Hospitals eTool
Hospital-wide Hazards » Biological Hazards – Infectious Diseases
Workers in hospital settings may be exposed to a variety of common and emerging infectious disease hazards, particularly if proper infection prevention and control measures are not implemented in the workplace. Examples of infectious disease hazards include seasonal and pandemic influenza; norovirus; Ebola; Middle East Respiratory Syndrome (MERS), tuberculosis, methicillin-resistant Staphylococcus Aureus (MRSA), and other potentially drug-resistant organisms.
Infectious diseases are caused by agents that are transmissible through one or more different routes, including the contact, droplet, airborne, and bloodborne routes. The transmission of infectious agents through the bloodborne route—a specific subset of contact transmission—is defined in the Bloodborne Pathogens (BBP) standard, 29 CFR 1910.1030 (See the Bloodborne Pathogens section below).
An effective infection control program normally relies upon a multi-layered and overlapping strategy of engineering, administrative and work practice controls, and PPE. It is OSHA's intent in this eTool to highlight some – not all – of the controls that would be necessary to the development and implementation of an effective program. Implementing the controls highlighted here alone will not typically protect workers from infection hazards.
Follow standard and transmission-based precautions to prevent worker infections (see also the OSHA page: Worker protections against occupational exposure to infectious diseases). Early identification and isolation of sources of infectious agents (including sick patients), proper hand hygiene, worker training, effective engineering and administrative controls, safer work practices, and appropriate personal protective equipment (PPE), among other controls, help reduce the risk of transmission of infectious agents to workers.
Employers must comply with the BBP standard to the extent that there is "occupational exposure" (i.e., to the extent workers should reasonably anticipate contact with blood or other potentially infectious materials (OPIM) that may result from the performance of duties). Employers must also comply with the PPE Standard, 29 CFR 1910 Subpart I, and the OSH Act's General Duty Clause, 29 U.S.C. 654(a)(1), to protect their workers from infectious disease hazards. The General Duty Clause requires each employer to "furnish to each of his employees employment and a place of employment which are free from recognized hazards that are causing or are likely to cause death or serious physical harm to his employees."
OSHA provides agent-specific guidance for a variety of pathogens that workers in hospital settings may encounter. See OSHA's Safety and Health Topics Pages for Biological Agents and Bloodborne Pathogens and Needlestick Prevention for additional information.
Hazard
Exposure of workers to pathogens, e.g., Methicillin-resistant Staphylococcus aureus (MRSA). Nosocomial infections are infections that occur from exposure to infectious organisms found in facilities such as hospitals. Healthcare workers are exposed to these organisms and can then become infected and/or become carriers and spread the infection to other staff and patients.
Requirements under OSHA's Bloodborne Pathogens Standard, 29 CFR 1910.1030
- Establish Universal Precautions:
- Universal Precautions: An approach to infection control that treats all human blood and certain human bodily fluids as if they were infectious for HIV and HBV or other bloodborne pathogens. [29 CFR 1910.1030(b)]
- The requirement to use Universal Precautions in the Bloodborne Pathogens Standard [29 CFR 1910.1030(d)(1)] means implementing the precautions required by the standard (e.g., engineering and work practice controls, appropriate PPE such as gloves, masks, and gowns) whenever there is exposure to blood or OPIM (or in some cases other body fluids).
- Alternative concepts in infection control are called Body Substance Isolation and Standard Precautions. These alternatives define all body fluids and substances as infectious, and OSHA permits the implementation of these approaches, as an alternative to universal precautions, provided that facilities utilizing them adhere to all other provisions of the Bloodborne Pathogens Standard.
- All procedures involving blood or other potentially infectious materials shall be performed in such a manner as to minimize splashing, spraying, spattering, and generation of droplets of these substances. [29 CFR 1910.1030(d)(2)(xi)]
- Ensure that employees use appropriate personal protective equipment (PPE), (e.g., gloves, gowns, face masks), as required by the standard, when there is anticipated blood or OPIM exposure. [29 CFR 1910.1030(d)(2)(i), 29 CFR 1910.1030(d)(3)(ii)]
- Provide handwashing facilities (see definition in standard) which are readily accessible to employees. Ensure that employees wash their hands immediately or as soon as feasible after removal of gloves or other personal protective equipment, and that employees wash hands and any other skin with soap and water, or flush mucous membranes with water immediately or as soon as feasible following contact of such body areas with blood or other potentially infectious materials. [29 CFR 1910.1030(d)(2)(iii), 29 CFR 1910.1030(d)(2)(v), 29 CFR 1910.1030(d)(2)(vi)]
- If there has been no occupational exposure to blood or OPIM,(as defined in 29 CFR 1910.1030(b)), the use of alcohol-based hand cleansers, as described in the 2002 CDC “Guideline for Hand Hygiene in Health-Care Settings,” would be appropriate.
OSHA requires employers to ensure that the biosafety officer or other responsible person conducts an exposure determination to determine the exposure of workers to blood or OPIM throughout the hospital setting. [29 CFR 1910.1030(c)(2)(i)].
Recognized Controls and Work Practices (For Use Even When the Bloodborne Pathogens Standard Does Not Apply)
- Use appropriate handwashing.
- According to the CDC, appropriate handwashing results in a reduced incidence of both nosocomial and community infections. Guidelines from national and international infection prevention and control organizations have repeatedly acknowledged that handwashing is the single most important procedure for preventing infections (Canadian Centre for Occupational Health & Safety; CDC). Despite this, compliance with handwashing by healthcare providers is poor (CDC).
- Handwashing with plain soap (detergents) effectively removes most transient microbial flora. The components of good handwashing include using an adequate amount of soap, rubbing the hands together to create some friction, and rinsing under running water. The mechanical action of washing and drying removes most of the transient bacteria present.
- Washing hands as promptly and thoroughly as possible between patient contacts and after contact with blood, body fluids, secretions, excretions, and equipment or articles contaminated by them is an important component of infection control and isolation precautions.
- Wear gloves. In addition to handwashing, gloves play an important role in reducing the risks of transmission of microorganisms. Gloves are worn for three important reasons in hospitals:
- First, gloves are worn to provide a protective barrier to prevent contamination of the workers' hands.
- Second, gloves are worn to reduce the likelihood that microorganisms present on the hands of personnel will be transmitted to patients during invasive patient-care procedures, as well as patient-care procedures that involve touching a patient's non-intact skin.
- Third, gloves are worn to reduce the likelihood that hands of personnel contaminated with microorganisms from a patient or object can transmit these microorganisms to another patient. To prevent this, gloves must be changed between patient contacts and hands washed after gloves are removed.
- Wearing gloves does not replace the need for handwashing, because gloves may have small, non-apparent defects or may be torn during use, and hands can become contaminated during removal of gloves.
- Failure to change gloves between patient contacts is an infection control hazard.
Additional Information
- Healthcare-associated Infections. Centers for Disease Control and Prevention (CDC).
- Infection Control. Centers for Disease Control and Prevention (CDC).
- Healthcare. OSHA Safety and Health Topics Page.
- Hand Hygiene in Healthcare Settings. Centers for Disease Control and Prevention (CDC).
- Guideline for Hand Hygiene in Health-Care Settings. Centers for Disease Control and Prevention (CDC), Morbidity and Mortality Weekly Report (MMWR) 51(RR16);1-44, (October 25, 2002).
- Hand Hygiene When and How. World Health Organization (WHO).
- Larsen EL. APIC guideline for handwashing and hand antisepsis in health care settings. Am J Infect Control, (Aug 1995); 23(4):251-69. Association for Professionals in Infection Control and Epidemiology (APIC) guideline.
- Guidance for the Selection and Use of Personal Protective Equipment (PPE) in Healthcare Settings. Centers for Disease Control and Prevention (CDC).
In this module, OSHA provides additional guidance specifically for:
Bloodborne pathogens are pathogenic microorganisms present in human blood that can cause disease in humans. These pathogens include, but are not limited to, Hepatitis B Virus (HBV), Hepatitis C Virus (HCV), Human Immunodeficiency Virus (HIV) and Viral Hemorrhagic Fevers (e.g. Ebola). [29 CFR 1910.1030(b)]
Hazard
Exposure of workers to blood or other potentially infectious materials (OPIM) in the hospital.
Requirements under OSHA's Bloodborne Pathogens Standard, 29 CFR 1910.1030
The Bloodborne Pathogens Standard requires precautions when there is occupational exposure to blood or OPIM (as defined by the standard). Under the standard, OPIM means (1) the following human body fluids: semen, vaginal secretions, cerebrospinal fluid, synovial fluid, pleural fluid, pericardial fluid, peritoneal fluid, amniotic fluid, saliva in dental procedures, any body fluid that is visibly contaminated with blood, and all body fluids in situations where it is difficult or impossible to differentiate between body fluids; (2) any unfixed tissue or organ (other than intact skin) from a human (living or dead); and (3) HIV-containing cell or tissue cultures, organ cultures, and HIV- or HBV-containing culture medium or other solutions; and blood, organs, or other tissues from experimental animals infected with HIV or HBV.
OSHA requires employers to:
- Ensure that the biosafety officer or other responsible person conducts an exposure determination to determine the exposure of workers to blood or OPIM throughout the hospital setting. [29 CFR 1910.1030(c)(2)(i)].
- Use engineering and work practice controls
- Engineering (e.g., engineered safer needle devices and sharps) and work practice controls must be the primary means to eliminate or minimize exposure to bloodborne pathogens. Where engineering controls, including SESIP (Sharps with Engineered Sharps Injury Protection) will eliminate or minimize employee exposure, either by removing or isolating the hazard, they must be used. [29 CFR 1910.1030(d)(2)(i)]
- All procedures involving blood or other potentially infectious materials shall be performed in such a manner as to minimize splashing, spraying, spattering, and generation of droplets of these substances. [29 CFR 1910.1030(d)(2)(xi)]
- Ensure that employees use appropriate personal protective equipment (PPE), (e.g., gloves, gowns, face masks), as required by the standard, when there is anticipated blood or OPIM exposure. [29 CFR 1910.1030(d)(2)(i), 29 CFR 1910.1030(d)(3)(ii)]
- Provide handwashing facilities (see definition in standard) which are readily accessible to employees. Ensure that employees wash their hands immediately or as soon as feasible after removal of gloves or other personal protective equipment, and that employees wash hands and any other skin with soap and water, or flush mucous membranes with water immediately or as soon as feasible following contact of such body areas with blood or other potentially infectious materials. [29 CFR 1910.1030(d)(2)(iii), 29 CFR 1910.1030(d)(2)(v), 29 CFR 1910.1030(d)(2)(vi)]
- If there has been no occupational exposure to blood or OPIM, (as defined in 29 CFR 1910.1030(b)), the use of alcohol-based hand cleansers, as described in the 2002 CDC "Guideline for Hand Hygiene in Health-Care Settings," would be appropriate.
