[Updated: September 14, 2023]
Personal Sampling for Air Contaminants
Table of Contents:
- Introduction
- Pre-Inspection Activities
- On-Site Inspection Activities
- Develop Documentation
- On-Site Sampling Plan Adjustments and Protocol
- Short Term Exposure Limits, Ceiling Limits, and Peak Exposure Values
- Overview of the Sampling Process
- Extended Work Shifts
- Air Contaminants Related to Heating and Combustion
- Chemical Mixtures
- Field Blanks
- Total Dust
- Respirable Dust
- Crystalline Silica
- Metals
- Asbestos
- Organic Vapors and Gases
- Post-Inspection Activities
- Bibliography
- Appendix A Additional Sampling and Exposure Assessment Support
- Appendix B Pre-Weighed Filters
- Appendix C Shelf-Life of Sampling Media
- Appendix D Sampling Pump Calibration
- Appendix E Chain of Custody and How to Apply Form OSHA-21 to Sampling Media
- Appendix F Example Calculations for Mixtures
- Appendix G Conversion Equations (mg/m3 to ppm)
- Appendix H Health Effects Codes
- Appendix J Example Calculations to Determine Compliance Using Full-Period Continuous Single Samples and Full-Period Consecutive Samples
I. Introduction
This chapter provides basic information related to sampling air contaminants. Other relevant reference resources include OSHA's Occupational Chemical Database (OCD) and the OSHA Field Operations Manual (FOM). Sampling and analytical methods that have been validated or approved by the OSHA Salt Lake Technical Center (SLTC) are to be used. To maximize defensibility of sampling results the use of sampling methods not approved by SLTC may require re-sampling with an approved sampling procedure. Unique sampling situations will arise during some inspections, and it is essential that OSHA Compliance Safety and Health Officers (CSHOs) contact and work closely with SLTC whenever sampling questions arise.
The systematic process for pre-inspection activities includes the development of a sampling plan and collection of sampling media and required sampling equipment. Sampling strategies should be planned for a meaningful evaluation of air contaminants and workplace contamination as appropriate, with the prudent use of limited resources, and with some flexibility to account for unexpected issues that frequently arise once onsite. Screening techniques and devices such as detector tubes, direct-reading meters, and thermal desorption tubes for collection of instantaneous samples may provide valuable information. These techniques can inform a sampling strategy to assess exposures for employees with the highest exposure potential (see discussion in II.B of this chapter and OSHA Technical Manual (OTM) Chapter 3, Section II: Technical Equipment: On-Site Measurements).
Knowledge of sampling procedures, including sampling media, recommended air sample volumes, and sample storage precautions are essential in planning for defensible sampling. The most up to date sampling and sample shipment guidance can be obtained by searching based on chemical name or Integrated Management Information System (IMIS) code in OSHA's OCD. For OSHA personnel with Department of Labor intranet access, the internal version of the OCD also serves as an interface to the approved sampling media for a given analyte in the Agency Expendable Supplies Program (AESP) catalog. Consistent and correct use of the OCD will help ensure that air samples are taken on the correct media, that correct flow rates and sample volumes are used, and that samples are shipped in such a way to ensure the best possible quality of analytical results.
Administrative details regarding worksite inspections and sampling may be found in the FOM.
II. Pre-Inspection Activities
A. Background Information and Pre-Inspection Sampling Plan
-
Review and follow the inspection procedures in the FOM.
-
As part of the pre-inspection review, use available information to determine whether sampling may be required (and later verify during the on-site walk-around). Also determine whether exposure to more than one chemical may occur. When this is the case, consider using the vapor hazard ratio (VHR) for air contaminants that are likely to be present as vapors. The VHR is the ratio of a chemical's equilibrium concentration to its occupational airborne exposure limit which can be used as an indication of the relative hazard potentials of different chemicals. Prioritize sampling for contaminants to which overexposures are likely to occur because of a higher VHR. See Equation 3 in section III.G for instruction on how to calculate VHR. When more than one chemical is present determine if those chemicals have an additive or synergetic health effect (see section III.G).
-
After identifying target analytes for sampling, refer to the OCD for the required sampling media, recommended sampling volume and flow rate, and potential interferences to assess exposure to chemical substances. Consider ordering extra media in case overloading occurs during sampling (see section III.D.6).
-
Determine whether special handling or shipping requirements exist for sampling media to be used, and for collected samples, prior to requesting sampling media or collecting samples. Some types of samples need to be shipped quickly and/or on ice. As an example, sampling media for isocyanates need to be stored refrigerated and protected from light until used. This information is available in the OCD. Contact SLTC for further guidance if necessary. The SLTC Duty Senior Analyst can be reached at 801-233-5001.
-
Consider direct-reading instruments and other screening tools that may be used to identify areas and individuals in a workplace with the highest exposure potential. Refer to OTM Chapter 3, Section II for technical equipment options available to complete on-site measurements. To identify unknown organic compounds SLTC offers a qualitative analysis with a quick turn-around-time. This analysis is further discussed in section III.B.
-
Specialized expertise and equipment are available to support inspection activities, along with support for routine sampling and analysis questions. See Appendix A for details.
B. Obtain Sampling Media, Equipment and Supplies
- The Cincinnati Technical Center (CTC) provides sampling media and supplies as part of the Agency Expendable Supplies Program (AESP), and equipment through the Agency Loan Equipment Program (ALEP), the Agency Excess Equipment Program (AEEP), and the Agency Technical Equipment Procurement Program (ATEPP).
-
Agency Expendable Supplies Program (AESP)
The following are some examples of sampling supply categories that may be found in the AESP:
- Detector tubes
- Sampling - Test Media (Sorbent Tubes and filters)
- Sampling - Tube Holder
- Equipment - Bags
- Gas - Accessories
- Inspection - Supplies
- Labels and Forms
- Quick Reference Cards
- Sampling - Accessories
- Sampling -Bags
- Sampling - Cyclone
- Equipment Supplies
- Shipping - Supplies
- Sampling - Adapters
- Sampling - Cassettes
- Sampling – Backup Pads
SLTC provides some specialized sampling media such as pre-weighed filter/cassette units for gravimetric sampling and analysis. Any special sampling media prepared at SLTC is ordered through the same process as other sampling media using the AESP but is shipped directly from the SLTC. Gravimetric filters are weighed at the SLTC and shipped to the field assembled in special cassettes to be used for sampling. The cassette/filter units are returned to the SLTC after sampling for gravimetric determinations and other analyses. See Appendix B for a discussion of pre-weighed filters. Refer to the OCD for individual chemicals for which gravimetric determination may be appropriate or to Appendix C for a list of substances quantified through gravimetry.
A listing of supplies available through the AESP may be found and ordered from the OSHA CTC AESP intranet site.
Offices may also place an order for expendable supplies through CTC via e-mail at CTCservices@dol.gov. The requesting office is charged for the items delivered. When placing an order, please include "AESP ORDER" in the subject line and the following information in the body of the message:
- CSHO name and telephone number
- Office name and address
- For each item ordered:
- AESP System ID Number (FES #)
- Ground or Rush Shipment
- Size or Color
- Quantity
-
Agency Loan Equipment Program (ALEP)
The ALEP allows field offices to borrow specialized monitoring equipment and other technical equipment from CTC. The typical loan period is 30 days, which can be extended, if necessary, depending on demand. Equipment can be shipped overnight if the need is urgent. The following are some examples of monitoring equipment categories available for loan equipment through the ALEP program:
- Sampling Pump/ Sampling Pump Calibrator
- Air Velocity
- Indoor Air Quality
- Dust and Fiber
A list of typical sampling and monitoring equipment available through the ALEP may be found on the OSHA CTC intranet site.
Orders for ALEP technical equipment may be made from the OSHA CTC ALEP intranet page but requires users to register first through the same email at CTCservices@dol.gov used for expendable supplies. When placing an order, please include "ALEP ORDER" in the subject line and for each item requested include manufacturer and model, a description of the item(s), and quantity.
-
Agency Excess Equipment Program (AEEP)
The AEEP program can be checked for available free sampling equipment. The AEEP enables field offices to turn in excess technical equipment to CTC so that it might be shared and used by other offices. If a field office requests a piece of equipment, CTC will check the equipment for proper operation and, if working, calibrate the equipment. It is then sent to the field office with all the corresponding property inventory paperwork. The requesting field office will receive a working piece of technical equipment to meet their needs at no cost.
A list of typical sampling equipment available through the AEEP may be found on the OSHA CTC AEEP intranet site.
Orders for AEEP technical equipment may be made through the same email at CTCServices@dol.gov used for expendable supplies. When placing an order, please include "AEEP ORDER" in the subject line and for each item requested include manufacturer and model, a description of the item(s), and quantity.
-
Agency Technical Equipment Procurement Program (ATEPP)
The ATEPP program can be used to order new equipment during an open order period which occurs annually, usually in the late spring. The ATEPP provides OSHA field offices with centralized shopping capability to buy technical equipment for their office. In addition, the ATEPP enables OSHA to standardize the Agency's equipment purchasing which results in streamlining equipment training and usage as well as improving equipment servicing efficiency.
A catalog and order form of ATEPP offered equipment and supplies can be found on the OSHA CTC ATEPP intranet site. For more information email CTCServices@dol.gov.
C. Prepare Personal Air Sampling Equipment
-
Active Sampling is performed by using a pump to draw air through a sampling media. Sampling media types include sorbent tubes, filter cassettes, gas bags, impingers, and bubblers. Prior to sampling, verify that any media to be used are not expired (see Appendix C). Assemble filter cassettes prior to the site visit when practical. Verify that the solid cassette components are firmly and completely seated against each other to prevent sample material from bypassing the designated flow path. If a filter is not firmly held within a cassette by firmly seating the cassette components that hold it in place, sampling material can bypass the filter. Do not mix brands of cassette components. A hand press can be used to ensure a good seal between the filter and the solid cassette components. Examine the assembled cassette to make certain that all joints fit together securely. Use shrink-tape or gel bands around the cassette to cover joints.
-
Ensure sampling pump batteries are fully charged. Battery care is discussed in OTM Section II: Chapter 3. Also, refer to the relevant pump manual for specific battery care guidance.
-
Calibrate personal sampling pumps before and after each day of sampling as described in Appendix D. Disconnect a pump from the charger and allow it to run for a few minutes before calibration. Use the same specific type of sample media in line that will be used for sampling in the field (e.g., a filter or sorbent tube), but do not use the calibration media to collect an actual sample in the field that will be submitted for analysis, nor as a blank to be analyzed by the laboratory. Where more than one pump will be used in the field, label the pumps to avoid confusion. Calibrate extra pumps to have available in case of a pump failure during sampling.
-
All sampling pump and flow calibration equipment should be operated within the manufacturer's stated operating specifications unless otherwise approved by CTC or SLTC. When possible, it is good practice to calibrate sampling pumps to the environment where they will be used to reduce possible sampling flow rate uncertainties. Some newer sampling pumps have a function called "automatic flow correction" for temperature and barometric pressure. This provides a pump the ability to automatically correct the flow rate of the sampling pump after calibration to within the sampling pump's operational specifications for temperature and or barometric pressure changes. Some sampling pumps either don't have automatic flow correction, have partial capability, or can operate with this feature disabled. Ensure that devices being used for sampling are acclimated to the operational sampling site environment by giving them at least 15 minutes of time to equilibrate to the temperature conditions at the site. If a sampling event will be performed at temperatures below 41 °F or above 95 °F, always verify the temperature operating range specifications in the sampling pump and flow calibrator manuals before going to the sampling site. Most air sampling pump or flowmeter manufacturers do not recommend operating below 32 °F and only a few sampling pump models, and non-primary standard flow calibrators are currently rated for use below 32 °F. See the CTC document for using air sampling equipment in extreme temperature on the OSHA CTC Information for Equipment and Accessories Intranet or contact CTC for further details. To avoid confusion in sample identification, each sample (i.e., cassette, sorbent tube, impinger liquid) must be labeled with a unique sample number.
Either label each sampler before use or prepare the OSHA-21 seals beforehand by writing in the sample numbers, and then affix an OSHA-21 seal after removing the sampler from the pump. OSHA-21 seals are shown in Appendix E. Pre-weighed gravimetric filters have pre-assigned bar code numbers that must be used for sampler identification.
Record pre-sampling calibration data (including pump serial number and initial flow rate) and the temperature and pressure of the calibration location using the OIS sampling worksheet. The OIS sampling worksheet is used to create the required sample submission document for samples requiring analysis by SLTC.
-
Diffusive (Passive) Sampling
Diffusive samplers are convenient air samplers that sample gases and vapors and do not require the use of a sampling pump. They are discussed further in section III.N.2 of this chapter. Also refer to the OCD file for diffusive sampling applications and guidance.
When using diffusive samplers, it is very important to record sampling site temperature and pressure on the OIS sampling worksheet and indicate the source of the information. See section III.N for additional information on obtaining pressure and temperature data. If this is not done, the uncertainty of the exposure assessment will be much greater and higher concentration values will be needed in excess of an exposure limit to sustain a citation.
Diffusive samplers start sampling immediately when opened and exposed to the atmosphere. Therefore, they should remain completely sealed in the manufacturer's packaging before sampling (i.e., while travelling to the site) and must be re-sealed following the manufacturer's directions immediately after sampling.
III. On-Site Inspection Activities
A. Develop Documentation Throughout Onsite Activities
-
Document accurate and complete sampling pump calibration records, sampling pump checks, and field sampling notes using the OIS air sampling worksheet.
-
Ensure accurate and consistent spelling of the inspected establishment name to facilitate future database searches.
-
Follow any special sample handling instructions provided by the OCD or in the sampling method (see section II.A.4).
-
Take photographs and/or videos (as appropriate) and detailed notes to document any factor that can assist in evaluating employee exposures. These may include sources of airborne contaminants, work practices, potential chemical interferences to exposure assessment sampling, movement of employees around the workplace during the performance of their duties, engineering and administrative controls, and other relevant factors. Document the use and availability of personal protective equipment.
-
Ventilation and/or smoke tube measurements may be helpful in assessing engineering controls, as described in OTM Section II: Chapter 3.
-
Be certain to observe and document that employees wear sampling equipment properly. Correct and document any improper placement or misuse of sampling media and equipment as soon as possible. This can be an important issue in litigation.
-
Refer to the FOM for a thorough discussion of inspection documentation procedures.
B. On-Site Sampling Plan Adjustments and Sampling Protocol
As part of the establishment walkthrough, identify the:
-
Processes/operations with exposure potential.
-
Tasks performed.
-
Materials used/materials employees are exposed to.
-
Work practices.
-
Exposure controls in place and how effective they appear to be.
Confirm the information gathered in preparing the pre-inspection sampling plan by evaluating the chemicals used on-site. Observe, to the degree possible the quantities available and utilization rates. For chemicals not anticipated in the pre-inspection sampling plan, consider indicators of volatility (e.g., boiling point and vapor pressure) and update the sampling plan with VHR data when applicable, to prioritize the actual samples to be collected. Consider whether handling practices and engineering controls are being used that would increase or decrease exposure. Ensure that the work operation being sampled are representative of the typical work activities at the facility (i.e., the employer hasn't decreased production or implemented other administrative controls during sampling).
Update the sampling plan as soon as possible after the start of the inspection by using the information obtained during the walkthrough (including any screening samples, such as detector tube or other direct read detection results) and information obtained through interviews with employees and supervisors. Sample those individuals likely to have the highest exposures (i.e., highest-risk employees) due to the materials and processes with which they work, the conditions in which they work (e.g., distance to exposure source and air movement), the tasks they perform, the frequency and duration of the tasks, and the way in which they perform the tasks (e.g., work habits and employee mobility). For example, during the initial walkthrough in a frozen food processing facility that uses dry ice, a direct-reading instrument measures an area with carbon dioxide concentrations just above the permissible exposure limit (PEL) and concentrations about 2 times that value near a pallet of dry ice. Personal samples should be collected for individuals working in the area that are closest to the dry ice for the longest duration. CSHO should also consider personal sampling for affected individuals who are experiencing adverse health effects. Sampling of unaffected individuals who enter the area for short durations but spend most of a workday at another location would be a low priority. Also consider sampling different work shifts as exposures may differ between shifts.
