Hospitals eTool
Laboratory » Biological Hazards – Infectious Diseases
Workers in hospital settings may be exposed to a variety of common and emerging infectious disease hazards, particularly if proper infection prevention and control measures are not implemented in the workplace. Examples of infectious disease hazards include seasonal and pandemic influenza; norovirus; Ebola; Middle East Respiratory Syndrome (MERS), tuberculosis, methicillin-resistant Staphylococcus Aureus (MRSA), and other potentially drug-resistant organisms.
Infectious diseases are caused by agents that are transmissible through one or more different routes, including the contact, droplet, airborne, and bloodborne routes. The transmission of infectious agents through the bloodborne route—a specific subset of contact transmission—is defined in the Bloodborne Pathogens (BBP) standard, 29 CFR 1910.1030 (See the Bloodborne Pathogens page below).
An effective infection control program normally relies upon a multi-layered and overlapping strategy of engineering, administrative and work practice controls, and PPE. It is OSHA's intent in this eTool to highlight some – not all – of the controls that would be necessary to the development and implementation of an effective program. Implementing the controls highlighted here alone will not typically protect workers from infection hazards.
Follow standard and transmission-based precautions to prevent worker infections (see also the OSHA page: Worker protections against occupational exposure to infectious diseases). Early identification and isolation of sources of infectious agents (including sick patients), proper hand hygiene, worker training, effective engineering and administrative controls, safer work practices, and appropriate personal protective equipment (PPE), among other controls, help reduce the risk of transmission of infectious agents to workers.
Employers must comply with the BBP standard to the extent that there is "occupational exposure" (i.e., to the extent workers should reasonably anticipate contact with blood or other potentially infectious materials (OPIM) that may result from the performance of duties). Employers must also comply with the PPE Standard, 29 CFR 1910 Subpart I, and the OSH Act's General Duty Clause, 29 U.S.C. 654(a)(1), to protect their workers from infectious disease hazards. The General Duty Clause requires each employer to "furnish to each of his employees employment and a place of employment which are free from recognized hazards that are causing or are likely to cause death or serious physical harm to his employees."
OSHA provides agent-specific guidance for a variety of pathogens that workers in hospital settings may encounter. See OSHA's Safety and Health Topics Pages for Biological Agents and Bloodborne Pathogens and Needlestick Prevention for additional information.
In this module, OSHA provides additional guidance specifically for
Bloodborne pathogens are pathogenic microorganisms present in human blood that can cause disease in humans. These pathogens include, but are not limited to, Hepatitis B Virus (HBV), Hepatitis C Virus (HCV), Human Immunodeficiency Virus (HIV) and Viral Hemorrhagic Fevers (e.g. Ebola). [29 CFR 1910.1030(b)]
Hazards
Laboratory workers are at particular risk for exposure to blood or other potentially infectious materials (OPIM) because of the handling of clinical specimens in the laboratory.
Requirements under OSHA's Bloodborne Pathogens Standard, 29 CFR 1910.1030
The Bloodborne Pathogens Standard requires precautions when there is occupational exposure to blood or OPIM (as defined by the standard). Under the standard, OPIM means (1) the following human body fluids: semen, vaginal secretions, cerebrospinal fluid, synovial fluid, pleural fluid, pericardial fluid, peritoneal fluid, amniotic fluid, saliva in dental procedures, any body fluid that is visibly contaminated with blood, and all body fluids in situations where it is difficult or impossible to differentiate between body fluids; (2) any unfixed tissue or organ (other than intact skin) from a human (living or dead); and (3) HIV-containing cell or tissue cultures, organ cultures, and HIV- or HBV-containing culture medium or other solutions; and blood, organs, or other tissues from experimental animals infected with HIV or HBV.
Have the laboratory director, biosafety officer, or other responsible person conduct an exposure determination to determine all tasks and procedures in the laboratory in which there is exposure to bloodborne pathogens (e.g., where there is a potential for sprays, splashes, or aerosol generated during laboratory procedures). [29 CFR 1910.1030(c)(2)(i)]
Under the Bloodborne Pathogens Standard:
- Mouth pipetting/suctioning of blood or other potentially infectious materials is NOT allowed. [29 CFR 1910.1030(d)(2)(xii)]
- Eating, drinking, smoking, applying cosmetics or lip balm, or handling contact lenses is NOT allowed in work areas where there is a reasonable likelihood of occupational exposure to bloodborne pathogens. [29 CFR 1910.1030(d)(2)(ix)]
- Food and drink is NOT to be kept in refrigerators, freezers, shelves, cabinets or on countertops or bench tops where blood or other potentially infectious materials are present. [29 CFR 1910.1030(d)(2)(x)].
