST
CANCER AND LUNG DISEASE HAZARD
(B) Labels shall also contain a warning statement against breathing
asbestos fibers.
* * * * *
0
47. Revise Sec. 1926.1126 paragraphs (g)(2)(iv) and (j)(1) to read as
follows:
Sec. 1926.1126 Chromium (VI).
* * * * *
(g) * * *
(2) * * *
(iv) The employer shall ensure that bags or containers of
contaminated protective clothing or equipment that are removed from
change rooms for laundering, cleaning, maintenance, or disposal shall
be labeled in accordance with the requirements of the Hazard
Communication Standard, Sec. 1910.1200.
* * * * *
(j) * * *
(1) Hazard communication. The employer shall include chromium (VI)
in the program established to comply with the Hazard Communication
Standard (HCS) (Sec. 1910.1200). The employer shall ensure that each
employee has access to labels on containers of chromium and safety data
sheets, and is trained in accordance with the provisions of Sec.
1910.1200 and paragraph (j)(2) of this section. The employer shall
provide information on at least the following hazards: Cancer; eye
irritation; and skin sensitization.
* * * * *
0
48. Revise Sec. 1926.1127 paragraphs (i)(2)(iv), (k)(7), and (m)(1),
(m)(2), and (m)(3), to read as follows:
Sec. 1926.1127 Cadmium.
* * * * *
(i) * * *
(2) * * *
(iv) The employer shall ensure that containers of contaminated
protective clothing and equipment that are to be taken out of the
change rooms or the workplace for laundering, cleaning, maintenance or
disposal shall bear labels in accordance with paragraph (m)(3)(ii) of
this section.
(k) * * *
(7) Waste, scrap, debris, bags, and containers, personal protective
equipment and clothing contaminated with cadmium and consigned for
disposal shall be collected and disposed of in sealed impermeable bags
or other closed, impermeable containers. These bags and containers
shall be labeled in accordance with paragraph (m)(3)(ii) of this
section.
* * * * *
(m) * * *
(1) Hazard communication. The employer shall include cadmium in the
program established to comply with the Hazard Communication Standard
(HCS) (Sec. 1910.1200). The employer shall ensure that each employee
has access to labels on containers of cadmium and safety data sheets,
and is trained in accordance with the provisions of HCS and paragraph
(m)(4) of this section. The employer shall provide information on at
least the following hazards: Cancer; lung effects; kidney effects; and
acute toxicity effects.
(2) Warning signs. (i) Warning signs shall be provided and
displayed in regulated areas. In addition, warning signs shall be
posted at all approaches to regulated areas so that an employee may
read the signs and take necessary protective steps before entering the
area.
(ii) Warning signs required by paragraph (m)(2)(i) of this section
shall bear the following legend:
DANGER
CADMIUM
MAY CAUSE CANCER
CAUSES DAMAGE TO LUNGS AND KIDNEYS
WEAR RESPIRATORY PROTECTION IN THIS AREA
AUTHORIZED PERSONNEL ONLY
(iii) The employer shall ensure that signs required by this
paragraph (m)(2) are illuminated, cleaned, and maintained as necessary
so that the legend is readily visible.
(iv) Prior to June 1, 2016, employers may use the following legend
in lieu of that specified in paragraph (m)(2)(ii) of this section:
DANGER
CADMIUM
CANCER HAZARD
CAN CAUSE LUNG AND KIDNEY DISEASE
AUTHORIZED PERSONNEL ONLY
RESPIRATORS REQUIRED IN THIS AREA
(3) Warning labels. (i) Shipping and storage containers containing
cadmium or cadmium compounds shall bear appropriate warning labels, as
specified in paragraph (m)(1) of this section.
(ii) The warning labels for containers of cadmium-contaminated
protective clothing, equipment, waste, scrap, or debris shall include
at least the following information:
DANGER
CONTAINS CADMIUM
MAY CAUSE CANCER
CAUSES DAMAGE TO LUNGS AND KIDNEYS
AVOID CREATING DUST
(iii) Where feasible, installed cadmium products shall have a
visible label or other indication that cadmium is present.
(iv) Prior to June 1, 2015, employers may include the following
information on shipping and storage containers containing cadmium,
cadmium compounds, or cadmium-contaminated clothing, equipment, waste,
scrap, or debris in lieu of the labeling requirements specified in
paragraphs (m)(3)(i) and (m)(3)(ii) of this section:
DANGER
CONTAINS CADMIUM
CANCER HAZARD
AVOID CREATING DUST
CAN CAUSE LUNG AND KIDNEY DISEASE
* * * * *
[FR Doc. 2012-4826 Filed 3-20-12; 11:15 am]
BILLING CODE 4510-26-P
Where:
ni = number of peroxygen groups per molecule of organic peroxide i;
ci = concentration (mass %) of organic peroxide i;
mi = molecular mass of organic peroxide i.
B.15.2.2 Organic peroxides shall be classified in one of the
seven categories of "Types A to G" for this class, according to
the following principles:
(a) Any organic peroxide which, as packaged, can detonate or
deflagrate rapidly shall be defined as organic peroxide TYPE A;
(b) Any organic peroxide possessing explosive properties and
which, as packaged, neither detonates nor deflagrates rapidly, but
is liable to undergo a thermal explosion in that package shall be
defined as organic peroxide TYPE B;
(c) Any organic peroxide possessing explosive properties when
the chemical as packaged cannot detonate or deflagrate rapidly or
undergo a thermal explosion shall be defined as organic peroxide
TYPE C;
(d) Any organic peroxide which in laboratory testing meets the
criteria in (d)(i), (ii), or (iii) shall be defined as organic
peroxide TYPE D:
(i) Detonates partially, does not deflagrate rapidly and shows
no violent effect when heated under confinement; or
(ii) Does not detonate at all, deflagrates slowly and shows no
violent effect when heated under confinement; or
(iii) Does not detonate or deflagrate at all and shows a medium
effect when heated under confinement;
(e) Any organic peroxide which, in laboratory testing, neither
detonates nor deflagrates at all and shows low or no effect when
heated under confinement shall be defined as organic peroxide TYPE
E;
(f) Any organic peroxide which, in laboratory testing, neither
detonates in the cavitated state nor deflagrates at all and shows
only a low or no effect when heated under confinement as well as low
or no explosive power shall be defined as organic peroxide TYPE F;
(g) Any organic peroxide which, in laboratory testing, neither
detonates in the cavitated state nor deflagrates at all and shows no
effect when heated under confinement nor any explosive power,
provided that it is thermally stable (self-accelerating
decomposition temperature is 60 [deg]C (140 [deg]F) or higher for a
50 kg (110 lb) package), and, for liquid mixtures, a diluent having
a boiling point of not less than 150 [deg]C (302 [deg]F) is used for
desensitization, shall be defined as organic peroxide TYPE G. If the
organic peroxide is not thermally stable or a diluent having a
boiling point less than 150 [deg]C (302 [deg]F) is used for
desensitization, it shall be defined as organic peroxide TYPE F.
B.15.3 Additional Classification Considerations
B.15.3.1 For purposes of classification, the properties of
organic peroxides shall be determined in accordance with test series
A to H as described in Part II of the UN ST/SG/AC.10 (incorporated
by reference; See Sec. 1910.6).
B.15.3.2 Self-accelerating decomposition temperature (SADT)
shall be determined in accordance with the UN ST/SG/AC.10
(incorporated by reference; See Sec. 1910.6), Part II, section 28.
B.15.3.3 Mixtures of organic peroxides may be classified as the
same type of organic peroxide as that of the most dangerous
ingredient. However, as two stable ingredients can form a thermally
less stable mixture, the SADT of the mixture shall be determined.
B.16 CORROSIVE TO METALS
B.16.1 Definition
A chemical which is corrosive to metals means a chemical which
by chemical action will materially damage, or even destroy, metals.
B.16.2 Classification Criteria
A chemical which is corrosive to metals shall be classified in a
single category for this class, using the test in Part III, sub-
section 37.4 of the UN ST/SG/AC.10 (incorporated by reference; See
Sec. 1910.6), in accordance with Table B.16.1:
Table B.16.1--Criteria for Chemicals Corrosive to Metal
----------------------------------------------------------------------------------------------------------------
Category Criteria
----------------------------------------------------------------------------------------------------------------
1........................................... Corrosion rate on either steel or aluminium surfaces exceeding
6.25 mm per year at a test temperature of 55 [deg]C (131 [deg]F)
when tested on both materials.
----------------------------------------------------------------------------------------------------------------
Note: Where an initial test on either steel or aluminium
indicates the chemical being tested is corrosive, the follow-up test
on the other metal is not necessary.
B.16.3 Additional Classification Considerations
The specimen to be used for the test shall be made of the
following materials:
(a) For the purposes of testing steel, steel types S235JR+CR
(1.0037 resp.St 37-2), S275J2G3+CR (1.0144 resp.St 44-3), ISO 3574,
Unified Numbering System (UNS) G 10200, or SAE 1020;
(b) For the purposes of testing aluminium: Non-clad types 7075-
T6 or AZ5GU-T6.
APPENDIX C TO Sec. 1910.1200--ALLOCATION OF LABEL ELEMENTS (MANDATORY)
C.1 The label for each hazardous chemical shall include the
product identifier used on the safety data sheet.
C.1.1 The labels on shipped containers shall also include the
name, address, and telephone number of the chemical manufacturer,
importer, or responsible party.
C.2 The label for each hazardous chemical that is classified
shall include the signal word, hazard statement(s), pictogram(s),
and precautionary statement(s) specified in C.4 for each hazard
class and associated hazard category, except as provided for in
C.2.1 through C.2.4.
C.2.1 Precedence of Hazard Information
C.2.1.1 If the signal word "Danger" is included, the signal
word "Warning" shall not appear;
C.2.1.2 If the skull and crossbones pictogram is included, the
exclamation mark pictogram shall not appear where it is used for
acute toxicity;
C.2.1.3 If the corrosive pictogram is included, the exclamation
mark pictogram shall not appear where it is used for skin or eye
irritation;
C.2.1.4 If the health hazard pictogram is included for
respiratory sensitization, the exclamation mark pictogram shall not
appear where it is used for skin sensitization or for skin or eye
irritation.
C.2.2 Hazard Statement Text
C.2.2.1 The text of all applicable hazard statements shall
appear on the label, except as otherwise specified. The information
in italics shall be included as part of the hazard statement as
provided. For example: "causes damage to organs (state all organs
affected) through prolonged or repeated exposure (state route of
exposure if no other routes of exposure cause the hazard)". Hazard
statements may be combined where appropriate to reduce the
information on the label and improve readability, as long as all of
the hazards are conveyed as required.
C.2.2.2 If the chemical manufacturer, importer, or responsible
party can demonstrate that all or part of the hazard statement is
inappropriate to a specific substance or mixture, the corresponding
statement may be omitted from the label.
C.2.3 Pictograms
C.2.3.1 Pictograms shall be in the shape of a square set at a
point and shall include a black hazard symbol on a white background
with a red frame sufficiently wide to be clearly visible. A square
red frame set at a point without a hazard symbol is not a pictogram
and is not permitted on the label.
BILLING CODE 4510-26-P
[GRAPHIC] [TIFF OMITTED] TR26MR12.069
[GRAPHIC] [TIFF OMITTED] TR26MR12.070
BILLING CODE 4510-26-C
C.2.4 Precautionary Statement Text
C.2.4.1 There are four types of precautionary statements
presented, "prevention," "response," "storage," and
"disposal." The core part of the precautionary statement is
presented in bold print. This is the text, except as otherwise
specified, that shall appear on the label. Where additional
information is required, it is indicated in plain text.
C.2.4.2 When a backslash or diagonal mark (/) appears in the
precautionary statement text, it indicates that a choice has to be
made between the separated phrases. In such cases, the chemical
manufacturer, importer, or responsible party can choose the most
appropriate phrase(s). For example, "Wear protective gloves/
protective clothing/eye protection/face protection" could read
"wear eye protection".
C.2.4.3 When three full stops (* * *) appear in the
precautionary statement text, they indicate that all applicable
conditions are not listed. For example, in "Use explosion-proof
electrical/ventilating/lighting/* * */equipment", the use of "* *
*" indicates that other equipment may need to be specified. In such
cases, the chemical manufacturer, importer, or responsible party can
choose the other conditions to be specified.
C.2.4.4 When text in italics is used in a precautionary
statement, this indicates specific conditions applying to the use or
allocation of the precautionary statement. For example, "Use
explosion-proof electrical/ventilating/lighting/* * */equipment" is
only required for flammable solids "if dust clouds can occur".
Text in italics is intended to be an explanatory, conditional note
and is not intended to appear on the label.
C.2.4.5 Where square brackets ([ ]) appear around text in a
precautionary statement, this indicates that the text in square
brackets is not appropriate in every case and should be used only in
certain circumstances. In these cases, conditions for use explaining
when the text should be used are provided. For example, one
precautionary statement states: "[In case of inadequate
ventilation] wear respiratory protection." This statement is given
with the condition for use "- text in square brackets may be used
if additional information is provided with the chemical at the point
of use that explains what type of ventilation would be adequate for
safe use". This means that, if additional information is provided
with the chemical explaining what type of ventilation would be
adequate for safe use, the text in square brackets should be used
and the statement would read: "In case of inadequate ventilation
wear respiratory protection." However, if the chemical is supplied
without such ventilation information, the text in square brackets
should not be used, and the precautionary statement should read:
"Wear respiratory protection."
C.2.4.6 Precautionary statements may be combined or consolidated
to save label space and improve readability. For example, "Keep
away from heat, sparks and open flame," "Store in a well-
ventilated place" and "Keep cool" can be combined to read "Keep
away from heat, sparks and open flame and store in a cool, well-
ventilated place."
C.2.4.7 In most cases, the precautionary statements are
independent (e.g., the phrases for explosive hazards do not modify
those related to certain health hazards, and products that are
classified for both hazard classes shall bear appropriate
precautionary statements for both). Where a chemical is classified
for a number of hazards, and the precautionary statements are
similar, the most stringent shall be included on the label (this
will be applicable mainly to preventive measures). An order of
precedence may be imposed by the chemical manufacturer, importer or
responsible party in situations where phrases concern "Response."
Rapid action may be crucial. For example, if a chemical is
carcinogenic and acutely toxic, rapid action may be crucial, and
first aid measures for acute toxicity will take precedence over
those for long-term effects. In addition, medical attention to
delayed health effects may be required in cases of incidental
exposure, even if not associated with immediate symptoms of
intoxication.
C.2.4.8 If the chemical manufacturer, importer, or responsible
party can demonstrate that a precautionary statement is
inappropriate to a specific substance or mixture, the precautionary
statement may be omitted from the label.
C.3 Supplementary Hazard Information
C.3.1 To ensure that non-standardized information does not lead
to unnecessarily wide variation or undermine the required
information, supplementary information on the label is limited to
when it provides further detail and does not contradict or cast
doubt on the validity of the standardized hazard information.
C.3.2 Where the chemical manufacturer, importer, or distributor
chooses to add supplementary information on the label, the placement
of supplemental information shall not impede identification of
information required by this section.
C.3.3 Where an ingredient with unknown acute toxicity is used in
a mixture at a concentration >=1%, and the mixture is not classified
based on testing of the mixture as a whole, a statement that X% of
the mixture consists of ingredient(s) of unknown acute toxicity is
required on the label.
BILLING CODE 4510-26-P
[GRAPHIC] [TIFF OMITTED] TR26MR12.071
[GRAPHIC] [TIFF OMITTED] TR26MR12.072
[GRAPHIC] [TIFF OMITTED] TR26MR12.073
[GRAPHIC] [TIFF OMITTED] TR26MR12.074
[GRAPHIC] [TIFF OMITTED] TR26MR12.075
[GRAPHIC] [TIFF OMITTED] TR26MR12.076
[GRAPHIC] [TIFF OMITTED] TR26MR12.077
[GRAPHIC] [TIFF OMITTED] TR26MR12.078
[GRAPHIC] [TIFF OMITTED] TR26MR12.079
[GRAPHIC] [TIFF OMITTED] TR26MR12.080
[GRAPHIC] [TIFF OMITTED] TR26MR12.081
[GRAPHIC] [TIFF OMITTED] TR26MR12.082
[GRAPHIC] [TIFF OMITTED] TR26MR12.083
[GRAPHIC] [TIFF OMITTED] TR26MR12.084
[GRAPHIC] [TIFF OMITTED] TR26MR12.085
[GRAPHIC] [TIFF OMITTED] TR26MR12.086
[GRAPHIC] [TIFF OMITTED] TR26MR12.087
[GRAPHIC] [TIFF OMITTED] TR26MR12.088
[GRAPHIC] [TIFF OMITTED] TR26MR12.089
[GRAPHIC] [TIFF OMITTED] TR26MR12.090
[GRAPHIC] [TIFF OMITTED] TR26MR12.091
[GRAPHIC] [TIFF OMITTED] TR26MR12.092
[GRAPHIC] [TIFF OMITTED] TR26MR12.093
[GRAPHIC] [TIFF OMITTED] TR26MR12.094
[GRAPHIC] [TIFF OMITTED] TR26MR12.095
[GRAPHIC] [TIFF OMITTED] TR26MR12.096
[GRAPHIC] [TIFF OMITTED] TR26MR12.097
[GRAPHIC] [TIFF OMITTED] TR26MR12.098
[GRAPHIC] [TIFF OMITTED] TR26MR12.099
[GRAPHIC] [TIFF OMITTED] TR26MR12.100
[GRAPHIC] [TIFF OMITTED] TR26MR12.101
[GRAPHIC] [TIFF OMITTED] TR26MR12.102
[GRAPHIC] [TIFF OMITTED] TR26MR12.103
[GRAPHIC] [TIFF OMITTED] TR26MR12.104
[GRAPHIC] [TIFF OMITTED] TR26MR12.105
[GRAPHIC] [TIFF OMITTED] TR26MR12.106
[GRAPHIC] [TIFF OMITTED] TR26MR12.107
[GRAPHIC] [TIFF OMITTED] TR26MR12.108
[GRAPHIC] [TIFF OMITTED] TR26MR12.109
[GRAPHIC] [TIFF OMITTED] TR26MR12.110
[GRAPHIC] [TIFF OMITTED] TR26MR12.111
[GRAPHIC] [TIFF OMITTED] TR26MR12.112
[GRAPHIC] [TIFF OMITTED] TR26MR12.113
[GRAPHIC] [TIFF OMITTED] TR26MR12.114
[GRAPHIC] [TIFF OMITTED] TR26MR12.115
[GRAPHIC] [TIFF OMITTED] TR26MR12.116
[GRAPHIC] [TIFF OMITTED] TR26MR12.117
[GRAPHIC] [TIFF OMITTED] TR26MR12.118
[GRAPHIC] [TIFF OMITTED] TR26MR12.119
[GRAPHIC] [TIFF OMITTED] TR26MR12.120
[GRAPHIC] [TIFF OMITTED] TR26MR12.121
[GRAPHIC] [TIFF OMITTED] TR26MR12.122
[GRAPHIC] [TIFF OMITTED] TR26MR12.123
[GRAPHIC] [TIFF OMITTED] TR26MR12.124
[GRAPHIC] [TIFF OMITTED] TR26MR12.125
[GRAPHIC] [TIFF OMITTED] TR26MR12.126
[GRAPHIC] [TIFF OMITTED] TR26MR12.127
BILLING CODE 4510-26-C
Appendix D to Sec. 1910.1200--Safety Data Sheets (Mandatory)
A safety data sheet (SDS) shall include the information
specified in Table D.1 under the section number and heading
indicated for sections 1-11 and 16. If no relevant information is
found for any given subheading within a section, the SDS shall
clearly indicate that no applicable information is available.
Sections 12-15 may be included in the SDS, but are not mandatory.
Table D.1--Minimum Information for an SDS
------------------------------------------------------------------------
Heading Subheading
------------------------------------------------------------------------
1. Identification............ (a) Product identifier used on the label;
(b) Other means of identification;
(c) Recommended use of the chemical and
restrictions on use;
(d) Name, address, and telephone number
of the chemical manufacturer, importer,
or other responsible party;
(e) Emergency phone number.
2. Hazard(s) identification.. (a) Classification of the chemical in
accordance with paragraph (d) of Sec.
1910.1200;
(b) Signal word, hazard statement(s),
symbol(s) and precautionary statement(s)
in accordance with paragraph (f) of Sec.
1910.1200. (Hazard symbols may be
provided as graphical reproductions in
black and white or the name of the
symbol, e.g., flame, skull and
crossbones);
(c) Describe any hazards not otherwise
classified that have been identified
during the classification process;
(d) Where an ingredient with unknown
acute toxicity is used in a mixture at a
concentration >=1% and the mixture is
not classified based on testing of the
mixture as a whole, a statement that X%
of the mixture consists of ingredient(s)
of unknown acute toxicity is required.
3. Composition/information on Except as provided for in paragraph (i)
ingredients. of Sec. 1910.1200 on trade secrets:
For Substances
(a) Chemical name;
(b) Common name and synonyms;
(c) CAS number and other unique
identifiers;
(d) Impurities and stabilizing additives
which are themselves classified and
which contribute to the classification
of the substance.
For Mixtures
In addition to the information required
for substances:
(a) The chemical name and concentration
(exact percentage) or concentration
ranges of all ingredients which are
classified as health hazards in
accordance with paragraph (d) of Sec.
1910.1200 and
(1) Are present above their cut-off/
concentration limits; or
(2) Present a health risk below the cut-
off/concentration limits.
(b) The concentration (exact percentage)
shall be specified unless a trade secret
claim is made in accordance with
paragraph (i) of Sec. 1910.1200, when
there is batch-to-batch variability in
the production of a mixture, or for a
group of substantially similar mixtures
(See A.0.5.1.2) with similar chemical
composition. In these cases,
concentration ranges may be used.
For All Chemicals Where a Trade Secret is
Claimed
Where a trade secret is claimed in
accordance with paragraph (i) of Sec.
1910.1200, a statement that the specific
chemical identity and/or exact
percentage (concentration) of
composition has been withheld as a trade
secret is required.
4. First-aid measures........ (a) Description of necessary measures,
subdivided according to the different
routes of exposure, i.e., inhalation,
skin and eye contact, and ingestion;
(b) Most important symptoms/effects,
acute and delayed.
(c) Indication of immediate medical
attention and special treatment needed,
if necessary.
5. Fire-fighting measures.... (a) Suitable (and unsuitable)
extinguishing media.
(b) Specific hazards arising from the
chemical (e.g., nature of any hazardous
combustion products).
(c) Special protective equipment and
precautions for fire-fighters.
6. Accidental release (a) Personal precautions, protective
measures. equipment, and emergency procedures.
(b) Methods and materials for containment
and cleaning up.