Determine if employees are exposed to more than one chemical, either simultaneously or sequentially. This topic is discussed in section III.G. Chemical Mixtures.
For cases where unknown organic air contaminants could be present, a CSHO may consider collecting a sample for submission to SLTC for qualitative thermal desorption analysis to determine the presence of unexpected organic compounds, or to confirm the presence of expected organic compounds. The sampling kit for this analysis may be obtained before the onsite inspection along with a next day air shipping label for return of the kit to SLTC for expedited analysis. SLTC will prioritize next-day CSHO communication by e-mail or phone with preliminary results identifying major or high priority organic air contaminants present in such samples. Through this expedited thermal desorption analysis, a CSHO can use the results to correctly sample for initially unanticipated analytes during a subsequent site visit, potentially the same week as the initial walk-through survey. This technique can also be useful during incident and fatality investigations where airborne organic chemicals are suspected to be a root cause or contributing factor. Sampling information for these qualitative samples and their analysis can be found in the Intranet OCD under "Qualitative Volatile Organic Compounds in Air, by TD-GC-MS."
Conduct representative full-shift sampling for air contaminants when determining compliance with an 8-hour time-weighted average (TWA) permissible exposure limit (PEL) standards. Full-shift sampling is defined as a minimum of the total time of the work shift less one hour (e.g., seven hours of an 8-hour work shift or nine hours of a ten-hour work shift). Make every attempt to sample as much of the work shift as possible, including segments of the greatest exposure. However, no more than eight hours of sampling can be used in the 8-hour TWA calculation (for extended work shifts refer to Section III. E.). It is best to sample for multiple time segments in each instance where there is adequate time to capture sufficient analyte to produce valid quantitative results. Following analysis, the time segments adding up to 8 hours or less, with the greatest TWA value should be used. A representative exposure sample period may be less than eight hours, although the unsampled time will be averaged assuming zero exposure.
Where relatively high airborne concentrations are anticipated or when the recommended sampling time for a sampling media is less than the duration of a work shift, collect multiple samples to cover the full shift. The collection of multiple samples can also be used to avoid filter overloading and/or sorbent saturation (refer to Section III.D.6.). The flow rate and the minimum and maximum sample volumes needed for each sample should have been pre-determined in pre-inspection sample planning. Determine these parameters as needed for the updated on-site sampling plan. Based on the limit for quantification, flow rate, occupational exposure limit, and other sampling instructions of the method, a minimum sampling time and volume can be determined (Equation 1). The recommended sampling duration listed on the method is the maximum sampling time at that flow rate and should not be exceeded. The latter period is based on analyte breakthrough as discussed in specific sampling and analytical methods, noticeable filter loading that could impair pump flow, and consideration of the need to capture the exposure durations with the highest analyte concentrations while not exceeding an 8-hour sampling time. If exposure at the relevant occupational exposure limit (e.g., PEL when applicable) can be assumed, the recommended sampling time, sample volume, and flow rate can be found on the OCD page for each specific contaminant.
Equation (1)
| Tmin/sample = | LOQ |
| OEL × r |
Where:
Tmin/sample is the minimum sampling time in minutes,
LOQ is the limit of quantitation (in many cases stated as a reliable quantitation limit (RQL)) from the sampling and analytical method, with units of mass per sample (e.g., ug/sample),
OEL is the relevant occupational exposure limit with units of mass per volume (e.g., ug/L),
r is the sampling rate with units of volume per minute (e.g., L/min).
Note: unit conversions may be necessary for unit agreement. In the case of gas or vapor analytes, use the respective "mg/m3" OEL values. For either gas phase or aerosol analytes convert to appropriate units of mass and volume as needed: 1,000 ug = 1 mg, and 1,000 L = 1 m3. When the example units are used, a ug/L value is identical to the corresponding mg/m3 value.
Do not sample for more than the recommended sampling time. In cases where the sampling time needed to evaluate the process or work shift exceeds the method's recommended sampling time, multiple samples should be collected to cover the process or shift. Sampling for more than the recommended time can overwhelm the capacity of sampling media and lead to loss of analyte. It can also cause decreased flow as filters become blocked with particulate matter. When sampling for high concentrations of contaminants, these problems also occur, and sampling time can be shortened accordingly.
C. Short Term Exposure Limits, Ceiling Limits, and Peak Exposure Values
Some OSHA expanded health standards, such as those for beryllium, formaldehyde, and methylene chloride, include permissible short term exposure limits (STEL), or in the case of asbestos and ethylene oxide, an excursion limit. A STEL is a 15-minute TWA exposure limit, while the 30-minute TWA excursion limit averaging period for asbestos exposure is unique. The excursion limit for ethylene oxide is functionally identical to a STEL, as it integrates exposures over a 15-minute period. Sampling for enforcement of any standard with a TWA type exposure limit is conducted by taking a breathing zone air sample over the specified TWA period (e.g., 15 minutes in the case of a 15-minute STEL), in accordance with the applicable sampling method found in the OCD. The 15-minute period (or periods) with the greatest exposure potential should be sampled.
Several air contaminants listed in Table Z-1 of 29 CFR 1910.1000, Table 1 of 29 CFR 1926.55, and Table Z-Shipyards of 29 CFR 1915.1000 have ceiling exposure limits that should never be exceeded, instead of 8-hour TWA PEL values. In the respective tables these are noted by a (C) designation, while Table Z-2 of the General Industry Air Contaminant Standard (1910.1000) contains a separate column for acceptable ceiling concentrations. The ceiling standards in Table Z-2 may be exceeded, but only for a specified period, and exposures for Table Z-2 air contaminants may never exceed the respective acceptable maximum peak concentration values found in the same table. If instantaneous monitoring is not feasible, such as using a direct reading instrument, then a ceiling exposure for Table Z-1 of 1910.1000, Table 1 of 1926.55 or Table Z-shipyard of 1915.1000 is measured by sampling for up to 15-minute duration, if possible, and is assessed as a 15-minute TWA. Unlike Table Z-1 ceiling standards, the Table Z-2 peak standards (analogous to ceiling standards) have no minimum sampling time.
D. Overview of the Sampling Process
- Select the employees to be monitored and discuss the purpose of sampling with them, how the equipment will be placed, and when and where the sampling equipment will be put on and removed, and when and how sampling results will be provided to them. When appropriate (e.g., full-shift sampling) try to monitor employees that will be working the entire shift.
- Stress the importance of not removing, covering, or tampering with the sampling equipment. Instruct the employees to notify a supervisor or the CSHO if the sampler requires temporary removal (e.g going to the bathroom, taking a break, or leaving the jobsite for lunch).
- If employee is wearing a cyclone, stress the importance of always keeping the cyclone vertical to protect sample integrity. If this cannot be done, another type of sampler should be considered (see section III.J. Respirable Dust).
- Resources for CSHO self-sampling can be found in OSHA Field Safety and Health Management System (SHMS) Manual, Chapter 27 - Exposure Monitoring. Due to difficulties of attaching pumps and sampling media when working alone, consider using a sleeveless vest (see Figure 6) to slip sampling equipment on and off. All self-sampling information must be entered in the OIS in accordance with OSHA Information System User Guide - Self Sampling.
- Assemble the sampling train by attaching one end of the sample tubing to the sampling pump (if needed), and the appropriate attachments or sampling tube holders to the other end. Use the minimum length of tubing necessary to connect the sample pump inlet with any attachments needed, accounting for the need to maintain some slack that will allow the sampled worker to move and complete anticipated tasks.
- Place the calibrated sampling equipment on the employee so that it does not interfere with the employee's work performance or safety.
- Attach the sampling pump to the employee's belt (with the flexible sample tubing already attached to the pump). A CSHO may need to supply a sturdy adjustable-length belt in some cases to allow for pump attachment, and these are available through CTC AESP. Secure tubing to the employee to prevent snagging and to avoid interfering with the employee's work. For example, use a collar clip to attach the sampler to the employee's lapel or as appropriate to another area of clothing within the breathing zone (i.e., in a hemispheric area forward of the shoulders within a 6-to-9-inch radius of a worker's nose and mouth), and tape the tubing to the employee's back using duct tape. Collar clips and duct tape are available through CTC AESP. The CSHO should take care when using clips or duct tape on worker's personal clothing if the material is deemed to be fragile. Neck lanyards, medical tape, or other options should be considered to avoid damage to employee clothing.
- Attach the appropriate sampling media (filter cassette, charcoal tube, etc.) to the flexible tubing after removing the outlet plug or cap. For flame-sealed sorbent tubes, safely break open both ends before attaching a sorbent tube to the sample tubing. Do not allow glass fragments to fall onto surfaces but contain these in a manner which will allow for proper disposal. Protective plastic caps shipped with such tubes may be briefly placed over the open sorbent tube ends if the tubes must be opened in an area away from the sample collection site before they are placed in a sampling train. This will likely be required when sampling in any establishment where food products are processed or served, or in other situations where the possible presence of small glass fragments will pose a problem. If this approach is followed, be sure to retain the plastic caps to seal sorbent tubes immediately after sampling as discussed below.
- Adjust the attachment of the sample collection device (use a tube holder for glass sampling tubes) to place the sample inlet in the employee's breathing zone. When possible, the collection device inlet should be oriented in a downward vertical position to avoid gross contamination from airborne debris falling into the collection device. Some sampling tubes are collected in series or use a large tube holder and it is impractical to have the tubes facing down. In this case try to get the sampler to fit comfortably on the employee with the inlet near the breathing zone. Except for the sampling of whole air into bags (e.g., carbon dioxide or carbon monoxide), sampled air should not pass through any tubing before entering the collection device to avoid loss of the contaminant of interest to the tubing walls.
- For an employee wearing a respirator (including a supplied-air hood for welding or abrasive blasting), place the sampler outside of the respirator. This action is necessary to determine whether the respirator's Assigned Protection Factor (APF) is adequate. For an employee wearing a welding helmet (which is not a respirator), the collection device shall be placed under the helmet.
- Open the inlet to the collection device. Retain the cap, plug, or cassette face cover as appropriate, and turn on the air sampling pump. Verify that the pump is operating correctly. This can be done by visually checking the pump rotameter (if equipped) or digital flow readout, or by touching the pump to feel for vibration.
- Document the sampling pump start time and other required information. For diffusive samplers be sure to record the sampling site temperature and pressure.
- CSHOs must monitor and document the status of the sampling equipment throughout the employee's work shift to assure the pump continues to work properly, and that the sampler being used is not overloaded (in the case of a filter collecting aerosol). Overloading of a filter is characterized by the presence of loose material in the filter cassette, and/or by a reduction in the sampling pump flow rate. For adsorbent media, overloading occurs when the ability of the sampling media to effectively collect a gas phase analyte is compromised. In practice, overloading of a sorbent tube used for sampling gas phase analytes is difficult to detect. In general, overloading of such media can be avoided by replacing the sample media several times during the work shift once the minimum sample volume is achieved. Refer to the OCD and the sampling method for minimum sampling times. A CSHO shall document if recommended sampling media replacement does not occur and describe the reason for this, for example, due to the inability to approach a sampled worker to change out the sampling media.
If detectable overloading does occur, immediately replace the sampling media. The sample may still be analyzed, although the reported results may be lower than the actual air concentration sampled if the sampling flow was reduced due to the overloading. - CSHOs should periodically monitor a sampled employee throughout the workday to ensure that sample integrity is maintained, and that cyclical activities and work practices are identified. Do not enter areas where sampling is being conducted without the appropriate PPE. Frequent pump checks may be necessary, especially when heavy filter loading is possible. For air sampling filters and sorbent tubes, verify downward orientation of the sampler inlet and for aerosol sampling verify symmetrical deposition of particulate on the filter. There should be no large particles on a filter, since these do not move with the airstream. Check for evidence of tampering with the sample or pump. Ensure that the sampling train remains properly assembled, and that the tubing does not become pinched or detached from the collection device or from the pump. Thicker tubing may help prevent pinching or kinking and is available through CTC AESP. Check the pump flow readout or rotameter to be sure the pump is still running. In case of pump failure, document conditions and time and replace with a new pump and new sampling media, if available. Record any relevant observations. Turn off or remove sampling pumps immediately prior to an employee leaving a potentially contaminated area (such as when they shower, go to lunch, or on a break in a clean area). If these areas also appear contaminated and are considered part of the workplace, continue sampling and assess the need for surface contamination measurements (see Section II, Chapter 2, Surface Contaminants, Skin Exposure, Biological Monitoring and Other Analyses). If the pump is turned on and off during the day and/or if the sampling media is changed, document subsequent start/stop times (time on/time off).
- Before removing the pump at the end of the sampling period, check the pump flow readout or rotameter to be sure it has remained operational throughout the sampling period. If the pump shows a fault or lower than expected sampling flow rate or volume, document this in the OIS air sampling worksheets. Note that appropriate precautions (e.g. PPE) should be used when handling potentially contaminated sampling equipment.
- Turn off the pump and document the stop time to the nearest minute (time off).
- Remove the sample collection device from the sample tubing and close off both the inlet and the outlet of the sampling media as appropriate, for example using the retained caps or plugs. For all other samplers, after sampling has concluded, follow instructions found in the OCD regarding sample handling that were noted previously in the pre-inspection sampling plan.
- Seal the collection device with a Form OSHA-21 as soon as possible after sampling (see Appendix E regarding Form OSHA-21 seals, sample integrity, and chain of custody requirements).
E. Extended Work Shifts
For sampling work shifts that extend beyond eight hours, CSHOs can choose one of two approaches.
The first approach is to sample what the CSHO believes to be the worst continuous 8-hour work period of the entire extended work shift (e.g., two consecutive four-hour work periods separated by a lunch break).
The second approach is to collect multiple samples over the entire work shift. Multiple samples are thus collected during smaller consecutive time intervals throughout the whole work shift. The employee's exposure will then be calculated based upon the worst case 8-hour period of exposure during the entire work shift, which need not be contiguous. For example, on a 10-hour work shift, following an established sampling protocol specified in the OCD, ten one-hour samples or five two-hour samples could be taken, and the eight highest one-hour sample results or the four highest two-hour sample result could be used to calculate the employee's 8-hour TWA exposure for compliance purposes. This approach requires that the sample duration for each individual sample is long enough to meet the minimum sample time for the analytical method specified by the laboratory.
Some standards will require PEL adjustments if the work shift is longer than 8 hours. For example, the lead standards for construction (29 CFR 1926.62) and general industry (29 CFR 1910.1025) require PEL adjustments with respect to extended work shifts (longer than eight hours). Similarly, under the Cotton Dust standard (29 CFR 1910.1043), the PEL must be proportionately reduced for extended work shifts for the purpose of determining appropriate use of respiratory protection.
For substances with ceiling limits (e.g., butylamine), no adjustment is needed for work shifts beyond 8 hours, since ceiling limits do not depend on the length of time worked.
The PEL values for other substances have been set either by technologic feasibility (e.g., vinyl chloride) or good hygiene practices (e.g., methyl acetylene). These factors are independent of the length or frequency of work shifts. The PEL for substances in this category also should not be adjusted.
F. Air Contaminants Related to Heating and Combustion
Combustion and thermal breakdown products can include those originating from volatilization, reaction, or oxidation of base materials, metals, and coatings. Certain air contaminants are associated with combustion processes. Carbon monoxide (CO) exposures should be suspected whenever combustion-powered equipment, particularly gasoline-powered equipment (but also equipment powered by natural gas or propane), is used in areas with limited ventilation. Without a catalytic converter, gasoline-powered equipment typically produces thousands of parts per million (ppm) of tailpipe CO concentrations, as compared to a few hundred ppm produced by propane-powered equipment. The current PEL value for CO is 50 ppm. Another contaminant produced in combustion processes is nitrogen dioxide (NO2), which has a ceiling PEL value of 5 ppm and is produced by propane-and diesel-fueled equipment.
