Ethanol Processing
Table of Contents:
- Introduction
- Ethanol Industry and Process Descriptions
- Ethanol Manufacturing Health and Safety Hazards
- Safety Measures
- Engineering Controls for Flammable Liquids
- Safe Work Practices When Working with Flammable Liquids
- Engineering Controls to Prevent Equipment Ruptures
- Safe Work Practices to Prevent Equipment Ruptures
- Engineering Controls for Combustible Dust Hazards
- Safe Work Practices for Combustible Dust Environments
- Engineering Controls for Engulfment Hazards
- Safe Work Practices for Engulfment Hazards
- Personal Protective Equipment for Engulfment Hazards
- Engineering Controls for Hazardous Noise Levels
- Hearing Conservation Program
- Hearing Protection Devices
- Engineering Controls for Hazardous Substances
- Safe Work Practices to Protect Workers from Hazardous Substances
- Personal Protective Equipment (PPE) to Protect Workers from Hazardous Substances
- Engineering Controls for Confined Spaces
- Safe Work Practices for Confined Space Hazards
- Personal Protective Equipment for Confined Space Hazards
- Engineering Controls for Motor Vehicle Hazards (e.g., loading, unloading, operating)
- Safe Work Practices for Motor Vehicle Operation
- Lockout/Tagout of Hazardous Energy
- Emergency Planning
- Investigating/Inspecting Ethanol Processing Facilities
List of Appendices
- Appendix A Summary of Hazards and Controls
- Appendix B List of Some of the Standards Applicable to Ethanol Manufacturing Facilities
- Appendix C Safe Entry Requirements for Above Ground Storage Tanks
- Appendix D Producing Ethanol from Cellulosic Materials
List of Figures
- Figure II.1. Ethanol Characteristics
- Figure II.2. Map of Ethanol Production Facilities in the U.S. (USDA, 2013b)
- Figure II.3. Growth in U.S. Ethanol Production 1980-2013 (RFA, 2014)
- Figure II.4. Current Breakdown of Domestic Ethanol Manufacturing by Feedstock
- Figure II.5. Image of a Corn Kernel
- Figure II.6. Nutritional Makeup of Shelled Corn
- Figure II.7. Chemical Process in Corn Dry-Milling
- Figure II.8. Corn Dumped into Receiving Pits
- Figure II.9. Bucket Elevator and Storage Silo
- Figure II.10. Corn Cleaning Unit Operation
- Figure II.11. A Typical Corn Milling Device
- Figure II.12. Ethanol Fermentation Tanks
- Figure II.13. Ethanol Distillation Columns
- Figure II.14. Molecular Sieve Packed Bed Tanks
- Figure II.15. Product Storage Tanks
- Figure II.16. A Typical Ethanol Load-Out Area
- Figure II.17. A Rotary Drum Dryer
- Figure II.18. A Ring Dryer Solids Recovery Cyclone
- Figure II.19. Transferring Co-Products in Load-Out Area
- Figure II.20. General Overview of the Corn Wet-Milling Process
- Figure II.21. Steep Tanks
- Figure II.22. Germ, Fiber, and Starch Separation During Corn Wet-Milling
- Figure II.23. Biochemical Conversion of Cellulosic Feedstocks
- Figure II.24. Chemical Process During Biochemical Conversion of Cellulosic Feedstocks
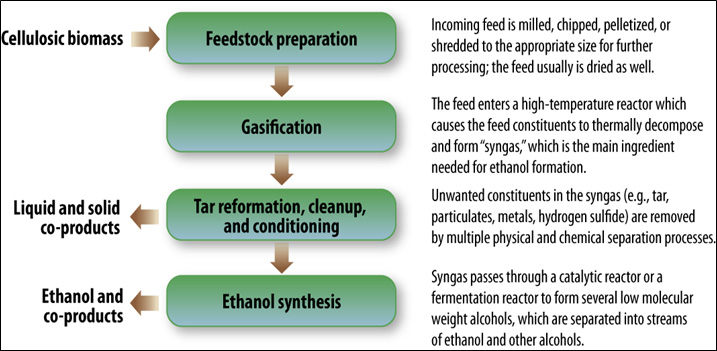
- Figure II.25. Thermochemical Conversion of Cellulosic Biomass to Ethanol and Co-products
- Figure II.26. Chemical Process During Thermochemical Conversion of Cellulosic Feedstocks
- Figure II.27. Estimated Mixed Alcohol Product Distributions for Thermochemical Conversion of Cellulosic Feedstock
- Figure II-5.1 Various Uses of Corn (EPA, 1995)
- Figure II-7.1 General Overview of the Corn Dry-Milling Process
- Figure II-8.1 Flow Diagram of Corn Dry-Milling Process
- Figure II-20.1 Simplified Flow Diagram of Corn Wet-Milling Process (Ramirez, 2008)
List of Tables
- Table II.1. Production Steps in Corn Dry-Milling
- Table II.2. Production Steps in Corn Wet-Milling
- Table II.3. Production Steps in Biochemical Conversion
- Table II.4. Production Steps in Thermochemical Conversion
- Table III.1. Examples of Hazardous Chemicals Found at Ethanol Manufacturing Facilities
Abbreviations
- AFEX
- ammonia fiber explosion
- ANPR
- Advanced Notice of Proposed Rulemaking
- ARRA
- American Recovery and Reinvestment Act
- Ca(OH)2
- calcium hydroxide
- CGF
- corn gluten feed
- CO
- carbon monoxide
- CO2
- carbon dioxide
- DD&E
- distillation, drying, and evaporation
- DDGS
- dried distillers' grain with solubles
- DOE
- United States Department of Energy
- EISA
- Energy Independence and Security Act
- E10
- ethanol/gasoline blend containing 10 percent ethanol
- E85
- ethanol/gasoline blend containing 85 percent ethanol
- E98
- ethanol/gasoline blend containing 98 percent ethanol
- HAZWOPER
- Hazardous Waste Operations and Emergency Response
- H2
- hydrogen gas
- H2S
- hydrogen sulfide
- H2SO4
- sulfuric acid
- HCS
- Hazard Communication Standard
- LFL
- lower flammable limit
- mg/m3
- milligrams per cubic meter
- mm
- millimeter
- MOC
- management of change
- MPa
- megapascals
- NEC
- National Electric Code
- NEP
- National Emphasis Program
- NFPA
- National Fire Protection Association
- PEL
- permissible exposure limit
- PHA
- process hazard analysis
- ppm
- parts per million
- POx
- partial oxidation
- PPE
- personal protective equipment
- psi
- pounds per square inch
- PSM
- Process Safety Management
- RFS2
- Renewable Fuels Standard, 2007 issue
- SDS
- Safety Data Sheet
- SO2
- sulfur dioxide
- SSF
- simultaneous saccharification and fermentation
- STEL
- short-term exposure limit
- TWA
- time-weighted average
- USDA
- United States Department of Agriculture
- WDGS
- wet distillers grain with solubles
- USCSB
- United States Chemical Safety and Hazard Investigation Board
I. Introduction
Ethanol is an organic chemical used in medical products, industrial solvents, alcoholic beverages, and many other applications. In recent years, ethanol has been used increasingly in motor vehicle fuels, and this particular use now dominates the demand for ethanol in the United States. The ethanol manufacturing industry has changed considerably and rapidly in response to this increased demand:
- Between 2000 and 2010, the number of ethanol manufacturing facilities in the U.S. nearly quadrupled.
- Over the same time frame, total nationwide ethanol production increased more than eight-fold. In 2010, U.S. facilities manufactured more than 13 billion gallons of ethanol, compared to just over 1.6 billion gallons in 2000.
- Corn is currently the primary feedstock for most ethanol production. However, to meet demands for alternative fuels, ongoing research and development is investigating how to manufacture ethanol from a much broader array of feedstocks.
As with any growing industry, it is important to ensure that health and safety management procedures keep up with the changing profile of the underlying manufacturing processes. This OSHA Technical Manual (OTM) chapter was prepared to educate readers about some of the safety and health hazards associated with the current and anticipated future production technologies for ethanol manufacturing facilities in the United States.
Information in this OTM chapter is organized as follows:
- Section II presents a profile of the U.S. ethanol manufacturing industry and describes the most commonly used production technologies, including information on emerging technologies.
- Section III identifies selected safety and health hazards associated with ethanol manufacturing.
- Section IV identifies some of the controls (engineering, administrative and personal protective equipment) typically used to prevent or mitigate these hazards.
- Section V outlines emergency planning requirements.
- Section VI discusses inspecting/investigating ethanol processing facilities.
- Appendix A summarizes hazards and controls discussed in this OTM chapter.
- Appendix B is a list of some OSHA standards relevant to workplace hazards in ethanol manufacturing facilities.
- Appendix C reviews safe entry requirements for aboveground storage tanks.
- Appendix D discusses various options for producing ethanol from cellulosic materials.
- Last are the references and glossary sections, respectively. The glossary is a list of definitions for all terms shown in bold.
The contents of this OTM chapter reflect conditions in the ethanol manufacturing industry at the time of its composition. It is likely that this industry will continue to change in the future, particularly as viable production technologies are developed for feedstocks other than corn. General information is presented on production processes likely to be found at most ethanol manufacturing facilities. However, process design and safety systems are expected to vary from one facility to the next. An extensive account of unit operations and safety and health hazards for ethanol manufacturing facilities is presented, but should not be viewed as a comprehensive compilation of the equipment and hazards that exist at every facility in this industry.
In addition to complying with the requirements of the Hazard Communication Standard (HCS; 29 CFR 1910.1200) when hazardous chemicals exist in the workplace, ethanol processing facilities must also be evaluated to determine if the requirements of the Process Safety Management of Highly Hazardous Chemicals standard (PSM; 29 CFR 1910.119(a)(1)(i) & (ii)) apply. PSM requirements must be implemented when a process involves a highly hazardous chemical at or above the specified threshold quantities, listed in Appendix A of the standard and/or involves 10,000 pounds or more of a flammable gas or liquid (e.g., ethanol, gasoline). However, there is an exemption (OSHA, 1997) for flammable liquids stored in atmospheric tanks or transferred and kept below their normal boiling point without the benefit of chilling or refrigeration. This exemption is applicable to flammable liquids in tanks, containers and pipes used only for storage and transfer. Similarly, stored flammable liquids in containers, including cans, barrels and drums are exempt from coverage by the PSM standard.
Process means any activity involving a highly hazardous chemical including any use, storage, manufacturing, handling, or on-site movement of such chemicals, or any combination of these activities. For purposes of this definition, any group of vessels which are interconnected and separate vessels which are located such that a highly hazardous chemical could be involved in a potential release, shall be considered a single process. Citations under 29 CFR 1910.119 will continue to be issued when the quantity of flammables in the process, not counting atmospheric storage, exceeds 10,000 pounds, or where the quantities in storage do not fall within the exception for other reasons (i.e., non-atmospheric storage, storage that relies on refrigeration, quantities in process and not actually in storage). 29 CFR 1910.119 Appendix C provides non-mandatory compliance guidelines and recommendations for PSM.
II. Ethanol Industry and Process Descriptions
A. Profile of the U.S. Ethanol Industry
Figure II.1. Ethanol Characteristics

Figure II.1. Ethanol Characteristics
Text version of Figure II.1.
Physical properties of C2H6O:
- Boiling point: 173°F
- Explosive limit in air: 3.3-19 in percent, by volume
- Clear, colorless liquid
- Weak and winelike odor
Ethanol or ethyl alcohol (Figure II.1), has three primary U.S. markets, the most recognized being the alcohol found in alcoholic beverages. Industrial applications are another major market, since ethanol is widely used as a formulation component or solvent in the manufacturing of pharmaceuticals, paints, personal care products, cleaning products, and flavorings. Finally, ethanol can be used as an automotive fuel or fuel additive, and this is by far the largest market for ethanol today. In the United States, ethanol is typically mixed with gasoline in a 10 percent ethanol blend (E10), but vehicle engines can be designed to run on blends of up to 85 percent ethanol (E85) or even pure ("neat") ethanol.
Historically, ethanol is produced in two different ways: as a fermentation product (i.e., bioethanol) or from the petroleum by-product ethylene (i.e., synthetic ethanol). Synthetic ethanol accounts for less than 10 percent of ethanol production and is used almost exclusively in industrial applications (ORNL, 2010). Bioethanol accounts for the vast majority of non-petroleum based ethanol production. Specifically, ethanol formed by fermentation of starch-based or cellulosic-based feedstock accounts for nearly all of today's ethanol market, with only minimal amounts currently manufactured via thermochemical production. However, this breakdown may change in the future, as further research is conducted on the viability of thermochemical production processes.
The U.S. ethanol industry has changed dramatically in recent years as it adjusts to increasing demand, emerging production technologies, and shifting government policy. This section characterizes the current state of the industry and highlights some of the issues influencing the future of the industry. The future profile of the U.S. ethanol industry may differ considerably from current conditions. Major growth in the U.S. ethanol industry within the last 10 years has propelled ethanol into a significant player in meeting America's demand for motor fuels. This growth has been spurred in part by interest in reducing reliance on costly, foreign, nonrenewable energy sources.
From 2000 to 2010, total U.S. ethanol production increased from 1.63 billion gallons a year to 13.3 billion gallons a year. U.S. corn-based ethanol production was 13.3 billion gallons in 2010, accounting for almost 40 percent of total U.S. corn use in the 2010 crop year. In the same period, ethanol was blended into over 90 percent of the nation's gasoline supply, replacing about 445 million barrels of imported oil. As gasoline prices rise, ethanol's appeal as a blend increases, as does the profitability of ethanol production, and the demand for corn (USDA, 2012). In 2013, ethanol (an estimated 13.3 billion gallons) accounted for 10 percent of U.S. gasoline supply; more than 98 percent of the ethanol production was made from corn (RFA, 2014). Corn used for ethanol generates co-products, for example, dried distillers grains with solubles (DDGS), which is sold as livestock feed (USDA, 2013). Due to the dominance of corn in the U.S. ethanol industry, II.B focuses on producing ethanol from corn.
From 2000 to 2011, the total number of ethanol production facilities rose from 54 facilities in 17 states to 204 facilities in 29 states. Thus, a large portion of the nation's ethanol manufacturing facilities were constructed in the last 10 years. As of January, 2014, the number of ethanol plants rose to 210 in 28 states. The vast majority of biorefineries are concentrated in the "corn belt", the top six ethanol producing states as of January 2011 were, in order, Iowa, Nebraska, Illinois, Minnesota, South Dakota, and Indiana. New construction is expanding to other areas of the country, with facilities underway in North Carolina, Georgia, and California (RFA, 2011a). As of December 2013, the top ten ethanol producing facilities were in North Dakota, South Dakota, Nebraska, Kansas, Minnesota, Iowa, Wisconsin, Illinois, Indiana and Ohio (not in order of production; RFA, 2014). Figure II.2. shows the locations of ethanol production facilities in the U.S. and Figure II.3 shows the growth rate in U.S. ethanol production.
Figure II.2. Map of Ethanol Production Facilities in the U.S. (USDA, 2013b)

Figure II.3. Growth in U.S. Ethanol Production 1980-2013 (RFA, 2014)

Text version of Figure II.2.
This figure shows a map of counties in the United States shaded different colors depending on how many bushels of corn are produced in the county.
Figure II.3. Growth in U.S. Ethanol Production 1980-2013 (RFA, 2014)
Text version of Figure II.3. Listed below are years of 1980-2013 and the corresponding amount of U.S. Ethanol Production (in millions of gallons):
- 1980
- 175
- 1981
- 215
- 1982
- 350
- 1983
- 415
- 1984
- 510
- 1985
- 617
- 1986
- 712
- 1987
- 819
- 1988
- 831
- 1989
- 843
- 1990
- 848
- 1991
- 866
- 1992
- 985
- 1993
- 1,154
- 1994
- 1,289
- 1995
- 1,358
- 1996
- 1,088
- 1997
- 1,288
- 1998
- 1,405
- 1999
- 1,465
- 2000
- 1,622
- 2001
- 1,765
- 2002
- 2,140
- 2003
- 2,810
- 2004
- 3,404
- 2005
- 3,904
- 2006
- 4,884
- 2007
- 6,521
- 2008
- 9,309
- 2009
- 10,938
- 2010
- 13,298
- 2011
- 13,929
- 2012
- 13,218
- 2013
- 13,300
Ethanol can be produced from a wide variety of feedstocks. Traditionally, ethanol is produced from materials rich in simple sugars or starches. These materials are broken down and fermented with yeast or other organisms to produce ethanol. The U.S. industry is dominated by corn, but other possible sugar and starch feedstocks include sugarcane, sugar beets, grain sorghum (milo), wheat, barley, and cheese whey.
Appendix D focuses on producing ethanol from cellulosic materials because of future expansion into this method of production by the ethanol industry. As of January 2014, five of the seven plants under construction or expansion will use cellulosic or waste feedstocks (RFA, 2014). More recently, the industry has been developing new methods to handle cellulosic feedstocks, which refer to materials where energy is stored as complex sugar polymers called cellulose. Cellulosic feedstocks include materials such as agricultural residues (e.g., corn stover, sugarcane bagasse), forestry waste, and municipal solid waste. Cellulosic ethanol can be produced similarly to sugar/starch ethanol, except that additional chemical pretreatments are required to break down the cellulose into simple sugars prior to fermentation. Alternatively, cellulosic material can undergo thermochemical processing where the feedstock is gasified and then converted to ethanol through catalysis or fermentation. Cellulosic technologies promise to substantially increase the quantity and types of feedstock available for ethanol production, as well as use materials that were previously viewed as waste products. However, some of these technologies are still in development and have not yet been realized at commercial scales.
Integrated biorefineries are envisioned as a key model for future ethanol production. Corn wet-milling facilities, described below, are one example of this model. Biorefineries utilize all of the components of a feedstock to produce multiple products, such as fuels, chemicals, animal feed, and power. By generating their own power, these facilities reduce costs and emissions, and the production of high-value chemicals and animal feeds increases profitability when compared to facilities that produce fuel alone (NREL, 2009). The government is investing heavily in biorefineries: in December 2009, the U.S. Department of Energy (DOE) and the U.S. Department of Agriculture (USDA) awarded $564 million in American Recovery and Reinvestment Act (ARRA) funding to 19 integrated biorefinery projects at pilot, demonstration, and commercial scales (DOE, 2009). The biorefinery approach is viewed as integral to the industry's development and economic success.
B. Producing Ethanol from Starch- and Sugar-Based Materials
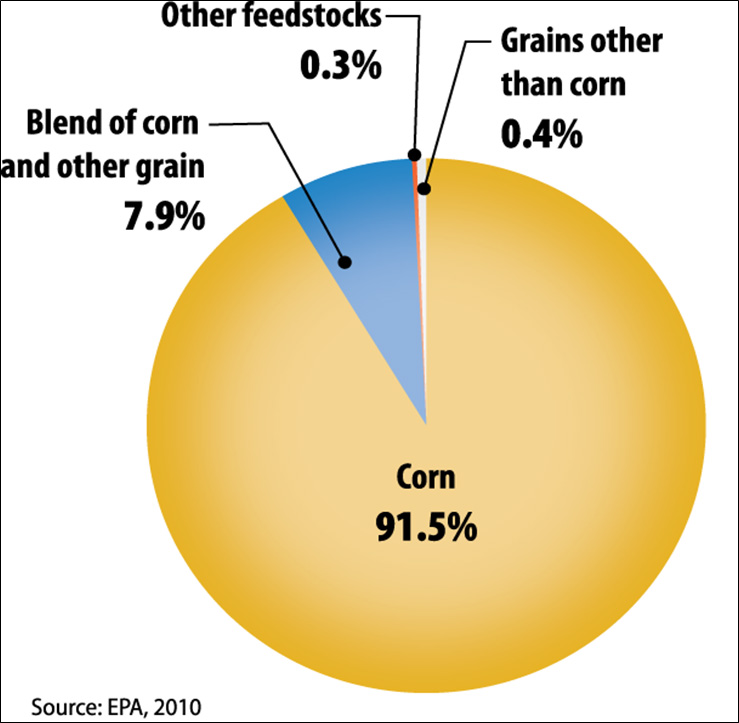
Figure II.4. Current Breakdown of Domestic Ethanol Manufacturing by Feedstock
Text version of Figure II.4.
- 91.5% - Corn
- 7.9% - Blend of corn and other grain
- 0.3% - Other feedstocks
- 0.4% - Grains other than corn
Current commercial production of ethanol is based almost exclusively on starch- and sugar-based feedstocks. As shown in Figure II.4, in the United States, the ethanol industry is dominated by corn, with 91.5 percent of production capacity from facilities using corn alone and another 7.9 percent of capacity from facilities using a blend of corn and other grain (e.g., corn and milo), with corn as the primary feedstock (EPA, 2010). Facilities using other grains (e.g., wheat, milo) without corn make up an additional 0.4 percent of capacity. The remaining U.S. production capacity (0.3 percent) comes from facilities processing other feedstocks, such as cheese whey (lactose fermentation), potato waste, and beverage or brewery waste.
Figure II.5. Image of a Corn Kernel showing the various components - starch, bran, starch and gluten, and germ.

While not yet in commercial use in the U.S., other feedstocks can also be utilized for ethanol production. Brazil, the world's second-largest producer of ethanol, used sugarcane to produce 6.9 billion gallons of ethanol in 2010 (RFA, 2011a).
In Europe, the most common feedstock is wheat, although other cereal based grains can be used (e.g., barley, maize, rye), two-thirds of all raw materials used are cereal grains, while the rest of the feedstock is mainly derived from sugar beets (ePURE, 2011).
Although the United States grows small amounts of sugarcane and sugar beets, these crops are used primarily in the sugar industry; because sugarcane is a tropical plant, in the United States it is grown primarily in Florida, Texas, Louisiana, and Hawaii. In contrast, sugar beets are harvested primarily in northern states, with production concentrated in Minnesota and North Dakota. A 2006 assessment found that domestic production of ethanol from sugarcane and sugar beets was not cost competitive at that time (USDA, 2006).
Figure II.5.1 Various Uses of Corn (EPA, 1995)

A corn kernel—or shelled corn (Figure II.5) —is sometimes viewed as consisting of three main structural elements, each of which contains a different subset of the kernel's nutrients (Figure II.5.1). The first element, the bran (or hull), includes the outer skin of the kernel and is high in fiber. The second element, the endosperm, makes up most of the volume of the corn kernel and contains protein-rich gluten and sugar-rich starch. The last element, the germ, is the embryo of the seed and is located inside the endosperm. The germ is small but oil-rich. By dry weight, shelled corn's nutritional makeup is dominated by starch (72 percent, dry weight). Figure II.6. illustrates the remaining nutritional constituents of corn. The high starch content makes corn a good feedstock for ethanol production, while its widespread cultivation in the United States makes it economically efficient. The challenge in corn ethanol production is to separate the starch from the other components of shelled corn, and then to convert the starch into ethanol.
Text version of Figure II.5.1.
This diagram shows varous parts of corn and what they can be used for.
- Endosperm
- Corn Meal
- Cereals
- Gluten
- Cattle Feed
- Raw Starch
- Edible Starch
- Corn Starch
- Jellies
- Candies
- Dextrin
- Mucilage
- Glue
- Textile Sizing
- Food Sauces
- Fireworks
- Industrial Starch
- Laundry Starch
- Textile Sizing Manufacture
- Filler in Paper
- Cosmetics
- Explosives
- Corn Syrup
- Mixed Table Syrups
- Candies
- Confectionery
- Ice Cream
- Shoe Polishes
- Corn Sugar
- Infant Feeding
- Diabetic Diet
- Caramel Coloring
- Vinegar
- Lactic Acid
- Tanning Mixtures
- Brewing
- Artificial Silk
- Edible Starch
- Hull
- Bran
- Cattle Feed
- Bran
- Germ
- Oil Cake (or Meal)
- Cattle Feed
- Crude Corn Oil
- Soap
- Glycerin
- Soluble Corn Oil
- Textile Sizing
- Cloth Coloring
- Refined Corn Oil
- Salad Oils
- Cooking Oils
- Medicinal Oils
- Plastic Resin
- Rubber Substitutes
- Erasers
- Elastic
- Heels
- Oil Cake (or Meal)
Figure II.6. Nutritional Makeup of Shelled Corn

Figure II.6. Nutritional Makeup of Shelled Corn
Text version of Figure II.6.
- 72% - Starch
- 9.5% - Fiber
- 9.5% - Protein
- 4.3% - Oils
- 2.6% - Simple Sugars
- 1.4% - Minerals
- 0.7% - Others
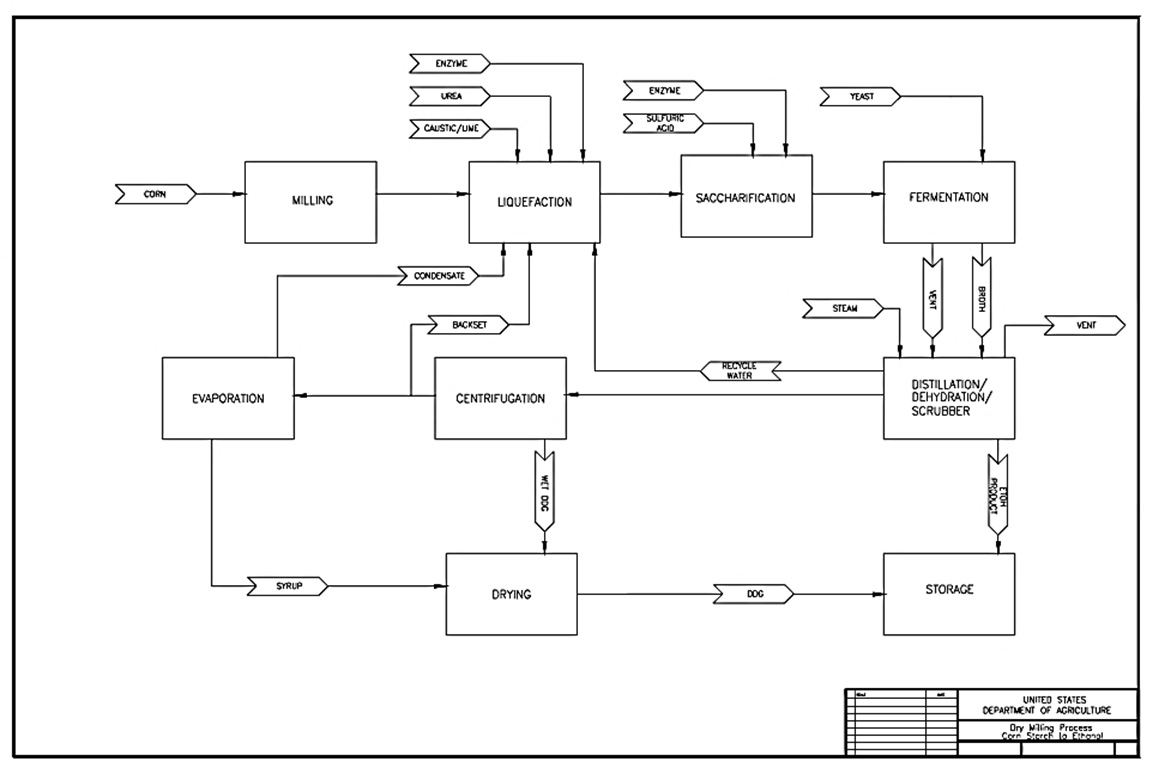
There are two main processes for producing ethanol from corn: dry-milling (II.B.1) and wet-milling (II.B.2). Both processes involve breaking down the starch in the corn kernel into simple sugars and then fermenting the sugars to create ethanol. The primary difference between the two methods is whether the entire kernel is processed, as in corn dry-milling; or if the corn kernel is first broken down into its individual components (i.e., germ, fiber, gluten, and starch) prior to processing, as in corn wet-milling. Dry mill ethanol plants generally produce only one primary co-product, distillers' grain with solubles (DGS), which can be sold wet (WDGS) or dried (DDGS) for use as animal feed. In contrast, the corn wet-milling process of separating shelled corn into its components, prior to processing, increases the number of co-products realized, usually gluten feed, gluten meal, food-grade corn oil, and DGS. There are currently far fewer wet milling facilities than dry milling facilities in the United States. Data available in November, 2009 indicated that there were 180 corn or starch ethanol plants operating in the U.S. 173 of these plants processed corn and/or other similar grains, and only 11 of these used the wet milling process (EPA, 2010).
-
Corn Dry-Milling
This section reviews the typical production steps found at ethanol manufacturing facilities that use the corn dry-milling process. Figure II.7 is an overview of the chemical processes that occur during corn dry-milling. The processing steps (Figure II.7.1, Figure II.8.1) are shown in the chronological order observed at these facilities. However, corn dry-milling facilities will not necessarily employ every one of these steps in the order shown, and some facilities may use different terminology when referring to these processing steps. Compare the steps of the corn dry-milling process in Figure II.8.1 with the corn wet-milling process in Figure II.20. Table II.1 summarizes each of the production steps, primary inputs, primary outputs and the main operations discussed.
This section is based on information from many references (e.g., BeMiller and Whistler, 2009; ICM, 2011; Jacques et al., 2003; Mosier and Ileleji, 2006; Rausch et al., 2005 and RFA, 2011b).
Figure II.7. Chemical Process in Corn Dry-Milling

Figure II.7.1 General Overview of the Corn Dry-Milling Process

Figure II.8.1 Flow Diagram of Corn Dry-Milling Process (NREL, 2000)
Text version of Figure II.7.
This chart shows an overview of chemical processes during corn dry-milling.
The chemical process begins with starch and water being added together. After enzymes are introduced, the combination becomes glucose. Adding yeast to the glucose results in ethanol and carbon dioxide.
The chemical equations for the steps are as follows: (C6H10O5)n + H2O becomes C6H12O6 after adding enzymes. After the yeast step, the result is 2C2H5OH + 2CO2.
As an example, these are example masses during the various steps: 100 lbs of starch plus 10 lbs of water becomes 105 lbs of glucose after the addition of enzymes. 53 lbs of ethanol and 51 lbs of carbon dioxide are the result after the addition of yeast.
Notes:
- The masses shown in the third row indicate anticipated quantities of intermediates and products assuming a 100-pound charge of starch and a 100 percent reaction yield. Actual yields at ethanol manufacturing facilities are considerably lower.
- lbs = pounds
- Adapted from Dale and Tyner, 2006
Text version of Figure II.7.1.
The chart begins with shelled corn going to the receipt, storage, and inspection stage. After that, the process goes to cleaning, then milling, followed by liquefaction, then saccharification, and fermentation. After the next step, distillation and dehydration, ethanol is formed, and the rest of the product goes to the co-product processing stage, after which it becomes animal feeds and other co-products.
The following are descriptions given after various stages of the chart:
- Receipt, storage, and inspection
- Large corn husks and forein material are removed from the incoming corn, and the kernels are stored for further processing.
- Cleaning
- Screeners or scalpers remove additional unwanted material (e.g., stones, glass, sticks) from the corn kernels.
- Milling
- Corn kernels are crushed and ground, forming a dry, fine corn flour.
- Liquefaction
- Corn flour, water, enzymes, and other ingredients are mixed in a tank, where corn starch breaks into simple sugars.
- Saccharification
- Another enzyme is added to break the simple sugars down further into glucose.
- Fermentation
- Yeast is added to the mixture to help convert glucose into ethanol and carbon dioxide.
- Distillation and dehydration
- The ethanol that is formed is separated from other constituents and purified to product specifications
- Co-product processing
- Products other than ethanol are further processed to meet end-user needs.
Text version of Figure II.8.1.
This diagram shows the steps that corn goes through in a Dry-Milling process.
NOTE: The table below is best viewed on a tablet device, notebook, or desktop computer.
Table II-1. Production Steps in Corn Dry-Milling
Production Steps Primary Inputs Primary Outputs Operations Performed Receipt, storage, and inspection
Shelled corn
Stored corn
- - Receive shelled corn by truck or rail
- - Unload corn into receiving pits
- - Transfer corn to storage bins or silos
- - Inspect corn upon receipt
Cleaning
Shelled corn from storage
Cleaned whole corn kernels
- - Pass corn through screeners or scalpers to remove oversized and smaller material
- - Pass corn through other steps (e.g., destoner, magnet) to remove other unwanted objects
Milling
Cleaned whole corn kernels
Fine corn flour
- - Transfer whole kernels to a hammer mill, impact mill, or other milling operation
- - Crush and grind shelled corn into a fine flour
Liquefaction
Fine corn flour
"Mash" (liquid mixture of corn flour and other corn parts)
- - Mix fine corn flour with water in large cook or slurry tanks
- - Add chemicals to adjust the slurry's pH
- - Add enzymes (alpha amylase) to solution to break down corn starch into dextrins
Saccharification
"Mash"
Mash with starch broken down into simple sugars
- - Add enzymes (glucoamylase) to mash to breakdown dextrins into glucose
Fermentation
Mash with starch broken down into simple sugars
"Beer" mixture containing ethanol and solids from grain and yeast; and carbon dioxide
- - Add yeast to convert glucose in mash into ethanol and carbon dioxide
- - Pump beer to separate storage vessel
Distillation and dehydration
"Beer" mixture (fermented mash)
Denatured ethanol
- - Pump beer mixture into a continuous distillation system, which may contain multiple columns
- - Collect purified ethanol from the vapor portion of columns and spent solids (stillage) from the bottom
- - Purify ethanol stream in rectifying column, molecular sieve, or other production equipment
- - Add denaturant to ethanol in cases where ethanol is not used for human consumption
- - Store denatured ethanol in tanks until distribution
Co-product processing
Stillage from distillation columns
Wet distillers' grain and/or dried distillers' grain with solubles (DDGS)
- - Reduce moisture content in stillage by using centrifuges, dryers, or other equipment
- - Make selected co-products by mixing varying quantities of dry products with other materials
- - Load dry products into trucks, railcars, or other means of transport for distribution
-
Receipt, storage and inspection
Corn dry-milling facilities typically receive corn kernels or shelled corn by truck or rail from farms. The shelled corn is separated from husks and other unwanted material during harvesting, though some of this material is still present in the shelled corn shipments that arrive at ethanol manufacturing facilities. The shelled corn shipments arriving at manufacturing facilities typically have moisture contents of 15 percent or less, which helps prevent heating and microbial mold activity in the grain.At ethanol manufacturing facilities, incoming shelled corn is dumped from trucks or railcars into receiving pits – typically grated chutes (Figure II.8) in designated unloading areas. The kernels fall through the grates and typically onto a conveying system which takes the kernels to a storage area. At most facilities, a bucket elevator lifts the shelled corn to the top of large storage bins or silos (Figure II.9). It is not uncommon to encounter concrete silos that are more than 100 feet tall. A bucket elevator is designed for the vertical transport of materials. The elevator consists of a series of buckets attached to a continuous, rotating belt driven by a motor, and the entire system is usually enclosed. The buckets pick up material at the bottom of the elevator and empty the same material at the top of the elevator via the movement of the belt. Storage bins and silos are filled from the top (by bucket elevator) and emptied from the bottom so that the oldest material is continually emptied, thus ensuring that shelled corn does not remain in the silos for unacceptably long time frames. Facilities typically keep enough stored corn on hand so that production could continue for several days in the event that scheduled deliveries are interrupted.
Upon receipt in storage bins or silos, the corn is inspected for quality. Typically, milling facilities use U.S. No. 2 grade yellow dent corn, which has specifications for weight, damaged kernels, and foreign material (Corn Refiners Association, 2011; USDA, 2013a). Facilities may also perform quality assurance testing to check for infestations or fungal toxins (e.g., aflatoxin, which is a by-product of mold).
Figure II.8. Corn Dumped into Receiving Pits

Figure II.9. Bucket Elevator and Storage Silo

-
Figure II.10. Corn Cleaning Unit Operation

Cleaning
The corn kernels must be cleaned prior to processing. The incoming stream of corn kernels, as received, is typically passed through a screener or scalper (Figure II.10.), or multiple screeners or scalpers arranged to remove oversized materials (e.g., corn cobs, husks, sticks) and smaller materials. The unwanted materials tend to account for a very small portion of the incoming corn stream. Cleaning operations, which are typically operated continuously but can also be operated in batches, involve passing the shelled corn over multiple vibrating, perforated sheets, with different sized holes on each sheet. The screeners are sized so that corn kernels can pass through some sheets, thereby removing oversized materials, but do not pass through others, thereby removing undersized materials. Ultimately, only materials of a certain size range (i.e., a standard corn kernel) will make it through the screening process. The corn may also pass through a destoner, which separates objects based on weight and can be used to remove heavier objects (e.g., stones, glass) from the process stream. During the cleaning process, pressurized air may be used to remove chaff and dust. In addition, the process stream will usually pass through a magnetic separator to remove any tramp metal that may be remaining. The principal output from the cleaning operation is a "dry" process stream of shelled corn. -
Figure II.11. A Typical Corn Milling Device

Milling
Once the corn has been cleaned, the whole kernels are conveyed to a milling operation (Figure II.11), typically a hammer mill or impact mill that breaks down the hard exterior of the shelled corn to expose the starch that was previously encapsulated in it. This makes the starch in the milled corn flour more suitable for further processing at the next step, liquefaction. Milling processes typically crush and grind shelled corn into a fine flour. In a hammer mill, for example, a series of hammers are mounted on a rotating drum. The drum rotates at a high speed, and the incoming kernels are shattered by the impact of hammers, other particles, and the walls of the hammer mill. Size-selective screens allow milled corn flour to exit the mill while retaining larger particles requiring further size reduction. Typical screens are sized between 0.13 and 0.2 inches in diameter (3.2 and 4.8 millimeter (mm) in diameter). The output from milling operations is a "dry" fine corn flour that can be readily mixed with water, whereas shelled corn cannot. -
Liquefaction
The previous three production steps involved a "dry" process stream. In the liquefaction step, the milled corn flour is poured into water, thus entering a "wet" process stream for the remainder of the ethanol manufacturing operations. The primary functions of the liquefaction step are to generate a slurry suitable for further processing and to begin breaking down the corn starch into simpler sugars.In liquefaction, the fine corn flour is mixed with water in large cookers (e.g., a steam injection heater called a jet-cooker) or slurry tanks. Facilities will also add alpha-amylase, an enzyme, to the solution. Alpha-amylase helps break down the corn starch into shorter carbohydrate chains known as dextrins. The most effective use of alpha-amylase occurs when the pH of the slurry is between 6.0 and 6.5, and the pH is kept in this range from the time the alpha-amylase is added until liquefaction is complete. Anhydrous ammonia (refer to 29 CFR 1910.119 Appendix A for PSM applicability), used caustic from the cleaning system, and various other bases (e.g., lime) may be added if the pH falls below the optimal range, whereas sulfuric acid might be added if the pH starts to exceed the optimal range. Other chemicals (e.g., urea) may be added at some facilities to optimize enzymatic activity (NREL, 2000). The vessel temperature is also adjusted throughout the liquefaction cycle to optimize the enzymatic breakdown of starches, with temperatures ranging anywhere from 180°F to 220°F. Hot condensate water from other production areas is used to achieve this temperature range. The liquefaction process can take several hours.
To ensure a relatively steady flow of product, facilities with corn dry-milling operations will often operate multiple liquefaction vessels in parallel. Overall, the cooking process that occurs during liquefaction uses physical processes (heat and high-shear mixing) to further break apart the starch granules so that the alpha-amylase can access the starch polymers to break them down biochemically. At the end of this process, all the starch should have been converted to dextrins. The liquefied slurry is a yellow, watery mixture of corn solubles and insolubles called "mash." In the next step, saccharification, the dextrins will be broken down into glucose.
-
Saccharification
At the end of liquefaction, the mash is cooled to 86°F and the enzyme glucoamylase is added as the mash is being pumped into fermentation tanks. Glucoamylase breaks down the dextrins into glucose, which completes the breakdown of the starch into simple sugars. The most effective use of glucoamylase occurs when the solution pH is between 4.0 and 5.5, which is considerably lower than the pH in the liquefaction process. Therefore, the solution pH must be lowered to achieve optimal enzymatic activity. The pH reduction at this stage is typically accomplished by blending in a lower pH stillage solution generated later in the process or by adding sulfuric acid. Saccharification usually occurs as the mash is filling the fermentation tanks and continues throughout fermentation in a process known as simultaneous saccharification and fermentation (SSF). -
Fermentation
Fermentation is the step that produces ethanol; it occurs in large fermentation tanks (Figure II.12). In addition to the mash and glucoamylase, yeast is added to the tanks in proper quantities to convert the glucose in the mash into ethanol and carbon dioxide (CO2). Fermentation lasts for 40 to 60 hours and during this time the mixture is agitated to ensure that the yeast and sugars remain well mixed. The purpose of this step is to biochemically convert as much of the glucose in the mash as possible to ethanol.Figure II.12. Ethanol Fermentation Tanks.