7. Handling and storage...... (a) Precautions for safe handling.
(b) Conditions for safe storage,
including any incompatibilities.
8. Exposure controls/personal (a) OSHA permissible exposure limit
protection. (PEL), American Conference of
Governmental Industrial Hygienists
(ACGIH) Threshold Limit Value (TLV), and
any other exposure limit used or
recommended by the chemical
manufacturer, importer, or employer
preparing the safety data sheet, where
available.
(b) Appropriate engineering controls.
(c) Individual protection measures, such
as personal protective equipment.
9. Physical and chemical (a) Appearance (physical state, color,
properties. etc.);
(b) Odor;
(c) Odor threshold;
(d) pH;
(e) Melting point/freezing point;
(f) Initial boiling point and boiling
range;
(g) Flash point;
(h) Evaporation rate;
(i) Flammability (solid, gas);
(j) Upper/lower flammability or explosive
limits;
(k) Vapor pressure;
(l) Vapor density;
(m) Relative density;
(n) Solubility(ies);
(o) Partition coefficient: n-octanol/
water;
(p) Auto-ignition temperature;
(q) Decomposition temperature;
(r) Viscosity.
10. Stability and reactivity. (a) Reactivity;
(b) Chemical stability;
(c) Possibility of hazardous reactions;
(d) Conditions to avoid (e.g., static
discharge, shock, or vibration);
(e) Incompatible materials;
(f) Hazardous decomposition products.
11. Toxicological information Description of the various toxicological
(health) effects and the available data
used to identify those effects,
including:
(a) Information on the likely routes of
exposure (inhalation, ingestion, skin
and eye contact);
(b) Symptoms related to the physical,
chemical and toxicological
characteristics;
(c) Delayed and immediate effects and
also chronic effects from short- and
long-term exposure;
(d) Numerical measures of toxicity (such
as acute toxicity estimates).
(e) Whether the hazardous chemical is
listed in the National Toxicology
Program (NTP) Report on Carcinogens
(latest edition) or has been found to be
a potential carcinogen in the
International Agency for Research on
Cancer (IARC) Monographs (latest
edition), or by OSHA.
12. Ecological information (a) Ecotoxicity (aquatic and terrestrial,
(Non-mandatory). where available);
(b) Persistence and degradability;
(c) Bioaccumulative potential;
(d) Mobility in soil;
(e) Other adverse effects (such as
hazardous to the ozone layer).
13. Disposal considerations Description of waste residues and
(Non-mandatory). information on their safe handling and
methods of disposal, including the
disposal of any contaminated packaging.
14. Transport information (a) UN number;
(Non-mandatory).
(b) UN proper shipping name;
(c) Transport hazard class(es);
(d) Packing group, if applicable;
(e) Environmental hazards (e.g., Marine
pollutant (Yes/No));
(f) Transport in bulk (according to Annex
II of MARPOL 73/78 and the IBC Code);
(g) Special precautions which a user
needs to be aware of, or needs to comply
with, in connection with transport or
conveyance either within or outside
their premises.
15. Regulatory information Safety, health and environmental
(Non-mandatory). regulations specific for the product in
question.
16. Other information, The date of preparation of the SDS or the
including date of last change to it.
preparation or last revision.
------------------------------------------------------------------------
Appendix F to Sec. 1910.1200--Guidance for Hazard Classifications Re:
Carcinogenicity (Non-Mandatory)
The mandatory criteria for classification of a chemical for
carcinogenicity under HCS (Sec. 1910.1200) are found in Appendix
A.6 to this section. This non-mandatory Appendix provides additional
guidance on hazard classification for carcinogenicity. Part A of
Appendix F includes background guidance provided by GHS based on the
Preamble of the International Agency for Research on Cancer (IARC)
"Monographs on the Evaluation of Carcinogenic Risks to Humans"
(2006). Part B provides IARC classification information. Part C
provides background guidance from the National Toxicology Program
(NTP) "Report on Carcinogens" (RoC), and Part D is a table that
compares GHS carcinogen hazard categories to carcinogen
classifications under IARC and NTP, allowing classifiers to be able
to use information from IARC and NTP RoC carcinogen classifications
to complete their classifications under the GHS, and thus the HCS.
Part A: Background Guidance 1
---------------------------------------------------------------------------
\1\ The text of Appendix F, Part A, on the IARC Monographs, is
paraphrased from the 2006 Preamble to the "Monographs on the
Evaluation of Carcinogenic Risks to Humans"; the Classifier is
referred to the full IARC Preamble for the complete text. The text
is not part of the agreed GHS text on the harmonized system
developed by the OECD Task Force-HCL.
---------------------------------------------------------------------------
As noted in Footnote 6 of Appendix A.6. to this section, the GHS
includes as guidance for classifiers information taken from the
Preamble of the International Agency for Research on Cancer (IARC)
"Monographs on the Evaluation of Carcinogenic Risks to Humans"
(2006), providing guidance on the evaluation of the strength and
evidence of carcinogenic risks to humans. This guidance also
discusses some additional considerations in classification and an
approach to analysis, rather than hard-and-fast rules. Part A is
consistent with Appendix A.6, and should help in evaluating
information to determine carcinogenicity.
Carcinogenicity in humans:
The evidence relevant to carcinogenicity from studies in humans
is classified into one of the following categories:
(a) Sufficient evidence of carcinogenicity: A causal
relationship has been established between exposure to the agent and
human cancer. That is, a positive relationship has been observed
between the exposure and cancer in studies in which chance, bias and
confounding could be ruled out with reasonable confidence.
(b) Limited evidence of carcinogenicity: A positive association
has been observed between exposure to the agent and cancer for which
a causal interpretation is considered by the Working Group to be
credible, but chance, bias or confounding could not be ruled out
with reasonable confidence.
In some instances, the above categories may be used to classify
the degree of evidence related to carcinogenicity in specific organs
or tissues.
Carcinogenicity in experimental animals:
The evidence relevant to carcinogenicity in experimental animals
is classified into one of the following categories:
(a) Sufficient evidence of carcinogenicity: A causal
relationship has been established between the agent and an increased
incidence of malignant neoplasms or of an appropriate combination of
benign and malignant neoplasms in two or more species of animals or
two or more independent studies in one species carried out at
different times or in different laboratories or under different
protocols. An increased incidence of tumors in both sexes of a
single species in a well-conducted study, ideally conducted under
Good Laboratory Practices, can also provide sufficient evidence.
Exceptionally, a single study in one species and sex might be
considered to provide sufficient evidence of carcinogenicity when
malignant neoplasms occur to an unusual degree with regard to
incidence, site, type of tumor or age at onset, or when there are
strong findings of tumors at multiple sites.
(a) Limited evidence of carcinogenicity: The data suggest a
carcinogenic effect but are limited for making a definitive
evaluation because, e.g. the evidence of carcinogenicity is
restricted to a single experiment; there are unresolved questions
regarding the adequacy of the design, conduct or interpretation of
the studies; the agent increases the incidence only of benign
neoplasms or lesions of uncertain neoplastic potential; or the
evidence of carcinogenicity is restricted to studies that
demonstrate only promoting activity in a narrow range of tissues or
organs.
Guidance on How To Consider Important Factors in Classification of
Carcinogenicity (See Reference Section)
The weight of evidence analysis called for in GHS and the HCS
(Sec. 1910.1200) is an integrative approach that considers
important factors in determining carcinogenic potential along with
the strength of evidence analysis. The IPCS "Conceptual Framework
for Evaluating a Mode of Action for Chemical Carcinogenesis"
(2001), International Life Sciences Institute (ILSI) "Framework for
Human Relevance Analysis of Information on Carcinogenic Modes of
Action" (Meek, et al., 2003; Cohen et al., 2003, 2004), and
Preamble to the IARC Monographs (2006; Section B.6. (Scientific
Review and Evaluation; Evaluation and Rationale)) provide a basis
for systematic assessments that may be performed in a consistent
fashion. The IPCS also convened a panel in 2004 to further develop
and clarify the human relevance framework. However, the above
documents are not intended to dictate answers, nor provide lists of
criteria to be checked off.
Mode of Action
Various documents on carcinogen assessment all note that mode of
action in and of itself, or consideration of comparative metabolism,
should be evaluated on a case-by-case basis and are part of an
analytic evaluative approach. One must look closely at any mode of
action in animal experiments, taking into consideration comparative
toxicokinetics/toxicodynamics between the animal test species and
humans to determine the relevance of the results to humans. This may
lead to the possibility of discounting very specific effects of
certain types of substances. Life stage-dependent effects on
cellular differentiation may also lead to qualitative differences
between animals and humans. Only if a mode of action of tumor
development is conclusively determined not to be operative in humans
may the carcinogenic evidence for that tumor be discounted. However,
a weight of evidence evaluation for a substance calls for any other
tumorigenic activity to be evaluated, as well.
Responses in Multiple Animal Experiments
Positive responses in several species add to the weight of
evidence that a substance is a carcinogen. Taking into account all
of the factors listed in A.6.2.5.2 and more, such chemicals with
positive outcomes in two or more species would be provisionally
considered to be classified in GHS Category 1B until human relevance
of animal results are assessed in their entirety. It should be
noted, however, that positive results for one species in at least
two independent studies, or a single positive study showing
unusually strong evidence of malignancy may also lead to Category
1B.
Responses Are in One Sex or Both Sexes
Any case of gender-specific tumors should be evaluated in light
of the total tumorigenic response to the substance observed at other
sites (multi-site responses or incidence above background) in
determining the carcinogenic potential of the substance.
If tumors are seen only in one sex of an animal species, the
mode of action should be carefully evaluated to see if the response
is consistent with the postulated mode of action. Effects seen only
in one sex in a test species may be less convincing than effects
seen in both sexes, unless there is a clear patho-physiological
difference consistent with the mode of action to explain the single
sex response.
Confounding Effects of Excessive Toxicity or Localized Effects
Tumors occurring only at excessive doses associated with severe
toxicity generally have doubtful potential for carcinogenicity in
humans. In addition, tumors occurring only at sites of contact and/
or only at excessive doses need to be carefully evaluated for human
relevance for carcinogenic hazard. For example, forestomach tumors,
following administration by gavage of an irritating or corrosive,
non-mutagenic chemical, may be of questionable relevance. However,
such determinations must be evaluated carefully in justifying the
carcinogenic potential for humans; any occurrence of other tumors at
distant sites must also be considered.
Tumor Type, Reduced Tumor Latency
Unusual tumor types or tumors occurring with reduced latency may
add to the weight of evidence for the carcinogenic potential of a
substance, even if the tumors are not statistically significant.
Toxicokinetic behavior is normally assumed to be similar in
animals and humans, at least from a qualitative perspective. On the
other hand, certain tumor types in animals may be associated with
toxicokinetics or toxicodynamics that are unique to the animal
species tested and may not be predictive of carcinogenicity in
humans. Very few such examples have been agreed internationally.
However, one example is the lack of human relevance of kidney tumors
in male rats associated with compounds causing [alpha]2u-globulin
nephropathy (IARC, Scientific Publication N[deg] 147 \2\). Even when
a particular tumor type may be discounted, expert judgment must be
used in assessing the total tumor profile in any animal experiment.
---------------------------------------------------------------------------
\2\ While most international agencies do not consider kidney
tumors coincident with [alpha]2u-globulin nephropathy to be a
predictor of risk in humans, this view is not universally held.
(See: Doi et al., 2007).
---------------------------------------------------------------------------
Part B: International Agency for Research on Cancer (IARC) 3
---------------------------------------------------------------------------
\3\ Preamble of the International Agency for Research on Cancer
(IARC) "Monographs on the Evaluation of Carcinogenic Risks to
Humans" (2006).
---------------------------------------------------------------------------
IARC Carcinogen Classification Categories:
Group 1: The agent is carcinogenic to humans
This category is used when there is sufficient evidence of
carcinogenicity in humans. Exceptionally, an agent may be placed in
this category when evidence of carcinogenicity in humans is less
than sufficient but there is sufficient evidence of carcinogenicity
in experimental animals and strong evidence in exposed humans that
the agent acts through a relevant mechanism of carcinogenicity.
Group 2:
This category includes agents for which, at one extreme, the
degree of evidence of carcinogenicity in humans is almost
sufficient, as well as those for which, at the other extreme, there
are no human data but for which there is evidence of carcinogenicity
in experimental animals. Agents are assigned to either Group 2A
(probably carcinogenic to humans) or Group 2B (possibly carcinogenic
to humans) on the basis of epidemiological and experimental evidence
of carcinogenicity and mechanistic and other relevant data. The
terms probably carcinogenic and possibly carcinogenic have no
quantitative significance and are used simply as descriptors of
different levels of evidence of human carcinogenicity, with probably
carcinogenic signifying a higher level of evidence than possibly
carcinogenic.
Group 2A: The agent is probably carcinogenic to human.
This category is used when there is limited evidence of
carcinogenicity in humans and sufficient evidence of carcinogenicity
in experimental animals. In some cases, an agent may be classified
in this category when there is inadequate evidence of
carcinogenicity in humans and sufficient evidence of carcinogenicity
in experimental animals and strong evidence that the carcinogenesis
is mediated by a mechanism that also operates in humans.
Exceptionally, an agent may be classified in this category solely on
the basis of limited evidence of carcinogenicity in humans. An agent
may be assigned to this category if it clearly belongs, based on
mechanistic considerations, to a class of agents for which one or
more members have been classified in Group 1 or Group 2A.
Group 2B: The agent is possibly carcinogenic to humans.
This category is used for agents for which there is limited
evidence of carcinogenicity in humans and less than sufficient
evidence of carcinogenicity in experimental animals. It may also be
used when there is inadequate evidence of carcinogenicity in humans
but there is sufficient evidence of carcinogenicity in experimental
animals. In some instances, an agent for which there is inadequate
evidence of carcinogenicity in humans and less than sufficient
evidence of carcinogenicity in experimental animals together with
supporting evidence from mechanistic and other relevant data may be
placed in this group. An agent may be classified in this category
solely on the basis of strong evidence from mechanistic and other
relevant data.
Part C: National Toxicology Program (NTP), "Report on Carcinogens",
Background Guidance
NTP Listing Criteria \4\:
---------------------------------------------------------------------------
\4\ See: http://ntp.niehs.nih.gov/go/15209.
---------------------------------------------------------------------------
The criteria for listing an agent, substance, mixture, or
exposure circumstance in the Report on Carcinogens (RoC) are as
follows:
Known To Be A Human Carcinogen: There is sufficient evidence of
carcinogenicity from studies in humans \5\ that indicates a causal
relationship between exposure to the agent, substance, or mixture,
and human cancer.
---------------------------------------------------------------------------
\5\ This evidence can include traditional cancer epidemiology
studies, data from clinical studies, and/or data derived from the
study of tissues or cells from humans exposed to the substance in
question that can be useful for evaluating whether a relevant cancer
mechanism is operating in people.
---------------------------------------------------------------------------
Reasonably Anticipated To Be A Human Carcinogen: There is
limited evidence of carcinogenicity from studies in humans that
indicates that a causal interpretation is credible, but that
alternative explanations, such as chance, bias, or confounding
factors, could not adequately be excluded,
or
there is sufficient evidence of carcinogenicity from studies in
experimental animals that indicates there is an increased incidence
of malignant and/or a combination of malignant and benign tumors in
multiple species or at multiple tissue sites, or by multiple routes
of exposure, or to an unusual degree with regard to incidence, site,
or type of tumor, or age at onset,
or
there is less than sufficient evidence of carcinogenicity in humans
or laboratory animals; however, the agent, substance, or mixture
belongs to a well-defined, structurally-related class of substances
whose members are listed in a previous Report on Carcinogens as
either known to be a human carcinogen or reasonably anticipated to
be a human carcinogen, or there is convincing relevant information
that the agent acts through mechanisms indicating it would likely
cause cancer in humans.
Conclusions regarding carcinogenicity in humans or experimental
animals are based on scientific judgment, with consideration given
to all relevant information. Relevant information includes, but is
not limited to, dose response, route of exposure, chemical
structure, metabolism, pharmacokinetics, sensitive sub-populations,
genetic effects, or other data relating to mechanism of action or
factors that may be unique to a given substance. For example, there
may be substances for which there is evidence of carcinogenicity in
laboratory animals, but there are compelling data indicating that
the agent acts through mechanisms that do not operate in humans and
would therefore not reasonably be anticipated to cause cancer in
humans.
Part D: Table Relating Approximate Equivalences Among IARC, NTP RoC,
and GHS Carcinogenicity Classifications
The following table may be used to perform hazard
classifications for carcinogenicity under the HCS (Sec. 1910.1200).
It relates the approximated GHS hazard categories for
carcinogenicity to the classifications provided by IARC and NTP, as
described in Parts B and C of this Appendix.
Approximate Equivalences Among Carcinogen Classification Schemes
----------------------------------------------------------------------------------------------------------------
IARC GHS NTP RoC
----------------------------------------------------------------------------------------------------------------
Group 1................................. Category 1A................ Known.
Group 2A................................ Category 1B................ Reasonably Anticipated.
Group 2B................................ Category 2................. (See Note 1).
----------------------------------------------------------------------------------------------------------------
Note 1:
1. Limited evidence of carcinogenicity from studies in humans (corresponding to IARC 2A/GHS 1B);
2. Sufficient evidence of carcinogenicity from studies in experimental animals (again, essentially corresponding
to IARC 2A/GHS 1B);
3. Less than sufficient evidence of carcinogenicity in humans or laboratory animals; however:
c. The agent, substance, or mixture belongs to a well-defined, structurally-related class of substances whose
members are listed in a previous RoC as either "Known" or "Reasonably Anticipated" to be a human
carcinogen, or
d. There is convincing relevant information that the agent acts through mechanisms indicating it would likely
cause cancer in humans.
*References
Cohen, S.M., J. Klaunig, M.E. Meek, R.N. Hill, T. Pastoor, L.
Lehman-McKeeman, J. Bucher, D.G. Longfellow, J. Seed, V. Dellarco,
P. Fenner-Crisp, and D. Patton. 2004. Evaluating the human relevance
of chemically induced animal tumors. Toxicol. Sci. 78(2):181-186.
Cohen, S.M., M.E. Meek, J.E. Klaunig, D.E. Patton, P.A. Fenner-
Crisp. 2003. The human relevance of information on carcinogenic
modes of action: Overview. Crit. Rev. Toxicol. 33(6):581-9.
Meek, M.E., J.R. Bucher, S.M. Cohen, V. Dellarco, R.N. Hill, L.
Lehman-McKeeman, D.G. Longfellow, T. Pastoor, J. Seed, D.E. Patton.
2003. A framework for human relevance analysis of information on
carcinogenic modes of action. Crit. Rev. Toxicol. 33(6):591-653.
Sonich-Mullin, C., R. Fielder, J. Wiltse, K. Baetcke, J. Dempsey, P.
Fenner-Crisp, D. Grant, M. Hartley, A. Knapp, D. Kroese, I.
Mangelsdorf, E. Meek, J.M. Rice, and M. Younes. 2001. The conceptual
framework for evaluating a mode of action for chemical
carcinogenesis. Reg. Toxicol. Pharm. 34:146-152.
International Programme on Chemical Safety Harmonization Group.
2004. Report of the First Meeting of the Cancer Working Group. World
Health Organization. Report IPCS/HSC-CWG-1/04. Geneva.
International Agency for Research on Cancer. IARC Monographs on the
Evaluation of Carcinogenic Risks to Human. Preambles to Volumes.
World Health Organization. Lyon, France.
Cohen, S.M., P.A. Fenner-Crisp, and D.E. Patton. 2003. Special
Issue: Cancer Modes of Action and Human Relevance. Critical Reviews
in Toxicology, R.O. McClellan, ed., Volume 33/Issue 6. CRC Press.
Capen, C.C., E. Dybing, and J.D. Wilbourn. 1999. Species differences
in thyroid, kidney and urinary bladder carcinogenesis. International
Agency for Research on Cancer, Scientific Publication N[deg] 147.
Doi, A.M., G. Hill, J. Seely, J.R. Hailey, G. Kissling, and J.R.
Buchera. 2007. [alpha]2u-Globulin nephropathy and renal tumors in
National Toxicology Program studies. Toxicol. Pathol. 35:533-540.
* * * * *
0
33. Amend Sec. 1910.1450 as follows:
0
A. Remove the definitions of Combustible liquid, Compressed gas,
Explosive, Flammable, Flashpoint, Organic peroxide, Oxidizer, Unstable
(reactive), and Water-reactive from paragraph (b);
0
B. Revise the definitions of Hazardous chemical, Physical hazard, and
Reproductive toxins in paragraph (b);
0
C. Add definitions of Health hazard and Mutagen in alphabetical order
in paragraph (b);
0
D. In paragraphs (f)(3)(v), (h)(1) introductory text, (h)(1)(ii) and
(h)(2)(iii), remove the phrases "Material Safety Data Sheets" and
"material safety data sheets" and add in their place "safety data
sheets";
0
E. In Appendix A to Sec. 1910.1450, in the Table of Contents (item
"G") remove "Material Safety Data Sheets" and add in its place
"Safety Data Sheets";
0
F. In Appendix A to Sec. 1910.1450, revise the heading "G. Material
Safety Data Sheets" and revise the text following the heading.
The revisions read as follows:
Sec. 1910.1450 Occupational exposure to hazardous chemicals in
laboratories.
* * * * *
(b) * * *
Hazardous chemical means any chemical which is classified as health
hazard or simple asphyxiant in accordance with the Hazard Communication
Standard (Sec. 1910.1200).
Health hazard means a chemical that is classified as posing one of
the following hazardous effects: Acute toxicity (any route of
exposure); skin corrosion or irritation; serious eye damage or eye
irritation; respiratory or skin sensitization; germ cell mutagenicity;
carcinogenity; reproductive toxicity; specific target organ toxicity
(single or repeated exposure); aspiration hazard. The criteria for
determining whether a chemical is classified as a health hazard are
detailed in Appendix A of the Hazard Communication Standard (Sec.
1910.1200) and Sec. 1910.1200(c) (definition of "simple
asphyxiant").
* * * * *
Mutagen means chemicals that cause permanent changes in the amount
or structure of the genetic material in a cell. Chemicals classified as
mutagens in accordance with the Hazard Communication Standard (Sec.
1910.1200) shall be considered mutagens for purposes of this section.
* * * * *
Physical hazard means a chemical that is classified as posing one
of the following hazardous effects: Explosive; flammable (gases,
aerosols, liquids, or solids); oxidizer (liquid, solid, or gas); self
reactive; pyrophoric (gas, liquid or solid); self-heating; organic
peroxide; corrosive to metal; gas under pressure; in contact with water
emits flammable gas; or combustible dust. The criteria for determining
whether a chemical is classified as a physical hazard are in Appendix B
of the Hazard Communication Standard (Sec. 1910.1200) and Sec.