Other operations that should be evaluated for the generation of hazardous air contaminants from heating are welding, brazing, torch cutting, and plasma cutting. The composition and quantity of chemicals present in welding and brazing fumes or from oxy/acetylene and plasma cutting are dependent upon the metal being heated, the process used, the presence of paint or plated coatings in an area to be heated, and the flux and electrode material used. Fumes from plasma cutting may contain carbon monoxide when carbon dioxide is used in the plasma gas. Welding fumes may contain complex oxides or compounds of the following: amorphous silica, calcium, chromium, iron, fluoride, manganese, molybdenum, nickel, strontium, titanium, vanadium, beryllium, and zinc. In cases where lead-containing paint or corrosion-resistant plating is present in material being heated in any of the processes noted above, the fumes generated may contain lead and cadmium. Ozone, and nitrogen oxides may be produced during welding. Other conditions, which influence the composition and quantity of the fumes and gases to which workers may be exposed include the number of welders, the air volume of the work area, and the quality and amount of exhaust ventilation. Safety data sheets (SDS) for welding electrodes, wire, and fluxes should be consulted to determine if components are present that present a need to evaluate employee exposures. In cases where the composition of base metals or other items being heated is not known or when plated or painted metal is heated, select a sampling and analytical method that includes quantitative measurement of a wide range of metals, including lead and cadmium. "Weldable paints" may thermally degrade to aldehydes, butyric acid, bisphenol A, and numerous other organic molecules. Sampling for welding is discussed in Section III.L., Metals.
Where heated processes are present in a workplace, it may be necessary to sample for thermal decomposition products derived from carbon-containing (organic) materials. In some cases, these are discussed in the SDS for a product used at the establishment. In other cases, guidance is available from SLTC for specific industrial processes. For example, in the polymer resin and plastics industries, machining, torch or laser cutting, or overheating of molding equipment may produce toxic decomposition products such as carbon monoxide or cyanide. The following thermal decomposition products are associated with specific types of plastic: hydrogen chloride from polyvinyl chloride (PVC); styrene from polystyrene; fluoride compounds from polytetrafluoroethylene (PTFE or Teflon®); cyanide compounds from urethanes, nylon, and acrylonitrile. Further information may be found in industrial hygiene references such as Patty's Industrial Hygiene and Toxicology.1
[1]Rose, V.E., and B. Cohrssen: Patty's industrial hygiene and Toxicology, 6th ed.; John Wiley & Sons Inc: New York, 2011.
G. Chemical Mixtures
-
Chemical Interactions
Employees may be exposed to a variety of chemical substances simultaneously - due to the use of several chemicals in an operation, the presence of impurities, or from chemical reactions and byproducts. Such exposures may result in different effects than would be experienced with exposure to only one chemical and, in many cases, can lead to an increase in the severity of adverse health effects experienced by employees. When exposure to multiple chemicals exists in a workplace, CSHOs should review the SDSs and the health effects and target organ information in the OCD to determine whether the chemicals affect the same body organ or physiologic system.
Simultaneous exposure to multiple chemicals can cause health effects in different ways: An additive effect occurs when the combined health effect of the simultaneous exposures is equal to the sum of the effects of each individual substance alone. For example, the cholinesterase inhibition of two organophosphate pesticides is usually additive when exposure occurs together. Similarly, many solvents have narcotic effects that are considered additive in nature. Below are additional examples of chemicals which have additive effects when exposure occurs together:
- acetonitrile + cyanides
- n-hexane + hexone (methyl isobutyl ketone [MIBK]); 2,5 hexanedione or 2,5 hexanediol (all cause peripheral neuropathy)
-
carbon monoxide + methylene chloride
A synergistic effect occurs when the combined effect of the exposures is much greater than the sum of the individual effects. Examples include the synergistic effect of carbon tetrachloride and ethanol on liver toxicity and the synergistic effect on the lungs of smoking and exposure to asbestos.
Potentiation describes a condition in which the target organ toxicity of a particular chemical is markedly increased by exposure to another chemical which does not ordinarily have toxic effects on that organ or system. For example, isopropanol is not a liver toxin, but when combined with exposure to carbon tetrachloride (liver toxin), the liver toxicity is much greater than with carbon tetrachloride exposure alone. Ethanol also potentiates the toxicity of many chlorinated hydrocarbons.
Antagonism refers to the toxic effects of two chemicals interfering with one another, or when the effects of one chemical are reduced by exposure to another chemical. This is the basis for many antidotes. Antagonism can occur by several different mechanisms. When chemical antagonism takes place, for example with chelating agents, two chemicals react in the body to a less toxic form, or one that is more readily eliminated from the body. Functional antagonism refers to two chemicals having opposite effects on the same system, such as central nervous system (CNS) stimulants and depressants. Competitive antagonism refers to chemicals acting on the same receptor, such as nicotine and ganglionic blocking agents. Noncompetitive antagonism refers to the toxic effect being blocked by some other means, such as atropine reducing the toxicity of cholinesterase inhibitors.
-
Mixture Formula
OSHA's Air Contaminants standards provide a formula for assessing exposures to chemicals having additive effects [for general industry see 29 CFR 1910.1000(d)(2) and for shipyards see 29 CFR 1915.1000(d)(2)]. This calculation should be used when multiple components in a mixture affect/target the same body organ or physiological system. This formula calculates equivalent exposures occurring simultaneously or for TWA exposures occurring consecutively within the same work shift.
The mixture calculation is expressed in Equation 2:
Equation (2)
Em = C1 + C2 + ··· + Cn L1 L2 Ln Where:
Em is the minimum equivalent exposure for the mixture (Em should be less than or equal to 1 for compliance);
Cn is the measured concentration of a particular substance;
Ln is the corresponding occupational exposure limit for a particular substance in the same units as the concentration.Section IV.D. describes sampling and analytical error (SAE) calculations for use of the mixture formula, and example calculations are provided in Appendix G. In addition, an online calculator is available to CSHOs on the OSHA Directorate of Technical Support and Emergency Management's Intranet page which will calculate a mixture control limit. Simply input the exposures, occupational exposure limits, and SAE values, and the program will calculate values using Equation 2 and the equations in Appendix F.
The mixture formula may be used to assess employee exposures to chemicals having synergistic effects. However, since the health effects are generally more severe in this scenario, it may be appropriate to apply an increased penalty. As per FOM Chapter 4, all such cases should be discussed with the supervisor and referred to the Regional Administrator. The following resource may be used to determine whether there is evidence for synergistic effects: Chemical Mixture Risk Calculation IRSST.
-
Air Sampling for Mixtures (Sampling Strategies)
The following three examples present SDS information for products containing mixtures. They illustrate processes to determine which ingredients should be evaluated for employee exposures.
Example 1
Sample Safety Data Sheet
Section 1: Product Name: Aromatic Cleaning Solvent
Section 3 Composition: Ingredient
CAS No.
Percent
Hazardous
Toluene
108-88-3
>50%
Yes
Proprietary solvent
-----
40-49%
No
Benzene
71-43-2
1%
Yes
Section 8: Exposure Controls / Personal Protection
OSHA Permissible Exposure Limits:
Toluene:
200 ppm TWA PEL
300 ppm Acceptable Ceiling Concentration
500 ppm Acceptable Maximum Peak Above the Acceptable Ceiling Concentration for an 8-hr ShiftBenzene:
1 ppm TWA*
5 ppm STEL**Except industry segments for which 29 CFR 1910.1000, Table Z-2 exposure limits apply
Section 9: Physical and Chemical Properties:
Vapor Pressure of the Mixture (mmHg): 30-40
Since the SDS does not report the physical properties for the individual ingredients, it is necessary to look at other reference information to determine the relative volatility of the components. Physical properties for specific chemicals may be found in either the OCD file for each chemical, or in the NIOSH Pocket Guide to Chemical Hazards.
Excerpts from NIOSH Pocket Guide:
Toulene:
Boiling point: 232°F
Vapor Pressure: 21 mmHg
IDLH: 500 ppmBenzene:
Boiling point: 176°F
Vapor Pressure: 75 mmHg
IDLH: 500 ppmSampling priority can be determined by calculating the Vapor Hazard Ratio (VHR) of each component using the following equation:
Equation (3)
VHR = P·106 OEL·760 mm Hg Where:
P is the chemical's vapor pressure in mm Hg;
OEL is the occupational exposure limit in ppm.Using the vapor pressures and occupational exposure limits (8-hour TWA PEL) provided above for benzene and toluene yields VHRs of 9.9 × 104 and 1.4 × 102, respectively. Because the VHR for benzene is higher that the VHR for toluene sampling in this example should be conducted for benzene.
Example 2
Sample Safety Data Sheet
Section 1: Product Name: Gravure Ink
Section 3: Composition: Ingredient
CAS No.
Percent
OSHA PEL (8-hr TWA, ppm)
Other Exposure Limits (ppm)
Toluene
108-88-3
29%
200
300 ceiling(OSHA)
500 peak (OSHA)1,2-propanediol
57-55-6
5%
None
not found
Xylene (mixed)
1330-20-7
31%
100
150 STEL
(NIOSH and ACGIH)Section 9 – Physical Properties: % Volume Volatile: 88.6
Again, the physical property information on the SDS does not indicate the relative volatility of the components, so it is helpful to refer to the OCD file, which includes information taken from the NIOSH Pocket Guide.
Excerpts from OCD and/or NIOSH Pocket Guide: Chemical
Boiling Point
Vapor Pressure
Toluene
232°F
21 mmHg
1,2-propanediol
188°C
0.05 mmHg
m-xylene
282°F
9 mmHg
Review of the OCD file for 1,2-propanediol (CAS number 57-55-6) reveals the more common name, propylene glycol. Because propylene glycol does not have a PEL or TLV, direct citation for exceeding an OEL may not be possible. In fact, propylene glycol is a Food and Drug Administration (FDA)-approved food additive which is "generally recognized as safe." Due to its low concentration, low volatility, and low toxicity, sampling for this material is not a priority.
Sampling for both toluene and the xylenes is recommended if significant quantities are used without adequate local exhaust ventilation. Additionally, toluene and xylenes have similar target organ effects, so the exposures should be evaluated as a mixture using Equation 2. Toluene and xylenes share the following target organs: central nervous system, eyes, skin, respiratory system, liver and kidneys. The VHR values for each are respectively 1.4 x 102 and 1.2 x 102. The VHR value for m-xylene accounts for a lower PEL value (which tends to increase the relative VHR), which is somewhat balanced by a lower vapor pressure (which tends to decrease the VHR). These calculations confirm that both solvents pose a roughly equal risk for overexposure using the simple VHR model, if all other factors are equal.
Note that this SDS includes references to non-OSHA occupational exposure limits – in particular, limits set by NIOSH and American Conference of Governmental Industrial Hygienists (ACGIH). NIOSH sets Recommended Exposure Limits (RELs), while ACGIH sets Threshold Limit Values (TLVs). Note that there are cases where no OSHA ceiling value exists for a chemical (xylene), but there is a NIOSH or ACGIH STEL. Such cases should be referred to the Regional Administrator if exposure exceeds an ACGIH or NIOSH STEL or ceiling value (FOM Chapter 4).
Example 3
Sample Safety Data Sheet
Section 1: Product Name: Indoor/Outdoor Spray Paint – True Blue
Section 3: Composition: Ingredient
CAS No.
Percent
Exposure Limits
Vapor Pressure
Propane
74-98-6
25%
PEL 1,000 ppm
760 mmHg
VM & P Naptha
8032-32-4
12%
TLV 300 ppm
12 mmHg
Toluene
108-88-3
15%
PEL 200 ppm
TLV 20 ppm22 mmHg
Light Aromatic Hydrocarbons
64742-95-6
1%
Not available
4 mmHg
*1,2,4-Trimethylbenzene
95-63-6
2%
PEL 25 ppm
2 mmHg
Acetone
67-64-1
30%
PEL 1,000 ppm
180 mmHg
Titanium Dioxide (Total Dust)
13463-67-7
0.1%
PEL 15 mg/m3 TLV 10 mg/m3
n/a
*Regulated under Construction and Shipyard standards only
Section 5: Fire Fighting Measures:
Flash Point of Propane: <0°F
LEL 0.7%
UEL 12.8%The PEL for propane is 1,000 ppm and it constitutes 25% of the mixture. Propane is essentially a "simple asphyxiant," meaning it has low toxicity, with its flammability hazard a greater concern. It is relevant to monitor as a flammable gas safety issue but is a low priority for sampling to enforce the air contaminant standard. The VHR value for a gas can be approached by assigning it a vapor pressure of 760, which is equal to atmospheric pressure. It is sufficiently volatile that there are no limitations to its atmospheric concentration (unlike solvents such as toluene or benzene). Following this approach, propane will have a VHR of 1.0 x 103 which makes it a candidate for sampling in situations where large amounts of the paint are used in a relatively enclosed environment.
Among the solvents known to be present, the greatest VHR value is calculated for acetone (2.4 x 102), owing to its relatively high vapor pressure despite its high PEL value. This chemical is also present in the mixture at a relatively high percentage of the total product and should be considered for air sampling.
The VHR value for toluene (1.4 x 102) and its presence as 15% of the mixture also make it a good candidate for sampling. The VHR value obtained for 1,2,4-trimethylbenzene is 1.1 x 102, although its presence at only 1% of the total mixture tends to lower its exposure potential. Examination of OCD information for either toluene or 1,2,4-trimethylbenzene shows that both are members of Organic Vapor Sampling Group 1 and may be sampled simultaneously using OSHA Sampling and Analytical Method 5000. This resolves any questions regarding the need to sample for 1,2,4-trimethylbenzene if toluene is already considered, as sampling for both chemicals can occur simultaneously using a single coconut shell charcoal sorbent tube.
Since all these solvents are likely to have similar narcotic effects, the OCD should be reviewed to determine whether they have the same target organs, in which case the mixture calculation should be applied.
The titanium dioxide (TiO2) is present at a very low concentration, has a very high PEL value, and will be released in a wetted form. Gravimetric sampling for the TiO2 is not a priority.
Information regarding the exact identities of the light aromatic hydrocarbons may be obtained using thermal desorption tube sampling kits. These kits may be rush delivered from SLTC along with a next-day return shipping label. Preliminary qualitative results are typically available one day after receipt of the returned sampling kits. Thermal desorption tube sampling can further guide decisions regarding sampling that may be warranted for the light aromatic hydrocarbons which likely include xylenes and other trimethylbenzene isomers, as well as information on any undisclosed volatile organic compound that may be detected using the kit.
H. Field Blanks
Field blanks are used by SLTC to determine if contamination has occurred at any point beginning with the manufacture or preparation of a sampling media and the final analysis of a sample, including sample handling, shipping, and storage. Field blanks (e.g., sorbent tubes, filters, absorbing solution) are clean sample media that are taken and opened in a clean area at the sampling site, but they are not used to take samples. Field blanks are required for each requested analysis and for each lot number of sampling media. Prepare field blanks during the sampling period for each type of sample collected. One field blank will usually suffice for each requested analysis. Asbestos is an exception and requires a minimum of two field blanks, even for a single asbestos sample.
Field blanks should be taken at the same worksite and during the same period as employee sampling. They should be handled, stored, and shipped in the same manner as other sampling media used in sampling air contaminants, except no air is drawn through them. To prepare a field blank, take an unused sample media and open the outlet and inlet of the device in a location where no exposure to a contaminant is expected. Reposition the caps or plugs over the outlet and inlet of the media immediately after it has been opened. For bubbler or impinge solutions, the solution should be opened and placed into a clean impinger or bubbler as appropriate, and then decanted into the container to be used for shipping. Seal all field blanks using OSHA-21 Forms as described in Appendix E. Ship the blank sample with the exposure samples.
I. Total Dust
Total dust sampling is used to evaluate exposures to a variety of aerosols. The General Industry Air Contaminants standard notes the following: "All inert or nuisance dusts, whether mineral, inorganic, or organic, not listed specifically by substance name are covered by the Particulates Not Otherwise Regulated (PNOR) limit which is the same as the inert or nuisance dust limit of Table Z-3." Use total dust sampling for toxicologically inert, or nuisance dusts for which no PEL exists, or which have PEL values identical to those for PNOR. The term "particulates not otherwise regulated" is used in 29 CFR 1910.1000 Table Z-1, in 29 CFR 1915.1000 Table Z-Shipyards, and in 29 CFR 1926.55 Table 1.
Total dust sampling uses pre-weighed PVC filters to determine the total mass of dust collected during the sampling period. The OCD pages for various analytes typically sampled using these filters link to CTC AESP where pre-weighed filters can be ordered. For total dust sampling, use a maximum flow rate of 2 L/min for a maximum sampling time of 480 minutes or eight hours. Visually check the filter during the sampling period to avoid overloading. Overloading may be evidenced by the presence of loose material in the filter cassette, by a darkening of the filter, and/or by a reduction in the sampling pump flow rate. Check for overloading by looking into the inlet of the sampling cassette, using a flashlight if needed.
J. Respirable Dust
Respirable dust sampling requires the use of a particle size-selector to separate and capture those particles in the size range which would be deposited in the gas-exchange region of the lung. When these samplers are operated at their designed flow rate, they will separate dust particles according to size; larger particles are removed while the respirable fraction is captured on a pre-weighed filter on the device.
The size-selective convention for sampling respirable particulates is specified by the International Organization for Standardization (ISO) in ISO 7708:1995: Air Quality-Particle Size Fraction Definitions for Health-Related Sampling. The American Conference of Governmental Industrial Hygienists (ACGIH) and the European Committee for Standardization (CEN) have adopted identical criteria. This ISO/ACGIH/CEN convention utilizes a mathematical model with a sampling efficiency curve shown in Figure 1 below. Note that particles with an aerodynamic diameter of 4 µm are collected at 50% efficiency, often referred to as the "50% cut point".