Note: Appropriate labels/markings (e.g., HazCom (29 CFR 1910.1200), confined space warning sign (29 CFR 1910.146)) must be displayed as required. Spill containment mechanism must be installed.
Unlike the upstream processes (e.g., milling, liquefaction, saccharification) that operate continuously, fermentation typically operates as a batch process. Most facilities have numerous tanks dedicated to fermentation and they typically operate in groups of three: while one tank is being filled, another is fermenting, and the third is emptying and made ready for filling. With this arrangement, upstream production never needs to halt between batches. Larger facilities can have multiple groupings of three tanks.
At the end of a fermentation batch, the vessel contains a mixture known as "beer," which is a complex mixture containing yeast, bran, gluten, and liquids. Ideally, the beer should contain very little, if any, glucose because the purpose of fermentation is to convert all glucose to ethanol. The liquid phase of the beer is typically 8 to 12 percent ethanol by weight. Facilities will pump the beer into a beer well, which serves as a reservoir of material for the subsequent downstream processes.
CO2 produced during fermentation is handled differently from one facility to the next. Some facilities collect the gas and sell it, typically to off-site vendors (e.g., for beverage carbonation). Other facilities will vent the gas to the atmosphere, particularly when no buyers are available. However, facilities will generally include scrubbers and other devices to ensure that any ethanol vapor in the CO2 exhaust is captured and returned to the process.
-
Distillation and Dehydration
After fermentation is completed, the next step is to separate and purify the ethanol from other beer constituents. The beer is pumped through a continuous, multicolumn distillation system. Distillation takes advantage of the fact that different liquids have different boiling points: while water boils at 212 degrees Fahrenheit (°F), ethanol boils at approximately 173°F. The temperature in the column will vary from approximately 212°F in the base (almost pure water at modest pressure) to about 172°F at the top, where an ethanol-water solution is recovered as an azeotrope. The boiling point of the mixture is lower than the boiling point of either ethanol or water, although very close to that of ethanol. Thus, when the ethanol-water mixture is heated, the vapors generated contain a greater percentage of ethanol than water. To purify the ethanol, the process is repeated multiple times in series, inside distillation columns. After each distillation step (or contacting step) in the columns, the vapors contain a higher percentage of ethanol.Figure II.13. Ethanol Distillation Columns.
 Note: Appropriate labels/markings (e.g., HazCom (29 CFR 1910.1200), confined space warning sign (29 CFR 1910.146)) must be displayed as required. Spill containment mechanism must be installed.
Note: Appropriate labels/markings (e.g., HazCom (29 CFR 1910.1200), confined space warning sign (29 CFR 1910.146)) must be displayed as required. Spill containment mechanism must be installed.Most corn dry-milling facilities have at least two types of distillation columns (Figure II.13): beer columns and rectifying columns. The beer column generally separates the liquids (water and ethanol) from the spent solids (whole stillage). By heating the column, liquids in the beer evaporate and ethanol-containing vapors are collected at the top of the column, while the stillage is drained from the bottom. The vapors collected from the first column are then condensed and sent to the rectifying column. Again, the liquid is heated, and the ethanol-rich vapors are collected at the top of the column. Vapors collected from the rectifying column are approximately 91 to 95 percent ethanol. Water from the bottom of the rectifying column is diverted to a side stripper to extract the remaining ethanol. The side stripper works on the same principle as the distillation columns and continues to extract purified ethanol based on the 173°F boiling point. Water from the side stripper is recycled back to the cook process.
Figure II.14. Molecular Sieve Packed Bed Tanks

Note: Appropriate labels/markings (e.g., HazCom (29 CFR 1910.1200), confined space warning sign (29 CFR 1910.146)) must be displayed as required. Spill containment mechanism is not visible.
At this point, the purified ethanol still contains approximately 5 percent water. However, ethanol used for fuel must be anhydrous (i.e., waterless) to be blended with gasoline. To remove the remaining water, the ethanol-water azeotrope is typically passed through a molecular sieve to adsorb water from the mixture. The molecular sieve is a bed of specialized beads that selectively adsorb water based on molecule size (Figure II.14). The beads are commonly made from zeolite, a type of aluminosilicate. Similar to fermentation tanks, facilities typically operate multiple molecular sieves so that when one sieve needs to be regenerated (removal of the adsorbate (water)) after it becomes saturated with water another is always available to handle the ethanol stream. Ethanol exiting the molecular sieve is over 99 percent pure.
The final step in production is denaturing the ethanol. The purpose of denaturing is to intentionally put additives in the ethanol that render it unusable for human consumption, which makes the manufactured ethanol exempt from the beverage tax. At facilities that manufacture ethanol for fuels, the ethanol is denatured with conventional gasoline (5 percent by volume) prior to storage. The denatured ethanol is stored in tanks (Figure II.15) until distribution via tanker truck or rail to large terminals for further fuel blending and storage (Figure II.16).
Figure II.15. Product Storage Tanks

Note: Appropriate labels/markings (e.g., HazCom (29 CFR 1910.1200), confined space warning sign (29 CFR 1910.146)) must be displayed as required.
Figure II.16. A Typical Ethanol Load-Out Area

Note: Appropriate labels/markings (e.g., HazCom (29 CFR 1910.1200), confined space warning sign (29 CFR 1910.146)) must be displayed as required on rooftop storage tanks; transportation vehicles must also have the required labels/markings. Spill containment mechanism must be installed.
-
Co-Product Processing
The stillage from the distillation columns is centrifuged to separate the liquid from the solids. The solids, known as wet cake or distillers' grain, are made up of the remaining non-starch components of the corn kernels (e.g., bran, gluten) and added yeast. The wet cake collected from the distillation columns and centrifuge initially contains about 65 percent moisture. The liquid, known as thin stillage, is water containing 5 to 10 percent solids. The thin stillage is either diverted back to the cook process as makeup water or is sent to an evaporator and concentrated into syrup containing 25 to 50 percent solids (condensed distillers' solubles).The syrup is mixed back into the wet cake or distillers' grain to form what is commonly referred to as wet distillers' grain. Wet distillers' grain is a viable co-product, but has poor flow properties that make it difficult to handle. As a result, wet distillers' grain is often dried to 10 percent moisture. This can be done in a rotary drum dryer (Figure II.17) or in a ring dryer (Figure II.18) to form dried distillers' grain with solubles (DDGS). Dryer temperatures can vary considerably (e.g., from 220°F to 380°F) depending on many factors, such as the type of dryer used, the dryer residence time and feed rate, and the target moisture content. DDGS has the advantage of being more readily handled and transported, but the necessary drying step makes it more expensive to produce. Wet distillers' grain and DDGS are both sold as high-quality, high-protein animal feed products. These products are typically shipped to customers via railcar or truck at the facility's product load-out area (Figure II.19).
Figure II.17. A Rotary Drum Dryer

Note: Appropriate labels/markings (e.g., HazCom (29 CFR 1910.1200), confined space warning sign (29 CFR 1910.146)) must be displayed as required. Improper installation of explosion vent panel near work platform.
Figure II.18. A Ring Dryer and Solids Recovery Cyclone

Figure II.19a. Transferring Co-Products in Load-Out Area

Figure II.19b. Transferring Co-Products in Load-Out Area

-
Corn Wet-Milling
Corn wet-milling shares many of the same processes with corn dry-milling. The main difference is the addition of several steps at the beginning of the process that separate the corn kernel into its components. More co-products are typically created because of the corn kernel fractionation. Compare the steps of the corn wet-milling process in Figure II.20. and Figure II.20.1 with the steps of the corn dry-milling process in Figure II.7.1 and Figure II.8.1. Figure II.20.1 is a simplified flow diagram of the corn wet-milling process showing only the processing steps from the cleaning of the corn to the separation of the kernel into its component parts (germ, fiber, protein and starch; II.B.).Table II.2. summarizes each of the production steps, primary inputs, primary outputs, and main operations discussed in this section. While this section documents the common processing steps for corn wet-milling facilities, every facility differs: some facilities might not employ every step shown in this section, and other facilities might use different terminology when referring to these processing steps. This section is based on information from many references (e.g., Corn Refiners Association, 2011; Davis, 2001; EPA, 1995, 2010; and Galitsky et al., 2003).
Figure II.20. General Overview of the Corn Wet-Milling Process

Figure II.20.1 Simplified Flow Diagram of Corn Wet-Milling Process (Ramirez, 2008)

Figure II.20. Simplified Flow Diagram of Corn Wet-Milling Process
Text version of Figure II.20. Simplified Flow Diagram of Corn Wet-Milling Process.
The chart starts with shelled corn going to receipt, storage, inspection, and cleaning. From that step, it moves to steeping, then germ separation, followed by fiber separation, and finally starch separation. After starch separation, the two independent resulting steps are saccharification, fermentation, distillation, and dehydration, and co-product processing. Saccharification, fermentation, distillation, and dehydration results in ethanol, and the final products after co-product processing are animal feeds and other co-products.
The following are descriptions given after various stages of the chart:
- Receipt, storage, inspection, and cleaning
- Corn kernels are first stored in silos and other vessels and then are passed through screeners or scalpers to remove unwanted material (e.g., stones, glass, sticks).
- Steeping
- Corn kernels are added to large tanks of water containing sulfur dioxide; this softens the kernels and begins to separate their constituents.
- Germ separation
- Mills coarsely grind the softened corn kernels in order to separate the oil-rich germ from the steeped kernels.
- Fiber separation
- Mills further grind the mixture and screens help separate the bran-rich fiber from the starch and gluten.
- Starch separation
- Centrifuges and other processes separate the starch from the gluten.
- Saccharification, fermentation, distillation, and dehydration
- Enzymes and yeast are added to break the starch into simple sugars and eventually to convert glucose into ethanol and carbon dioxide.
- Co-product processing
- Products other than ethanol are further processed to meet end-user needs.
NOTE: The table below is best viewed on a tablet device, notebook, or desktop computer.
Table II-2. Production Steps in Corn Wet-Milling Production Steps Primary Inputs Primary Outputs Operations Performed Receipt, storage, and inspection
Shelled corn
Stored corn
- - Receive shelled corn by truck, rail, or grain elevators
- - Move shelled corn from truck or railcar into receiving pits and transfer it to storage area
- - Transfer corn in bucket elevators to storage bins or silos
- - Inspect corn upon receipt
Cleaning
Shelled corn from storage
Cleaned whole corn kernels
- - Pass corn through screener(s) or scalper(s) to remove oversized and smaller material
- - Pass corn through other steps (e.g., destoner)
- - Use pressurized air to remove chaff and dust
Steeping
Cleaned whole corn kernels
- Steeped corn kernels
- Spent steepwater
- - Add corn kernels to water tanks with sulfur dioxide and soak them for 28 to 48 hours
- - Empty tank and recharge it with fresh corn
- - Add fresh liquid to longest steeping tank
- - Recycle excess water
Germ separation
Steeped corn kernels
- Germ
- Fiber, starch, and gluten slurry
- - Grind steeped corn kernels into slurry
- - Separate oil-rich germ from steeped kernels (i.e., fiber, starch, and gluten slurry)
- - Wash extracted germ repeatedly to remove starch and gluten from mixture
Fiber separation
Fiber, starch, and gluten slurry
- Fiber
- Starch and gluten mixture (mill starch)
- - Pump fiber, starch, and gluten to impact mill for grinding to remove bran-rich fiber from starch and gluten
- - Separate fiber from gluten and starch using fixed, concave screens
- - Wash and screen fiber again to remove additional starch and gluten
Starch separation
Mill starch
- Starch (suspension)
- Gluten
- - Pump mill starch to centrifuge to separate gluten
- - Wash starch 8 to 14 times in water and hydroclones to remove remaining gluten
Saccharification, fermentation, distillation, and dehydration
Starch suspension
Denatured ethanol
- - Treat starch suspension with acid and enzymes to break down starch into dextrins and then into glucose
- - Add yeast to convert glucose into ethanol
- - Distill ethanol
- - Remove remaining water with molecular sieve
- - Add denaturant to ethanol
Co-product processing
- Spent steepwater
- Germ
- Gluten
- Starch
- Animal feed products
- Oils, starches, and syrups
- - Reduce moisture content in steepwater and starch suspension using centrifuges, dryers, or other equipment
- - Generate selected co-products by mixing varying quantities of dry products with other materials
- - Load dry products into trucks, railcars, or other packaging for distribution into commerce
-
Receipt, Storage, Inspection and Cleaning
This process is the same as that described for corn dry-milling (II.B.1.i. and II.B.1.ii). -
Steeping
The purpose of steeping is to soften the corn kernels for subsequent processing. The kernels are added to large tanks of water (Figure II.21), held at 125°F and contain approximately 0.1 percent sulfur dioxide (SO2). For the SO2-treated process water, a pH range of 4.0 to 5.0 is optimal to prevent microorganism growth and to facilitate separation of starch and proteins. As the corn soaks for 28 to 48 hours, the kernels increase in moisture content from 15 to 45 percent and approximately double in size. The kernels soften and the acidity of the steepwater helps to release some of the gluten and starch.Figure II.21. Steep Tanks

Note: Appropriate labels/markings (e.g., HazCom (29 CFR 1910.1200), confined space warning sign (29 CFR 1910.146)) must be displayed as required. Spill containment mechanism must be installed.
Steeping is often operated as a "continuous-batch" process, so that multiple steep tanks are connected in series and operated by the counterflow principle. When one tank of corn has finished steeping, the tank is emptied and it is refilled with fresh corn. Fresh liquid is added, not to the tank with the fresh corn, but rather to the steep tank that has been steeping the longest. The excess water from that tank is recycled back through the series of steep tanks (in order of descending steep time) until finally the liquid reaches the steep tank with fresh corn. This approach minimizes the water used by the process, reducing operating costs.
The principal outputs of this step are steeped corn kernels and spent steepwater. The sequence of processing steps for the steeped corn kernels are discussed in II.B.1.iii and II.B.1.iv, and further processing of the steepwater is described in II.B.2.v.
-
Germ, Fiber, and Starch Separation
Steeped corn kernels are sent through degerminating mills which tear the kernels apart to free the germ and about half of the starch and gluten. The resultant slurry is pumped through hydrocyclones to extract the germ from the mixture of fiber, starch, and gluten (EPA, 1995). The purpose of this step is to separate the oil-rich germ from the rest of the steeped kernels, which is essentially a mixture of fiber, starch, and gluten. This separation is performed because the germ does not contain the starch that is needed to produce ethanol. The extracted germ is washed repeatedly to remove any starch and gluten from the mixture. The germ is further processed into co-products II.B.2.v.The fiber, starch, and gluten slurry is pumped to an impact mill, where further grinding completely frees the bran-rich fiber from the starch and gluten. The fiber is then physically separated from the gluten and starch using a series of fixed, concave screens. The collected fiber is washed with water and screened again to remove any additional starch and gluten. Processing of the fiber is discussed in II.B.2.v. The remaining starch and gluten mixture is known as mill starch.
Figure II.22. Germ, Fiber, and Starch Separation During Corn Wet-Milling
Text version of Figure II.22. Germ, Fiber, and Starch Separation During Corn Wet-Milling.
Various production processes, intermediates, and co-products are listed in this flowchart.
The chart starts with steeped corn in the production processes category. From here, it moves on to germ separation, and can go further onwards to fiber separation and starch separation in the production processes category.
From germ separation, in addition to fiber separation, the germ is a resulting intermediate. From germ, the co-products of corn oil and feed products are the final steps.
The intermediate of the fiber separation step is fiber. This fiber can become a resulting co-product in the form of feed products.
Starch separation produces the intermediates of gluten and starch. Gluten can be a feed product, and various starches are co-products on their own, but can also be used in ethanol production.
The mill starch is pumped to a centrifuge where the low-density gluten is spun out of the mixture, leaving a starch-rich stream ready for further processing. II.B.2.v describes how facilities typically handle the gluten. The starch is washed 8 to 14 times in water, re-diluted, and passed through hydrocyclones to completely remove any remaining gluten. The starch is over 99.5 percent pure at this point and can be used to produce ethanol; the starch can also be used to manufacture various other products, including pure corn starch and corn syrups (described in II.B.2.v). Some corn wet-milling facilities are designed to manufacture multiple products from the starch, while others will manufacture just a single product. Figure II.22. illustrates the process of germ, fiber, and starch separation during corn wet-milling.
-
Saccharification, Fermentation, Distillation, and Dehydration
At this point, the wet milling process proceeds similarly to the dry milling process. Detailed descriptions of each of these process steps, including the chemicals and microorganisms that are typically added are discussed in II.B.1.iv to II.B.1.viii. The starch suspension is treated with acid and enzymes to break down the starch into dextrins, which are further broken down into glucose. Yeast fermentation then converts the glucose into ethanol. The ethanol is distilled to concentrate the alcohol content, remaining water is removed via molecular sieve, and the product is denatured prior to shipment. -
Co-product Processing
The major co-products from corn wet-milling include: animal feed products, corn oil, ordinary and modified starches, and corn syrups and sugars (Figure II.5.1). The exact combination of co-products will vary from one manufacturing facility to the next and each facility will tailor the range of co-products it produces to current market demands. Every U.S. ethanol-producing wet milling facility produces starches and corn gluten feed or meal; and most, but not all, of these facilities produce corn syrups in addition to ethanol. Most corn wet-milling facilities in the United States will have some of the following operations to process co-products:- The water drawn off the steeping process, known as light steepwater, contains approximately 6 percent of the original dry weight of the grain, of which 35 to 40 percent is protein. At most facilities, the light steepwater is sent to an evaporator where it is concentrated to 30 to 55 percent solids. The concentrated steeping liquor is then added to the fibrous milling residue produced by fiber separation. After the fiber has been screened and washed, it is sent through a drying operation. The steep liquor is blended with the dried fiber to form wet corn gluten feed (CGF). CGF can be sold wet or it can be dried first to extend its shelf life. CGF is widely used by farmers to feed dairy and beef cattle, swine, and poultry.
- The extracted germ is routinely processed for corn oil. The oil is extracted from the germ using a combination of mechanical (i.e., washing, dewatering, drying) and proprietary solvent-based extraction processes and is then refined. The remaining germ residue, or germ meal, is used as a component of animal feed. It is sometimes blended with CGF and is valued for use in poultry and swine diets.
- The extracted gluten is a protein-rich stream that is oftentimes dried and sold as another type of animal feed (corn gluten meal). Corn gluten meal contains about 60 percent protein compared to approximately 20 percent protein in CGF. Corn gluten meal is often used in poultry feed formulations.
- Finally, in addition to being converted to ethanol, the starch component of the corn kernel can be used to manufacture other starch or sweetener products. To produce ordinary starch, the starch suspension is dewatered mechanically (by vacuum filters or centrifuge) and thermally (by drier). To produce modified starches, such as acid-modified or oxidized starches, the starch suspension is treated with chemicals (e.g., hydrochloric acid, sodium hypochlorite) prior to dewatering. Alternatively, to produce sweeteners, the starch is broken down with acids and enzymes to form various syrups with different mixtures of sugars and levels of sweetness. The syrup is refined and then concentrated in evaporators. Some syrup is crystallized to form solid sugars.
- Receipt, storage, inspection, and cleaning
III. Ethanol Manufacturing Health and Safety Hazards
The principal hazards in ethanol manufacturing are associated with processing dry feedstocks and handling ethanol; therefore, ethanol manufacturing facilities combine some of the attributes of grain handling facilities and chemical processing facilities. Individually, these two types of facilities are accompanied by distinct hazards, but when they are combined, hazards associated with both grain handling and flammable liquid processing, storage, and transport come into play (RFA, 2007). Sources of information on past incidents in the ethanol industry include: OSHA IMIS accident investigation summaries, the media, and trade association publications (e.g., Jessen, 2011a).
While individual ethanol manufacturing facilities vary in terms of layout and their sequence of operations, safety and health hazards at these facilities typically occur in various production areas common to most facilities, such as the feedstock receiving area, process buildings where feedstocks are prepared for processing, the ethanol bulk storage area, and the ethanol load-out area (IAFC, 2008).
The remainder of this section provides an overview of some of the common types of hazards found at ethanol manufacturing facilities. Section IV discusses some of the preventive measures that may be implemented to assure workers' safety. Preventive measures in OSHA's PSM standard (29 CFR 1910.119) apply when a process involves a chemical at or above the specified threshold quantities, listed in Appendix A of the PSM standard and/or involves 10,000 pounds or more of a flammable gas or liquid, if the exemption discussed in Section I does not apply.
A. Flammable Liquids
Two Employees are Burned in Distillery Explosion
On September 13, 2002, Employees #1 and #2 were working in the vicinity of the A-still [where initial distillation took place] when it was started. One of the manhole covers in the absorption column was apparently open and flammable alcohol vapors escaped and exploded. Employees #1 and #2 both sustained burns and were hospitalized. The absorption column had been previously opened to inspect the corn grits process and apparently one of the manholes had not been closed. No lockout and tagout procedures (IV.U) were used when the absorption column was opened, and there was no verification process in place to ensure that all the manhole covers were closed before the still was activated.
(Modified for clarity)
Large ethanol manufacturing facilities produce more than 100 million gallons of ethanol each year and store large quantities of the chemical on site at any given time; because ethanol is flammable, the production and storage quantities have the potential to cause catastrophic fires and explosions. Ethanol manufacturing facilities typically take numerous precautions to prevent ethanol fires (IV.A; IV.B), which are perhaps the most well-known occupational hazard for this industry. They must also ensure compliance with emergency planning requirements (Section V).
In addition to ethanol, these facilities process and handle many other hazardous chemicals (III.F), but usually in much lower quantities. Table III.1. below lists some chemicals that are commonly found at ethanol manufacturing facilities. One example is the gasoline (which also contains benzene, 29 CFR 1910.1028; 29 CFR 1926.1128) that is added to ethanol as a denaturant. While gasoline and many other substances in the table exhibit their own hazardous properties, the total amount of gasoline used at these facilities is minimal in comparison to the quantities of ethanol.
| Chemical | Associated Type of Production | Associated Process at Facility | |||
|---|---|---|---|---|---|
|
Corn Dry-Milling |
Corn Wet-Milling |
Biochemical Conversion |
Thermochemical Conversion |
||
|
Alcohols other than ethanol |
|
|
|
May be found at all kinds of facilities, but primarily produced during catalysis of syngas at thermochemical conversion facilities. |
|
|
Ammonia |
Used for various purposes, such as to adjust solution pH, as a nutrient for yeast and other beneficial microorganisms, and for pretreatment in thermochemical conversion. |
||||
|
Carbon dioxide |
|
Formed as a by-product in all fermentation processes and either captured for further processing or vented to the atmosphere. Used in some pretreatment processes for biochemical conversion of cellulose. |
|||
|
Ethanol |
Formed in fermentation tanks and thermochemical conversion reactors, separated from water and other chemicals in multiple unit operations, and kept in storage tanks. |
||||
|
Gasoline |
Added to ethanol product in storage tanks. Benzene is an important hazardous constituent of gasoline (29 CFR 1910.1028; 29 CFR 1926.1128). |
||||
|
Hydrochloric acid |
|
|
|
Used to treat starch suspension to produce modified starches and sweeteners. |
|
|
Sodium hydroxide |
|
|
Used in fermentation tanks for pH control and as a yeast nutrient |
||
|
Sulfur dioxide |
|
|
Added to large tanks of water for steeping corn kernels, and used to treat starch suspension to produce modified sweeteners. |
||
|
Sulfuric acid |
|
Used to control bacteria; added to slurry mixtures to separate corn into starch, germ, fiber, and protein; and used during steam explosion or acid hydrolysis. |
|||
|
Syngas |
|
|
|
Primary product from process in gasification reactor and used to form ethanol in catalytic reactors. |
|
Note: This table presents a partial list of raw materials, intermediates, and products that may be encountered at ethanol manufacturing facilities. However, individual facilities are different, and they can use different chemicals and chemical combinations in their production processes. The table was adapted from Weston Solutions, Inc., 2008 and Section A; and the table does not consider typical process emissions.
Both OSHA and the National Fire Protection Association (NFPA) have classified ethanol as a flammable liquid (29 CFR 1910.106; NFPA 30). Ethanol meets the criteria for a Category 2 flammable liquid, according to OSHA's Flammable Liquids standard and the HCS (29 CFR 1910.106; 29 CFR 1910.1200 Appendix B). This is because ethanol ignites at normal room temperatures, has a flash point of 55°F, and has a boiling point of 173°F (OSHA/EPA, 2011).
Figure III.1. Fire Triangle

When ethanol vapor combines with air in the presence of ignition sources, fires and explosions can result. The lower and upper explosive limits of ethanol are 3.3 percent and 19 percent, respectively, by volume in air. This range spans air concentrations of ethanol that can ignite and burn in the presence of an ignition source (OSHA/EPA, 2011). As the ethanol is blended or denatured (e.g., using gasoline) the explosive limits of the product mixture change slightly. As an example, a blend of 85 percent ethanol and 15 percent gasoline has lower and upper explosive limits of 1.4 percent and 19 percent, respectively (Shaw, 2011). Another key factor is that high-ethanol fuels can form flammable mixtures in the headspace of storage tanks at ordinary temperatures. Gasoline, by way of contrast is usually too rich (above its upper flammable limit) to support combustion in gasoline storage tank headspaces (ullage). Another important physical property of ethanol is that its vapors are heavier than air, this is apparent from ethanol's vapor density (approximately 1.6). Thus, ethanol vapors do not rise in air and tend to accumulate at ground level until dispersed by wind or ventilation (if inside a structure).
At ethanol manufacturing facilities, fire and explosion hazards are present from the time ethanol is first formed through product purification, storage, and transport. As shown in the fire triangle in Figure III.1, three components must be present for a fire to occur: fuel, oxygen (oxidizing agent), and heat (All About Fire, NFPA, 2011b). For ethanol fires, the ethanol vapor represents the "fuel" in the fire triangle; oxygen is the "oxidizing agent;" and, the "heat" component comes from ignition sources that cause the ethanol vapor to first burn.
Several factors can contribute to the release of ethanol, thus providing the fuel for an ethanol fire. These factors include improper storage, accidental releases (e.g., spills, containment failures), undetected leaks, inadequate venting of gases, equipment malfunctions, human error, and transportation accidents. Ethanol spills must be carefully addressed, because flammable vapors will form above all areas where the liquid ethanol travels. Examples of typical ignition sources in areas where ethanol is handled include electric arcs, human activities (e.g., smoking), heating equipment (e.g., furnaces and ovens), open flames, static electricity, and frictional heat. One of the most common ignition sources encountered at these facilities is "hot work" activities, such as welding, cutting, and grinding.
Fires fueled by pure ethanol generate no visible smoke and have a blue flame that may be difficult to see. Fires fueled by denatured ethanol produce no smoke or minimal smoke, but have a faint orange flame that may be noticeable. Fires fueled by ethanol are particularly challenging because they are not easily extinguished by traditional firefighting methods. Some commonly used fire suppression foams (e.g., those used to extinguish gasoline fires) are ineffective on ethanol fires, so special alcohol-resistant foams must be used.
Saccharification Tank Explodes
A contractor was killed and a worker suffered acid burns when an ethanol manufacturing facility's saccharification tank exploded. The 50-foot tall tank contained 40,000 gallons of corn mash. A welding contractor was cutting a hole in the top of the tank so workers could remove and load material from the top. Reportedly, atmospheric conditions had not been checked before the worker began welding, and flammable ethanol vapors had built up inside the tank. The welding ignited the vapors, and the resulting explosion threw the entire tank 75 feet. This event ruptured several interconnecting pipes, which in turn discharged an estimated 1,700 gallons of sulfuric acid. In addition, the saccharification tank landed on an empty railcar and an ethanol-containing tanker truck, causing the ethanol to catch fire. The facility's saccharification tank and fuel load-out area were destroyed during the incident, and other equipment was heavily damaged.
The following are some common areas at ethanol manufacturing facilities that present flammability hazards (Gardiner et al., 2008, 2010).
- Distillation process area. Ethanol is initially formed at manufacturing facilities prior to distillation. However, the distillation process area is typically where the liquid contains high concentrations of ethanol and becomes flammable. This part of the facility, therefore, has significant flammability hazards due to the presence of highly concentrated ethanol vapors and heat. Overpressure or mechanical damage in the distillation columns can cause ethanol vapors to leak, which presents a hazardous situation due to the various ignition sources present and the potential for workers to be exposed beyond OSHA's permissible exposure limit (PEL) for ethanol (1,000ppm for a time-weighted average, 29 CFR 1910.1000 Table Z1). This hazard also exists for other equipment (e.g., piping, molecular sieve) typically found in the distillation process area.
- Fuel storage tank area. Ethanol manufacturing facilities usually store their finished products–both ethanol and denatured ethanol– in large storage tanks before the product is distributed into commerce. Proper handling of the ethanol is critical to avoid leaks in tanks and interconnecting equipment and to prevent more catastrophic ruptures. Some important concerns include pipe ruptures, tank ruptures, and lightning strikes.
-
Storage tank headspace. The headspace is the gas-phase area above liquids inside a tank or other vessel. Recent research found the headspace vapors of denatured ethanol to be flammable at room temperature (64°F) and all temperatures down to approximately 22°F (NREL, 2008). No upper flammability limit was established for the denatured ethanol evaluated in the study and no tests were conducted at elevated temperatures. Instead, the denatured ethanol approached its lower (lean) flammability limit as the ambient temperature was lowered and eventually would not ignite if it was too cold (NREL, 2008).
Fire occurs when there is an ignitable vapor-air mixture and a source of ignition, such as a static discharge (for some examples of previous incidents refer to the United States Chemical Safety and Hazard Investigation Board (USCSB) reports for: Barton Flammable Liquid Explosion and Fire (9/18/2008) and Barton Solvents Explosions and Fire (6/26/2008)). Static electricity may be generated as liquid flows through ungrounded pipes, valves, and filters while being transferred. It can also be produced by en-trained water or air, splashing or agitation, and when sediment in the bottom of the tank becomes suspended.
At normal handling temperatures, flammable storage tanks, like those containing materials such as gasoline, may contain vapor-air mixtures that typically cannot be ignited because the vapor-air mixture is too "rich" (i.e., contains too much fuel and not enough oxygen or is above the gasoline's upper flammability limit) to burn. However, other flammable liquids, including ethanol and high ethanol content fuels, may form ignitable vapor-air mixtures inside tanks at normal handling temperatures.
-
Heated storage tanks. There are specific hazards related to storing flammable liquids in heated tanks. For example, draining flammable liquids below high temperature tank heating elements can result in the ignition of the flammable headspace vapors, as the liquid is pumped out of the tank and oxygen flows into the tank, if the maximum temperature of the heating element can exceed the ignition temperature of the mixture.
Also, excessive tank heating in general (with the heating elements submerged or with the use of heating jackets) can cause boiling and release large quantities of flammable vapor, which is unsafe if the vapors are venting inside a building or close to ignition sources.
Examples of incidents involving deflagrations inside buildings are the Universal Form Clamp Company Explosion and Fire (6/14/2006), Synthron Explosion and Fire (1/31/2006), and CAI/Arnel Chemical Plant Explosion (11/22/2006), all of which involved flammable vapors venting from heated tanks inside structures. All of these incidents were investigated by the USCSB.
- Ethanol loading areas. Ethanol manufacturing facilities usually have loading docks or loading zones where flammable chemicals are transferred to the facility (e.g., the facility receives gasoline for use as a denaturant) and where the denatured ethanol is loaded into trucks or railcars for distribution into commerce. Overfilling, leaking equipment, and other unanticipated releases in the loading areas are extremely hazardous, especially when ignition sources are present (e.g., idling vehicles).
B. Equipment Ruptures
Leaking Valve Causes Fire
Four workers at an ethanol manufacturing facility suffered first- and second-degree burns after a leaking valve caused a stream of pure ethanol to pour from process equipment onto a floor, out an open door, and into a separate production area where hot work operations were taking place. A spark from nearby cutting and welding activities ignited ethanol vapor from the accidental release and caused the fire, which rapidly spread over a large area.
(Cahill, 2000; OSHA, 2011a)
Ruptures can occur in fermentation vessels, product storage tanks, or pipes for various reasons. For example, due to the lack of safety systems or improperly functioning safety systems (e.g., failure of a rupture disk or pressure relief device on a fire protection line, failure of a safety valve to open when a heated storage tank is overheated). Ruptures may also be the result of fires; age deterioration; cracks (e.g., at the bottom and welding edges of storage tanks); corrosion (e.g., of a defective weld); or a lack of proper maintenance (Chang and Lin, 2005; Nolan, 1996).
If hazardous substances (e.g., anhydrous ammonia, ethanol, gasoline, sulfuric acid) released during ruptures are not controlled and contained this may result in fires, explosions, and workers' exposure to hazardous air contaminants.
C. Combustible Dusts
Figure III.2 Combustible Dust Explosion Pentagon