1910.1200(c) (definitions of "combustible dust" and "pyrophoric
gas").
* * * * *
Reproductive toxins mean chemicals that affect the reproductive
capabilities including adverse effects on sexual function and fertility
in adult males and females, as well as adverse effects on the
development of the offspring. Chemicals classified as reproductive
toxins in accordance with the Hazard Communication Standard (Sec.
1910.1200) shall be considered reproductive toxins for purposes of this
section.
* * * * *
Appendix A to Sec. 1910.1450--National Research Council
Recommendations Concerning Chemical Hygiene in Laboratories (Non-
Mandatory)
* * * * *
G. Safety Data Sheets
Safety data sheets are presented in "Prudent Practices" for the
chemicals listed below. (Asterisks denote that comprehensive safety
data sheets are provided).
* * * * *
PART 1915--OCCUPATIONAL SAFETY AND HEALTH STANDARDS FOR SHIPYARD
EMPLOYMENT
0
34. Revise the authority citation for part 1915 to read as follows:
Authority: Section 41, Longshore and Harbor Workers'
Compensation Act (33 U.S.C. 941); Sections. 4, 6, and 8 of the
Occupational Safety and Health Act of 1970 (29 U.S.C. 653, 655,
657); Secretary of Labor's Order No. 12-71 (36 FR 8754), 8-76 (41 FR
25059), 9-83 (48 FR 35736), 1-90 (55 FR 9033), 6-96 (62 FR 111), 3-
2000 (65 FR 50017), 5-2002 (67 FR 65008), 5-2007 (72 FR 31160), 4-
2010 (75 FR 55355), or 1-2012 (77 FR 3912), as applicable; 29 CFR
Part 1911.
Section 1915.100 also issued under 49 U.S.C. 1801-1819 and 5
U.S.C. 553.
Sections 1915.120 and 1915.152 of 29 CFR also issued under 29
CFR part 1911.
Subpart Z--[Amended]
0
35. Revise Sec. 1915.1001 paragraphs (i)(3), (k)(7), and (k)(8) to
read as follows:
Sec. 1915.1001 Asbestos.
* * * * *
(i) * * *
(3) The employer shall ensure that contaminated clothing is
transported in sealed impermeable bags, or other closed, impermeable
containers, and labeled in accordance with paragraph (k) of this
section.
* * * * *
(k) * * *
(7) Hazard communication. (i) Labels shall be affixed to all
products containing asbestos and to all containers containing such
products, including waste containers. Where feasible, installed
asbestos products shall contain a visible label.
(ii) General. The employer shall include asbestos in the program
established to comply with the Hazard Communication Standard (HCS)
(Sec. 1910.1200). The employer shall ensure that each employee has
access to labels on containers of asbestos and safety data sheets, and
is trained in accordance with the provisions of the HCS and paragraph
(k)(9) of this section. The employer shall ensure that at least the
following hazards are addressed: Cancer and lung effects.
(iii) Labels. (A) The employer shall ensure that labels of bags or
containers of protective clothing and equipment, scrap, waste, and
debris containing asbestos fibers bear the following information:
DANGER
CONTAINS ASBESTOS FIBERS
MAY CAUSE CANCER
CAUSES DAMAGE TO LUNGS
DO NOT BREATHE DUST
AVOID CREATING DUST
(B)(1) Prior to June 1, 2015, employers may include the following
information on raw materials, mixtures or labels of bags or containers
of protective clothing and equipment, scrap, waste, and debris
containing asbestos fibers in lieu of the labeling requirements in
paragraphs (k)(7)(ii) and (k)(7)(iii)(A) of this section:
DANGER
CONTAINS ASBESTOS FIBERS
AVOID CREATING DUST
CANCER AND LUNG DISEASE HAZARD
(2) Labels shall also contain a warning statement against breathing
asbestos fibers.
(iv) The provisions for labels required in paragraph (k)(7) of this
section do not apply where:
(A) Asbestos fibers have been modified by a bonding agent, coating,
binder, or other material, provided that the manufacturer can
demonstrate that, during any reasonably foreseeable use, handling,
storage, disposal, processing, or transportation, no airborne
concentrations of asbestos fibers in excess of the permissible exposure
limit and/or excursion limit will be released, or
(B) Asbestos is present in a product in concentrations less than
1.0 percent.
(8) Signs. (i) Warning signs that demarcate the regulated area
shall be provided and displayed at each location where a regulated area
is required to be established by paragraph (e) of this section. Signs
shall be posted at such a distance from such a location that an
employee may read the signs and take necessary protective steps before
entering the area marked by the signs.
(ii) The warning signs required by this paragraph (k)(8) shall bear
the following legend:
DANGER
ASBESTOS
MAY CAUSE CANCER
CAUSES DAMAGE TO LUNGS
AUTHORIZED PERSONNEL ONLY
(iii) In addition, where the use of respirators and protective
clothing is required in the regulated area under this section, the
warning signs shall include the following:
WEAR RESPIRATORY PROTECTION
AND PROTECTIVE CLOTHING IN THIS AREA
(iv) The employer shall ensure that employees working in and
contiguous to regulated areas comprehend the warning signs required to
be posted by paragraph (k)(8) of this section. Means to ensure employee
comprehension may include the use of foreign languages, pictographs,
and graphics.
(v) When a building/vessel owner or employer identifies previously
installed PACM and/or ACM, labels or signs shall be affixed or posted
so that employees will be notified of what materials contain PACM and/
or ACM. The employer shall attach such labels in areas where they will
clearly be noticed by employees who are likely to be exposed, such as
at the entrance to mechanical room/areas. Signs required by paragraph
(k)(6) of this section may be posted in lieu of labels, so long as they
contain information required for labeling. The employer shall ensure,
to the extent feasible, that employees who come in contact with these
signs or labels can comprehend them. Means to ensure employee
comprehension may include the use of foreign languages, pictographs,
graphics, and awareness training.
(vi) Prior to June 1, 2016, employers may use the following legend
in lieu of that specified in paragraph (k)(8)(ii) of this section:
DANGER
ASBESTOS
CANCER AND LUNG DISEASE HAZARD
AUTHORIZED PERSONNEL ONLY
(vii) Prior to June 1, 2016, employers may use the following legend
in lieu of that specified in paragraph (k)(8)(iii) of this section:
RESPIRATORS AND PROTECTIVE CLOTHING ARE REQUIRED IN THIS AREA
* * * * *
0
36. Revise Sec. 1915.1026 paragraphs (g)(2)(iv) and (j)(1), to read as
follows;
Sec. 1915.1026 Chromium (VI).
* * * * *
(g) * * *
(2) * * *
(iv) The employer shall ensure that bags or containers of
contaminated protective clothing or equipment that are removed from
change rooms for laundering, cleaning, maintenance, or disposal are
labeled in accordance with the requirements of the Hazard Communication
Standard, Sec. 1910.1200.
* * * * *
(j) * * *
(1) Hazard communication. The employer shall include chromium (VI)
in the program established to comply with the Hazard Communication
Standard (HCS) (Sec. 1910.1200). The employer shall ensure that each
employee has access to labels on containers of chromium (VI) and safety
data sheets, and is trained in accordance with the provisions of HCS
and paragraph (j)(2) of this section. The employer shall ensure that at
least the following hazards are addressed: Cancer; skin sensitization;
and eye irritation.
* * * * *
PART 1926--SAFETY AND HEALTH REGULATIONS FOR CONSTRUCTION
Subpart D--[Amended]
0
37. The authority citation for subpart D is revised to read as follows:
Authority: Section 107 of the Contract Work Hours and Safety
Standards Act (40 U.S.C. 3704); Sections 4, 6, and 8 of the
Occupational Safety and Health Act of 1970 (29 U.S.C. 653, 655, and
657); and Secretary of Labor's Order No. 12-71 (36 FR 8754), 8-76
(41 FR 25059), 9-83 (48 FR 35736), 1-90 (55 FR 9033), 6-96 (62 FR
111), 3-2000 (65 FR 50017), 5-2002 (67 FR 65008), 5-2007 (72 FR
31159), 4-2010 (75 FR 55355), or 1-2012 (77 FR 3912) as applicable;
and 29 CFR part 1911.
Sections 1926.58, 1926.59, 1926.60, and 1926.65 also issued
under 5 U.S.C. 553 and 29 CFR part 1911.
Section 1926.61 also issued under 49 U.S.C. 1801-1819 and 6
U.S.C. 553.
Section 1926.62 also issued under section 1031 of the Housing
and Community Development Act of 1992 (42 U.S.C. 4853).
Section 1926.65 also issued under section 126 of the Superfund
Amendments and Reauthorization Act of 1986, as amended (reprinted at
29 U.S.C.A. 655 Note), and 5 U.S.C. 553.
0
38. Revise Sec. 1926.60 paragraphs (l)(1) and (l)(2) to read as
follows:
Sec. 1926.60 Methylenedianiline.
* * * * *
(l) * * *
(1) Hazard communication. The employer shall include
Methylenedianiline (MDA) in the program established to comply with the
Hazard Communication Standard (HCS) (Sec. 1910.1200). The employer
shall ensure that each employee has access to labels on containers of
MDA and safety data sheets, and is trained in accordance with the
provisions of HCS and paragraph (l)(3) of this section. The employer
shall ensure that at least the following hazards are addressed: Cancer;
liver effects; and skin sensitization.
(2) Signs and labels-- (i) Signs. (A) The employer shall post and
maintain legible signs demarcating regulated areas and entrances or
access-ways to regulated areas that bear the following legend:
DANGER
MDA
MAY CAUSE CANCER
CAUSES DAMAGE TO THE LIVER
RESPIRATORY PROTECTION AND PROTECTIVE CLOTHING MAY BE REQUIRED IN
THIS AREA
AUTHORIZED PERSONNEL ONLY
(B) Prior to June 1, 2016, employers may use the following legend
in lieu of that specified in paragraph (l)(2)(i)(A) of this section:
DANGER
MDA
MAY CAUSE CANCER
LIVER TOXIN
AUTHORIZED PERSONNEL ONLY
RESPIRATORS AND PROTECTIVE CLOTHING MAY BE REQUIRED TO BE WORN IN
THIS AREA
(ii) Labels. (A) The employer shall ensure that labels or other
appropriate forms of warning are provided for containers of MDA within
the workplace. The labels shall comply with the requirements of Sec.
1910.1200(f) and shall include at least the following information for
pure MDA and mixtures containing MDA:
DANGER
CONTAINS MDA
MAY CAUSE CANCER
CAUSES DAMAGE TO THE LIVER
(B) Prior to June 1, 2015, employers may include the following
information workplace labels in lieu of the labeling requirements in
paragraph (l)(2)(ii)(A) of this section:
(1) For Pure MDA:
DANGER
CONTAINS MDA
MAY CAUSE CANCER
LIVER TOXIN
(2) For mixtures containing MDA:
DANGER
CONTAINS MDA
CONTAINS MATERIALS WHICH MAY CAUSE CANCER
LIVER TOXIN
* * * * *
0
39. Amend Sec. 1926.62 by revising paragraph (g)(2)(vii), the heading
of paragraph (l), paragraph (l)(1)(i), and paragraph (m), and Appendix
B to Sec. 1926.62 section XI, to read as follows:
Sec. 1926.62 Lead.
* * * * *
(g) * * *
(2) * * *
(vii)(A) The employer shall ensure that the containers of
contaminated protective clothing and equipment required by paragraph
(g)(2)(v) of this section are labeled as follows:
DANGER: CLOTHING AND EQUIPMENT CONTAMINATED WITH LEAD. MAY DAMAGE
FERTILITY OR THE UNBORN CHILD. CAUSES DAMAGE TO THE CENTRAL NERVOUS
SYSTEM. DO NOT EAT, DRINK OR SMOKE WHEN HANDLING. DO NOT REMOVE DUST
BY BLOWING OR SHAKING. DISPOSE OF LEAD CONTAMINATED WASH WATER IN
ACCORDANCE WITH APPLICABLE LOCAL, STATE, OR FEDERAL REGULATIONS.
(B) Prior to June 1, 2015, employers may include the following
information on bags or containers of contaminated protective clothing
and equipment required by paragraph (g)(2)(v) in lieu of the labeling
requirements in paragraph (g)(2)(vii)(A) of this section:
Caution: Clothing contaminated with lead. Do not remove dust by
blowing or shaking. Dispose of lead contaminated wash water in
accordance with applicable local, state, or federal regulations.
* * * * *
(l) Communication of hazards.
(1) * * *
(i) Hazard communication. The employer shall include lead in the
program established to comply with the Hazard Communication Standard
(HCS) (Sec. 1910.1200). The employer shall ensure that each employee
has access to labels on containers of lead and safety data sheets, and
is trained in accordance with the provisions of HCS and paragraph (l)
of this section. The employer shall ensure that at least the following
hazards are addressed:
(A) Reproductive/developmental toxicity;
(B) Central nervous system effects;
(C) Kidney effects;
(D) Blood effects; and
(E) Acute toxicity effects.
* * * * *
(m) Signs.
(1) General.
(i) The employer shall post the following warning signs in each
work area where an employee's exposure to lead is above the PEL.
DANGER
LEAD WORK AREA
MAY DAMAGE FERTILITY OR THE UNBORN CHILD
CAUSES DAMAGE TO THE CENTRAL NERVOUS SYSTEM
DO NOT EAT, DRINK OR SMOKE IN THIS AREA
(ii) The employer shall ensure that no statement appears on or near
any sign required by this paragraph (m) that contradicts or detracts
from the meaning of the required sign.
(iii) The employer shall ensure that signs required by this
paragraph (m) are illuminated and cleaned as necessary so that the
legend is readily visible.
(iv) The employer may use signs required by other statutes,
regulations or ordinances in addition to, or in combination with, signs
required by this paragraph (m).
(v) Prior to June 1, 2016, employers may use the following legend
in lieu of that specified in paragraph (m)(1)(i) of this section:
WARNING
LEAD WORK AREA
POISON
NO SMOKING OR EATING
* * * * *
Appendix B to Sec. 1926.62--Employee Standard Summary
* * * * *
XI. Signs--Paragraph (M)
The standard requires that the following warning sign be posted
in work areas when the exposure to lead is above the PEL:
DANGER
LEAD WORK AREA
MAY DAMAGE FERTILITY OR THE UNBORN CHILD
CAUSES DAMAGE TO THE CENTRAL NERVOUS SYSTEM
DO NOT EAT, DRINK OR SMOKE IN THIS AREA
Prior to June 1, 2016, employers may use the following legend in
lieu of that specified above:
WARNING
LEAD WORK AREA
POISON
NO SMOKING OR EATING
* * * * *
0
40. Revise Sec. 1926.64 paragraphs (a)(1)(ii) introductory text,
(a)(1)(ii)(B), and (d)(1)(vii), and the note following paragraph
(d)(1)(vii), to read as follows:
Sec. 1926.64 Process safety management of highly hazardous chemicals.
* * * * *
(a) * * *
(1) * * *
(ii) A process which involves a Category 1 flammable gas (as
defined in Sec. 1910.1200(c)) or flammable liquid with a flashpoint
below 100 [deg]F (37.8 [deg]C) on site in one location, in a quantity
of 10,000 pounds (4535.9 kg) or more except for:
* * * * *
(B) Flammable liquids with a flashpoint below 100 [deg]F (37.8
[deg]C) stored in atmospheric tanks or transferred that are kept below
their normal boiling point without benefit of chilling or
refrigeration.
* * * * *
(d) * * *
(1) * * *
(vii) Hazardous effects of inadvertent mixing of different
materials that could foreseeably occur.
Note to paragraph (d)(1): Safety data sheets meeting the
requirements of Sec. 1910.1200(g) may be used to comply with this
requirement to the extent they contain the information required by
this paragraph (d)(1).
* * * * *
0
41. Amend Sec. 1926.65 paragraph (a)(3) by revising the definition of
"Health hazard" to read as follows:
Sec. 1926.65 Hazardous waste operations and emergency response.
(a) * * *
(3) * * *
Health hazard means a chemical or a pathogen where acute or chronic
health effects may occur in exposed employees. It also includes stress
due to temperature extremes. The term health hazard includes chemicals
that are classified in accordance with the Hazard Communication
Standard, Sec. 1910.1200, as posing one of the following hazardous
effects: acute toxicity (any route of exposure); skin corrosion or
irritation; serious eye damage or eye irritation; respiratory or skin
sensitization; germ cell mutagenicity; carcinogenicity; reproductive
toxicity; specific target organ toxicity (single or repeated exposure);
aspiration toxicity or simple asphyxiant. (See Appendix A to Sec.
1910.1200--Health Hazard Criteria (Mandatory) for the criteria for
determining whether a chemical is classified as a health hazard.)
* * * * *
Subpart F--[Amended]
0
42. Revise the authority citation for subpart F to read as follows:
Authority: Section 107 of the Contract Work Hours and Safety
Standards Act (40 U.S.C. 3704); Sections 4, 6, and 8 of the
Occupational Safety and Health Act of 1970 (29 U.S.C. 653, 655,
657); Secretary of Labor's Order No. 12-71 (36 FR 8754), 8-76 (41 FR
25059), 9-83 (48 FR 35736),1-90 (55 FR 9033), 6-96 (62 FR 111), 3-
2000 (62 FR 50017), 5-2002 (67 FR 650008), 5-2007 (72 FR 31159), 4-
2010 (75 FR 55355), or 1-2012
(77 FR 3912), as applicable; and 29 CFR part 1911.
* * * * *
0
43. Amend Sec. 1926.152 as follows:
0
A. Revise the section heading;
0
B. Remove the words "and combustible" from the first sentence in
paragraph (a)(1), the heading of paragraph (b), and paragraphs (b)(2)
introductory text, (b)(4)(viii), (h) introductory text, and (h)(1);
0
C. Remove the words "or combustible" wherever it appears in
paragraphs (a)(2), (b)(1), (b)(4)(iii), (b)(5), and (c)(3);
0
D. Remove the words "or combustible" in paragraphs (d) (the heading),
(d)(1), (d)(4), (e)(1), (e)(3), (f)(2), (g)(1), and (g)(8);
0
E. Remove the words "or combustible" wherever it appears in
paragraphs (i)(1)(i)(D) and (F), (i)(1)(iii)(D), (i)(2)(ii)(A), (D),
and (F), (i)(2)(vii)(B)(2), (i)(4)(iv)(C), (i)(5)(vi)(A), (D), (G), (V)
introductory text, and (i)(5)(vi)(V)(1); (j)(1)(i), (j)(2)(ii), (j)(5),
and (k)(4);
0
F. Amend the fifth sentence of paragraph (b)(4)(vi) by adding the words
"Category 1, 2, or 3" before the words "flammable liquids";
0
G. Amend paragraphs (e)(2), (e)(5), (g)(7)(i), and (g)(7)(ii), by
adding the words "Category 1, 2, or 3" before the words "flammable
liquids" ;
0
H. Amend paragraphs (f)(1) and (f)(3) by removing the words "Flammable
liquids" and adding in their place the words "Category 1, 2, or 3
flammable liquids";
0
I. Revise paragraphs (b)(2)(iii), (b)(3), (h) introductory text,
(i)(2)(iv)(F) and (G), (i)(2)(vi)(B), (i)(2)(viii)(E), (i)(3)(i),
(i)(3)(iv)(A) and (C), (i)(3)(v)(D), and (i)(4)(iv)(E);
0
J. Revise Table F-19 and paragraph (k)(3)(iv).
The revisions read as follows:
Sec. 1926.152 Flammable liquids.
* * * * *
(b) * * *
(2) * * *
(iii) Cabinets shall be labeled in conspicuous lettering,
"Flammable-Keep Away from Open Flames."
(3) Not more than 60 gallons of Category 1, 2 and/or 3 flammable
liquids or 120 gallons of Category 4 flammable liquids shall be stored
in any one storage cabinet. Not more than three such cabinets may be
located in a single storage area. Quantities in excess of this shall be
stored in an inside storage room.
* * * * *
(h) Scope. This section applies to the handling, storage, and use
of flammable liquids with a flashpoint at or below 199.4 [deg]F (93
[deg]C). This section does not apply to:
* * * * *
(i) * * *
(2) * * *
(iv) * * *
(F) Tanks and pressure vessels storing Category 1 flammable liquids
shall be equipped with venting devices that shall be normally closed
except when venting to pressure or vacuum conditions. Tanks and
pressure vessels storing Category 2 flammable liquids, or Category 3
flammable liquids with a flashpoint below 100 [deg]F (37.8 [deg]C),
shall be equipped with venting devices that shall be normally closed
except when venting under pressure or vacuum conditions, or with
approved flame arresters.
Exemption: Tanks of 3,000 bbls (barrels) (84 m(3)) capacity or less
containing crude petroleum in crude-producing areas; and, outside
aboveground atmospheric tanks under 1,000 gallons (3,785 L) capacity
containing other than Category 1 flammable liquids may have open vents.
(See paragraph (i)(2)(vi)(B) of this section.)
(G) Flame arresters or venting devices required in paragraph
(i)(2)(iv)(F) of this section may be omitted for Category 2 flammable
liquids or Category 3 flammable liquids with a flashpoint below 100
[deg]F (37.8 [deg]C) where conditions are such that their use may, in
case of obstruction, result in tank damage.
* * * * *
(vi) * * *
(B) Where vent pipe outlets for tanks storing Category 1 or 2
flammable liquids, or Category 3 flammable liquids with a flashpoint
below 100 [deg]F (37.8 [deg]C), are adjacent to buildings or public
ways, they shall be located so that the vapors are released at a safe
point outside of buildings and not less than 12 feet (3.658 m) above
the adjacent ground level. In order to aid their dispersion, vapors
shall be discharged upward or horizontally away from closely adjacent
walls. Vent outlets shall be located so that flammable vapors will not
be trapped by eaves or other obstructions and shall be at least 5 feet
(1.52 m) from building openings.
(viii) * * *
(E) For Category 2 flammable liquids or Category 3 flammable
liquids with a flashpoint below 100 [deg]F (37.8 [deg]C), other than
crude oils, gasolines, and asphalts, the fill pipe shall be so designed
and installed as to minimize the possibility of generating static
electricity. A fill pipe entering the top of a tank shall terminate
within 6 inches (15.24 cm) of the bottom of the tank and shall be
installed to avoid excessive vibration.
* * * * *
(3) * * *
(i) Location. Evacuation for underground storage tanks shall be
made with due care to avoid undermining of foundations of existing
structures. Underground tanks or tanks under buildings shall be so
located with respect to existing building foundations and supports that
the loads carried by the latter cannot be transmitted to the tank. The
distance from any part of a tank storing Category 1 or 2 flammable
liquids, or Category 3 flammable liquids with a flashpoint below 100
[deg]F (37.8 [deg]C), to the nearest wall of any basement or pit shall
be not less than 1 foot (0.304 m), and to any property line that may be
built upon, not less than 3 feet (0.912 m). The distance from any part
of a tank storing Category 3 flammable liquids with a flashpoint at or
above 100 [deg]F (37.8 [deg]C) or Category 4 flammable liquids to the
nearest wall of any basement, pit or property line shall be not less
than 1 foot (0.304 m).