- Ensure that employees discard contaminated needles and other sharp instruments into appropriate containers immediately or as soon as feasible after use. [29 CFR 1910.1030(d)(4)(iii)(A)(1)]
- Establish a written Exposure Control Plan (ECP). The ECP must contain, among other elements, annual documentation of consideration and implementation of appropriate commercially available and effective safer medical devices designed to eliminate or minimize exposure to blood and OPIM. Solicit input from non-managerial employees responsible for direct patient care who are potentially exposed to injuries from contaminated sharps in the identification, evaluation, and selection of effective engineering and work practice controls. Document the solicitation in the Exposure Control Plan. Any change to the use of engineering controls (and any other change affecting exposure) must also be reflected in the ECP. [29 CFR 1910.1030(c)(1)]
- Establish Universal Precautions:
- Universal Precautions: An approach to infection control that treats all human blood and certain human bodily fluids as if they were infectious for HIV and HBV or other bloodborne pathogens. [29 CFR 1910.1030(b)]
- The requirement to use Universal Precautions in the Bloodborne Pathogens Standard [29 CFR 1910.1030(d)(1)] means implementing the precautions required by the standard (e.g., engineering and work practice controls, appropriate PPE such as gloves, masks, and gowns) whenever there is exposure to blood or OPIM (or in some cases other body fluids).
- Alternative concepts in infection control are called Body Substance Isolation and Standard Precautions. These alternatives define all body fluids and substances as infectious, and OSHA permits the implementation of these approaches, as an alternative to universal precautions, provided that facilities utilizing them adhere to all other provisions of the Bloodborne Pathogens Standard.
- Establish and maintain a sharps injury log for recording needlestick/sharps injuries. [29 CFR 1910.1030(h)(5)] The confidentiality of the injured employee must be protected.
- Make immediately available to an exposed employee a confidential medical evaluation and follow-up, after a report of a needlestick injury or other exposure incident. [29 CFR 1910.1030(f)(3)]
- Obtain and provide the employee with a copy of the evaluating healthcare professional’s written opinion for post-exposure evaluation and follow-up within 15 days of the completion of the evaluation, as required by the Bloodborne Pathogens Standard. [29 CFR 1910.1030(f)(5)] OSHA provides in this eTool a non-mandatory sample form to help employers comply with this requirement: Written Opinion for Post-Exposure Evaluation.
- Offer the Hepatitis B vaccination, and ensure vaccination is performed by or under the supervision of a licensed physician or by or under the supervision of another licensed healthcare professional, at no cost, to all employees who have occupational exposure to blood or OPIM, as required by the Bloodborne Pathogens Standard. [29 CFR 1910.1030(f)(1), 29 CFR 1910.1030(f)(2)]
- Provide BBP training to employees at the time of initial assignment where occupational exposure may take place and at least annually thereafter. [29 CFR 1910.1030(g)(2)]
Additional Information
- 29 CFR 1910.1030, Bloodborne Pathogens Standard. OSHA.
- Most frequently asked questions concerning the bloodborne pathogens standard. OSHA.
- Quick Reference Guide to the Bloodborne Pathogens Standard. OSHA.
- Enforcement Procedures for the Occupational Exposure to Bloodborne Pathogens. OSHA Directive CPL 02-02-069, (November 27, 2001). Contains information concerning OSHA's general enforcement policy and procedures for conducting inspections and issuing citations related to bloodborne pathogen hazards.
- Inspection Guidance for Inpatient Healthcare Settings. (June 25, 2015). OSHA memorandum establishing guidance for inspections conducted in inpatient healthcare settings.
- Bloodborne Pathogens and Needlestick Prevention. OSHA Safety and Health Topics Page.
- Bloodborne Pathogen Exposure Incidents. OSHA Fact Sheet.
- OSHA's Bloodborne Pathogens Standard. OSHA Fact Sheet.
- Personal Protective Equipment (PPE) Reduces Exposure to Bloodborne Pathogens. OSHA Fact Sheet.
- Model Plans and Programs for the OSHA Bloodborne Pathogens and Hazard Communications Standards. OSHA Publication 3186, (2003).
- Updated U.S. Public Health Service Guidelines for the Management of Occupational Exposures to HIV and Recommendations for Postexposure Prophylaxis. Centers for Disease Control and Prevention (CDC), (September 25, 2013).
- Updated U.S. Public Health Service Guidelines for the Management of Occupational Exposures to HBV, HCV, and HIV and Recommendations for Postexposure Prophylaxis. Centers for Disease Control and Prevention (CDC), (June 29, 2001).
- Workbook for Designing, Implementing, and Evaluating a Sharps Injury Prevention Program. Centers for Disease Control and Prevention (CDC).
- FDA, NIOSH and OSHA Joint Safety Communication: Blunt-Tip Surgical Suture Needles Reduce Needlestick Injuries and the Risk of Subsequent Bloodborne Pathogen Transmission to Surgical Personnel. (May 30, 2012).
- Protecting Yourself When Handling Contaminated Sharps. OSHA Fact Sheet.
- Boyce, JM et al. (2002). Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Society for Healthcare Epidemiology of America/Association for Professionals in Infection Control/Infectious Diseases Society of America. MMWR, 51(RR16): 1-45.
- Acceptable use of antiseptic-hand cleansers for bloodborne pathogen decontamination and as an appropriate handwashing practice. [1910.1030; 1910.1030(d)(2)(v); 1910.1030(d)(2)(vi)]. OSHA Letter of Interpretation, (March 31, 2003).
- Biological Agents. OSHA Safety and Health Topics Page.
- Hepatitis B Vaccination Protection. OSHA Fact Sheet.
- Ebola. OSHA Safety and Health Topics Page.
Also see Hospital-wide Hazards - Universal Precautions.
Universal Precautions
Hazard
Increased risk of infection associated with employee exposure to bloodborne pathogens from blood and other potentially infectious materials (OPIM) because of a failure to follow Universal Precautions.
- Some infections that can be transmitted through exposure to blood and body fluids include:
- HIV, Hepatitis B, Hepatitis C, and Ebola.
Requirements under OSHA's Bloodborne Pathogens Standard, 29 CFR 1910.1030
OSHA requires employers to:
- Establish Universal Precautions:
- Universal Precautions: An approach to infection control that treats all human blood and certain human bodily fluids as if they were infectious for HIV and HBV or other bloodborne pathogens. [29 CFR 1910.1030(b)]
- The requirement to use Universal Precautions in the Bloodborne Pathogens Standard [29 CFR 1910.1030(d)(1)] means implementing the precautions required by the standard (e.g., engineering and work practice controls, appropriate PPE such as gloves, masks, and gowns) whenever there is exposure to blood or OPIM (or in some cases other body fluids).
- Alternative approaches to Universal Precautions in infection control are called Body Substance Isolation and Standard Precautions. These alternatives define all body fluids and substances as infectious, and OSHA permits the implementation of these approaches, as an alternative to universal precautions, provided that facilities utilizing them adhere to all other provisions of the Bloodborne Pathogens Standard.
OSHA requires employers to ensure that the biosafety officer or other responsible person conducts an exposure determination to determine the exposure of workers to blood or OPIM throughout the hospital setting. [29 CFR 1910.1030(c)(2)(i)].
The CDC recommends Standard Precautions for the care of all patients, regardless of their diagnosis or presumed infection status.
- Standard Precautions apply to (1) blood; (2) all body fluids, secretions, and excretions, except sweat, regardless of whether or not they contain visible blood; (3) non-intact skin; and (4) mucous membranes. Standard precautions are designed to reduce the risk of transmission of microorganisms from both recognized and unrecognized sources of infection in hospitals.
- Standard precautions include, among other protections, the use of hand hygiene, appropriate personal protective equipment (such as gloves, gowns, masks) and eye protection whenever touching or exposure to patients' body fluids is anticipated.
- Transmission-Based Precautions (i.e., Contact Precautions, Droplet Precautions, and Airborne Precautions), are recommended for further protection beyond Standard Precautions to interrupt transmission of pathogens in hospitals.
- Transmission-based precautions can be used for patients who are known or suspected to be infected or colonized with epidemiologically important pathogens that can be transmitted by airborne or droplet transmission or by contact with dry skin or contaminated surfaces. Use these precautions in addition to standard precautions.
- Contact Precautions are used for infections, such as herpes simplex virus, which are spread by skin-to-skin contact or contact with contaminated surfaces.
- Droplet Precautions are used for infections, such as influenza, which are spread in large droplets by coughing, talking, or sneezing.
- Airborne Precautions are used for infections, such as chicken pox, which are spread in small particles in the air
- Transmission-based precautions can be used for patients who are known or suspected to be infected or colonized with epidemiologically important pathogens that can be transmitted by airborne or droplet transmission or by contact with dry skin or contaminated surfaces. Use these precautions in addition to standard precautions.
- Contact Precautions, Droplet Precautions and Airborne Precautions may be combined for diseases that have multiple routes of transmission. When used either singularly or in combination, they are to be used in addition to Standard Precautions.
Additional Information
- 2007 Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings. Centers for Disease Control and Prevention (CDC), (2007).
- Guideline for infection control in health care personnel, 1998. Centers for Disease Control and Prevention (CDC), (1998).
- Bloodborne Infectious Diseases HIV/AIDS, Hepatitis B, and Hepatitis C. National Institute for Occupational Safety and Health (NIOSH) Workplace Safety and Health Topic.