Handling and size reduction of feedstock materials can generate combustible dusts. Combustible dusts are fine dust particles that when present in air at certain amounts and under the right conditions, can cause dangerous flash fires1, deflagrations2, and explosions3 (OSHA Fact Sheet: Combustible Dust Explosions [OSHA, 2008a]; Combustible Dust National Emphasis Program (NEP), CPL 03-00-008 [OSHA 2008b]). Although some exceptions occur, the hazard potential of a given dust material increases as particle size decreases and as moisture content decreases. NFPA has published standards that present far more detailed information on the hazards associated with combustible dusts (e.g., Standard for the Prevention of Fires and Dust Explosions in Agricultural and Food Processing Facilities, NFPA 61; Standard for the Prevention of Fire and Dust Explosions from the Manufacturing, Processing, and Handling of Combustible Particulate Solids, NFPA 654).
Flash fires, deflagrations, and explosions are the primary hazards related to combustible dusts at ethanol manufacturing facilities and can result in death, injury, and substantial property damage. As shown in the combustible dust explosion pentagon above, five elements must be present for a combustible dust explosion to occur: fuel (i.e., combustible dust), an ignition source (e.g., heat), confinement (e.g., a building, a room, vessel, or process equipment), oxygen, and dispersion in sufficient quantity and concentration (OSHA, 2008a). Removing any of these elements prevents an explosion from occurring.
The initial flash fire, deflagration, or explosion which occurs during a combustible dust incident is known as a primary event. A primary explosion has the potential to launch even more severe secondary explosions. Secondary explosions may occur when additional dust gets lofted into the air following an initial incident, providing the fuel necessary for multiple additional explosions throughout a facility. The dust fueling a secondary explosion may be dust released as a result of damage to a containment structure by the primary event; or, the release of dust that had previously settled, for example, on the floor, on top of equipment, on overhead structures, and on other visible and hidden surfaces. The secondary explosion may cause more damage than the primary explosion because they may be fueled by larger amounts and higher concentrations of dispersed dusts (OSHA, 2008a). Dust incidents may also lead to other hazardous events in other operating or storage areas of the facility, for example, the release of flammable liquids from storage areas due to fires or explosions spreading to those areas.
Many different types of combustible dusts can be found at ethanol manufacturing facilities. The largest quantities and accumulations of combustible dusts are expected to occur at facility locations that handle dry materials or processes that dry wet materials, such as in areas where feedstocks are received and processed and co-products are dried and loaded into trucks and railcars. Several different materials are expected to present combustible dust hazards at ethanol manufacturing facilities:
Corn Dust Explosion Injures Three Workers
Three workers at an ethanol manufacturing facility endured serious first- and second-degree burns following a corn dust explosion. The explosion resulted from welding activities near a grain elevator, which caused hot metal to come into contact with corn dust near the base of the elevator. Following the initial explosion, a secondary explosion emitted smoke and dust into the atmosphere. Nearly 50 firefighters responded to the explosion, which was so powerful it raised a semi-truck hauling 80,000 pounds of grain.
(Galvan, 2008)
- Grain dusts (e.g., from corn, wheat, sorghum), which are present in every load of grain received at typical dry and wet milling operations. Grain dusts are also released from unit operations that transfer, crush, grind, and otherwise handle the agricultural feedstocks.
- Dusts from various co-products produced at dry and wet milling operations. These dusts are typically first formed in dryers and are present at all downstream process locations through eventual product load-out.
- Wood dusts from cellulosic materials, as well as other dusts from cellulosic operations (e.g., agricultural residue, municipal solid waste) that may be combustible.
The list below identifies some typical unit operations and production areas that most commonly present combustible dust hazards at ethanol manufacturing facilities:
- Cleaning area. Screeners and scalpers are designed to remove oversized objects and finer material (e.g., dusts) from shelled corn. When operating properly, these devices should collect and control dusts. However, older, leaking, and malfunctioning devices can release significant quantities of dust into the workplace.
- Bulk storage. Dust clouds can form when loading materials into silos, bins, hoppers, and other bulk storage locations, including large storage piles. If fine dust in these areas contact an ignition source (e.g., nearby welding, or electrical equipment not rated for combustible dust environments), fires and explosions are possible.
-
Transfer points. Transfer points, such as enclosed belt conveyors, which are dust ignition-proof (Figure III.3.) and bucket elevators, move large quantities of solid materials and dusts. Ordinarily, these devices effectively move material from one production area to the next. However, blockages, leakages, and other operational conditions can cause large quantities of solids to fall to the ground and high concentrations of dust particles to enter the air. Dust clouds can ignite at transfer points if an ignition source is present, such as a hot bearing along a belt conveyor or use of a conventional vacuum cleaner that is not dust ignition-proof.
Figure III.3. Enclosed Belt Conveyor

Failure to Protect Workers' from Fire and Explosion Hazards
- General Duty Clause Violation at a Grain Handling Facility: There were multiple locations where dust collectors, cyclones, hoppers, heat exchangers, bag houses, and other equipment did not have adequate fire protection or meet the venting requirements. There were also storage bins that did not have an explosion suppression system.
- Abatement note: Among other methods, one feasible and acceptable abatement method to correct these hazards is to comply with NFPA 61 (Prevention of Fires and Dust Explosions in Agricultural and Food Processing Facilities) and NFPA 69 (Standard on Explosion Prevention Systems). Equip dust collectors with deflagration venting (vented to the outside). Install deflagration suppression system and deflagration pressure containment system for the Soymeal, Wheat and Dry Soy Powder locations in accordance with NFPA 69. Install spark detection devices inside pneumatic conveyor systems. Install proper fans and blowers in accordance with NFPA 61 chapter 10. Install proper explosion panels and explosion suppression system in wheat storage bins in accordance with NFPA 61 chapter 6.
(OSHA IMIS Inspection #310768262)
-
Dryers. Ethanol manufacturing facilities use dryers primarily to prepare co-products, for example, dried distillers' grain with solubles (DDGS), which is high in protein and sold as animal feed (II.B.1.viii). Facilities use dryers to reduce the moisture content of solid streams to the desired levels to meet product quality standards.
Dryers can lead to fires through various means. For example, if the moisture content of the inlet stream unexpectedly drops, the material inside the dryer can become excessively dry producing conditions that can lead to smoldering and fires inside the dryer itself. Fires may also be caused by dead spots in fluidized bed dryers, which usually occur when material stops flowing, accumulates, or fluidization is lost in some or all of the beds, resulting in heat buildup and combustion.
-
Dust collectors. Dust collectors remove and collect dust particles from air streams before venting "clean" air for recirculation or exhausting it to the atmosphere. Air material separators are designed to separate the conveying air from the material being conveyed (NFPA 654), they may be used for dust collection and removal (Figure III.4). Fires and explosions can result from dust collectors that are not well maintained; malfunctioning; or poorly designed, for example, not complying with design requirements in applicable standards, such as NFPA 654.
Figure III.4. Air Material Separator (Cyclone)

Dust collector problems should be suspected when there is a sustained dust cloud in the workplace. A common cause of dust collector incidents occurs when smoldering material is inadvertently conveyed to the dust collector, where it can ignite the accumulated dust.
- Grinding or milling area. Grinding and milling reduces feedstock materials into smaller particle sizes (corn flour). The inherent nature of these unit operations causes high levels of combustible dust to form inside the equipment and also generates heat. The key to avoid fires and explosions is to prevent ignition sources from entering the equipment, for example, tramp metal that might be inadvertently conveyed inside the mill along with the feedstock.
- Load-out and cooling areas. Facilities transfer dried co-products to interim storage areas and eventually to railcars or trucks for distribution into commerce. The transfer and loading of the dried product leads to dust formation. Fires and explosions may result if dust clouds are not adequately controlled and ignition sources are present.
D. Engulfment
(Controls: IV.G, IV.H, IV.I, and IV.U)
Figure III.5 Image of a Grain Engulfment Hazard

Text version of Figure III.5.
Text in the image reads "It takes only two to three seconds to become helpless in flowing grain." Additionally, there is an inset illustration of a grain silo with grain on the surface having crusted, leaving empty voids beneath.
In addition to receiving, handling, and processing grain, ethanol manufacturing facilities also store grain on site in very large silos, and some facilities temporarily keep much smaller quantities of dry co-products in large storage piles that might reach heights of 20 feet or more. A significant safety issue associated with grain storage and large storage piles is the possibility of workers becoming engulfed and entrapped by the materials (Inspection of Grain Handling Facilities, 29 CFR 1910.272, CPL-02-01-004, OSHA 1996). Although sometimes used interchangeably, these terms do have subtle differences in meaning (Anku, 1993; Purdue University, 2011):
- Engulfment describes situations in which a worker is entirely buried and submerged below the grain surface.
- Entrapment describes situations in which a worker is physically stuck in grain or co-products, but still has his or her head above the surface.
Workers Engulfed in Storage Bin
Two teenage workers suffocated and died and another young worker was seriously injured when they were engulfed in corn in a storage bin at a grain elevator facility. Reportedly, the storage bin's unloading system was operating as the three workers stood in corn that was more than 30 feet deep. While a conveyor system underneath the bin was removing corn, the workers were trying to make the corn flow by walking down the grain, a practice in which workers enter storage bins and other structures and literally walk on top of encrusted grain to break it up and facilitate the transfer. (Note: OSHA standards specifically prohibit this practice (29 CFR 1910.272(g)(1)(iv).) Upon doing this, one worker began to sink in the corn, and the other two workers attempted to rescue him. Two of the workers became entirely engulfed in corn, while one worker was able to keep his head above the surface and survive until he was rescued.
For the purposes of this document, the term engulfment will be used to encompass both engulfment and entrapment hazards. Engulfment hazards (Figure III.5) may be present when grain is stored in silos, bins, and other large vessels. Grain engulfment presents a suffocation risk for workers and is a leading cause of deaths and injuries in the grain handling industry. Once a worker is engulfed in grain, suffocation quickly follows as the grain fills the mouth, nose, and throat of the submerged individual (Danger of Engulfment and Suffocation in Grain Bins, Hazard Alert, OSHA 20011f). This hazard also applies to large storage piles of co-products that can collapse without warning, especially in instances when workers use heavy-duty equipment (e.g., front-end loaders) to scoop up and move material from around the base of a storage pile. Specific examples of hazardous activities involving workers include (OSHA, 2010):
- Standing on flowing or moving grain in silos. Grain flows downward when unloaded, which results in a funnel-type effect. This moving or flowing grain is like quicksand, and can cover and suffocate a worker within seconds. It is for this reason that workers must never enter silos and large storage vessels that contain moving grain (29 CFR 1910.272(g)(1)(iv)).
- Standing on a grain bridge in silos (OSHA 2011f). A grain bridge forms when mold or moisture causes grain to stick together. As a result, the grain surface in a silo might appear to be stable, but an empty space below the grain surface can form as bin unloading occurs. The grain bridge will readily collapse under the weight of a worker and instantly bury the individual.
- Standing on or next to a grain mass in a storage pile. Facilities can have piles of grain along the side of a storage bin and poorly conditioned grain inside a storage bin. If a worker tries to dislodge the grain in the pile or in a bin, the grain pile can quickly fall and cover the worker.
The aforementioned scenarios are extremely dangerous because workers simply cannot extricate themselves after being engulfed in grain. Engulfment typically has fatal results.
E. Hazardous Noise Levels
(Controls: IV.J, IV.K, and IV.L)
Noise is a prevalent potential hazard throughout many grain handling and processing operations. However, noise levels may not translate into high exposure levels because of workers' mobility between operations and tasks, and equipment not being operated continuously. Excessive noise can hinder communication between workers. It can also damage workers hearing due to long-term repeated exposure. Varying levels of exposure to noise may occur during grain unloading operations; in grain elevators; and grain processing areas, where the most significant exposure to noise is possible (excerpt from Cralley, 1985 (continues below)).
- Grain unloading. Sources of noise include grain dumping, pneumatic blowers or internal combustion-powered vehicles. Sometimes, during the unloading of powdery grain-related products, vibrators may be attached to the metal hoppers of vehicles to facilitate complete dumping of the product which may adhere to the inside surfaces of the vehicle. Noise levels vary depending on the type of vibrator, amount of grain in the vehicle, and its location. As the vehicle empties, noise levels immediately adjacent could easily increase to more than 115 decibels on the A scale (dBA), even for outdoor operations.
- Grain elevators. Noise levels typically exceed 90dBA on the gallery and basement floors of elevators, although full-shift exposures may not reflect these high levels because of worker mobility and the intermittent nature of equipment operation. The primary noise sources include conveying equipment, dust collection equipment, and compressed air used for housekeeping.
- Grain processing. Noise occurs at almost every processing stage and seems to be an inherent, undesirable side effect associated with the operation of most grain processing machinery (e.g., hammer mill). Other major sources of noise include exhaust ventilation equipment, blowers for pneumatic transport, and compressed air usage and generation.
F. Exposure to Hazardous Substances
OSHA standards address employers responsibilities when there is a potential for workers to be exposed to harmful concentrations of toxic and other hazardous substances in the workplace (e.g., 29 CFR 1910.1200; 29 CFR 1910.119; 29 CFR 1910.119 Appendix A; Section I). OSHA standards specifying permissible exposure limits (PELs) for air contaminants include: 29 CFR 1926 Subpart Z; 29 CFR 1926 Subpart D (e.g., 29 CFR 1926.55, 29 CFR 1926.60, and 29 CFR 1926.62). Although OSHA's standards are the regulatory requirements, employers are encouraged to adopt stricter measures to ensure the maximum protection for workers (Permissible Exposure Limits Annotated Tables, OSHA 2014).
Chemicals, feedstocks, catalysts, enzymes, intermediates, products, and other substances (Table III.1) involved in ethanol manufacturing may be hazardous to workers. If appropriate preventive measures are not implemented exposure can occur during handling, storing, processing, and transporting activities. Some of the safety precautions that can be implemented to protect workers from exposure to hazardous chemicals are discussed in IV.M. Examples of hazardous substances likely to be found at ethanol manufacturing facilities include, but are not limited to the following (Brisman et al., 2004; Grisso et al., 2005; LaPrade, 2008; NIOSH, 2010b; Pahwa et al., 2006):
- Enzymes. As noted in II.B.1.iv, facilities add the enzyme alpha-amylase to the solution/slurry during liquefaction to convert corn starch into dextrins. Exposure to alpha-amylase is associated with increased risk for respiratory illnesses, such as occupational asthma, in exposed workers. Also reported are exposure-related symptoms in the eyes (e.g., itchiness) and nose (e.g., sneezing), as well as allergic reactions in those who are more sensitized to its effects (Houba, 1996; HSE, 2010). At this time, OSHA has no permissible exposure limit (PEL) for alpha-amylase.
- Gases. For example, carbon dioxide (CO2) may build up in stored grain, CO2 is also produced during the fermentation process, and other gases can be produced from decomposing and fermenting grain, for example, hydrogen sulfide, ammonia, sulfuric acid, and methane.
- Combustion by-products. For example, carbon monoxide can be generated when workers use machinery within or close to confined spaces.
- PSM covered chemicals. Some hazardous chemicals that may be used in ethanol processing facilities in quantities that necessitate compliance with OSHA's PSM standard include: sulfur dioxide, ammonia, and anhydrous hydrochloric acid (29 CFR 1910.119, 29 CFR 1910.119 Appendix A, Section I).
- Fumigants. Fumigants, for example, phosphine, used for pest control can be present in stored grains. Many of the fumigants used in grain handling and processing facilities are hazardous chemicals. Workers exposed to fumigants can develop serious chronic health effects, such as heart disease and cancer.
- Molds in stored grain. Mold spores can form in spoiled stored grain. Exposure to molds can cause adverse effects.
- Grain dusts. Workers exposed to grain dusts generated throughout ethanol manufacturing unit operations can experience respiratory effects.
- Metallic catalysts. Metallic catalysts are used at facilities that thermochemically convert syngas into ethanol. Potential worker exposures to the metal constituents will depend on the physical state of the catalyst, the extent of catalyst handling, and other parameters.
G. Confined Spaces
(Controls: IV.P, IV.Q and IV.R)
Some examples of confined spaces at ethanol manufacturing facilities include silos, process vessels, storage tanks, grain storage bins, and feed hoppers. The safety and health of workers entering confined spaces in grain handling facilities is addressed by both the Grain Handling Facilities standard, 29 CFR 1910.272, and the Permit-Required Confined Spaces standard, 29 CFR 1910.146. However, confined space work, such as grain bin entry, that is regulated by 29 CFR 1910.272, is not subject to the provisions of 29 CFR 1910.146, as long as the provisions of 29 CFR 1910.272 protect workers against all the hazards within the grain bins (OSHA Letter of Interpretation: Questions regarding the PRCS standard, 29 CFR 1910.146, and the Grain Handling standard, 29 CFR 1910.272. [02/08/2005]). CSHOs should also refer to OSHA's directive, Application of the Permit-Required Confined Spaces standard, CPL-02-00-100, for additional information.
Adverse health effects are possible if workers enter confined spaces containing toxic atmospheres. These are atmospheres containing gases, vapors, or fumes known to have poisonous physiological effects (OSHA, 1993). Examples of some, but not all, of the potential hazards in confined spaces at ethanol manufacturing facilities include the following (OSHA, 1998, 2011b; Porter, 2010):
Employee Dies after Falling into a Fermentation Tank
On November 20, 2011, Employee #1 opened an access door on the top of a fermentation tank to perform tank prewash activities utilizing a high-pressure water hose to presoak the sides and agitators inside of the tank. The tank was energized and the agitators were turning (III.I). The tank was approximately 18 ft. deep and contained approximately 4 ft. of finished liquid product the employer described as "13 percent beer". Sometime during the presoaking activity, Employee #1 fell into the tank for an unknown reason. There were no witnesses to his activities. Coworkers found Employee #1 unresponsive on the bottom of the tank. He was pronounced dead at the scene by a local physician.
(OSHA IMIS Inspection #316034545)
- Hazardous chemicals. Employers must prevent workers exposure to concentrations of toxic and other hazardous substances (III.F.; 29 CFR 1910 Subpart Z; 29 CFR 1910.119, 29 CFR 1910.119 Appendix A, Section I.) capable of causing acute health effects (e.g. within seconds or minutes) that can prevent workers from effecting self-rescue or being able to request help when working in a confined space.
- Oxygen-deficiency. Some confined spaces can be oxygen-deficient, especially when oxygen is displaced by other gases (e.g., CO2) formed by the decomposition of stored grain or other processes in the confined space.
- Fire and explosion hazards.For example, combustible dusts (e.g., in bucket elevators (III.A.)) and flammable gas or vapor in excess of 10% of its lower flammable limit (III.C.).
- Physical hazards. These include: falls from heights; drowning; pipes that could cause workers to trip and fall; crushing or laceration injuries from moving mechanical parts, for example, sweep augers capable of causing severe laceration or crushing injuries or death; and, exposure to hazardous energy (III.I., IV.U.).
H. Motor Vehicles
Heavy-duty trucks and railcars have a ubiquitous presence at ethanol manufacturing facilities. At larger facilities, a nearly constant stream of truck traffic delivers corn to receiving areas, and a separate set of trucks and railcars are routinely filled with denatured ethanol at product load-out areas. Hazards that could affect safe transportation and safe loading and unloading of motor vehicles include: a flammable atmosphere; static electricity which could act as a source of ignition during loading and unloading of flammable substances or combustible dust; poorly maintained roads (e.g., pot holes, uneven road surfaces); severe weather conditions (e.g., lightning, snow storm); unsafe driving; and, operating motor vehicles in poor working condition. Unsafe driving habits and/or improperly planned routes may also result in motor vehicle collisions with pedestrians, equipment, pipes, etc. Safety measures must be implemented to preclude motor vehicle incidents.
I. Exposure to Hazardous Energy
(Control: IV.U.)
Sources of hazardous energy include mechanical, electrical, hydraulic, and pneumatic equipment, which present a danger to workers if the energy isolating source (e.g., circuit breaker, disconnect switch) is not properly shut down and if appropriate measures are not taken to prevent the equipment from starting up unexpectedly while employees are still working (IV.U; 29 CFR 1910.147).
J. Other Hazards
Ethanol manufacturing facilities, like many chemical manufacturing and processing operations, have numerous additional potentially hazardous situations. To name a few, hazards can be associated with the boiler operation; use of hand tools; electrical fixtures; walking and working surfaces; elevated platforms; and, brazing, cutting and welding operations. For example, employers are required to protect workers from exposure to hexavalent chromium (above the PEL) that may be produced from the welding or torch cutting of piping and vessels made of steel (29 CFR 1910.1026; 29 CFR 1926.1126; OSHA 2013b).
Some ethanol manufacturing facilities will have specific hazards that are not common across the industry. For instance, the small number of facilities that operate coal-fired boilers may have hazards associated with coal dust, while wood dust may be an issue at facilities that process certain cellulosic feedstocks. It is, therefore, necessary to identify the complete set of site specific hazards when developing and implementing worker safety and health programs at ethanol manufacturing facilities.
IV. Safety Measures
This section reviews some safety measures that could be implemented to prevent workers exposure to the hazards that were discussed in Section III (IAFC, 2008). Often-times facilities have many different types of controls available to them and will typically adopt those best suited for specific applications. The basic concept behind engineering controls is that, to the extent feasible, the work environment and the job itself should be designed to eliminate hazards or reduce workers exposure to an acceptable measure. Where engineering controls are not feasible, or do not completely eliminate or reduce workers exposure to permissible levels, other safety measures must be implemented; these include (in order of precedence) safe work practices, administrative controls (e.g., rotation of workers), and PPE (OSHA Hazard Prevention and Controls)
PSM requirements must be implemented when a process involves a chemical at or above the specified threshold quantities, listed in Appendix A of the PSM standard and/or involves 10,000 pounds or more of a flammable gas or liquid if the exemption discussed in Section I does not apply.
A. Engineering Controls for Flammable Liquids
Several different engineering controls are available to prevent hazards related to flammable liquids such as ethanol and gasoline (III.A.). Generally speaking, there are two types of these controls: (1) those that prevent or control the release of flammable vapors, and (2) those that eliminate ignition sources (29 CFR 1910.106(h)(7)(i)(a)). PSM covered facilities have specific safety requirements that employers must implement (OSHA, 2000; 29 CFR 1910.119). The following are examples of the types of controls that might be seen at these facilities:
- Control of ethanol releases (e.g., in production areas and ethanol loading areas). Equipment must be designed and arranged to prevent the unintentional escape of liquids and vapors and to minimize the quantity escaping in the event of accidental release (29 CFR 1910.106(h)(4)(iv)(a)). There are several ways to minimize the amount of ethanol vapors in the open workspace. This includes process equipment that is designed to handle process upsets, for example, the incorporation of safety relief valves that avoid equipment failure by venting excess ethanol streams directly to safe areas. Drains in certain production areas can be designed to quickly move spilled chemicals from surfaces into enclosed tanks. Similarly, tanks and railcars can be equipped with vapor recovery systems that collect the ethanol vapors that would otherwise be released when tanks are filled with liquids. Overfill protection devices can also minimize spills from tanks and other vessels. Dikes may be used to contain any spilled ethanol with drainage to a safe area (e.g., where sources of ignition are absent or prohibited). Other safety measures to prevent fires and/or explosions in these areas include proper bonding and grounding (discussed below) and the installation of Hazard Class I electrical equipment and wiring.
Figure IV.1 A Telephone Rated for Class I Hazardous Location

- Controls for electrical wiring and equipment. One of the principal engineering controls for preventing electrical equipment and wiring used in ethanol production areas from constituting a source of ignition is to ensure that they are rated for environments containing flammable vapors (29 CFR 1910.307(a)(1); 29 CFR 1926.407). Specifically, processing and handling areas involving ethanol vapors are referred to as Hazard Class I locations according to the National Electrical Code (NEC, NFPA 70). A Hazard Class I location is an area where the existence of flammable gases or vapors in air pose a hazard because they could ignite or explode. Each area within the facility must be rated appropriately, for example, Class I Division I or Class I Division II (NFPA 497; NFPA 70). However, there may be locations within the facility that may not qualify to be classified as a hazardous location, for example, office areas, where the hazards of a classified location do not exist.
Electrical equipment and wiring used in hazardous classified locations must be of a type and design that the employer demonstrates will provide protection from the hazards arising from the flammable vapors involved (29 CFR 1910.307(c)(3)), for example, electrical design specified by and installed in accordance with the NEC. These include explosion proof equipment and intrinsically safe electrical equipment (Figure IV.1) that is rated for the specific hazardous classified location. Equipment must be identified not only for the class of the location but also for the explosive or ignitable properties of the specific vapor that will be present (NFPA 70; 29 CFR 1910.307(a)(1)). The electrical equipment's rating is based on the performance of the equipment when tested in a specific flammable atmosphere by a nationally recognized testing laboratory (e.g., Underwriters Laboratories, Canadian Standards Association, etc.).
- Controls for static electricity. Another source of ignition is static electricity. Ignition by static electricity can be prevented in the design and operation of equipment, and by using proper bonding and grounding mechanisms (described below).
- Control of Flammability Hazards in Storage Tanks Headspace. Controls include:
Figure IV.2 Illustration of bonding and grounding