For more information, see Hospital-wide Hazards - Bloodborne Pathogens
- Needlestick/Sharps Injuries
-
Hazard
Exposure to blood and OPIM from needlestick injuries due to:
- Unsafe needle devices
- Improper handling and disposal of needles
Requirements under Bloodborne Pathogens Standard, 29 CFR 1910.1030
- Use safer needle devices and needleless devices to decrease needlestick or other sharps exposures. See Safer Needle Devices section. In addition, proper handling and disposal of needles and other sharps according to the Bloodborne Pathogens Standard can help prevent needlestick injuries.
- Use engineering controls (e.g., safer needle devices) and work practice controls (e.g., altering the way a task is performed to reduce chance of injury such as prohibiting recapping of needles by a two-handed technique) to eliminate or minimize exposure to bloodborne pathogens [29 CFR 1910.1030(c), 29 CFR 1910.1030(d)].
- Examples of engineering controls
- Safer needle/other sharps devices.
- Blunt-tip suture needles.
- Needleless IV connectors.
- Proper containers for sharps.
- Non-glass capillary tubes. OSHA, FDA and NIOSH warn healthcare workers about the hazards from breakage of glass capillary tubes and sanction using non-glass capillary tubes.
- The risk of sharps injuries must be eliminated or minimized. Follow the applicable provisions of the standard. For example:
- Contaminated needles and other contaminated sharps shall be discarded immediately or as soon as feasible into appropriate containers, as required by the standard. [29 CFR 1910.1030(d)(4)(iii)(A)(1)]
- Sharps containers shall be easily accessible and located as close as is feasible to the immediate location where sharps are used or can be reasonably anticipated to be found. [29 CFR 1910.1030(d)(4)(iii)(A)(2)(i)]
- Contaminated needles and other contaminated sharps must not be bent, recapped, or removed except as noted in paragraphs 29 CFR 1910.1030(d)(2)(vii)(A) and (d)(2)(vii)(B). Shearing or breaking contaminated needles is prohibited. [29 CFR 1910.1030(d)(2)(vii)]
- Do not allow sharps containers to overfill. Replace sharps containers routinely. [29 CFR 1910.1030(d)(4)(iii)(A)(2)(iii)]
Additional Information
- Workbook for Designing, Implementing, and Evaluating a Sharps Injury Prevention Program. Centers for Disease Control and Prevention (CDC).
- Emergency Sharps Information (CDC) also provides immediate access to treatment protocols following blood exposures involving HIV, HBV and HCV, including the Clinicians' Post Exposure Prophylaxis Hotline (PEPline) at 1-888-448-4911.
- Bloodborne Infectious Diseases: HIV/AIDS, Hepatitis B, and Hepatitis C. National Institute for Occupational Safety and Health (NIOSH) Workplace Safety and Health Topic.
- EPINet®. International Safety Center.
- Blunt-Tip Surgical Suture Needles Reduce Needlestick Injuries and the Risk of Subsequent Bloodborne Pathogen Transmission to Surgical Personnel: FDA, NIOSH and OSHA Joint Safety Communication. NIOSH, (May 30, 2012).
- Other Sharps
-
Take precautions with respect to all contaminated sharps, not just needles. "Contaminated Sharps" means any contaminated object that can penetrate the skin including, but not limited to, needles, scalpels, broken glass, broken capillary tubes, and exposed ends of dental wires. [29 CFR 1910.1030(b)]
Hazard
Exposure to blood and OPIM through contaminated sharps, such as:
- I.V. Connectors that use needle systems.
- Broken Capillary Tubes.
Follow requirements of the Bloodborne Standard (29 CFR 1910.1030) with respect to all "contaminated sharps." For example:
- Implement engineering and work practice controls to eliminate or minimize exposure to bloodborne pathogens [29 CFR 1910.1030(c), 29 CFR 1910.1030(d)].
- Dispose of contaminated sharps immediately or as soon as feasible into appropriate containers, as required by the standard. [29 CFR 1910.1030(d)(4)(iii)(A)(1)]
Additional Information
- Protecting Yourself When Handling Contaminated Sharps. OSHA Fact Sheet.
- Also see Hospital-wide Hazards - Needlesticks/Sharps Injuries.
- Engineering Controls
-
OSHA's Bloodborne Pathogens Standard requires that engineering and work practice controls be used to eliminate or minimize exposures to blood and other potentially infectious materials (OPIM). [29 CFR 1910.1030(c), 29 CFR 1910.1030(d)].