Figure 1: ISO/ACGIH/CEN Respirable Particulates Collection Efficiency Versus Aerodynamic Diameter Showing 50% Cumulative Cut Point at 4.0 µm. Bartley, D.L.; Chen, C.C.; Song, R.; Fischbach, T.J. Respirable Aerosol Sampler Performance Testing. Am. Ind. Hyg. Assoc. J. 1994, 55, 1035-1046.
Some examples of respirable dust samplers are shown in Figure 2 below. Such devices must be calibrated and used according to the manufacturer's instructions to ensure that they sample correctly. For instance, the Dorr-Oliver cyclone set to a flow rate of 1.7 L/min will separate larger particles which are captured in the grit pot of the cyclone, while the respirable fraction is deposited on a pre-weighed PVC filter for gravimetric analysis. The Dorr-Oliver cyclone has been shown to demonstrate reasonable agreement to the ISO/ACGIH/CEN criteria and may be used for respirable dust sampling. Other devices may also be used if verified to show agreement to the same criteria.

Zefon Dorr-Oliver Cyclone Assembly

SKC Aluminum Cyclone

SKC Parallel Particle Impactor (PPI)
Figure 2: Example Respirable Dust Size-Selective Samplers.
Appendix B provides additional information for pre-weighed filters. The intranet OCD pages for specific respirable dusts contain additional information per analyte and link to the AESP where pre-weighed filters can be ordered. The OCD pages also include links to sampling methods and information on samplers that may be utilized.
The Dorr-Oliver cyclone requires cleaning, leak testing, and calibration procedures that must be followed to ensure proper operation. Refer to the CTC intranet page for additional information.
There may be work activities where the employee must stoop, crawl, climb, or do similar activities and where a cyclone, if utilized, could become inverted and cause larger particulate from the grit pot to deposit onto the sampling filter and cause an inaccurate result. In these cases, a different type of samplers that can be inverted during use, such as the SKC PPI device, should be utilized.
K. Crystalline Silica
Crystalline silica samples are collected using a Dorr-Oliver or other suitable size-selecting device as described previously for respirable dust samples. A silica sample collected without a size-selector would be considered a total dust sample and analyzed accordingly. If the collected sample is non-respirable, SLTC must be advised on the OIS air sampling worksheet. The silica PEL is for respirable crystalline silica and cannot be applied to total dust samples.
Request analysis for "silica, crystalline, mixed respirable (quartz, cristobalite, tridymite)". Note that most respirable crystalline silica is quartz. All samples submitted to SLTC will be analyzed for quartz. Cristobalite is a polymorph of respirable crystalline silica that occurs in workplaces where silica is heated to extremely hot temperatures (>1400 °C). If it is suspected that cristobalite may be present, note this on the OSHA Sampling Sheet. Cristobalite is rare and will usually only be analyzed if there is reason to suspect its presence. Tridymite, which is even rarer, cannot be analyzed by SLTC until a suitable standard reference material is readily available.
X-ray diffraction (XRD) is the preferred analytical method for silica because of its sensitivity, its minimum requirements for sample preparation, and its ability to identify polymorphs (different crystalline forms) of free silica. Polymorphs are initially identified by major (primary) x-ray diffraction peaks. If significant levels of polymorph are identified, presence is confirmed using secondary, tertiary, and/or quaternary peaks to eliminate the possibility of interfering crystalline substances. CSHOs should notify SLTC if any of the following substances are known to be present in the workplace:
Aluminum Phosphate (Berlinite)
Biotite
Clinoferrosilite
Copper
Graphite
High Albite
Iron carbide
Kaolinite
Lead sulfate
Leucite
Microcline
Muscovite
Orthoclase
Potassium Hydroxide
Sanidine
Sillimanite
Wollastonite
Zircon
When a sample is reported as not detected, this indicates that the quantity of quartz (or cristobalite) present in the sample is not greater than the detection limit of the instrument. The detection limit is usually 10 μg for quartz and 20 μg for cristobalite. If less than a full-shift sample was collected, CSHOs should evaluate a not-detected result to determine whether adequate sampling was performed. If the presence of quartz (or cristobalite) is suspected, CSHOs may want to sample for a longer time to increase the amount of sample collected. A sampling collector with a higher flow rate, such as an SKC PPI device, may also be considered to increase sample amount collected.
When employees are exposed to silica during abrasive blasting, the sampling collector should be placed outside of the abrasive blasting hood respirator. In other operations where an employee is wearing protective head/face gear such as a hood or face shield (not a respirator), the sampling collector should be placed under the hood/face shield, as applicable (but always outside of any respiratory protection worn). A smaller sampling collector, rather than a cyclone, may be necessary in this circumstance for it to fit under the protective gear.
L. Metals
SLTC can analyze a variety of metals in specific combinations, which are defined in Metals Sampling Groups. Metals that can be simultaneously digested in the same acid matrix and that are stable when collected on a specific sampling media can be analyzed together. Other metals, such as beryllium and hexavalent chromium, must be sampled and analyzed individually. Visit the OCD for detailed information on the specific methods used for metal analysis.
SLTC has three major sampling groups for metal analysis. CSHOs can take advantage of these groups by sampling and requesting analysis of the suite of analytes based on the metals suspected to be most hazardous and predominant in the workplace.
| METALSSG-1 | METALSSG-2 | METALSSG-3 |
|---|---|---|
|
Arsenic |
Antimony |
Antimony |
All metal results are reported as an elemental composition rather than as a metal compound. The spectroscopy-based analytical techniques only detect and measure the presence of elemental metals, and the final identification of actual workplace contaminants present should be determined by the compliance officer through knowledge of the workplace conditions/operations where such samples have been collected. To assist compliance officers in converting metal elemental results to a corresponding compound, the OCD has been updated with stoichiometric factors for metal compounds that have OSHA PELs.
For example, a sample for zinc oxide (ZnO) and zinc chloride (ZnCl2) will have results reported as zinc (Zn). Both ZnO and ZnCl2 have PELs, and results for Zn can be converted to ZnO by multiplying the Zn result by 1.245. Results for Zn can also be converted to ZnCl2 by multiplying the zinc result by 2.084. The individual collecting the sample must determine whether Zn, ZnO, or ZnCl2 was sampled based on the materials and conditions at a worksite, and any operations being performed. Once this is known, apply the appropriate stoichiometric factor to process the data.
When sampling welding operations, the following should be considered:
-
When sampling for welding fumes, CSHOs must consider what type of PPE employees are using. Studies have shown that the use of a welding helmet alone typically results in an exposure reduction in the wearer's breathing zone.2 If an employee is using a welding helmet, the filter cassette used for sampling must be placed in the inside of the welding helmet to obtain an accurate measurement of the employee's breathing zone exposure. The sampler is placed on the collar or shoulder of the employee's breathing zone but beneath the helmet when it is positioned for use. Alternatively, sampling attachments for welding helmets are available through the AESP.
-
Welding fume samples are normally taken using a 37-mm mixed cellulose ester filter (MCEF) mounted in a cassette. If a standard cassette for a 37-mm filter will not fit inside the helmet, a 25-mm MCEF and cassette may be used. Extra care must be taken not to overload the smaller 25-mm MCEF when sampling. Figure 3 provides a photo of the 25-mm and 37-mm filter cassettes to demonstrate their relative size difference.

Figure 3. 25-mm (left) and 37-mm (right) filter cassettes.
-
If the employee is using respiratory protection, the sampler must be placed outside the respirator in the employees breathing zone. If the employee is wearing both a respirator and a welding helmet, the sampler must be placed outside the respirator but under the welding helmet in the employee's breathing zone.
-
If the welder is using a supplied air or Powered Air Purifying Respirator (PAPR) welding hood, the sampler should be placed outside of the hood and not in a position directly below the hood where the fresh air may be blowing. Position the sampler on or near the shoulder.
-
Some metals, such as vanadium, will have different and specific exposure limits for both dust and fume. In those situations, the physical form of a sample (dust, mist, or fume) is identified by the compliance officer through observation and available documentation of materials and processes.
-
Gravimetric determination is conducted using pre-weighed-filters (see Appendix B) including some metals, such as aluminum. Pre-weighed filters are used for gravimetric determination and can be submitted for other analysis after gravimetric determination has been performed. See OSHA's OCD for more details.
-
Bulk samples can be used by CSHOs to document sources of contamination, or as a screening tool to evaluate analytes of interest for personal air sampling. This qualitative analysis can be done by selecting analysis for one of the different sampling groups, or for a specific analyte. Results are reported back as a percent composition by weight. Bulk samples should be representative of the worksite conditions and where possible should consist of a minimum of approximately 300 mg of homogenous material. Bulk samples for metal analysis are generally shipped in glass 20-mL scintillation vials with PTFE-lined caps. See Figure 4 for an example of a 20-Ml scintillation vial containing approximately 300 mg of bulk sample material.

Figure 4. 20-mL scintillation vial containing approximately 300 mg of bulk sample material.
[2] Welding helmet airborne fume concentrations compared to personal breathing zone sampling
D Liu 1, H Wong, P Quinlan, P D Blanc, Am Ind Hyg Assoc J. 1995 Mar;56(3):280-3.
M. Asbestos
Collect samples for asbestos using 0.8-μm, 25-mm diameter MCEF cassettes which have been designated by the manufacturer for asbestos analysis. The filters must be contained in an electrically conductive cassette assembly that includes a 50-mm extension cowl (see Figure 5 below). An electrically conductive cassette is necessary to prevent loss of fibers to the walls of the cassette due to electrostatic forces. Ensure that the bottom joint (between the extension and the conical black piece) of the cassette is sealed tightly and that a shrink band or electrical tape is used to hold the cassette pieces together. Fasten the cassette to the worker's lapel and connect the pump to the base of a sampling cassette with flexible tubing. Remove the entire end cap cassette piece and take air samples with the cassette face open. Assure that each sample cassette is held with the open face inlet pointing downward in the employee's breathing zone during sampling.