* * * * *
(iv) * * *
(A) Location and arrangement of vents for Category 1 or 2 flammable
liquids, or Category 3 flammable liquids with a flashpoint below 100
[deg]F (37.8 [deg]C). Vent pipes from tanks storing Category 1 or 2
flammable liquids, or Category 3 flammable liquids with a flashpoint
below 100 [deg]F (37.8 [deg]C), shall be so located that the discharge
point is outside of buildings, higher than the fill pipe opening, and
not less than 12 feet (3.658 m) above the adjacent ground level. Vent
pipes shall discharge only upward in order to disperse vapors. Vent
pipes 2 inches (5.08 cm) or less in nominal inside diameter shall not
be obstructed by devices that will cause excessive back pressure. Vent
pipe outlets shall be so located that flammable vapors will not enter
building openings, or be trapped under eaves or other obstructions. If
the vent pipe is less than 10 feet (3.04 m) in length, or greater than
2 inches (5.08 cm) in nominal inside diameter, the outlet shall be
provided with a vacuum and pressure relief device or there shall be an
approved flame arrester located in the vent line at the outlet or
within the approved distance from the outlet.
* * * * *
(C) Location and arrangement of vents for Category 3 flammable
liquids with a flashpoint at or above 100 [deg]F (37.8 [deg]C) or
Category 4 flammable liquids. Vent pipes from tanks storing Category 3
flammable liquids with a flashpoint at or above 100 [deg]F (37.8
[deg]C) or Category 4
flammable liquids shall terminate outside of the building and higher
than the fill pipe opening. Vent outlets shall be above normal snow
level. They may be fitted with return bends, coarse screens or other
devices to minimize ingress of foreign material.
* * * * *
(v) * * *
(D) For Category 2 flammable liquids, or Category 3 flammable
liquids with a flashpoint below 100 [deg]F (37.8 [deg]C), other than
crude oils, gasolines, and asphalts, the fill pipe shall be so designed
and installed as to minimize the possibility of generating static
electricity by terminating within 6 inches (15.24 cm) of the bottom of
the tank.
* * * * *
(4) * * *
(iv) * * *
(E) For Category 2 flammable liquids, or Category 3 flammable
liquids with a flashpoint below 100 [deg]F (37.8 [deg]C), other than
crude oils, gasolines, and asphalts, the fill pipe shall be so designed
and installed as to minimize the possibility of generating static
electricity by terminating within 6 inches (15.24 cm) of the bottom of
the tank.
* * * * *
(k) * * *
(3) * * *
* * * * *
BILLING CODE 4510-26-P
[GRAPHIC] [TIFF OMITTED] TR26MR12.128
[GRAPHIC] [TIFF OMITTED] TR26MR12.129
BILLING CODE 4510-26-C
(iv) Piping handling Category 1 or 2 flammable liquids, or Category
3 flammable liquids with a flashpoint below 100 [deg]F (37.8 [deg]C),
shall be grounded to control stray currents.
* * * * *
0
44. Amend Sec. 1926.155 as follows:
0
A. Remove and reserve paragraph (c);
0
B. Revise paragraphs (h) and (i)(1) and (2).
The revisions read as follows:
Sec. 1926.155 Definitions applicable to this subpart.
* * * * *
(h) Flammable liquid means any liquid having a vapor pressure not
exceeding 40 pounds per square inch (absolute) at 100 [deg]F (37.8
[deg]C) and having a flashpoint at or below 199.4 [deg]F (93 [deg]C).
Flammable liquids are divided into four categories as follows:
(1) Category 1 shall include liquids having flashpoints below 73.4
[deg]F (23 [deg]C) and having a boiling point at or below 95 [deg]F (35
[deg]C).
(2) Category 2 shall include liquids having flashpoints below 73.4
[deg]F (23 [deg]C) and having a boiling point above 95 [deg]F (35
[deg]C).
(3) Category 3 shall include liquids having flashpoints at or above
73.4 [deg]F (23 [deg]C) and at or below 140 [deg]F (60 [deg]C).
(4) Category 4 shall include liquids having flashpoints above 140
[deg]F (60 [deg]C) and at or below 199.4 [deg]F (93 [deg]C).
(i) * * *
(1) The flashpoint of liquids having a viscosity less than 45
Saybolt Universal Second(s) at 100 [deg]F (37.8 [deg]C) and a
flashpoint below 175 [deg]F (79.4 [deg]C) shall be determined in
accordance with the Standard Method of Test for Flash Point by the Tag
Closed Tester, ASTM D-56-69 (incorporated by reference; See Sec.
1926.6), or an equivalent method as defined by Sec. 1910.1200 appendix
B.
(2) The flashpoints of liquids having a viscosity of 45 Saybolt
Universal Second(s) or more at 175 [deg]F (79.4 [deg]C) or higher shall
be determined in accordance with the Standard Method of Test for Flash
Point by the Pensky Martens Closed Tester, ASTM D-93-69 (incorporated
by reference; See Sec. 1926.6), or an equivalent method as defined by
Sec. 1910.1200 appendix B.
* * * * *
Subpart Z--[Amended]
0
45. Revise the authority citation for subpart Z to read as follows:
Authority: Section107 of the Contract Work Hours and Safety
Standards Act (40 U.S.C. 3704); Sections 4, 6, and 8 of the
Occupational Safety and Health Act of 1970 (29 U.S.C. 653, 655,
657); and Secretary of Labor's Order No. 12-71 (36 FR 8754), 8-76
(41 FR 25059), 9-83 (48 FR 35736), 1-90 (55 FR 9033), 6-96 (62 FR
111), 3-2000 (65 FR 50017), 5-2002 (67 FR 65008), 5-2007 (72 FR
31159), 4-2010 (75 FR 55355), or 1-2012 (77 FR 3912) as applicable;
and 29 CFR part 1911.
Section 1926.1102 not issued under 29 U.S.C. 655 or 29 CFR part
1911; also issued under 5 U.S.C. 553.
0
46. Amend Sec. 1926.1101 as follows:
0
A. Redesignate paragraph (k)(1) as (k)(1)(i) and add a new heading to
paragraph (k)(1);
0
B. Add new paragraphs (k)(1)(ii), (k)(7)(ii)(C), (k)(7)(ii)(D), and
(k)(8)(iv);
0
C. Amend paragraphs (k)(2)(i) and (k)(3)(i) by removing the references
to "(k)(1)" and adding in their place "(k)(1)(i)";
0
D. Revise paragraphs (k)(7)(ii)(A) and (B), and (k)(8)(ii) and (iii);
The revisions read as follows:
Sec. 1926.1101 Asbestos.
* * * * *
(k) * * *
(1) Hazard communication.
* * * * *
(ii) The employer shall include asbestos in the program established
to comply with the Hazard Communication Standard (HCS) (Sec.
1910.1200). The employer shall ensure that each employee has access to
labels on containers of asbestos and safety data sheets, and is trained
in accordance with the provisions of HCS and paragraphs (k)(9) and (10)
of this section. The employer shall provide information on at least the
following hazards: Cancer and lung effects.
* * * * *
(7) * * *
(ii) * * *
(A) The warning signs required by paragraph (k)(7) of this section
shall bear the following information.
DANGER
ASBESTOS
MAY CAUSE CANCER
CAUSES DAMAGE TO LUNGS
AUTHORIZED PERSONNEL ONLY
(B) In addition, where the use of respirators and protective
clothing is required in the regulated area under this section, the
warning signs shall include the following:
WEAR RESPIRATORY PROTECTION AND PROTECTIVE CLOTHING IN THIS AREA
(C) Prior to June 1, 2016, employers may use the following legend
in lieu of that specified in paragraph (k)(7)(ii)(A) of this section:
DANGER
ASBESTOS
CANCER AND LUNG DISEASE HAZARD
AUTHORIZED PERSONNEL ONLY
(D) Prior to June 1, 2016, employers may use the following legend
in lieu of that specified in paragraph (k)(7)(ii)(B) of this section:
RESPIRATORS AND PROTECTIVE CLOTHING ARE REQUIRED IN THIS AREA
* * * * *
(8) * * *
(ii) The employer shall ensure that such labels comply with
paragraphs (k) of this section.
(iii) The employer shall ensure that labels of bags or containers
of protective clothing and equipment, scrap, waste, and debris
containing asbestos fibers bear the following information:
DANGER
CONTAINS ASBESTOS FIBERS
MAY CAUSE CANCER
CAUSES DAMAGE TO LUNGS
DO NOT BREATHE DUST
AVOID CREATING DUST
(iv) (A) Prior to June 1, 2015, employers may include the following
information on raw materials, mixtures or labels of bags or containers
of protective clothing and equipment, scrap, waste, and debris
containing asbestos fibers in lieu of the labeling requirements in
paragraphs (k)(8)(ii) and (k)(8)(iii) of this section:
DANGER
CONTAINS ASBESTOS FIBERS
AVOID CREATING DUST
CANCER AND LUNG DISEASE HAZARD
(B) Labels shall also contain a warning statement against breathing
asbestos fibers.
* * * * *
0
47. Revise Sec. 1926.1126 paragraphs (g)(2)(iv) and (j)(1) to read as
follows:
Sec. 1926.1126 Chromium (VI).
* * * * *
(g) * * *
(2) * * *
(iv) The employer shall ensure that bags or containers of
contaminated protective clothing or equipment that are removed from
change rooms for laundering, cleaning, maintenance, or disposal shall
be labeled in accordance with the requirements of the Hazard
Communication Standard, Sec. 1910.1200.
* * * * *
(j) * * *
(1) Hazard communication. The employer shall include chromium (VI)
in the program established to comply with the Hazard Communication
Standard (HCS) (Sec. 1910.1200). The employer shall ensure that each
employee has access to labels on containers of chromium and safety data
sheets, and is trained in accordance with the provisions of Sec.
1910.1200 and paragraph (j)(2) of this section. The employer shall
provide information on at least the following hazards: Cancer; eye
irritation; and skin sensitization.
* * * * *
0
48. Revise Sec. 1926.1127 paragraphs (i)(2)(iv), (k)(7), and (m)(1),
(m)(2), and (m)(3), to read as follows:
Sec. 1926.1127 Cadmium.
* * * * *
(i) * * *
(2) * * *
(iv) The employer shall ensure that containers of contaminated
protective clothing and equipment that are to be taken out of the
change rooms or the workplace for laundering, cleaning, maintenance or
disposal shall bear labels in accordance with paragraph (m)(3)(ii) of
this section.
(k) * * *
(7) Waste, scrap, debris, bags, and containers, personal protective
equipment and clothing contaminated with cadmium and consigned for
disposal shall be collected and disposed of in sealed impermeable bags
or other closed, impermeable containers. These bags and containers
shall be labeled in accordance with paragraph (m)(3)(ii) of this
section.
* * * * *
(m) * * *
(1) Hazard communication. The employer shall include cadmium in the
program established to comply with the Hazard Communication Standard
(HCS) (Sec. 1910.1200). The employer shall ensure that each employee
has access to labels on containers of cadmium and safety data sheets,
and is trained in accordance with the provisions of HCS and paragraph
(m)(4) of this section. The employer shall provide information on at
least the following hazards: Cancer; lung effects; kidney effects; and
acute toxicity effects.
(2) Warning signs. (i) Warning signs shall be provided and
displayed in regulated areas. In addition, warning signs shall be
posted at all approaches to regulated areas so that an employee may
read the signs and take necessary protective steps before entering the
area.
(ii) Warning signs required by paragraph (m)(2)(i) of this section
shall bear the following legend:
DANGER
CADMIUM
MAY CAUSE CANCER
CAUSES DAMAGE TO LUNGS AND KIDNEYS
WEAR RESPIRATORY PROTECTION IN THIS AREA
AUTHORIZED PERSONNEL ONLY
(iii) The employer shall ensure that signs required by this
paragraph (m)(2) are illuminated, cleaned, and maintained as necessary
so that the legend is readily visible.
(iv) Prior to June 1, 2016, employers may use the following legend
in lieu of that specified in paragraph (m)(2)(ii) of this section:
DANGER
CADMIUM
CANCER HAZARD
CAN CAUSE LUNG AND KIDNEY DISEASE
AUTHORIZED PERSONNEL ONLY
RESPIRATORS REQUIRED IN THIS AREA
(3) Warning labels. (i) Shipping and storage containers containing
cadmium or cadmium compounds shall bear appropriate warning labels, as
specified in paragraph (m)(1) of this section.
(ii) The warning labels for containers of cadmium-contaminated
protective clothing, equipment, waste, scrap, or debris shall include
at least the following information:
DANGER
CONTAINS CADMIUM
MAY CAUSE CANCER
CAUSES DAMAGE TO LUNGS AND KIDNEYS
AVOID CREATING DUST
(iii) Where feasible, installed cadmium products shall have a
visible label or other indication that cadmium is present.
(iv) Prior to June 1, 2015, employers may include the following
information on shipping and storage containers containing cadmium,
cadmium compounds, or cadmium-contaminated clothing, equipment, waste,
scrap, or debris in lieu of the labeling requirements specified in
paragraphs (m)(3)(i) and (m)(3)(ii) of this section:
DANGER
CONTAINS CADMIUM
CANCER HAZARD
AVOID CREATING DUST
CAN CAUSE LUNG AND KIDNEY DISEASE
* * * * *
[FR Doc. 2012-4826 Filed 3-20-12; 11:15 am]
BILLING CODE 4510-26-P
Where: [Delta]Hc = chemical heat of combustion (kJ/g); wi% = mass fraction of component i in the product; [Delta]Hc(i) = specific heat of combustion (kJ/g) of component i in the product; The chemical heats of combustion shall be found in literature, calculated or determined by tests (See ASTM D240-02, ISO 13943, Sections 86.1 to 86.3, and NFPA 30B (incorporated by reference; See Sec. 1910.6)). B.3.3.3 The Ignition Distance Test, Enclosed Space Ignition Test and Aerosol Foam Flammability Test shall be performed in accordance with sub-sections 31.4, 31.5 and 31.6 of the of the UN ST/SG/AC.10 (incorporated by reference; See Sec. 1910.6). B.4 OXIDIZING GASES B.4.1 Definition Oxidizing gas means any gas which may, generally by providing oxygen, cause or contribute to the combustion of other material more than air does. Note: "Gases which cause or contribute to the combustion of other material more than air does" means pure gases or gas mixtures with an oxidizing power greater than 23.5% (as determined by a method specified in ISO 10156 or 10156-2 (incorporated by reference, See Sec. 1910.6) or an equivalent testing method.) B.4.2 Classification Criteria An oxidizing gas shall be classified in a single category for this class in accordance with Table B.4.1: Table B.4.1--Criteria for Oxidizing Gases ---------------------------------------------------------------------------------------------------------------- Category Criteria ---------------------------------------------------------------------------------------------------------------- 1........................................... Any gas which may, generally by providing oxygen, cause or contribute to the combustion of other material more than air does. ---------------------------------------------------------------------------------------------------------------- B.4.3 Additional Classification Considerations Classification shall be in accordance with tests or calculation methods as described in ISO 10156 (incorporated by reference; See Sec. 1910.6) and ISO 10156-2 (incorporated by reference; See Sec. 1910.6). B.5 GASES UNDER PRESSURE B.5.1 Definition Gases under pressure are gases which are contained in a receptacle at a pressure of 200 kPa (29 psi) (gauge) or more, or which are liquefied or liquefied and refrigerated. They comprise compressed gases, liquefied gases, dissolved gases and refrigerated liquefied gases. B.5.2 Classification Criteria Gases under pressure shall be classified in one of four groups in accordance with Table B.5.1: Table B.5.1--Criteria for Gases Under Pressure ---------------------------------------------------------------------------------------------------------------- Group Criteria ---------------------------------------------------------------------------------------------------------------- Compressed gas.............................. A gas which when under pressure is entirely gaseous at -50 [deg]C (-8 [deg]F), including all gases with a critical temperature\1\ <=-50 [deg]C (-58 [deg]F). Liquefied gas............................... A gas which when under pressure is partially liquid at temperatures above -50 [deg]C (-58 [deg]F). A distinction is made between: (a) High pressure liquefied gas: A gas with a critical temperature \1\ between -50 [deg]C (-58 [deg]F) and +65 [deg]C (149 [deg]F); and (b) Low pressure liquefied gas: A gas with a critical temperature \1\ above +65 [deg]C (149 [deg]F). Refrigerated liquefied gas.................. A gas which is made partially liquid because of its low temperature. Dissolved gas............................... A gas which when under pressure is dissolved in a liquid phase solvent. ---------------------------------------------------------------------------------------------------------------- \1\ The critical temperature is the temperature above which a pure gas cannot be liquefied, regardless of the degree of compression. B.6 FLAMMABLE LIQUIDS B.6.1 Definition Flammable liquid means a liquid having a flash point of not more than 93 [deg]C (199.4 [deg]F). Flash point means the minimum temperature at which a liquid gives off vapor in sufficient concentration to form an ignitable mixture with air near the surface of the liquid, as determined by a method identified in Section B.6.3. B.6.2 Classification Criteria A flammable liquid shall be classified in one of four categories in accordance with Table B.6.1: Table B.6.1--Criteria for Flammable Liquids -------------------------------------------------------------------------------------------------------------------------------------------------------- Category Criteria -------------------------------------------------------------------------------------------------------------------------------------------------------- 1........................................... Flash point <23 [deg]C (73.4 [deg]F) and initial boiling point <=35 [deg]C (95 [deg]F). 2........................................... Flash point <23 [deg]C (73.4 [deg]F) and initial boiling point >35 [deg]C (95 [deg]F). 3........................................... Flash point >=23 [deg]C (73.4 [deg]F) and <=60 [deg]C (140 [deg]F). 4........................................... Flash point >60 [deg]C (140 [deg]F) and <=93 [deg]C (199.4 [deg]F). -------------------------------------------------------------------------------------------------------------------------------------------------------- B.6.3 Additional Classification Considerations The flash point shall be determined in accordance with ASTM D56- 05, ASTM D3278, ASTM D3828, ASTM D93-08 (incorporated by reference; See Sec. 1910.6), or any other method specified in GHS Revision 3, Chapter 2.6. The initial boiling point shall be determined in accordance with ASTM D86-07a or ASTM D1078 (incorporated by reference; See Sec. 1910.6). B.7 FLAMMABLE SOLIDS B.7.1 Definitions Flammable solid means a solid which is a readily combustible solid, or which may cause or contribute to fire through friction. Readily combustible solids are powdered, granular, or pasty chemicals which are dangerous if they can be easily ignited by brief contact with an ignition source, such as a burning match, and if the flame spreads rapidly. B.7.2 Classification Criteria B.7.2.1 Powdered, granular or pasty chemicals shall be classified as flammable solids when the time of burning of one or more of the test runs, performed in accordance with the test method described in the UN ST/SG/AC.10 (incorporated by reference; See Sec. 1910.6), Part III, sub-section 33.2.1, is less than 45 s or the rate of burning is more than 2.2 mm/s (0.0866 in/s). B.7.2.2 Powders of metals or metal alloys shall be classified as flammable solids when they can be ignited and the reaction spreads over the whole length of the sample in 10 min or less. B.7.2.3 Solids which may cause fire through friction shall be classified in this class by analogy with existing entries (e.g., matches) until definitive criteria are established. B.7.2.4 A flammable solid shall be classified in one of the two categories for this class using Method N.1 as described in Part III, sub-section 33.2.1 of the UN ST/SG/AC.10 (incorporated by reference; See Sec. 1910.6), in accordance with Table B.7.1: Table B.7.1--Criteria for Flammable Solids ------------------------------------------------------------------------ Category Criteria ------------------------------------------------------------------------ 1...................................... Burning rate test: Chemicals other than metal powders: (a) Wetted zone does not stop fire; and (b) Burning time <45 s or burning rate >2.2 mm/s. Metal powders: Burning time <=5 min. 2...................................... Burning rate test: Chemicals other than metal powders: (a) Wetted zone stops the fire for at least 4 min; and (b) Burning time <45 s or burning rate >2.2 mm/s. Metal powders: Burning time >5 min and <=10 min. ------------------------------------------------------------------------ Note: Classification of solid chemicals shall be based on tests performed on the chemical as presented. If, for example, for the purposes of supply or transport, the same chemical is to be presented in a physical form different from that which was tested and which is considered likely to materially alter its performance in a classification test, classification must be based on testing of the chemical in the new form. B.8 SELF-REACTIVE CHEMICALS B.8.1 Definitions Self-reactive chemicals are thermally unstable liquid or solid chemicals liable to undergo a strongly exothermic decomposition even without participation of oxygen (air). This definition excludes chemicals classified under this section as explosives, organic peroxides, oxidizing liquids or oxidizing solids. A self-reactive chemical is regarded as possessing explosive properties when in laboratory testing the formulation is liable to detonate, to deflagrate rapidly or to show a violent effect when heated under confinement. B.8.2 Classification Criteria B.8.2.1 A self-reactive chemical shall be considered for classification in this class unless: (a) It is classified as an explosive according to B.1 of this appendix; (b) It is classified as an oxidizing liquid or an oxidizing solid according to B.13 or B.14 of this appendix, except that a mixture of oxidizing substances which contains 5% or more of combustible organic substances shall be classified as a self- reactive chemical according to the procedure defined in B.8.2.2; (c) It is classified as an organic peroxide according to B.15 of this appendix; (d) Its heat of decomposition is less than 300 J/g; or (e) Its self-accelerating decomposition temperature (SADT) is greater than 75 [deg]C (167 [deg]F) for a 50 kg (110 lb) package. B.8.2.2 Mixtures of oxidizing substances, meeting the criteria for classification as oxidizing liquids or oxidizing solids, which contain 5% or more of combustible organic substances and which do not meet the criteria mentioned in B.8.2.1 (a), (c), (d) or (e), shall be subjected to the self-reactive chemicals classification procedure in B.8.2.3. Such a mixture showing the properties of a self-reactive chemical type B to F shall be classified as a self- reactive chemical. B.8.2.3 Self-reactive chemicals shall be classified in one of the seven categories of "types A to G" for this class, according to the following principles: (a) Any self-reactive chemical which can detonate or deflagrate rapidly, as packaged, will be defined as self-reactive chemical TYPE A; (b) Any self-reactive chemical possessing explosive properties and which, as packaged, neither detonates nor deflagrates rapidly, but is liable to undergo a thermal explosion in that package will be defined as self-reactive chemical TYPE B; (c) Any self-reactive chemical possessing explosive properties when the chemical as packaged cannot detonate or deflagrate rapidly or undergo a thermal explosion will be defined as self-reactive chemical TYPE C; (d) Any self-reactive chemical which in laboratory testing meets the criteria in (d)(i), (ii), or (iii) will be defined as self- reactive chemical TYPE D: (i) Detonates partially, does not deflagrate rapidly and shows no violent effect when heated under confinement; or (ii) Does not detonate at all, deflagrates slowly and shows no violent effect when heated under confinement; or (iii) Does not detonate or deflagrate at all and shows a medium effect when heated under confinement; (e) Any self-reactive chemical which, in laboratory testing, neither detonates nor deflagrates at all and shows low or no effect when heated under confinement will be defined as self-reactive chemical TYPE E; (f) Any self-reactive chemical which, in laboratory testing, neither detonates in the cavitated state nor deflagrates at all and shows only a low or no effect when heated under confinement as well as low or no explosive power will be defined as self-reactive chemical TYPE F; (g) Any self-reactive chemical which, in laboratory testing, neither detonates in the cavitated state nor deflagrates at all and shows no effect when heated under confinement nor any explosive power, provided that it is thermally stable (self-accelerating