- Some infections that can be transmitted through exposure to blood and body fluids include:
Needlestick/Sharps Injuries
Needlesticks and other sharps-related injuries that expose workers to bloodborne pathogens (BBP) continue to be a significant hazard for hospital employees. BBP are pathogenic microorganisms that are present in human blood and can cause disease in humans. These pathogens include Human Immunodeficiency Virus (HIV), Hepatitis B Virus (HBV), Hepatitis C Virus (HCV), and others.
Any worker handling sharp devices or equipment, such as scalpels, sutures, hypodermic needles, blood collection devices, or phlebotomy devices, is at risk. Nursing staff are most frequently injured. Exposure Prevention Information Network (EPINet) data show that needlestick injuries occur most frequently in the operating room and in patient rooms.
According to the Centers for Disease Control and Prevention (CDC), about 385,000 sharps injuries occur annually among healthcare workers in hospital employees.
Hazard
Exposure to blood and OPIM from needlestick injuries due to:
- Unsafe needle devices
- Improper handling and disposal of needles
Requirements under Bloodborne Pathogens Standard, 29 CFR 1910.1030
- Use safer needle devices and needleless devices to decrease needlestick or other sharps exposures. See Safer Needle Devices section. In addition, proper handling and disposal of needles and other sharps according to the Bloodborne Pathogens Standard can help prevent needlestick injuries.
- Use engineering controls (e.g., safer needle devices) and work practice controls (e.g., altering the way a task is performed to reduce chance of injury such as prohibiting recapping of needles by a two-handed technique) to eliminate or minimize exposure to bloodborne pathogens [29 CFR 1910.1030(c), 29 CFR 1910.1030(d)].
- Examples of engineering controls
- Safer needle/other sharps devices.
- Blunt-tip suture needles.
- Needleless IV connectors.
- Proper containers for sharps.
- Non-glass capillary tubes. OSHA, FDA and NIOSH warn healthcare workers about the hazards from breakage of glass capillary tubes and sanction using non-glass capillary tubes.
- Examples of engineering controls
- The risk of sharps injuries must be eliminated or minimized. Follow the applicable provisions of the standard. For example:
- Contaminated needles and other contaminated sharps shall be discarded immediately or as soon as feasible into appropriate containers, as required by the standard. [29 CFR 1910.1030(d)(4)(iii)(A)(1)]
- Sharps containers shall be easily accessible and located as close as is feasible to the immediate location where sharps are used or can be reasonably anticipated to be found. [29 CFR 1910.1030(d)(4)(iii)(A)(2)(i)]
- Contaminated needles and other contaminated sharps must not be bent, recapped, or removed except as noted in paragraphs 29 CFR 1910.1030(d)(2)(vii)(A) and (d)(2)(vii)(B). Shearing or breaking contaminated needles is prohibited. [29 CFR 1910.1030(d)(2)(vii)]
- Do not allow sharps containers to overfill. Replace sharps containers routinely. [29 CFR 1910.1030(d)(4)(iii)(A)(2)(iii)]
Sharps Containers

[29 CFR 1910.1030(d)(4)(iii)(A)(1), 29 CFR 1910.1030(d)(4)(iii)(A)(2)] Sharps containers must be:
- Closable, puncture-resistant, and leak-proof on sides and bottom.
- Easily accessible, maintained upright, and replaced routinely and not be allowed to overfill.
- Labeled or color-coded according to 29 CFR 1910.1030(g)(1)(i). Labels must:
- Include the required biohazard symbol.
- Be fluorescent orange or orange-red or predominately so, with lettering and symbols in a contrasting color. [29 CFR 1910.1030(g)(1)(i)(C)]
Additional Information
- Workbook for Designing, Implementing, and Evaluating a Sharps Injury Prevention Program. Centers for Disease Control and Prevention (CDC).
- CDC: Emergency Sharps Information also provides immediate access to treatment protocols following blood exposures involving HIV, HBV and HCV, including the Clinicians' Post Exposure Prophylaxis Hotline (PEPline) at 1-888-448-4911.
- Bloodborne Infectious Diseases: HIV/AIDS, Hepatitis B, and Hepatitis C. National Institute for Occupational Safety and Health (NIOSH) Workplace Safety and Health Topic.
- B. Stringer, T. Haines. "Hands-free technique: preventing occupational exposure during surgery." Journal of Perioperative Practice 16.10(October 2006): 495.
- Blunt-Tip Surgical Suture Needles Reduce Needlestick Injuries and the Risk of Subsequent Bloodborne Pathogen Transmission to Surgical Personnel: FDA, NIOSH and OSHA Joint Safety Communication. U.S. Food and Drug Administration (FDA), (May 30, 2012).
- Use of Blunt-Tip Suture Needles to Decrease Percutaneous Injuries to Surgical Personnel (PDF). OSHA and the National Institute for Occupational Safety and Health (NIOSH) Publication No. 2008-101, (October 2007). Supersedes NIOSH Publication 2007–132.
- Engineering Controls for Contaminated Sharps. OSHA Letter of Interpretation, (June 3, 2005).
Other Sharps Injury
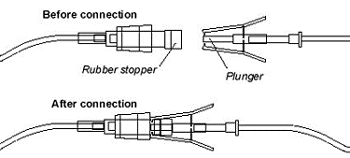
Take precautions with respect to all contaminated sharps, not just needles. "Contaminated Sharps" means any contaminated object that can penetrate the skin including, but not limited to, needles, scalpels, broken glass, broken capillary tubes, and exposed ends of dental wires. [29 CFR 1910.1030(b)]
Hazard
Exposure to blood and OPIM through contaminated sharps, such as:
- I.V. Connectors that use needle systems.
- Broken Capillary Tubes.
Follow requirements of the Bloodborne Standard (29 CFR 1910.1030) with respect to all "contaminated sharps." For example:
- Implement engineering and work practice controls to eliminate or minimize exposure to bloodborne pathogens [29 CFR 1910.1030(c), 29 CFR 1910.1030(d)].
- Dispose of contaminated sharps immediately or as soon as feasible into appropriate containers, as required by the standard. [29 CFR 1910.1030(d)(4)(iii)(A)(1)]
Additional Information
- Workbook for Designing, Implementing, and Evaluating a Sharps Injury Prevention Program. Centers for Disease Control and Prevention (CDC).
- Protecting Yourself When Handling Contaminated Sharps. OSHA Fact Sheet.
- CDC: Emergency Sharps Information also provides immediate access to treatment protocols following blood exposures involving HIV, HBV and HCV, including the Clinicians' Post Exposure Prophylaxis Hotline (PEPline) at 1-888-448-4911.
- Most frequently asked questions concerning the bloodborne pathogens standard. OSHA.
- Quick Reference Guide to the Bloodborne Pathogens Standard. OSHA.
- Bloodborne Pathogens and Needlestick Prevention. OSHA Safety and Health Topics Page.
- Engineering Controls for Contaminated Sharps. OSHA Letter of Interpretation, (June 3, 2005).
- Also see Hospital-wide Hazards - Needlesticks/Sharps Injuries.
Safer Needle Devices
Hazard
According to the Centers for Disease Control and Prevention (CDC), approximately 385,000 sharps-related injuries occur annually among healthcare workers in hospitals. It has been estimated about half or more of sharps injuries go unreported (CDC). Most reported sharps injuries involve nursing staff, but laboratory staff, physicians, housekeepers, and other healthcare workers are also injured.
Controls
These injuries in the hospital setting could be prevented by using safer needle devices and following proper handling and disposal procedures. Safer needle devices, such as those that use a self-sheathing needle, have safer design features and built-in safety control devices to help prevent injuries before, during, and after use.
Following the Needlestick Safety and Prevention Act of 2000, sharps-related injuries in nonsurgical hospital settings decreased 31.6% during 2001–2006 (CDC). However, injuries in surgical settings increased 6.5% in the same period, where adoption of safety devices was limited compared to nonsurgical settings (Jagger, et al., 2010).
Under the Bloodborne Pathogens Standard, employers must ensure employees use engineering controls, including safer needle devices, to eliminate or minimize occupational exposure.
- There are different types of safety features that are available for safer needle devices, such as:
- Needleless devices
- Passive safety features, which remain in effect before, during and after use.
- Devices with passive safety features typically have integrated safety design, a safety feature that is built in as an integral part of the device and cannot be removed. This design feature is usually preferred.
- Active devices, which require the worker to activate the safety mechanism.
- Active devices typically have accessory safety devices, safety features that are external to the device and must be carried to, or be temporarily or permanently fixed to, the point of use. This design is dependent on employee compliance and is less desirable.
- The most desirable characteristics in safety devices include:
- A device that is needleless.
- The safety feature is an integral part of the device.
- The device is easy to use and practical.
- The device performs reliably.
- The safety feature cannot be deactivated and remains protective through disposal.
- The device works effectively and reliably, is acceptable to the healthcare worker, and does not adversely affect patient care.
- The Food and Drug Administration (FDA) is responsible for clearing medical devices for marketing in the U.S. It recommends sharps injury prevention devices that:
- Can be activated with a single-handed technique, allowing the user’s hands to remain behind the exposed sharp.
- Are easy to tell whether the sharps injury prevention feature is activated.
- Fully retracts the sharp within the housing of the device; and
- Cannot be deactivated once activated and remains in effect through disposal to protect users and trash handlers, and for environmental safety.
- Examples of safety device designs
- Needleless connector systems: Needleless connectors for I.V. delivery systems (e.g., blunt cannula for use with pre-pierced ports and valved connectors that accept tapered or luer ends of I.V. tubing) (Figure 1).
- Self-sheathing safety feature: Sliding needle shields attached to disposable syringes and vacuum tube holders (Figures 2A and 2B).
- Disposable scalpels with safety features such as a sliding blade shield (Figure 6).
- Retractable technology: Needles or sharps that retract into a syringe, vacuum tube holder, or back into the device.
- Self-blunting technology: Self-blunting phlebotomy and winged steel "butterfly" needles (a blunt cannula seated inside the phlebotomy needle is advanced beyond the needle tip before the needle is withdrawn from the vein) (Figure 4), (Figure 5).
- Hinged safety feature: Hinged or sliding shields attached to phlebotomy needles, winged steel needles, and blood gas needles (Figure 7).