Figure 5. 0.8-μm pore size, 25-mm diameter MCEF cassette in an electrically conductive cassette assembly with 50-mm extension cowl. The end cap of the cassette visible on the right includes a blue Luer type plug. The entire end cap must be removed to allow open face sampling.
Use a flow rate in the range of 0.5 to 5 L/min. 1 L/min is required for general sampling. For office environments use flow rates up to 5 L/min.
Calibrate as discussed in Appendix D. Do not use nylon or metal (e.g., stainless steel or plated brass) adapters if in-line calibration is done. Do not use the same filter cassette intended to be used for field sampling for sampling pump calibration.
Samples can be taken for between 30 and 480 minutes and should be taken for as long as possible, but with care to not overload the filter. Overloading can lead to an unreadable sample. In a dusty environment, smaller air volumes may be necessary to prevent obscuring the filter (see the discussions on filter overloading in sections III.D. and III.I.). Instruct the employee sampled to avoid bumping or striking the cassette, and if possible, to avoid using a compressed air source directed towards the open face of the sampler that might dislodge the collected contaminant. After sampling, replace the end cap and secure the Form OSHA-21 seal on the sampler, then post-calibrate the sampling pump.
Approximately 10% of all samples submitted should be blanks, with a minimum of two blanks in all cases.
Where possible, collect and submit a bulk sample of the material suspected to be the source of air contamination. CSHOs must avoid destructive testing of in-place building components. Priority should be placed on obtaining bulk samples from waste, dust, or debris created by the employer's work activities. Use an appropriate wet method for bulk sampling and wear respiratory protection in accordance with regional policy. Submit approximately 0.5 to 1 gram of material in a 20 mL glass scintillation vial with a PTFE-lined cap. Be sure to collect samples from all layers and phases (visually distinct types) of the material. A knife or cork-borer may be used. For larger samples, such as roofing cores or layered flooring, submit in a 1-liter polypropylene bottle. Ship bulk samples and air samples separately to avoid cross-contamination.
Secure and handle the samples so that they will not rattle during shipment or be exposed to static electricity. Do not ship samples in expanded polystyrene peanuts, vermiculite, paper shreds, or excelsior. Tape sample cassettes to sheet bubbles and place in a container that will cushion the samples without rattling.
Asbestos air samples are analyzed using phase contrast microscopy (PCM) enhanced by polarized light microscopy (PLM) to determine fiber counts. PCM alone does not identify fiber type. OSHA asbestos samples are analyzed using a technique called differential counting to exclude non-asbestos fibers, when possible, and the concentrations are reported as fibers/cc. List any known fibrous interferences present during sampling in the OIS air sampling worksheet such as cellulose (e.g., paper and wood), fiberglass, fur, or refractory ceramic fiber. Also, note the workplace operations sampled. Bulk samples are analyzed by polarized light microscopy (PLM) to identify asbestos species.
For unusual sampling conditions or the possible use of flow rates outside the range specified in the corresponding sampling and analytical method, contact SLTC for more detailed instructions.
N. Organic Vapors and Gases
Organic vapors and gases can be collected using several different sampling media including sorbent tubes, diffusive samplers, impingers, or bubblers. Gas bags or canisters can also be useful for the collection of whole air samples.
-
Solid Sorbent Sampling Tubes
-
Sorbent sampling tubes containing various types of sampling media (e.g., coconut shell charcoal) together with low-flow sampling pumps can be used to collect many contaminants present as vapors and gases (See Figure 6). Refer to the OCD for required sampling media, flow rates, and air volumes for specific chemicals.
-
Sorbent tube sampling is generally conducted at much lower flow rates than particulate sampling to allow sufficient residence time for the contaminant of interest to adsorb to the sorbent in the tube. Sorbent sampling tubes typically contain two sections of sorbent separated by a spacer, such as foam or glass wool. A smaller sorbent section in a single tube (or a second tube in some instances) is placed behind the primary section and is analyzed separately from the primary section to determine if analyte has broken through the primary section. The secondary (back-up) section is always oriented behind the primary sorbent bed, between the primary section and the sampling pump. As air is drawn through the sorbent tube, the contaminant of interest will pass into the primary section and bind to the sorbent. When the sorbent surface in the primary section becomes saturated with target analyte molecules or other chemicals present in the sampled air (possibly including water vapor), contaminant will begin to pass into the back-up section. This is known as breakthrough. The lab analyzes the two sorbent sections separately. If greater than 25% of the total analyte recovered from both sections is found in the back-up section, this may indicate that sample was lost due to breakthrough. Breakthrough may result in an underestimation of the employee exposure. The lab will report breakthrough with the sampling results if it has occurred.

Figure 6. Charcoal tube with flame-sealed ends and end caps
-
Contaminant migration may also occur—where contaminant bound in the primary section desorbs and passes into the back-up section after sample collection is completed. There is no way for the lab to distinguish whether material found in the back-up section is the result of breakthrough or migration. To avoid migration, ship samples to the lab without delay. In some cases, refrigeration of samples is recommended to reduce migration. For methanol and other alcohols, OSHA Sampling and Analytical Method 5001 addresses the problem of migration by using two sorbent tubes attached in series (see Figures 7 and 8). The two tubes must be separated from each other and sealed (capped) immediately after sampling.
-
Note that other airborne contaminants, including moisture, will compete for binding sites on the sorbent. Sample volumes (flow rate and/or sample duration) may need to be adjusted for conditions of high or low humidity (as is the case for OSHA 5001) or when competing contaminants are present in relatively high concentrations. Follow directions found in the OSHA sampling and analytical method to be used or contact SLTC as necessary.

Figure 7. Two sorbent tubes in series
-
Series sampling may also be used where the contaminant of interest must be chemically converted to a more stable form to be retained on the sorbent.

Figure 8. Large protective tube cover for sorbent tubes in series (photo courtesy of NIOSH)
-
Sampling tubes may also be used in parallel. Sampling in parallel allows simultaneous sampling for multiple chemicals using different sampling media with the same sampling pump. This would generally be done when multiple airborne contaminants are suspected to be present, and the analytical methods do not allow for simultaneous sampling on a single tube. Sorbent tubes are manifolded together using adjustable flow controllers and tube holders available through CTC AESP. The airflow through each tube must be adjusted separately, and the combined flow cannot exceed the flow range of the sampling pump. When considering sampling for multiple contaminants operating from the same sampling pump, contact CTC for further guidance.
-
Immediately before sampling, use a tube opener to break off the ends of the flame-sealed tube to provide an opening approximately half the internal diameter of the tube. Wear eye protection when breaking ends and be careful not to cut yourself. Do not use the charging inlet or the exhaust outlet of the pump to break the ends of the tube. Insert the sorbent tube into the tube holder, attach the tube cover over the sorbent tube to shield the sampled person from the sharp ends, then connect the tube holder to pump using an appropriate length of tubing. Tube openers (also called tube breakers), tube holders, and low flow controllers (if needed) are available through CTC AESP.
-
Draw air to be sampled directly into the inlet of the tube. To avoid sample loss, air is not to be passed through any hose or tubing before entering the sorbent tube (except in cases where a very short piece of tubing is used to connect two tubes together that are used in series). Position the sampling pump, sampler, and tubing so it does not impede work performance or safety of employees.
-
Immediately after sampling, cap the tube with the supplied plastic caps, and seal the tube with a Form OSHA-21 (see Appendix E, Figures E-1 and E-2). The Form OSHA-21 should cover the end caps. If the seal does not cover the end caps because the tube is too long, tape the ends of the seal, using clear plastic tape, so that it is secure and tamper resistant.
-
After the samples are properly sealed, post-calibrate the sampling pumps. See Appendix D for more details.
-
Submit the samples for analysis. Ship sorbent tube samples and bulk samples separately to avoid cross contamination.
-
-
Diffusive (Passive) Sampling
-
Diffusive samplers, also known as passive monitors or badges, can be useful for compliance sampling. A diffusive sampler collects organic vapors using a diffusion process onto sorbent materials within the sampler and does not require the use of an air sampling pump. There are several disadvantages associated with diffusive samplers as well. They are frequently less accurate than active sampling. They generally require longer sampling times, and they are more expensive. Limits of detection for diffusive samplers are not always low enough for compliance monitoring, particularly for STEL sampling. A major disadvantage is that few analytical methods are validated for passive samplers so such samplers should only be used when recommended by the OCD. Figure 9 shows an example of one style of diffusive sampler.