decomposition temperature is 60 [deg]C (140 [deg]F) to 75 [deg]C (167 [deg]F) for a 50 kg (110 lb) package), and, for liquid mixtures, a diluent having a boiling point greater than or equal to 150 [deg]C (302 [deg]F) is used for desensitization will be defined as self-reactive chemical TYPE G. If the mixture is not thermally stable or a diluent having a boiling point less than 150 [deg]C (302 [deg]F) is used for desensitization, the mixture shall be defined as self-reactive chemical TYPE F. B.8.3 Additional Classification Considerations B.8.3.1 For purposes of classification, the properties of self- reactive chemicals shall be determined in accordance with test series A to H as described in Part II of the UN ST/SG/AC.10 (incorporated by reference; See Sec. 1910.6). B.8.3.2 Self-accelerating decomposition temperature (SADT) shall be determined in accordance with the UN ST/SG/AC.10, Part II, section 28 (incorporated by reference; See Sec. 1910.6). B.8.3.3 The classification procedures for self-reactive substances and mixtures need not be applied if: (a) There are no chemical groups present in the molecule associated with explosive or self-reactive properties; examples of such groups are given in Tables A6.1 and A6.2 in the Appendix 6 of the UN ST/SG/AC.10 (incorporated by reference; See Sec. 1910.6); or (b) For a single organic substance or a homogeneous mixture of organic substances, the estimated SADT is greater than 75 [deg]C (167 [deg]F) or the exothermic decomposition energy is less than 300 J/g. The onset temperature and decomposition energy may be estimated using a suitable calorimetric technique (See 20.3.3.3 in Part II of the UN ST/SG/AC.10 (incorporated by reference; See Sec. 1910.6)). B.9 PYROPHORIC LIQUIDS B.9.1 Definition Pyrophoric liquid means a liquid which, even in small quantities, is liable to ignite within five minutes after coming into contact with air. B.9.2 Classification Criteria A pyrophoric liquid shall be classified in a single category for this class using test N.3 in Part III, sub-section 33.3.1.5 of the UN ST/SG/AC.10 (incorporated by reference; See Sec. 1910.6), in accordance with Table B.9.1: Table B.9.1--Criteria for Pyrophoric Liquids ---------------------------------------------------------------------------------------------------------------- Category Criteria ---------------------------------------------------------------------------------------------------------------- 1........................................... The liquid ignites within 5 min when added to an inert carrier and exposed to air, or it ignites or chars a filter paper on contact with air within 5 min. ---------------------------------------------------------------------------------------------------------------- B.9.3 Additional Classification Considerations The classification procedure for pyrophoric liquids need not be applied when experience in production or handling shows that the chemical does not ignite spontaneously on coming into contact with air at normal temperatures (i.e., the substance is known to be stable at room temperature for prolonged periods of time (days)). B.10 PYROPHORIC SOLIDS B.10.1 Definition Pyrophoric solid means a solid which, even in small quantities, is liable to ignite within five minutes after coming into contact with air. B.10.2 Classification Criteria A pyrophoric solid shall be classified in a single category for this class using test N.2 in Part III, sub-section 33.3.1.4 of the UN ST/SG/AC.10 (incorporated by reference; See Sec. 1910.6), in accordance with Table B.10.1: Table B.10.1--Criteria for Pyrophoric Solids -------------------------------------------------------------------------------------------------------------------------------------------------------- Category Criteria -------------------------------------------------------------------------------------------------------------------------------------------------------- 1............................................. The solid ignites within 5 min of coming into contact with air. -------------------------------------------------------------------------------------------------------------------------------------------------------- Note: Classification of solid chemicals shall be based on tests performed on the chemical as presented. If, for example, for the purposes of supply or transport, the same chemical is to be presented in a physical form different from that which was tested and which is considered likely to materially alter its performance in a classification test, classification must be based on testing of the chemical in the new form. B.10.3 Additional Classification Considerations The classification procedure for pyrophoric solids need not be applied when experience in production or handling shows that the chemical does not ignite spontaneously on coming into contact with air at normal temperatures (i.e., the chemical is known to be stable at room temperature for prolonged periods of time (days)). B.11 SELF-HEATING CHEMICALS B.11.1 Definition A self-heating chemical is a solid or liquid chemical, other than a pyrophoric liquid or solid, which, by reaction with air and without energy supply, is liable to self-heat; this chemical differs from a pyrophoric liquid or solid in that it will ignite only when in large amounts (kilograms) and after long periods of time (hours or days). Note: Self-heating of a substance or mixture is a process where the gradual reaction of that substance or mixture with oxygen (in air) generates heat. If the rate of heat production exceeds the rate of heat loss, then the temperature of the substance or mixture will rise which, after an induction time, may lead to self-ignition and combustion. B.11.2 Classification Criteria B.11.2.1 A self-heating chemical shall be classified in one of the two categories for this class if, in tests performed in accordance with test method N.4 in Part III, sub-section 33.3.1.6 of the UN ST/SG/AC.10 (incorporated by reference; See Sec. 1910.6), the result meets the criteria shown in Table B.11.1. Table B.11.1--Criteria for Self-Heating Chemicals ---------------------------------------------------------------------------------------------------------------- Category Criteria ---------------------------------------------------------------------------------------------------------------- 1........................................... A positive result is obtained in a test using a 25 mm sample cube at 140 [deg]C (284 [deg]F). 2........................................... A negative result is obtained in a test using a 25 mm cube sample at 140 [deg]C (284 [deg]F), a positive result is obtained in a test using a 100 mm sample cube at 140 [deg]C (284 [deg]F), and: (a) The unit volume of the chemical is more than 3 m\3\; or (b) A positive result is obtained in a test using a 100 mm cube sample at 120 [deg]C (248 [deg]F) and the unit volume of the chemical is more than 450 liters; or (c) A positive result is obtained in a test using a 100 mm cube sample at 100 [deg]C (212 [deg]F). ---------------------------------------------------------------------------------------------------------------- B.11.2.2 Chemicals with a temperature of spontaneous combustion higher than 50 [deg]C (122 [deg]F) for a volume of 27 m\3\ shall not be classified as self-heating chemicals. B.11.2.3 Chemicals with a spontaneous ignition temperature higher than 50 [deg]C (122 [deg]F) for a volume of 450 liters shall not be classified in Category 1 of this class. B.11.3 Additional Classification Considerations B.11.3.1 The classification procedure for self-heating chemicals need not be applied if the results of a screening test can be adequately correlated with the classification test and an appropriate safety margin is applied. B.11.3.2 Examples of screening tests are: (a) The Grewer Oven test (VDI guideline 2263, part 1, 1990, Test methods for the Determination of the Safety Characteristics of Dusts) with an onset temperature 80[deg]K above the reference temperature for a volume of 1 l; (b) The Bulk Powder Screening Test (Gibson, N. Harper, D. J. Rogers, R. Evaluation of the fire and explosion risks in drying powders, Plant Operations Progress, 4 (3), 181-189, 1985) with an onset temperature 60[deg]K above the reference temperature for a volume of 1 l. B.12 CHEMICALS WHICH, IN CONTACT WITH WATER, EMIT FLAMMABLE GASES B.12.1 Definition Chemicals which, in contact with water, emit flammable gases are solid or liquid chemicals which, by interaction with water, are liable to become spontaneously flammable or to give off flammable gases in dangerous quantities. B.12.2 Classification Criteria B.12.2.1 A chemical which, in contact with water, emits flammable gases shall be classified in one of the three categories for this class, using test N.5 in Part III, sub-section 33.4.1.4 of the UN ST/SG/AC.10 (incorporated by reference; See Sec. 1910.6), in accordance with Table B.12.1: Table B.12.1--Criteria for Chemicals Which, in Contact With Water, Emit Flammable Gases ---------------------------------------------------------------------------------------------------------------- Category Criteria ---------------------------------------------------------------------------------------------------------------- 1........................................... Any chemical which reacts vigorously with water at ambient temperatures and demonstrates generally a tendency for the gas produced to ignite spontaneously, or which reacts readily with water at ambient temperatures such that the rate of evolution of flammable gas is equal to or greater than 10 liters per kilogram of chemical over any one minute. 2........................................... Any chemical which reacts readily with water at ambient temperatures such that the maximum rate of evolution of flammable gas is equal to or greater than 20 liters per kilogram of chemical per hour, and which does not meet the criteria for Category 1. 3........................................... Any chemical which reacts slowly with water at ambient temperatures such that the maximum rate of evolution of flammable gas is equal to or greater than 1 liter per kilogram of chemical per hour, and which does not meet the criteria for Categories 1 and 2. ---------------------------------------------------------------------------------------------------------------- Note: Classification of solid chemicals shall be based on tests performed on the chemical as presented. If, for example, for the purposes of supply or transport, the same chemical is to be presented in a physical form different from that which was tested and which is considered likely to materially alter its performance in a classification test, classification must be based on testing of the chemical in the new form. B.12.2.2 A chemical is classified as a chemical which, in contact with water emits flammable gases if spontaneous ignition takes place in any step of the test procedure. B.12.3 Additional Classification Considerations The classification procedure for this class need not be applied if: (a) The chemical structure of the chemical does not contain metals or metalloids; (b) Experience in production or handling shows that the chemical does not react with water, (e.g., the chemical is manufactured with water or washed with water); or (c) The chemical is known to be soluble in water to form a stable mixture. B.13 OXIDIZING LIQUIDS B.13.1 Definition Oxidizing liquid means a liquid which, while in itself not necessarily combustible, may, generally by yielding oxygen, cause, or contribute to, the combustion of other material. B.13.2 Classification Criteria An oxidizing liquid shall be classified in one of the three categories for this class using test O.2 in Part III, sub-section 34.4.2 of the UN ST/SG/AC.10 (incorporated by reference; See Sec. 1910.6), in accordance with Table B.13.1: Table B.13.1--Criteria for Oxidizing Liquids ---------------------------------------------------------------------------------------------------------------- Category Criteria ---------------------------------------------------------------------------------------------------------------- 1........................................... Any chemical which, in the 1:1 mixture, by mass, of chemical and cellulose tested, spontaneously ignites; or the mean pressure rise time of a 1:1 mixture, by mass, of chemical and cellulose is less than that of a 1:1 mixture, by mass, of 50% perchloric acid and cellulose; 2........................................... Any chemical which, in the 1:1 mixture, by mass, of chemical and cellulose tested, exhibits a mean pressure rise time less than or equal to the mean pressure rise time of a 1:1 mixture, by mass, of 40% aqueous sodium chlorate solution and cellulose; and the criteria for Category 1 are not met; 3........................................... Any chemical which, in the 1:1 mixture, by mass, of chemical and cellulose tested, exhibits a mean pressure rise time less than or equal to the mean pressure rise time of a 1:1 mixture, by mass, of 65% aqueous nitric acid and cellulose; and the criteria for Categories 1 and 2 are not met. ---------------------------------------------------------------------------------------------------------------- B.13.3 Additional Classification Considerations B.13.3.1 For organic chemicals, the classification procedure for this class shall not be applied if: (a) The chemical does not contain oxygen, fluorine or chlorine; or (b) The chemical contains oxygen, fluorine or chlorine and these elements are chemically bonded only to carbon or hydrogen. B.13.3.2 For inorganic chemicals, the classification procedure for this class shall not be applied if the chemical does not contain oxygen or halogen atoms. B.13.3.3 In the event of divergence between test results and known experience in the handling and use of chemicals which shows them to be oxidizing, judgments based on known experience shall take precedence over test results. B.13.3.4 In cases where chemicals generate a pressure rise (too high or too low), caused by chemical reactions not characterizing the oxidizing properties of the chemical, the test described in Part III, sub-section 34.4.2 of the UN ST/SG/AC.10 (incorporated by reference; See Sec. 1910.6) shall be repeated with an inert substance (e.g., diatomite (kieselguhr)) in place of the cellulose in order to clarify the nature of the reaction. B.14 OXIDIZING SOLIDS B.14.1 Definition Oxidizing solid means a solid which, while in itself is not necessarily combustible, may, generally by yielding oxygen, cause, or contribute to, the combustion of other material. B.14.2 Classification Criteria An oxidizing solid shall be classified in one of the three categories for this class using test O.1 in Part III, sub-section 34.4.1 of the UN ST/SG/AC.10 (incorporated by reference; See Sec. 1910.6), in accordance with Table B.14.1: Table B.14.1--Criteria for Oxidizing Solids ---------------------------------------------------------------------------------------------------------------- Category Criteria ---------------------------------------------------------------------------------------------------------------- 1........................................... Any chemical which, in the 4:1 or 1:1 sample-to-cellulose ratio (by mass) tested, exhibits a mean burning time less than the mean burning time of a 3:2 mixture, by mass, of potassium bromate and cellulose. 2........................................... Any chemical which, in the 4:1 or 1:1 sample-to-cellulose ratio (by mass) tested, exhibits a mean burning time equal to or less than the mean burning time of a 2:3 mixture (by mass) of potassium bromate and cellulose and the criteria for Category 1 are not met. 3........................................... Any chemical which, in the 4:1 or 1:1 sample-to-cellulose ratio (by mass) tested, exhibits a mean burning time equal to or less than the mean burning time of a 3:7 mixture (by mass) of potassium bromate and cellulose and the criteria for Categories 1 and 2 are not met. ---------------------------------------------------------------------------------------------------------------- Note 1: Some oxidizing solids may present explosion hazards under certain conditions (e.g., when stored in large quantities). For example, some types of ammonium nitrate may give rise to an explosion hazard under extreme conditions and the "Resistance to detonation test" (IMO: Code of Safe Practice for Solid Bulk Cargoes, 2005, Annex 3, Test 5) may be used to assess this hazard. When information indicates that an oxidizing solid may present an explosion hazard, it shall be indicated on the Safety Data Sheet. Note 2: Classification of solid chemicals shall be based on tests performed on the chemical as presented. If, for example, for the purposes of supply or transport, the same chemical is to be presented in a physical form different from that which was tested and which is considered likely to materially alter its performance in a classification test, classification must be based on testing of the chemical in the new form. B.14.3 Additional Classification Considerations B.14.3.1 For organic chemicals, the classification procedure for this class shall not be applied if: (a) The chemical does not contain oxygen, fluorine or chlorine; or (b) The chemical contains oxygen, fluorine or chlorine and these elements are chemically bonded only to carbon or hydrogen. B.14.3.2 For inorganic chemicals, the classification procedure for this class shall not be applied if the chemical does not contain oxygen or halogen atoms. B.14.3.3 In the event of divergence between test results and known experience in the handling and use of chemicals which shows them to be oxidizing, judgements based on known experience shall take precedence over test results. B.15 ORGANIC PEROXIDES B.15.1 Definition B.15.1.1 Organic peroxide means a liquid or solid organic chemical which contains the bivalent -0-0- structure and as such is considered a derivative of hydrogen peroxide, where one or both of the hydrogen atoms have been replaced by organic radicals. The term organic peroxide includes organic peroxide mixtures containing at least one organic peroxide. Organic peroxides are thermally unstable chemicals, which may undergo exothermic self-accelerating decomposition. In addition, they may have one or more of the following properties: (a) Be liable to explosive decomposition; (b) Burn rapidly; (c) Be sensitive to impact or friction; (d) React dangerously with other substances. B.15.1.2 An organic peroxide is regarded as possessing explosive properties when in laboratory testing the formulation is liable to detonate, to deflagrate rapidly or to show a violent effect when heated under confinement. B.15.2 Classification Criteria B.15.2.1 Any organic peroxide shall be considered for classification in this class, unless it contains: (a) Not more than 1.0% available oxygen from the organic peroxides when containing not more than 1.0% hydrogen peroxide; or (b) Not more than 0.5% available oxygen from the organic peroxides when containing more than 1.0% but not more than 7.0% hydrogen peroxide. Note: The available oxygen content (%) of an organic peroxide mixture is given by the formula:
Where: ni = number of peroxygen groups per molecule of organic peroxide i; ci = concentration (mass %) of organic peroxide i; mi = molecular mass of organic peroxide i. B.15.2.2 Organic peroxides shall be classified in one of the seven categories of "Types A to G" for this class, according to the following principles: (a) Any organic peroxide which, as packaged, can detonate or deflagrate rapidly shall be defined as organic peroxide TYPE A; (b) Any organic peroxide possessing explosive properties and which, as packaged, neither detonates nor deflagrates rapidly, but is liable to undergo a thermal explosion in that package shall be defined as organic peroxide TYPE B; (c) Any organic peroxide possessing explosive properties when the chemical as packaged cannot detonate or deflagrate rapidly or undergo a thermal explosion shall be defined as organic peroxide TYPE C; (d) Any organic peroxide which in laboratory testing meets the criteria in (d)(i), (ii), or (iii) shall be defined as organic peroxide TYPE D: (i) Detonates partially, does not deflagrate rapidly and shows no violent effect when heated under confinement; or (ii) Does not detonate at all, deflagrates slowly and shows no violent effect when heated under confinement; or (iii) Does not detonate or deflagrate at all and shows a medium effect when heated under confinement; (e) Any organic peroxide which, in laboratory testing, neither detonates nor deflagrates at all and shows low or no effect when heated under confinement shall be defined as organic peroxide TYPE E; (f) Any organic peroxide which, in laboratory testing, neither detonates in the cavitated state nor deflagrates at all and shows only a low or no effect when heated under confinement as well as low or no explosive power shall be defined as organic peroxide TYPE F; (g) Any organic peroxide which, in laboratory testing, neither detonates in the cavitated state nor deflagrates at all and shows no effect when heated under confinement nor any explosive power, provided that it is thermally stable (self-accelerating decomposition temperature is 60 [deg]C (140 [deg]F) or higher for a 50 kg (110 lb) package), and, for liquid mixtures, a diluent having a boiling point of not less than 150 [deg]C (302 [deg]F) is used for desensitization, shall be defined as organic peroxide TYPE G. If the organic peroxide is not thermally stable or a diluent having a boiling point less than 150 [deg]C (302 [deg]F) is used for desensitization, it shall be defined as organic peroxide TYPE F. B.15.3 Additional Classification Considerations B.15.3.1 For purposes of classification, the properties of organic peroxides shall be determined in accordance with test series A to H as described in Part II of the UN ST/SG/AC.10 (incorporated by reference; See Sec. 1910.6). B.15.3.2 Self-accelerating decomposition temperature (SADT) shall be determined in accordance with the UN ST/SG/AC.10 (incorporated by reference; See Sec. 1910.6), Part II, section 28. B.15.3.3 Mixtures of organic peroxides may be classified as the same type of organic peroxide as that of the most dangerous ingredient. However, as two stable ingredients can form a thermally less stable mixture, the SADT of the mixture shall be determined. B.16 CORROSIVE TO METALS B.16.1 Definition A chemical which is corrosive to metals means a chemical which by chemical action will materially damage, or even destroy, metals. B.16.2 Classification Criteria A chemical which is corrosive to metals shall be classified in a single category for this class, using the test in Part III, sub- section 37.4 of the UN ST/SG/AC.10 (incorporated by reference; See Sec. 1910.6), in accordance with Table B.16.1: Table B.16.1--Criteria for Chemicals Corrosive to Metal ---------------------------------------------------------------------------------------------------------------- Category Criteria ---------------------------------------------------------------------------------------------------------------- 1........................................... Corrosion rate on either steel or aluminium surfaces exceeding 6.25 mm per year at a test temperature of 55 [deg]C (131 [deg]F) when tested on both materials. ---------------------------------------------------------------------------------------------------------------- Note: Where an initial test on either steel or aluminium indicates the chemical being tested is corrosive, the follow-up test on the other metal is not necessary. B.16.3 Additional Classification Considerations The specimen to be used for the test shall be made of the following materials: (a) For the purposes of testing steel, steel types S235JR+CR (1.0037 resp.St 37-2), S275J2G3+CR (1.0144 resp.St 44-3), ISO 3574, Unified Numbering System (UNS) G 10200, or SAE 1020; (b) For the purposes of testing aluminium: Non-clad types 7075- T6 or AZ5GU-T6. APPENDIX C TO Sec. 1910.1200--ALLOCATION OF LABEL ELEMENTS (MANDATORY) C.1 The label for each hazardous chemical shall include the product identifier used on the safety data sheet. C.1.1 The labels on shipped containers shall also include the name, address, and telephone number of the chemical manufacturer, importer, or responsible party. C.2 The label for each hazardous chemical