- There are different types of safety features that are available for safer needle devices, such as:
| Example Devices with Safety Features | |
|---|---|
 **  |
Self Re-sheathing Needles
As seen in this animation, initially the sleeve is located over the barrel of the syringe with the needle exposed for use.
|
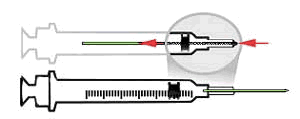 |
Syringe with Retractable Needles
As seen in this animation, after the needle is used, an extra push on the plunger retracts the needle into the syringe, removing the hazard of needle exposure.
|
 |
Blunt-Tipped Blood Drawing Needles
As seen in this animation, after blood is drawn, a push on the collection tube moves the blunt tip needle forward through the needle and past the sharp needle point. The blunt point tip of this needle can be activated before it is removed from the vein or artery.
|
 |
Winged Steel Needles
As seen in this animation, after placement, the third wing is rotated to flat position which blunts the needle point before it is removed from the patient.
|
 |
Re-sheathing Disposable Scalpels
As seen in this animation, single-use disposable scalpels have a shield that is advanced forward over the blade after use, containing and removing the hazard.
|
 |
"Add on" Safety Feature
As seen in this animation, hinged or sliding shields attached to phlebotomy needles, winged steel needles, and blood gas needles, act as an "add on" safety feature.
|
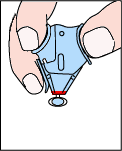 |
Retracting Finger Prick Lancets
As seen in this animation, this single use lancet retracts automatically after use, containing and removing the hazard.
|
(These drawings are presented for educational purposes only and do not imply endorsement of a particular product).
† Please note: These safety devices lock in place and do not reset in actual use situations. The animation resets for viewer convenience only.
According to NIOSH's Preventing Needlestick Injuries in Health Care Settings, the process for selecting and evaluating needle devices with safety features includes these steps:
- Form a multidisciplinary team that includes workers to:
- Develop, implement, and evaluate a plan to reduce needlestick injuries in the institution; and
- Evaluate needle devices with safety features. Solicit input from non-managerial employees responsible for direct patient care who are potentially exposed to injuries from contaminated sharps in the identification, evaluation, and selection of effective engineering and work practice controls. Document the solicitation in the Exposure Control Plan. [29 CFR 1910.1030(c)(1)-(2)]
- Identify priorities based on assessments of how needlestick injuries are occurring, patterns of device use in the institution, and local and national data on injury and disease transmission trends.
- Identify injury patterns and accident analysis to determine if other training, procedures, or safer needle devices need to be used to prevent future accidents.
- Give the highest priority to needle devices with safety features that will have the greatest impact on preventing occupational infection (such as hollow-bore needles used in veins and arteries).
- When selecting a safer device, identify its intended scope of use in the healthcare facility and any special techniques or design factors that will influence its safety, efficiency and user acceptability. Identify published, Internet, or other sources of data on the safety and overall performance of the device.
- Conduct a product evaluation, making sure that the participants represent the variety of eventual product users. The following steps will contribute to a successful product evaluation:
- Train healthcare workers in the correct use of the new device.
- Establish clear criteria and measures to evaluate the device for both healthcare worker safety and patient care.
- Conduct onsite follow-up to obtain informal feedback, identify problems, and provide further guidance.
- Monitor the use of a new device after it is implemented to determine the need for more training, solicit informal feedback on healthcare worker experience with the device (e.g., using a suggestion box), and identify possible adverse effects of the device on patient care.
Additional Information
- Bloodborne Pathogens and Needlestick Prevention. OSHA Safety and Health Topics Page.
- Preventing Needlestick Injuries in Health Care Settings. U.S. Department of Health and Human Services (DHHS), National Institute for Occupational Safety and Health (NIOSH) Publication No. 2000-108, (November 1999).
- What Every Worker Should Know – How to Protect Yourself from Needlestick Injuries. U.S. Department of Health and Human Services (DHHS), National Institute for Occupational Safety and Health (NIOSH) Publication No. 2000-135, (July 1997).
- Blunt-Tip Surgical Suture Needles Reduce Needlestick Injuries and the Risk of Subsequent Bloodborne Pathogen Transmission to Surgical Personnel: FDA, NIOSH and OSHA Joint Safety Communication. U.S. Food and Drug Administration (FDA), (May 30, 2012).
- Use of Blunt-Tip Suture Needles to Decrease Percutaneous Injuries to Surgical Personnel (PDF). OSHA and the National Institute for Occupational Safety and Health (NIOSH) Publication No. 2008-101, (October 2007). Supersedes NIOSH Publication 2007–132.
- CDC: Emergency Sharps Information also provides immediate access to treatment protocols following blood exposures involving HIV, HBV and HCV, including the Clinicians' Post Exposure Prophylaxis Hotline (PEPline) at 1-888-448-4911.
- Form a multidisciplinary team that includes workers to:
Recordkeeping requirements for Bloodborne Pathogens
Employers are required to establish and maintain both medical and training records in accordance with the Bloodborne Pathogens Standard, 29 CFR 1910.1030(h) and the Access to Employee Exposure and Medical Records Rule, 29 CFR 1910.1020]
- Medical records under the Bloodborne Pathogens Standard [29 CFR 1910.1030(h)(1)] must be preserved and maintained for each employee with occupational exposure to bloodborne pathogens for at least the duration of employment plus 30 years, in accordance with 29 CFR 1910.1020 and must include:
- The employee's name;
- A copy of the employee’s hepatitis B vaccination status including the dates of all vaccinations and any medical records related to the employee's ability to receive vaccinations (see 29 CFR 1910.1030(f)(2));
- A copy of all results of examinations, medical testing, and follow-up procedures conducted, following a report of an exposure incident, in connection with any post-exposure evaluations and follow-up procedures required by 29 CFR 1910.1030(f)(3);
- With respect to each exposure incident, a description of the exposed employee's duties as they relate to the exposure incident, documentation of the route(s) of exposure and circumstances under which exposure occurred, and results of the source individual's blood testing, if available (see 29 CFR 1910.1030(f)(4)(ii)(B), (C), and (D)); and
- The employer’s copy of the written opinion of the healthcare professional as required by 29 CFR 1910.1030(f)(5).
- Medical records under the Bloodborne Pathogens Standard [29 CFR 1910.1030(h)(1)] must also:
- be kept confidential;
- not be disclosed or reported to any person without written consent of the employee, except as required by the standard or by law);
- be maintained by a physician, nurse, or other healthcare professional, or technician [29 CFR 1910.1020(c)(6)], and kept separate from other personnel records;
- Training records under the Bloodborne Pathogens Standard [29 CFR 1910.1030(h)(2)] must be preserved and maintained for 3 years from the date the training occurred, and must include:
- The names and job titles of all persons attending the training sessions;
- The dates and contents or a summary of the training sessions; and
- The names and qualifications of all trainers.
- Whenever an employer is ceasing to do business and there is no successor employer to receive and maintain the records subject to this standard, the employer must notify affected current employees of their rights of access to records at least three (3) months prior to the cessation of the employer's business [29 CFR 1910.1020(h)(2)].
- The employer must ensure that all medical records required to be maintained under the Bloodborne Pathogens Standard [29 CFR 1910.1030(h)(3)(iii)] be made available upon request to the subject employee, to anyone having written consent of the subject employee, the Director of the National Institute for Occupational Safety and Health (NIOSH), and the Assistant Secretary of Labor for Occupational Safety and Health in accordance with 29 CFR 1910.1020.
- The employer must ensure that all employee training records required to be maintained under the Bloodborne Pathogens Standard [29 CFR 1910.1030(h)(3)(ii)] be made available upon request to employees, employee representatives, the Director of the National Institute for Occupational Safety and Health (NIOSH), and the Assistant Secretary of Labor for Occupational Safety and Health.
- The employer must establish and maintain a sharps injury log for the recording of percutaneous injuries from contaminated sharps [29 CFR 1910.1030(h)(5)]. The information in the sharps injury log must be recorded and maintained in such manner as to protect the confidentiality of the injured employee. The log must contain, at a minimum:
- The type and brand of device involved in the incident [29 CFR 1910.1030(h)(5)(i)(A)];
- The department or work area where the exposure incident occurred. [29 CFR 1910.1030(h)(5)(i)(B)]; and
- An explanation of how the incident occurred. [29 CFR 1910.1030(h)(5)(i)(C)]
NOTE: If an employer is not required to maintain a log of occupational injuries and illnesses under 29 CFR Part 1904, then this requirement does not apply [29 CFR 1910.1030(h)(5)(ii)].
Additional Information
- OSHA Injury and Illness Recordkeeping and Reporting Requirements. OSHA Safety and Health Topics Page.
- Medical records under the Bloodborne Pathogens Standard [29 CFR 1910.1030(h)(1)] must be preserved and maintained for each employee with occupational exposure to bloodborne pathogens for at least the duration of employment plus 30 years, in accordance with 29 CFR 1910.1020 and must include:
Personal Protective Equipment
Requirements under Bloodborne Pathogens Standard, 29 CFR 1910.1030
- Ensure that employees use appropriate personal protective equipment (PPE), (e.g., gloves, gowns, face masks), as required by the standard, when there is anticipated blood or OPIM exposure. [29 CFR 1910.1030(d)(2)(i), 29 CFR 1910.1030(d)(3)(ii)]
- Ensure that employees wear gloves when hand contact with blood, mucous membranes, OPIM, or non-intact skin can be reasonably anticipated, and when performing vascular access procedures, as required by the standard, or when handling or touching contaminated items or surfaces. [29 CFR 1910.1030(d)(3)(ix)]
- Ensure that employees remove all PPE before leaving the work area [29 CFR 1910.1030(d)(3)(vii)], and that, when PPE is removed, it is placed in an appropriately designated area or container for storage, washing, decontamination or disposal [29 CFR 1910.1030(d)(3)(viii)].
Requirements under OSHA's Respiratory Protection Standard, 29 CFR 1910.134
- When relevant hazards are present, employers must comply with applicable provisions of OSHA's Respiratory Protection standard, 29 CFR 1910.134, including:
- Following the hierarchy of controls required by the standard.
- Providing appropriate respirators when such equipment is necessary to protect the health of the employee(s).
- Establishing and implementing a written respiratory protective program that includes the elements required by 29 CFR 1910.134(c).