Figure 9. Diffusive Sampler
-
Record the temperature and barometric pressure at the sampling site in the OIS air sampling worksheet. Temperature and pressure are needed for proper calculation of exposure results for diffusive samplers. Results from samples without the sampling site temperature and pressure will have significantly higher sampling and analytical error values. Check the National Oceanic and Atmospheric Administration's (NOAA) website the same day as sampling to obtain the barometric pressure reported with the local weather forecast for that day. The barometric pressure for the period sampled can sometimes be obtained by contacting the local weather station or airport. If air pressures are obtained by these means, it is necessary to obtain the unadjusted barometric pressure (station pressure) for compliance applications. CSHO may also measure barometric pressure and temperature using National Institute of Standards and Technology (NIST)-traceable instruments. If the barometric pressure value cannot be found or measured, note the time and elevation where the samples were collected, and refer to Appendix G, Equation G-3.
-
Specific sampling instructions for each type of diffusive sampler are supplied with the sampler and included in the OSHA methods that permit diffusive sampling. Diffusive samplers should not be opened until just before sampling because they begin to sample as soon as they are opened. To terminate sampling, properly seal the samplers with the manufacturer's packaging materials. Apply the OSHA-21 seal as shown in Appendix E. Send the sealed sampler and all its accessories to SLTC for analysis. Interfering substances should be noted in the OIS air sampling worksheet. Contact SLTC for further information regarding diffusive sampler availability and use. Consult OSHA's OCD page for new methods as they become available.
-
-
Impingers and Bubblers
-
In many cases, newer methods, such as specially treated sorbents, have been developed that can be used in place of the methods calling for use of an impinger or bubbler. However, in specialized conditions, methods requiring an impinger or bubbler must still be used. It is always advisable to check the OCD to see if alternative methods can be used.
-
Examples of a midget impinger and of a midget bubbler are shown in Figure 10. The term midget refers to the volume of the sampler flask. The difference between an impinger and a bubbler is that the end of the inlet tube of an impinger is tapered and sized to allow sufficient velocity for particles to strike the bottom of the flask and become suspended in the liquid, while the stem of a bubbler is fritted to allow collection of vapors in the solution. Bubblers break incoming air into small bubbles to improve collection efficiency of vapors.
-
The following suggestions should be followed when using impingers and bubblers:
- Numbers are usually etched into flasks and stems, and matching numbers should be used whenever possible. Take care in preparing impingers and bubblers so that tips or frits are not damaged and so that joints can be securely tightened.
- Rinse the impinger or bubbler with the appropriate collection liquid (absorbing solution) (see the applicable sampling and analytical method). Then add the specified amount of this liquid to the bubbler or impinger flask. Contact SLTC to obtain the absorbing solutions.
- To prevent overflow, do not add more absorbing solution than is recommended by the corresponding OSHA method. Place an empty impinger in series after the impinger (or bubbler) to function as a trap to prevent impinger liquid from being drawn into the air sampling pump. Position this impinger just before the sampling pump; it can be taped to the pump. If an impinger holder or holster is available, tape or secure the holstered impinger to the sampling pump.
- The maximum sampling rate for both midget impingers and bubblers is usually 1.0 L/min but should be double-checked with the individual sampling method. Because bubblers tend to offer better collection efficiency than impingers, they are preferred over impingers for gas and vapor sampling. Impingers are used only when necessary for particle counting. Contact SLTC prior to collecting any samples for particle (dust) counting using impingers.
- The impinger or bubbler can be attached to the employee's clothing using a holster. It is very important that the impinger or bubbler does not tilt and cause the absorbing solution to flow down the side arm to the hose and into the pump. NOTE: Attach a trap in-line with the pump, if possible. Impinger and bubbler cases are available in CTC AESP.
- Sampling using a glass impinger or bubbler in a food processing facility (e.g., peracetic acid sampling at a poultry processing facility) may require the use of a protective plastic case around the glass sampler. This equipment is available for loan from SLTC.
- In some instances, it will be necessary to add additional absorbing solution during the sampling period to prevent the amount of liquid from dropping below one half of the original amount.
- After sampling, remove the glass stopper and stem from the impinger or bubbler flask. Rinse the absorbing solution adhering to the outside and inside of the stem directly into the impinger or bubbler flask with a small amount (1-2 mL) of the sampling liquid. Pour the contents of the flask into a 20-mL glass vial (preferably a scintillation vial with inert cap and liner). Avoid using metal cap liners or other materials that may react with the samples. PTFE cap liners with polypropylene caps are inert to most materials. Rinse the flask with a small amount (1-2 mL) of the absorbing solution and pour the rinse solution into the vial. Tape the cap shut by wrapping the tape in the direction of cap closure to prevent it from coming loose due to vibration. If electrical tape is used, do not stretch the tape too much because it could shrink and loosen the cap.
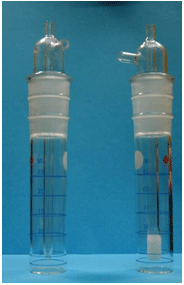
Figure 10. Midget Impinger (left) and Bubbler (right)
-
-
Gas Sampling Bags and Canister Samplers
-
OSHA uses gas sampling bags to collect whole-air samples for some analytes, such and carbon dioxide and carbon monoxide, and for sampling unknowns. CSHOs can obtain gas sampling bags from CTC AESP. Be certain not to fill the bag to more than 75% of its rated volume, and to close the sampling valve after sampling. Transport the gas sampling bag to SLTC by ground shipment if it contains particularly hazardous materials or if its odor is particularly offensive. If guidance is needed with shipping these samples, contact SLTC.
- Calibrate personal sampling pumps. Consult the OCD for recommended sampling time and rate according; 10-L sampling bags have a total volume capacity of approximately 7.5 L.
- If possible before sampling, evacuate and check gas sampling bags for leaks. The sampling bag can be evacuated and leak-tested by applying a vacuum to the bag. If a vacuum is applied to a leaky sampling bag, the bag will not fully collapse. If a vacuum pump is not available, gas sampling bags can be inflated, inspected for leaks, and then evacuated by hand rolling and flattening.
- Label each sampling bag. Attach one end of a piece of flexible tubing securely to the inlet hose-barb of the pump and place the other end in the breathing zone of the worker. Use another piece of tubing to securely connect the open metal valve sampling-bib of the sampling bag to the outlet hose-barb of the pump.
- For personal sampling attach the gas sampling bag to any loose-fitting clothing on the worker's back or side using tubing clamps.
- When ready to sample, open the gas sampling bag valve by rotating the metal valve counterclockwise until fully open. Attach the free end of the tubing connected to the bag to the outlet hose-barb. Turn on the pump.
- After sampling, rotate gas sampling bag valve clockwise until tight. Record the total air volume taken.
- Do not prepare or submit blank samples.
- Wrap an OSHA 21 (or equivalent) seal across the gas sampling bag valve.
-
When submitting the sampling bags to the laboratory for analysis, pack loosely and apply generous padding to minimize potential damage during shipment. Submit samples as soon as possible after sampling for laboratory analysis.
-
Gas sampling bags or canisters are sometimes used to collect whole air samples. The OSHA method PV2120 details the use of a 400-mL evacuated fused silica-lined stainless-steel canister with the recommended sampling time from a minute to 8 hours. Another method, OSHA 1021 (validated for toluene), specifies a 50-mL evacuated canister for instantaneous personal sampling of substances with ceiling and peak exposure values. The 50-mL evacuated canister can also be used for area sampling and IDLH (Immediately Dangerous to Life or Health) screening. Call SLTC for guidance.
-
IV. Post-Inspection Activities
A. Post-Calibration
CSHOs will perform post-calibration of all pumps used for sampling. Procedures for post-calibration are described in Appendix D. Record the post-calibration results in the respective OIS air sampling worksheet. Document any discrepancies in flow rates or faults noted on the pump.
B. Complete Documentation
All sampling information must be entered in the OIS Sampling module. CSHOs must fill out as much information as possible to accurately describe the working and exposure conditions employees were exposed to. When requesting an analysis, use the correct IMIS code as described in the OCD page for a specific analyte. Some OCD pages will have information about sampling groups. These are analytes that can be analyzed together from the same field sample.
SLTC will contact field personnel when a different analysis seems to better fit the working conditions and associated hazards as described in the documentation or when analytes requested cannot be analyzed simultaneously. Special attention should be placed on labeling and entered information in OIS. Samples must have the same name on the OSHA-21 seal and their corresponding OIS air sampling worksheet.
CSHOs must also indicate if they would like the results to be reported as an 8-hour TWA. SLTC will report results using actual time sampled unless otherwise requested.
C. Package and ship samples
Once the CSHO has entered all the information in OIS, a completed OIS air sampling worksheet is generated and mailed together with the corresponding samples to SLTC. The information in OIS is transferred to SLTC's information system and corroborated with the hard copy mailed with the samples.
Samples must be packed in a box, sturdy container, or padded envelope. Fill any space in the packaging with bubble wrap or other packaging material to protect the sample cassettes from breaking or scintillation vials from shattering. Samples should be placed inside plastic bags to prevent them from moving freely. Bulk samples and air samples must be shipped separately to prevent cross contamination.
Some samples have special shipping requirements. Asbestos samples cannot be packaged with material that produce static, including polystyrene packing material (Styrofoam). Other samples need to be shipped with cold packs (at cold temperatures) or overnight due to the sample degrading over time or when not refrigerated. Special shipping instructions for specific analytes can be found in the OCD.
For shipping of hazardous materials, specific instructions have been developed by SLTC for these types of samples. In some cases, a certified hazardous materials shipper will be the only person able to ship certain materials. For more information see the SLTC Hazardous Materials Shipping intranet page.
D. Receive sample results
Sampling results will be delivered to compliance officers via a designated area office email and uploaded to OIS. Exposure assessments to evaluate 8-hour TWAs for employee exposures can be performed in OIS. OIS air sampling worksheets containing laboratory raw results and exposure assessment results can also be generated when the exposure assessment is completed. SLTC reports mass per actual air volume sampled in milligrams per cubic meter (mg/m3). For gases and vapors, SLTC typically calculates concentration in mg/m3 and then converts it to ppm at 25°C and 760 mmHg using Equation G-1 in Appendix G. This ppm result is to be compared with the PEL without adjustment for temperature and pressure at the sampling site. Additional supporting equations are also found in Appendix J.
-
8-Hour TWA Calculation
OSHA's Permissible Exposure Limits (PELs) are based on an 8-hour time weighted average. Calculations must be performed on results that are based on actual time to compare them to the OSHA PEL.
Equation (4)
Z = C·T 480 Where:
Z is the 8-hour TWA;
C is sample result;
T is sample time (minutes);
480 minutes is 8 hours.When more than one sample is consecutively taken to determine an employee's exposure, the 8-hour TWA calculation must consider all individual results of each sample. It is OSHA's policy to assume zero exposure for all time not sampled.
Equation (5)
Z = C·T1+C2·T2+···Cn·Tn 480 Where:
Z is the 8-hour TWA;
Cn are sample results;
Tn are sample times for each respective sample in minutes;
480 minutes is 8 hours. -
Sampling and Analytical Error
Sampling results produced by SLTC have some degree of uncertainty. The total uncertainty depends on the combined effects of the contributing uncertainties inherent in sampling and analysis and has historically been called sampling and analytical error or SAE by OSHA. The SAE is used to determine the upper and lower confidence limits as described below. Correct application of the SAE enables CSHOs to make reliable exposure assessments. SAEs that provide a one-sided 95% confidence limit are developed by SLTC and are reported with the sampling results. Wipe samples and bulk sample results do not have an associated SAE.
-
Severity Calculation
Severity calculations will provide the ratio between the sampling results and the PEL. This shows how many times above the PEL the exposure was and can indicate a potential overexposure.
Equation (6)
Y = Z PEL Where:
Y is severity;
Z is the 8-hour TWA;
PEL is the permissible exposure limit for the analyte. -
Upper and Lower Confidence Limit Calculations
Confidence limits are values at each end of the confidence interval, which is the probable range of the true value. The Upper Confidence Limit (UCL) and the Lower Confidence Limit (LCL) are each termed one-sided because the main concern is with being confident that the true exposure is either less or greater than the PEL. OSHA applies the UCL and LCL with a 95% statistical confidence limit (expressed as UCL95% and LCL95% respectively); they are calculated as follows:
Equation (7)
UCL95% = Y + SAE
Where:
Y is the severity;
SAE is the sampling and analytical errorEquation (8)
LCL95% = Y – SAE
Where:
Y is the severity;
SAE is the sampling and analytical errorThe following parameters determine the existence of a potential overexposure, and/or a standard violation:
If the UCL95% ≤ 1.0, a violation does not exist.
If LCL95% ≤ 1.0 and the UCL95% > 1.0, classify as possible overexposure.
If LCL95% > 1.0, a violation exists.The following parameters determine compliance with an action level.
If the UCL95% < 1.0, the action level is not exceeded.
If LCL95% < 1.0 and the UCL95% ≥ 1.0, classify as the action level is possibly exceeded.
If LCL95% ≥ 1.0, the action level is exceeded.If the results are in the "possible overexposure" category, consider further sampling, taking into consideration the seriousness of the hazard and pending citations. If further sampling is not conducted, or if additional measured exposures still fall into the "possible overexposure" category, the CSHO can explain to the employer and employee representative at the closing conference that the sampled employee(s) may be overexposed, but that there is insufficient data to document noncompliance. The employer should be encouraged to voluntarily reduce the exposure and/or to conduct further sampling to ensure that exposures do not exceed the PEL.
For instances where consecutive samples were taken instead of a single sample, and the LCL95% < 1.0 and UCL95% > 1.0, the results are in the "possible overexposure" region, then the CSHO must analyze the data using the more exact calculation for full-period consecutive sampling, as follows:
Equation (9)
LCL95% = Y − SAE√(X1T1)2+(X2T2)2+···(XnTn)2 PEL(T1+T2+···Tn) Where,
Y is the severity,
PEL is the permissible exposure limit for the analyte,
SAE is the sampling and analytical error,
Xn is a sample result,
Tn is the respective sampling time.The LCL95% and UCL95% are calculated differently depending upon the type of sampling method used. Sampling procedures can be classified into Full-period, Continuous, Single Sampling or Full-period, Consecutive Sampling
-
Full-period, Continuous, Single Sampling. Full-period, continuous, single sampling is defined as sampling over the entire sample period with only one sample. The sampling may be for a full-shift sample or for a short period ceiling determination.
-
Full-period, Consecutive Sampling. Full-period, consecutive sampling is defined as sampling using multiple consecutive samples of equal or unequal duration that, if combined, equal the total duration of the sample period. An example would be taking four two-hour charcoal tube samples. There are several advantages to this type of sampling:
- If a single sample is lost during the sampling period due to pump failure, gross contamination, etc., at least some data will have been collected to evaluate the exposure.
- The use of multiple samples should result in slightly lower sampling and analytical errors.
- Collection of several samples allows conclusions to be reached concerning the way differing segments of the workday affect overall exposure.
- This practice also allows for monitoring peak and ceiling exposures for a more appropriate period. Note that there is some loss of sensitivity with consecutive sampling as compared to continuous sampling. Appendix J provides example calculations.
-
-
Flow Rate Calculations
If the initial and final sampling pump calibration flow rates are different, use of the higher of the two calibration flow rates will provide the lowest analytical results for compliance purposes. Generally, sampling is conducted at approximately the same temperature and pressure as calibration, in which case no correction for temperature and pressure is required and the sample volume reported to SLTC is the volume measured. Where sampling is conducted at a substantially different temperature or pressure than calibration, consult the operating manual for the sampling pump to determine if the air volume needs to be adjusted. If possible, calibrate the equipment at the site. The air volume reported by the CSHO is used in all subsequent calculations.
-
SAEs for Exposures to Chemical Mixtures
As described above in Section III, often an employee is simultaneously exposed to a variety of chemical substances, which may result in additive or synergistic health effects. 29 CFR 1910.1000(d)(2)(i) and 29 CFR 1915.1000(d)(2)(i) specify the computational approach for assessing exposure to a mixture. For mixtures, the CSHO must determine the SAE. These SAEs can be pooled and weighted to give a control limit for the additive mixture using Equation 2. If Em calculated using Equation 2 is greater than 1, indicating that an overexposure has occurred, then the SAE for each substance also needs to be considered. Example calculations for mixture SAE values are provided in Appendix F.
If the PEL violation is confirmed, apply the appropriate health effects provided in Appendix H. Whether using a single PEL or the mixture calculation, the SAE of the individual constituents must be considered before arriving at a final compliance decision.
V. Bibliography
American Conference of Governmental Industrial Hygienists. Air Sampling Instruments, Ninth Edition. Cincinnati, ACGIH, 2001.
Burgess, W.A. Recognition of Health Hazards in Industry: A Review of Materials Processes, Second Edition. Hoboken: John Wiley and Sons, Inc., 1995.
Hesketh, H.E. Fine Particles in Gaseous Media. Chelsea: Lewis Publishers, Inc., August 1986.
Lodge, J.P., Jr. (Ed.). Methods of Air Sampling and Analysis, Third Edition. Boca Raton: Taylor & Francis, 1989.
McDermott, H.J. Air Monitoring for Toxic Exposures, Second Edition. Hoboken: John Wiley and Sons, Inc., 2004
National Institute for Occupational Safety and Health. Occupational Exposure Sampling Strategy Manual. DHEW (NIOSH) Publication No. 77-173. Cincinnati: NIOSH, January 1977.
National Institute for Occupational Safety and Health. Mixed Exposures Research Agenda: A Report by the NORA Mixed Exposure Team. DHHS (NIOSH) Publication No. 2005-106. Cincinnati: NIOSH, December 2004.
Occupational Safety and Health Administration, U.S. Department of Labor. OSHA Instruction CPL 02-00-164, Field Operation Manual (FOM) (Online).
Occupational Safety and Health Administration, U.S. Department of Labor. OSHA Occupational Chemical Database (OCD) (Online).
Occupational Safety and Health Administration, U.S. Department of Labor. OSHA Instruction ADM 04-00-003, OSHA Field Safety and Health Management System (SHMS) Manual (Online).
Popendorf, W. Vapor pressure and solvent vapor hazards. Am. Ind. Hyg. Assoc. J. 45:719-26, 1984.
Appendix A
Additional Sampling and Exposure Assessment Support
Additional support is available at SLTC for unique exposure assessment questions, sampling of unusual airborne hazards, or where questions remain after reviewing sampling instructions found in a sampling and analytical method or the OCD.
The SLTC Industrial Hygiene Chemistry (IHC) Division Duty Senior Analyst is available to take calls during working hours (Mountain Time Zone) and may be contacted at 801-233-5001.
The IHC Duty Senior Analysist is available to help with question which include:
- Interpretation of sampling results
- Assistance with sampling methods
- Sample shipping requirements
- Media ordering and media questions
- Sample result status
- OCD questions
- Carboxyhemoglobin inspections
- Combustible dust sampling and analysis
- Material failures accident investigations
- Chemistry and chemical exposure questions
- Sampling for unknown chemicals
In addition, remote support and on-site support is provided by the Health Response Team (HRT) co-located at SLTC. HRT may be contacted at 801-233-4900 option 2 to request technical assistance with evaluating unusual exposures for situations which include:
- Suspected exposure to nanoparticles
- Sampling for radiological hazards
- Sampling for biological hazards
- Acute exposures for which ceiling or peak exposure standards exist
- Exposure to unknown chemicals
- Determination of general ventilation characteristics (air changes per hour)
- Movement of air contaminants within a structure
- Where information is needed regarding chemical reactivity and reactions
- On-site use of special sampling methods and field detection instruments
Appendix B
Pre-Weighed Filters
SLTC provides pre-weighed filters for gravimetric analysis. Filter/cassette units, when assembled in a cassette are tested for leaks. These filter/cassette units reduce sample preparation time by CSHOs because the filters are weighed at SLTC, and the units are shipped to the field fully assembled and ready for use. The filter/cassette units are returned to SLTC for gravimetric determinations and additional analyses as needed. The filter media is 5-μm, 37-mm diameter, low-ash PVC, or PTFE (TEFLON). The PVC filters should be used for silica (quartz) analysis, aluminum, and other appropriate substances having high PELs or requiring gravimetric analysis. The PTFE filters are used for asphalt fumes. The filters may be used with or without a cyclone. Other than for silica, if the gravimetric analysis yields a result less than the PEL for the requested substance(s), no further analysis will be provided unless specifically requested. The filter/cassette unit is shown below in Figure B-1.

Figure B-1. Filter/Cassette Unit
Check the filter frequently to avoid overloading. This can be accomplished by looking into the inlet sampling port of the cassette. Use a flashlight if necessary. Visual observation of the airborne dust in the workplace may assist in determining how frequently to check the filter for overloading. If used with a cassette, do not lift the cyclone in such a way that particles from the grit pot could be deposited on the filter.
As shown in Figure B-1, the inlet side of the cassette is marked on the polystyrene cassette. This is the side of the filter cassette with the aluminum cone antistatic shield. The stainless-steel support (Figures B-2) is visible from the outlet side of the assembly (Figure B-3). Each of the filter assemblies is bar coded for automated analysis (Figure B-4). To aid in tracking the filters, the barcode number must be used as the sample submission number when completing the OIS air sampling worksheet. A blank filter/cassette assembly should be included with every set of samples.

Figure B-2. Stainless Steel Filter Support

Figure B-3. Outlet View of a Filter Cassette (connect to sampling pump)

Figure B-4. Inlet View of a Filter Cassette (open to atmosphere, pointed downward during sampling)
The filter/cassette assembly can be used with both nylon cyclone and holder assemblies currently in field use; however, the standard MSA coupler (used with a standard 2- or 3-piece cassette) will not fit these cassettes. Another coupler available from MSA (part #457391), which is plastic instead of stainless steel, can be obtained from CTC.