- Bonding and Grounding (Figure IV.2). Bonding is the process of electrically connecting conductive objects, like tanker-trailers, to transfer pumps to equalize their individual electrical potentials and prevent sparking. Grounding (earthing) means connecting a conductive object to the earth to dissipate electricity (e.g., accumulated static, lightning strikes, and equipment faults) into the ground, away from workers, equipment, and ignitable mixtures. Bonding and grounding mechanisms must be implemented simultaneously to safely discharge static charges.
- Inerting the headspace. Using an inert gas such as nitrogen, if done correctly, is effective in reducing the potential for an ignitable incident (explosion) as it renders tank head spaces incapable of supporting ignition from a static spark. However, because this practice can produce oxygen-deficient environments inside tanks, safety procedures must be implemented when opening tanks for routine inspections and maintenance.
- Cross venting to keep oxygen out of the headspace.
- Installing flame arrestors on atmospheric vents.
- Controls for Heated Storage Tanks. Employers should only use tanks designed to comply with the required safety standards (Myers, 1997). Essential safeguards include: electrical equipment designed for use in processes involving flammable liquids (Hazard Class I); proper bonding and grounding to safely discharge static electricity; automatic temperature controls to prevent overheating; alarms and automatic shutdown systems that would detect hazardous conditions, such as high temperatures and safely turn off the system; ventilation systems in flammable storage and processing areas in compliance with the requirements of 29 CFR 1910.106 and designed in accordance with accepted engineering practices such as the Flammable and Combustible Liquids Code (NFPA 30) and the Standard for Exhaust Systems for Air Conveying of Vapors, Gases, Mists, and Noncombustible Particulate Solids (NFPA 91).
- Explosion relief venting. Areas where category 1 or unstable liquids are processed (in processing plants) must have explosion relief venting through one or more of the following methods: open air construction, lightweight walls and roof, light weight wall panels and roof hatches, and windows of explosion venting type (29 CFR 1910.106(h)(3)(iv)). Equipment shall be designed and arranged to prevent the unintentional escape of liquids and vapors and to minimize the quantity escaping in the event of accidental release (29 CFR 1910.106(h)(4)(iv)(a)). Where the vapor space of equipment is usually within the flammable range, the probability of explosion damage to the equipment can be limited by inerting, by providing an explosion suppression system, or by designing the equipment to contain the peak explosion pressure which may be modified by explosion relief. Where the special hazards of operation, sources of ignition, or exposures indicate a need, consideration must be given to providing protection by one or more of the above means (29 CFR 1910.106(h)(4)(iv)(b)).
- Design of piping, valves and fittings in processing plants. The design (including selection of materials) fabrication, assembly, testing, and inspection of piping systems containing flammable liquids must be suitable for the expected working pressures and structural stresses. Conformity with the applicable provisions of Pressure Piping, American National Standards Institute B31 series (American Society of Mechanical Engineers, ASME B31 series) and the provisions of 29 CFR 1910.106(c)(1), is considered prima facie evidence of compliance (29 CFR 1910.106(c)(1)(i)).
- Mechanical Integrity. It is important to ensure that critical process equipment is designed and installed correctly and operates properly. Employers must establish and implement written procedures to maintain the ongoing integrity of process equipment. Workers involved in maintaining process equipment must be trained in the overview of the process, its hazards, and the procedures applicable to their specific tasks. The requirements for maintaining the mechanical integrity of equipment in PSM covered facilities apply to the following (29 CFR 1910.119(j)):
- Pressure vessels and storage tanks;
- Piping systems (including piping components such as valves);
- Relief and vent systems and devices;
- Emergency shutdown systems;
- Controls (including monitoring devices and sensors, alarms, and interlocks); and,
- Pumps.
In constructing new plants and equipment, the employer must ensure that the fabricated equipment is suitable for the process that it will be used in (29 CFR 1910.119(j)(6)(i)). Appropriate checks and inspections must be performed to ensure that equipment is installed properly and is consistent with design specifications and the manufacturer's instructions (29 CFR 1910.119(j)(6)(ii)).
- Other engineering controls. Ethanol manufacturing facilities are typically equipped with many additional engineering controls designed to prevent or mitigate hazards from flammable chemicals. At some facilities, continuous ethanol vapor monitoring devices are installed in key production areas. The devices are equipped with multiple alarm levels designed to trigger increasingly serious actions as detected ethanol vapor concentrations increase (e.g., sounding audible alerts in process control rooms, shutting down process flows, initiating precautionary fire suppression). Similarly, automatic fire detection and suppression systems are usually installed at critical points within production areas, such as tank storage areas and ethanol load-out areas. Suppression systems typically can be activated through both automatic and manual means.
B. Safe Work Practices When Working with Flammable Liquids
The HCS (29 CFR 1910.1200) specifies that employers with hazardous chemicals in their workplaces must develop, implement, and maintain at each workplace, a written hazard communication program (29 CFR 1910.1200(e)) which at least describes how the criteria specified in paragraphs (f), (g), and (h) of the standard for labels and other forms of warning, safety data sheets, employee information, and training will be met (29 CFR 1910.1200(e)(1)).
There are also specific requirements for safe work practices in facilities covered by the PSM standard (29 CFR 1910.119). The employer must develop and implement safe work practices to provide for the control of hazards during work activities such as lockout/tagout; confined space entry; opening of process equipment or piping; and, control of the entrance into the facility by maintenance, contractor, laboratory, or other support personnel. These safe work practices must apply both to employees and contract workers (29 CFR 1910.119(f)(4)).
Examples of safe work practices at ethanol processing facilities include:
- Signage and barriers. All Class I areas where ethanol vapors may be present should be carefully delineated. Signage can be used to inform workers when they are entering Class I areas. In some cases, facilities may choose to erect fences and other barriers to ensure that only authorized personnel work in the designated Class I locations.
- Prohibit smoking. Smoking must not be permitted as a precaution to prevent fires/explosions (29 CFR 1910.106(h)(7)(i)(a)) in or near areas where flammable liquids are stored or processed.
- Hot work programs. Employers must implement rigorous hot work programs to ensure that workers and contractors follow important safety procedures before performing any cutting, welding, and other related activity near areas that may contain flammable liquids (29 CFR 1910.106(h)(7)(ii)(b).
A permit must be issued for hot work operations conducted on or near a PSM covered process. The permit must document that the fire prevention and protection requirements in OSHA standards have been implemented prior to beginning the hot work operations; it must indicate the date(s) authorized for hot work; and identify the object on which hot work is to be performed. The permit must be kept on file until completion of the hot work (29 CFR 1910.119(k); 29 CR 1910.252(a)).
Typical elements of an effective hot work program include: definitions of hot work and hazardous locations; inspections of affected work areas; multiple approvals needed before commencing work; and requirements that workers performing hot work operate continuous ethanol vapor detectors to ensure that no flammable vapors are present. These programs should also specify personal protective equipment (PPE) requirements, which may require workers performing hot work to wear flame-retardant clothing.
- Management of Change. A procedure should be established in the workplace to ensure that changes to any operation or process are safely implemented. Employers responsible for facilities that are covered by the PSM standard must establish and implement written procedures to manage changes (except for "replacements in kind") to process chemicals, technology, equipment, and procedures; and, changes to facilities that affect a process that is covered by the PSM standard (29 CFR 1910.119(l)(1)). The procedures must include the technical basis for the change; impact of the change on safety and health; modifications to operating procedures; necessary time period for the change; and, authorization requirements for the proposed change (29 CFR 1910.119(l)(2)). Operating procedures and process safety information must be updated as applicable (29 CFR 1910.119(l)(4) & (5)). Affected workers, including contractors, must be informed of the change and receive appropriate training before starting up the process or affected part of the process (29 CFR 1910.119(l)(3)).
- Training. The HCS (29 CFR 1910.1200) requires employers to provide workers with effective information and training on hazardous chemicals in their work area (29 CFR 1910.1200(h); 29 CFR 1926.59). The specific training requirements for facilities that are covered by the PSM standard are:
- Initial training. PSM requires that each worker presently involved in operating a process or a newly assigned process must be trained in an overview of the process and in its operating procedures. The training must include emphasis on the specific safety and health hazards of the process, emergency operations including shutdown, and other safe work practices that apply to the worker's job tasks (29 CFR 1910.119(g)(1)).
- Refresher training. PSM also specifies that refresher training must be provided at least every three years or more often if necessary to each worker involved in operating a process to ensure that the worker understands and adheres to the current operating procedures of the process. The employer, in consultation with the workers involved in operating the process, must determine the appropriate frequency of refresher training (29 CFR 1910.119(g)(2)).
- Training documentation. The employer must determine whether each worker operating a process has received and understood the training required by PSM. A record must be kept containing the identity of the worker, the date of the training, and how the employer verified that the worker understood the training (29 CFR 1910.119(g)(3)).
- Safe Operating procedures. Maintenance and operating practices in flammable liquid processing plants must be in accordance with established procedures which will tend to control leakage and prevent the accidental escape of flammable liquids (29 CFR 1910.106(h)(8)(i)). Employers covered by the PSM standard must develop and implement written operating procedures consistent with the process safety information. The operating procedures must provide clear instructions for safely conducting activities involved in each covered process (29 CFR 1910.119(f)(1)).
Operating procedures must be readily accessible to workers who work in or maintain a process (29 CFR 1910.119(f)(2)). This will form a foundation for needed training for workers and ensure that a ready and up-to-date reference is always available. The operating procedures must be reviewed as often as necessary to ensure that they reflect current operating practices, including changes in process chemicals, technology, equipment, and facilities. To guard against outdated or inaccurate operating procedures, the employer must certify annually that these operating procedures are current and accurate (29 CFR 1910.119(f)(3)).
The procedures must address at least the following elements:
- Steps for each operating phase:
- Initial startup;
- Normal operations;
- Temporary operations;
- Emergency shutdown, including the conditions under which emergency shutdown is required, and the assignment of shutdown responsibility to qualified operators to ensure that emergency shutdown is executed in a safe and timely manner;
- Emergency operations;
- Normal shutdown; and,
- Startup following a turnaround, or after an emergency shutdown (29 CFR 1910.119(f)(1)(i)).
- Operating limits:
Consequences of deviation, and steps required to correct or avoid deviation (29 CFR 1910.119(f)(1)(ii)).
- Safety and health considerations:
- Properties of, and hazards presented by, the chemicals used in the process;
- Precautions necessary to prevent exposure, including engineering controls, administrative controls, and personal protective equipment;
- Control measures to be taken if physical contact or airborne exposure occurs;
- Quality control for raw materials and control of hazardous chemical inventory levels; and,
- Any special or unique hazards (29 CFR 1910.119(f)(1)(iii)).
- Safety systems and their functions. For example, interlocks and fire detection or suppression systems (29 CFR 1910.119(f)(1)(iv)).
- Steps for each operating phase:
- Process hazard analysis. The process hazard analysis is a thorough, orderly, systematic approach for identifying, evaluating, and controlling the hazards of processes involving highly hazardous chemicals. Employers covered by the PSM standard must perform an initial process hazard analysis (hazard evaluation) on all processes covered by the standard (29 CFR 1910.119(d) & (e)). The process hazard analysis methodology selected must be appropriate to the complexity of the process and must identify, evaluate, and control the hazards involved in the process.
First, employers must determine and document the priority order for conducting process hazard analyses based on a rationale that includes such considerations as the extent of the process hazards, the number of potentially affected workers, the age of the process, and the operating history of the process (29 CFR 1910.119(e)). All process hazard analyses must be updated and revalidated, based on their completion date, at least every five years (29 CFR 1910.119(e)(6)).
The employer must use one or more of the following methods, as appropriate, to determine and evaluate the hazards of the process being analyzed:
- What-if,
- Checklist,
- Hazard and operability study (HAZOP),
- Failure mode and effects analysis (FMEA),
- Fault tree analysis, or
- An appropriate equivalent methodology (29 CFR 1910.119(e)(2)).
Whichever method(s) is used, the process hazard analysis must address the following:
- The hazards of the process;
- The identification of any previous incident that had a potential for catastrophic consequences in the workplace;
- Engineering and administrative controls applicable to the hazards and their interrelationships, such as appropriate application of detection methodologies to provide early warning of releases. Acceptable detection methods might include process monitoring and control instrumentation with alarms, and detection hardware such as hydrocarbon sensors;
- Consequences of failure of engineering and administrative controls;
- Facility siting;
- Human factors; and,
- A qualitative evaluation of a range of the possible safety and health effects on workers in the workplace if there is a failure of controls (29 CFR 1910.119(e)(3)).
The process hazard analysis must be performed by a team with expertise in engineering and process operations, and that the team must include at least one worker who has experience and knowledge of the process being evaluated. Also, one member of the team must be knowledgeable in the specific analysis methods being used (29 CFR 1910.119(e)(4)).
The employer must establish a system to promptly address the team's findings and recommendations; ensure that the recommendations are resolved in a timely manner and that the resolutions are documented; document what actions are to be taken; develop a written schedule of when these actions are to be completed; complete actions as soon as possible; and communicate the actions to operating, maintenance, and other workers whose work assignments are in the process and who may be affected by the recommendations or actions (29 CFR 1910.119(e)(5)).
Employers must keep on file and make available to OSHA, on request, process hazard analyses and updates or revalidation for each process covered by PSM, as well as the documented resolution of recommendations, for the life of the process (29 CFR 1910.119(e)(7)).
- Maintenance program. Facilities covered by 29 CFR 1910.106 are required to establish maintenance and operating practices in accordance with established procedures which will tend to control leakage and prevent the accidental escape of flammable liquids (29 CFR 1910.106(h)(8)(i)). Additionally, all plant fire protection facilities must be adequately maintained and periodically inspected and tested to ensure that they are always in satisfactory operating condition and will serve their purpose in an emergency (29 CFR 1910.106(h)(6)(iv)). When necessary to do maintenance work in a flammable liquid processing area, the work must be authorized by a responsible representative of the employer (29 CFR 1910.106(h)(7)(ii)(a)). OSHA requirements for maintenance and repairs involving hot work and electrical wiring and equipment are addressed in 29 CFR 1910.106(h)(7)(ii)(b) & (h)(7)(iii).
Maintenance procedures must be in place and adequate before introducing highly hazardous chemicals to a process in a PSM covered facility (29 CFR 1910.119(i)(2)(ii)).
PSM covered facilities must perform inspection, testing, and repairs to ensure the safe functioning of process equipment (29 CFR 1910.119(j)(4).
PSM covered facilities must ensure that inspection and testing is performed on process equipment, using procedures that follow recognized and generally accepted good engineering practices (29 CFR 1910.119(j)(4)(ii)). The frequency of inspections and tests of process equipment must conform with manufacturers' recommendations and good engineering practices, or more frequently if determined to be necessary by prior operating experience (29 CFR 1910.119(j)(4)(iii)). Each inspection and test on process equipment must be documented, identifying the date of the inspection or test, the name of the person who performed the inspection or test, the serial number or other identifier of the equipment on which the inspection or test was performed, a description of the inspection or test performed, and the results of the inspection or test (29 CFR 1910.119(j)(4)(iv)).
Equipment deficiencies outside the acceptable limits defined by the process safety information must be corrected before further use. In some cases, it may not be necessary that deficiencies be corrected before further use, as long as deficiencies are corrected in a safe and timely manner, when other necessary steps are taken to ensure safe operation (29 CFR 1910.119(j)(4)(v)). The employer also must ensure that maintenance materials, spare parts, and equipment are suitable for the process application for which they will be used (29 CFR 1910.119(j)(6)(iii)).
- Incident investigations. The employer should investigate incidents that happen in the workplace, including near misses, to identify and correct hazardous situations, to preclude a catastrophic or fatal event. Facilities subject to the PSM standard must initiate an incident investigation no later than 48 hours after occurrence, for each incident which resulted in, or could have resulted in a catastrophic release of highly hazardous chemical in the workplace. The employer must establish a system for implementing corrective actions (29 CFR 1910.119(m)).
- Contractor Safety. The requirements of the PSM standard in covered facilities also apply to contractors performing maintenance or repair, turnaround, major renovation, or specialty work on or adjacent to a covered process. (29 CFR 1910.119(f)(4) & (h). It does not apply, however, to contractors providing incidental services that do not influence process safety, such as janitorial, food and drink, laundry, delivery, or other supply services.
Employer responsibilities. When selecting a contractor, the employer must:
- Obtain and evaluate information regarding the contract employer's safety performance and programs.
- Inform contract employers of the known potential fire, explosion, or toxic release hazards related to the contractor's work and the process.
- Explain to contract employers the applicable provisions of the emergency action plan.
- Develop and implement safe work practices to control the presence, entrance, and exit of contract employers and contract employees in covered process areas.
- Evaluate periodically the performance of contract employers in fulfilling their obligations; and,
- Maintain a contract employee injury and illness log related to the contractor's work in the process areas (29 CFR 1910.119(h)(2)).
Contract employer responsibilities. The contract employer must:
- Ensure that contract employees are trained in the work practices necessary to perform their job safely;
- Ensure that contract employees are instructed in the known potential fire, explosion, or toxic release hazards related to their job and the process, and in the applicable provisions of the emergency action plan;
- Document that each contract employee has received and understood the training required by the standard by preparing a record that contains the identity of the contract employee, the date of training, and the means used to verify that the employee understood the training;
- Ensure that each contract employee follows the safety rules of the facility including the required safe work practices required in the operating procedures section of the standard; and
- Advise the employer of any unique hazards presented by the contract employer's work (29 CFR 1910.119(h)(3)).
C. Engineering Controls to Prevent Equipment Ruptures
- Fire Detection and Suppression Systems. Fires can be controlled or prevented by installing automatic fire detection and suppression systems at critical points within production areas such as tank storage areas and ethanol load-out areas.
- Electrical Equipment and wiring. Use of electrical equipment and wiring that is rated for the specific hazardous (classified) location (e.g., Hazard Class I).
- Pressure Relief. Equipment with design features such as relief valves or rupture discs preclude/limit the occurrence of ruptures by relieving excess pressure. ASME B31.3, Process Piping, provides the presumptive pipe and corrosion resistance specifications that must be met for relief system piping (29 CFR 1910.106(c)(1)(i)).
Hazards that lead to equipment ruptures are discussed in III.B.
D. Safe Work Practices to Prevent Equipment Ruptures
- Monitoring. Routinely inspecting tanks, piping and other equipment at regular intervals to identify cracks, leakages, corrosions, and worn parts that could potentially result in ruptures. Inspecting equipment systems to ensure that they are functioning properly, for example, monitoring equipment pressure and temperature, and establishing procedures for taking appropriate actions if the pressure/temperature is not within the normal limits of operation for the equipment.
- Maintenance Program. An adequate maintenance program will establish the procedures for routine maintenance and provide the resources for sustaining equipment (repairs/replacement) in optimal working condition at all times (IV.A, mechanical integrity; and IV.B, maintenance program).
E. Engineering Controls for Combustible Dust Hazards
Several different engineering controls are available to prevent hazards and exposure to combustible dusts (III.C.). Examples of some, but not all, of the engineering controls that ethanol manufacturing facilities could use are listed below. A more complete listing of available and recommended engineering controls can be found in industry guidance documents, NFPA consensus standards, and OSHA compliance guidelines (e.g., OSHA, 2005; OSHA, 2011a; FM Global, 2009a, 2009b, 2010a, 2010b; Combustible Dust: Safety and Injury Prevention Instructors Manual (2008); NFPA 61, NFPA 69). Again, facility-specific factors ultimately determine the most appropriate combination of controls.
- Ignition controls. Typical process areas involving the presence of combustible dusts are classified as Class II hazardous locations according to the NEC (29 CFR 1910.307(c)(2)(i)). A Class II hazardous location is an area where the dusts suspended in air are capable of causing an explosion. The use of electrical equipment and wiring classified for Class II locations is essential to preventing electrical devices from becoming sources of ignition. Another common ignition source is overheated bearings on belt conveyors and bucket elevators. This source can be effectively controlled by installing temperature sensors on bearings and other related controls, like belt alignment sensors. These monitors can have low- and high-alarm settings interlocked with audible alarms, automated shutdowns, and other actions based on the detection of increasingly unsafe conditions. Additionally, effective grounding and bonding should be considered on process equipment prone to electrostatic discharge during routine operation.
- Dust controls. An effective strategy for preventing combustible dust incidents is to prevent dust from being released, preclude the accumulation of dust that can be ignited, or that can provide additional fuel for a secondary explosion. This can be accomplished by designing and maintaining equipment (e.g., chutes, transfer points, bucket elevators, conveyor belts) to be as dust-tight as possible to minimize dust generation. Highly efficient dust collection and aspiration systems are critical to effectively controlling dust. If unexpected leaks or emissions occur, fast response and remediation will minimize the quantity of dust released.
- Equipment design. Engineering design that incorporates safety measures is vital to maintaining a safe workplace. For example, under the grain handling standard, direct-heat grain dryers installed after March 30, 1988 must be located outside the grain elevator; located in an area inside the grain elevator protected by a fire or explosion suppression system; or, located in an area inside the grain elevator which is separated from other areas of the facility by construction having at least a one hour fire-resistance rating (29 CFR 1910.272(p)(2)). Safeguards, monitoring systems and controls that are required (at a minimum) for bucket elevators are discussed in 29 CFR 1910.272(q).
- Other controls. Numerous other engineering controls have been designed specifically to prevent combustible dust explosions or to mitigate their consequences, and NFPA has developed consensus standards that specifically address controls most suitable for agricultural dusts (NFPA 61).
- Explosion suppression systems. Automatic explosion suppression systems (NFPA 69) can be used to detect pressure rises indicative of explosions and promptly suppress these events, for example, by injecting large volumes of a suppression agent (suppressant) into the operation being controlled. These systems are most commonly applied to dust collectors, milling rooms, and other areas believed to present the greatest risk for explosions.
- Fire detection and suppression systems. Fire detection and suppression systems are common in dryers, dust collectors, and other operations prone to fire. In the case of dryers, sensors placed inside dryers and their outlets are used to detect elevated and rapidly increasing temperatures, with the measured levels triggered to various actions of increasing severity, such as audible alarms, steam suppression, and full water deluge.
Similarly, other automatic systems can be used to detect sparks and smoldering materials in certain dust-laden air streams, with detections triggering fire suppression at downstream locations. These devices are particularly useful in ductwork on the outlet of unit operations (e.g., dryers, mills) suspected of causing sparks or smoldering material.
- Explosion relief venting. Yet another commonly encountered feature is explosion-relief venting, which is designed to control deflagration pressure (NFPA 68) by venting combustion products from an enclosure (e.g., dust collector, bucket elevator) to a safe location, rather than having a confined deflagration result in a devastating explosion. Should pressure inside the equipment rise rapidly and unexpectedly, explosion relief panels are designed to open to safely relieve the developing pressure and any material discharged from a deflagration outside to a safe location. This minimizes structural and mechanical damage, and protects workers from severe injuries and/or fatalities that could have occurred in an explosion.
F. Safe Work Practices for Combustible Dust Environments
Various safe work practices are commonly used to help prevent dust-related fires and explosions in general industry, and many of these would also apply to ethanol manufacturing facilities. Examples of such administrative controls are listed below (OSHA, 2011a; Combustible Dust: Safety and Injury Prevention Instructors Manual (2008)).
- Housekeeping. One of the most important administrative controls for combustible dust is an effective housekeeping program. These programs must establish the frequency and methods to best reduce dust accumulations on ledges, floors, equipment, and other exposed surfaces (29 CFR 1910.272(j)(1)). For priority housekeeping areas, employers must immediately remove any grain dust accumulation that exceeds 1/8th of an inch or demonstrate that equivalent protection is provided through the housekeeping program (29 CFR 1910.272(j)(2)(ii)). Priority housekeeping include areas near grain handling equipment; for example, floor areas within 35 feet of inside bucket elevators, floors of enclosed areas containing grinding equipment, and enclosed areas with grain dryers (29 CFR 1910.272(j)(2)(i)).
The methods used for housekeeping should be based on the assessed hazard potential. For instance, employers might determine that only non-sparking tools (e.g., soft bristle brooms, bronze hand tools) and intrinsically safe hazard class II rated vacuums are acceptable in certain facility areas. The use of compressed air to blow dust from surfaces is only permitted when all machinery that presents an ignition source in the area is shut down, or all other known potential ignition sources in the area are removed or controlled (29 CFR 1910.272(j)(3)). Water or steam washes might also be preferred in some instances. Frequent housekeeping audits should be conducted to assess the effectiveness of cleaning procedures, measure dust accumulation rates, and identify hidden accumulations of combustible dusts (e.g., above drop ceilings).
- Safety programs. Another essential element of preventing combustible dust hazards is by developing and implementing safety programs required by other OSHA standards, such as hot work programs, confined space entry permit programs, and lockout-tagout programs (Section IV). These should all specifically acknowledge combustible dust hazards associated with performing hot work or conducting other maintenance activity in enclosed spaces.
- Training. Many dust explosions result from workers simply not being aware of a hazardous situation. This is a root cause that can readily be prevented through effective training programs. Thorough initial and refresher training on combustible dust hazards is essential for all workers whose jobs involve working with or near these materials. For example, workers who conduct routine housekeeping activities need to learn what types of cleaning equipment should be used and avoided in different production areas(e.g., use of non-sparking tools, dust ignition-proof vacuums). The employer must provide training to workers at least annually and when changes in job assignment will expose them to new hazards. This training must include general safety precautions for the facility, including recognizing and preventing the hazards associated with dust accumulations and common ignition sources. In addition, it must include specific procedures associated with the employee's job including cleaning procedures for grinding equipment, clearing procedures for choked legs, housekeeping procedures, hot work procedures, preventative maintenance procedures, and lockout/tagout procedures.
- Facility dust hazard assessment. Past incidents have revealed that company representatives, managers, and workers are often not aware of existing dust explosion hazards and effective mitigation measures. In these cases, a systematic evaluation of potential dust explosion hazards associated with all processes, equipment, and work areas could have helped identify dangerous situations before major incidents occurred. A periodic review is necessary to identify new hazards and/or safety measures that may not be functioning effectively, and to implement effective measures.
- Management commitment. Previous incident investigations at selected facilities have identified a need for stronger commitment from management to prevent combustible dust hazards. For example, some facilities experienced fires and explosions where similar events had previously occurred but had not been fully investigated.
- Permit System. The employer shall institute an effective permit system that prevents workers from performing operations without complying with the required safety precautions. For instance, when required by 29 CFR 1910.272, a hot work permit must be issued (29 CFR 1910.272(f)), certifying that the fire prevention and control measures specified in 29 CFR 1910.252(a) have been met before starting work.
Similarly, when required by 29 CFR 1910.272, a permit must be issued for workers entering bins, silos or tanks certifying that the precautions contained in 29 CFR 1910.272(g) (e.g., isolation of hazardous energy, atmospheric testing, and the use of the right type of respirator when needed) have been implemented.
- Maintenance Program. Employers can further prevent hazards through routine inspection, testing, and maintenance of process equipment to keep it in optimal working condition. The grain handling standard specifies minimum requirements for preventative maintenance in facilities it covers, which includes conducting regularly scheduled inspections, lubrication, correcting malfunctioning dust collection systems, and maintaining records of each inspection (29 CFR 1910.272(m)).
- Incident Investigation. Investigation of all incidents that happen in the workplace, including near misses, to correct hazardous situations in order to prevent a catastrophic or fatal event from occurring.
G. Engineering Controls for Engulfment Hazards
Engulfment hazards (III.D.) at ethanol manufacturing facilities can be avoided through various approaches.
- Equipment design. Silos, bins, and other storage vessels can be equipped with design features that reduce the need for workers to enter the equipment in the first place. These features,which are particularly important for grain elevator facilities that handle corn that is more prone to spoilage, are thoroughly documented in the literature (e.g., NIOSH, 1987; Purdue University, 2011).
H. Safe Work Practices for Engulfment Hazards
Administrative controls are particularly effective for preventing engulfment hazards at ethanol manufacturing facilities (OSHA, 2011a; Purdue University, 2011).
- Operating procedures. Above all else, operating procedures that specifically prohibit employees from entering stored grain–whether in silos, truck beds, or other locations–can effectively eliminate the hazard. Operating procedures must prohibit workers from "walking down the grain" and performing similar activities expected to make grain flow (29 CFR 1910.272(g)(1)(iv)). Workers are forbidden from entering onto or below a grain bridging condition or anywhere that a grain accumulation could fall and engulf a worker, for example, grain built up along the side of the bin (29 CFR 1910.272(g)(6)). Measures should also be implemented to ensure that workers do not need to work around the perimeter of tall storage piles of solid co-products.
- Training. Every worker must be provided with the required safety training before they start work, at least annually and whenever changes in job assignment (29 CFR 1910.272(e)) or processes (e.g., equipment, chemicals) will expose them to new hazards. Employees working with grain need to be properly trained in handling, storing, and processing these materials. Training must at least cover general safety precautions and specific procedures and safety practices applicable to their tasks, including any special assignments, such as, bin entry. The majority of entrapment and engulfment incidents result when workers enter bins in unsafe conditions; therefore, workers must be trained on the hazards they face when performing tasks inside silos, bins and other grain storage units. The training must address engulfment, mechanical hazards and means of avoiding these hazards. Workers should not be allowed to perform tasks until they can demonstrate a complete knowledge of all equipment involved in their work activities.
- Permits. In grain handling facilities where workers must enter bins, silos, or tanks, safe work entry permits must be issued before entry (unless the employer or employer's representative responsible for authorizing the permit would be present for the entire duration of the work). The permit must certify that the standards required precautionary measures are implemented before workers are allowed to enter the silo, bin, or tank (29 CFR 1910.272(g)(1)(i)).
I. Personal Protective Equipment for Engulfment Hazards
- Body harness and lifeline. Workers who must enter grain storage structures with engulfment hazards must be equipped with body harnesses and lifelines, or a boatswain's chair (29 CFR 1910.272(g)(2)), with a designated observer present who is in communication with the worker at all times and can assist with rescue procedures in the event of an unexpected engulfment (29 CFR 1910.272(g)(3)). The lifeline must be long enough so that a worker does not sink further than waist-deep in grain. If the required PPE poses a greater hazard or is not feasible, employers must use alternate means that prevent workers from sinking beyond waist deep in grain (29 CFR 1910.272(g)(2)).
J. Engineering Controls for Hazardous Noise Levels
Safety measures to minimize workers exposure to hazardous noise levels in grain processing areas include (excerpt from Cralley, 1985):
- Grain unloading areas. Locating pneumatic blowers separately from work areas or enclosed in acoustic booths.
- Grain elevators. Using quieter conveyor equipment, shutting off conveyor systems when not in use, and minimizing the use of compressed air (29 CFR 1910.272(j)(3)). Using low-speed, high-volume conveyors can also greatly reduce noise and dust. If such methods cannot be used effectively, it may be necessary for operators to monitor grain flow from inside acoustical booths.
- Grain processing. Reducing noise levels at their source through engineering controls may be extremely difficult because of the large size, sanitation requirements, and number of machines in a typical facility. However, in some workers can be separated from noise exposures by isolation techniques. Pneumatic blowers, air compressors, and other extremely loud noise sources should be located in separate rooms away from major work areas. Control panels should be consolidated in a centrally located acoustically treated control booth, which functions both as a work station and relief area from noise exposure.
Additional information on noise exposure controls is discussed in the OTM, Section III, Chapter 5, Noise.
K. Hearing Conservation Program
OSHA requires employers in the general industry to administer a continuing, effective hearing conservation program, as described in paragraphs (c) through (o) of 29 CFR 1910.95, whenever employee noise exposures equal or exceed an 8-hour time-weighted average (TWA) sound level of 85 decibels measured on the A scale (dBA) or, equivalently, a dose of fifty percent (29 CFR 1910.95(c)).
A continuing, effective hearing conservation program is also required in the construction industry when sound levels exceed the values shown in Table D-2 of 29 CFR 1926.52. This table includes a permissible exposure of 90 dBA for an 8-hour TWA.
Additional information on noise exposure controls is discussed in the OTM, Section III, Chapter 5, Noise.
L. Hearing Protection Devices
OSHA requires employers to provide workers with hearing protection devices whenever noise exposure levels equals or exceeds OSHA's specifications, unless the costs of engineering and/or administrative controls are less than the cost of an effective hearing conservation program (29 CFR 1910.95; 29 CFR 1926.52; CPL 02-00-148, OSHA's Field Operations Manual, p. 4-41 [Updated to be CPL 02-00-160, August 2, 2016].
Additional information on noise exposure controls is discussed in the OTM, Section III, Chapter 5, Noise.
M. Engineering Controls for Hazardous Substances
In general, the standards in subparts Z of 29 CFR 1910 and 29 CFR 1926 require employers to use engineering and administrative controls, to the extent feasible, to control worker exposure above the specified PEL. Engineering controls include:
- Ventilation. Industrial ventilation generally involves the use of supply and exhaust ventilation to control emissions, exposures, and chemical hazards in the workplace (OSHA Technical Manual [OTM], Section III, Chapter 3, Ventilation Investigation, OSHA 1999).
- Enclosures. Emission sources may be enclosed to protect workers from exposure, for example, processing toxic chemicals in closed piping systems or hoods.
- Substitution. Hazardous chemicals or processes should be replaced with less hazardous chemicals or processes when feasible.
- Elimination. Hazardous chemicals and/or processes should be removed from the operation when practicable.
N. Safe Work Practices to Protect Workers from Hazardous Substances
- Hazard Communication Standard (HCS). The requirements of the HCS (29 CFR 1910.1200) must be implemented (OSHA's Hazard Communication Page) to protect workers from exposure to hazardous chemicals (III.F.). These include:
- Hazard Communication Program. A site specific Hazard Communication Program must be implemented for each applicable workplace, this includes employee training (29 CFR 1910.1200(e)).
- Material Safety Data Sheets (MSDSs) or Safety Data Sheets4 (SDSs). MSDSs/SDSs for hazardous substances must contain information required by the HCS, for example, it must document physical and health hazards associated with exposure and the appropriate controls that must be utilized in the work place to protect workers. The MSDS/SDS must be easily accessible to workers and workers must receive the necessary training, including how to use the hazard information (29 CFR 1910.1200(g)).
- Container labeling. The HCS requires hazardous chemicals to display the appropriate labeling information and for workers to receive training that explains the label information (29 CFR 1910.1200(f)).
- Employee training. Employers must train workers on methods that may be used to detect the presence of hazardous chemicals in the work area; the hazards of the chemicals in the work area; the safety measures that have been implemented to protect them from hazardous chemicals in the workplace, including appropriate work practices, emergency procedures, and personal protective equipment to be used; and the details of the employers hazard communication program (29 CFR 1910.1200(h)(3)).