Additional Information
- Personal Protective Equipment (PPE) Reduces Exposure to Bloodborne Pathogens. OSHA Fact Sheet.
- Hospital Respiratory Protection Program Toolkit: Resources for Respirator Program Administrators, (May 2015).
- OSHA's Personal Protective Equipment Standard, 29 CFR 1910 Subpart I.
- Respiratory Protection Program Training and Resources. American Association of Occupational Health Nurses.
- Ensure that employees use appropriate personal protective equipment (PPE), (e.g., gloves, gowns, face masks), as required by the standard, when there is anticipated blood or OPIM exposure. [29 CFR 1910.1030(d)(2)(i), 29 CFR 1910.1030(d)(3)(ii)]
Labeling and Signs
Requirements under Bloodborne Pathogens Standard, 29 CFR 1910.1030
- Biohazardous Waste Containers. Regulated waste (such as contaminated sharps; I.V. tubing which is used to administer blood and could release blood if compacted in a waste container; and personal protective equipment that is being discarded and is caked with dried blood or OPIM and is capable of releasing these materials during handling, see definition in standard) must be disposed of into biohazardous waste containers that are appropriately labeled or color-coded in accordance with 29 CFR 1910.1030(g)(1)(i). [29 CFR 1910.1030(d)(4)(iii)]
- Biohazard Labels. Warning labels must be affixed to containers of regulated waste, refrigerators and freezers containing blood or OPIM, and other containers used to store, transport or ship blood or other potentially infectious materials, except as provided in 29 CFR 1910.1030(g)(1)(i)(E), (F) and (G). [29 CFR 1910.1030(g)(1)(i)(A)
- Labels required by the standard must:
- Bear the biohazard symbol and legend, as shown in the standard. [29 CFR 1910.1030(g)(1)(i)(B)]
- Be fluorescent orange or orange-red, or predominantly so, with lettering and symbols in a contrasting color. [29 CFR 1910.1030(g)(1)(i)(C)]
- Labels required by the standard must:
- Exceptions under 29 CFR 1910.1030(g)(1)(i)(E), (F) and (G).
- Red bags or red containers may be substituted for labels. [29 CFR 1910.1030(g)(1)(i)(E)
- Containers of blood, blood components, or blood products that are labeled as to their contents and have been released for transfusion or other clinical use are exempted from the labeling requirements of paragraph (g). [29 CFR 1910.1030(g)(1)(i)(F)
- Individual containers of blood or other potentially infectious materials that are placed in a labeled container for storage, transport, shipment or disposal are also exempted from the labeling requirement. [29 CFR 1910.1030(g)(1)(i)(G)]
Hepatitis B Virus (HBV)
Hepatitis is an inflammation of the liver that can lead to liver damage and death. The annual number of occupational infections has decreased 95% since the Hepatitis B vaccine became available in 1982 (CDC, 2003). Hepatitis B vaccine immunizations and compliance with other provisions of OSHA's Bloodborne Pathogens Standard may reduce infections.
Hazard
Exposure to potentially fatal bloodborne illness Hepatitis B Virus (HBV).
- Hepatitis B is 50–100 times more infectious than HIV
- Many people with HBV infection are unaware that they have the virus.
- According to the CDC, HBV can survive outside the body for at least one week. People can become infected from activities such as exposure to blood from contaminated needles and other sharp instruments. Even dried blood on environmental surfaces poses a risk. For more information, see Contaminated Work Environments.
Requirements under OSHA's Bloodborne Pathogens Standard, 29 CFR 1910.1030
The Bloodborne Pathogens Standard requires precautions when there is occupational exposure to blood or OPIM (as defined by the standard). Under the standard, OPIM means (1) the following human body fluids: semen, vaginal secretions, cerebrospinal fluid, synovial fluid, pleural fluid, pericardial fluid, peritoneal fluid, amniotic fluid, saliva in dental procedures, any body fluid that is visibly contaminated with blood, and all body fluids in situations where it is difficult or impossible to differentiate between body fluids; (2) any unfixed tissue or organ (other than intact skin) from a human (living or dead); and (3) HIV-containing cell or tissue cultures, organ cultures, and HIV- or HBV-containing culture medium or other solutions; and blood, organs, or other tissues from experimental animals infected with HIV or HBV. OSHA requires employers to:
- Ensure that the biosafety officer or other responsible person conducts an exposure determination to determine the exposure of workers to blood or OPIM throughout the hospital setting. [29 CFR 1910.1030(c)(2)(i)].
- Establish a written Exposure Control Plan (ECP). The ECP must contain, among other elements, annual documentation of consideration and implementation of appropriate commercially available and effective safer medical devices designed to eliminate or minimize exposure to blood and OPIM. Solicit input from non-managerial employees responsible for direct patient care who are potentially exposed to injuries from contaminated sharps in the identification, evaluation, and selection of effective engineering and work practice controls. Document the solicitation in the Exposure Control Plan. Any change to the use of engineering controls (and any other change affecting exposure) must also be reflected in the ECP. [29 CFR 1910.1030(c)(1)]
- Establish and maintain a sharps injury log for recording needlestick/sharps injuries. [29 CFR 1910.1030(h)(5)] The confidentiality of the injured employee must be protected.
- Make immediately available to an exposed employee a confidential medical evaluation and follow-up, after a report of a needlestick injury or other exposure incident. [29 CFR 1910.1030(f)(3)].
- Obtain and provide the employee with a copy of the evaluating healthcare professional’s written opinion for post-exposure evaluation and follow-up within 15 days of the completion of the evaluation, as required by the Bloodborne Pathogens Standard. [29 CFR 1910.1030(f)(5)] OSHA provides a non-mandatory sample form to help employers comply with this requirement: Written Opinion for Post-Exposure Evaluation.
- Hepatitis B Vaccination:
- Offer the hepatitis B vaccine and vaccination series to all employees who have occupational exposure to blood or OPIM, under the supervision of a licensed physician, as required by the standard [29 CFR 1910.1030(f)(1), 29 CFR 1910.1030(f)(2)(i)]. The hepatitis B vaccine must be:
- Made available at no cost to employee, at a reasonable time and place [29 CFR 1910.1030(f)(1)];
- Performed by or under the supervision of a licensed physician or by or under the supervision of another licensed healthcare professional [29 CFR 1910.1030(f)(1)];
- Made available after the employee has received the required training in 29 CFR 1910.1030(g)(2)(vii)(I) [29 CFR 1910.1030(f)(2)(i)]; and
- Made available within 10 working days of initial assignment [29 CFR 1910.1030(f)(2)(i)].
- Ensure that those employees declining the hepatitis B vaccine sign the declination statement in 29 CFR 1910.1030 Appendix A [29 CFR 1910.1030(f)(2)(iv)].
- This eTool contains a Hepatitis B Declination Statement that is in accord with the Bloodborne Pathogens standard’s requirements
- The healthcare professional's written opinion for Hepatitis B vaccination shall be limited to whether Hepatitis B vaccination is indicated for an employee, and if the employee has received such vaccination. [29 CFR 1910.1030(f)(5)(i)] OSHA provides in this eTool a non-mandatory sample form to help employers comply with this requirement: Written Opinion for Hepatitis B Vaccination.
- 29 CFR 1910.1030(f)(1)(ii)(D) takes into consideration the changing nature of medical treatment relating to hepatitis B. OSHA requires use of the U.S. Public Health Service (USPHS) guidelines current at the time of the evaluation or procedure.
- The current guidelines regarding hepatitis B vaccine as of the publication of this eTool are the Updated U.S. Public Health Service Guidelines for the Management of Occupational Exposures to HBV, HCV, and HIV and Recommendations for Postexposure Prophylaxis in MMWR, Vol. 50, No.11, June 29, 2001 and the CDC guidelines on the 2-dose Hepatitis B vaccine, found at https://www.cdc.gov/mmwr/volumes/67/wr/mm6715a5.htm, dated April 20, 2018.
- The hepatitis B vaccination must be given in the standard dose and through the standard route of administration, per the recommendations in the guidelines. Employees who have ongoing contact with patients or blood and are at ongoing risk for percutaneous injuries must be tested for antibody to hepatitis B surface antigen, one to two months after completing the final dose of either the two- or three-dose vaccination series. Employees who do not respond to the primary vaccination series must be fully revaccinated with a second two-dose or three-dose vaccine series and retested. Non-responders to the second series must be medically evaluated.
- Also per the guidelines, timely hepatitis B post-exposure prophylaxis must include hepatitis B immune globulin and/or hepatitis B vaccine series after evaluation of the hepatitis B surface antigen of the source and the vaccination and vaccine-response status of the exposed person. [29 CFR 1910.1030(f)(1)(ii)(D)]
- Offer the hepatitis B vaccine and vaccination series to all employees who have occupational exposure to blood or OPIM, under the supervision of a licensed physician, as required by the standard [29 CFR 1910.1030(f)(1), 29 CFR 1910.1030(f)(2)(i)]. The hepatitis B vaccine must be:
Additional Information
- Hepatitis B Vaccination Protection. OSHA Fact Sheet.
- Hepatitis B Virus Antibody Testing. OSHA Letter of Interpretation, (August 17, 2015).
- HBV antibody testing is required after vaccination series; HBV booster not required. OSHA Letter of Interpretation, (March 10, 2000).
- Bloodborne Pathogen Exposure Incidents. OSHA Fact Sheet.
- A Comprehensive Immunization Strategy to Eliminate Transmission of Hepatitis B Virus Infection in the United States. Centers for Disease Control and Prevention (CDC), Morbidity and Mortality Weekly Report (MMWR) 55(RR16);1-25, (December 8, 2006).
- Immunization of Health-Care Workers: Recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Hospital Infection Control Practices Advisory Committee (HICPAC). Centers for Disease Control and Prevention (CDC) Mortality Weekly Report (MMWR) 46(RR-18);1-42, (December 26, 1997).
- Updated U.S. Public Health Service Guidelines for the Management of Occupational Exposures to HBV, HCV, and HIV and Recommendations for Postexposure Prophylaxis (PDF). Centers for Disease Control and Prevention (CDC) Morbidity and Mortality Weekly Report (MMWR) 50(RR11);1-42, (June 29, 2001).