The Bloodborne Pathogens Standard requires that Universal Precautions be observed. This means that all specimens of human blood and certain human bodily fluids must be treated as if known to be infectious for bloodborne pathogens. Of course, the protections of the standard also apply if the specimens are from a patient who is known or suspected to be infectious with bloodborne pathogens, such as Ebola. Laboratories must therefore:
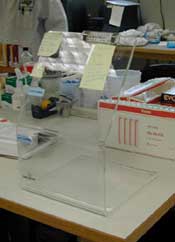


- Use effective engineering and safe work practice controls to minimize or eliminate exposure in connection with collecting, transporting, storing, packaging and shipping these specimens. [29 CFR 1910.1030(d)(2)]
- Use effective engineering controls (e.g., biological safety cabinets) and safe work practice controls to minimize or eliminate exposure in connection with potential splashes of, or aerosol-generating laboratory procedures on, these specimens. [29 CFR 1910.1030(d)(2)]
Where occupational exposure remains after institution of engineering and work practice controls, personal protective equipment must also be used. [29 CFR 1910.1030(d)(2)]
The basic concept behind engineering controls is that, to the extent feasible, the work environment and the job itself should be designed to eliminate hazards or reduce exposure to hazards. Some examples are:
- Splatter guards to prevent splashing from reaching employee, (e.g., plexiglass barriers).
- Sensor-controlled automatic sinks or foot, knee, or elbow controls that are available on sinks to operate handwashing facilities without using hands so that the contaminants are not spread from the hands to sink handles.
- Ventilation systems (e.g. laboratory hoods). Be sure to properly maintain ventilation systems and keep associated maintenance records.
- Centrifuge tubes with caps.
- Biological safety cabinets.
- Biological Safety Cabinets (BSCs), when properly maintained and used in conjunction with good microbiological techniques, provide an effective containment system for safe manipulation of moderate- and high-risk infectious agents [Biosafety (BSL) Level 2 and 3 agents].
- BSCs protect laboratory workers and the immediate environment from infectious aerosols generated within the cabinet. BSCs must be certified when installed, whenever they are moved and at least annually. [29 CFR 1910.1030(e)(2)(iii)(B)]
Additional Information
- Biosafety in Microbiological and Biomedical Laboratories (BMBL), 5th Edition (PDF). Centers for Disease Control and Prevention (CDC), National Institutes of Health (NIH), (December 2009).
- Laboratory Safety Biosafety Cabinets (BSCs). OSHA Fact Sheet, (August 2011).
- FDA, NIOSH and OSHA Joint Safety Communication: Blunt-Tip Surgical Suture Needles Reduce Needlestick Injuries and the Risk of Subsequent Bloodborne Pathogen Transmission to Surgical Personnel. (May 30, 2012).
- Workbook for Designing, Implementing, and Evaluating a Sharps Injury Prevention Program. Centers for Disease Control and Prevention (CDC).
- B. Stringer, T. Haines. "Hands-free technique: preventing occupational exposure during surgery." Journal of Perioperative Practice 16.10(October 2006): 495.
- Acceptable use of antiseptic-hand cleansers for bloodborne pathogen decontamination and as an appropriate handwashing practice [1910.1030; 1910.1030(d)(2)(v); 1910.1030(d)(2)(vi)]. OSHA Letter of Interpretation, (March 31, 2003).
- Guideline for Hand Hygiene in Health-Care Settings. Centers for Disease Control and Prevention (CDC), Morbidity and Mortality Weekly Report (MMWR) 51(RR16), (October 15, 2002).
- Bloodborne Infectious Diseases: HIV/AIDS, Hepatitis B, and Hepatitis C - Emergency Sharps Information. National Institute for Occupational Safety and Health (NIOSH) Workplace Safety and Health Topic.
- Also see Hospital-wide Hazards - Bloodborne Pathogens.
The CDC considers workers in medical laboratories that handle M. Tuberculosis to be at high risk for occupational transmission of TB. The potential of contracting TB among persons who work with TB in the laboratory is three times greater than among lab personnel that do not work with TB bacterium (CDC).
Hazard
Exposure of laboratory employees to M. tuberculosis from working with specimens (e.g., acid fast bacilli smears), from patients who have tuberculosis. Other potential sources of exposure are sputum, cerebrospinal fluid, urine, and fluids collected from gastric or bronchial lavage.
Recognized Controls and Work Practices
All cultures or specimens known to contain, or suspected of containing, TB bacilli must be manipulated in settings where specific engineering controls, administrative procedures, appropriate personal work practices, and use of PPE ensure containment of the organism and protection of the workers. Recognized practices include:
- Biosafety Level: For a laboratory to handle TB sputum and TB materials, the laboratory must operate at a biosafety level of 2 or 3, as specified.
- Controlled access, anterooms, sealed windows, directional airflow, preventing recirculation of laboratory exhaust air, filtration of exhaust air before discharge to the outside, and thimble exhaust connections for biological safety.