- PSM covered facilities. The PSM standard requires employers to have process safety information for highly hazardous chemicals (29 CFR 1910.119(d)). A highly hazardous chemical means a substance possessing toxic, reactive, flammable (e.g., ethanol, gasoline), or explosive properties and specified by 29 CFR 1910.119(a)(1). The PSM standard covers toxic and reactive highly hazardous chemicals which are listed with their threshold quantities in Appendix A of 29 CFR 1910.119 (e.g., sulfur dioxide (1,000 pounds); anhydrous ammonia (10,000 pounds); and anhydrous hydrochloric acid (5,000 pounds)). The process safety information must include information on the hazards of the highly hazardous chemicals used or produced by the process, information on the technology of the process, and information on the equipment in the process.
Information on the hazards of the highly hazardous chemicals in the process must consist of at least the following:
- Toxicity,
- Permissible exposure limits,
- Physical data,
- Reactivity data,
- Corrosivity data, and
- Thermal and chemical stability data, and hazardous effects of inadvertent mixing of different materials (29 CFR 1910.119(d)(1)(i)).
Information on the technology of the process must include at least the following:
- A block flow diagram or simplified process flow diagram,
- Process chemistry,
- Maximum intended inventory,
- Safe upper and lower limits for such items as temperatures, pressures, flows or compositions, and
- An evaluation of the consequences of deviations, including those affecting the safety and health of employees (29 CFR 1910.119(d)(2)(i)).
Where the original technical information no longer exists, such information may be developed in conjunction with the process hazard analysis in sufficient detail to support the analysis.
Information on the equipment in the process must include the following:
- Materials of construction,
- Piping and instrument diagrams (P&IDs),
- Electrical classification,
- Relief system design and design basis,
- Ventilation system design,
- Design codes and standards employed,
- Material and energy balances for processes built after May 26, 1992, and
- Safety systems (e.g., interlocks, detection, or suppression systems) (29 CFR 1910.119(d)(3)(i).
The employer shall document that equipment complies with recognized and generally accepted good engineering practices (29 CFR 1910.119(d)(3)(ii)). For existing equipment designed and constructed in accordance with codes, standards, or practices that are no longer in general use, the employer shall determine and document that the equipment is designed, maintained, inspected, tested, and operated in a safe manner (29 CFR 1910.119(d)(3)(iii)).
The compilation of the above described process safety information provides the basis for identifying and understanding the hazards of a process and is necessary in developing the process hazard analysis and may be necessary for complying with other provisions of PSM such as management of change and incident investigations.
O. Personal Protective Equipment (PPE) to Protect Workers from Hazardous Substances
Whenever workers' exposure to hazardous chemicals cannot be eliminated or reduced to acceptable/safe levels, employers must select and provide personal protective equipment of the appropriate type to affected workers, including training and implementation of any required safety program, such as the respiratory protection program. For example, when there are pesticides or fumigants used in the workplace that can penetrate the skin, only trained workers using clean, well-maintained PPE (e.g., protective clothing, gloves) resistant to the chemical's penetration can be allowed to handle the pesticides or fumigants.
P. Engineering Controls for Confined Spaces
The safety and health of workers entering confined spaces in grain handling facilities is addressed by both the Grain Handling Facilities standard, 29 CFR 1910.272 and the Permit Required Confined Spaces standard, 29 CFR 1910.146. However, confined space work, such as grain bin entry, that is regulated by 29 CFR 1910.272, is not subject to the provisions of 29 CFR 1910.146, as long as the provisions of 29 CFR 1910.272 protect workers against all the hazards within the grain bins (OSHA Letter of Interpretation: Questions regarding the PRCS standard, 1910.146, and the Grain Handling standard, 1910.272. [02/08/2005]).
Refer to Appendix C for safety and health information on above ground storage tanks.
- Atmospheric Testing. Atmospheric testing (pre-entry testing) is conducted to assess the oxygen content, potential explosive atmospheres, and to detect any hazardous gases before entering into confined spaces such as grain storage units. Continuous atmospheric testing and monitoring is done while workers are in the confined space. Supplemental ventilation or respirator use may also be required, depending on the gas concentrations measured.
- Ventilation. Continuous ventilation combined with initial purging and local exhaust ventilation can be implemented to ensure that the atmospheric conditions are safe for workers. If unable to ensure safe air quality, then workers should don appropriate respirators.
- Barriers. Barriers are used to prevent external hazards from harming confined space entrants (OSHA, 2011a).
Q. Safe Work Practices for Confined Space Hazards
- Warning signs. Warning signs at confined space entry points are used to discourage unauthorized entry.
- Permits. Confined space programs specify the safety procedures that must be implemented before a confined space entry permit can be obtained.
- Housekeeping. For example, housekeeping programs for grain elevators shall address fugitive grain dust accumulations at priority housekeeping areas. These include floor areas within 35 feet (10.7 m) of inside bucket elevators (29 CFR 1910.272(j)(2)).
- Maintenance Program. Regularly scheduled inspections of at least the mechanical and safety control equipment associated with dryers, grain stream processing equipment, dust collection equipment including filter collectors, and bucket elevators (29 CFR 1910.272(m)(1)(i)). The employer must promptly correct dust collection systems which are malfunctioning or which are operating below designed efficiency. Additionally, the employer must promptly correct, or remove from service, overheated bearings and slipping or misaligned belts associated with inside bucket elevators (29 CFR 1910.272(m)(2)).
R. Personal Protective Equipment for Confined Space Hazards
If needed, the appropriate types of personal protective equipment (PPE) for the identified hazards must be provided to confined space entrants. PPE may include a body harness and lifeline.
S. Engineering Controls for Motor Vehicle Hazards (e.g., loading, unloading, operating)
- Grounding and bonding. Grounding and bonding precautions prevent static electricity discharges that could serve as a source of ignition, e.g., when flammable liquids or combustible dusts are present during loading/unloading operations, from motor vehicles in product load-out areas.
- Hazardous Classified Location. Use of equipment and wiring rated for the hazardous classified location, including intrinsically safe tools to eliminate sources of ignition.
- Lightning Early Warning Systems. Lightning early warning systems are used to provide advance warning of lightning approaching an area. This will alert workers to suspend loading and unloading of flammable and/or combustible dust materials in advance of an impending lightning storm.
- Road Maintenance. It is crucial to maintain roads in good condition around the workplace to facilitate the safe flow of traffic.
T. Safe Work Practices for Motor Vehicle Operation
- Preventive Maintenance Program. Inspecting and maintaining vehicles in optimal working condition is essential to ensure that they function properly.
- Pedestrian safety. Planning travel routes to avoid intersections between truck routes and frequently-used pedestrian walkways wherever possible and clearly marking truck access routes and crosswalks. Alarm systems, signs, and barricades can be used to warn pedestrians of trucks pulling out or into parking areas, loading or unloading zones, etc.
- Speed limits. Setting and enforcing safe speed limits to manage safe movement of traffic for truck deliveries.
- Protecting equipment, pipes, etc. It is critical to carefully plan transportation routes away from equipment and pipes to avoid collisions that could cause serious injuries or death to workers, equipment ruptures, facility damage, etc.
- Awareness campaigns. Launching awareness campaigns to target unsafe driving practices, such as driving while fatigued or under the influence of alcohol and drugs.
- Loading and unloading. Establishing procedures for the safe loading and unloading of motor vehicle contents. For example, not allowing vehicles to idle during loading or unloading operations, prohibiting the loading or unloading of motor vehicles to occur during an electrical storm, and establishing lightning approach distances to stop loading and unloading operations during such storms. Pedestrians must be prohibited in loading and unloading areas.
- Repairs. No repairs or maintenance activities should be done on trucks within the loading or unloading areas; disabled vehicles should be towed.
- Road conditions. Transporting goods in inclement weather should be avoided.
- Ignition Sources. Sources of ignition (e.g., smoking, lightning, cutting, welding, hot surfaces) in areas where flammable vapors may be present must be eliminated or controlled (29 CFR 1910.106(b)(6)). For example, smoking and open flames inside or near vehicles should be prohibited. Designated smoking areas must be at safe distances from motor vehicles and flammable liquid processing or storage areas.
- Training. A driver training program should be established to promote safe driving.
- Scheduling. Driver schedules should be set to allow for breaks, rest periods and ensure compliance with the Department of Transportation's Hours of Service Regulation.
U. Lockout/Tagout of Hazardous Energy
Energy sources found throughout ethanol processing facilities include electrical, mechanical, hydraulic, pneumatic, chemical, thermal or other sources in machines and equipment which can be hazardous to workers. During the servicing and maintenance of machines and equipment, the unexpected startup or release of stored energy could cause injury to employees. Workers servicing or maintaining machines or equipment may be seriously injured or killed if hazardous energy is not properly controlled. Injuries resulting from the failure to control hazardous energy during maintenance activities can be serious or fatal. Injuries may include electrocution, burns, crushing, cutting, lacerations, amputations, fractures and others.
Failure to control hazardous energy accounts for nearly 10 percent of the serious accidents in many industries. Proper Lockout/Tagout (LOTO) practices and procedures protect workers from the release of hazardous energy. OSHA's Lockout/Tagout fact sheet describes the practices and procedures necessary to disable machinery or equipment to prevent the release of hazardous energy.
The OSHA standard for the Control of Hazardous Energy (Lockout/Tagout), 29 CFR 1910.147, specifies that when other general industry standards (29 CFR 1910) require the use of lockout or tagout, they shall be used and supplemented by the procedural and training requirements of 29 CFR 1910.147 (29 CFR 1910.147(a)(3)(ii)). The LOTO standard establishes the employer's responsibility to protect workers from hazardous energy and to train each worker to know, understand, and be able to follow the applicable provisions of the hazardous energy control procedures in their workplace (Control of Hazardous Energy (Lockout/Tagout), Safety and Health Topics Page, OSHA, 2011e.
The requirement to utilize LOTO procedures in grain storage structures are specified in 29 CFR 1910.272(g) & (h). It states that all mechanical, electrical, hydraulic, and pneumatic equipment that presents a danger, particularly grain-moving equipment, must be de-energized (turned off) and disconnected, locked out and tagged, or blocked off, or otherwise prevented from operating by other equally effective means, before permitting workers to enter (29 CFR 1910.272(g)(1)(ii) & (h)(2)(i); Worker Entry into Grain Storage Bins fact sheet, OSHA 2010).
V. Emergency Planning
A. PSM Emergency Preparedness
Each employer must address actions that workers will take when there is an unwanted release of highly hazardous chemicals. Employers will need to decide: if they want workers to handle and stop small or minor incidental releases; whether they wish to mobilize the available resources at the plant and have them brought to bear on a more significant release; or whether they want their workers to evacuate the danger area and promptly escape to a preplanned safe zone area and allow the local community emergency response organizations to handle the release; or whether the employer wants to use some combination of these actions. Employers will need to select how many different emergency preparedness or lines of defense they plan to have, develop the necessary plans and procedures, properly train workers in their emergency duties and responsibilities, and effectively implement the selected plans and procedures (29 CFR 1910.119 Appendix C).
One effective way for medium to large facilities to enhance coordination and communication during emergencies for on plant operations and with local community organizations is for employers to establish and equip an emergency control center. The emergency control center would be sited in a safe zone area so that it could be occupied throughout the duration of an emergency. The center would serve as the major communication link between the on-scene incident commander and plant or corporate management as well as with local community officials. The communication equipment in the emergency control center should include a network to receive and transmit information by telephone, radio or other means. It is important to have a backup communication network in case of a power failure or if a means of communication fails. The center should also be equipped with the plant layout and community maps, utility drawings (including fire and water), emergency lighting, appropriate reference materials such as a government agency notification list, company personnel phone list, Superfund Amendments and Reauthorization Act (SARA) Title III reports and safety data sheets, emergency plans and procedures manual, a list of emergency response equipment and their location(s), mutual aid information, access to meteorological or weather condition data and any dispersion modeling data (29 CFR 1910.119 Appendix C).
B. Emergency Action Plans and Incident Prevention Planning
- To prepare for any contingencies, an emergency action plan (EAP) establishes procedures to prevent fatalities, injuries, and property damage (IAFC, 2008). An EAP (29 CFR 1910.38) is a workplace requirement when another applicable OSHA standard requires it, for example, 29 CFR 1910.119 (PSM standard) and 29 CFR 1910.272 (Grain Handling Facilities standard).
- The host employer for a facility that must implement the requirements of the PSM standard is responsible for training its workers, this includes additional training to workers who provide emergency response actions covered by OSHA's Hazardous Waste Operations and Emergency Response standard, 29 CFR 1910.120(q). The host employer must also review the facility's emergency shutdown and response procedures with workers and clearly communicate EAPs to contractors. Contract employers are responsible for ensuring that their workers are instructed in potential fire, explosion, or toxic release hazards related to their jobs.
- Employers at a minimum must have an EAP which will facilitate the prompt evacuation of workers when an unwanted release of highly hazardous chemical occurs. This means that the employer will have a plan that will be activated by an alarm system to alert workers when to evacuate and, that workers who are physically impaired will have the necessary support and assistance to get them to the safe zone as well. The intent of these requirements is to alert and move workers to a safe zone quickly. Delayed or confusing alarms are to be avoided. The use of process control centers or similar process buildings in the process area as safe areas is discouraged because these structures may not be properly sited and may not be designed to withstand over-pressures from shockwaves resulting from explosions in the process area (29 CFR 1910.119 Appendix C).
- If the Process Safety Management of Highly Hazardous Chemicals standard (29 CFR 1910.119(n)) applies to the facility:
- The employer must establish and implement an EAP for the entire plant in accordance with the provisions of 29 CFR 1910.38. In addition, the EAP must include procedures for handling small releases of hazardous chemicals.
- At a minimum, employers must implement the requirements of 29 CFR 1910.119 for preventing or minimizing the consequences of catastrophic releases of toxic, reactive, flammable, or explosive chemicals. These releases may result in toxic, fire or explosion hazards (Section III).
- Employers must address the actions that workers will take when there is an incidental release of highly hazardous chemicals in the process area. If the employer wants workers to evacuate the area, then the EAP will be activated. For outdoor processes where wind direction is important for selecting the safe route to a refuge area, the employer should place a wind direction indicator (such as a wind sock or pennant) at the highest point that can be seen throughout the process area. Workers can move in the direction of cross wind to upwind to gain safe access to the refuge area by knowing the wind direction (29 CFR 1910.119 Appendix C).
- If the employer wants specific workers in the release area to control or stop minor emergencies or incidental releases, these actions must be planned for in advance and procedures developed and implemented. Appropriate equipment for the hazards must be provided and training conducted for workers that will perform the emergency work before they respond to handle an actual release. The employer's training program will address the training needs of workers that are expected to handle incidental or minor releases (29 CFR 1910.119 Appendix C), and must include the training requirements of the Hazard Communication Standard (HCS, 29 CFR 1910.1200).
- Employers covered by the 29 CFR 1910.119 standard may also be subject to the hazardous waste and emergency response provisions contained in 29 CFR 1910.120(a), (p) and (q) (the Hazardous Waste Operations and Emergency Response (HAZWOPER) standard; CPL 02-02-073)). Preplanning for releases that are more serious than incidental releases is another important line of defense to be used by the employer. When a serious release of a highly hazardous chemical occurs, the employer - through preplanning - would have determined in advance the actions that workers will take. The evacuation of the immediate release area and other areas (as necessary) would be accomplished under the EAP. If the employer wishes to use plant personnel such as a fire brigade, spill control team, a hazardous materials team, or to have workers render aid to those in the immediate release area and control or mitigate the incident, these actions are covered by 29 CFR 1910.120. If outside assistance is necessary, such as through mutual aid agreements between employers or local government emergency response organizations, these emergency responders are also covered by 29 CFR 1910.120. The safety and health protections required for emergency responders are the responsibility of their employers and of the on-scene incident commander. Responders may be working under very hazardous conditions and therefore the objective is to have them competently led by an on-scene incident commander and the commander's staff, properly equipped to do their assigned work safely, and fully trained to carry out their duties safely before they respond to an emergency. Drills, training exercises, or simulations with the local community emergency response planners and responder organizations are ways of attaining better preparedness. This close cooperation and coordination between plant and local community emergency preparedness managers will also aid the employer in complying with the Environmental Protection Agency's Risk Management Plan criteria (29 CFR 1910.119 Appendix C).
- The fire prevention and protection requirements in 29 CFR 1910.252(a) must be implemented and documented in the hot work permit, prior to beginning any hot work operations (29 CFR 1910.119(k)(2)).
- If the Grain Handling Facilities standard (29 CFR 1910.272(d); 29 CFR 1910.272 Appendix A) applies:
- At a minimum, employers must implement the requirements contained in 29 CFR 1910.272 for the control of grain dust fires and explosions (IV.E; IV.F).
- The employer must develop and implement an EAP in compliance with 29 CFR 1910.38. It is also recommended that employers seek the assistance of the local fire department for the purpose of preplanning for emergencies. Preplanning is encouraged to facilitate coordination and cooperation between facility personnel and those who may be called upon for assistance during an emergency. It is important for emergency service units to be aware of the usual work locations of employees at the facility (29 CFR 1910.272 Appendix A).
- The employer must train workers who serve as observers for entry into grain storage structures on rescue procedures, including how to request for additional assistance (29 CFR 1910.272(g)(5)). It is also important to train workers in the recognition and prevention of hazards associated with grain facilities, especially those hazards specific to their own tasks. Workers must understand the factors necessary to produce a fire or explosion (29 CFR 1910.272 Appendix A; III.C).
- The use of floor plans or workplace maps which clearly show the emergency escape routes should be included in the EAP; color coding will aid workers in determining their route assignments. The employer should designate a safe area outside the facility where workers can congregate after an emergency evacuation and implement procedures to account for all workers (29 CFR 1910.272 Appendix A).
- It is important that the type of employee alarm used to notify workers of an emergency is distinguishable and distinct from all other signals and alarms used in the workplace (29 CFR 1910.272 Appendix A).
- The employer must explain pertinent provisions of the EAP to contractors. The employer must inform contractors performing work at the grain handling facility of known potential fire and explosion hazards related to the contractor's work and work area (29 CFR 1910.272(i)). Also, in the event of an emergency, contractors should be able to take appropriate action as a part of the overall facility EAP. Contractors should also be aware of the employer's permit systems. Contractors should develop specified procedures for performing hot work and for entering bins, silos, and tanks, and these activities should be coordinated with the facility employer (29 CFR 1910.272 Appendix A).
- The employer must provide at least two means of emergency escape from galleries/bin decks (29 CFR 1910.272(o)(1)).
- The employer must provide at least one means of emergency escape in tunnels of existing grain elevators. Tunnels in grain elevators constructed after the effective date of the 29 CFR 1910.272 standard, must be provided with at least two means of emergency escape (29 CFR 1910.272(o)(2)).
- Grain handling facilities must implement a permit system for hot work as specified in 29 CFR 1910.252 (29 CFR 1910.272 Appendix A).
- The employer must equip the employee with a body harness and lifeline or boatswain's chair for entry into grain storage structures when the worker enters at or above the grain level, or whenever a worker walks or stands on, or in stored grain, of a depth which poses an engulfment hazard (29 CFR 1910.272(g)(2)).
- The employer must ensure that a properly-equipped observer maintains communication with a worker who enters a bin, silo, or tank (29 CFR 1910.272(g)(3)).
- The employer must provide rescue equipment designed for the bin, silo, or tank being entered (29 CFR 1910.272(g)(4)).
- Fire control measures in process plants that are covered by the flammable liquid standard must include (29 CFR 1910.106(h)(6)):
- Portable extinguishers. Approved portable fire extinguishers of the appropriate size, type, and number must be provided.
- Other controls. Where the special hazards of operation or exposure indicate a need, the following fire control provisions must be provided:
- A reliable water supply must be available within the pressure and quantity adequate to meet the probable firefighting demands.
- Hydrants must be provided in accordance with accepted good practice.
- Hose connected to a source of water must be installed so that all vessels, pumps, and other equipment containing flammable liquids can be reached with at least one hose stream. Nozzles that are capable of discharging a water spray must be provided.
- Processing plants must be protected by an approved automatic sprinkler system or equivalent extinguishing system. If special extinguishing systems (including but not limited to those employing foam, carbon dioxide, or dry chemical) are provided, approved equipment must be used and installed in an approved manner.
- Alarm systems. An approved means for prompt notification of a fire to those within the plant and any public fire department available must be provided. It may be advisable to connect the plant system with the public system where a public fire alarm system is available.
- Maintenance. All plant fire protection facilities shall be adequately maintained and periodically inspected and tested to make sure they are always in satisfactory operating condition and that they will serve their purpose in an emergency.
C. Fire Extinguishers
OSHA's requirements regarding portable fire extinguishers in workplaces are covered by 29 CFR 1910.157, Portable Fire Extinguishers.
- Where the employer has established and implemented a written fire safety policy which requires the immediate and total evacuation of workers from the workplace upon the sounding of a fire alarm/signal and which includes an EAP and a fire prevention plan that meet the requirements of 29 CFR 1910.38 and 29 CFR 1910.39 respectively, and when extinguishers are not available in the workplace, the employer is exempt from all the requirements of 29 CFR 1910.157 unless a specific standard in 29 CFR Part 1910 requires that a portable fire extinguisher be provided (29 CFR 1910.157(b)(1)).
- Where the employer has an EAP meeting the requirements of 29 CFR 1910.38, which designates certain employees to be the only workers authorized to use the available portable fire extinguishers, and which requires all other workers in the fire area to immediately evacuate the affected work area upon the sounding of the fire alarm, the employer is exempt from the distribution requirements in the selection and distribution section of 29 CFR 1910.157 (29 CFR 1910.157(b)(2)).
VI. Investigating/Inspecting Ethanol Processing Facilities
Ethanol processing facilities contain specialized processes that can produce catastrophic consequences such as fire, explosion, engulfment, or asphyxiation (III.A) if the proper preventive measures (Section IV) are not implemented. Consequently, Compliance Safety and Health Officers (CSHOs) must be properly trained and equipped to recognize hazards and appropriate safety measures at these facilities.
A. Pre-inspection or Investigation Planning
Proper planning and preparation is needed to conduct an inspection or investigation at an ethanol processing facility.
-
OSHA Directives Review
The following is a list of resources that can be consulted when conducting inspections/investigations (additional resources are listed in Appendix B):
- PSM Covered Chemical Facilities National Emphasis Program (CPL 03-00-021).
- Grain Handling (29 CFR 1910.272).
- Permit-Required Confined Spaces (29 CFR 1910.146).
- Application of the Permit-Required Confined Spaces Standard (CPL-02-00-100).
- The Control of Hazardous Energy (Lockout/Tagout) (29 CFR 1910.147; IV.U).
- Hazard Communication Standard (29 CFR 1910.1200).
- Respiratory Protection (29 CFR 1910.134).
- Although 29 CFR 1910.272 takes precedence inside grain handling facilities, it is not intended to address all the hazards that can be found in workplaces handling grains. Additional standards (as relevant) within 29 CFR 1910 for General Industry and 29 CFR 1917 for Marine Terminals will apply to the grain handling portion of these facilities. For example, bin entry requirements will be found in 29 CFR 1910.272(g) & (h), and permit- required confined space requirements will be found in 29 CFR 1910.146. Requirements for the control of hazardous energy will be found in 29 CFR 1910.272(e) & (m) and 29 CFR 1910.147 as applicable.
- Some construction requirements are included in Appendix B.
-
Research the Facility
Most states have a state agency with a website that provides information on local ethanol production facilities. Information such as a description of the milling process (dry or wet), the feedstock (corn, other), production capacity, and number of permanent workers may be available for each operational facility at these websites. Additional information about the facility can be obtained on the OSHA website, if it has an OSHA Establishment History. A search for the facility's history can be conducted by using the Standard Industrial Classification (SIC) for ethanol processing facilities, 2869, industrial organic chemicals not elsewhere classified and the North American Industry Classification System (NAICS) 325193, ethanol, non-potable manufacturing.
Each Regional Office library should have ethanol industry reference documents accessible to CSHOs. Area Office jurisdictions that conduct a large number of Process Safety Management (PSM) inspections should have relevant reference documents in their libraries. CSHOs may also access documents available online through OSHA's Technical Data Center. Similarly, jurisdictions that cover a large number of ethanol processing facilities should have libraries with relevant resources covering appropriate subjects, such as: pressure vessel inspection, rating, repair and alteration, ANSI piping inspection code, guidelines for the integrity of mechanical systems and engineering design of the process, etc.
Relevant American Petroleum Institute (API) publications (e.g., standards (STD), recommended practices (RP), and technical reports (TR)) include:
- API 510, Pressure Vessel Inspection Code: In-Service Inspection, Rating, Repair, and Alteration - Tenth Edition, 2014.
- API RP 520 part (PT) I, Sizing, Selection, and Installation of Pressure-relieving Devices in Refineries Part I - Sizing and Selection – Eight Edition, 2008.
- API RP 520 PT II, Sizing, Selection, and Installation of Pressure-Relieving Devices in Refineries Part II–Installation - Fifth Edition, 2003.
- API STD 521, Pressure-relieving and Depressuring Systems - Sixth Edition, 2014.
- API 570, Piping Inspection Code: In-service Inspection, Rating, Repair, and Alteration of Piping Systems - Third Edition, 2009.
- API RP 574, Inspection Practices for Piping System Components - Third Edition, 2009.
- API STD 653, Tank Inspection, Repair, Alteration, and Reconstruction - Fourth Edition, 2009; Incorporating Addendum 1: August 2010, Addendum 2: January 2012, Addendum 3: November 2013.
- API RP 752, Management of Hazards Associated with Location of Process Plant Permanent Buildings - Third Edition, 2009.
- API RP 753, Management of Hazards Associated with Location of Process Plant Portable Buildings - First Edition, 2007, reaffirmed 2012.
- API TR 939-D, Stress Corrosion Cracking of Carbon Steel in Fuel-Grade Ethanol: Review, Experience Survey, Field Monitoring, and Laboratory Testing - SECOND EDITION, ADDENDUM 1: OCTOBER 2013.
- API Bull 939-E, Identification, Repair, and Mitigation of Cracking of Steel Equipment in Fuel Ethanol Service - SECOND EDITION, 2013.
Another source of information is the Right to Know Network; it provides access to various databases (e.g., Hazardous Waste, Toxic Releases), including the Environmental Protection Agency's (EPA) Risk Management Plan (RMP) database, which collects information on large amounts of highly hazardous substances used at facilities An example is the use of sulfur dioxide, gasoline and ammonia at a facility in Cedar Rapids, IA.
-
Select Equipment
- Prior to the initial walkaround inspections (if feasible), CSHOs should review the employer's procedures for PPE selection and allowable electronic equipment in the selected unit(s) and/or areas of the facility that will be inspected. The right type of PPE must be made available to the CSHO at all times, regardless of the urgency of the inspection/investigation circumstances.
- Equipment rated for the hazardous classified location must be used (if applicable). Process areas with the possibility of flammable vapors require Class 1 electrical equipment, while process areas with the possibility of combustible dusts require Class 2 electrical equipment.
- Required equipment includes cameras/or video cameras. CSHOs may use non-intrinsically safe cameras equipped with a telephoto lens from outside classified areas (OSHA 2011g; OSHA 2008b) and/or still cameras without batteries or a flash (OSHA 2011g). If the employer allows the use of non-intrinsically safe cameras in hazardous (classified) locations, CSHOs may use this type of equipment when: (1) the employer issues a hot work permit for the use of the camera; and (2) continuous combustible gas metering, which has been calibrated prior to use, is provided in the areas where the camera will be used (OSHA 2011g).
- Properly calibrated four-gas meters and/or other appropriate detection equipment is recommended to determine the condition of the atmosphere.
- Flame-retardant coveralls for protection from flash fires must be available if needed. Clothing made of hazardous synthetic fabrics must not be worn underneath flame-retardant coveralls. CSHOs must wear natural fiber non-spark producing clothing and underwear (such as 100% cotton) for protection from ignitable combustible dusts and/or flammable vapors (OSHA 2011g; OSHA 2008b). Avoid jackets or other clothing with nylon liners.
- NIOSH-approved emergency escape respirators must be carried (the CSHO must be trained and certified to use them) in case an atmosphere that is Immediately Dangerous to Life and Health (IDLH; 29 CFR 1910.134(d)(2)) is encountered.
- Other necessary PPE may include: steel-toed shoes, hard hats, safety glasses, rubber/nitrile gloves, leather gloves, hearing protection, etc.
B. On-site Investigations or Inspections
On-site investigations/inspections follow a similar pattern. The CSHO must present official identification as soon as he/she arrives at the site and request to speak with the appropriate employer management representative. The CSHO conducts a preliminary opening conference, followed by a walkthrough of the facility. If there is union representation at the facility, a union representative should be at the opening conference and participate in the walk through or workplace evaluation.
The CSHO will walk through the facility to conduct a physical assessment and interview workers. This requires an examination of the processes involved (VI.B.1; OSHA 2000; OSHA 2011g), floor plans or drawings, maintenance procedures, written confined space program, and other appropriate safety and health information (Section III).
The inspection ends with the closing conference, during which the CSHO will discuss the findings of the walkaround evaluation. The CSHO will conduct the closing conference with the employer's management representative and the workers union representative (if applicable). The purpose is to discuss the standards violated based on the hazards observed, corrective actions to implement, and possible citations that would be recommended to the OSHA Area Director (Employer Rights and Responsibilities Following a Federal OSHA Inspection, 2011 [Publication OSHA-3000]; OSHA Field Operations Manual (FOM), 2011 [CPL-02-00-150], [Updated Field Operations Manual (FOM), 2016 CPL 02-00-160].
Prior to starting the walk-through evaluation, the CSHO should:
- Establish the applicability of the PSM standard (I) at the opening conference; for example, by reviewing the inventory of chemicals on site and their respective maximum intended quantities, including the amount of ethanol and any other flammable liquids, and determining if there are exemptions that apply as specified in the PSM NEP, OSHA 2011g. Due to its flashpoint of 55 degrees Fahrenheit, ethanol is a flammable liquid. In accordance with 29 CFR 1910.119(a)(1)(ii), the on-site threshold quantity for the implementation of PSM is 10,000 pounds or more of a flammable liquid. Exemptions may apply (I).
- Establish if an inspection for combustible dust hazards is warranted. During the opening conference and after a preliminary walkthrough of the facility, if the CSHO determines that the employer's operation does not have combustible dust explosion, deflagration, or other fire hazards, then the CSHO may terminate the combustible dust hazards inspection, or contact the Area Office on whether to continue. Additionally, if the CSHO determines that the facility has undergone an OSHA consultation visit in the past three years and verifies through a basic walkthrough and evaluation of any changes made by the employer that the combustible dust explosion hazards have sufficiently been addressed by the employer, then the CSHO shall normally terminate the combustible dusts hazard inspection (OSHA 2008b).
- Obtain a facility site map for PSM covered facilities (29 CFR 1910.119(e)(3)(v)). Facility means the buildings, containers or equipment that comprise a process (29 CFR 1910.119(b); API RP 752, Management of Hazards Associated with Location of Process Plant Permanent Buildings - Third Edition, 2009; API RP 753, Management of Hazards Associated with Location of Process Plant Portable Buildings - First Edition, 2007, reaffirmed 2012).
- API RP 752 (2009), which discusses recommended practices for siting permanent buildings in process plants, is based on the following guiding principles:
- Locate personnel away from process areas consistent with safe and effective operations.
- Minimize the use of buildings intended for occupancy in close proximity to process areas.
- Manage the occupancy of buildings in close proximity to process areas.
- Design, construct, install, modify, and maintain buildings intended for occupancy to protect occupants against explosion, fire, and toxic material releases.
- Manage the use of buildings intended for occupancy as an integral part of the design, construction, maintenance, and operation of a facility.
- API 753 (2007 R 2012) which provides guidance for reducing the risk to personnel located in portable buildings from potential explosion, fire, and toxic release hazards is based on the following guiding principles:
- Locate personnel away from covered process areas consistent with safe and effective operations.
- Minimize the use of occupied portable buildings in close proximity to covered process areas.
- Manage the occupancy of portable buildings, especially during periods of increased risk, including unit start-up or planned shut-down operations.
- Design, construct, install, and maintain occupied portable buildings to protect occupants against potential hazards.
- Manage the use of portable buildings as an integral part of the design, construction, maintenance, and operation of a facility.
- API RP 752 (2009), which discusses recommended practices for siting permanent buildings in process plants, is based on the following guiding principles:
- Information on the equipment used in the process must include the following:
- relief system design and design basis;
- piping and instrument diagrams (including piping components such as valves);
- ventilation system design;
- materials of construction;
- design codes and standards employed;
- materials and energy balances for processes built after May 26, 1992; and,
- safety systems (e.g., interlocks, detection/monitoring devices, sensors, alarms, or suppression systems).
- Determine the amount and type of grain on site. Obtain a process flow diagram during the opening conference. Refer to Production Steps in Corn Dry-Milling (Table II.1) or Production Steps in Corn Wet-Milling (Table II.2) as a guide for reviewing the specific process used. The tables summarize each of the production steps used, the primary inputs and outputs, and the main operations performed in each step. While common processing steps are documented, every facility differs, so some facilities may not employ every step while other facilities may use different terminology for a step in the process than those used in the tables.
- Obtain information on what parts of the process flow are operating at the time of the on-site inspection. This may include all steps in the process, or segments of the process may be operational if it is a batch facility.
- The employer's emergency planning and response should be reviewed (V.A). Evaluate the evacuation route(s), assembly area(s), available emergency services and contact information. Observe if trucks or railcars block emergency access to a unit. Assess any emergency response facilities, fire pumps, emergency isolation valves, etc. Since ethanol processing facilities are often found in rural, remote locations, it would be prudent to evaluate the facility's contingencies for the provision of medical services and first aid (29 CFR 1910.151), permit-required confined space rescue (29 CFR 1910.146), and response to incidents or emergencies involving Hazardous Waste Operations (HAZWOPER) (29 CFR 1910.120).
- Review the employer's records (OSHA 2000):
- Employee training documentation (e.g., PSM, HCS, confined space, PPE).
- Safety procedures for contractors performing maintenance or repair, turnaround, major renovation, or specialty work on or adjacent to a covered process.
- Injury and illness records including OSHA-300 and OSHA-300A.
- Review the employers PSM records (if applicable) in accordance with 29 CFR 1910.119.
- Request other safety documentation, such as hot work permits and procedures (e.g., shutdown of ducts and conveyor systems during welding), confined space entry permits, and procedures and contractor information.
-
Walkaround Evaluation
It is highly recommended that inspections of these industrial sites follow the process flow described in sections 1 and 2 (Figure VI.1; Figure VI.2). Some of the hazards that may be encountered in certain areas of the facility are summarized in this section. Refer to sections Section III and Section V for a more detailed discussion of the hazards and safety measures respectively. Be prepared to draw and write an accurate description of the hazards that cannot be photographed and identify their locations.
Observe workers' use of the appropriate PPE. Interview workers to determine if they understand job hazards, safeguards, control measures, PPE, and other safety-related issues that the employer is required to ensure they fully understand. A noise exposure evaluation may be conducted (e.g., in grain receiving and processing areas; III.E; IV.J, IV.K, IV.L). Atmospheric testing may also be conducted to establish concentration levels for any hazardous chemicals used in the workplace (OSHA Technical Manual [OTM], Section III, Chapter 3, Ventilation Investigation, OSHA 1999).
Figure VI.1 Example of a Process Building Map (IAFC, 2008)