- Recommendations of the Advisory Committee on Immunization Practices for Use of a Hepatitis B Vaccine with a Novel Adjuvant, Weekly / April 20, 2018 / 67(15);455–458.
- Viral Hepatitis Prevention in Health Care. Centers for Disease Control and Prevention (CDC), National Center for HIV, STD, & TB Prevention.
Human Immunodeficiency Virus (HIV)
HIV infection has been reported following occupational exposures to HIV-infected blood through needlesticks (0.23% risk of infection if untreated, according to the CDC). Currently no vaccine exists to prevent HIV infection, and no treatment exists to cure it.
Hazard
Worker exposure to potentially fatal bloodborne pathogen Human Immunodeficiency Virus (HIV).
Requirements under Bloodborne Pathogens Standard, 29 CFR 1910.1030
OSHA requires employers to:
- Ensure that the biosafety officer or other responsible person conducts an exposure determination to determine the exposure of workers to blood or OPIM throughout the hospital setting. [29 CFR 1910.1030(c)(2)(i)].
- Establish a written Exposure Control Plan (ECP). The ECP must contain, among other elements, annual documentation of consideration and implementation of appropriate commercially available and effective safer medical devices designed to eliminate or minimize exposure to blood and OPIM. Solicit input from non-managerial employees responsible for direct patient care who are potentially exposed to injuries from contaminated sharps in the identification, evaluation, and selection of effective engineering and work practice controls. Document the solicitation in the Exposure Control Plan. Any change to the use of engineering controls (and any other change affecting exposure) must also be reflected in the ECP. [29 CFR 1910.1030(c)(1)]
- Establish and maintain a sharps injury log for recording needlestick/sharps injuries. [29 CFR 1910.1030(h)(5)] The confidentiality of the injured employee must be protected.
- Make immediately available to an exposed employee a confidential medical evaluation and follow-up, after a report of a needlestick injury or other exposure incident. The initial medical evaluation often occurs in the emergency department. [29 CFR 1910.1030(f)(3)]
- A healthcare professional's written opinion is required after an exposure incident. [29 CFR 1910.1030(f)(5)(ii)]
- The following non-mandatory sample form is available: Written Opinion for Post-Exposure Evaluation.
- A healthcare professional's written opinion is required after an exposure incident. [29 CFR 1910.1030(f)(5)(ii)]
- Under certain circumstances, provide post-exposure prophylaxis for HIV to healthcare workers who have an exposure incident. [29 CFR 1910.1030(f)(3)(iv)]
Additional Information
- Updated U.S. Public Health Service Guidelines for the Management of Occupational Exposures to HIV and Recommendations for Postexposure Prophylaxis. Centers for Disease Control and Prevention (CDC), (September 25, 2013). Updates US Public Health Service recommendations for the management of the health-care personnel (HCP) who have occupational exposure to blood and other body fluids that might contain human immunodeficiency virus.
- Occupational HIV Transmission and Prevention among Health Care Workers. Centers for Disease Control and Prevention (CDC).
- Bloodborne Pathogen Exposure Incidents. OSHA Fact Sheet.
Hepatitis C Virus (HCV)
Hepatitis C Virus (HCV) infection is the most common chronic bloodborne infection in the United States. After a needlestick or sharps exposure to HCV-positive blood, the risk of HCV infection is 0.1%. HCV infection often occurs with no symptoms, however chronic infection can develop that may lead to active liver disease. Currently there is no vaccine available for hepatitis C.
Hazard
Worker exposure to potentially fatal bloodborne pathogen Hepatitis C Virus (HCV), which is a major cause of chronic liver disease.
Requirements under OSHA’s Bloodborne Pathogens Standard, 29 CFR 1910.1030
- Establish a written Exposure Control Plan (ECP). The ECP must contain, among other elements, annual documentation of consideration and implementation of appropriate commercially available and effective safer medical devices designed to eliminate or minimize exposure to blood and OPIM. Solicit input from non-managerial employees responsible for direct patient care who are potentially exposed to injuries from contaminated sharps in the identification, evaluation, and selection of effective engineering and work practice controls. Document the solicitation in the Exposure Control Plan. Any change to the use of engineering controls (and any other change affecting exposure) must also be reflected in the ECP. [29 CFR 1910.1030(c)(1)]
- Establish and maintain a sharps injury log for recording needlestick/sharps injuries. [29 CFR 1910.1030(h)(5)] The confidentiality of the injured employee must be protected.
- Make immediately available to an exposed employee a confidential medical evaluation and follow-up, after a report of a needlestick injury or other exposure incident. The initial medical evaluation often occurs in the emergency department. [29 CFR 1910.1030(f)(3)]
- A healthcare professional's written opinion is required as part of the medical evaluation and follow-up. [29 CFR 1910.1030(f)(5)(ii)]
- OSHA provides a non-mandatory sample form to help employers comply with this requirement: Written Opinion for Post-Exposure Evaluation.
- A healthcare professional's written opinion is required as part of the medical evaluation and follow-up. [29 CFR 1910.1030(f)(5)(ii)]
OSHA requires employers to ensure that the biosafety officer or other responsible person conducts an exposure determination to determine the exposure of workers to blood or OPIM throughout the hospital setting. [29 CFR 1910.1030(c)(2)(i)].
Additional Information
- Hepatitis C FAQs for Health Professionals. Centers for Disease Control and Prevention (CDC).
- Bloodborne Pathogen Exposure Incidents. OSHA Fact Sheet.
- Recommendations for Prevention and Control of Hepatitis C Virus (HCV) Infection and HCV-Related Chronic Disease. Centers for Disease Control and Prevention (CDC), Morbidity and Mortality Weekly Report (MMWR) 47(RR19); 1-39, (October 16, 1998).
- Updated U.S. Public Health Service Guidelines for the Management of Occupational Exposures to HBV, HCV, and HIV and Recommendations for Postexposure Prophylaxis (PDF). Centers for Disease Control and Prevention (CDC) Morbidity and Mortality Weekly Report (MMRW) 50(RR11); 1-42, (June 29, 2001).
- Viral Hepatitis. Centers for Disease Control and Prevention (CDC).
Ebola
Ebola hemorrhagic fever (EHF) (sometimes called Ebola Virus Disease, or EVD) is a type of viral hemorrhagic fever (VHF) brought on by any of several strains of viruses in the Ebola virus genus. Ebola viruses are capable of causing severe, life-threatening disease. The average case fatality rate for EHF is approximately 50%.
Workers performing tasks involving close contact with symptomatic individuals with EHF or in environments contaminated or reasonably anticipated to be contaminated with infectious body fluids are at risk of exposure. The most common routes of transmission of Ebola viruses are:
- Contact of the eyes or other mucous membranes with blood or body fluids of a person or animal with Ebola Hemorrhagic Fever (EHF);
- Contact with contaminated equipment or other objects; and
- Ingestion of infectious blood or body fluids, e. g., after splatter.
It may be possible for Ebola virus to be aerosolized under certain conditions (CDC).
Hazard
Worker exposure to bloodborne pathogen with a high fatality rate Ebola (EHF).
Requirements under Bloodborne Pathogens Standard, 29 CFR 1910.1030
- OSHA's Bloodborne Pathogens Standard (29 CFR 1910.1030) covers occupational exposure to Ebola virus via the bloodborne route. Ebola is among the subset of contact-transmissible diseases to which the Bloodborne Pathogens Standard applies, as it can be transmitted by blood or other potentially infectious materials (OPIM) as defined in the standard.
Other OSHA Requirements
- OSHA's Respiratory Protection Standard (29 CFR 1910.134) also applies in situations where workers may be exposed to bioaerosols containing Ebola virus.
- Other elements of infection control for Ebola, including a number of precautions for contact-transmissible diseases, are covered under OSHA's Personal Protective Equipment (PPE) Standard (29 CFR Part 1910 Subpart I) and the General Duty Clause, Section 5(a)(1) of the Occupational Safety and Health (OSH) Act of 1970, which requires employers to provide their employees with a workplace free of recognized hazards likely to cause death or serious physical harm.
CDC has established interim guidance for healthcare workers on Ebola, as well as information on specimen handling:
- CDC's Ebola page provides the most up-to-date information on medical aspects of Ebola virus infection and EHF.
- Ebola Virus Disease (EVD) Information for Clinicians in U.S. Healthcare Settings. Centers for Disease Control and Prevention (CDC).
- Guidance on Personal Protective Equipment (PPE) To Be Used By Healthcare Workers during Management of Patients with Confirmed Ebola or Persons under Investigation (PUIs) for Ebola who are Clinically Unstable or Have Bleeding, Vomiting, or Diarrhea in U.S. Hospitals, Including Procedures for Donning and Doffing PPE. Centers for Disease Control and Prevention (CDC).
- Infection Prevention and Control Recommendations for Hospitalized Patients Under Investigation (PUIs) for Ebola Virus Disease (EVD) in U.S. Hospitals. Centers for Disease Control and Prevention (CDC).
- Guidance for U.S. Laboratories for Managing and Testing Routine Clinical Specimens When There is a Concern About Ebola Virus Disease. Centers for Disease Control and Prevention (CDC).
Hazard
Exposure of employees to Multidrug Resistant Organisms (MDROs) in hospital settings.
Common examples of these organisms include:
- Carbapenem-resistant Enterobacteriaceae (CRE) are a family of germs that can become carbapenem-resistant, including Klebsiella pneumoniae and Escherichia coli (E. coli).
- Clostridium difficile (C. Diff). Clostridium difficile is a spore-forming, Gram-positive anaerobic bacillus. It is a common cause of antibiotic-associated diarrhea
- Extended-spectrum beta-lactamases (ESBLs). ESBLs of concern are resistant to cephalosporins and monobactams.
- Methicillin/Oxacillin-resistant Staphylococcus aureus (MRSA), which includes Vancomycin-intermediate Staphylococcus aureus (VISA) and Vancomycin-resistant Staphylococcus aureus (VRSA) is addressed below and also in Hospital Wide Hazards – MRSA.
- Multidrug-resistant Acinetobacter baumannii (MDR-Ab) is a gram-negative bacterium that can colonize the skin of infected patients.