- The use of biological safety cabinets (BSCs) whenever working with infectious materials that have a chance of splashing or aerosolizing. Processes that can expose employees to splashes or aerosolized materials include:
- Pouring liquid cultures
- Using fixed-volume automatic pipettes
- Mixing liquid cultures with a pipette
- Preparing specimens and culture smears
- Dropping and spilling tubes containing suspensions of bacilli
- Centrifugation of specimens without tops
- Ensure the use of appropriate PPE, including respiratory protection (e.g., N-95 respirator).
Additional Information
- Biosafety in Microbiological and Biomedical Laboratories (BMBL), 5th Edition (PDF). Centers for Disease Control and Prevention (CDC), National Institutes of Health (NIH), (December 2009).
- Enforcement Procedures and Scheduling for Occupational Exposure to Tuberculosis. OSHA Directive CPL 02-02-078, (June 30, 2015). Contains information concerning OSHA's general enforcement policy and procedures for conducting inspections and issuing citations related to occupational tuberculosis (TB) hazards.
- Inspection Guidance for Inpatient Healthcare Settings. (June 25, 2015). OSHA memorandum establishing guidance for inspections conducted in inpatient healthcare settings.
- Fit Testing Requirements for Employees Who Wear Respirators to Protect against M. Tuberculosis, SARS, Smallpox, and Monkeypox. (February 5, 2004). Employers must comply with applicable provisions of OSHA's Respiratory Protection standard, 29 CFR 1910.134, for using respirators to protect against TB hazards.
- Tuberculosis. OSHA Safety and Health Topics Page.
- Tuberculosis. Centers for Disease Control and Prevention.
- Lewinsohn, D.M., et al. (2017). Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of Tuberculosis in Adults and Children. Clinical Infectious Diseases, 64(2): e1-e33.
- Respiratory Protection Program Training and Resources. American Association of Occupational Health Nurses (AAOHN). This online training program prepares hospital staff for respiratory protection in the workplace.
- Also see Hospital-wide Hazards – Tuberculosis.
Hazard
Exposure of laboratory staff to seasonal or pandemic influenza. The CDC has documented cases of laboratory-acquired influenza among laboratory staff who handle influenza specimens.
Influenza can be transmitted by both symptomatic and asymptomatic individuals through infected respiratory tract secretions, mucus, cough aerosols, and contaminated hands and materials.
Recognized Controls and Work Practices
- Encourage workers to get vaccinated and make vaccinations available to workers. The Centers for Disease Control and Prevention (CDC), the Advisory Committee on Immunization Practices (ACIP), and the Healthcare Infection Control Practices Advisory Committee (HICPAC) recommend that all U.S. healthcare workers get vaccinated annually against influenza.
- Use the appropriate Biosafety Level when handling specimens from flu patients (i.e., patients known to have, or suspected of having, influenza).
- Use gloves, gowns, eye protection, and other appropriate PPE for any tasks that might cause contamination or create splashes.
- Put on and take off protective equipment in the correct order to prevent contamination.
- Use proper respiratory and cough etiquette and encourage hand washing by laboratory staff.
- Follow standard cleaning and disinfection methods.
Additional Information
Seasonal Flu
- Seasonal Flu. OSHA Safety and Health Topics Page.
- Prevention Strategies for Seasonal Influenza in Healthcare Settings. Centers for Disease Control and Prevention.
- Biosafety in Microbiological and Biomedical Laboratories (BMBL) 5th Edition. Centers for Disease Control and Prevention. 2009.
- Seasonal Influenza Vaccination – Important Protection for Healthcare Workers. OSHA Fact Sheet.
- Influenza Vaccination Information for Health Care Workers. Centers for Disease Control and Prevention.
Pandemic Influenza
- Pandemic Influenza OSHA Safety and Health Topics Page.
- How to Protect Yourself in the Workplace during a Pandemic. OSHA QuickCard™.
- What Employers Can Do to Protect Workers from Pandemic Influenza. OSHA Fact Sheet.
- Also see Hospital-wide Hazards – Influenza.
The following resources offer information on the potential for exposure to Zika virus:
- Preventing and Managing Laboratory Worker Exposure to Zika Virus. OSHA Fact Sheet.
- Interim Guidance for Protecting Workers from Occupational Exposure to Zika Virus. The Occupational Safety and Health Administration (OSHA) and the National Institute for Occupational Safety and Health (NIOSH).
- Laboratory Safety when Working with Zika Virus. Centers for Disease Control and Prevention (CDC).
- Interim Guidance for Managing Occupational Exposures to Zika Virus for Healthcare Personnel. Centers for Disease Control and Prevention (CDC).
- Zika: Protecting Healthcare and Laboratory Workers. Centers for Disease Control and Prevention (CDC).