Figure VI.2 Common Components of a Typical Ethanol Production Facility (IAFC, 2008)

Legend
X Approximate location of fire hydrants O Approximate location of fire department sprinkler connections 1 Administrative building 2 Cooling towers 3 Anhydrous ammonia tank 4 Maintenance building 5 Process area 6 Grain storage 7 Ethanol rail loadout 8 Tank farm -
Grain receiving areas
Grain is received by truck or rail (II.B.2.i) and dumped into receiving pits. Observe the various activities in this area carefully: pedestrian/vehicular safety (IV.S), idling vehicles (which are sources of ignition), trucks or railcars that may be blocking emergency access to a unit/building, etc. Inspect bonding and grounding equipment. For example, check to see if they are properly connected and in optimal working condition to properly dissipate electrostatic charges. Electrical equipment and wiring should be rated for a Class II hazardous environment (where there is a potential for dusts to be suspended in the air).
Grain elevators may be used to transport grains to trucks. They are made up of buckets attached to a continuous rotating belt driven by a motor with the entire system enclosed. Dust clouds may form if equipment is in poor condition (e.g., leakage from bucket elevators or if the dust collector is not functioning properly), if poor housekeeping is practiced, etc.
Other safety considerations in this area include confined spaces (III.G; IV.P; IV.Q), combustible dust hazards (III.C; IV.E; IV.F), and engulfment hazards (III.D; IV.G; IV.H; IV.I).
Prohibited practices in grain storage bins or areas include:standing in flowing grain in silos, or on bridged grain in silos, standing next to a grain mass in a storage pile, or standing next to large piles of grain (III.D). Review lockout/tagout procedures (IV.U) for sources of hazardous energy (III.I).
-
Load-out/cooling areas for grains
In these areas (Figure II.16; Figure II.19), dried co-products are transferred to interim storage areas and eventually to railcars or trucks for distribution into commerce. Transfer and loading of dry products may lead to dust formation. Safety considerations would generally be the same as for grain receiving areas discussed above (VI.B.1.i; III.C; IV.E). Electrical equipment must be rated for class II hazardous location (29 CFR 1910.307(c)(2)(i); NFPA 70).
-
Ethanol loading areas
Hazards in these areas include: overfilling; leaking equipment; and, presence of ignition sources (III.A; IV.A) such as smoking, lightning, idling vehicles (III.H; IV.S), and open flame operations (e.g., welding). Check for controls (e.g., bonding and grounding mechanism) and safeguards (e.g., fire suppression systems and vapor recovery systems). Inspect tanks, piping and other equipment for cracks and other signs of wear and tear (III.B). Electrical equipment and wiring must be rated for a Class I (Division I or II, as appropriate) hazardous environment where there is a potential for flammable gases and vapors (29 CFR 1910.307(c)(2)(i); NFPA 70).
-
Distillation
The distillation process area (II.B.1.vii; Figure II.13) is typically where ethanol first becomes highly concentrated by heating the process stream. This part of the facility, therefore, has significant flammable vapor hazards (III.A; IV.A; IV.B) due to the presence of highly-concentrated ethanol vapors and heat. Overpressure or mechanical damage in the distillation columns can cause ethanol vapors to leak and this may present a fire and/or explosion hazard and may expose workers to concentrations above OSHA's permissible exposure limit (PEL) for ethanol (1,000ppm for an 8-hour time-weighted average, 29 CFR 1910.1000 Table Z1). This hazard also exists for other equipment (e.g., piping, molecular sieve) typically found in the distillation process area. Process controls must be adequate to maintain the required temperatures and pressures in the distillation columns.
Process equipment must be designed to handle process upsets (29 CFR 1910.119; 29 CFR 1910.106), for example, incorporating safety relief valves that avoid equipment failure by venting excess ethanol streams directly to safe areas; designing drains to quickly move spilled chemicals from surfaces into enclosed tanks; and, overfill protection devices that minimize spills from tanks and other vessels. Other safety measures include ethanol vapor detectors and automatic fire detection and suppression systems.
Sources of ignition must be eliminated in distillation areas, for example, by prohibiting smoking, implementing bonding and grounding mechanisms, utilizing electrical equipment and wiring that is classified for the hazardous location, and implementing a hot work program.
-
Fuel storage tank area
Hazards include: pipe and equipment ruptures (III.B; IV.C; IV.D), lightning strikes, potential for electrostatic discharge, leaks, improper storage, etc. Some fuel storage tank hazards are also discussed in III.A. Safety measures include: flame arrestors; bonding and grounding mechanisms; ethanol vapor monitoring devices; inerting headspaces; availability of drains designed to quickly remove spills from surfaces into tanks; vapor recovery systems; overfill protection devices; Class I electrical equipment, wiring and fixtures; installing a Lightning Early Warning System; posted signs; fences/barriers to keep unauthorized workers out of fuel storage areas; and, prohibiting the use of open flames and smoking in the area.
Evaluate the flammable liquids dispensing system, the design and capacity of containers, and the design and construction of any inside storage rooms, such as having fire protective walls (where required) and an appropriate ventilation system (OSHA Technical Manual [OTM], Section III, Chapter 3, Ventilation Investigation, OSHA 1999).
-
Cleaning areas
In these areas (II.B.1.ii) screeners and scalpers (Figure II.10) are designed to remove oversized objects and finer material (e.g., dusts) from shelled corn. Corn kernels may also be passed through a destoner. Older and malfunctioning devices can release a significant amount of dust that can create a dust cloud which could cause fires or explosions when disturbed and confined in the presence of an ignition source. Dust collectors must be maintained in optimal working condition to function effectively in removing any generated dust (III.C). The operating limits for the dust collector may be obtained by reviewing a standard operating procedure for the equipment or its manual to determine if it is functioning as designed.
-
Bulk storage areas for grains
These include silos and other bulk storage areas (Figure II.9; Figure II.15). Dust clouds can be generated if equipment is not in good working order. Highly efficient dust collection and aspiration systems are needed (III.C; IV.E; IV.F), Class II electrical equipment and wiring must be installed, a hot work program must be implemented, smoking must be prohibited, and proper bonding and grounding for processing equipment must be utilized, if applicable. Safety precautions to protect workers from engulfment hazards (III.D; IV.G; IV.H; IV.I) and confined space hazards (III.G; IV.P; IV.Q; IV.R) must be implemented in these areas (as applicable).
-
Transfer points
Transfer points, such as enclosed belt conveyors (Figure III.3) and bucket elevators move large quantities of solid materials and dusts (III.C; IV.E; IV.F). Safety measures that can be implemented to prevent fires/explosions at transfer points include installing temperature sensors to monitor equipment temperature; maintaining a proper belt alignment on the bearing to help increase efficiency and reduce heat caused by friction; and equipping conveyor belts that are completely enclosed with a dust collection system (Imperial Sugar Company Dust Explosion and Fire (2/7/08), U.S. CSB Investigation). Electrical wiring and equipment (e.g., chutes, bucket elevators, and conveyor belts) must be designed for Hazard Class II locations (dust ignition proof) to prevent an explosion and/or ignition (29 CFR 1910.307(c)).
-
Dryers
Safety measures include automatic suppression systems that detect pressure rises in the equipment (Figure II.18) and inject inert materials to suppress an explosion; explosion relief venting that detects a rise in pressure and opens to vent heated gases outside (NFPA 69); dust tight equipment design; automatic system to detect sparks and smoldering materials, for example, in the ductwork on the outlet of unit operations suspected of causing sparks or smoldering; and, a control system for maintaining the dryer's temperature within prescribed limits.
-
Grinding/milling area
Grinding/milling (II.B.1.iii) reduces feedstock materials into smaller particle sizes of corn flour, therefore there is a potential to generate large amounts of dust. The key to avoiding fires and explosions is to prevent ignition sources from entering the equipment (Figure II.11), such as tramp metal that might be inadvertently conveyed inside the mill along with the feedstock. Implement the same precautions as for dryers in VI.B.1.ix above.
-
Liquefaction
Toxic enzyme, alpha-amylase is used in this phase (II.B.1.iv) to convert corn starch into dextrins. Exposure to alpha-amylase is associated with increased risk for respiratory effects, such as occupational asthma, in exposed workers. Also reported are exposure-related symptoms in the eyes (e.g., itchiness) and nose (e.g., blockage, sneezing), as well as allergic reactions in those who are more sensitized to its effects. At this time, OSHA has no permissible exposure limit (PEL) for alpha-amylase.
-
Saccharification
There are various hazardous chemicals (III.F; IV.M; IV.N; IV.O) used in this phase (II.B.1.v) including sulfuric acid. Proper procedures should be in place to protect workers from exposure to hazardous chemicals (VI.B.1.xv, below).
-
Fermentation
Carbon dioxide (CO2) may be released into the atmosphere during an overpressure event in the fermentation phase (II.B.1.vi). This can be prevented by properly venting the equipment (Figure II.12) and piping network. However, facilities will generally include scrubbers and other devices to ensure that any gaseous ethanol in the CO2 exhaust system is captured and returned to the process. Failure to control potentially dangerous chemical reactions may lead to the rupture of equipment and pipes; cause explosions and fires; and, expose workers to hazardous chemicals. Equipment may rupture (III.B; IV.C; IV.D) due to age deterioration, if it is not properly designed or maintained in good condition. Smoking must be prohibited in work areas where flammability and/or explosion hazards exist. It is also critical that there is an effective permit issuing process in areas where flammability and/or explosion hazards exist to ensure that safety procedures are implemented before starting any hot work, for example, welding operations (IV.B). Other engineering controls include: controlling the rate and sequence of chemical addition; providing robust cooling; segregating incompatible materials to prevent inadvertent mixing; and using detailed operating procedures to keep the process within safe limits. The appropriate PPE must also be provided to workers when needed.
-
Denaturing of ethanol
At facilities that manufacture ethanol for fuels, the ethanol is denatured with conventional gasoline before storing it in tanks. Workers should be cautious of flammable headspace vapors in tanks (III.A; IV.A).
-
Hazardous chemicals
Biofuels and the chemicals used (Table III.1) in the manufacturing process present potential exposure hazards (III.F; IV.M; IV.N; IV.O) that must be eliminated or carefully controlled to safe levels to protect workers' safety and health SDSs/MSDSs must be consulted to determine the hazards of exposure to feedstocks, products, and other hazardous chemicals used in biofuel processes, including, but not limited to, methanol, sulfuric acid, and ethanol, as well as hydrocarbons used for blending and alcohol denaturing, e.g., gasoline. Employers must also protect workers from exposure to benzene, an important hazardous constituent of gasoline (29 CFR 1910.1028; 29 CFR 1926.1128).
-
Temporary Structures and Permanent Buildings
Temporary employee-occupied structures include trailers that by virtue of their location expose workers to potential hazards (e.g., fires, explosions, overpressures, toxic or corrosive materials) or that risk being damaged by process equipment from other locations (e.g., toppling of equipment onto occupied structures). Both temporary and permanent structures within the facility must be evaluated for compliance with proper facility layout/siting. PSM covered facilities must implement process hazard analysis that includes facility siting information, such as a blast study (VI.B).
-
Dehydration
There are significant hazards associated with operating the molecular sieve beds, especially in regenerating them, which is generally done using hot nitrogen or another inert gas. It is easy to have fires or explosions in these vessels unless their operation is precisely sequenced. It is necessary to review appropriate documentation (e.g., operating manuals, equipment manuals, operating procedures, incident reports, and maintenance records) to determine if the equipment is being properly operated and maintained in good working condition by trained workers.
-
Appendix A--Summary of Hazards and Control
|
Hazards |
Potential Hazardous Event include |
Causes include |
Effects include |
Controls include |
|---|---|---|---|---|
|
(1) Flammable liquids (e.g., ethanol, gasoline) |
Fire/Explosion |
Sources of ignition in contact with a flammable liquid or atmosphere. The release of flammable liquid or vapor could be due to:
Sources of ignition include:
|
Injuries Death Equipment/facility damage |
Process Safety Management requirements must be implemented if applicable (29 CFR 1910.119) as discussed in IV.A:
Engineering controls to prevent the accidental release of ethanol include:
Engineering controls for ignition sources:
Safe work practices:
|
|
(2) Combustible Dusts |
Fires/Explosions |
Combustible dust release can be due to:
Ignition sources include:
|
Injuries Death Damage and/or destruction of property and equipment |
Engineering controls for combustible dust release:
Engineering controls for ignition sources:
Safe work practices:
|
|
(3) Engulfment |
Collapse of a bridged surface Avalanche of a vertical wall |
|
Injuries Suffocation Death |
Engineering controls:
Safe work practices:
Personal Protective Equipment (PPE; 29 CFR 1910.272(g)(2):
|
|
(4) Confined spaces with hazards that can incapacitate a worker such that he/she cannot self-rescue or ask for assistance; cause serious injuries; or death E.g., silos, process vessels, grain storage bins, and feed hoppers |
Accidental exposure to a hazardous atmosphere, e.g., oxygen deficient atmosphere, or to acutely toxic and hazardous substances Worker is crushed in equipment Laceration or crushing injuries from moving mechanical parts, e.g., sweep augers Fires/Explosions Employee falls from a height within the space |
|
Asphyxiation Incapacitation Loss of consciousness Entrant is unable to self-rescue or request assistance Death Damage/destruction of property and equipment Serious injuries |
Engineering controls (29 CFR 1910.272(g); 29 CFR 1910.146):
Safe work practices:
PPE:
|
|
(5) Equipment Ruptures |
Release of volatile flammable liquids or combustible dusts due to equipment rupture |
|
Workers exposure to hazardous chemicals can cause: asphyxiation; incapacitation; loss of consciousness; etc. Release of flammable liquids in the presence of an ignition source can cause: Fires Explosions Injuries Death |
Engineering controls:
Safe work practices:
|
|
(6) Motor Vehicles (e.g., loading, unloading, operating) |
Release of flammable vapor in loading/unloading area Smoking or open flames inside or newar vehicles with flammable/combustible materials Static electricity discharge during loading or unloading of flammable/ combustible materials Vehicle failure/ malfunction Motor vehicle collision with another motor vehicle, pedestrian, piping, or other structures Lightning strikes motor |
|
Fires/explosions Injuries/Death Vehicle/property damage |
Engineering controls:
Safe work practices:
|
|
(7) Exposure to hazardous substances |
Uncontrolled release of hazardous substances |
|
Asphyxiation Death Injuries Inability to self-rescue or request assistance in a confined space |
Engineering controls:
Safe work practices:
PPE: Whenever workers' exposure to hazardous substances cannot be eliminated or reduced to acceptable/safe levels, employers must select and provided the proper PPE to affected workers, including training and implement any required safety program, e.g., respiratory protection program. |
|
(8) Hazardous Noise Levels |
Repeated exposure to hazardous noise levels |
Sources of noise include:
|
Impaired hearing Loss of hearing Loss of balance |
Engineering controls:
Hearing protection devices:
|
|
(9) Exposure to Hazardous Energy |
Unexpected start-up of equipment or machinery during maintenance operation |
|
Injury Death |
|
Appendix B--List of Some of the Standards Applicable to Ethanol Manufacturing Facilities
|
Applicable Standards |
Relevancy to Ethanol Manufacturing Facilities |
Example Requirements in Applicable Standards |
|---|---|---|
|
29 CFR 1926.1101- Asbestos |
If construction, repairs or modification of an ethanol processing facility involves the demolition or renovation of structures, the asbestos standard or National Emission Standards for Hazardous Air Pollutants (NESHAP) regulation (40 CFR 61 Subpart M) may apply. |
Regulates asbestos exposure in all construction work including but not limited to construction, alteration, repair, maintenance, or renovation of structures, substrates, or portions thereof, that contain asbestos. Construction work means work for construction, alteration, and/or repair, including painting and decorating (29 CFR 1910.12(b)). |
|
29 CFR 1910.1020 - Access to Employee Exposure and Medical Records |
Some ethanol manufacturing facilities are expected to keep medical records of their employees. |
Requires employers to provide workers and their representatives with the right to access any relevant exposure and medical records. |
|
29 CFR 1910.1028 - Benzene 29 CFR 1926.1128 - Benzene |
Benzene is a component of gasoline. Gasoline is used as a denaturant in ethanol manufacturing facilities. Applicability will depend on the benzene content in the gasoline used and other possible exemptions. |
Includes requirements for monitoring, engineering controls, respiratory protection, hazard communication, recordkeeping, medical surveillance, work practices, permissible exposure limit, and short-term exposure limit. |
|
29 CFR 1910.1026 - Chromium VI 29 CFR 1926.1126 - Chromium VI |
Hexavalent chromium may be produced from the welding or torch cutting of piping and vessels made of steel. |
Includes requirements for the permissible exposure limit (PEL), action level, employee monitoring, exposure determination and more. |
|
All types of ethanol manufacturing facilities will have combustible dusts, with the types of dust present dependent upon the feedstock used. The chief concerns for combustible dusts are the increased risk for major fires and explosions. |
OSHA has issued an Advance Notice of Proposed Rulemaking for combustible dust (Combustible Dust; Advance notice of proposed rulemaking, 74:54333-54347 (10/21/2009)) NFPA has published consensus standards (e.g., NFPA 61) that outline administrative and engineering controls for preventing grain dust fires and explosions. |
|
|
29 CFR 1910.101 - Compressed Gases |
Some ethanol manufacturing facilities use compressed gases. Some facilities capture carbon dioxide formed during fermentation and may compress the gas on site. |
Requires employers to manage hazards by ensuring that gas cylinders are stored and handled safely and by equipping certain compressed gas containers with pressure relief devices. Several other requirements also apply. |
|
29 CFR 1910.147 - The Control of Hazardous Energy (lockout/tagout) |
This standard covers the servicing and maintenance of machines and equipment in which the unexpected energization or startup of the machines or equipment, or release of stored energy, could harm employees. Ethanol manufacturing facilities use several equipment and machines that must be maintained/repaired to ensure that they function properly. |
Specifies that employers must establish a program consisting of energy control procedures, worker training, and periodic inspections to ensure that before any worker performs any servicing or maintenance on a machine or equipment where the unexpected energizing, startup, or release of stored energy could occur and cause injury, the machine or equipment must be isolated from the energy source and rendered inoperative. |
|
29 CFR 1910 Subpart L - Fire Protection 29 CFR 1926 Subpart F - Fire Protection and Prevention |
Standards pertain to fire detection, fire suppression, and employee alarm systems–all of which are relevant to ethanol manufacturing facilities given the presence of flammable chemicals. |
Outlines requirements for fire brigades, automatic sprinkler systems, portable and fixed fire extinguishing systems, fire detection systems, and alarm systems. |
|
29 CFR 1910. Subpart S - Electrical |
Electrical equipment always has the potential for presenting a fire hazard. This is particularly important in production areas with ethanol vapors and grain dusts. |
Includes four types of requirements to ensure the use of safe electrical installations: (1) design safety standards, (2) safety-related work practices, (3) safety-related maintenance requirements, and (4) requirements for special equipment. |
|
29 CFR 1910.307 - Electrical Hazardous (Classified) Locations |
Electric equipment and wiring pose an ignition source for areas known or suspected to contain flammable vapors or combustible dusts. |
Defines the classification scheme for hazardous locations and requires electrical equipment, wiring, and installations to be of the type approved for the hazardous location classification. |
|
29 CFR 1926 Subpart M - Fall protection 29 CFR 1926 Subpart X - Stairways and Ladders 29 CFR 1910 Subpart D - Walking-Working Surfaces |
Requirements to protect workers from slips, trips and falls hazards on working and walking surfaces and safety requirements on ladders and stairways. |
Includes provisions for fall protection, such as guard rails and covers. |
|
29 CFR 1910.106 - Flammable Liquids 29 CFR 1926.152- Flammable Liquids |
Ethanol and several other liquids used in these processes are flammable. The flammable liquids are stored, formed, and transferred between ethanol manufacturing facilities. |
Provides requirements for storage containers, ventilation near flammable liquids, control of ignition sources, and housekeeping and maintenance. |
|
29 CFR 1910.272 - Grain Handling Facilities |
Ethanol manufacturing facilities that process grain feedstock (e.g., corn) receive, handle, and store large quantities of grain on a daily basis. This material poses dust fire, explosion, and engulfment hazards. |
Requires administrative and engineering controls to prevent engulfment hazards and fire and explosion hazards from combustible dusts. Controls include training, hot work permits, housekeeping, proper design and installation of equipment, preventive equipment maintenance and inspections, emergency escape plans, and various engulfment prevention measures. |
|
29 CFR 1910 Subpart P - Hand and Portable Powered Tools and Other Hand-Held Equipment |
Many workers at ethanol manufacturing facilities, especially maintenance personnel, use a variety of hand and portable power tools, which present physical hazards. |
Requires tools be in safe condition, properly used and fit for the task, equipped with guards (as necessary), and equipped with automatic power shutoffs. |
|
29 CFR 1910.1200 - Hazard Communication |
The Hazard Communication Standard (HCS) requires chemical manufacturers or importers to classify the hazards of chemicals which they produce or import, and all employers to provide information to their workers about the hazardous chemicals they are exposed to. |
Requires employers to provide information to their workers about the hazardous chemicals they are exposed to by means of a hazard communication program, labels, safety data sheets, and information and training. |
|
29 CFR 1910.120 - Hazardous Waste Operations and Emergency Response (HAZWOPER) |
Specifies OSHA's requirements for emergency response operations involving releases of hazardous substances. |
Provides requirements on decontamination, training, emergency response plans, PPE, an incident command system, and site safety and control plans. |
|
International Code Council Codes and Standards (Ethanol Fixed Facilities: Assessment and Guide, 1st Edition, IAFC, 2008) |
ICC makes every effort to provide current, accurate code adoption information, but in some cases jurisdictions do not notify ICC of adoptions, amendments, or changes to their codes. To ensure you have accurate information, please contact the jurisdiction directly. The International Code State and Jurisdiction Adoption Charts are works in progress. The information contained herewith has been provided by individuals involved in local jurisdictions and state legislatures. |
Chapter 9 Fire Protection Systems:
|
|
29 CFR 1910.212 - Machinery and Machine Guarding |
Some machines used in ethanol processing facilities require point of operation guarding, e.g., milling machine. |
Specifies that one or more methods of machine guarding must be provided to protect operators and other workers in the machine area from hazards such as those created by the point of operation, ingoing nip points, rotating parts, flying chips and sparks. Examples of guarding methods are: barrier guards, two-hand tripping devices, and electronic safety devices. |
|
Managing Your Environmental Responsibilities: A Planning Guide for Construction and Development A Planning Guide for Construction and Development, EPA/305-B-04-003, EPA, 2005 |
General planning guide for construction and development. |
Provides information on environmental obligations for:
|
|
29 CFR 1910 Subpart E - Means of Egress 29 CFR 1910 Subpart L - Fire Protection |
These standards address hazards arising from the possibility of fires and explosions in ethanol manufacturing and grain handling facilities, |
Includes various requirements to ensure that all workers have a safe means of egress from facilities during fires, explosions, and other emergencies. Specific requirements address fire prevention plans, emergency action plans, safe exit routes, alarm systems, and employee training. |
|
29 CFR 1910.151- Medical Services and First Aid |
Employees can be injured while handling hazardous materials, working with process equipment, and performing other job duties. |
Requires employers to ensure that medical personnel are available to discuss facility health-related issues. Includes requirements for individuals trained in first aid, eyewash stations, and first-aid supplies. |
|
National Fire Protection Association (NFPA) Codes, Standards, Recommended Practices, and Guides |
NFPA codes, standards, recommended practices, and guides are developed through a consensus standards development process approved by the American National Standards Institute (ANSI). |
|
|
29 CFR 1910.95 - Occupational Noise Exposure 29 CFR 1926.52 - Occupational Noise Exposure |
Protect workers from the effects of noise exposure, e.g., in grain processing areas, from noisy equipment. |
Includes permissible noise exposure levels, preventive, and protective measures, e.g., hearing protection devices. |
|
29 CFR 1910.146 - Permit-Required Confined Spaces |
Ethanol manufacturing facilities have numerous unit operations that employees must enter for cleaning, inspection, and maintenance purposes. These include, but are not limited to, distillation columns, tanks, and large fermentation vessels. |
Requires a workplace assessment to determine whether spaces are permit-required. Further requires atmospheric testing and monitoring, ventilation, barriers to prevent external hazards, PPE, and an observer placed outside if assistance is needed. |
|
29 CFR 1910 Subpart I - Personal Protective Equipment |
Workers at ethanol manufacturing facilities potentially come into contact with various hazardous materials and can be exposed through inhalation, absorption, or direct contact. |
Specifies circumstances in which PPE is required to prevent or reduce exposure to hazards of chemical liquids, gases, and vapors. Requirements apply to protecting eyes, face, head, feet, and hands. Respirator requirements are also covered. |
|
29 CFR 1910.178 - Powered Industrial Trucks |
Powered industrial trucks pose various safety hazards. Collisions can result in injuries and damage process equipment. Vehicles can also be ignition sources when used in the presence of flammable liquids and combustible dust. |
Outlines safety requirements for powered industrial trucks, including their use, design, maintenance, and fire protection. Equipment should be rated for the specific environments in which they are used. |
|
29 CFR 1910.119 - Process Safety Management of Highly Hazardous Chemicals 29 CFR 1910.119 App A - List of Highly Hazardous Chemicals, Toxics and Reactives (Mandatory) 29 CFR 1910.119 App B - Block Flow Diagram and Simplified Process Flow Diagram (Nonmandatory). 29 CFR 1910.119 App C - Compliance Guidelines and Recommendations for Process Safety Management (Nonmandatory). 1910.119 App D - Sources of Further Information (Nonmandatory). |
Preventive measures in OSHA's PSM standard (29 CFR 1910.119) apply when a process involves a chemical at or above the specified threshold quantities, listed in Appendix A of the PSM standard and/or involves 10,000 pounds or more of a flammable gas or liquid, if the exemption discussed in Section I does not apply. |
Contains numerous requirements to help facilities avoid catastrophic incidents. Examples include: gathering process safety information, conducting process hazard analyses, implementing operating practices and procedures, managing change, and assessing mechanical integrity of process equipment. |
|
29 CFR 1910.111 - Storage and Handling of Anhydrous Ammonia |
Some ethanol manufacturing facilities use ammonia in corn dry and wet milling processes. It can also be used to pretreat cellulosic feedstocks. |
Outlines safety requirements for containers and components for ammonia handling and storage. Permissible exposure limit for ammonia is 50 ppm (35 mg/m3 TWA). |
|
29 CFR 1910 Subpart Z - Toxic and Hazardous Substances 29 CFR 1910.1000 - Air Contaminants 29 CFR 1926 Subpart Z - Toxic and Hazardous Substances |
Workers at ethanol manufacturing facilities are potentially exposed to various air contaminants, depending on the processes with which they are involved. |
Contains tables with substance-specific exposure limits that must not be exceeded in an 8-hour work shift over a 40-hour work week; also includes substances with ceiling values. |
|
U.S. Environmental Protection Agency: Environmental Laws Applicable to Construction and Operation of Ethanol Plants, November 2007 (EPA-907-B-07-001), EPA, 2007a |
This compliance assistance manual serves as a road map of information on federal environmental programs and federal and state agency roles. Air, water, hazardous waste, accident prevention and release reporting are examples of requirements that might apply. This manual, like a road map, does not contain all the details of the federal and state statutes and regulations. Ethanol facility operators need to review the applicable statutes and regulations. |
Includes information on environmental laws that apply when constructing or modifying an ethanol plant. |
|
29 CFR 1910 Subpart D - Walking-Working Surfaces 29 CFR 1910.22 - General Requirements |
Applies to virtually every workplace. Thorough and effective housekeeping programs are an effective administrative control for preventing hazardous combustible dust accumulations. |
Requires all places of employment to be clean, orderly, and in a sanitary condition. Requires employers to maintain walking and working surfaces free of hazards, including accumulations of combustible dust. |
|
29 CFR 1910 Subpart Q - Welding, Cutting, and Brazing |
Welding, cutting, brazing, and other heating (i.e., "hot work") activities occur throughout ethanol manufacturing facilities, which increases fire risks when performed in the presence of flammable liquids (ethanol) and combustible dusts. |
Addresses fire prevention and protection, worker protection, health protection and ventilation, and industrial applications. Covers specific aspects of these processes: oxygen-fuel gas welding and cutting, arc welding and cutting, and resistance welding. |
|
Additional standards are listed throughout this technical manual in appropriate sections (e.g., VI.A.1; VI.A.2). |
||
Appendix C--Safe Entry Requirements for Above Ground Storage Tanks
What are the characteristics of gasoline/ethanol storage tanks?
Typically, aboveground gasoline/ethanol storage tanks are "confined spaces" with limited entry through "manways" (relatively small openings in the side or top of the tank). The following potential hazards trigger Permit Required Confined Space (PRCS) characterization:
- Top entry can present a falling hazard.
- The internal structure of tanks can be complex, which may not be apparent from the outside.
- Some tanks have internal floating roofs, which can move and trap workers.
- Tripping hazards may be present.
- The petroleum products typically stored in these tanks pose flammability and toxicity hazards that must be recognized.
Are empty tanks hazardous?
Historically, numerous incidents have involved "empty" tanks.
- Welding in and near tanks can ignite flammable vapors.
- Tanks that do not contain enough oxygen (less than 19.5%) can cause asphyxiation.
- Just because a tank has been "emptied" of its contents does not mean it is free of hazards.
- A tank is never safe for entry until properly and thoroughly evaluated to ensure that there are no actual or potential atmospheric hazards that can affect the oxygen content, flammability, or toxicity.
What keeps entrants safe?
Safe tank entry requires an evaluation of hazards (both physical and atmospheric) at the job site.
- The MSDS/SDS of the tank contents will provide useful information on the hazards of the chemical and recommended control measures.
- Each permit-required confined space atmosphere must be tested using properly calibrated equipment.
- Entry supervisors must know OSHA regulations, industry standards and the specific work to be performed in the tank.
- Understanding safe tank entry requirements and procedures leads to proper preparation and verified hazard control, such as tank isolation, ventilation and proper use of PPE.
- Confined spaces that are mobile, such as a tanker trucks used for transporting fuels must be properly secured, bonded, and grounded.
What are safe entry priorities?
- Do as much work as possible without entry.
- Eliminate or isolate all potential hazards prior to entry.
- Use a written entry program, permits and trained personnel.
- Understand hazards before entry.
- Ventilate if needed, using forced mechanical ventilation.
- Test and inspect to evaluate and verify tank entry conditions.
- Communicate needs and requirements with employees, contractors and others working in the vicinity of the tank.
- Coordinate all activities in accordance with 29 CFR 1910.146(c)(8).
Good Practices for Tank Entry
- Comply with OSHA's Permit Required Confined Space (PRCS) Standard, 29 CFR 1910.146.
- NEVER assume an "empty" tank is free of atmospheric hazards.
- Assume hazards are present until evaluated and verified.
- Evaluate all confined spaces to determine if they are PRCSs.
- Prior to any hot work, ensure that the tank is cleaned and gas-free.
- Check seals.
- Blind or blank piping connected to the tank or use "double block and bleed" to isolate systems. Assure the blind or blank is appropriately sized (includes thickness) to withstand pump pressure.
- If using a bleed line, ensure that it is sized to fully bleed the line it serves (ANSIZ117.1).
- Lockout/tagout of electrical connections. Lockout/tagout rectifiers for any cathodic protection systems connected to tank.
- Use approved low-voltage or ground fault circuit interrupter electrical equipment to reduce potential hazards.
- Disable tank heating and mixers, stirrers and similar mechanical equipment in accordance with lockout/tagout procedures (29 CFR 1910.147).
- Verify that Entry Permit conditions have been achieved.
- Restrict entry into tank.
- Calibrate gas detection meters often, at least before each day's use.
- If tank oxygen content differs from outside air find out why before entry and consider using forced air ventilation to maintain oxygen content between 19.5-23.5%.
- Use approved lighting (electrically certified) to illuminate the interior space.
- Make sure that rescue services equipment is at the site and that personnel are ready, available, and have been evaluated in accordance with 29 CFR 1910.146(k) and 29 CFR 1910.146 Appendix F.
- Make sure that contractors know, understand, and follow safe tank entry procedures and coordinate entry as required by 29 CFR 1910.146(c)(8).
- Use the correct PPE properly when required.
- Enter tanks from ground level where possible.
- Keep track of who is in and out of the tank.
- Look for activities in adjacent areas that could affect confined space conditions.
- Notify nearby areas when doing tank entry.
- Use barriers to keep unauthorized personnel away.
- Consider cutting a large door sheet opening into the side of tank, if feasible.
The Do's and Don'ts of Safe Tank Entry
DO:
- Obtain all necessary permits.
- Post a copy of the permit at the job site.
- Understand the type of tank and its contents before entry.
- Get copies of MSDSs/SDSs for chemicals/products and review them.
- Identify hazards and conduct atmospheric testing of the tank before entry.
- Expect to find product under a tank's bottom or wherever product may become trapped (columns, legs, interstitial spaces between double walls, double bottoms, etc.).
- Use proper ventilation. Ventilate with air flow away from workers and directed away from outside sources of ignition.
- Maintain two-way communication between entrant and attendant.
- Evacuate personnel and cancel permits if conditions change or new hazards are found.
- Ensure that entrants are trained.
- Have competent supervision (29 CFR 1910.146(d)(8)).
- Have a trained PRCS attendant (29 CFR 1910.146(d)(8)).
- Choose "cold work" over "hot work".
- Use approved power tools.
- Know that welding generates fumes and use effective safety measures to preclude workers' exposure.
- Keep all compressed gas cylinders outside tanks.
- Avoid clutter, use good housekeeping.
- Identify any ignition sources.
- Have rescue services available to respond in a timely manner.
DON'T:
- DO NOT enter PRCS without proper permits.
- DO NOT perform hot work without a permit.
- DO NOT work where the concentration of a flammable chemical is "too rich to burn".
- DO NOT depend on 19.5% oxygen as a "safe" atmosphere.
- DO NOT ignore health concerns.
- DO NOT enter tanks without training and authorization.
Key References for Work Inside Aboveground Storage Tanks
OSHA Standards:
- OSHA 29 CFR 1910.146: Permit-Required Confined Spaces and 29 CFR 1910.1200, Hazard Communication standard.
Other Resources:
- OSHA Publication 3138: Permit-Required Confined Spaces
- API STD 2015: Requirements for Safe Entry and Cleaning of Petroleum Storage Tanks
- API RP 2016: Guidelines and Procedures for Entering and Cleaning Petroleum Storage Tanks
- NFPA 326: Safeguarding of Tanks and Containers for Entry, Cleaning, or Repair
- STI/SPFA SP001: Standard for Inspection of Aboveground Storage Tanks
- ANSI/ASSE Z117: Safety Requirements for Confined Spaces
For more information on regulatory guidance, visit OSHA Standards and Publications at www.osha.gov or visit your state's Department of Labor. Non-regulatory confined space entry information can be found in the standards, publications or books from API (www.api.org), the National Fire Protection Association (www.nfpa.org), the Steel Tank Institute (www.steeltank.com) the American National Standards Institute (www.ansi.org), the American Society of Safety Engineers (www.asse.org), the American Industrial Hygiene Association (www.aiha.org) and the National Institute for Occupational Safety and Health (www.cdc.gov/niosh).
Through OSHA's Alliance Program, this Fact Sheet was developed as a product of the OSHA and Safe Tank Alliance for informational purposes only. It does not necessarily reflect the official views of OSHA or the U.S. Department of Labor. Safe Tank Alliance Fact Sheets are intended only as a guide and should not be relied upon for complying with any federal, state, or local laws, regulations, codes, ordinances, or other requirements. Fact Sheets are not exhaustive and are not intended to be a substitute for sound engineering or work practices.
The information in this appendix was adopted (with some modifications) from Safe Tank Entry When Entering Aboveground Storage Tanks (Fact Sheet, API, 2008).
Appendix D--Producing Ethanol from Cellulosic Materials
Cellulose is the primary component of plant cell walls and the most common organic compound on earth (RFA, 2011c). As of January 2014, five of the seven ethanol production facilities under construction or expansion will use cellulosic or waste feedstocks (RFA, 2014). Some types of cellulosic feedstocks being considered for ethanol production include:
- Agricultural residues. This includes material leftover in fields, such as stalks and leaves (e.g., corn stover, sugarcane bagasse), after a crop has been harvested.
- Wood residues. This includes woody material generated by the forestry industry (i.e., logging operations), primary wood processing mills (i.e., sawmills), secondary mills (i.e., paper mills), and urban wood residues (i.e., yard trimmings, construction debris).
- Municipal solid waste. This refers to household garbage, much of which is currently sent to landfills or incinerated. The portions of municipal solid waste that can be used as cellulosic feedstocks include wood, paper and paperboard products, yard trimmings, and many food scraps.
- Other energy crops. These include fast-growing trees and grasses grown specifically as renewable sources of cellulosic materials. The most commonly considered energy crops are tall, perennial grasses (e.g., switchgrass, miscanthus) and hybrid poplar trees. Energy crops can be planted on marginal lands unsuitable for food crop production.
Based on the above descriptions, cellulosic biomass has many potential advantages: it does not compete with the food supply; many sources are already available as waste streams from existing processes; and, it has the potential for creating new, dedicated sources of feedstocks.
Cellulosic biomass consists of three main components: cellulose, hemicellulose, and lignin (NREL, 2000 ). Cellulose is a complex polysaccharide and makes up 40 to 60 percent of cellulosic biomass by weight. Like starch, cellulose is a glucose polymer that can be broken down into glucose monomers. Unlike starch, the organization of the cellulose chains into strong, rigid, water-insoluble microfibrils makes it much more difficult to break down into fermentable sugars. Hemicellulose (20 to 40 percent of cellulosic biomass, by weight) is another polysaccharide that binds with cellulose microfibrils and links them together. Although hemicellulose is relatively easy to hydrolyze with its branched structure, its component sugars (which include five-carbon sugars and minor six-carbon sugars) are more difficult to ferment to ethanol. Lignin (10 to 24 percent by weight) surrounds the cellulose and hemicellulose and serves as a protective barrier, providing structure, impermeability, and resistance to bacteria and fungi. It also inhibits hydrolysis. Lignin is not a sugar-based molecule and therefore contributes no fermentable sugars to the process; however, residual lignin can be burned as a fuel (DOE, 2013; EPA, 2010).
Despite the challenges of biochemical fermentation of cellulosic feedstocks, many researchers are dedicated to finding ways to make this an efficient and cost-effective ethanol conversion pathway. To bypass the challenges associated with preparing cellulosic feedstocks for fermentation, other researchers are working on a radically different approach: harnessing the thermochemical gasification process originally developed to convert coal to liquid fuels. However, optimizing this technology for use with cellulosic biomass presents many challenges of its own. This section discusses both approaches–biochemical and thermochemical conversion–for producing ethanol from cellulosic feedstocks.
Compared to producing ethanol from corn, producing ethanol from cellulosic biomass is a more recently developed process. Thus, as of 2011, most of the technologies discussed in this section are only just being commercialized or are still in various pilot or research and development phases. A 2010 report identified 28 cellulosic ethanol production facilities operating in the United States and Canada. However, at the time that report was published, 20 of these facilities were pilot plants, five were demonstration plants, and only three were commercial demonstration plants. Of these facilities, 10 are using thermochemical technologies while the other 18 are pursuing biochemical technologies (EPA, 2010). The rest of this section will provide a detailed discussion of the production processes at both biochemical and thermochemical cellulosic ethanol facilities. However, note that because these technologies are not well established, the specifics of the unit operations may be more variable compared to corn milling facilities.
A. Biochemical Conversion
Biochemical conversion refers to processes that break down the cellulose into simple sugars and then ferment the sugars into ethanol. The sequence of manufacturing operations involves many of the same steps as the typical fermentation process utilized by corn milling facilities. However, increased chemical treatment is required to break the raw cellulosic materials down into fermentable sugars. Any of the cellulosic feedstocks described previously (e.g., corn stover, wood residues) could be used as raw materials in biochemical conversion processes. Table II.3 summarizes each of the production steps, primary inputs, primary outputs, and main operations discussed in this section. Figure II.23 illustrates the biochemical conversion process, while Figure II.24 shows conceptually how cellulosic material (and lignin) is broken down before arriving at fermentable sugars. This section is based on information from many references (e.g., EPA, 2010; Hahn-Hägerdal et al., 2006; Kumar et al., 2009; NREL, 2007a, 2010; and Wyman, 1999).
| Production Steps | Primary Inputs | Primary Outputs | Operations Performed |
|---|---|---|---|
|
Feedstock handling |
Cellulosic feedstock |
Milled, chipped, or shredded biomass |
- Mill, chip, or shred biomass to reduce its size |
|
Pretreatment (one of three approaches is applied) |
Milled, chipped, or shredded biomass |
Hydrolyzate |
Steam explosion approach: - Disrupt cellulosic structure by treating biomass with high-pressure, saturated steam |
|
Liquid hot water approach: - Break down cellulosic structure by treating biomass in a pressurized liquid vessel |
|||
|
Acid hydrolysis approach: - Use dilute sulfuric acid and high temperatures to hydrolyze hemicellulose while improving enzymatic access to cellulose. |
|||
|
Neutralization |
Hydrolyzate |
Neutralized hydrolyzate |
- Remove or inactivate fermentation inhibitors produced during pretreatment - Condition stream for further processing |
|
Cellulase production |
Enzymes |
Cellulase |
- Purchase cellulase from an outside vendor; or - Operate an on-site bioreactor to produce cellulase |
|
Saccharification |
Neutralized hydrolyzate |
Saccharified hydrolyzate |
- Add cellulase to break cellulose into glucose and other fermentable sugars |
|
Fermentation |
Saccharified hydrolyzate |
Fermented hydrolyzate |
- Direct saccharified hydrolyzate to fermentation tanks - Add microorganisms to ferment sugars into ethanol |
|
Distillation and dehydration |
Fermented hydrolyzate |
Denatured ethanol |
- Pump mixture into a continuous distillation system - Purify ethanol stream in rectifying column, molecular sieve, and other production equipment - Add denaturant to ethanol in cases where ethanol is not used for human consumption - Store denatured ethanol in tanks until distribution |
|
Stillage processing |
Stillage from distillation columns |
Liquid and solid co-products |
- Process stillage to form liquid and solid co-products for end users or for on-site energy recovery purposes |
Figure II.23. Biochemical Conversion of Cellulosic Feedstocks