- Multi-drug-resistant Tuberculosis (MDR-TB) is addressed in Hospital-Wide Hazards – Tuberculosis.
- Penicillin-resistant Streptococcus pneumoniae (PRSP). Pneumococcal disease is an infection caused by Streptococcus pneumoniae bacteria, sometimes referred to as pneumococcus. Pneumococcus can cause many types of illnesses, including ear infections and meningitis.
- Vancomycin-resistant enterococci (VRE). Enterococci are bacteria that can live in the human intestines and female genital tract without causing disease or infection (often called colonization). However, enterococci can cause infections of the urinary tract, the bloodstream, or of wounds associated with catheters or surgical procedures. Vancomycin is usually the drug of choice for treating these bacteria. However, VRE are resistant to Vancomycin.
The CDC provides guidelines and recommends controls for MDRO hazards.
Additional Information
- Management of Multidrug-Resistant Organisms in Healthcare Settings. Centers for Disease Control and Prevention (CDC).
- Healthcare: Infectious Disease. OSHA Safety and Health Topics Page.
- Healthcare-associated Infections (HAI). Centers for Disease Control and Prevention (CDC).
- Antibiotic / Antimicrobial Resistance. Centers for Disease Control and Prevention (CDC).
- Understanding Antimicrobial (Drug) Resistance. National Institute of Allergy and Infectious Diseases (NIAID).
- Methicillin-resistant Staphylococcus aureus (MRSA)
-
Staphylococcus aureus (S. Aureus) is a type of bacteria commonly carried on the skin or in the noses of healthy people. Methicillin-resistant Staphylococcus aureus (MRSA) refers to types of S. Aureus that are resistant to methicillin, a type of antibiotic. MRSA is often resistant to other antibiotics as well. While 33% of the population is colonized with S. Aureus (meaning that the bacteria are present, but are not causing an infection), approximately 1% is colonized with MRSA. MRSA has emerged as one of the leading pathogens in healthcare-associated infections. Treatment options for MRSA are more limited and less effective than options available for S. aureus infections and result in higher morbidity and mortality.
Modes of Transmission of MRSA:
- Skin-to-skin contact with someone who has an MRSA infection
- Contact with items and surfaces that have been exposed to MRSA
After exposure to MRSA, the employee can become infected or become a carrier. A person can carry MRSA, without symptoms, for a long time before getting sick. The distinction between MRSA colonization and infection is important.
Colonization and Infection:
- Colonization means that the organism is present in or on the body but is not causing illness. Many patients who are admitted to the hospital may already be colonized. Exposure to these patients may result in colonization of healthcare workers having direct contact with these patients. A hospital employee who has been colonized can become a carrier and spread infection to other healthcare workers and patients.
- Infection means that the organism is present and is causing illness.
Risk factors for increasing the chance of both colonization and infection of these organisms include:
- Severity of illness
- Previous exposure to anti-microbial agents
- Underlying diseases or conditions, particularly:
- Chronic renal disease
- Insulin-dependent diabetes mellitus
- Peripheral vascular disease
- Dermatitis or skin lesions
- Invasive procedures, such as:
- Dialysis
- Presence of invasive devices
- Urinary catheterization
- Repeated contact with the healthcare system
- Previous colonization by a multidrug-resistant organism
- Advanced age
- Compromised skin, such as cuts or abrasions
- Crowded living conditions
- Poor personal hygiene or a lack of cleanliness
Hazard
Exposure of employees to infections from multidrug-resistant organisms such as MRSA in the workplace. Employees can become infected or become carriers and spread infection to other healthcare workers and patients
Recognized Controls and Work Practices
Prevention of MRSA Infections
Take steps to decrease or minimize the spread of MRSA at the workplace as part of a comprehensive Safety and Health Management Program. Some steps are:
- Place importance on worker safety and health in the workplace.
- Ensure the availability of adequate facilities and supplies that encourage workers to practice good hygiene.
- Ensure that routine housekeeping in the workplace is followed.
- Ensure that contaminated equipment and surfaces are cleaned with detergent-based cleaners.
- Disinfect with EPA-registered disinfectants where applicable.
Infection control is the key to stopping MRSA spread in healthcare settings. Appropriate controls include, but are not limited to:

- Ensuring that hospital employees practice good hand hygiene as well as good personal hygiene.
- Keeping hands clean by washing thoroughly with soap and water or by using an alcohol-based sanitizer when soap and water is not immediately accessible;
- Proper use of gloves and other personal protective equipment;
- Keeping cuts and scrapes clean and covered with a bandage until healed;
- Avoiding direct contact with patients’ wounds or bandages; and
- Cleaning contaminated equipment and surfaces with detergent-based cleaners and Environmental Protection Agency (EPA)-registered disinfectants that are effective at removing MRSA from the environment.
The CDC's recommendations for infection control for all patient care in hospitals consist of Standard Precautions. The CDC also recommends Contact Precautions in special cases, when the facility (based on national or local regulations) deems the multidrug-resistant microorganism is of special clinical and epidemiologic significance, until patients are culture-negative for the target MDRO. Also included are: improvements in hand hygiene, active surveillance cultures, education, enhanced environmental cleaning, and improvements in communication about patients with MDROs within and between healthcare facilities.

Additional Information:
- 2007 Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings. Centers for Disease Control and Prevention (CDC), (2007).
- General Information About MRSA in Healthcare Settings. Centers for Disease Control and Prevention (CDC)
- MRSA and the Workplace. Centers for Disease Control and Prevention (CDC).
- Precautions to Prevent Spread of MRSA. Centers for Disease Control and Prevention (CDC).
- Multidrug-resistant organisms (MDRO) Management. Centers for Disease Control and Prevention (CDC).
- Healthcare-associated Infections (HAI). Centers for Disease Control and Prevention (CDC).
- Infectious Diseases. OSHA Safety and Health Topics Page.
Seasonal influenza is a respiratory illness that typically occurs every fall and winter in the United States. Outbreaks are typically limited, as most people have some immunity to the circulating strains of the virus. A vaccine is prepared in advance of influenza season that is designed to match the influenza viruses most likely to be circulating in the community.
Pandemic influenza refers to a worldwide outbreak of influenza that occurs when a new strain of the virus emerges that has the ability to infect humans and to spread from person to person. During the early phases of an influenza pandemic, when people might not have any natural immunity to the new strain, the disease could spread rapidly among the population. A vaccine to protect people against illness from a pandemic influenza virus may not be widely available until many months after an influenza pandemic begins.
It is important to emphasize that, as of the date of publication of this eTool, there is no current influenza pandemic. However, pandemics have occurred throughout history. Pandemics can vary in severity from something that seems simply like a bad flu season to an especially severe influenza pandemic that could lead to high levels of illness, death, social disruption and economic loss. The last pandemic occurred in 2009 and was mild compared to a severe pandemic that occurred in 1918. Although it is impossible to predict when the next pandemic will occur or whether it will be mild or severe, many scientists believe that it is only a matter of time before a severe pandemic occurs.
Emerging influenza strains may have pandemic potential. Pandemic influenza remains a concern for employers and workers. A pandemic can occur at any time and can be mild, moderate, or severe. For more information, see the OSHA webpage on pandemic influenza.
The contribution of each route of exposure to influenza transmission (droplet, airborne, contact) is uncertain at this time and may vary based upon the characteristics of the influenza strain.
- Influenza is thought to be primarily spread through large droplets (droplet transmission) that directly contact the nose, mouth or eyes. These droplets are produced when infected people cough, sneeze or talk, sending the relatively large infectious droplets and very small sprays (aerosols) into the nearby air and into contact with other people. Large droplets can only travel a limited range; therefore, to help avoid transmission during flu season and a pandemic, limit close contact (within 6 feet) with others when possible.
- To a lesser degree, human influenza is spread by touching objects contaminated with influenza viruses and then transferring the infected material from the hands to the nose, mouth or eyes.
- Influenza may also be spread by very small infectious particles (aerosols) traveling in the air.
Hazard
Workers who perform certain types of healthcare tasks for patients who may have the flu may be at a higher risk for exposure to the influenza virus, and additional precautions are needed. In addition to workers engaged in direct patient care, these tasks include workers engaged in aerosol-generating procedures, specimen analysis, and other patient support such as dietary and housekeeping services.
Recognized Controls and Work Practices
Take steps to decrease or minimize the spread of influenza at the workplace as part of a comprehensive Safety and Health Management Program. Some steps are:
- Encourage workers to get vaccinated and make vaccinations available to workers. The Centers for Disease Control and Prevention (CDC), the Advisory Committee on Immunization Practices (ACIP), and the Healthcare Infection Control Practices Advisory Committee (HICPAC) recommend that all U.S. healthcare workers get vaccinated annually against influenza.
- Modify patient intake, triage, and other service areas to increase space between workers, coworkers, and patients and provide barriers against transmission when applicable (e.g., install sneeze guards or partitions).
- If available, use airborne infection isolation rooms (AIIRs), for aerosol-generating procedures and limit the number of people present during the procedure.
- Isolate and group flu patients when possible.
- Limit patient transport. Conduct exams and procedures at the bedside, instead of transporting the patient to other areas of the facility. Place a surgical mask on the patient, if possible, when they are being transported out of the room.
- Use closed suctioning systems to suction a patient's airways and use high quality filters on the expiratory port of ventilators, when available.
- Limit the staff entering patient isolation rooms to only those necessary for patient care.
- Restrict visits for patients in isolation.
- Use proper respiratory and cough etiquette and encourage hand washing by patients and visitors.
- Wash hands with soap and water for at least 20 seconds before and after contact with patients, after using PPE, and after touching contaminated surfaces; use an alcohol-based hand rub if soap and water are not available.
- When using soap and water, rub soapy hands together for at least 20 seconds, rinse hands with water, and dry completely.
- If soap and water are not available, use of an alcohol-based hand rub is helpful as an interim measure until hand washing is possible. When using an alcohol-based hand rub, apply liquid to palm of hand, cover all surfaces of the hands with the liquid, and rub hands together until dry.
- Monitor yourself for symptoms of the flu, such as fever or feeling feverish/chills, cough, sore throat, runny or stuffy nose, muscle or body aches, headaches, and fatigue (tiredness).
- Follow standard cleaning and disinfection methods.