that is classified shall include the signal word, hazard statement(s), pictogram(s), and precautionary statement(s) specified in C.4 for each hazard class and associated hazard category, except as provided for in C.2.1 through C.2.4. C.2.1 Precedence of Hazard Information C.2.1.1 If the signal word "Danger" is included, the signal word "Warning" shall not appear; C.2.1.2 If the skull and crossbones pictogram is included, the exclamation mark pictogram shall not appear where it is used for acute toxicity; C.2.1.3 If the corrosive pictogram is included, the exclamation mark pictogram shall not appear where it is used for skin or eye irritation; C.2.1.4 If the health hazard pictogram is included for respiratory sensitization, the exclamation mark pictogram shall not appear where it is used for skin sensitization or for skin or eye irritation. C.2.2 Hazard Statement Text C.2.2.1 The text of all applicable hazard statements shall appear on the label, except as otherwise specified. The information in italics shall be included as part of the hazard statement as provided. For example: "causes damage to organs (state all organs affected) through prolonged or repeated exposure (state route of exposure if no other routes of exposure cause the hazard)". Hazard statements may be combined where appropriate to reduce the information on the label and improve readability, as long as all of the hazards are conveyed as required. C.2.2.2 If the chemical manufacturer, importer, or responsible party can demonstrate that all or part of the hazard statement is inappropriate to a specific substance or mixture, the corresponding statement may be omitted from the label. C.2.3 Pictograms C.2.3.1 Pictograms shall be in the shape of a square set at a point and shall include a black hazard symbol on a white background with a red frame sufficiently wide to be clearly visible. A square red frame set at a point without a hazard symbol is not a pictogram and is not permitted on the label. BILLING CODE 4510-26-P [GRAPHIC] [TIFF OMITTED] TR26MR12.069 [GRAPHIC] [TIFF OMITTED] TR26MR12.070 BILLING CODE 4510-26-C C.2.4 Precautionary Statement Text C.2.4.1 There are four types of precautionary statements presented, "prevention," "response," "storage," and "disposal." The core part of the precautionary statement is presented in bold print. This is the text, except as otherwise specified, that shall appear on the label. Where additional information is required, it is indicated in plain text. C.2.4.2 When a backslash or diagonal mark (/) appears in the precautionary statement text, it indicates that a choice has to be made between the separated phrases. In such cases, the chemical manufacturer, importer, or responsible party can choose the most appropriate phrase(s). For example, "Wear protective gloves/ protective clothing/eye protection/face protection" could read "wear eye protection". C.2.4.3 When three full stops (* * *) appear in the precautionary statement text, they indicate that all applicable conditions are not listed. For example, in "Use explosion-proof electrical/ventilating/lighting/* * */equipment", the use of "* * *" indicates that other equipment may need to be specified. In such cases, the chemical manufacturer, importer, or responsible party can choose the other conditions to be specified. C.2.4.4 When text in italics is used in a precautionary statement, this indicates specific conditions applying to the use or allocation of the precautionary statement. For example, "Use explosion-proof electrical/ventilating/lighting/* * */equipment" is only required for flammable solids "if dust clouds can occur". Text in italics is intended to be an explanatory, conditional note and is not intended to appear on the label. C.2.4.5 Where square brackets ([ ]) appear around text in a precautionary statement, this indicates that the text in square brackets is not appropriate in every case and should be used only in certain circumstances. In these cases, conditions for use explaining when the text should be used are provided. For example, one precautionary statement states: "[In case of inadequate ventilation] wear respiratory protection." This statement is given with the condition for use "- text in square brackets may be used if additional information is provided with the chemical at the point of use that explains what type of ventilation would be adequate for safe use". This means that, if additional information is provided with the chemical explaining what type of ventilation would be adequate for safe use, the text in square brackets should be used and the statement would read: "In case of inadequate ventilation wear respiratory protection." However, if the chemical is supplied without such ventilation information, the text in square brackets should not be used, and the precautionary statement should read: "Wear respiratory protection." C.2.4.6 Precautionary statements may be combined or consolidated to save label space and improve readability. For example, "Keep away from heat, sparks and open flame," "Store in a well- ventilated place" and "Keep cool" can be combined to read "Keep away from heat, sparks and open flame and store in a cool, well- ventilated place." C.2.4.7 In most cases, the precautionary statements are independent (e.g., the phrases for explosive hazards do not modify those related to certain health hazards, and products that are classified for both hazard classes shall bear appropriate precautionary statements for both). Where a chemical is classified for a number of hazards, and the precautionary statements are similar, the most stringent shall be included on the label (this will be applicable mainly to preventive measures). An order of precedence may be imposed by the chemical manufacturer, importer or responsible party in situations where phrases concern "Response." Rapid action may be crucial. For example, if a chemical is carcinogenic and acutely toxic, rapid action may be crucial, and first aid measures for acute toxicity will take precedence over those for long-term effects. In addition, medical attention to delayed health effects may be required in cases of incidental exposure, even if not associated with immediate symptoms of intoxication. C.2.4.8 If the chemical manufacturer, importer, or responsible party can demonstrate that a precautionary statement is inappropriate to a specific substance or mixture, the precautionary statement may be omitted from the label. C.3 Supplementary Hazard Information C.3.1 To ensure that non-standardized information does not lead to unnecessarily wide variation or undermine the required information, supplementary information on the label is limited to when it provides further detail and does not contradict or cast doubt on the validity of the standardized hazard information. C.3.2 Where the chemical manufacturer, importer, or distributor chooses to add supplementary information on the label, the placement of supplemental information shall not impede identification of information required by this section. C.3.3 Where an ingredient with unknown acute toxicity is used in a mixture at a concentration >=1%, and the mixture is not classified based on testing of the mixture as a whole, a statement that X% of the mixture consists of ingredient(s) of unknown acute toxicity is required on the label. BILLING CODE 4510-26-P [GRAPHIC] [TIFF OMITTED] TR26MR12.071 [GRAPHIC] [TIFF OMITTED] TR26MR12.072 [GRAPHIC] [TIFF OMITTED] TR26MR12.073 [GRAPHIC] [TIFF OMITTED] TR26MR12.074 [GRAPHIC] [TIFF OMITTED] TR26MR12.075 [GRAPHIC] [TIFF OMITTED] TR26MR12.076 [GRAPHIC] [TIFF OMITTED] TR26MR12.077 [GRAPHIC] [TIFF OMITTED] TR26MR12.078 [GRAPHIC] [TIFF OMITTED] TR26MR12.079 [GRAPHIC] [TIFF OMITTED] TR26MR12.080 [GRAPHIC] [TIFF OMITTED] TR26MR12.081 [GRAPHIC] [TIFF OMITTED] TR26MR12.082 [GRAPHIC] [TIFF OMITTED] TR26MR12.083 [GRAPHIC] [TIFF OMITTED] TR26MR12.084 [GRAPHIC] [TIFF OMITTED] TR26MR12.085 [GRAPHIC] [TIFF OMITTED] TR26MR12.086 [GRAPHIC] [TIFF OMITTED] TR26MR12.087 [GRAPHIC] [TIFF OMITTED] TR26MR12.088 [GRAPHIC] [TIFF OMITTED] TR26MR12.089 [GRAPHIC] [TIFF OMITTED] TR26MR12.090 [GRAPHIC] [TIFF OMITTED] TR26MR12.091 [GRAPHIC] [TIFF OMITTED] TR26MR12.092 [GRAPHIC] [TIFF OMITTED] TR26MR12.093 [GRAPHIC] [TIFF OMITTED] TR26MR12.094 [GRAPHIC] [TIFF OMITTED] TR26MR12.095 [GRAPHIC] [TIFF OMITTED] TR26MR12.096 [GRAPHIC] [TIFF OMITTED] TR26MR12.097 [GRAPHIC] [TIFF OMITTED] TR26MR12.098 [GRAPHIC] [TIFF OMITTED] TR26MR12.099 [GRAPHIC] [TIFF OMITTED] TR26MR12.100 [GRAPHIC] [TIFF OMITTED] TR26MR12.101 [GRAPHIC] [TIFF OMITTED] TR26MR12.102 [GRAPHIC] [TIFF OMITTED] TR26MR12.103 [GRAPHIC] [TIFF OMITTED] TR26MR12.104 [GRAPHIC] [TIFF OMITTED] TR26MR12.105 [GRAPHIC] [TIFF OMITTED] TR26MR12.106 [GRAPHIC] [TIFF OMITTED] TR26MR12.107 [GRAPHIC] [TIFF OMITTED] TR26MR12.108 [GRAPHIC] [TIFF OMITTED] TR26MR12.109 [GRAPHIC] [TIFF OMITTED] TR26MR12.110 [GRAPHIC] [TIFF OMITTED] TR26MR12.111 [GRAPHIC] [TIFF OMITTED] TR26MR12.112 [GRAPHIC] [TIFF OMITTED] TR26MR12.113 [GRAPHIC] [TIFF OMITTED] TR26MR12.114 [GRAPHIC] [TIFF OMITTED] TR26MR12.115 [GRAPHIC] [TIFF OMITTED] TR26MR12.116 [GRAPHIC] [TIFF OMITTED] TR26MR12.117 [GRAPHIC] [TIFF OMITTED] TR26MR12.118 [GRAPHIC] [TIFF OMITTED] TR26MR12.119 [GRAPHIC] [TIFF OMITTED] TR26MR12.120 [GRAPHIC] [TIFF OMITTED] TR26MR12.121 [GRAPHIC] [TIFF OMITTED] TR26MR12.122 [GRAPHIC] [TIFF OMITTED] TR26MR12.123 [GRAPHIC] [TIFF OMITTED] TR26MR12.124 [GRAPHIC] [TIFF OMITTED] TR26MR12.125 [GRAPHIC] [TIFF OMITTED] TR26MR12.126 [GRAPHIC] [TIFF OMITTED] TR26MR12.127 BILLING CODE 4510-26-C Appendix D to Sec. 1910.1200--Safety Data Sheets (Mandatory) A safety data sheet (SDS) shall include the information specified in Table D.1 under the section number and heading indicated for sections 1-11 and 16. If no relevant information is found for any given subheading within a section, the SDS shall clearly indicate that no applicable information is available. Sections 12-15 may be included in the SDS, but are not mandatory. Table D.1--Minimum Information for an SDS ------------------------------------------------------------------------ Heading Subheading ------------------------------------------------------------------------ 1. Identification............ (a) Product identifier used on the label; (b) Other means of identification; (c) Recommended use of the chemical and restrictions on use; (d) Name, address, and telephone number of the chemical manufacturer, importer, or other responsible party; (e) Emergency phone number. 2. Hazard(s) identification.. (a) Classification of the chemical in accordance with paragraph (d) of Sec. 1910.1200; (b) Signal word, hazard statement(s), symbol(s) and precautionary statement(s) in accordance with paragraph (f) of Sec. 1910.1200. (Hazard symbols may be provided as graphical reproductions in black and white or the name of the symbol, e.g., flame, skull and crossbones); (c) Describe any hazards not otherwise classified that have been identified during the classification process; (d) Where an ingredient with unknown acute toxicity is used in a mixture at a concentration >=1% and the mixture is not classified based on testing of the mixture as a whole, a statement that X% of the mixture consists of ingredient(s) of unknown acute toxicity is required. 3. Composition/information on Except as provided for in paragraph (i) ingredients. of Sec. 1910.1200 on trade secrets: For Substances (a) Chemical name; (b) Common name and synonyms; (c) CAS number and other unique identifiers; (d) Impurities and stabilizing additives which are themselves classified and which contribute to the classification of the substance. For Mixtures In addition to the information required for substances: (a) The chemical name and concentration (exact percentage) or concentration ranges of all ingredients which are classified as health hazards in accordance with paragraph (d) of Sec. 1910.1200 and (1) Are present above their cut-off/ concentration limits; or (2) Present a health risk below the cut- off/concentration limits. (b) The concentration (exact percentage) shall be specified unless a trade secret claim is made in accordance with paragraph (i) of Sec. 1910.1200, when there is batch-to-batch variability in the production of a mixture, or for a group of substantially similar mixtures (See A.0.5.1.2) with similar chemical composition. In these cases, concentration ranges may be used. For All Chemicals Where a Trade Secret is Claimed Where a trade secret is claimed in accordance with paragraph (i) of Sec. 1910.1200, a statement that the specific chemical identity and/or exact percentage (concentration) of composition has been withheld as a trade secret is required. 4. First-aid measures........ (a) Description of necessary measures, subdivided according to the different routes of exposure, i.e., inhalation, skin and eye contact, and ingestion; (b) Most important symptoms/effects, acute and delayed. (c) Indication of immediate medical attention and special treatment needed, if necessary. 5. Fire-fighting measures.... (a) Suitable (and unsuitable) extinguishing media. (b) Specific hazards arising from the chemical (e.g., nature of any hazardous combustion products). (c) Special protective equipment and precautions for fire-fighters. 6. Accidental release (a) Personal precautions, protective measures. equipment, and emergency procedures. (b) Methods and materials for containment and cleaning up. 7. Handling and storage...... (a) Precautions for safe handling. (b) Conditions for safe storage, including any incompatibilities. 8. Exposure controls/personal (a) OSHA permissible exposure limit protection. (PEL), American Conference of Governmental Industrial Hygienists (ACGIH) Threshold Limit Value (TLV), and any other exposure limit used or recommended by the chemical manufacturer, importer, or employer preparing the safety data sheet, where available. (b) Appropriate engineering controls. (c) Individual protection measures, such as personal protective equipment. 9. Physical and chemical (a) Appearance (physical state, color, properties. etc.); (b) Odor; (c) Odor threshold; (d) pH; (e) Melting point/freezing point; (f) Initial boiling point and boiling range; (g) Flash point; (h) Evaporation rate; (i) Flammability (solid, gas); (j) Upper/lower flammability or explosive limits; (k) Vapor pressure; (l) Vapor density; (m) Relative density; (n) Solubility(ies); (o) Partition coefficient: n-octanol/ water; (p) Auto-ignition temperature; (q) Decomposition temperature; (r) Viscosity. 10. Stability and reactivity. (a) Reactivity; (b) Chemical stability; (c) Possibility of hazardous reactions; (d) Conditions to avoid (e.g., static discharge, shock, or vibration); (e) Incompatible materials; (f) Hazardous decomposition products. 11. Toxicological information Description of the various toxicological (health) effects and the available data used to identify those effects, including: (a) Information on the likely routes of exposure (inhalation, ingestion, skin and eye contact); (b) Symptoms related to the physical, chemical and toxicological characteristics; (c) Delayed and immediate effects and also chronic effects from short- and long-term exposure; (d) Numerical measures of toxicity (such as acute toxicity estimates). (e) Whether the hazardous chemical is listed in the National Toxicology Program (NTP) Report on Carcinogens (latest edition) or has been found to be a potential carcinogen in the International Agency for Research on Cancer (IARC) Monographs (latest edition), or by OSHA. 12. Ecological information (a) Ecotoxicity (aquatic and terrestrial, (Non-mandatory). where available); (b) Persistence and degradability; (c) Bioaccumulative potential; (d) Mobility in soil; (e) Other adverse effects (such as hazardous to the ozone layer). 13. Disposal considerations Description of waste residues and (Non-mandatory). information on their safe handling and methods of disposal, including the disposal of any contaminated packaging. 14. Transport information (a) UN number; (Non-mandatory). (b) UN proper shipping name; (c) Transport hazard class(es); (d) Packing group, if applicable; (e) Environmental hazards (e.g., Marine pollutant (Yes/No)); (f) Transport in bulk (according to Annex II of MARPOL 73/78 and the IBC Code); (g) Special precautions which a user needs to be aware of, or needs to comply with, in connection with transport or conveyance either within or outside their premises. 15. Regulatory information Safety, health and environmental (Non-mandatory). regulations specific for the product in question. 16. Other information, The date of preparation of the SDS or the including date of last change to it. preparation or last revision. ------------------------------------------------------------------------ Appendix F to Sec. 1910.1200--Guidance for Hazard Classifications Re: Carcinogenicity (Non-Mandatory) The mandatory criteria for classification of a chemical for carcinogenicity under HCS (Sec. 1910.1200) are found in Appendix A.6 to this section. This non-mandatory Appendix provides additional guidance on hazard classification for carcinogenicity. Part A of Appendix F includes background guidance provided by GHS based on the Preamble of the International Agency for Research on Cancer (IARC) "Monographs on the Evaluation of Carcinogenic Risks to Humans" (2006). Part B provides IARC classification information. Part C provides background guidance from the National Toxicology Program (NTP) "Report on Carcinogens" (RoC), and Part D is a table that compares GHS carcinogen hazard categories to carcinogen classifications under IARC and NTP, allowing classifiers to be able to use information from IARC and NTP RoC carcinogen classifications to complete their classifications under the GHS, and thus the HCS. Part A: Background Guidance 1 --------------------------------------------------------------------------- \1\ The text of Appendix F, Part A, on the IARC Monographs, is paraphrased from the 2006 Preamble to the "Monographs on the Evaluation of Carcinogenic Risks to Humans"; the Classifier is referred to the full IARC Preamble for the complete text. The text is not part of the agreed GHS text on the harmonized system developed by the OECD Task Force-HCL. --------------------------------------------------------------------------- As noted in Footnote 6 of Appendix A.6. to this section, the GHS includes as guidance for classifiers information taken from the Preamble of the International Agency for Research on Cancer (IARC) "Monographs on the Evaluation of Carcinogenic Risks to Humans" (2006), providing guidance on the evaluation of the strength and evidence of carcinogenic risks to humans. This guidance also discusses some additional considerations in classification and an approach to analysis, rather than hard-and-fast rules. Part A is consistent with Appendix A.6, and should help in evaluating information to determine carcinogenicity. Carcinogenicity in humans: The evidence relevant to carcinogenicity from studies in humans is classified into one of the following categories: (a) Sufficient evidence of carcinogenicity: A causal relationship has been established between exposure to the agent and human cancer. That is, a positive relationship has been observed between the exposure and cancer in studies in which chance, bias and confounding could be ruled out with reasonable confidence. (b) Limited evidence of carcinogenicity: A positive association has been observed between exposure to the agent and cancer for which a causal interpretation is considered by the Working Group to be credible, but chance, bias or confounding could not be ruled out with reasonable confidence. In some instances, the above categories may be used to classify the degree of evidence related to carcinogenicity in specific organs or tissues. Carcinogenicity in experimental animals: The evidence relevant to carcinogenicity in experimental animals is classified into one of the following categories: (a) Sufficient evidence of carcinogenicity: A causal relationship has been established between the agent and an increased incidence of malignant neoplasms or of an appropriate combination of benign and malignant neoplasms in two or more species of animals or two or more independent studies in one species carried out at different times or in different laboratories or under different protocols. An increased incidence of tumors in both sexes of a single species in a well-conducted study, ideally conducted under Good Laboratory Practices, can also provide sufficient evidence. Exceptionally, a single study in one species and sex might be considered to provide sufficient evidence of carcinogenicity when malignant neoplasms occur to an unusual degree with regard to incidence, site, type of tumor or age at onset, or when there are strong findings of tumors at multiple sites. (a) Limited evidence of carcinogenicity: The data suggest a carcinogenic effect but are limited for making a definitive evaluation because, e.g. the evidence of carcinogenicity is restricted to a single experiment; there are unresolved questions regarding the adequacy of the design, conduct or interpretation of the studies; the agent increases the incidence only of benign neoplasms or lesions of uncertain neoplastic potential; or the evidence of carcinogenicity is restricted to studies that demonstrate only promoting activity in a narrow range of tissues or organs. Guidance on How To Consider Important Factors in Classification of Carcinogenicity (See Reference Section) The weight of evidence analysis called for in GHS and the HCS (Sec. 1910.1200) is an integrative approach that considers important factors in determining carcinogenic potential along with the strength of evidence analysis. The IPCS "Conceptual Framework for Evaluating a Mode of Action for Chemical Carcinogenesis" (2001), International Life Sciences Institute (ILSI) "Framework for Human Relevance Analysis of Information on Carcinogenic Modes of Action" (Meek, et al., 2003; Cohen et al., 2003, 2004), and Preamble to the IARC Monographs (2006; Section B.6. (Scientific Review and Evaluation; Evaluation and Rationale)) provide a basis for systematic assessments that may be performed in a consistent fashion. The IPCS also convened a panel in 2004 to further develop and clarify the human relevance framework. However, the above documents are not intended to dictate answers, nor provide lists of criteria to be checked off. Mode of Action Various documents on carcinogen assessment all note that mode of action in and of itself, or consideration of comparative metabolism, should be evaluated on a case-by-case basis and are part of an analytic evaluative approach. One must look closely at any mode of action in animal experiments, taking into consideration comparative toxicokinetics/toxicodynamics between the animal test species and humans to determine the relevance of the results to humans. This may lead to the possibility of discounting very specific effects of certain types of substances. Life stage-dependent effects on cellular differentiation may also lead to qualitative differences between animals and humans. Only if a mode of action of tumor development is conclusively determined not to be operative in humans may the carcinogenic evidence for that tumor be discounted. However, a weight of evidence evaluation for a substance calls for any other tumorigenic activity to be evaluated, as well. Responses in Multiple Animal Experiments Positive responses in several species add to the weight of evidence that a substance is a carcinogen. Taking into account all of the factors listed in A.6.2.5.2 and more, such chemicals with positive outcomes in two or more species would be provisionally considered to be classified in GHS Category 1B until human relevance of animal results are assessed in their entirety. It should be noted, however, that positive results for one species in at least two independent studies, or a single positive study showing unusually strong evidence of malignancy may also lead to Category 1B. Responses Are in One Sex or Both Sexes Any case of gender-specific tumors should be evaluated in light of the total tumorigenic response to the substance observed at other sites (multi-site responses or incidence above background) in determining the carcinogenic potential of the substance. If tumors are seen only in one sex of an animal species, the mode of action should be carefully evaluated to see if the response is consistent with the postulated