Figure B-5. Barcoded lot number and expiration date of filter cassette (discard prior to sampling)
Appendix C
Shelf-Life of Sampling Media
SLTC prepares and ships certain media and will provide an expiration date for sampling media shipped to the field. The date will be printed either on the media itself, on its container, or on its packaging. Return liquid media to SLTC in the same outer packaging in which it was received.
| SLTC Prepared Media |
Analyte |
SLTC Part No. |
AESP Part No. |
Time Between Preparation and Expiration |
|---|---|---|---|---|
|
37 mm Glass fiber filter coated with veratrylamine (3,4-dimethoxybenzylamine) and di-n-octyl phthalate |
Anhydrides |
SLTC100 |
FES0002215 |
30 days** |
|
37 mm Glass fiber filter Nitrite impregnated GFF |
Ozone |
SLTC101 |
FES0002216 |
28 days** |
|
37 mm Glass fiber filter (used open-face) coated with 1 mg 1-(2-pyridyl)piperazine |
Diisocyanates (MDI, HDI, TDI, etc.) |
SLTC103 |
FES0002218 |
6 months |
|
37 mm PVC filter, hexavalent chromium sampler |
Chromium VI Compounds (hexavalent chromium) |
SLTC104 |
FES0002219 |
As provided |
|
25 mm PVC filter, hexavalent chromium sampler |
Chromium VI Compounds (hexavalent chromium) |
SLTC105 |
FES0002220 |
As provided |
|
37 mm Quartz fiber filter coated with 1% NaOH for chrome plating operations |
Chromium VI Compounds (hexavalent chromium) |
SLTC106 |
FES0002221 |
As provided |
|
37 mm Binderless quartz fiber filter |
Chromium VI Compounds (hexavalent chromium) |
SLTC107 |
FES0002222 |
4 months |
|
Tared low-ash 37 mm PVC filter, 5 microns |
Gravimetric analyses |
SLTC108 |
FES0000161 |
5 years |
|
37 mm Prewashed glass fiber filter |
Coal tar pitch volatiles |
SLTC109 |
FES0002223 |
As provided** |
|
10 mL 0.003 M NaHCO₃/0.0024 M Na₂CO₃* |
Bromine |
SLTC110SOL |
FES0002224 |
12 months** |
|
15 mL of 0.02% potassium iodide in a buffer mixture of sodium carbonate and sodium bicarbonate (1.5 mM of each) - Chlorine Dioxide Sampling Solution* |
Chlorine dioxide |
SLTC111SOL |
FES0002225 |
12 months** |
|
Sulfamic acid solution (0.1%)* |
Chlorine |
SLTC112SOL |
FES0002226 |
12 months** |
|
10 mL 0.1 N NaOH* |
Cyanides (as CN) |
SLTC113SOL |
FES0002227 |
12 months** |
|
Tared low-ash 37 mm PVC filter, 5 microns in 3 piece cassette |
Cotton Dust (Raw) |
SLTC114 |
FES0002329 |
5 years |
|
Thermal desorption tubes (TD air toxics) |
Thermal Desorption with Qualitative GC-MS Analysis |
SLTC115 |
FES0002383 |
Use as soon as possible |
|
25-mm quartz fiber filter, coated with titanium oxysulfate, followed by an impinger containing methyl p-tolyl sulfide (MTS) and 4-chlorophenyl methyl sulfone in acetonitrile (ACN)* |
Peracetic Acid |
SLTC116 |
FES0002395 |
2 months** |
|
Tared 37 mm PTFE (2 µm) |
Asphalt Fumes |
SLTC117 |
FES0002382 |
5 years |
* Give SLTC at least two days' notice to allow time for reagent preparation.
**Hazardous goods shipment as a Corrosive Liquid, Toxic, UN 2922, Class 8, PGIII may be required both from SLTC to the field and from the field to SLTC depending on quantity.
Appendix D
Sampling Pump Calibration
A. Calibration
Sampling pumps are used to evaluate air contaminants in the workplace. Pump sampling rate and sample media is determined by the sampling method for the substance being sampled. The chemical specific sampling methods are provided in the OSHA OCD. To ensure sample accuracy, pump flow rates must be calibrated before and after each sampling event. Additionally, operation of the pump and flow rate of the pump should be checked during each sampling event. OSHA approved sampling pump calibration methods/procedures, approved sampling pumps/calibrators, calibrating in temperature extremes resources, maintaining pump calibration equipment, tutorial videos, and quick reference guides are located on the OSHA CTC intranet page under Information for Equipment and Accessories in the Air Sampling. All sampling pump and flow calibration equipment should be in good working order, with fully charged batteries, and operated within the manufacturer's stated operating specifications. Sampling pump inlet filters should be replaced whenever they appear discolored or dirty prior to calibration.
Pump flow calibrators should have a certified accuracy that is ±1% at flow rates of 0.050 LPM and above and have an annual calibration traceable to the National Institute of Standards and Technology (NIST) where a traceable calibration back to time and volume standards are performed. Sampling pumps currently have no calibration due date and are serviced as needed due to being calibrated before and after sampling events in the field. Assure calibration equipment is within its prescribed calibration interval and record the serial number of the calibration equipment in your case file and the OIS air sampling worksheet.
If the sampling pump is equipped with a rotameter or digital flow readout, record the reading in the OIS air sampling worksheet. The accuracy of a pump rotameter or digital readout is only approximate; it is intended primarily as a reference, and not a replacement for flow rate calibration using approved methods.
NOTE: Precision rotameters are no longer normally used by OSHA for calibration due to the potential for measurement error (e.g., tests with precision rotameters have indicated substantial error due to pump pulsation at lower flow rates and require temperature corrections). Special approved circumstances may require the use of a rotameter or other devices for below freezing calibrations. Rotameters may also have applications for qualitative type mold testing devices or kits. Inverted field burets with handheld stop watches may still be useful, but their use is discouraged because they are no longer considered one of the most accurate pump flow field calibration devices available due to uncertainties caused by the human eye with regard to stopping and starting the bubble on the burette graduations.
Place the same type of sampling media, representative media, or valve with similar backpressure adjusted load in-line during sampling pump calibration that will be used to sample in the field. Do not use the actual cassette and filter intended for sampling use to perform calibration.
B. Pump Calibration for use with Size-Selective Devices
Sampling pumps for use with size-selective devices for respirable dust sampling (see sections III.J. and III.K.) can be calibrated via various methods. It is important that all manufacturer's recommendations are followed for the specific pump, calibrator, and size-selective device utilized, as applicable.
Regarding the Dorr-Oliver cyclone, there are several options available for calibrating pumps for use with the sampler. Devices such as the dry piston calibrators (see Section D below) simplify the process significantly and are the recommended method for such calibration. These calibrators are equipped with both a flow inlet and outlet. To perform calibration, the pump is connected to the calibrator outlet while the cyclone with sampling media is connected to the calibrator inlet. Also, depending on the type of pump and calibrator utilized, it may be necessary to utilize an inline device such as an orifice or laminar flow element. Refer to the pump and calibrator manufacturer's instructions for details, as well as the CTC Technical Equipment - Air Sampling intranet site for additional information and procedures.
An additional method available for calibrating Dorr-Oliver type cyclones is the OSHA "jarless" method. This method utilizes a jarless calibration kit available from CTC and determines whether the pump will be able to maintain the required flow rate as the drop in static pressure grows due to particulate loading on the filter. Refer to the CTC Information of Equipment and Accessories intranet site for additional information and procedures regarding the jarless method, as well as an instructional video.
It should be noted that the original "jar" method for pump and cyclone calibration is not recommended due to technical issues. Some commonly observed issues include air leakage at the jar lid as well as conflict with manufacturer's recommendations for such calibrations.
Other issues with the Dorr-Oliver cyclone involve leaks in the cyclone assembly which can cause significant calibration flow errors. Cyclones require proper maintenance, such as cleaning and leak testing to ensure proper functioning. Leak test the cyclone before use unless it has been leak tested within the past month. CTC also provides a cyclone leak test kit for this purpose. Information regarding the leak test kit, procedures, as well as an instructional video can also be found on the CTC Information of Equipment and Accessories intranet site.
C. Sampling Pump Devices
There are many different types and models of sampling pumps approved for use by the CTC. Sampling pump models can offer various flow rates, backpressure capabilities, and other features, depending on the model. The CTC may evaluate and approve sampling pumps with manufacturer constant flow control rated accuracy of ± 5% of set flow or ±3 mL/min whichever is greater. This means the sampling pump should maintain its constant flow rate to within ±5% or 3 mL/min of its calibrated set flow rate within the backpressure rated specifications for that flow rate. If a sampling pump varies by more than 5% or 3 mL/min (whichever is greater) between calibration and post calibration check then note the difference on the sampling sheet for SLTC.
Flow Rates and Backpressure Specifications
Sampling pumps usually come in three distinct categories for flow rate; they can be low (1 ml/min up to 500 ml/min), medium/high (450 mL/min up to 5 L/min), and high (4 L/min up to 30 L/min). There could also be combinations of flow ranges or ranges outside these listed for models. Backpressure maximum capabilities for most sampling pumps can vary from 20" of water up to almost 96" of water depending on flow rate and model. It is important to read the manufacturer's pump specifications to determine if the sampling pump is the right choice for your sampling method.
Constant Pressure
Sampling pumps can also come with features for constant pressure sampling. This is mostly for low flow type pumps, but some other higher flow pumps have this option as well. This option allows the user to place the pump into a constant pressure mode instead of the normal constant flow mode. In this mode, a constant vacuum pressure is created by the pump allowing the user to place optional user adjustable manifolds or paralleled adjustable flow needle valve devices in line with the pump, so that multiple samples can be taken at one time. Flow of each sample is then calibrated by adjusting each needle valve. The user should understand the difference in constant flow mode and constant pressure mode to make certain the sampling pump settings are correct for the sampling method.
Automatic Flow Correction for Changes in Temperature or Barometric Pressure
The latest sampling pumps may have automatic flow correction capability for changes in temperature or barometric pressure (or altitude). Some sampling pumps either don't have flow correction for temperature and barometric pressure changes, or they have this capability, but have limited or no ability to turn it off. It is important to know if your sampling pump has this option, what the range of operation is, and if it is turned on. Even though some pumps compensate flow rate for changes in temperature and barometric pressure, the sampling pumps being used for sampling should be acclimated to the operational sampling site environment by giving them 15 minutes of time to equilibrate to the temperature conditions at the site. For example, when a sampling pump is calibrated in a warm environment and then taken to a cold environment for a sampling event, the pump may no longer stay within its rated constant flow pump accuracy. This could be true if the pump does not have automatic flow correction for temperature, or it is outside the manufacturer specification limits for this option.
Sampling Data
A sampling pump is to be calibrated before and after a sampling event, but sometimes the user needs to know what flow characteristics occurred during the sampling event. Data acquisition can be a feature of some of the latest pumps, to save sampling data that happened during a sampling event. The sampling event is recorded for sampling event time, flow rate, and sometimes other optional information such as faults or backpressures, depending on the model. Each applicable pump model sampling event data is saved to the maximum events allowed by the model's specifications, and then later overwritten and lost in most cases. The data can be deleted by the user as well. Some model's data can be downloaded to a computer using approved data collection software and cables. Special identified cables are usually required to download data from the pump to a computer or from the pumps docking/charging station to a computer, depending on the pump model. Charging docks for this use are usually identified as enhanced, or as communication docks. Some pump models also have a Bluetooth option which allows data to transmit to an application on the phone and allows some of the sampling pump features to be operated remotely, usually up to 40 feet.
Sampling Pump Field Maintenance
Sampling pumps require very little field maintenance except for battery care and inlet filter changes. Batteries should be charged only with chargers or charging docks that were intended for use with the sampling pump per manufacturer recommendations. Most modern chargers have trickle or smart charging technology, which safely allows the battery to remain on the charger after the initial full charge is complete. Do not overcharge the battery if the charger does not have trickle or smart charging technology. Avoid charging and storing pumps or batteries in extreme heat. Inlet filters should be changed before calibration, if they appear dirty, as a preventative measure. If the inlet filters get excessively dirty, the pump may no longer be able to operate within manufacturer specifications and may fault during sampling due to flow restriction or a blockage. Some other steps may be taken to test pump operation or working condition in the field.
Decontamination
Sampling pumps that were used in environments where the possibility of contamination with hazardous substances may have occurred should be decontaminated using kits available from CTC AESP. Plastic pump covers and pump bags are also available for some models in the AESP to help protect the sampling pump during a sampling event.
National Recognized Testing Laboratory (NRTL) Rated
All low flow and medium/high flow sampling pumps evaluated and serviced by CTC should have an NRTL rating for hazardous locations. Some newer pumps are not rated for all areas, and the NRTL Class ratings for the pump should be reviewed prior to using the sampling pump for sampling in hazardous locations.
D. Sampling Pump Flow Calibrator Devices
There are many types and models of sampling pump flow calibrators approved for use or serviced by CTC. These calibrators can utilize various designs to verify the flow rate of a sampling pump. Bubble type and dry piston type calibrators are approved for use in OSHA due to them being a volume and time design type which has traceability to NIST. They can come in different models for different flow ranges and features. The user should select a model that has a flow range suitable for the expected sampling pump flow rate. Flow calibrators should be manufacturer rated at minimum ±1% of reading accurate for use by OSHA when used to calibrate sampling pumps for a sampling event. It is recommended to use the flow calibrator range closest to the sampling event flow rate for best results.
Flow calibrator devices should be fully charged with the proper charger before use or have known good batteries. If the battery display is showing low battery indication for units with disposable batteries, then the battery or batteries should be replaced.
Approved software is available for some models that will allow the user to extract the data from the flow calibrator during its use.
No sampling pump flow calibrators evaluated or serviced by CTC are currently rated for use in hazardous locations. All calibrations with these devices should be performed outside of the hazardous locations, as close to the sampling site environmental conditions as possible.
Bubble Type Flow Calibrator Devices
These are electronic bubble type design flow meters, used to calibrate sampling pump flow rate, which can provide instantaneous air-flow readings and cumulative averaging of multiple measurements. These calibrators calculate the flow rate by measuring the time it takes a bubble to pass between two sensor points and display the results as volume per unit of time (e.g., mL/min and (L/min). Flow cells or calibrator models of various volumes are used to accommodate different flow ranges. The middle-sized flow cell or model is typically used for personal sampling for particulates, while the largest cell or model is used for high volume area sampling, and the smallest cell or model may be needed for certain low flow sorbent tube methods. The total range with the different flow cells is from 1 mL/min to 30 L/min.
Charging procedures should be reviewed in the manufacturer's manual, as some units should not be left plugged into the charger for extended time periods because doing so will decrease the service life of the battery.
Bubble type flow calibrators utilize a bubble solution to create the bubble used in the operation. Only CTC available bubble solution should be used, as other types such as soap may damage the cells. The bubble solution should be changed monthly when used often and filled to the proper level as indicated in the manufacturer's manuals. Cell inlet and outlet boss ports should have a tube placed between them when not in use or in storage, to prevent contaminants from getting in the fluid, and to keep the fluid from evaporating and drying out. When in operation, always allow time between readings taken to allow the solution to return to the base before another reading is taken. For some low flow cells, the time delay between readings can be as much as 7 seconds to get repeatable results.
CTC also recommends that the bubble type calibrator not be used in corrosive or otherwise contaminated environments. When removing the cells from a base always twist from the bottom of the cell otherwise the cell could crack. If cracks appear in the cell, it could indicate a leak in which the flow calibrator would not produce accurate results. Units with suspected cracks should be leak checked with less than 20" of water pressure vacuum if possible and/or sent to CTC for repair.
Dry Piston Type Flow Calibrator Devices
Electronic dry-piston-type design flow meters are used to calibrate sampling pump flow rates and provide immediate and average readings. These calibrators calculate the flow rate by measuring the time it takes a piston to pass between sensor points and display the results as selectable volume per unit of time (e.g., mL/min) and (L/min). The device can be used to calibrate either pressure (labeled inlet) or vacuum (labeled outlet) flow sources. The vacuum port is used to calibrate sampling pumps, and the pressure port can be used to calibrate the outlet of sampling pumps used to fill gas sampling bags. Different models and flow cells can be available, like the bubble type devices, for typical ranges from 5 mL/min to 30 L/min.
Charging procedures should be reviewed in the manufacturer's manual, as some units should not be left plugged into the charger for extended time periods because doing so will decrease the service life of the battery.
CTC recommends that the dry piston type flow calibrators not be used in a very dusty environment because dust that flows through the calibrator piston area has the potential to scratch the glass and piston inside the calibrator. CTC also recommends that the calibrator not be used in corrosive or otherwise contaminated environments. Dry piston type flow calibrator ports should be capped when not in use to prevent debris from entering the piston chamber. If debris gets into the piston chamber the piston can stick, causing either no readings or faulty readings. If you notice the piston sticking, the unit should be sent to CTC for service. These type of flow calibrators have more setting options, and it can be easy to not have a setting correct for your application. Make sure to check the unit settings to make certain they are correct for your application. It is recommended that the flow rates obtained from these devices be reported to significant figures and units which would offer the most precision for the flow rate displayed.
E. Calibration Process for Open-Face Filters
Open-face cassettes are used for asbestos and certain chemicals such as isocyanates, crotonaldehyde, and glutaraldehyde.
A way to calibrate an open-face cassette is to use the cover section which comes with the cassette and attach the tubing directly from the electronic flow calibrator to the inlet port on the cassette cover. Be certain there are no leaks and do not use a Luer adapter. This set-up will provide the least amount of flow resistance and represent the open-face conditions while actually sampling.
Perform the pump calibration at the pressure (altitude) and temperature where sampling is to be conducted. If this is not possible, consult the operating manual for the sampling pump to determine if the air volume needs to be adjusted for temperature and pressure.
Appendix E
Chain of Custody and How to Apply Form OSHA-21 to Sampling Media
SLTC uses OSHA's established chain-of-custody procedures to track whether official Form OSHA-21 seals were properly used to ensure the integrity of samples collected by OSHA CSHOs. The procedure also tracks the history and control of samples received at SLTC. The chain of custody includes the following dates: the date the sample was collected, the date the sample was shipped to SLTC, the date the sample was received at SLTC, the date the SLTC analyst received the sample, the date the analysis was completed, the date the analytical results were checked by another analyst, and the date the sample results were released by a supervisor or his/her representative. It is important to follow chain-of-custody requirements to document the proper handling of OSHA samples for litigation purposes and sample integrity.
Proper Form OSHA-21 Application examples are provided below:

Figure E-1. Correctly sealed charcoal tube inside Form OSHA-21

Figure E-2. Incorrectly sealed charcoal tube. End caps can be removed allowing sample integrity to be jeopardized without disturbing the seal.