Figure II.23. Biochemical Conversion of Cellulosic Feedstocks
Text version of Figure II.23. Biochemical Conversion of Cellulosic Feedstocks.
This chart shows cellulosic biomass being changed into feedstock handling, which goes to pretreatment, and then to neutralization. From neutralization, the next step is saccharification - there is also a path to saccharification immediately from cellulase production. After saccharification, the next step is fermentation, followed by distillation and dehydration. There are two paths from distillation and dehydration - one to ethanol, and another to stillage processing. From stillage processing, the last step is liquid and solid co-products.
The following are descriptions given after various stages of the chart:
- Feedstock handling
- Incoming feed is milled, chipped, or shredded to the appropriate size for further processing.
- Pretreatment
- Physical, chemical, or biological processing steps are applied to isolate the cellulose from the hemicellulose and lignin. The output of this step is a mixture known as hydrolyzate.
- Neutralization
- Fermentation inhibitors in the hydrolyzate are inactivated or removed to optimize the downstream yield.
- Cellulase production
- Cellulase - the enzyme used to break down cellulose - is either purchased from an outside vendor or produced and harvested onsite.
- Saccharification
- Cellulase breaks the cellulose down into glucose, and other simple sugars are also formed.
- Fermentation
- Different strains of yeast are used to convert the glucose and simple sugars into ethanol and carbon dioxide.
- Distillation and dehydration
- The ethanol is separated from other constituents and purified to product specifications.
- Stillage processing
- Products other than ethanol are further processed to meet end-user needs or are used (i.e., burned) onsite for purposes of energy recovery.
Figure II.24. Chemical Process During Biochemical Conversion of Cellulosic Feedstocks
Text version of Figure II.24. Chemical Process During Biochemical Conversion of Cellulosic Feedstocks.
The chart shows Cellulosic biomass being changed by enzymes into intermediates, and then by yeast and other additives into products.
Cellulosic biomass is a mixture of cellulose, hemicellulose, and lignin. Intermediates are a mixture of fermentable 6-carbon sugars (e.g., glucose), fermentable 5-carbon sugars (e.g., xylose), and residual lignin. Products are a mixture of ethanol, other alcohols, carbon dioxide, and residual lignin.
-
Feedstock Handling
Incoming feedstock typically undergoes a size-reduction step for ease of material handling and increased efficiency during production. Depending on the feedstock, the biomass might be milled, chipped, or shredded. -
Pretreatment
Pretreatment, in the context of cellulosic-based ethanol manufacturing, refers to physical, chemical, or biological treatment applied to the biomass for the purposes of disrupting the lignin structure and exposing the cellulose for subsequent enzyme hydrolysis. Pretreatment typically removes or hydrolyzes the hemicellulose. Ideally, pretreatment will be conducted in a manner that minimizes the loss of fermentable sugars and avoids forming by-products that inhibit downstream processes, all while improving the ultimate sugar yield without being cost-prohibitive. Fermentation inhibitors are produced during pretreatment due to sugar degradation, lignin degradation, and the release of natural compounds from the feedstock. Categories of fermentation inhibitors include weak acids, furan derivatives, and phenolic compounds. These inhibitors are generally toxic to fermenting microorganisms and decrease their viability and productivity. The types and quantities of fermentation inhibitors produced are highly variable based on the type of pretreatment selected. The output of pretreatment is known as a hydrolyzate, which refers to the fact that some of its components are a product of hydrolysis. The hydrolyzate is typically a slurry of liquid and solid components. There are multiple methods of pretreatment, and the most appropriate method depends on the type and composition of the cellulosic raw material. A few of the most common types of pretreatment are discussed below (although many other methods are under development).-
Steam Explosion
This method involves first treating the biomass with high-pressure, saturated steam. The temperature of the steam is typically 160°C to 260°C, and the pressure is usually between 100 to 700 pounds per square inch (psi). This treatment lasts for up to several minutes. The pressure is then rapidly reduced to atmospheric pressure, causing the material to undergo an explosive decompression. The high temperature and mechanical turbulence disrupts the cellulosic structure. However, this process also creates fermentation inhibitors. There are several variants of this process, which all take advantage of the same explosive reduction in pressure:- Substituting liquid ammonia for steam is known as ammonia fiber explosion (AFEX). The liquid ammonia (refer to 29 CFR 1910.119 Appendix A for PSM applicability) is typically applied at a lower temperature (90°C) and for a longer period of time (30 minutes) than the steam. AFEX is very effective at hydrolyzing cellulose and hemicellulose (e.g., grasses) but has relatively poor performance with high-lignin materials (e.g., wood). This process does not produce fermentation inhibitors.
- Substituting steam with supercritical CO2 is cost-effective and does not form inhibitory compounds. In addition, CO2 explosion operates at low temperatures, which reduces sugar degradation. However, this pretreatment approach does not modify the hemicellulose or lignin.
-
Liquid Hot Water
This method uses high pressure to keep water in a liquid state at temperatures ranging between 200°C and 230°C. The biomass is treated for around 15 minutes, which dissolves between 40 and 60 percent of the total biomass, including all of the hemicellulose, 35 to 60 percent of the lignin, and 4 to 22 percent of the cellulose.
-
Acid Hydrolysis
This method uses dilute sulfuric acid to effectively hydrolyze hemicellulose while improving enzymatic access to the cellulose. As hemicellulose is removed, glucose yields from cellulose hydrolysis increase. However, this method is more expensive than steam explosion and may interfere with subsequent enzymatic digestion. Acid hydrolysis typically is conducted at relatively high temperatures.
-
-
Neutralization
The purpose of neutralization is to condition the product of pretreatment, the hydrolyzate, and remove or inactivate compounds produced in the pretreatment step that are fermentation inhibitors (e.g., organic acids, furans, phenolic compounds). The extent to which neutralization is required depends on the type of pretreatment conducted. Different types of pretreatment create different types of fermentation inhibitors and require different modes of detoxification or conditioning. Some pretreatments, such as AFEX, create no fermentation inhibitors as by-products.In a common type of neutralization known as "over liming," calcium hydroxide [Ca(OH)2] is added to the liquid fraction of the hydrolyzate to counter the effects of added acid. The reaction between the lime and H2SO4 precipitates gypsum, which can then be extracted. Acetic acid generated during pretreatment can be neutralized by adding ammonia, and ion-exchange columns may also be used to remove selected contaminants. Overall, neutralization increases process costs and may cause some sugar loss. However, it can improve ethanol yield from fermentation because the fermenting microorganisms are more productive in the absence of toxic fermentation inhibitors.
-
Cellulase Production
The enzyme that breaks down cellulose, known as cellulase, can either be purchased from commercial suppliers or produced and harvested on site. Enzymes are typically very expensive, so producing cellulase as part of the production process may ultimately reduce costs. Although research and development efforts have dramatically lowered enzyme costs in recent years, the cellulase enzyme is still expensive compared to the enzymes used for corn ethanol production.Cellulase can be produced in a bioreactor using an organism such as the fungus Trichoderma reesei. The fungus (or other organism) is supplied with a portion of the neutralized hydrolyzate, and it consumes those sugars to produce cellulase. Ammonia is used to control the pH and provide fixed nitrogen, a compressor pumps air into the bioreactor (providing the necessary oxygen), and other nutrients are added as needed. Rather than purifying the cellulase, the entire bioreactor broth is diverted to cellulose hydrolysis. This eliminates additional enzyme processing costs and returns unconsumed sugars to the process stream.
-
Saccharification (Cellulose Hydrolysis)
During saccharification, cellulase catalyzes the hydrolysis of cellulose into glucose. As in the production of corn ethanol, saccharification involves breaking down a polysaccharide into simple, fermentable sugars. Saccharification typically begins at higher temperatures, at which the cellulase enzymes are most active. Saccharification can take up to five days. The product of saccharification continues to be called hydrolyzate. This hydrolyzate contains a greater proportion of soluble sugars compared to the hydrolyzate from pretreatment, but solids such as lignin are still present. -
Fermentation
The hydrolyzate from saccharification is directed to fermentation tanks. During fermentation, microorganisms convert sugars to ethanol. Unlike in starch fermentation where glucose is the only sugar in the hydrolyzate, cellulosic hydrolyzate contains a mix of glucose, other six-carbon sugars (e.g., mannose and galactose), and five-carbon sugars (e.g., xylose and arabinose). This presents a challenge because the yeast Saccharomyces cerevisiae, the most commonly used microorganism for industrial fermentation, does not metabolize five-carbon sugars. In addition, there may be inhibiting compounds that interfere with the fermenting microorganisms. These inhibitors would be present if they were not removed during neutralization or if they were inadvertently formed during saccharification. These and other factors make fermentation much more difficult for cellulosic feedstocks compared to sugar or starch feedstocks.Thus, research efforts have focused on developing special (recombinant) strains of yeast and bacteria that can convert all of the mixed sugars into ethanol. Scientists have successfully engineered strains of the bacteria Escherichia coli, Klebsiella oxytoca, and Zymomona mobilis as well as a strain of S. cerevisiae that can ferment the five-carbon sugars, xylose, and arabinose. Some of these strains have also been engineered for resistance to certain inhibitory compounds, such as acetic acid. However, as an alternative to using genetically engineered fermenting microorganisms to co-ferment five- and six-carbon sugars, the two types of sugars can be fermented separately. Following pretreatment, the liquid hydrolyzate (containing the five-carbon sugars) can be separated from the solids (containing six-carbon sugars). Fermenting these streams separately increases ethanol yield, but additional equipment and water are required.
Some facilities use simultaneous saccharification and fermentation (SSF). During SSF, cellulose hydrolysis and fermentation occur in the same reactor. Both the cellulase enzyme and the fermenting organism are working simultaneously so that the fermenting organism can metabolize sugars as soon as they become available. A benefit to this approach is that, because glucose actually inhibits cellulase action, immediately metabolizing glucose allows enzymatic action to proceed more quickly than it would in a separate hydrolysis step. This is true despite non-optimal temperatures for the enzymes (due to the lower temperature requirements of the fermenting organism). In addition, the fermentation process actually detoxifies some types of inhibitors (e.g., carbonyl compounds), thereby reducing the inhibitory pressure on the enzymes.
-
Distillation and Dehydration
The process stream from the fermentation tank follows a similar distillation and dehydration process as described earlier for corn dry-milling (II.B.1.viii). -
Stillage Processing
The spent solids removed during the first step of the distillation process (stillage) can be used for energy recovery, typically by collecting the stillage, drying it, and burning it. The stillage, consisting primarily of insoluble lignin but also including enzymes and fermenting organisms, is pumped to an evaporator where much of the water is removed. The solids are then mechanically dewatered (e.g., using a pressure filter or a screw press) to form a dry cake. The liquid portion from mechanical dewatering is concentrated in a series of two evaporators to form highly concentrated syrup. The syrup and cake are used as boiler fuel to generate heat and electricity for the facility.Wastewater from the stillage and other processes undergoes aerobic and anaerobic waste treatment processes. The clarified water is recycled back into the process, and the sludge is dewatered and also used as boiler fuel.
B. Thermochemical Conversion
The three ethanol production methods reviewed earlier (corn dry-milling, corn wet-milling, and biochemical conversion) all share the common feature of breaking raw materials down to simple sugars, and then relying on yeast to convert glucose into ethanol. Thermochemical conversion takes an entirely different approach than the three methods discussed so far. Table II.4 summarizes each of the production steps, primary inputs, primary outputs, and main operations discussed in this section. This section is based on information from many references (e.g., BRI Energy, LLC and Bioengineering Resources, Inc., 2005; NETL 2002, 2011; and NREL, 2007a, 2007b).
Thermochemical conversion operates under the following premise: Rather than breaking down starch or cellulose into their constituent sugars, the biomass is converted to a mixture of gases at high temperatures, and these gases are then fed to a reactor equipped with catalysts that convert the gases into ethanol and other products (the process diagram in Figure II.25 and the conceptual reaction illustration in Figure II.26. Traditionally, thermochemical conversion was developed for use with coal, but any carbonaceous feedstock can be used in this process. Thus, any of the cellulosic feedstocks described previously (e.g., corn stover, wood residues) could be implemented in this process. However, thermochemical conversion is particularly valuable for lignin-rich forest products (since lignin is not utilized by the biochemical process) as well as for heterogeneous feedstocks, such as municipal solid waste.
| Production Steps | Primary Inputs | Primary Outputs | Operations Performed |
|---|---|---|---|
|
Feedstock preparation |
Cellulosic biomass |
Reduced-sized feedstock |
- Reduce incoming feedstock size and moisture content to meet the requirements of the gasification system |
|
Gasification |
Reduced-size feedstock |
Syngas |
- Thermally decompose processed feedstock in high-temperature fixed bed or fluidized bed reactors |
|
Tar reformation, cleanup, and conditioning |
Syngas |
Clean syngas |
- Use multiple separation processes to remove impurities (e.g., tar, ammonia) from syngas and to condition syngas for further processing - Compress syngas for further processing |
|
Ethanol synthesis (two approaches used) |
Clean syngas |
Ethanol |
Use of metal catalysts: - Use a metallic catalyst to convert syngas constituents to mixed alcohols in a fixed bed reactor - Separate condensed alcohols from unconverted syngas and recycle syngas to a reactor - Use multiple separation processes to separate methanol, ethanol, and higher alcohols |
|
Use of fermentation: - Feed syngas to bioreactor to form ethanol using certain anaerobic bacteria strains - Remove dilute ethanol stream through a membrane - Distill and dehydrate the liquid process stream to purify the ethanol and separate co-products |
Figure II.25. Thermochemical Conversion of Cellulosic Biomass to Ethanol and Co-products
Text version of Figure II.25, Thermochemical Conversion of Cellulosic Biomass to Ethanol and Co-products.
A chart shows a cellulosic biomass with an arrow to feedstock preparation. After that, an arrow leads to gasification, and then tar reformation, cleanup, and conditioning. From that stage, one arrow diverges to become liquid and solid co-products, and another continues to ethanol synthesis. Ethanol synthesis leads to ethanol and co-products.
The following are descriptions given after various stages of the chart:
- Feedstock preparation
- Incoming feed is milled, chipped, pelletized, or shredded to the appropriate size for further processing; the feed usually is dried as well.
- Gasification
- The feed enters a high-temperature reactor which causes the feed constituents to thermally decompose and form "syngas," which is the main ingredient needed for ethanol formation.
- Tar reformation, cleanup, and conditioning
- Unwanted constituents in the syngas (e.g., tar, particulates, metals, hydrogen sulfide) are removed by multiple physical and chemical separation processes.
- Ethanol synthesis
- Syngas passes through a catalytic reactor or fermentation reactor to form several low molecular weight alcohols, which are separated into streams of ethanol and other alcohols.
Figure II.26. Chemical Process During Thermochemical Conversion of Cellulosic Feedstocks
Text version of Figure II.26. Chemical Process During Thermochemical Conversion of Cellulosic Feedstocks.
The chemical process shows the cellulosic biomass undergoing a change to a syngas due to a gasifying agent. After that, a metallic catalyst or bacteria changes it into products.
Cellulosic biomass can be a mixture of cellulose, hemicellulose, and lignin. Syngas can be a mixture of Carbon monoxide gas, hydrogen gas, and many other minor constituents. Products can be a mixture of ethanol, other alcohols, and many other minor constituents.
-
Feedstock Preparation
Incoming feedstock is reduced to the required size for handling by the gasification system. Depending on the type of gasifier (i.e., gasification reactor) that will be used, the required particle size can vary from fine to coarsely chipped. Some types of feedstock such as straw might need to be pelletized to be accommodated by mechanical handling systems. Typically, the feedstock also passes through a drier (oftentimes fed by recycled process air) to reduce the moisture content. High moisture content reduces gasification efficiency. -
Gasification
Gasification refers to the thermal decomposition of organic materials at elevated temperatures (around 1,600°F) and in reducing conditions. Oxygen, air, or steam can be used as a gasifying agent. The primary product of gasification is syngas (a mixture of carbon monoxide [CO] and hydrogen [H2] gases). Numerous minor products can be found in syngas, and these generally include water, char, and other condensibles. This OTM chapter does not discuss the complex chemistry of the numerous reactions involved in syngas formation.There are two main classes of gasifiers: direct and indirect. Directly heated or partial oxidation (POx) gasifiers generate the heat required for gasification inside the reactor from the partial combustion of the feedstock. In other words, the exothermic reactions between oxygen and biomass (combustion) are used to fuel the endothermic gasification reactions. This type of reactor typically requires the direct injection of oxygen when operating with biomass in order to avoid excessive nitrogen buildup.
Indirect gasifiers transfer heat to the gasifier from an external source. For this process to be efficient, the external source of heat is generated through the combustion of by-product char or product gas created during gasification. To manage heat transfer, an inert solid (such as olivine sand) can be circulated between the gasification reactor and the char combustor. Indirect gasifiers usually operate with steam. Eliminating the need to supply oxygen reduces capital costs and efficiency losses.
Most gasification occurs in one of two general types of reactors. In a fixed-bed reactor, the gasifying agent (oxygen or air) flows up or down through a fixed pile of biomass. In a fluidized-bed reactor, biomass is added to a fluid bed: small particles of an inert material suspended in air through the injection of the gasifying agent (i.e., air, oxygen, or steam). The bed is considered "fluidized" because the particles in gas behave like a liquid. In a bubbling fluidized-bed reactor, the fluid bed is located in one region of the reactor, and particles are unable to escape from the fluid bed due to low gas velocities. In a circulating fluidized-bed reactor, higher gas velocities carry the fluid bed throughout the reactor. Particles remain entrained with the gas so that inert bed material exits with the syngas, where it is separated via cyclone and recirculated to the reactor. In an entrained flow reactor, the biomass is finely ground so that it becomes entrained with the flow of gas in the reactor.
Different gasifier designs have different advantages and disadvantages–such as cost, tar yield, sensitivity to moisture, and volume potential–and may be better suited to some feedstocks more than others. Overall, fluidized bed reactors have greater potential for use with biomass conversion than fixed bed reactors.
-
Syngas Processing
–Syngas processing occurs after the syngas exits the reactor, and includes three stepstar reformation, gas cleanup, and gas conditioning that is designed to remove unwanted constituents from the syngas before alcohol synthesis occurs. Specifically, the syngas must be cleaned and conditioned before being converted to ethanol or other types of fuels. Contaminants in the syngas include tars (i.e., heavy hydrocarbons), particulates, acid gases, ammonia, and alkali metals.Tars can be removed via scrubbers or filters, but this is costly and creates an additional waste stream. Current research on tar reformation is focused on developing a cost-efficient catalytic tar cracking process that converts tar into additional syngas. This process takes place in a high-temperature reactor, with the catalyst forming a fluidized bed. The catalyst must be continually regenerated due to partial deactivation by sulfur poisoning. A catalytic tar reformer can also convert ammonia to nitrogen and hydrogen gas.
Following tar reformation, the gas is cooled and passed through a wet scrubber system to remove particulates and any residual tar or ammonia. The syngas is then compressed in a centrifugal compressor. Next, the syngas must be conditioned to remove hydrogen sulfide (H2S) and CO2. Acceptable levels of these gases in the syngas vary depending on the fuel synthesis catalyst used. The gases are typically removed in an acid gas scrubber or absorber column using physical or chemical solvents. Typically, the sulfur is extracted from the isolated H2S for purification and sale. Finally, the syngas may undergo water-gas shift to optimize the ratio of hydrogen to carbon monoxide prior to alcohol synthesis. The syngas remains in a gaseous state throughout the cleanup and conditioning process.
-
Ethanol Synthesis
Syngas is typically converted to ethanol using a catalytic reactor, although the more recently developed process of syngas fermentation is also briefly discussed.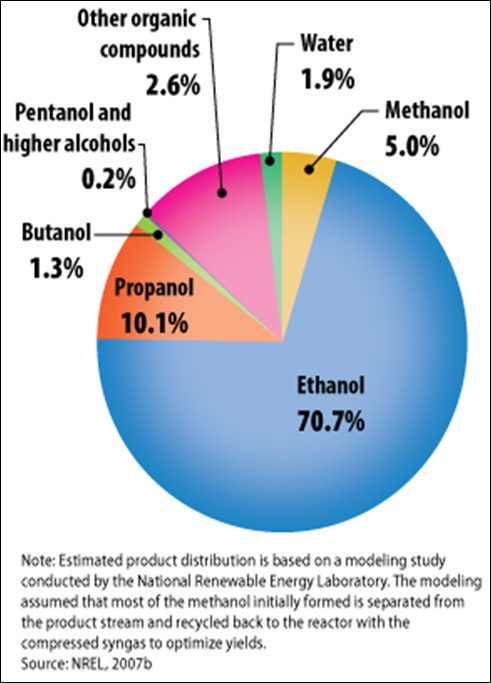
Figure II.27. Estimated Mixed Alcohol Product Distributions for Thermochemical Conversion of Cellulosic Feedstock
Figure II.27. Estimated Mixed Alcohol Product Distributions for Thermochemical Conversion of Cellulosic Feedstock
Text version of Figure II.27, Estimated Mixed Alcohol Product Distributions for Thermochemical Conversion of Cellulosic Feedstock.
- Methanol - 5.0%
- Ethanol - 70.7%
- Propanol - 10.1%
- Butanol - 1.3%
- Pentanol and higher alcohols - 0.2%
- Other organic compounds - 2.6%
- Water - 1.9%
Note: Estimated product distribution is based on a modeling study conducted by the National Renewable Energy Laboratory. The modeling assumed that most of the methanol initially formed is separated from the product stream and recycled back to the reactor with the compressed syngas to optimize yields.
Source: NREL, 2007b
-
Catalysis
Prior to entering the alcohol synthesis reactor, the clean syngas is further compressed and heated to 570°F. The syngas enters a fixed-bed reactor where it is converted to mixed alcohols with a metallic catalyst. Many metallic catalysts are being investigated for this process, and some known examples include catalysts containing iron, nickel, cobalt, and rhodium in various amounts. The product gas exits the reactor where it is cooled so that the condensed alcohols can be separated from unconverted syngas. The mixed alcohols are sent to a purification area while the unconverted syngas is recycled to the tar reformer (in order to prevent buildup of CO2 in the synthesis reactor).The composition of the mixed alcohol product varies depending on the type of feedstock and catalyst used (see an estimated product distribution in Figure II.27). Some types of catalysts have greater ethanol selectivity than others, although no catalyst creates pure ethanol. Other alcohols produced include methanol, propanol, butanol, and pentanol. To increase the proportions of ethanol and higher alcohols that are created, research is underway on the potential impact of recycling the methanol to the alcohol synthesis reactor. Recycling methanol would theoretically yield a final product mixture containing 5 percent methanol and 71 percent ethanol, by weight, whereas a traditional approach has proven to yield a mixture containing 31 percent methanol and 46 percent ethanol, by weight. In both cases, propanol makes up an additional 10 to 13 percent of the mixture, and remaining components include higher alcohols, acetates, water, and other (unspecified) compounds. Another consideration is that different catalysts have different tolerances to contaminants such as sulfur, which affects how thoroughly the syngas must be conditioned and the rate at which the catalyst must be recharged or replaced.
Besides mixed alcohols, catalytic conversion of syngas can also be used to generate certain hydrocarbon mixtures, including diesel and gasoline (Fisher-Tropsch Synthesis). In some cases, the same catalyst can produce variable products depending on physical parameters such as pressure in the reactor; for example, molybdenum-disulfide catalysts help produce alcohols when operating under high pressures, but they produce hydrocarbon mixtures when operating under lower pressures.
Following catalysis, the mixed alcohols are depressurized and then dehydrated with vapor-phase molecular sieves. The dehydrated alcohol feed is then separated into a methanol/ethanol stream and a higher alcohol stream in an alcohol separation column. The ethanol is subsequently separated from the methanol. As described previously, development of a methanol recycling process is underway to increase ethanol and higher alcohol production. Higher alcohols are valuable commodity chemicals and fuel additives.
-
Syngas Fermentation
As an alternative to the catalytic conversion of syngas to alcohols, syngas can be converted to ethanol via fermentation. Certain strains of anaerobic bacteria, such as Clostridium ljungdahlii, are able to metabolize syngas into ethanol. Syngas is bubbled into the bioreactor and other nutrients are added as needed. The dilute ethanol stream produced is removed through a membrane, which retains the bacteria. The liquid process stream undergoes conventional distillation and dehydration to purify the ethanol, while the water and nutrients are recycled to the bioreactor.
References
Anku, L.R. 1993. The Meaning of the Word "Entrapment" as in the Permit-Required Confined Space (PRCS) Standard [1910.146]. Standard Interpretation Letter from Linda R. Anku, Regional Administrator, OSHA, to Michael J. Shanshala, Safety Director, United Refining Company. October 8, 1993. Available at /laws-regs/standardinterpretations/1993-10-08.
Associated Press. 2009a. Workers Hurt in Ethanol Plant Explosion. Available at www.1011now.com/news/headlines/40849542.html.
Bartok, J.W., Jr. 2004. Approximate Heating Value of Common Fuels. Storrs, CT: University of Connecticut. Available at www.hrt.msu.edu/energy/pdf/heating%20value%20of%20common%20fuels.pdf.
BeMiller, J. and R. Whistler. 2009. Starch Chemistry and Technology. 3rd edition. Burlington, MA: Elsevier, Inc.
BRI Energy, LLC and Bioengineering Resources, Inc. 2005. Gasification-Fermentation Pilot Facility (Arkansas). Available at www.lacity-alternativetechnology.org/PDF/GasificationFermentation_Arkansas.pdf
Brisman, J., M.J. Nieuwenhuijsen, K.M. Venables, V. Putcha, S. Gordon, and A.J.N. Taylor. 2004. Exposure-response Relations for Work Related Respiratory Symptoms and Sensitisation in a Cohort Exposed to a-Amylase. Occupational & Environmental Medicine 61(6):551-553. Available at www.ncbi.nlm.nih.gov/pmc/articles/PMC1763635/pdf/v061p00551.pdf.
Cahill, B. Huron, S.D. 2000. Identifies victim of ethanol plant fire. American News. 23 August.
[CCPS] Center for Chemical Process Safety. 2006. The Business Case for Process Safety. Available at www.aiche.org
Chambliss, R.P. 2009. Bartow Ethanol Reopens After Explosion, Fire. The Ledger; 12 November. Available at http://www.theledger.com/news/20091112/bartow-ethanol-reopens-after-explosion-fire.
Chang, J.I., and C-C. Lin. 2005. A Study of Storage Tank Accidents. Journal of Loss Prevention in the Process Industries 19:51-59. Available at htpp://xa.yimg.com/kq/groups/3862917/1523127472/name/StorageTankFiresStudy.pdf.
Cralley, L.V., L.J. Cralley. 1985. Industrial Hygiene Aspects of Plant Operations.
Corn Refiners Association. 2011. The Corn Refining Process. Available at www.corn.org/wp-content/uploads/2009/11/CornRefiningProcess.pdf.
Dale, R.T. and W.E. Tyner. 2006. Economic and Technical Analysis of Ethanol Dry Milling: Model Description. Staff Paper #06-04. West Lafayette, IN: Department of Agricultural Economics, Purdue University. Available at www.ageconsearch.umn.edu/bitstream/28674/1/sp060004.pdf.
Davis, K.S. 2001. Corn Milling, Processing and Generation of Co-products. Paper presented at the Minnesota Nutrition Conference. Available at www.distillersgrains.org/files/grains/k.davis--dry&wetmillprocessing.pdf.
[DOE] Department of Energy. 2009. Secretaries Chu and Vilsack Announce More Than $600 Million Investment in Advanced Biorefinery Projects. Available at https://energy.gov/articles/secretaries-chu-and-vilsack-announce-more-600-million-investment-advanced-biorefinery.
[DOE] Department of Energy. 2013. Biofuels Basics. Available at https://www.energy.gov/eere/bioenergy/biofuels-basics.
[EPA] Environmental Protection Agency. 1995. Corn Wet Milling. AP-42, Fifth edition, Volume I. Available at http://www.epa.gov/ttn/chief/ap42/ch09/final/c9s09-7.pdf.
[EPA] Environmental Protection Agency. 2005. Managing Your Environmental Responsibilities: A Planning Guide for Construction and Development A Planning Guide for Construction and Development. Available at https://www.epa.gov/nps.
[EPA] Environmental Protection Agency. 2007a. Environmental Laws Applicable to Construction and Operation of Ethanol Plants. EPA-907-B-07-001. Available at https://nepis.epa.gov/Exe/ZyPDF.cgi?Dockey=P100L386.txt.
[EPA] Environmental Protection Agency. 2007b. Control of Hazardous Air Pollutants from Mobile Sources: Final Rule to Reduce Mobile Source Air Toxics. EPA-420-F-07-017. Available at https://www.epa.gov/mobile-source-pollution/final-rule-control-hazardous-air-pollutants-mobile-sources.
[EPA] Environmental Protection Agency. 2010. Renewable Fuel Standard Program (RFS2) Regulatory Impact Analysis. EPA-420-R-10-006. Assessment and Standards Division, Office of Transportation and Air Quality, Washington, D.C. Available at https://www.epa.gov/oms/fuels/renewablefuels/regulations.htm.
[EPA] Environmental Protection Agency. 2011. Connecticut Chemical Distributor Agrees to Pay $164,000 for Clean Air and Right-to-Know Violations. Available at https://yosemite.epa.gov/opa/admpress.nsf/1e5ab1124055f3b28525781f0042ed40/9af2066507a341be85257856005b686d!OpenDocument.
[ePURE] European Renewable Ethanol. 2011. Renewable Ethanol: Producing Food and Fuel (fact sheet). Available at https://www.epure.org.
[ERG] Eastern Research Group, Inc. 2010. Combustible Dust Stakeholder Meeting, Washington, D.C., Meeting Summary Report, December 14, 2009. Prepared for U.S. Department of Labor, Occupational Safety and Health Administration, Directorate of Standards and Guidance. Available at www.osha.gov/combustible-dust/meeting-summary-12-14-2009.
[ERG] Eastern Research Group, Inc. 2011. Combustible Dust Expert Forum, Washington, D.C., May 13, 2011, Meeting Summary Report. Prepared for U.S. Department of Labor, Occupational Safety and Health Administration, Directorate of Standards and Guidance. Available at www.osha.gov/combustible-dust/meeting-summary-05-13-2011.
FM Global. 2009a. Prevention and Mitigation of Explosible Dust Explosion and Fire. Data sheet no. 7-76; March.
FM Global. 2009b. Belt conveyors. Data sheet no. 7-11; October.
FM Global. 2010a. Grain storage and milling. Data sheet no. 7-75; May.
FM Global. 2010b. Dust collectors and collection systems. Data sheet no. 7-73; May.
Galitsky, C., E. Worrell, and M. Ruth. 2003. Energy Efficiency Improvement and Cost Saving Opportunities for the Corn Wet Milling Industry: An ENERGY STAR® Guide for Energy and Plant Managers. Sponsored by the U.S. Environmental Protection Agency. LBNL-52307. Berkeley, CA: Ernest Orlando Lawrence Berkeley National Laboratory. Available at https://www.energystar.gov/buildings/tools-and-resources/energy-efficiency-improvement-and-cost-saving-opportunities-corn-wet-milling.
Galvan, A. 2008. 3 Men Burned in Corn Dust Explosion. The Arizona Republic; 29 December. Available at http://archive.azcentral.com/community/chandler/articles/2008/12/29/20081229gr-explosion1229.html.
Gardiner, D.P., M.F. Bardon, and M. LaViolette. 2010. An Experimental and Modeling Study of the Flammability of Fuel Tank Headspace Vapors from Ethanol/Gasoline Fuels. Phase 2: Evaluations of Field Samples and Laboratory Blends. NREL/SR-540-47819. Golden, CO: National Renewable Energy Laboratory. Available at www.nrel.gov/docs/fy10osti/47819.pdf.
Gardiner, D.P., M.F. Bardon, and G. Pucher. 2008. An Experimental and Modeling Study of the Flammability of Fuel Tank Headspace Vapors from High Ethanol Content Fuels. NREL/SR-540-44040. Golden, CO: National Renewable Energy Laboratory. Available at www.nrel.gov/docs/fy09osti/44040.pdf.
Geddie, J.E. 2009. A Guide to Combustible Dusts. Raleigh, NC: N.C. Department of Labor, Occupational Safety and Health Division. Available at www.nclabor.com/osha/etta/indguide/ig43.pdf.
Grisso, R., S. Gay, G. Hetzel, and B. Stone. 2005. Farmer's Lung: Causes and Symptoms of Mold and Dust Induced Respiratory Illness. Publication 442-602. Blacksburg, VA: Virginia Cooperative Extension, Biological Systems Engineering. Available at nasdonline.org/static_content/documents/1862/d001796.pdf.
Hahn-Hägerdal, B., M. Galbe, M.F. Gorwa-Grauslund, G. Lidén, and G. Zacchi. 2006. Bio-ethanol – the Fuel of Tomorrow from the Residues of Today. Trends in Biotechnology 24(12):549-556. Available at pubmed.ncbi.nlm.nih.gov/17050014.
Hood, E. 2004. Lessons Learned? Chemical Plant Safety since Bhopal. Environmental Health Perspectives 112(6):A352-A359. Available at www.ncbi.nlm.nih.gov/pmc/articles/PMC1241982/pdf/ehp0112-a00352.pdf.
Houba, R., D.J. Heederik, G. Doekes, P.E. van Run. 1996. Exposure Sensitization Relationship for alpha-Amylase Allergens in the Baking Industry, Am J Respir Crit Care Med 1996; 154, 130-6. Available at pubmed.ncbi.nlm.nih.gov/8680668.
[HSE] Health and Safety Executive. 2010. A Study to Investigate Ways to Reduce the Dustiness of Bakery Ingredients and Exposure to Allergens, HSE RR8302010. Available at www.hse.gov.uk.
[IAFC] International Association of Fire Chiefs. 2008. Ethanol Fixed Facilities: Assessment and Guide. 1st Edition. Available at www.iafc.org/files/progsEERC_EthanolFixedFacilitiesGuide.pdf.
Jacques, K.A., T.P. Lyons, and D.R. Kelsall. 2003. The Alcohol Textbook: A Reference for the Beverage, Fuel and Industrial Alcohol Industries. 4th edition. Nottingham, UK: Nottingham University Press.
Jessen, H. 2011a. Explosion at Illinois Ethanol Plant Injures Three Employees. Ethanol Producer Magazine; 12 April. Available at www.ethanolproducer.com/articles/7655/explosion-at-illinois-ethanol-plant-injures-three-employees.
Kirkwood Community College. 2008. Combustible Dust: Safety and Injury Prevention Instructors Manual. Version 1.0. Produced under OSHA grant number SH-17797-08-60-F-19. Available at www.osha.gov/sites/default/files/2018-12/fy08_sh-h-08_cd_instructor_manual.pdf.
Kotz, J.C., P. Treichel, J.R. Townsend. 2009. Chemistry and Chemical Reactivity. 7th Edition. Canada: Thomson Brooks/Cole.
Kumar, P., D.M. Barrett, M.J. Delwiche, and P. Stroeve. 2009. Methods for Pretreatment of Lignocellulosic Biomass for Efficient Hydrolysis and Biofuel Production. Industrial Engineering and Chemistry Research 48:3713-3729. Available at ucce.ucdavis.edu/files/datastore/234-1388.pdf.
LaPrade, J. 2008. Farm Safety Series: Grain Bin Hazards and Safety Consideration. ANR-1332. Alabama Cooperative Extension System; June. Available at www.aces.edu/pubs/docs/A/ANR-1332/ANR-1332.pdf.
Mosier, N.S., and K. Ileleji. 2006. How Fuel Ethanol is Made from Corn. ID-328. Purdue Extension. Available at https://www.extension.purdue.edu/extmedia/ID/ID-328.pdf.
Myers, P.E. 1997. Above Ground Storage Tanks. McGraw-Hill Companies, Inc.
[NETL] National Energy Technology Laboratory. 2002. Benchmarking Biomass Gasification Technologies for Fuels, Chemicals and Hydrogen Production. Available at http://www.netl.doe.gov.
[NETL] National Energy Technology Laboratory. 2010. Archer Daniels Midland Company: CO2 Capture from Biofuels Production and Sequestration into the Mt. Simon Sandstone. Available at www.netl.doe.gov/publications/factsheets/project/FE0001547.pdf.
[NETL] National Energy Technology Laboratory. 2011. Gasifipedia. Available at http://www.netl.doe.gov/.
[NFPA] National Fire Protection Association. 2010. NFPA 91 Standard for Exhaust Systems for Air Conveying of Vapors, Gases, Mists, and Noncombustible Particulate Solids, 2004 edition. Available at www.nfpa.org.
[NFPA] National Fire Protection Association. 2011a. FAQ – NFPA 704. Identification of the Hazards of Materials for Emergency Response. Available at www.nfpa.org
[NFPA] National Fire Protection Association. 2011b. All About Fire (from "A Reporter's Guide to Fire and the NFPA"). Available at www.nfpa.org.
[NFPA] National Fire Protection Association. 2012. NFPA 30: Flammable and Combustible Liquids Code. Available at www.nfpa.org.
[NFPA] National Fire Protection Association. 2012a. NFPA 497. Recommended Practice for the Classification of Flammable Liquids, Gases, or Vapors and of Hazardous (Classified) Locations for Electrical Installations in Chemical Process Areas
[NFPA] National Fire Protection Association. 2013. NFPA 654: Standard for the Prevention of Fire and Dust Explosions from the Manufacturing, Processing, and Handling of Combustible Particulate Solids, 2006 edition. Available at www.nfpa.org.
[NFPA] National Fire Protection Association. 2013a. NFPA 61: Standard for the prevention of fires and dust explosions in agricultural and food processing facilities, 2008 edition. Available at www.nfpa.org.
[NFPA] National Fire Protection Association 2013b. NFPA 68: Standard on Explosion Protection by Deflagration Venting, 2007 edition. Available at www.nfpa.org.
[NFPA] National Fire Protection Association. 2014. NFPA 69: Standard on Explosion Prevention Systems, 2008 edition. Available at www.nfpa.org.
[NFPA] National Fire Protection Association. 2014. NFPA 70: National Electric Code, 2008 edition. Available at www.nfpa.org.