mode of action. Effects seen only in one sex in a test species may be less convincing than effects seen in both sexes, unless there is a clear patho-physiological difference consistent with the mode of action to explain the single sex response. Confounding Effects of Excessive Toxicity or Localized Effects Tumors occurring only at excessive doses associated with severe toxicity generally have doubtful potential for carcinogenicity in humans. In addition, tumors occurring only at sites of contact and/ or only at excessive doses need to be carefully evaluated for human relevance for carcinogenic hazard. For example, forestomach tumors, following administration by gavage of an irritating or corrosive, non-mutagenic chemical, may be of questionable relevance. However, such determinations must be evaluated carefully in justifying the carcinogenic potential for humans; any occurrence of other tumors at distant sites must also be considered. Tumor Type, Reduced Tumor Latency Unusual tumor types or tumors occurring with reduced latency may add to the weight of evidence for the carcinogenic potential of a substance, even if the tumors are not statistically significant. Toxicokinetic behavior is normally assumed to be similar in animals and humans, at least from a qualitative perspective. On the other hand, certain tumor types in animals may be associated with toxicokinetics or toxicodynamics that are unique to the animal species tested and may not be predictive of carcinogenicity in humans. Very few such examples have been agreed internationally. However, one example is the lack of human relevance of kidney tumors in male rats associated with compounds causing [alpha]2u-globulin nephropathy (IARC, Scientific Publication N[deg] 147 \2\). Even when a particular tumor type may be discounted, expert judgment must be used in assessing the total tumor profile in any animal experiment. --------------------------------------------------------------------------- \2\ While most international agencies do not consider kidney tumors coincident with [alpha]2u-globulin nephropathy to be a predictor of risk in humans, this view is not universally held. (See: Doi et al., 2007). --------------------------------------------------------------------------- Part B: International Agency for Research on Cancer (IARC) 3 --------------------------------------------------------------------------- \3\ Preamble of the International Agency for Research on Cancer (IARC) "Monographs on the Evaluation of Carcinogenic Risks to Humans" (2006). --------------------------------------------------------------------------- IARC Carcinogen Classification Categories: Group 1: The agent is carcinogenic to humans This category is used when there is sufficient evidence of carcinogenicity in humans. Exceptionally, an agent may be placed in this category when evidence of carcinogenicity in humans is less than sufficient but there is sufficient evidence of carcinogenicity in experimental animals and strong evidence in exposed humans that the agent acts through a relevant mechanism of carcinogenicity. Group 2: This category includes agents for which, at one extreme, the degree of evidence of carcinogenicity in humans is almost sufficient, as well as those for which, at the other extreme, there are no human data but for which there is evidence of carcinogenicity in experimental animals. Agents are assigned to either Group 2A (probably carcinogenic to humans) or Group 2B (possibly carcinogenic to humans) on the basis of epidemiological and experimental evidence of carcinogenicity and mechanistic and other relevant data. The terms probably carcinogenic and possibly carcinogenic have no quantitative significance and are used simply as descriptors of different levels of evidence of human carcinogenicity, with probably carcinogenic signifying a higher level of evidence than possibly carcinogenic. Group 2A: The agent is probably carcinogenic to human. This category is used when there is limited evidence of carcinogenicity in humans and sufficient evidence of carcinogenicity in experimental animals. In some cases, an agent may be classified in this category when there is inadequate evidence of carcinogenicity in humans and sufficient evidence of carcinogenicity in experimental animals and strong evidence that the carcinogenesis is mediated by a mechanism that also operates in humans. Exceptionally, an agent may be classified in this category solely on the basis of limited evidence of carcinogenicity in humans. An agent may be assigned to this category if it clearly belongs, based on mechanistic considerations, to a class of agents for which one or more members have been classified in Group 1 or Group 2A. Group 2B: The agent is possibly carcinogenic to humans. This category is used for agents for which there is limited evidence of carcinogenicity in humans and less than sufficient evidence of carcinogenicity in experimental animals. It may also be used when there is inadequate evidence of carcinogenicity in humans but there is sufficient evidence of carcinogenicity in experimental animals. In some instances, an agent for which there is inadequate evidence of carcinogenicity in humans and less than sufficient evidence of carcinogenicity in experimental animals together with supporting evidence from mechanistic and other relevant data may be placed in this group. An agent may be classified in this category solely on the basis of strong evidence from mechanistic and other relevant data. Part C: National Toxicology Program (NTP), "Report on Carcinogens", Background Guidance NTP Listing Criteria \4\: --------------------------------------------------------------------------- \4\ See: http://ntp.niehs.nih.gov/go/15209. --------------------------------------------------------------------------- The criteria for listing an agent, substance, mixture, or exposure circumstance in the Report on Carcinogens (RoC) are as follows: Known To Be A Human Carcinogen: There is sufficient evidence of carcinogenicity from studies in humans \5\ that indicates a causal relationship between exposure to the agent, substance, or mixture, and human cancer. --------------------------------------------------------------------------- \5\ This evidence can include traditional cancer epidemiology studies, data from clinical studies, and/or data derived from the study of tissues or cells from humans exposed to the substance in question that can be useful for evaluating whether a relevant cancer mechanism is operating in people. --------------------------------------------------------------------------- Reasonably Anticipated To Be A Human Carcinogen: There is limited evidence of carcinogenicity from studies in humans that indicates that a causal interpretation is credible, but that alternative explanations, such as chance, bias, or confounding factors, could not adequately be excluded, or there is sufficient evidence of carcinogenicity from studies in experimental animals that indicates there is an increased incidence of malignant and/or a combination of malignant and benign tumors in multiple species or at multiple tissue sites, or by multiple routes of exposure, or to an unusual degree with regard to incidence, site, or type of tumor, or age at onset, or there is less than sufficient evidence of carcinogenicity in humans or laboratory animals; however, the agent, substance, or mixture belongs to a well-defined, structurally-related class of substances whose members are listed in a previous Report on Carcinogens as either known to be a human carcinogen or reasonably anticipated to be a human carcinogen, or there is convincing relevant information that the agent acts through mechanisms indicating it would likely cause cancer in humans. Conclusions regarding carcinogenicity in humans or experimental animals are based on scientific judgment, with consideration given to all relevant information. Relevant information includes, but is not limited to, dose response, route of exposure, chemical structure, metabolism, pharmacokinetics, sensitive sub-populations, genetic effects, or other data relating to mechanism of action or factors that may be unique to a given substance. For example, there may be substances for which there is evidence of carcinogenicity in laboratory animals, but there are compelling data indicating that the agent acts through mechanisms that do not operate in humans and would therefore not reasonably be anticipated to cause cancer in humans. Part D: Table Relating Approximate Equivalences Among IARC, NTP RoC, and GHS Carcinogenicity Classifications The following table may be used to perform hazard classifications for carcinogenicity under the HCS (Sec. 1910.1200). It relates the approximated GHS hazard categories for carcinogenicity to the classifications provided by IARC and NTP, as described in Parts B and C of this Appendix. Approximate Equivalences Among Carcinogen Classification Schemes ---------------------------------------------------------------------------------------------------------------- IARC GHS NTP RoC ---------------------------------------------------------------------------------------------------------------- Group 1................................. Category 1A................ Known. Group 2A................................ Category 1B................ Reasonably Anticipated. Group 2B................................ Category 2................. (See Note 1). ---------------------------------------------------------------------------------------------------------------- Note 1: 1. Limited evidence of carcinogenicity from studies in humans (corresponding to IARC 2A/GHS 1B); 2. Sufficient evidence of carcinogenicity from studies in experimental animals (again, essentially corresponding to IARC 2A/GHS 1B); 3. Less than sufficient evidence of carcinogenicity in humans or laboratory animals; however: c. The agent, substance, or mixture belongs to a well-defined, structurally-related class of substances whose members are listed in a previous RoC as either "Known" or "Reasonably Anticipated" to be a human carcinogen, or d. There is convincing relevant information that the agent acts through mechanisms indicating it would likely cause cancer in humans. *References Cohen, S.M., J. Klaunig, M.E. Meek, R.N. Hill, T. Pastoor, L. Lehman-McKeeman, J. Bucher, D.G. Longfellow, J. Seed, V. Dellarco, P. Fenner-Crisp, and D. Patton. 2004. Evaluating the human relevance of chemically induced animal tumors. Toxicol. Sci. 78(2):181-186. Cohen, S.M., M.E. Meek, J.E. Klaunig, D.E. Patton, P.A. Fenner- Crisp. 2003. The human relevance of information on carcinogenic modes of action: Overview. Crit. Rev. Toxicol. 33(6):581-9. Meek, M.E., J.R. Bucher, S.M. Cohen, V. Dellarco, R.N. Hill, L. Lehman-McKeeman, D.G. Longfellow, T. Pastoor, J. Seed, D.E. Patton. 2003. A framework for human relevance analysis of information on carcinogenic modes of action. Crit. Rev. Toxicol. 33(6):591-653. Sonich-Mullin, C., R. Fielder, J. Wiltse, K. Baetcke, J. Dempsey, P. Fenner-Crisp, D. Grant, M. Hartley, A. Knapp, D. Kroese, I. Mangelsdorf, E. Meek, J.M. Rice, and M. Younes. 2001. The conceptual framework for evaluating a mode of action for chemical carcinogenesis. Reg. Toxicol. Pharm. 34:146-152. International Programme on Chemical Safety Harmonization Group. 2004. Report of the First Meeting of the Cancer Working Group. World Health Organization. Report IPCS/HSC-CWG-1/04. Geneva. International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Human. Preambles to Volumes. World Health Organization. Lyon, France. Cohen, S.M., P.A. Fenner-Crisp, and D.E. Patton. 2003. Special Issue: Cancer Modes of Action and Human Relevance. Critical Reviews in Toxicology, R.O. McClellan, ed., Volume 33/Issue 6. CRC Press. Capen, C.C., E. Dybing, and J.D. Wilbourn. 1999. Species differences in thyroid, kidney and urinary bladder carcinogenesis. International Agency for Research on Cancer, Scientific Publication N[deg] 147. Doi, A.M., G. Hill, J. Seely, J.R. Hailey, G. Kissling, and J.R. Buchera. 2007. [alpha]2u-Globulin nephropathy and renal tumors in National Toxicology Program studies. Toxicol. Pathol. 35:533-540. * * * * * 0 33. Amend Sec. 1910.1450 as follows: 0 A. Remove the definitions of Combustible liquid, Compressed gas, Explosive, Flammable, Flashpoint, Organic peroxide, Oxidizer, Unstable (reactive), and Water-reactive from paragraph (b); 0 B. Revise the definitions of Hazardous chemical, Physical hazard, and Reproductive toxins in paragraph (b); 0 C. Add definitions of Health hazard and Mutagen in alphabetical order in paragraph (b); 0 D. In paragraphs (f)(3)(v), (h)(1) introductory text, (h)(1)(ii) and (h)(2)(iii), remove the phrases "Material Safety Data Sheets" and "material safety data sheets" and add in their place "safety data sheets"; 0 E. In Appendix A to Sec. 1910.1450, in the Table of Contents (item "G") remove "Material Safety Data Sheets" and add in its place "Safety Data Sheets"; 0 F. In Appendix A to Sec. 1910.1450, revise the heading "G. Material Safety Data Sheets" and revise the text following the heading. The revisions read as follows: Sec. 1910.1450 Occupational exposure to hazardous chemicals in laboratories. * * * * * (b) * * * Hazardous chemical means any chemical which is classified as health hazard or simple asphyxiant in accordance with the Hazard Communication Standard (Sec. 1910.1200). Health hazard means a chemical that is classified as posing one of the following hazardous effects: Acute toxicity (any route of exposure); skin corrosion or irritation; serious eye damage or eye irritation; respiratory or skin sensitization; germ cell mutagenicity; carcinogenity; reproductive toxicity; specific target organ toxicity (single or repeated exposure); aspiration hazard. The criteria for determining whether a chemical is classified as a health hazard are detailed in Appendix A of the Hazard Communication Standard (Sec. 1910.1200) and Sec. 1910.1200(c) (definition of "simple asphyxiant"). * * * * * Mutagen means chemicals that cause permanent changes in the amount or structure of the genetic material in a cell. Chemicals classified as mutagens in accordance with the Hazard Communication Standard (Sec. 1910.1200) shall be considered mutagens for purposes of this section. * * * * * Physical hazard means a chemical that is classified as posing one of the following hazardous effects: Explosive; flammable (gases, aerosols, liquids, or solids); oxidizer (liquid, solid, or gas); self reactive; pyrophoric (gas, liquid or solid); self-heating; organic peroxide; corrosive to metal; gas under pressure; in contact with water emits flammable gas; or combustible dust. The criteria for determining whether a chemical is classified as a physical hazard are in Appendix B of the Hazard Communication Standard (Sec. 1910.1200) and Sec. 1910.1200(c) (definitions of "combustible dust" and "pyrophoric gas"). * * * * * Reproductive toxins mean chemicals that affect the reproductive capabilities including adverse effects on sexual function and fertility in adult males and females, as well as adverse effects on the development of the offspring. Chemicals classified as reproductive toxins in accordance with the Hazard Communication Standard (Sec. 1910.1200) shall be considered reproductive toxins for purposes of this section. * * * * * Appendix A to Sec. 1910.1450--National Research Council Recommendations Concerning Chemical Hygiene in Laboratories (Non- Mandatory) * * * * * G. Safety Data Sheets Safety data sheets are presented in "Prudent Practices" for the chemicals listed below. (Asterisks denote that comprehensive safety data sheets are provided). * * * * * PART 1915--OCCUPATIONAL SAFETY AND HEALTH STANDARDS FOR SHIPYARD EMPLOYMENT 0 34. Revise the authority citation for part 1915 to read as follows: Authority: Section 41, Longshore and Harbor Workers' Compensation Act (33 U.S.C. 941); Sections. 4, 6, and 8 of the Occupational Safety and Health Act of 1970 (29 U.S.C. 653, 655, 657); Secretary of Labor's Order No. 12-71 (36 FR 8754), 8-76 (41 FR 25059), 9-83 (48 FR 35736), 1-90 (55 FR 9033), 6-96 (62 FR 111), 3- 2000 (65 FR 50017), 5-2002 (67 FR 65008), 5-2007 (72 FR 31160), 4- 2010 (75 FR 55355), or 1-2012 (77 FR 3912), as applicable; 29 CFR Part 1911. Section 1915.100 also issued under 49 U.S.C. 1801-1819 and 5 U.S.C. 553. Sections 1915.120 and 1915.152 of 29 CFR also issued under 29 CFR part 1911. Subpart Z--[Amended] 0 35. Revise Sec. 1915.1001 paragraphs (i)(3), (k)(7), and (k)(8) to read as follows: Sec. 1915.1001 Asbestos. * * * * * (i) * * * (3) The employer shall ensure that contaminated clothing is transported in sealed impermeable bags, or other closed, impermeable containers, and labeled in accordance with paragraph (k) of this section. * * * * * (k) * * * (7) Hazard communication. (i) Labels shall be affixed to all products containing asbestos and to all containers containing such products, including waste containers. Where feasible, installed asbestos products shall contain a visible label. (ii) General. The employer shall include asbestos in the program established to comply with the Hazard Communication Standard (HCS) (Sec. 1910.1200). The employer shall ensure that each employee has access to labels on containers of asbestos and safety data sheets, and is trained in accordance with the provisions of the HCS and paragraph (k)(9) of this section. The employer shall ensure that at least the following hazards are addressed: Cancer and lung effects. (iii) Labels. (A) The employer shall ensure that labels of bags or containers of protective clothing and equipment, scrap, waste, and debris containing asbestos fibers bear the following information: DANGER CONTAINS ASBESTOS FIBERS MAY CAUSE CANCER CAUSES DAMAGE TO LUNGS DO NOT BREATHE DUST AVOID CREATING DUST (B)(1) Prior to June 1, 2015, employers may include the following information on raw materials, mixtures or labels of bags or containers of protective clothing and equipment, scrap, waste, and debris containing asbestos fibers in lieu of the labeling requirements in paragraphs (k)(7)(ii) and (k)(7)(iii)(A) of this section: DANGER CONTAINS ASBESTOS FIBERS AVOID CREATING DUST CANCER AND LUNG DISEASE HAZARD (2) Labels shall also contain a warning statement against breathing asbestos fibers. (iv) The provisions for labels required in paragraph (k)(7) of this section do not apply where: (A) Asbestos fibers have been modified by a bonding agent, coating, binder, or other material, provided that the manufacturer can demonstrate that, during any reasonably foreseeable use, handling, storage, disposal, processing, or transportation, no airborne concentrations of asbestos fibers in excess of the permissible exposure limit and/or excursion limit will be released, or (B) Asbestos is present in a product in concentrations less than 1.0 percent. (8) Signs. (i) Warning signs that demarcate the regulated area shall be provided and displayed at each location where a regulated area is required to be established by paragraph (e) of this section. Signs shall be posted at such a distance from such a location that an employee may read the signs and take necessary protective steps before entering the area marked by the signs. (ii) The warning signs required by this paragraph (k)(8) shall bear the following legend: DANGER ASBESTOS MAY CAUSE CANCER CAUSES DAMAGE TO LUNGS AUTHORIZED PERSONNEL ONLY (iii) In addition, where the use of respirators and protective clothing is required in the regulated area under this section, the warning signs shall include the following: WEAR RESPIRATORY PROTECTION AND PROTECTIVE CLOTHING IN THIS AREA (iv) The employer shall ensure that employees working in and contiguous to regulated areas comprehend the warning signs required to be posted by paragraph (k)(8) of this section. Means to ensure employee comprehension may include the use of foreign languages, pictographs, and graphics. (v) When a building/vessel owner or employer identifies previously installed PACM and/or ACM, labels or signs shall be affixed or posted so that employees will be notified of what materials contain PACM and/ or ACM. The employer shall attach such labels in areas where they will clearly be noticed by employees who are likely to be exposed, such as at the entrance to mechanical room/areas. Signs required by paragraph (k)(6) of this section may be posted in lieu of labels, so long as they contain information required for labeling. The employer shall ensure, to the extent feasible, that employees who come in contact with these signs or labels can comprehend them. Means to ensure employee comprehension may include the use of foreign languages, pictographs, graphics, and awareness training. (vi) Prior to June 1, 2016, employers may use the following legend in lieu of that specified in paragraph (k)(8)(ii) of this section: DANGER ASBESTOS CANCER AND LUNG DISEASE HAZARD AUTHORIZED PERSONNEL ONLY (vii) Prior to June 1, 2016, employers may use the following legend in lieu of that specified in paragraph (k)(8)(iii) of this section: RESPIRATORS AND PROTECTIVE CLOTHING ARE REQUIRED IN THIS AREA * * * * * 0 36. Revise Sec. 1915.1026 paragraphs (g)(2)(iv) and (j)(1), to read as follows; Sec. 1915.1026 Chromium (VI). * * * * * (g) * * * (2) * * * (iv) The employer shall ensure that bags or containers of contaminated protective clothing or equipment that are removed from change rooms for laundering, cleaning, maintenance, or disposal are labeled in accordance with the requirements of the Hazard Communication Standard, Sec. 1910.1200. * * * * * (j) * * * (1) Hazard communication. The employer shall include chromium (VI) in the program established to comply with the Hazard Communication Standard (HCS) (Sec. 1910.1200). The employer shall ensure that each employee has access to labels on containers of chromium (VI) and safety data sheets, and is trained in accordance with the provisions of HCS and paragraph (j)(2) of this section. The employer shall ensure that at least the following hazards are addressed: Cancer; skin sensitization; and eye irritation. * * * * * PART 1926--SAFETY AND HEALTH REGULATIONS FOR CONSTRUCTION Subpart D--[Amended] 0 37. The authority citation for subpart D is revised to read as follows: Authority: Section 107 of the Contract Work Hours and Safety Standards Act (40 U.S.C. 3704); Sections 4, 6, and 8 of the Occupational Safety and Health Act of 1970 (29 U.S.C. 653, 655, and 657); and Secretary of Labor's Order No. 12-71 (36 FR 8754), 8-76 (41 FR 25059), 9-83 (48 FR 35736), 1-90 (55 FR 9033), 6-96 (62 FR 111), 3-2000 (65 FR 50017), 5-2002 (67 FR 65008), 5-2007 (72 FR 31159), 4-2010 (75 FR 55355), or 1-2012 (77 FR 3912) as applicable; and 29 CFR part 1911. Sections 1926.58, 1926.59, 1926.60, and 1926.65 also issued under 5 U.S.C. 553 and 29 CFR part 1911. Section 1926.61 also issued under 49 U.S.C. 1801-1819 and 6 U.S.C. 553. Section 1926.62 also issued under section 1031 of the Housing and Community Development Act of 1992 (42 U.S.C. 4853). Section 1926.65 also issued under section 126 of the Superfund Amendments and Reauthorization Act of 1986, as amended (reprinted at 29 U.S.C.A. 655 Note), and 5 U.S.C. 553. 