- Use a facemask when entering a flu patient's room. A facemask is not a respirator. It will not protect workers during aerosol-generating procedures, which may create very fine aerosol sprays. A facemask can only be used to protect workers from contact with the large droplets made by patients when they cough, sneeze, talk or breathe.
- Use a respirator during aerosol-generating procedures; a fit tested N95 disposable respirator or better is needed.
- Use gloves, gowns, and eye protection for any tasks that might cause contamination or create splashes.
- Put on and take off protective equipment in the correct order to prevent contamination.
- Use the appropriate Biosafety Level, in laboratories, when handling specimens from flu patients (i.e., patients known to have, or suspected of having, influenza).
While the recognized control and work practices that protect workers from exposure to seasonal and pandemic influenza are basically the same, consult the CDC's "Interim Guidance for Infection Control Within Healthcare Settings When Caring for Confirmed Cases, Probable Cases, and Cases Under Investigation for Infection with Novel Influenza A Viruses Associated with Severe Disease," in the event of an influenza pandemic to determine if any higher level precautions should be implemented (i.e., the use of respirators rather than surgical masks when HCWs are engaged in direct patient care).
Additional Information
- Seasonal Flu
- Seasonal Flu. OSHA Safety and Health Topics Page.
- Prevention Strategies for Seasonal Influenza in Healthcare Settings. Centers for Disease Control and Prevention.
- Seasonal
- Influenza Vaccination – Important Protection for Healthcare Workers. OSHA Fact Sheet
- Influenza Vaccination Information for Health Care Workers. Centers for Disease Control and Prevention.
- Pandemic Influenza
- Pandemic Influenza. OSHA Safety and Health Topics Page.
- How to Protect Yourself in the Workplace during a Pandemic. OSHA QuickCard™.
- What Employers Can Do to Protect Workers from Pandemic Influenza. OSHA Fact Sheet.
Legionnaires' Disease is a bacterial disease commonly associated with water-based aerosols and is often a result of poorly maintained air conditioning cooling towers and potable water systems. It can occur where water, contaminated with the legionella organism, is aerosolized and then breathed in by workers or patients. Legionnaires' Disease is not contagious but is of environmental origin. Consequently, only those who are directly exposed to the contaminated aerosolized water source can get the disease.
Hazard
Inhalation of the Legionella bacteria located in contaminated aerosolized water may cause Legionnaires' Disease. Exposure to the Legionella bacteria could occur in shower or whirlpool areas, or areas that use spray nozzles. Cooling towers, evaporative condensers, fluid coolers, and hot-water systems are water sources that frequently provide optimal conditions for growth of the legionella organism.
Recognized Controls and Work Practices
Maintenance of water systems:
- Hot-water systems:
- Store water at 60°C (140°F) and deliver it at a minimum of 50°C (122°F) to all outlets. (To avoid scalding problems install fail-safe scald protection equipment, such as preset thermostatic mixing valves.)
- Where building cannot be retrofitted, consider periodically increasing the temperature to at least 70°C (158°F) for 24 hours, or chlorination followed by flushing for at least 20 minutes.
- Inspect systems annually to ensure that equipment functions properly.
- Cold-water systems:
- Maintain cold-water lines below 20°C (68°F).
- Eliminate water tanks that allow water to remain uncirculated for long periods or design them to reduce storage time to a day or less. Cover water tanks and protect them from temperature extremes.
- Prevent cross-contamination of the domestic cold water system with other systems.
- Use hyperchlorination to eradicate legionella if the cold-water lines have significant contamination.
- Clean and disinfect cooling towers at least twice a year.
- Use biocides periodically to control bacteria growth.
- Provide visual inspection and periodic maintenance of the system to prevent buildup of scale and sediment and biofouling (the accumulation of microorganisms, plants, algae, or animals on wet surfaces), all of which support legionella growth.
Additional Information
- Legionellosis (Legionnaires' Disease and Pontiac Fever). OSHA Safety and Health Topics Page.
- Environmental Care: Utility System and Clinical Impact. Joint Commission, Environment of Care Standards, (November 24, 2008). Addresses issues of improperly designed and maintained aerosolizing water systems (controlling pathogenic biological agents such as legionella in cooling towers, domestic hot water systems, etc.).
- Legionella (Legionnaires' Disease and Pontiac Fever). Centers for Disease Control and Prevention (CDC), (June 27, 2008).
- Veterans Health Administration Directive 1061, Prevention of Healthcare-Associated Legionella Disease and Scald Injury from Potable Water Distribution Systems. (August 13, 2014).
Hazard
Tuberculosis (TB) is caused by a bacterium called Mycobacterium tuberculosis. TB is caused by a bacterium called Mycobacterium tuberculosis and is spread by airborne droplets generated when a person with TB disease coughs, speaks, sneezes, etc. Infection occurs when a susceptible person inhales droplet nuclei containing the bacteria, which then become established in the body.
TB transmission has been documented in healthcare settings where healthcare workers and patients come in contact with people who have TB disease (CDC). An additional hazard is present because of multidrug-resistant (MDR) TB. MDR organisms are resistant to the drugs that are normally used to treat TB, such as Isoniazid and Rifampin (CDC).
Recognized Controls and Work Practices
The CDC discusses three levels of controls for TB infection in healthcare settings:
- Administrative controls to minimize the number of areas where exposure to TB can occur
- Environmental controls to reduce the concentration of TB
- The use of respiratory protection in situations that pose a high risk for exposure
Recognized controls and work practices include:
- Providing and practicing early patient screening to identify potentially infectious patients, and provide isolation to prevent employee exposures. Consistent with CDC guidelines, "a diagnosis of respiratory TB disease should be considered for any patient with symptoms or signs of infection in the lung, pleura, or airways (including larynx), including coughing for ≥3 weeks, loss of appetite, unexplained weight loss, night sweats, bloody sputum or hemoptysis, hoarseness, fever, fatigue, or chest pain."
- Providing an area in the hospital that is ventilated separately for TB patients (i.e., patients with suspected or confirmed TB) in facilities in which TB patients are frequently treated. If this is not possible, ensuring that TB patients wear surgical masks, stay in general-use areas the minimum amount of time possible, and be transferred promptly to isolation rooms.
- Healthcare facilities serving populations that have a high prevalence of TB may need to supplement the general ventilation or use additional engineering controls in general-use areas where TB patients are likely to go (e.g., waiting-room areas, emergency departments, and radiology suites). These engineering controls include:
- A single-pass, non-recirculating system that exhausts air directly to the outside.
- A recirculation system that passes air through HEPA (High Efficiency Particulate Air) filters before re-circulating it to the general ventilation system.
- Providing worker education, informational materials, and training about TB relevant to employees' work (e.g., symptoms, transmission, controls, and post-exposure protocols), as well as training specifically required by OSHA standards, as applicable. For more information see OSHA Directive CPL 02-02-078.
Requirements under OSHA's Respiratory Protection Standard, 29 CFR 1910.134
- Employers must comply with applicable provisions of OSHA's Respiratory Protection standard, 29 CFR 1910.134, for using respirators to protect against TB hazards, including:
- Following the hierarchy of controls required by the standard and providing appropriate respirators when such equipment is necessary to protect the health of the employee(s). The minimally acceptable level of respiratory protection for TB is the Type N95 Respirator. [29 CFR 1910.134(a)]
- Establishing and implementing a written respiratory protective program that includes the elements required by 29 CFR 1910.134(c).
- Training employees in the respiratory hazards to which they are potentially exposed during routine and emergency situations. [29 CFR 1910.134(c)(1)(vii)]
- Complying with other provisions of the standard, including those provisions requiring respirator training, medical evaluations, fit testing, a written respiratory protection program, and recordkeeping, at no cost to the employee.
Other OSHA Requirements
- Post a warning sign outside the ED respiratory isolation room to prevent accidental entry. [29 CFR 1910.145(a)(1)] 29 CFR 1910.145(e)(4) requires that a biological hazard warning shall be used to signify the actual or potential presence of a biohazard and to identify equipment, containers, rooms, materials, experimental animals, or combinations thereof, which contain, or are contaminated with, viable hazardous agents.
OSHA's enforcement information:
- Enforcement Procedures and Scheduling for Occupational Exposure to Tuberculosis. OSHA Directive CPL 02-02-078, (June 30, 2015). Contains information concerning OSHA's general enforcement policy and procedures for conducting inspections and issuing citations related to occupational tuberculosis (TB) hazards.
- Inspection Guidance for Inpatient Healthcare Settings. (June 25, 2015). OSHA memorandum establishing guidance for inspections conducted in inpatient healthcare settings.
Additional Information
- Respiratory Protection Program Guidelines. OSHA Directive CPL 02-02-054, (July 14, 2000). Sets forth guidelines for establishing and implementing an OSHA respirator program to ensure that all OSHA employees are protected from exposure to respiratory hazards.
- Inspection Procedures for the Respiratory Protection Standard. OSHA Directive CPL 02-00-158, (June 26, 2014). Establishes agency interpretations and enforcement policies, and provides instructions to ensure uniform enforcement of the Respiratory Protection Standard (29 CFR 1910.134).
- Tuberculosis Respiratory Protection Program in Health Care Facilities Administrator's Guide. Centers for Disease Control and Prevention (CDC).
- Respiratory Protection. OSHA eTool.
- Respiratory Protection. OSHA Safety and Health Topics Page.
- Hospital Respiratory Protection Program Toolkit: Resource for Respirator Program Administrator. OSHA Publication 3767, (May 2015).
- Respiratory Protection Program Training and Resources. American Association of Occupational Health Nurses (AAOHN). This online training program prepares hospital staff for respiratory protection in the workplace.
- Lewinsohn, D.M., et al. (2017). Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of Tuberculosis in Adults and Children. Clinical Infectious Diseases, 64(2): e1-e33.
- Whether annual Tuberculosis skin testing is required for low risk personnel. OSHA Letter of Interpretation, (June 23, 2009).
- Tuberculosis. OSHA Safety and Health Topics page.
Respirator Training Videos
- The Difference between Respirators and Surgical Masks. U.S. Department of Labor Video, (December 16, 2009). (Spanish)
- Respirator Safety. U.S. Department of Labor Video, (December 16, 2009). (Spanish)