[NIOSH] National Institute for Occupational Safety and Health. 1987. Preventing Entrapment and Suffocation Caused by the Unstable Surfaces of Stored Grain and Other Materials. NIOSH Alert; December. DHHS (NIOSH) Publication No. 88-102. Available at www.cdc.gov/niosh/docs/88-102/.
[NIOSH] National Institute for Occupational Safety and Health. 1994. Assistant Grain Elevator Supervisor Dies After Being Engulfed in Shelled Corn, North Carolina, September 11, 1994. FACE Report No. 1994-16. Available at www.cdc.gov/niosh/face/in-house/full9416.html.
[NIOSH] National Institute for Occupational Safety and Health. 2002. Feed Mill Worker Dies in Grain Bin. FACE Report No. 02NY012. Available at www.cdc.gov/niosh/face/stateface/ny/02ny012.html.
[NIOSH] National Institute for Occupational Safety and Health. 2010a. Catastrophic Incidents in Manufacturing. DHHS (NIOSH) Publication No. 2010-149. Available at www.cdc.gov/niosh/docs/2010-149/.
[NIOSH] National Institute for Occupational Safety and Health. 2010b. Asthma and Allergies, Prevention of Occupational Asthma: Primer. Available at www.cdc.gov/niosh/topics/asthma/OccAsthmaPrevention-primer.html.
Nolan, D.P. 1996. Handbook of Fire and Explosion Protection Engineering Principles for Oil, Gas, Chemical, and Related Facilities. Westwood, NJ: Noyes Publications. 310 pp.
[NREL] National Renewable Energy Laboratory. 2000. Determining the Cost of Producing Ethanol from Corn Starch and Lignocellulosic Feedstocks. NREL/TP-580-28893. Available at www.nrel.gov/docs/fy01osti/28893.pdf.
[NREL] National Renewable Energy Laboratory. 2007a. Research Advances Cellulosic Ethanol, NREL Leads the Way. NREL/BR-510-40742. Available at www.nrel.gov/docs/fy07osti/40742.pdf.
[NREL] National Renewable Energy Laboratory. 2007b. Thermochemical Ethanol via Indirect Gasification and Mixed Alcohol Synthesis of Lignocellulosic Biomass. Technical Report NREL/TP-510-41168. Available at http://www.nrel.gov/docs/fy07osti/41168.pdf.
[NREL] National Renewable Energy Laboratory. 2008. An Experimental and Modeling Study of the Flammability of Fuel Tank Headspace Vapors from High Ethanol Content Fuels. Subcontract Report NREL/SR-540-44040. Available at: www.nrel.gov/docs/fy09osti/44040.pdf.
[NREL] National Renewable Energy Laboratory. 2009. What Is a Biorefinery? Available at www.nrel.gov/biomass/biorefinery.html.
[NREL] National Renewable Energy Laboratory. 2010. Techno-Economic Analysis of Biochemical Scenarios for production of Cellulosic Ethanol. Technical Report NREL/TP-6A2-46588. Available at www.nrel.gov/docs/fy10osti/46588.pdf.
[ORNL] Oak Ridge National Laboratory. 2010. Biomass Energy Data Book, 3rd Edition. Available at http://info.ornl.gov/sites/publications/files/Pub28524.pdf.
[OSHA] Occupational Safety & Health Administration. Date unknown. Integrated Management Information System (IMIS). Available at www.osha.gov/pls/imis/accidentsearch.html.
[OSHA] Occupational Safety & Health Administration. Date unknown. Flammable Liquids. 29 CFR 1910.106. Available at www.osha.gov/dte/library/flammable_liquids/flammable_liquids.html.
[OSHA] Occupational Safety & Health Administration. Date unknown. Safety and Health Management eTools: Hazard Prevention and Control. Available at www.osha.gov/SLTC/etools/safetyhealth/comp3.html.
[OSHA] Occupational Safety & Health Administration. 2015. Process Safety Management of Highly Hazardous Chemicals–Compliance Guidelines and Enforcement Procedures. CPL 2-2.45A CH-1. Available at www.osha.gov/enforcement/directives/cpl-2-245a-ch-1.
[OSHA] Occupational Safety & Health Administration. 1993. Preamble to Final Rule, Permit Required Confined Spaces standard, Section II, Hazards. Available at www.osha.gov/laws-regs/federalregister/1993-01-14.
[OSHA] Occupational Safety & Health Administration. 1996. Inspection of Grain Handling Facilities. CPL 02-01-004. Available at www.osha.gov/enforcement/directives/cpl-02-01-004.
[OSHA] Occupational Safety & Health Administration. 1997. Coverage of Stored Flammables Under the Process Safety Management Standard. Available at www.osha.gov/laws-regs/standardinterpretations/1997-05-12-1.
[OSHA] Occupational Safety & Health Administration. 1998. CFR 1910.146. Permit Required Confined Spaces. Available at www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.146.
[OSHA]Occupational Safety & Health Administration. 1999. OSHA Technical Manual (OTM), Ventilation Investigation, Section III, Chapter 3. Available at www.osha.gov/otm/section-3-health-hazards/chapter-3.
[OSHA] Occupational Safety & Health Administration. 2000. Process Safety Management Publication #3132. Available at www.osha.gov/Publications/osha3132.html.
[OSHA] Occupational Safety & Health Administration. 2002. Exit Routes, Emergency Action Plans, and Fire Prevention Plans; Final Rule. 7 November. Available at https://www.osha.gov/laws-regs/federalregister/2002-11-07-0.
[OSHA] Occupational Safety & Health Administration. 2002a. Lockout Tagout. OSHA Fact Sheet. Available at www.osha.gov/OshDoc/data_General_Facts/factsheet-lockout-tagout.pdf.
[OSHA] Occupational Safety & Health Administration. 2003. Safety and Health Topics: Ammonia. Available at www.osha.gov/dts/chemicalsampling/data/CH_218300.html.
[OSHA] Occupational Safety & Health Administration. 2005. Combustible Dust in Industry: Preventing and Mitigating the Effects of Fires and Explosions. Safety and Health Information Bulletin; 31 July. Available at www.osha.gov/dts/shib/shib073105.html.
[OSHA] Occupational Safety & Health Administration. 2007. Inspection Procedures for 29 CFR 1910.120 and 1926.65, Paragraph (q): Emergency Response to Hazardous Substance Releases, CPL 02-02-073. Available at www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=DIRECTIVES&p_id=3671.
[OSHA] Occupational Safety & Health Administration. 2008a. Hazard Alert: Combustible Dust Explosions. OSHA Fact Sheet. Available at www.osha.gov/Publications/OSHA3878.pdf.
[OSHA] Occupational Safety & Health Administration. 2008b. Combustible Dust National Emphasis Program. Available at www.osha.gov/enforcement/directives/cpl-03-00-008.
[OSHA] Occupational Safety & Health Administration. 2009a. Part 1910: Combustible Dust, Proposed Rule. Docket No. OSHA-2009-0023. Federal Register: October 21, 2009, Volume 74, Number 202. Available at https://www.osha.gov/laws-regs/federalregister/2009-10-21.
[OSHA] Occupational Safety & Health Administration. 2009b. Hazard Communication Guidance for Combustible Dusts. OSHA 3371-08. Available at www.osha.gov/Publications/3371combustible-dust.html.
[OSHA] Occupational Safety & Health Administration. 2009c. Combustible Dust; Advance Notice of Proposed Rulemaking, 74:54333-54347. Available at https://www.osha.gov/laws-regs/federalregister/2009-10-21
[OSHA] Occupational Safety & Health Administration. 2010. Worker Entry into Grain Storage Bins. OSHA Fact Sheet. Available at www.osha.gov/Publications/grainstorageFACTSHEET.pdf.
[OSHA]Occupational Safety & Health Administration. 2011a. OSHA Safety and Health Topics Page: Grain Handling. Available at www.osha.gov/grain-handling.
[OSHA] Occupational Safety & Health Administration. 2011b. Letter from David Michaels, Assistant Secretary of Labor, OSHA, to Grain Storage Facility Operators. 1 February. Available at www.osha.gov/memos/2011-02-01/grain-storage-bins.
[OSHA] Occupational Safety & Health Administration. 2011c. US District Court Upholds OSHA Subpoena in Grain Engulfment Case. National News Release 11-706-CHI; 12 May. Available at https://www.osha.gov/news/newsreleases/national/05122011.
[OSHA] Occupational Safety & Health Administration. 2011d. OSHA Safety and Health Topics Page: Control of Hazardous Energy Lockout/Tagout. Available at www.osha.gov/control-hazardous-energy.
[OSHA] Occupational Safety & Health Administration. 2011e. Youth in Agriculture: Confined Spaces. Available at www.osha.gov/SLTC/youth/agriculture/confinedspaces.html.
[OSHA] Occupational Safety & Health Administration. 2011f. Hazard Alert: Dangers of Engulfment and Suffocation in Grain Bins. Last updated August 11, 2011. Available at www.osha.gov/Publications/hazard-alert_grain_bins.pdf.
[OSHA] Occupational Safety & Health Administration. 2011g. PSM Covered Chemical Facilities National Emphasis Program CPL 03-00-021. Available at www.osha.gov/enforcement/directives/cpl-03-00-021.
[OSHA] Occupational Safety & Health Administration. 2012. Hazard Communication Standard, Appendix B. Available at www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.1200AppB.
[OSHA] Occupational Safety & Health Administration. 2013. Hazard Communication Page. Available at www.osha.gov/hazcom.
[OSHA] Occupational Safety & Health Administration. 2013a. OSHA Quick Card: Comparison of NFPA 704 and HazCom 2012 Labels. Available at www.osha.gov/Publications/OSHA3678.pdf.
[OSHA] Occupational Safety & Health Administration. 2013b. OSHA Safety and Health Topics Page: Hexavalent Chromium. Available at www.osha.gov/hexavalent-chromium.
[OSHA/EPA] Occupational Safety & Health Administration/U.S. Environmental Protection Agency. 2011. OSHA/EPA Occupational Chemical Database. Available at www.osha.gov/chemicaldata/.
[OSHA] Occupational Safety & Health Administration. 2014. Permissible Exposure Limits Annotated Tables. Available at: www.osha.gov/annotated-pels.
Pahwa, P., H.H. McDuffie, and J.A. Dosman. 2006. Longitudinal Changes in Prevalence of Respiratory Symptoms Among Canadian Grain Elevator Workers. Chest 129:1605-1613. Available at http://pubmed.ncbi.nlm.nih.gov/16778282.
Perry, R.H., D. Green. 1984. Perry's Chemical Engineers' Handbook, Sixth Edition.
Porter, D.O. 2010. Handling and Storage of Grain and Silage. Hazard Alert! Morgantown, WV: Extension Service, West Virginia University, Agriculture and Natural Resources. Available at anr.ext.wvu.edu/farm_management/handling_and_storage_of_grain_and_silage.
Potts, D.L. 2008. A Guide to Emergency Action Planning. Industry Guide 40. Prepared for N.C. Department of Labor. Available at www.nclabor.com/osha/etta/indguide/ig40.pdf.
Powell, J. 2003. After the Blast; Dollars Can't Measure the Damage in the Deadly Oct. 22 Explosion at a Benson, Minn., Ethanol Plant. Star Tribune (Minneapolis, MN); 6 November. Available at https://www.highbeam.com/doc/1G1-109866006.html.
Purdue University. 2011. Frequently Asked Questions About Flowing Grain Entrapment, Grain Rescue and Strategies, and Grain Entrapment Prevention Measures. Available at https://www.extension.entm.purdue.edu/grainlab/content/pdf/QuestionFlowingGrainEntrap.pdf.
Ramirez, E.C., D.B. Johnston, A. J. McAloon, Y. Winnie, V. Singh. 2008. Engineering Process and Cost Model for a Conventional Corn Wet Milling Facility, Industrial Crops and Products 2008; 27, 91-97. Available at: https://naldc.nal.usda.gov.
Rausch, K.D., Belyea, R.L., Ellersieck, M.R., Singh, V., Johnston, D.B., and Tumbleson. M.E., 2005. Particle Size Distributions of Ground Corn and DDGS from Dry Grind Processing. Transactions of the American Society of Agricultural Engineers 48(1):273-277. Available at https://naldc.nal.usda.gov/naldc/home.xhtml.
[RFA] Renewable Fuels Association. Date unknown. The Complete Training Guide to Ethanol Emergency Response. Available at https://ethanolrfa.org/producers/safety/.
[RFA] Renewable Fuels Association. 2007. Ethanol Production: A Safety Perspective. Available at ethanolrfa.3cdn.net/f2515a419d1f99f3cc_21m6iigrb.pdf.
[RFA] Renewable Fuels Association. 2011a. Building Bridges to a More Sustainable Future: 2011 Ethanol Industry Outlook. Available at https://ethanolrfa.org/pages/annual-industry-outlook.
[RFA] Renewable Fuels Association. 2011b. How Ethanol Is Made. Available at https://ethanolrfa.org/how-ethanol-is-made/.
[RFA] Renewable Fuels Association. 2011c. Cellulosic Ethanol. Available at https://ethanolrfa.org/.
[RFA] Renewable Fuels Association. 2014. Building Bridges to a More Sustainable Future: 2014 Ethanol Industry Outlook. Available at https://ethanolrfa.org/pages/annual-industry-outlook.
Riedel, S. and Field, B. 2011. 2010 Summary of Grain Entrapments in the United States. West Lafayette, IN: Agricultural Safety and Health Program, Purdue University. Available at https://extension.entm.purdue.edu/grainlab/content/pdf/2010GrainEntrapments.pdf.
Sanders, R.E. 2005. Chemical Process Safety: Learning from Case Histories. Third edition. Oxford, UK: Elsevier Inc. 342 pp.
Shaw. 2011. Large Volume Ethanol Spills – Environmental Impacts and Response Options. Prepared for Massachusetts Department of Environmental Protection. Available at www.mass.gov/eopss/docs/dfs/emergencyresponse/special-ops/ethanol-spill-impacts-and-response-7-11.pdf.
Tomas, J-C., and Field, A. 2004. Workers Flee Ethanol Explosion. The Sydney Morning Herald; 29 January. Available at www.smh.com.au/articles/2004/01/28/1075088091566.html.
[USCSB] United States Chemical Safety and Hazard Investigation Board. Completed Investigations. Available at www.csb.gov/investigations/completed-investigations/.
[USCSB] United States Chemical Safety and Hazard Investigation Board. 2007. Universal Form Clamp Co. Explosion and Fire, Bellwood, Illinois (Mixing and Heating a Flammable Liquid in an Open Top Tank). Investigation No. 2006-08-I-IL. Available at www.csb.gov/investigations.
[USCSB] United States Chemical Safety and Hazard Investigation Board. 2009. Imperial Sugar Company Dust Explosion and Fire, Port Wentworth, GA (Sugar Dust Explosion and Fire). Report No. 2008-05-I-GA. Available at www.csb.gov/investigations/.
[USCSB] United States Chemical Safety and Hazard Investigation Board. 2010. Seven Key Lessons to Prevent Worker Deaths During Hot Work In and Around Tanks: Effective Hazard Assessment and Use of Combustible Gas Monitoring Will Save Lives. Safety Bulletin No. 2009-01-SB; February. Available at http://www.csb.gov/.
[USDA] United States Department of Agriculture. 2006. The Economic Feasibility of Ethanol Production from Sugar in the United States. Available at https://www.fsa.usda.gov/Internet/FSA_File/ethanol_fromsugar_july06.pdf.
[USDA] United States Department of Agriculture. 2012. Ethanol Strengthens the Link Between Agriculture and Energy Markets. Available at www.ers.usda.gov/amber-waves/2012-june/ethanol-strengthens-the-link.aspx Accessed on April 2, 2014.
[USDA] United States Department of Agriculture. 2013. Agriculture's Supply and Demand for Energy and Energy Products. Economic Information Bulletin #112. Available at www.ers.usda.gov/publications/eib-economic-information-bulletin/eib112.aspx.
[USDA] United States Department of Agriculture. 2013a. Grain Inspection Handbook, Book II, Chapter 4. Available at www.usda.gov/.
[USDA] United States Department of Agriculture. 2013b. Map: Production by County and Location of Ethanol Plants. Available at www.nass.usda.gov/Charts_and_Maps/Ethanol_Plants/U._S._Ethanol_Plants/index. Accessed on May 22, 2014.
Weston Solutions, Inc. 2008. Ethanol Manufacturing Facility Response Overview. Revision no. 2. Prepared for United States Environmental Protection Agency, Region 5. Available at www.aneep.org/pdf/r5_ethanol_response_manual.pdf.
Wyman, C.E. 1999. Biomass Ethanol: Technical Progress, Opportunities, and Commercial Challenges. Annual Review of Energy and the Environment 24:189-226. Available at www.cert.ucr.edu/research/ses/wymanpublications/Biomass%20Ethanol%20Technical.pdf.
Glossary
- Administrative controls:
- Policies, operating procedures, training programs, safe work practices, maintenance campaigns, and other actions taken to prevent or mitigate workplace hazards.
- Alpha-amylase:
- An enzyme that breaks corn starch down into simpler carbohydrates called dextrins. The enzyme is typically added to corn and water during the liquefaction step of the corn dry and wet milling processes.
- Ammonia fiber explosion (AFEX):
- A pre-treatment process used at facilities that biochemically convert cellulosic material into ethanol. The process involves mixing the feedstock (typically grasses) with liquid ammonia under high pressure, which prepares cellulose and hemicelluloses for further processing.
- Azeotrope:
- A liquid mixture that exhibits a maximum or minimum boiling point relative to the boiling points of surrounding mixture compositions. Boiling points of the pure components in the mixture must be sufficiently close to permit formation of an azeotrope. A mixture of close-boiling components may form an azeotrope when only small deviations from ideal liquid solutions occur (Perry, 1984).
- Beer:
- A mixture of yeast, bran, gluten, and liquids (including ethanol) that forms during the fermentation step of the corn dry- and wet- milling processes. The beer is pumped to the distillation system to separate ethanol from the other constituents.
- Bioethanol:
- Ethanol that is made by fermenting plant sugars and starches, such as those found in corn, wheat, and wood residues. This includes ethanol manufactured at corn dry-milling, corn wet-milling, and biochemical conversion facilities.
- Bran:
- The high-fiber outer skin of a corn kernel. It is often milled and then mixed with other components of corn kernels to form various animal feed products.
- Bucket elevator:
- Sometimes called a "grain leg," a system designed for moving grains and other materials vertically. The elevator is usually enclosed and consists of a series of buckets attached to a continuous, rotating belt. The buckets scoop up material at the bottom of the elevator and then empty the material at the top, typically into a silo or some other storage vessel.
- Cellulase:
- An enzyme used to break down cellulose into glucose. Ethanol manufacturing facilities either purchase cellulase from commercial suppliers or produce and harvest the enzyme on site.
- Cellulose:
- The primary structural component of plant cell walls. The fibrous material is the most common organic compound on earth and is composed of long chains of linked sugar molecules. The long, rigid chains in cellulose must be broken down into simpler molecules before being used to manufacture ethanol.
- Cellulosic ethanol:
- Ethanol that is produced through the processing of cellulose, a complex sugar. Cellulosic ethanol can be produced by two general production technologies. In biochemical conversion, the cellulose is broken down into fermentable sugars, which are converted to ethanol by enzymes. In thermochemical conversion, the cellulose passes through a gasification process that forms syngas, which can then be converted to ethanol.
- Cellulosic feedstock:
- The input material used in the production of cellulosic ethanol. The fibrous plant matter can be found in nearly all nonedible plant material. Examples include agricultural residue (such as corn stover), grasses, forestry biomass, and municipal solid waste.
- Chaff:
- The agricultural residue left in fields following corn harvesting. Most chaff is separated from corn kernels directly in the fields during harvesting. The remainder is separated from corn kernels at ethanol manufacturing facilities during a cleaning step (see definition for scalper and screener below).
- Char:
- A solid carbonaceous by-product formed at thermochemical conversion facilities when cellulosic feedstocks are gasified at very high temperatures. Char can be burned for process heat and used as a soil amendment.
- Cheese whey:
- A by-product of cheese manufacturing that has traditionally been considered a waste product. The material, rich in fermentable sugars (particularly lactose), can be used as a feedstock for ethanol manufacturing.
- Combustible dust:
- A combustible particulate solid that presents a fire or deflagration hazard when suspended in air or some other oxidizing medium over a range of concentrations, regardless of particle size or shape (OSHA 2008b).
- Complex sugar:
- A large carbohydrate molecule in which multiple simple sugar molecules are linked in long chains. At ethanol manufacturing facilities, complex sugars are typically broken down into simple fermentable sugars, which are then used to make ethanol.
- Condensed distillers' solubles:
- A syrup-like intermediate product formed during corn dry-milling co-product processing. The syrup is typically either sold as a by-product or mixed with other materials to form wet distillers' grain.
- Confined space:
- Means a space that (29 CFR 1910.146(b)): (1) Is large enough and so configured that an employee can bodily enter and perform assigned work; and (2) Has limited or restricted means for entry or exit (for example, tanks, vessels, silos, storage bins, hoppers, vaults, and pits are spaces that may have limited means of entry.); and (3) Is not designed for continuous employee occupancy. Silos, process vessels, feed hoppers, and grain storage bins are all examples of confined spaces at ethanol manufacturing facilities.
- Corn dry-milling:
- One of the two main processes of manufacturing ethanol from corn. In corn dry-milling whole corn kernels are milled into fine flour prior to liquefaction and other processing steps. In the United States, corn dry-milling facilities are more common than corn wet-milling facilities.
- Corn gluten feed (CGF):
- A co-product formed at corn wet-milling facilities. The material, a mixture consisting of steep liquor and dried fiber, is sold in wet or dried form to farmers as a feed product for cattle, swine, and poultry.
- Corn gluten meal:
- A co-product formed at corn wet-milling facilities that is derived from extracted gluten. The dried, condensed material is sold as a protein-rich feed product typically used in poultry formulations.
- Corn stover:
- The agricultural residue left over in the field from the harvesting of corn kernels. Once considered a waste product, the cellulose-rich stalks and leaves can be used as cellulosic feedstock.
- Corn wet-milling:
- One of the two main processes of manufacturing ethanol from corn. In corn wet-milling, whole corn kernels are first steeped before being milled, which allows for separation of corn into its individual components (e.g., fiber, gluten, germ starch) before fermentation occurs, thus enabling corn wet-milling facilities to produce many different co-products that cannot be produced at corn dry-milling facilities. In the United States, corn wet-milling facilities are less common than corn dry-milling facilities.
- Deflagration:
- Propagating combustion that occurs at subsonic speeds (compared to detonation, which occurs at supersonic speeds). When deflagrations take place in confined areas, explosions can result.
- Denaturing:
- The final step in ethanol manufacturing prior to product distribution. Denaturing is the deliberate addition of ingredients to ethanol to make the product unusable for human consumption (and therefore exempt from the U.S. beverage tax). Gasoline is one of the most common denaturants used at ethanol manufacturing facilities.
- Dextrins:
- An intermediate product formed during the breakdown of starch and complex sugars into simple sugars. Certain enzymes break starch and complex sugars into shorter-chain dextrins, and other enzymes then further break dextrin into simple, fermentable sugars.
- Distillation:
- A very common unit operation at chemical manufacturing facilities that separates liquid mixtures. At ethanol manufacturing facilities, a series of distillation columns are used to separate ethanol from other components in the beer mixture.
- Dried distillers' grain with solubles (DDGS):
- A co-product formed in the corn dry-milling process, typically containing less than 10 percent moisture. High in protein, DDGS is a high-quality animal feed product.
- Endosperm:
- The starch- and gluten-rich matter comprising the majority of a corn kernel's volume. The starch in the endosperm is the source of fermentable sugars for manufacturing ethanol.
- Energy crops:
- Any crop grown specifically for their fuel value. These include crops with high starch content (e.g., corn, sugar cane) and plants, grasses, and trees used as cellulosic feedstocks. These plants are typically fast growing, cellulose-rich, and capable of flourishing on marginal lands that cannot support food crop production.
- Engineering controls:
- Permanent features built into facilities or production processes to automatically eliminate or mitigate hazards. Primary engineering controls prevent hazards from ever occurring, and secondary engineering controls minimize damage after events occur.
- Engulfment:
- The surrounding and effective capture of a person by a liquid or finely divided (flowable) solid substance that can be aspirated to cause death by filling or plugging the respiratory system or that can exert enough force on the body to cause death by strangulation, constriction, or crushing. Grain engulfments are very serious hazards because engulfed persons will asphyxiate if not rescued within a matter of minutes.
- Entrapment:
- The partial submersion of a person in a granular substance. Grain entrapment occurs when individuals are trapped in grain and cannot extricate themselves on their own. Entrapment can lead to engulfment.
- Enzyme:
- A protein that catalyzes (i.e., increases the rate) of a chemical reaction. Enzymes important to ethanol manufacturing include alpha-amylase, glucoamylase, and cellulase, all of which help break complex sugars into simple, fermentable sugars.
- Explosive range:
- A substance-specific property indicating the range of concentration in air that can result in an explosion when an ignition source is present. See also flammable range.
- Fermentation:
- A biological process in which yeast converts sugar molecules into ethanol and carbon dioxide. Complex sugars typically must be broken down into simple (fermentable) sugars before this process can take place.
- Fermentation inhibitors:
- Any substance that inhibits the fermentation process. In the context of ethanol manufacturing, fermentation inhibitors are of greatest concern in biochemical conversion of cellulosic feedstock, because certain pre-treatment steps are known to form fermentation inhibitors (e.g., weak acids, phenolic compounds).
- Five-carbon sugars:
- A simple sugar containing five carbons, such as xylose and arabinose. Commonly found after certain cellulosic feedstocks are hydrolyzed, five-carbon sugars present a challenge in ethanol manufacturing because the yeast most widely used for industrial fermentation is not able to metabolize such sugars. Other enzymes active on five-carbon sugars are available.
- Fixed-bed reactor:
- A reactor in which catalysts are fixed in place and do not move through the reactor mixture. Fixed bed reactors are one of two unit operations used in the gasification of certain cellulosic feedstocks. See also fluidized-bed reactor.
- Flammable range:
- A substance-specific property indicating the range of concentration in air that can result in a fire when an ignition source is present. See also explosive range.
- Flash point:
- The lowest temperature at which a particular substance can vaporize to create an ignitable hazard in air.
- Fluidized-bed reactor
- A reactor in which catalysts are suspended in the reactor mixture. Fluidized bed reactors are one of two unit operations used in the gasification of certain cellulosic feedstocks. See also fixed-bed reactor.
- Gasification:
- A high temperature process that thermally decomposes cellulosic feedstock into syngas and selected by-products (e.g., char). Gasification is an important step in thermochemical conversion of cellulosic material into ethanol.
- Germ:
- The oil-rich embryo of a corn seed, located inside the endosperm. Corn wet-milling facilities separate and process the germ as a co-product, which can be used as a food ingredient or as a feedstock for biodiesel manufacturing.
- Glucoamylase:
- An enzyme used to break dextrins down into glucose. This reaction is important, because glucose is readily converted to ethanol by the yeast most widely used for industrial fermentation.
- Glucose:
- A simple sugar that serves as the building block for more complex sugars such as dextrins and starch. When fermented by yeast, glucose forms ethanol and carbon dioxide.
- Gluten:
- A protein-rich component of the endosperm of a corn kernel. Gluten is a major component of several animal feed co-products manufactured at corn milling facilities.
- Grain elevator:
- This term has multiple meanings. First, a grain elevator is a facility engaged in the receipt, handling, storage, and shipment of bulk raw agricultural commodities such as corn, wheat, oats, barley, sunflower seeds, and soybeans. Second, grain elevator is also used to describe a specific component of these facilities: the tall structure containing a bucket elevator used to lift grain and distribute it among storage silos.
- Grain sorghum (milo):
- A type of feedstock that can be used for starch-based ethanol production. The maize-like grass is more commonly used as a human food source or livestock feed.
- Hammer mill:
- A size-reduction unit operation commonly used in industry to grind or crush solid materials. At corn dry-milling facilities, hammer mills crush corn into fine corn flour that is better suited for further processing.
- Hemicellulose:
- A long-chain molecule consisting of various five-carbon and six-carbon sugars. Hemicellulose binds to cellulose and is found in all cellulosic feedstocks. Hemicellulose readily hydrolyzes into simple sugars, but the resulting sugars are less easy to ferment than glucose.
- Hydrocyclone (also referred to as "hydroclones"):
- A device used at corn wet-milling facilities to separate the germ from other constituents (mixture of fiber, starch and gluten) in corn kernels.
- Hydrolysis:
- A type of chemical reaction involving water molecules. In the context of bioethanol, hydrolysis reactions help break complex sugars and cellulose into smaller molecules that are more suitable for further processing.
- Hydrolyzate:
- A general term used to describe products of hydrolysis. In the context of ethanol manufacturing, hydrolyzate refers to the slurry stream formed in the biochemical conversion of cellulosic feedstocks.
- Ignition source:
- A process or event that can ignite a material and trigger a fire or explosion. Sources range from open flames and excessive heat to static electricity, sparks, friction, electric arcs, and welding activities.
- Impact mill:
- A size-reduction unit operation commonly used in industry to grind or crush solid materials. At corn dry- milling facilities, hammer mills pulverize whole corn kernels into fine corn flour that is more suitable for further processing.
- Integrated biorefinery:
- A facility that uses all feedstock components, whether for manufacturing ethanol and co-products or for energy recovery purposes. By using the entire feedstock (including components that do not form ethanol), integrated biorefineries can reduce waste and improve energy and production efficiency. Corn wet-milling facilities are typically integrated biorefineries.
- Intrinsically safe:
- Typically refers to electrical equipment that is designed for the specific hazardous (classified) location, such that it cannot produce sparks or other electrostatic hazards.
- Lignin:
- A structural material in cellulosic feedstocks that surrounds cellulose and hemicellulose fibers. Because it is not sugar-based, lignin must be removed in pretreatment operations prior to fermentation steps.
- Liquefaction:
- A corn dry-milling production step in which fine corn flour is mixed with water and other chemicals in large tanks. An enzyme is added to break starches down into dextrins.
- Mash:
- A slurry-like, intermediate product in the corn dry-milling process. Mash is a yellow, watery mixture that contains corn solubles and insolubles. Complex sugars in the corn have begun to break down in the mash, but further processing is needed before fermentation can begin.
- Mill starch:
- An intermediate product formed during corn wet-milling that is composed primarily of starch and gluten. The mill starch is centrifuged and then rinsed multiple times in water and hydroclones to fully separate the gluten from the starch.
- Molecular sieve:
- A unit operation that essentially operates as a filter at the molecular level. At ethanol manufacturing facilities, molecular sieves are needed to remove the final traces of water from the ethanol product stream.
- Neutralization:
- A unit operation used in biochemical conversion of cellulosic feedstock to ethanol. The neutralization step removes or inactivates fermentation inhibitors that may have formed during the initial pretreatment steps. Neutralization methods may include conditioning through "over liming" and detoxification through the use of ion-exchange columns.
- Noncombustible:
- A material that cannot be ignited. Process equipment constructed from noncombustible material is particularly important in workplace areas with combustible dust hazards.
- Permissible Exposure Limit (PEL):
- OSHA sets enforceable permissible exposure limits (PELs) to protect workers against the health effects of exposure to hazardous substances. PELs are regulatory limits on the amount or concentration of a substance in the air. They may also contain a skin designation. OSHA PELs are based on an 8-hour time-weighted average (TWA) exposure. Permissible exposure limits (PELs) are addressed in specific standards for the general industry, shipyard employment, and the construction industry.
- Pretreatment:
- Various chemical, physical, and biological processes used to prepare cellulosic feedstocks for subsequent processing. The primary goals of pretreatment are to disrupt lignin and to begin breaking down hemicelluloses, such that enzymes added later in the process can break down cellulose.
- Primary explosion:
- An explosion following the initial ignition of a combustible material in a confined space. Primary explosions can cause settled combustible dusts to become airborne, a very dangerous situation that can trigger additional secondary explosions.
- Recombinant:
- A type of DNA engineered by scientists to synthesize desired characteristics from different organisms. Scientists are experimenting with recombinant forms of yeast in hopes of developing strains more effective at fermenting mixed sugars. Some engineered strains are already being used on the commercial scale at ethanol manufacturing facilities.
- Process:
- Process means any activity involving a highly hazardous chemical including any use, storage, manufacturing, handling, or the on-site movement of such chemicals, or combination of these activities. For purposes of this definition, any group of vessels which are interconnected and separate vessels which are located such that a highly hazardous chemical could be involved in a potential release shall be considered a single process.
- Saccharification:
- A production step during corn dry-milling that uses glucoamylase to break dextrins down into glucose, a fermentable sugar.
- Scalper and Screener:
- Unit operations commonly used to "clean" incoming corn kernels. Foreign materials are removed via particle-size criterion. Large materials are removed by passing the corn stream through a series of screens with opening sizes just large enough to allow kernels to pass. Corn kernels are then passed over screens with fine openings through which smaller foreign materials separate from the corn.
- Secondary explosion:
- An explosion, or series of explosions, that is caused when a primary explosion lofts settled combustible dusts into the air. Secondary explosions have the potential to be even more destructive than primary explosions, because larger quantities of dusts may be involved.
- Shelled corn:
- The name used to describe complete, intact corn kernels. Shelled corn looks like popcorn kernels before they are cooked.
- Silo:
- Typically a tall tower-like structure used for grain storage. Materials are input at the top and removed at the bottom to ensure sufficient product rotation.
- Simple sugars:
- The most basic form of sugar. A single carbohydrate molecule that typically contains five or six carbons. Simple sugars are formed when more complex sugars (e.g., cellulose, starch, dextrins) are broken apart. Fermentation can convert most simple sugars into ethanol and carbon dioxide.
- Six-carbon sugars:
- A simple sugar that has six carbons in its molecular structure. Glucose is the most common six-carbon sugar, and yeast fermentation readily transforms glucose into ethanol and carbon dioxide. Other six-carbon sugars (e.g., mannose, galactose) require different fermentation organisms for ethanol production.
- Starch:
- A long chain of glucose molecules that plants use to store energy. Materials rich in starch (e.g., corn) are good candidates for ethanol manufacturing feedstocks.
- Steeping:
- A unit operation in the corn wet-milling process in which corn soaks in tanks of warm water mixed with sulfur dioxide for 24 to 48 hours. This process causes starch molecules in the corn to begin to separate from the gluten.
- Stillage:
- The material separated from ethanol and water during distillation. At corn milling facilities, stillage is usually dried and mixed with other materials to form various co-products, such as wet distillers' grain and dried distillers' grain with solubles. Stillage at cellulosic ethanol manufacturing facilities is typically used (i.e., burned) for purposes of energy recovery.
- Sugarcane bagasse:
- Residual fibrous material after sugarcane has been processed for its juice. Similar to corn stover, the leftover sugarcane stalks are rich in cellulosic biomass and are a valuable cellulosic feedstock.
- Syngas:
- A gaseous mixture consisting primarily of carbon monoxide and hydrogen gas, but also containing numerous by-products (e.g., water, char, and other condensibles). Syngas is formed after cellulosic feedstocks thermally decompose inside high temperature fixed bed reactors or fluidized bed reactors. Metal catalysts can then convert syngas into ethanol and other products.
- Synthetic ethanol:
- Ethanol that is manufactured from ethylene, a by-product of petrochemical processing. Synthetic ethanol is predominantly used in the industrial sector and represents a minor market share in nationwide ethanol production.
- Tar:
- In the context of ethanol manufacturing, tar is a by-product of heavy hydrocarbons formed during high temperature gasification of cellulosic feedstocks. The tar constituents must be removed from syngas before the syngas is formed into ethanol and other by-products.
- Thermochemical processing:
- A method used for converting cellulosic feedstocks into ethanol. The distinguishing step in thermochemical processing is the thermal decomposition of cellulosic material in high temperature gasification reactors. The syngas that is formed can then be converted into ethanol via catalysis or fermentation.
- Walking down the grain:
- The act of individuals using their own body weight to dislodge and release grain stuck on sides and surfaces of grain storage vessels. OSHA prohibits this practice, because workers who "walk down the grain" risk becoming engulfed in the grain.
- Wet distillers' grain:
- A co-product typically produced at corn dry-milling facilities. Wet distillers' grain is an animal feed product rich in fiber and protein. Due to its high moisture content, it must be transferred to customers within several days of manufacture to avoid spoilage. Wet distillers' grain can be dried to form another co-product (dried distillers' grain with solubles).
1 Flash fire – A fire that spreads by means of a flame front rapidly through a diffuse fuel, such as dust, gas or the vapors of an ignitable liquid without the production of damaging pressure (NFPA 654).
2 Deflagration – Is the propagation of a combustion zone at a velocity that is less than the speed of sound in the unreacted medium (NFPA 654).
3 Explosion – Is the bursting or rupture of an enclosure or container due to the development of an internal pressure from a deflagration (NFPA 654).
4 OSHA's Hazard Communication standard was revised in 2012. Safety Data Sheets (SDSs) will replace MSDSs. SDSs have a standardized 16-section format with specific information required in each section. Manufacturers and importers have until June 1, 2015 to replace MSDSs with SDSs; until then either MSDSs or SDSs may be received by employers.