0 38. Revise Sec. 1926.60 paragraphs (l)(1) and (l)(2) to read as follows: Sec. 1926.60 Methylenedianiline. * * * * * (l) * * * (1) Hazard communication. The employer shall include Methylenedianiline (MDA) in the program established to comply with the Hazard Communication Standard (HCS) (Sec. 1910.1200). The employer shall ensure that each employee has access to labels on containers of MDA and safety data sheets, and is trained in accordance with the provisions of HCS and paragraph (l)(3) of this section. The employer shall ensure that at least the following hazards are addressed: Cancer; liver effects; and skin sensitization. (2) Signs and labels-- (i) Signs. (A) The employer shall post and maintain legible signs demarcating regulated areas and entrances or access-ways to regulated areas that bear the following legend: DANGER MDA MAY CAUSE CANCER CAUSES DAMAGE TO THE LIVER RESPIRATORY PROTECTION AND PROTECTIVE CLOTHING MAY BE REQUIRED IN THIS AREA AUTHORIZED PERSONNEL ONLY (B) Prior to June 1, 2016, employers may use the following legend in lieu of that specified in paragraph (l)(2)(i)(A) of this section: DANGER MDA MAY CAUSE CANCER LIVER TOXIN AUTHORIZED PERSONNEL ONLY RESPIRATORS AND PROTECTIVE CLOTHING MAY BE REQUIRED TO BE WORN IN THIS AREA (ii) Labels. (A) The employer shall ensure that labels or other appropriate forms of warning are provided for containers of MDA within the workplace. The labels shall comply with the requirements of Sec. 1910.1200(f) and shall include at least the following information for pure MDA and mixtures containing MDA: DANGER CONTAINS MDA MAY CAUSE CANCER CAUSES DAMAGE TO THE LIVER (B) Prior to June 1, 2015, employers may include the following information workplace labels in lieu of the labeling requirements in paragraph (l)(2)(ii)(A) of this section: (1) For Pure MDA: DANGER CONTAINS MDA MAY CAUSE CANCER LIVER TOXIN (2) For mixtures containing MDA: DANGER CONTAINS MDA CONTAINS MATERIALS WHICH MAY CAUSE CANCER LIVER TOXIN * * * * * 0 39. Amend Sec. 1926.62 by revising paragraph (g)(2)(vii), the heading of paragraph (l), paragraph (l)(1)(i), and paragraph (m), and Appendix B to Sec. 1926.62 section XI, to read as follows: Sec. 1926.62 Lead. * * * * * (g) * * * (2) * * * (vii)(A) The employer shall ensure that the containers of contaminated protective clothing and equipment required by paragraph (g)(2)(v) of this section are labeled as follows: DANGER: CLOTHING AND EQUIPMENT CONTAMINATED WITH LEAD. MAY DAMAGE FERTILITY OR THE UNBORN CHILD. CAUSES DAMAGE TO THE CENTRAL NERVOUS SYSTEM. DO NOT EAT, DRINK OR SMOKE WHEN HANDLING. DO NOT REMOVE DUST BY BLOWING OR SHAKING. DISPOSE OF LEAD CONTAMINATED WASH WATER IN ACCORDANCE WITH APPLICABLE LOCAL, STATE, OR FEDERAL REGULATIONS. (B) Prior to June 1, 2015, employers may include the following information on bags or containers of contaminated protective clothing and equipment required by paragraph (g)(2)(v) in lieu of the labeling requirements in paragraph (g)(2)(vii)(A) of this section: Caution: Clothing contaminated with lead. Do not remove dust by blowing or shaking. Dispose of lead contaminated wash water in accordance with applicable local, state, or federal regulations. * * * * * (l) Communication of hazards. (1) * * * (i) Hazard communication. The employer shall include lead in the program established to comply with the Hazard Communication Standard (HCS) (Sec. 1910.1200). The employer shall ensure that each employee has access to labels on containers of lead and safety data sheets, and is trained in accordance with the provisions of HCS and paragraph (l) of this section. The employer shall ensure that at least the following hazards are addressed: (A) Reproductive/developmental toxicity; (B) Central nervous system effects; (C) Kidney effects; (D) Blood effects; and (E) Acute toxicity effects. * * * * * (m) Signs. (1) General. (i) The employer shall post the following warning signs in each work area where an employee's exposure to lead is above the PEL. DANGER LEAD WORK AREA MAY DAMAGE FERTILITY OR THE UNBORN CHILD CAUSES DAMAGE TO THE CENTRAL NERVOUS SYSTEM DO NOT EAT, DRINK OR SMOKE IN THIS AREA (ii) The employer shall ensure that no statement appears on or near any sign required by this paragraph (m) that contradicts or detracts from the meaning of the required sign. (iii) The employer shall ensure that signs required by this paragraph (m) are illuminated and cleaned as necessary so that the legend is readily visible. (iv) The employer may use signs required by other statutes, regulations or ordinances in addition to, or in combination with, signs required by this paragraph (m). (v) Prior to June 1, 2016, employers may use the following legend in lieu of that specified in paragraph (m)(1)(i) of this section: WARNING LEAD WORK AREA POISON NO SMOKING OR EATING * * * * * Appendix B to Sec. 1926.62--Employee Standard Summary * * * * * XI. Signs--Paragraph (M) The standard requires that the following warning sign be posted in work areas when the exposure to lead is above the PEL: DANGER LEAD WORK AREA MAY DAMAGE FERTILITY OR THE UNBORN CHILD CAUSES DAMAGE TO THE CENTRAL NERVOUS SYSTEM DO NOT EAT, DRINK OR SMOKE IN THIS AREA Prior to June 1, 2016, employers may use the following legend in lieu of that specified above: WARNING LEAD WORK AREA POISON NO SMOKING OR EATING * * * * * 0 40. Revise Sec. 1926.64 paragraphs (a)(1)(ii) introductory text, (a)(1)(ii)(B), and (d)(1)(vii), and the note following paragraph (d)(1)(vii), to read as follows: Sec. 1926.64 Process safety management of highly hazardous chemicals. * * * * * (a) * * * (1) * * * (ii) A process which involves a Category 1 flammable gas (as defined in Sec. 1910.1200(c)) or flammable liquid with a flashpoint below 100 [deg]F (37.8 [deg]C) on site in one location, in a quantity of 10,000 pounds (4535.9 kg) or more except for: * * * * * (B) Flammable liquids with a flashpoint below 100 [deg]F (37.8 [deg]C) stored in atmospheric tanks or transferred that are kept below their normal boiling point without benefit of chilling or refrigeration. * * * * * (d) * * * (1) * * * (vii) Hazardous effects of inadvertent mixing of different materials that could foreseeably occur. Note to paragraph (d)(1): Safety data sheets meeting the requirements of Sec. 1910.1200(g) may be used to comply with this requirement to the extent they contain the information required by this paragraph (d)(1). * * * * * 0 41. Amend Sec. 1926.65 paragraph (a)(3) by revising the definition of "Health hazard" to read as follows: Sec. 1926.65 Hazardous waste operations and emergency response. (a) * * * (3) * * * Health hazard means a chemical or a pathogen where acute or chronic health effects may occur in exposed employees. It also includes stress due to temperature extremes. The term health hazard includes chemicals that are classified in accordance with the Hazard Communication Standard, Sec. 1910.1200, as posing one of the following hazardous effects: acute toxicity (any route of exposure); skin corrosion or irritation; serious eye damage or eye irritation; respiratory or skin sensitization; germ cell mutagenicity; carcinogenicity; reproductive toxicity; specific target organ toxicity (single or repeated exposure); aspiration toxicity or simple asphyxiant. (See Appendix A to Sec. 1910.1200--Health Hazard Criteria (Mandatory) for the criteria for determining whether a chemical is classified as a health hazard.) * * * * * Subpart F--[Amended] 0 42. Revise the authority citation for subpart F to read as follows: Authority: Section 107 of the Contract Work Hours and Safety Standards Act (40 U.S.C. 3704); Sections 4, 6, and 8 of the Occupational Safety and Health Act of 1970 (29 U.S.C. 653, 655, 657); Secretary of Labor's Order No. 12-71 (36 FR 8754), 8-76 (41 FR 25059), 9-83 (48 FR 35736),1-90 (55 FR 9033), 6-96 (62 FR 111), 3- 2000 (62 FR 50017), 5-2002 (67 FR 650008), 5-2007 (72 FR 31159), 4- 2010 (75 FR 55355), or 1-2012 (77 FR 3912), as applicable; and 29 CFR part 1911. * * * * * 0 43. Amend Sec. 1926.152 as follows: 0 A. Revise the section heading; 0 B. Remove the words "and combustible" from the first sentence in paragraph (a)(1), the heading of paragraph (b), and paragraphs (b)(2) introductory text, (b)(4)(viii), (h) introductory text, and (h)(1); 0 C. Remove the words "or combustible" wherever it appears in paragraphs (a)(2), (b)(1), (b)(4)(iii), (b)(5), and (c)(3); 0 D. Remove the words "or combustible" in paragraphs (d) (the heading), (d)(1), (d)(4), (e)(1), (e)(3), (f)(2), (g)(1), and (g)(8); 0 E. Remove the words "or combustible" wherever it appears in paragraphs (i)(1)(i)(D) and (F), (i)(1)(iii)(D), (i)(2)(ii)(A), (D), and (F), (i)(2)(vii)(B)(2), (i)(4)(iv)(C), (i)(5)(vi)(A), (D), (G), (V) introductory text, and (i)(5)(vi)(V)(1); (j)(1)(i), (j)(2)(ii), (j)(5), and (k)(4); 0 F. Amend the fifth sentence of paragraph (b)(4)(vi) by adding the words "Category 1, 2, or 3" before the words "flammable liquids"; 0 G. Amend paragraphs (e)(2), (e)(5), (g)(7)(i), and (g)(7)(ii), by adding the words "Category 1, 2, or 3" before the words "flammable liquids" ; 0 H. Amend paragraphs (f)(1) and (f)(3) by removing the words "Flammable liquids" and adding in their place the words "Category 1, 2, or 3 flammable liquids"; 0 I. Revise paragraphs (b)(2)(iii), (b)(3), (h) introductory text, (i)(2)(iv)(F) and (G), (i)(2)(vi)(B), (i)(2)(viii)(E), (i)(3)(i), (i)(3)(iv)(A) and (C), (i)(3)(v)(D), and (i)(4)(iv)(E); 0 J. Revise Table F-19 and paragraph (k)(3)(iv). The revisions read as follows: Sec. 1926.152 Flammable liquids. * * * * * (b) * * * (2) * * * (iii) Cabinets shall be labeled in conspicuous lettering, "Flammable-Keep Away from Open Flames." (3) Not more than 60 gallons of Category 1, 2 and/or 3 flammable liquids or 120 gallons of Category 4 flammable liquids shall be stored in any one storage cabinet. Not more than three such cabinets may be located in a single storage area. Quantities in excess of this shall be stored in an inside storage room. * * * * * (h) Scope. This section applies to the handling, storage, and use of flammable liquids with a flashpoint at or below 199.4 [deg]F (93 [deg]C). This section does not apply to: * * * * * (i) * * * (2) * * * (iv) * * * (F) Tanks and pressure vessels storing Category 1 flammable liquids shall be equipped with venting devices that shall be normally closed except when venting to pressure or vacuum conditions. Tanks and pressure vessels storing Category 2 flammable liquids, or Category 3 flammable liquids with a flashpoint below 100 [deg]F (37.8 [deg]C), shall be equipped with venting devices that shall be normally closed except when venting under pressure or vacuum conditions, or with approved flame arresters. Exemption: Tanks of 3,000 bbls (barrels) (84 m(3)) capacity or less containing crude petroleum in crude-producing areas; and, outside aboveground atmospheric tanks under 1,000 gallons (3,785 L) capacity containing other than Category 1 flammable liquids may have open vents. (See paragraph (i)(2)(vi)(B) of this section.) (G) Flame arresters or venting devices required in paragraph (i)(2)(iv)(F) of this section may be omitted for Category 2 flammable liquids or Category 3 flammable liquids with a flashpoint below 100 [deg]F (37.8 [deg]C) where conditions are such that their use may, in case of obstruction, result in tank damage. * * * * * (vi) * * * (B) Where vent pipe outlets for tanks storing Category 1 or 2 flammable liquids, or Category 3 flammable liquids with a flashpoint below 100 [deg]F (37.8 [deg]C), are adjacent to buildings or public ways, they shall be located so that the vapors are released at a safe point outside of buildings and not less than 12 feet (3.658 m) above the adjacent ground level. In order to aid their dispersion, vapors shall be discharged upward or horizontally away from closely adjacent walls. Vent outlets shall be located so that flammable vapors will not be trapped by eaves or other obstructions and shall be at least 5 feet (1.52 m) from building openings. (viii) * * * (E) For Category 2 flammable liquids or Category 3 flammable liquids with a flashpoint below 100 [deg]F (37.8 [deg]C), other than crude oils, gasolines, and asphalts, the fill pipe shall be so designed and installed as to minimize the possibility of generating static electricity. A fill pipe entering the top of a tank shall terminate within 6 inches (15.24 cm) of the bottom of the tank and shall be installed to avoid excessive vibration. * * * * * (3) * * * (i) Location. Evacuation for underground storage tanks shall be made with due care to avoid undermining of foundations of existing structures. Underground tanks or tanks under buildings shall be so located with respect to existing building foundations and supports that the loads carried by the latter cannot be transmitted to the tank. The distance from any part of a tank storing Category 1 or 2 flammable liquids, or Category 3 flammable liquids with a flashpoint below 100 [deg]F (37.8 [deg]C), to the nearest wall of any basement or pit shall be not less than 1 foot (0.304 m), and to any property line that may be built upon, not less than 3 feet (0.912 m). The distance from any part of a tank storing Category 3 flammable liquids with a flashpoint at or above 100 [deg]F (37.8 [deg]C) or Category 4 flammable liquids to the nearest wall of any basement, pit or property line shall be not less than 1 foot (0.304 m). * * * * * (iv) * * * (A) Location and arrangement of vents for Category 1 or 2 flammable liquids, or Category 3 flammable liquids with a flashpoint below 100 [deg]F (37.8 [deg]C). Vent pipes from tanks storing Category 1 or 2 flammable liquids, or Category 3 flammable liquids with a flashpoint below 100 [deg]F (37.8 [deg]C), shall be so located that the discharge point is outside of buildings, higher than the fill pipe opening, and not less than 12 feet (3.658 m) above the adjacent ground level. Vent pipes shall discharge only upward in order to disperse vapors. Vent pipes 2 inches (5.08 cm) or less in nominal inside diameter shall not be obstructed by devices that will cause excessive back pressure. Vent pipe outlets shall be so located that flammable vapors will not enter building openings, or be trapped under eaves or other obstructions. If the vent pipe is less than 10 feet (3.04 m) in length, or greater than 2 inches (5.08 cm) in nominal inside diameter, the outlet shall be provided with a vacuum and pressure relief device or there shall be an approved flame arrester located in the vent line at the outlet or within the approved distance from the outlet. * * * * * (C) Location and arrangement of vents for Category 3 flammable liquids with a flashpoint at or above 100 [deg]F (37.8 [deg]C) or Category 4 flammable liquids. Vent pipes from tanks storing Category 3 flammable liquids with a flashpoint at or above 100 [deg]F (37.8 [deg]C) or Category 4 flammable liquids shall terminate outside of the building and higher than the fill pipe opening. Vent outlets shall be above normal snow level. They may be fitted with return bends, coarse screens or other devices to minimize ingress of foreign material. * * * * * (v) * * * (D) For Category 2 flammable liquids, or Category 3 flammable liquids with a flashpoint below 100 [deg]F (37.8 [deg]C), other than crude oils, gasolines, and asphalts, the fill pipe shall be so designed and installed as to minimize the possibility of generating static electricity by terminating within 6 inches (15.24 cm) of the bottom of the tank. * * * * * (4) * * * (iv) * * * (E) For Category 2 flammable liquids, or Category 3 flammable liquids with a flashpoint below 100 [deg]F (37.8 [deg]C), other than crude oils, gasolines, and asphalts, the fill pipe shall be so designed and installed as to minimize the possibility of generating static electricity by terminating within 6 inches (15.24 cm) of the bottom of the tank. * * * * * (k) * * * (3) * * * * * * * * BILLING CODE 4510-26-P [GRAPHIC] [TIFF OMITTED] TR26MR12.128 [GRAPHIC] [TIFF OMITTED] TR26MR12.129 BILLING CODE 4510-26-C (iv) Piping handling Category 1 or 2 flammable liquids, or Category 3 flammable liquids with a flashpoint below 100 [deg]F (37.8 [deg]C), shall be grounded to control stray currents. * * * * * 0 44. Amend Sec. 1926.155 as follows: 0 A. Remove and reserve paragraph (c); 0 B. Revise paragraphs (h) and (i)(1) and (2). The revisions read as follows: Sec. 1926.155 Definitions applicable to this subpart. * * * * * (h) Flammable liquid means any liquid having a vapor pressure not exceeding 40 pounds per square inch (absolute) at 100 [deg]F (37.8 [deg]C) and having a flashpoint at or below 199.4 [deg]F (93 [deg]C). Flammable liquids are divided into four categories as follows: (1) Category 1 shall include liquids having flashpoints below 73.4 [deg]F (23 [deg]C) and having a boiling point at or below 95 [deg]F (35 [deg]C). (2) Category 2 shall include liquids having flashpoints below 73.4 [deg]F (23 [deg]C) and having a boiling point above 95 [deg]F (35 [deg]C). (3) Category 3 shall include liquids having flashpoints at or above 73.4 [deg]F (23 [deg]C) and at or below 140 [deg]F (60 [deg]C). (4) Category 4 shall include liquids having flashpoints above 140 [deg]F (60 [deg]C) and at or below 199.4 [deg]F (93 [deg]C). (i) * * * (1) The flashpoint of liquids having a viscosity less than 45 Saybolt Universal Second(s) at 100 [deg]F (37.8 [deg]C) and a flashpoint below 175 [deg]F (79.4 [deg]C) shall be determined in accordance with the Standard Method of Test for Flash Point by the Tag Closed Tester, ASTM D-56-69 (incorporated by reference; See Sec. 1926.6), or an equivalent method as defined by Sec. 1910.1200 appendix B. (2) The flashpoints of liquids having a viscosity of 45 Saybolt Universal Second(s) or more at 175 [deg]F (79.4 [deg]C) or higher shall be determined in accordance with the Standard Method of Test for Flash Point by the Pensky Martens Closed Tester, ASTM D-93-69 (incorporated by reference; See Sec. 1926.6), or an equivalent method as defined by Sec. 1910.1200 appendix B. * * * * * Subpart Z--[Amended] 0 45. Revise the authority citation for subpart Z to read as follows: Authority: Section107 of the Contract Work Hours and Safety Standards Act (40 U.S.C. 3704); Sections 4, 6, and 8 of the Occupational Safety and Health Act of 1970 (29 U.S.C. 653, 655, 657); and Secretary of Labor's Order No. 12-71 (36 FR 8754), 8-76 (41 FR 25059), 9-83 (48 FR 35736), 1-90 (55 FR 9033), 6-96 (62 FR 111), 3-2000 (65 FR 50017), 5-2002 (67 FR 65008), 5-2007 (72 FR 31159), 4-2010 (75 FR 55355), or 1-2012 (77 FR 3912) as applicable; and 29 CFR part 1911. Section 1926.1102 not issued under 29 U.S.C. 655 or 29 CFR part 1911; also issued under 5 U.S.C. 553. 0 46. Amend Sec. 1926.1101 as follows: 0 A. Redesignate paragraph (k)(1) as (k)(1)(i) and add a new heading to paragraph (k)(1); 0 B. Add new paragraphs (k)(1)(ii), (k)(7)(ii)(C), (k)(7)(ii)(D), and (k)(8)(iv); 0 C. Amend paragraphs (k)(2)(i) and (k)(3)(i) by removing the references to "(k)(1)" and adding in their place "(k)(1)(i)"; 0 D. Revise paragraphs (k)(7)(ii)(A) and (B), and (k)(8)(ii) and (iii); The revisions read as follows: Sec. 1926.1101 Asbestos. * * * * * (k) * * * (1) Hazard communication. * * * * * (ii) The employer shall include asbestos in the program established to comply with the Hazard Communication Standard (HCS) (Sec. 1910.1200). The employer shall ensure that each employee has access to labels on containers of asbestos and safety data sheets, and is trained in accordance with the provisions of HCS and paragraphs (k)(9) and (10) of this section. The employer shall provide information on at least the following hazards: Cancer and lung effects. * * * * * (7) * * * (ii) * * * (A) The warning signs required by paragraph (k)(7) of this section shall bear the following information. DANGER ASBESTOS MAY CAUSE CANCER CAUSES DAMAGE TO LUNGS AUTHORIZED PERSONNEL ONLY (B) In addition, where the use of respirators and protective clothing is required in the regulated area under this section, the warning signs shall include the following: WEAR RESPIRATORY PROTECTION AND PROTECTIVE CLOTHING IN THIS AREA (C) Prior to June 1, 2016, employers may use the following legend in lieu of that specified in paragraph (k)(7)(ii)(A) of this section: DANGER ASBESTOS CANCER AND LUNG DISEASE HAZARD AUTHORIZED PERSONNEL ONLY (D) Prior to June 1, 2016, employers may use the following legend in lieu of that specified in paragraph (k)(7)(ii)(B) of this section: RESPIRATORS AND PROTECTIVE CLOTHING ARE REQUIRED IN THIS AREA * * * * * (8) * * * (ii) The employer shall ensure that such labels comply with paragraphs (k) of this section. (iii) The employer shall ensure that labels of bags or containers of protective clothing and equipment, scrap, waste, and debris containing asbestos fibers bear the following information: DANGER CONTAINS ASBESTOS FIBERS MAY CAUSE CANCER CAUSES DAMAGE TO LUNGS DO NOT BREATHE DUST AVOID CREATING DUST (iv) (A) Prior to June 1, 2015, employers may include the following information on raw materials, mixtures or labels of bags or containers of protective clothing and equipment, scrap, waste, and debris containing asbestos fibers in lieu of the labeling requirements in paragraphs (k)(8)(ii) and (k)(8)(iii) of this section: DANGER CONTAINS ASBESTOS FIBERS AVOID CREATING DUST CANCER AND LUNG DISEASE HAZARD (B) Labels shall also contain a warning statement against breathing asbestos fibers. * * * * * 0 47. Revise Sec. 1926.1126 paragraphs (g)(2)(iv) and (j)(1) to read as follows: Sec. 1926.1126 Chromium (VI). * * * * * (g) * * * (2) * * * (iv) The employer shall ensure that bags or containers of contaminated protective clothing or equipment that are removed from change rooms for laundering, cleaning, maintenance, or disposal shall be labeled in accordance with the requirements of the Hazard Communication Standard, Sec. 1910.1200. * * * * * (j) * * * (1) Hazard communication. The employer shall include chromium (VI) in the program established to comply with the Hazard Communication Standard (HCS) (Sec. 1910.1200). The employer shall ensure that each employee has access to labels on containers of chromium and safety data sheets, and is trained in accordance with the provisions of Sec. 1910.1200 and paragraph (j)(2) of this section. The employer shall provide information on at least the following hazards: Cancer; eye irritation; and skin sensitization. * * * * * 0 48. Revise Sec. 1926.1127 paragraphs (i)(2)(iv), (k)(7), and (m)(1), (m)(2), and (m)(3), to read as follows: Sec. 1926.1127 Cadmium. * * * * * (i) * * * (2) * * * (iv) The employer shall ensure that containers of contaminated protective clothing and equipment that are to be taken out of the change rooms or the workplace for laundering, cleaning, maintenance or disposal shall bear labels in accordance with paragraph (m)(3)(ii) of this section. (k) * * * (7) Waste, scrap, debris, bags, and containers, personal protective equipment and clothing contaminated with cadmium and consigned for disposal shall be collected and disposed of in sealed impermeable bags or other closed, impermeable containers. These bags and containers shall be labeled in accordance with paragraph (m)(3)(ii) of this section. * * * * * (m) * * * (1) Hazard communication. The employer shall include cadmium in the program established to comply with the Hazard Communication Standard (HCS) (Sec. 1910.1200). The employer shall ensure that each employee has access to labels on containers of cadmium and safety data sheets, and is trained in accordance with the provisions of HCS and paragraph (m)(4) of this section. The employer shall provide information on at least the following hazards: Cancer; lung effects; kidney effects; and acute toxicity effects. (2) Warning signs. (i) Warning signs shall be provided and displayed in regulated areas. In addition, warning signs shall be posted at all approaches to regulated areas so that an employee may read the signs and take necessary protective steps before entering the area. (ii) Warning signs required by paragraph (m)(2)(i) of this section shall bear the following legend: DANGER CADMIUM MAY CAUSE CANCER CAUSES DAMAGE TO LUNGS AND KIDNEYS WEAR RESPIRATORY PROTECTION IN THIS AREA AUTHORIZED PERSONNEL ONLY (iii) The employer shall ensure that signs required by this paragraph (m)(2) are illuminated, cleaned, and maintained as necessary so that the legend is readily visible. (iv) Prior to June 1, 2016, employers may use the following legend in lieu of that specified in paragraph (m)(2)(ii) of this section: DANGER CADMIUM CANCER HAZARD CAN CAUSE LUNG AND KIDNEY DISEASE AUTHORIZED PERSONNEL ONLY RESPIRATORS REQUIRED IN THIS AREA (3) Warning labels. (i) Shipping and storage containers containing cadmium or cadmium compounds shall bear appropriate warning labels, as specified in paragraph (m)(1) of this section. (ii) The warning labels for containers of cadmium-contaminated protective clothing, equipment, waste, scrap, or debris shall include at least the following information: DANGER CONTAINS CADMIUM MAY CAUSE CANCER CAUSES DAMAGE TO LUNGS AND KIDNEYS AVOID CREATING DUST (iii) Where feasible, installed cadmium products shall have a visible label or other indication that cadmium is present. (iv) Prior to June 1, 2015, employers may include the following information on shipping and storage containers containing cadmium, cadmium compounds, or cadmium-contaminated clothing, equipment, waste, scrap, or debris in lieu of the labeling requirements specified in paragraphs (m)(3)(i) and (m)(3)(ii) of this section: DANGER CONTAINS CADMIUM CANCER HAZARD AVOID CREATING DUST CAN CAUSE LUNG AND KIDNEY DISEASE * * * * * [FR Doc. 2012-4826 Filed 3-20-12; 11:15 am] BILLING CODE 4510-26-P