Figure E-3. Incorrectly sealed cassette allows access to inlet/outlet ports after sample has been taken.

Figure E-4. Correctly sealed cassette with Form OSHA-21 covering inlet/outlet ports maintaining sample integrity.

Figure E-5. Standard asbestos cassette (25mm) correctly sealed with a Form OSHA-21.

Figure E-6. Passive monitors correctly sealed with a Form OSHA-21

Figure E-7. Correctly sealed 3M passive monitor
Appendix F
Example Calculations for Mixtures
A mixture calculator and an executable computer program are available on the OSHA DTSEM Resources and Tools Intranet page, which will calculate a control limit for any mixture according to the formulas described in this appendix.
For this example, three substances will be considered. The exposure measurements and associated information are given in Table F-1 below:
|
Material |
8-hr. Exposure (ppm) |
8-hr. TWA PEL (ppm) |
CV* |
Sampling Error |
SAE** |
|---|---|---|---|---|---|
|
Substance 1 |
500 |
1,000 |
0.021 |
0.05 |
0.089 |
|
Substance 2 |
80 |
200 |
0.044 |
0.05 |
0.11 |
|
Substance 3 |
70 |
200 |
0.097 |
0.05 |
0.18 |
* CV - coefficient of variation, calculated from the SAE and the assumed 5% sampling error
** SAE - sampling and analytical error, provided on the air sampling report
Using Equation (2) (from Section III.G.2.):
Equation (F-1)
| Em = ( | C1 | ) + ( | C2 | ) + ··· + ( | Cn | ) |
| L1 | L2 | Ln |
Where:
Em is the equivalent exposure severity for the mixture, Em should be ≤1 for compliance,
Cn is the concentration of analyte n, and
Ln is the OSHA exposure limit for analyte n.
For the example data shown above for three substances, Equation F-1 is applied as follows:
| Em = ( | 500 | ) + ( | 80 | ) + ··· + ( | 70 | ) = 1.25 |
| 1000 | 200 | 200 |
Since Em > 1 an overexposure appears to have occurred; however, the SAE for each substance also needs to be considered:
To understand how to calculate of an SAE value applicable to a mixture, it is necessary to understand how an SAE for a single substance is calculated. SAE values incorporate error from sampling and analysis. Recoveries from quality control spiked media samples and tests performed during method development provide data are used to estimate the errors associate with an analysis. Pump flow error and errors in measurements associated with sampling time can also be estimated and combined to produce an overall estimate of error associated with sampling. For simplicity, the sampling error is assumed to be 5% in the example below.
An SAE value for a single substance is calculated using the following equation.
Equation (F-2)
SAE = 1.645√CV2 + 0.052
where:
SAE is the sampling and analytical error,
CV is the coefficient of variation that represents the overall analytical error,
0.05 is the assumed overall sampling error for flow rates at 60 mL/min or higher, and
1.645 is a statistical factor used to achieve a 95% one-sided confidence estimate in the result.
Using the value for the CV provided in the table above for substance 1 the SAE value is calculated using Equation F-2 as follows.
SAE = 1.645√0.0212 + 0.052 = 0.089
Conversely, the CV can be calculated from the SAE provided on the air sampling sheet by rearranging Equation F-2
| CV = √( | SAE | )2 - 0.052 |
| 1.645 |
| CV = √( | 0.089 | )2 - 0.052 = 0.021 |
| 1.645 |
To calculate a combined SAE for a mixture, each coefficient of variation is weighted by multiplying it by its exposure severity ratio. The exposure severity ratio is the ratio of the exposure to the exposure limit.
Equation (F-3)
| Rn = ( | Cn | ) / Em |
| Ln |
where:
Rn is the severity ratio for analyte n,
Cn is the concentration for analyte n,
Ln is the OSHA exposure limit for analyte n, and
Em is the equivalent exposure severity for the mixture.
For substance 1
| R1 = ( | 500 | ) / 1.25 = 0.40 |
| 1000 |
An equation similar to equation F-2 above can be derived using these severity ratios to calculate the combined SAE for the measurement of the mixture.
Equation (F-4)
SAEm = 1.645√R1(CV12) + R2(CV22) + ··· + Rn(CVn2) + 0.052
Completing the example using all the data above:
| R2 = ( | 80 | ) / 1.25 = 0.32 |
| 200 |
| R3 = ( | 70 | ) / 1.25 = 0.28 |
| 200 |
SAEm = 1.645√0.40(0.0212) + 0.32(0.0442) + 0.28(0.0972) + 0.052 = 0.127
The SAE value for the mixture can now be used in a way similar to that obtained for a single substance. Upper and lower control limits can be calculated, and an overexposure may possibly be confirmed.
Equation (F-5)
UCL = 1 + SAEm = 1 + 0.127 = 1.127
Equation (F-6)
LCL = 1 – SAEm = 1 – 0.127 = 0.873
If Em≤LCL then no overexposure has occurred at the 95% confidence level.
If LCL< Em ≤ UCL then the exposure cannot be classified as either under or over the PEL at the 95% confidence level; further sampling may be necessary.
If Em > UCL then an overexposure has occurred (95% confidence).
Because Em > UCL;1.25 > 1.127, an overexposure has occurred within 95 the percent confidence limit.
In this example the three concentration measurements were made from a single sample and all the error associated with the sampling event is estimated as 5%. If multiple sampling events were used to produce the different concentration measurements, additional terms are needed to account for the additional sampling error (see Appendix J). When all sampling error is associated with a single sampling event, equation F-4 can be expressed as:
Equation (F-7)
SAEm = √R1(SAE12) + R2(SAE22) + ··· + Rn(SAEn2)
where:
SAEm is the sampling and analytical error for the mixture,
SAEn is the SAE for analyte n, and
Rn is the exposure severity ratio for analyte n.
Appendix G
Conversion Equations (mg/m3 to ppm)
Equation (G-1)
| ppmNTP = | (mg/m3)·(24.46) |
| M |
Where:
24.46 = molar volume at 25°C (298K) and 760 mmHg
M = molar mass (g/mol)
NTP = Normal Temperature and Pressure (25°C and 760 mmHg)
mmHg = millimeters of mercury
NOTE: PELs in ppm are provided as parts of vapor or gas per million parts of contaminated air by volume at NTP.
CSHOs will not usually need to calculate the exposure concentration in ppm at the sampling site (ppmPT) but, if necessary, it can be calculated from SLTC results reported in ppmNTP by using the following equation:
Equation (G-2)
| ppmPT = (ppmNTP) · ( | (760) | ) · ( | T | ) |
| P | 298 |
Where:
P = sampling site pressure (mmHg)
T = sampling site temperature (K)
298 = normal temperature in degrees Kelvin (273 + 25)
760 = normal atmospheric pressure in mmHg
NOTE: When a contaminant concentration is converted from mg/m3 and expressed as ppmPT, that value cannot be compared directly to the PEL table without first converting it to its corresponding ppmNPT value.
NOTE: The barometric pressure for the time period sampled can sometimes be obtained from the NOAA website or by calling the local weather station or airport. If air pressures are obtained by this route, it is necessary to obtain the unadjusted barometric pressure (station pressure) for compliance applications. The barometric pressure information most readily available from weather and aviation sources is the sea-level adjusted barometric pressure which tends to average about 760 mmHg and does not represent the actual air pressure of worksites at elevations that differ from sea level.
If the sources above are not readily available or cannot provide the actual station pressure, then the elevation (Elev) in feet of the worksite can be used to calculate the typical barometric pressure (P) in mmHg using the following equation:
Equation (G-3)
| P = 760 · [1 – | (Elev·1.6470 × 10-3) | ] 6.3222 |
| (295.20 · (1+(Elev·4.9787 × 10-8)) |
Equation G-3 is an adaptation of the atmospheric model equation used in the U.S. Standard Atmosphere (1976) using a higher average effective sea-level screen temperature (295.2K) and lower temperature lapse rate (5.4K/km) typically observed over land surfaces within the northern latitudes of the U.S. (19°N to 61°N). For most of the U.S., the barometric pressures obtained with this equation are better estimates of observed station pressures than the 1976 model and deviate from mean annual station pressures by about 0.24% RSD (percent relative standard deviation) for elevations below 4,300 feet and 0.52% RSD for elevations below 30,000 feet. These deviations are insignificant compared to the estimated 1.6% RSD for combined normal seasonal, storm, and diurnal station pressure variations observed at any elevation within the year. The 1.6% RSD may be assumed if the worksite elevation can be estimated to within 100 feet. A global positioning system (GPS) elevation measurement is typically within 100 feet of the actual elevation. GPS elevation measurements should be made outdoors and away from tall structures. Example calculations using the equation give 723.2 mmHg for an elevation of 1,400 feet above mean sea level and 569.5 mmHg for an elevation of 8,000 feet above mean sea level. Due to Alaska's high latitudes, Equation G-3 is biased high for significant elevations in Alaska; therefore, the station pressure of a nearby weather station is necessary to obtain accurate air pressures for most of Alaska.
Appendix H
Health Effects Codes
The intranet version of the OCD provides health effect information, including the applicable Health Effect Codes, for each chemical. The complete list of Health Effect Codes is shown below in Table H-1. The Health Effect Codes indicate the principal health effect of exposure to each substance and are used to determine the seriousness of a violation and severity of the penalty, based on the guidelines contained in Chapter 4 of the FOM.
|
Health Code |
Health Effects |
|---|---|
|
HE1 |
Cancer---Currently regulated by OSHA as carcinogen |
|
HE2 |
Chronic (Cumulative) Toxicity---Known or Suspected animal or human carcinogen, mutagen (except Code HE1 chemicals) |
|
HE3 |
Chronic (Cumulative) Toxicity---Long-term organ toxicity other than nervous, respiratory, hematologic, or reproductive |
|
HE4 |
Acute Toxicity---Short-term high-risk effects |
|
HE5 |
Reproductive Hazards---Teratogenesis or other reproductive impairment |
|
HE6 |
Nervous System Disturbances---Cholinesterase inhibition |
|
HE7 |
Nervous System Disturbances---Nervous system effects other than narcosis |
|
HE8 |
Nervous System Disturbances---Narcosis |
|
HE9 |
Respiratory Effects Other Than Irritation---Respiratory sensitization (asthma or other) |
|
HE10 |
Respiratory Effects Other Than Irritation---Cumulative lung damage |
|
HE11 |
Respiratory Effects---Acute lung damage/edema or other |
|
HE12 |
Hematologic (Blood) Disturbances---Anemias |
|
HE13 |
Hematologic (Blood) Disturbances---Methemoglobinemia |
|
HE14 |
Irritation-Eyes, Nose, Throat, Skin---Marked |
|
HE15 |
Irritation-Eyes, Nose, Throat, Skin---Moderate |
|
HE16 |
Irritation-Eyes, Nose, Throat, Skin---Mild |
|
HE17 |
Asphyxiants, Anoxiants |
|
HE18 |
Explosive, Flammable, Safety (No adverse effects encountered when good housekeeping practices are followed) |
|
HE19 |
Generally Low Risk Health Effects---Nuisance particulates, vapors or gases |
|
HE20 |
Generally Low Risk Health Effects---Odor |
Appendix J
Example Calculations to Determine Compliance Using Full-Period Continuous Single Samples and Full-Period Consecutive Samples
Example Calculation for Full-Period Continuous Single Sample
A single glass-fiber filter and personal sampling pump were used to sample for carbaryl for an 8-hour period. SLTC reported 6.07 mg/m3. The SAE for this method is 0.23. The PEL is 5.0 mg/m3.
Step 1. Calculate the exposure severity:
Equation (J-1)
| Y = | 6.07 mg/m3 | = 1.21 |
| 5.0 mg/m3 |
Step 2. Calculate confidence limits
Calculate the LCL95%:
Equation (J-2)
LCL95% = 1.21 - 0.23 = 0.98
Because the LCL95% does not exceed 1.0, noncompliance is not established.
Calculate the UCL95%:
Equation (J-3)
UCL95% = 1.21 + 0.23 = 1.44
Step 3. Classify the exposure.
Because the LCL95% < 1.0 and the UCL95% > 1.0, classify as possible overexposure.
Example Calculation for Full-Period Consecutive Sampling
Two consecutive samples were taken for carbaryl instead of one continuous sample, and the following results were obtained:
| Type |
Sample Results
|
Sample Results
|
|---|---|---|
|
Sampling rate (L/min) |
2.0 |
2.0 |
|
Time (min) |
240 |
240 |
|
Volume (L) |
480 |
480 |
|
Weight (mg) |
3.005 |
2.808 |
|
Concentration (mg/m3) |
6.26 |
5.85 |
The SAE for carbaryl is 0.23
Step 1. Calculate the UCL95% and the LCL95% from the sampling and analytical results.
Using Equation (4) from Section IV.D:
| TWA = | (6.26 mg/m3)(240 min) + (5.85 mg/m3)(240 min) | = 6.055 mg/m3 |
| 480 min |
Using Equation J-1:
| Exposure severity (Y) = | 6.055 mg/m3 | = | 6.055 mg/m3 | = 1.21 |
| PEL | 5.0 mg/m3 |
Using Equation J-2 assuming a continuous sample:
LCL95% = 1.21 - 0.23 = 0.98
Using Equation J-3:
UCL95% = 1.21 + 0.23 = 1.44
Step 2. Because the LCL95% < 1.0 and UCL95% > 1.0, the results are in the possible overexposure region. To document an overexposure, the CSHO must reanalyze the data using the more exact calculation for full-period consecutive sampling (Using Equation (8) from Section IV.D):
| LCL95% = 1.21 – | 0.23 · 2√(6.25 mg/m3 · 240 min)2 + (5.85 mg/m3 · 240 min)2 | = 1.21 - 0.20 |
| 5.0 mg/m3 · (240 min + 240 min) |
= 1.01
Since the LCL95% > 1.0, a violation is established.

